Abstract
Is morphology a discrete and independent element of lexical structure or does it simply reflect a fine-tuning of the system to the statistical correlation that exists among orthographic and semantic properties of words? Hebrew provides a unique opportunity to examine morphological processing in the brain because of its rich morphological system. In an fMRI masked priming experiment we investigated the neural networks involved in implicit morphological processing in Hebrew. In the lMFG and lIFG, activation was found to be significantly reduced when the primes were morphologically related to the targets. This effect was not influenced by the semantic transparency of the morphological prime, and was not found in the semantic or orthographic condition. Additional morphologically related decrease in activation was found in the lIPL although there, activation was significantly modulated by semantic transparency. Our findings regarding implicit morphological processing suggest that morphology is an automatic and distinct aspect of visually processing words. These results also coincide with the behavioral data previously obtained demonstrating the central role of morphological processing in reading Hebrew.
Introduction
Morphology concerns the internal structure of words and is reflected by systematic correlations of form (orthography, phonology) and meaning (semantics). Models of lexical organization and lexical processing provide different answers to the question of whether morphology should be regarded as a distinct level of lexical architecture. The Parallel-Distributed Processing (PDP) tripartite view of the mental lexicon, for example, focuses on patterns of activation over processing units that correspond to the orthographic, phonological, and semantic sublexical features of a word (e.g., Seidenberg & McClelland, 1989). Thus, the PDP approach argues that there is no level of explicit and discrete representation that corresponds to morphological units (Rueckl et al., 1997). Morphological effects, according to this view, reflect a fine-tuning of the reader or speaker to the correlations that exist between the phonological, orthographic and semantic properties of words (e.g., Plaut & Gonnerman, 2000). In contrast, the traditional ”localist” representational framework typically assumes that morphemic units are explicitly represented in the mental lexicon and are involved in the processing of print (see Taft, 1994 for a discussion).
Extensive research has been conducted to examine the role of morphology in lexical structure and to determine the question of the independence of morphological processing. A wide array of behavioral experimental paradigms such as cross-modal priming (e.g., Marslen-Wilson et al., 1994), repetition priming at various lags (e.g., Bentin & Feldman, 1990), masked-priming (e.g., Rastle & Davis, 2003), segment-shifting (e.g., Feldman et al., 1995), monitoring of eye-movements (e.g., Deutsch et al., 2003) and letter transpositions (Velan & Frost, 2007) have shown that effects of morphological structure cannot simply be reduced to shared form or shared meaning.
Recently, attempts to address this psycholinguistic question have expanded from behavioral research to neuro-imaging as well. This approach typically engages subjects in explicit or implicit morphological processing and searches for distinct patterns and sites of activation that do not overlap with those involved in orthographic, phonological, or semantic processing. The results, however, do not present a clear and unequivocal picture (for a review see: Marslen-Wilson & Tyler, 2007). Devlin et al. (2004) used masked priming in English and did not detect evidence of morphological processing that could not be explained by the joint effects of semantics and orthography. In contrast, Bozic et al., (2007) and Gold and Rastle (2007) did find sites of independent activation for morphological processing in English, in both masked and long-term priming. These two studies, however, differ in the extent and location of morphological activation found, which creates difficulties in interpreting their results. Several event-related potential (ERP) studies have investigated the relation between morphological processing and semantics, but have again reported contradicting results regarding the influence of semantics on morphological effects (Koester & Schiller, 2008; Lavric et al., 2007; Morris et al., 2007).
The question of whether morphological effects can or cannot be reduced to the correlation of orthography, phonology, and meaning may be language-dependent. In general, it is well accepted that some aspects of language processing are universal. However, languages do differ in their morphological structure, and significant differences in morphological effects have been found between languages. These findings suggest a graded sensitivity to morphological manipulations in languages with a rich morphological system as opposed to languages with an impoverished one (Frost, in press; Frost et al., 2005a).
Within this domain, research in Hebrew is particularly interesting because of the language’s morphological depth. Hebrew is a Semitic language characterized by a rich and systematic morphology. Most Hebrew words are morphologically complex, as they are composed of two abstract morphemes: the root and the word-pattern. Roots in most cases consist of three consonants, and they convey the core meaning of the word. Word-patterns can be either a sequence of vowels or a sequence consisting of both vowels and consonants, and they often convey morpho-syntactic information. These morphemes are not appended to one another linearly, which is the case in languages with a concatenated morphology, such as English. Rather, the consonants of the root are intertwined with the phonemes (and therefore, the corresponding letters) of the word-pattern. Roots and word patterns are abstract structures because only their joint combination results in specific phonological word-forms with specific meanings. These meanings cannot necessarily be predicted by analyzing the two morphemes independently, and often two derivations of the same root do not appear to be semantically related (see Frost et al. 1997 for a detailed description of Hebrew morphology).
Behavioral studies in Hebrew have consistently demonstrated that words are automatically decomposed during word recognition, and that the tri-consonantal root morpheme mediates lexical access (Deutsch et al., 1998; Frost et al., 2000a; Frost et al., 2000b; Frost et al., 1997; Velan et al., 2005). Taken together, these studies suggest that Hebrew readers and speakers are routinely engaged in morphological decomposition that is distinct from semantic and orthographic processing. Hebrew, therefore, provides a unique opportunity to examine how the brain processes morphological information.
In a recent study, the independence of morphological processing was clearly demonstrated using an explicit task in Hebrew (Bick et al., 2008). In this study, we identified two areas, the left middle frontal gyrus (lMFG) and the left intraparietal sulcus (lIPS) that showed significantly higher activity during a task that required explicit judgments of morphological relatedness, relative to other linguistic tasks such as judgments of semantic and orthographic similarity. Furthermore, we have shown that this activation is independent of the semantic properties of the stimuli. Both the lMFG and lIPS are known to be involved in reading (e.g., Seghier et al., 2004), and specifically in grammar-related tasks (Forkstam et al., 2006; Tyler et al., 2005). For example, Shapiro, Moo & Caramazza (2006) demonstrated differential activation in response to verbs and nouns, and Marangolo, Piras, Galati & Burani (2006) have shown these areas to be activated in a morphological derivation task.
However, demonstrating that at relatively long time-courses explicit morphological judgments can be differentiated from decisions regarding semantic and orthographic similarity may not necessarily reveal whether morphological computation is a primary process occurring in the initial stage of visual word recognition. Behavioral research thus often focuses on implicit tasks that tap early and automatic processing, which is independent of subjects’ explicit strategies or the conscious awareness of the morphological manipulation. The masked-priming paradigm (Forster & Davis, 1984) is particularly useful for exploring early processes of word recognition because the brief presentation of the prime combined with forward and backward masking prevents the full conscious identification of the prime. Consequently, the priming effect obtained in this procedure is not influenced by the participants’ conscious appreciation of the prime-target morphological or orthographic relation, as is the case with some long-term priming effects. Masked priming has been extensively used in behavioral studies as well as in neuro-imaging research. Consistent results show that priming under masked presentation modulates activation in relevant areas (for a review see Henson, 2003). For instance, imaging studies have found that priming with semantically related words creates changes in activation in areas related to semantic processing. In some studies, however, a reduction in activity was observed while in others, activity increased (i.e. Copland et al., 2003; Devlin et al., 2004; i.e. Gold et al., 2006; Raposo et al., 2006). The precise nature of the neuronal mechanisms involved in priming are still under investigation (James & Gauthier, 2006; Schnyer et al., 2002). Recent studies have shown that priming effects might be strongly influenced by task (Nakamura et al., 2007) and brain region (Horner & Henson, 2008; Wig et al., 2009), and different hypotheses such as cortical tuning, neural adaptation, and response learning have been offered to account for priming effects (see: Race et al., 2008).
In the present paper, we report fMRI results using implicit morphological manipulations in Hebrew. Subjects were engaged in a lexical decision task; target words were preceded by a brief and unconscious presentation of primes. Morphological, semantic, and orthographic manipulations were introduced by changing the relations between the target and its primes. This allowed us to identify brain regions that were sensitive to morphological relations between words and compare this effect to that caused when words were semantically or orthographically related to targets. Note that activity studied in this experiment is not explicitly aroused by the experimental demands, but rather implicitly elicited by the very brief presentation of the prime, thereby reflecting rapid and automatic decomposition. Our goal was two-fold: first, to identify areas that are involved in early and automatic morphological processing and to provide a necessary point of comparison for our previous neuro-imaging results of explicit morphological judgments in Hebrew. We were interested in finding whether both experimental procedures use the same or different networks. Our second aim was to provide a contrast to previous English studies. Because masked priming has been employed in imaging experiments in English (Gold & Rastle, 2007), using the same method in a language with a deep morphology like Hebrew will enable us to progress in understanding the influence of morphological depth on morphological processing.
Methods
Subjects
Twenty volunteers participated in this study (ten males and ten females, ages ranging from 18–31, mean age 26.5). All participants were right-handed without any neurological record or reading disorders. Hebrew was the mother tongue of all subjects and none of them were exposed to other languages in early childhood. Participants gave written consent before taking part in the study and were paid for participating. Ethical approval was granted by the Hadassah Hebrew University Medical Center.
Magnetic Resonance Protocol
The Blood Oxygenation Level Dependent (BOLD) fMRI measurements were performed in a whole-body 1.5-T, Siemens Avanto scanner. BOLD contrast was obtained with a gradient-echo echo-planar imaging sequence and a standard head coil. Functional data was obtained using TR = 2 s, TE = 50 ms, flip angle = 90°, imaging matrix = 64 × 64, FOV = 20x20 cm and 21 slices, 3 mm each with 1 mm gap between slices. Slices were placed oblique to cover most of the brain. High resolution three-dimensional spoiled gradient echo (SPGR) anatomical sequence was preformed in the same session as functional data.
Stimuli
160 target words were used in the experiment. Each target word was matched with five primes: (1) A prime derived from the same root as the target, with a similar meaning. Hence this prime was morphologically and semantically related to the target (MS). (2) A prime derived from the same root but not semantically related (M). As explained above, this condition is unique to a language such as Hebrew. (3) A prime orthographically similar to the target (sharing at least three letters in the same order, but not all of the root letters, unlike the morphological primes). We refer to this condition as O (4) A prime semantically related to the target, but not derived from the same root (S), and (5) a control prime not related to the target in any way (NR). The advantage of this within-stimulus design is that each target word served as its own control for assessing the effect of morphological relatedness. All words were common Hebrew nouns or adjectives. All words were derived from productive roots and had a three-letter root (i.e. weak roots were not employed, see Frost et al., 2000a).
Semantic questionnaires were used to control for semantic relatedness. Fifty subjects judged the relatedness of each prime-target pair on a 1 (not related) to 7 (strongly related) scale. All pairs considered “semantically related” had to have been ranked above 4.5 and all pairs considered “semantically unrelated” received a ranking of below 3.0. Mean ranks of semantic relatedness for each group of primes, of orthographic overlap (both shared letters and shared letters in the same position), and of phonological similarity (shared phonemes) are presented in Table 1b. In addition, prime conditions were balanced for word length, word frequency, and the difference in length and frequency between prime and target (Examples of the stimuli in the five conditions are presented in Table 1a).
Table 1b.
properties of stimuli:
| Length | Frequency | Semantic relation | Orthographic relation | Shared phonemes | ||
|---|---|---|---|---|---|---|
| Shared letters | Shared letters in the same position | |||||
| Morphologic +semantic (MS) | 5.13±0.77 | 8.97±12.99 | 6.12±0.48 | 3.59±0.62 | 1.75±1.3 | 4.16±0.94 |
| 4.94±0.81 | 8.59±14.09 | |||||
| Morphologic (M) | 5.13±0.78 | 8.97±12.99 | 1.74±0.49 | 3.61±0.68 | 1.44±1.12 | 4.06±0.95 |
| 5.14±0.89 | 8.31±12.26 | |||||
| Orthographic (O) | 5.13±0.78 | 8.97±±12.99 | 1.42±0.35 | 3.28±0.51 | 1.47±1.03 | 3.61±0.96 |
| 5.15±0.79 | 6.21±10.38 | |||||
| Semantic (S) | 5.13±0.78 | 8.97±±12.99 | 5.76±0.49 | 1.4±0.96 | 0.43±0.67 | 1.96±1.36 |
| 4.92±0.83 | 8.01±12.17 | |||||
| Control (NR) | 5.13±0.78 | 8.97±±12.99 | 1.3±0.25 | 1.03±0.79 | 0.23±0.49 | 1.66±0.96 |
| 4.84±0.72 | 8.03±11.1 | |||||
Since we were constrained to a reasonable length for an imaging experiment, we could not achieve a probability of 0.5 to a “yes” response in the lexical decision task, but rather employed one hundred target non-words in this experiment (as
Table 1a.
examples of stimuli
| Hebrew | Orthographic Trans. | Phonetic Trans. | Semantic meaning | |
|---|---|---|---|---|
| Morphologic +semantic (MS) |
|
cwpn | tzofen | code |
|
|
hcpnh | hatzpana | encryption | |
| Morphologic (M) |
|
cwpn | tzofen | code |
|
|
cpwny | tzfoni | northern | |
| Orthographic (O) |
|
cwpn | tzofen | code |
|
|
cwnn | tzonen | cool | |
| Semantic (S) |
|
cwpn | tzofen | code |
|
|
kydwd | kidud | encoding | |
| Control (NR) |
|
cwpn | tzofen | code |
|
|
rkdn | rakdan | dancer |
Since we were constrained to a reasonable length for an imaging experiment, we could not achieve a probability of 0.5 to a “yes” response in the lexical decision task, but rather employed one hundred target non-words in this experiment (as opposed to 160 target words). Sixty non-words were created by combining an existing Hebrew root with an existing word pattern, but the result was an illegal combination that does not exist in the Hebrew lexicon (not all roots combine with all word patterns of Hebrew). For each of these target non-words, three non-word primes were matched: (1) primes derived from the same root with a different word pattern, creating another non-existing combination. We refer to this non-word condition as “pseudo-morphological” (R-M); (2) primes that were orthographically related (R-O); (3) primes that were not related, (R-NR). An additional 40 non-word targets did not have an existing root and were created by simply changing letters in existing words (“rootless” non-words). They were matched with two kinds of primes: (1) orthographically related (O) and (2) unrelated controls (NR). Length of non-words and words was matched. Note that this structure of non-words allowed us to examine some aspects of morphological processing even for non-word stimuli.
Experimental Setup
Stimulus presentation and recording of responses were implemented with Presentation software (http://www.neurobs.com/presentation). All words were visually presented via an LCD projector onto a tangent screen located inside the scanner in front of the subject. Subjects viewed the screen through a tilted mirror. Behavioral performance was assessed during the fMRI scan using a computer mouse. Subjects responded with the left index finger for “yes” responses and with the left middle finger for ”no” responses.
Experimental Design
During the experiment, subjects were required to decide if a visually presented letter string was an existing word in Hebrew. Conforming with the forward-masking paradigm (Forster & Davis, 1984), trials consisted of a sequence of three events: a 500 msec forward mask (########), followed by a prime presented for 33 msec, replaced by the target which remained on the screen until response (maximal exposure was 4000 msec). Participants were told that a series of hash marks would precede targets but no mention was made of primes. The different trial types (10 conditions: 5 of words and 5 of non-words) were presented in pseudorandom order, with a fixation cross (+) presented during a variable inter-trial interval to enable fMRI jittering in an event-related design. The inter-trial interval range and pseudo-random ordering were customized for the present design using the optseq2 program (http://surfer.nmr.mgh.harvard.edu/optseq/), in order to achieve optimal experimental efficiency (Dale, 1999).
The experiment was divided into four runs, each containing 40 word targets (8 in each of the 5 conditions) and 25 non-word targets (5 in each of the 5 conditions) so that each run lasted about 9 minutes. The stimuli were divided into five lists. Each list contained 32 words and 20 non-words in each of the 5 experimental conditions. The stimuli were rotated within the five conditions in each list in a Latin square design, so that all targets were presented in the different conditions between subjects, but no stimulus was repeated within a subject to avoid repetition priming effects.
Data Analysis
Data analysis was performed using the BrainVoyager Qx software package (Brain Innovation, Maastricht, The Netherlands, 2000). Prior to the statistical analysis, the raw data were examined for motion and signal artifacts. Head motion correction and high-pass temporal filtering in the frequency domain (3 cycles/total scan time) were applied in order to remove drifts and to improve the signal-to-noise ratio. The complete data set was transformed into Talairach space (Talairach & Tournoux, 1988), Z-normalized, and concatenated.
A behavioral log file was used to identify trials with null or incorrect responses. Responses longer than 2500 msec were considered null. All of these responses were coded as a separate condition and were not included in the analysis. Similarly, trials which included presentation timing errors beyond 3 msec were excluded from further analysis. Motion parameters calculated during motion correction for each subject were included as predictors to eliminate noise created by motion.
Changes in BOLD contrast associated with the different conditions were assessed on a pixel-by-pixel basis, using the general linear model (Friston et al., 1995) with the standard hemodynamic response function (Boynton et al., 1996). Group analyses were performed using random-effect analysis (p<0.05). In order to correct for multiple comparisons, a cluster size threshold was calculated for each map separately, using the BrainVoyager cluster-size threshold plug-in, based on Monte Carlo simulation (p<0.05). An initial mask was applied to select areas involved in global word reading. Only these volumes were included in the analysis.
Functional images were incorporated into the three-dimensional data sets through trilinear interpolation. The statistical parametric maps were overlaid on a cortical inflated map of a representative subject. The inflated maps were reconstructed from the T1-weighted 3D images. The procedure included segmentation of the white matter using a grow-region function, the smooth covering of a sphere around the segmented region, and the expansion of the reconstructed white matter into the gray matter.
Regions of interest (ROIs) corresponding to morphological conditions were defined for each subject individually by contrasting the morphological conditions with the control condition (p<0.05, not corrected). Activated voxels, located within 25mm of the multi-subject activity center, were defined as morphological ROIs. In addition, regions corresponding to morphologically related activity identified in our previous explicit study (Bick et al., 2008) were used as an additional morphological ROI. In these ROIs, hemodynamic responses associated with the different conditions were estimated using deconvolution analysis (Glover, 1999). From these hemodynamic responses, averaged bar histograms for the different tasks were calculated (t=2–10 sec). Predictors covering the time range of 0–12 sec were used for ROI GLM contrast calculations.
Areas associated with morphological processing of words were used as ROIs for analysis of non-words. Analysis of non-words was identical to the analysis of words.
Results
Behavioral Results
The mean accuracy and reaction time (RT) are shown in Table 3. Overall, subjects displayed adequate performance (average error rate for all priming conditions: 3.22±1.61%). Further statistical analysis was done on reaction times for correct trials only. ANOVA of RTs identified a main effect of lexicality (F = 402.22, p<0.001). No main effect of condition was found for words (F = 1.891, n.s). A main effect of priming condition was found, however, for the non-word stimuli (p<0.001, F = 20.6). Planned comparisons revealed that non-words composed of roots were rejected more slowly than non-words that did not contain roots (t = 8.92, p<0.001).
Table 3.
Behavioral results
| Words | M | MS | S | O | NR | Mean words |
|---|---|---|---|---|---|---|
| % error | 2.7±3.7 | 4.4±3.9 | 3.8±4.1 | 3.5±3.7 | 4.3±4.7 | 3.8±4 |
| Reaction time (msec) | 705±172 | 685±155 | 695±161 | 706±171 | 706±159 | 700±164 |
| Non-words | With root | rootless | Mean non-words | |||
| M | O | NR | O | NR | ||
| % error | 5.3±6.6 | 8.6±6.5 | 8.5±6.3 | 2.8±4.4 | 2.5±6.2 | 5.5±6.5 |
| Reaction time (msec) | 847±235 | 851±226 | 835±208 | 766±193 | 748±179 | 808±213 |
Imaging Results
Areas involved in reading were identified by contrasting all word conditions versus fixation. A most lenient threshold of 0.05 (corrected) was used, as to include maximum regions in the more detailed analysis. Areas identified were used as a mask, and all brain regions mentioned hereinafter are within this mask.
Maps were calculated to identify voxels involved in semantic, orthographic and morphological processing. Results are shown in Table 3 and Figure 1. Areas involved in semantic processing were identified by contrasting the two semantic conditions (the semantically (S) and morphologically and semantically (MS) related primes) with the orthographic and control related primes that had no semantic relation between primes and targets. As the interaction between semantics and morphology is under debate, pure morphological primes were not used to generate this contrast. Overall, in the semantic conditions, we observed a bilateral activity increase in the middle temporal gyrus (MTG), around the left central sulcus, in the left supramarginal gyrus, left superior parietal lobe, and bilateral cuneus. A small region showing a decrease in activation was also observed in the claustrum and the insula. This pattern of activation concurs with previous results showing a pattern of both decrease and increase in activation in response to semantic priming.
Figure 1.
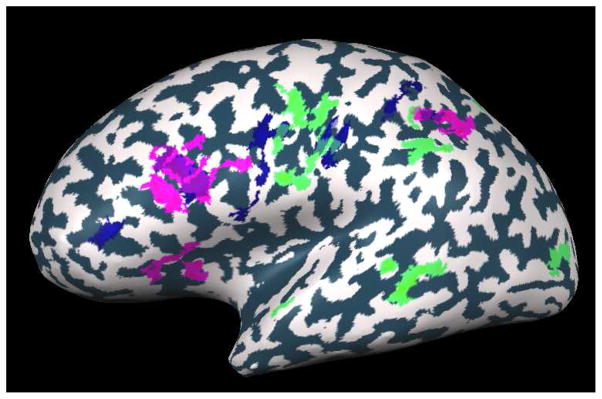
Statistical parametric map of 20 subjects using random effect GLM analysis (p<0.05, cluster size corrected) for the different conditions. The three maps overlaid (semantic – green, orthographic – blue, morphological – pink) on an inflated brain of one subject, presented from a lateral view.
Areas involved in orthographic processing were identified by contrasting the condition in which primes and targets were orthographically similar (i.e. shared at least three letters: the orthographic condition (O) and both morphological conditions (M, MS), with the conditions in which there was no clear orthographic relation between prime and target (the semantic (S) and control (NR) conditions). Results showed significant orthographic related decrease in various regions in the left frontal lobe including the inferior frontal gyrus, the middle frontal gyrus, the precentral gyrus and extending into the post central gyrus. Additional decrease in activation could be found in the left inferior parietal lobe and in the bilateral precuneus. Note however, that both orthographic and semantic effects were calculated using the morphological conditions, hence their maps may also include, to some extent, areas involved in morphological processing.
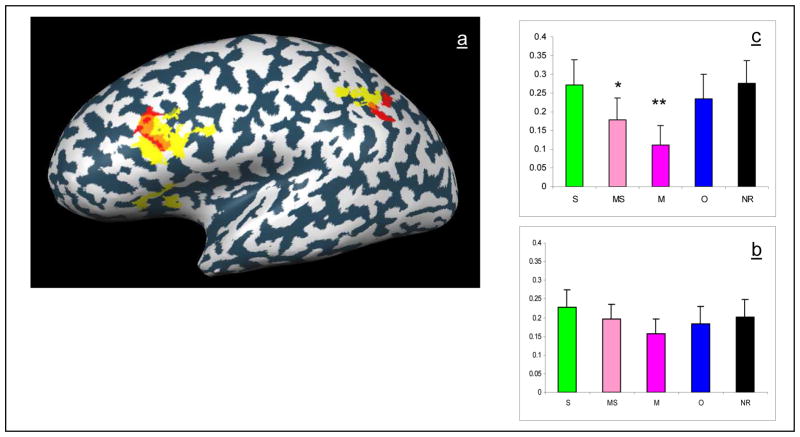
We were mostly concerned, however, with morphological processing. We therefore contrasted morphologically related activity (morphological (M) and morphological and semantic (MS) conditions) with morphologically unrelated activity (semantic (S), orthographic (O) and control (NR) conditions). Using this contrast, a decreased fMRI signal was observed in the left inferior frontal gyrus (IFG), in the left middle frontal gyrus (MFG) and in two regions in the left inferior parietal lobe (IPL): one in the angular gyrus (AG) and the other around the intraparietal sulcus (IPS) (see Table 3). In all of these areas the morphological effect was found bilaterally but to a stronger extent in the left hemisphere.
In order to compare temporal signal intensity between conditions, hemodynamic curves for each condition were calculated using deconvolution in the areas identified to be involved in morphological processing. Bar histograms were calculated including the average of the first seven components of the result of the deconvolution, covering 14 seconds from stimuli (see figure 2). This analysis revealed a significant reduction in activation for each of the morphological conditions in the lIFG (M vs O+S+NR: 0.001; MS vs O+S+NR: p<0.001) and in the lMFG (M vs O+S+NR: p<0.001; MS vs O+S+NR: p<0.001). In contrast, in the parietal lobe, decrease in activation was significant only when prime and target were not semantically related (lIPS: M vs O+S+NR: p<0.001; MS vs O+S+NR: t = 1.246 n.s.; lAG: M vs O+S+NR: p<0.001; MS vs O+S+NR: t = 0.71 n.s.). In all of these areas activation in the orthographic or semantic conditions did not differ significantly from activation in the unrelated conditions, suggesting that the morphological effect cannot be explained by the orthographic or semantic properties of the stimuli.
Figure 2.
Areas showing morphologically related decrease in activation: ROIs were selected from multi- subject maps using random-effect GLM analysis (p<0.05, corrected) contrasting both morphological conditions with other conditions (semantic, orthographic, and not related). For each ROI the average beta weights 2–12 sec after presentation of stimuli was calculated for the different conditions (morphologically and semantically related prime – light pink, morphologically and semantically unrelated prime – dark pink, semantically related prime - green, orthographically related prime – blue, non-related prime – black). Activation in the following regions in the left hemisphere is presented: a) lIFG b) lMFG c) lAG d) lIPS. Significant differences are marked (p<0.001).
To further validate these results, signal intensities between conditions in ROIs defined in the lIFG and lMFG were compared. ROIs were defined for each subject individually, by contrasting the morphological conditions with the control condition (see Methods section). Note that since significant decrease in activation was observed only for one of the morphological conditions, no ROI was used in the parietal lobe. An across-subjects paired t-test in these ROIs revealed a significant reduction in activation during each of the morphological conditions relative to the semantic and orthographic conditions (notice that these conditions were not used in defining the ROIs). Activation during the MS condition was significantly less than in the semantic condition (lIFG: p<0.0001, lMFG: p<0.0001) and in the orthographic condition (lIFG: p<0.0001, lMFG: p<0.0001). A similar result was obtained when morphologically related words were not semantically related - activation during the M condition was significantly less than in the semantic condition (lIFG: p<0.0001, lMFG: p<0.0001) and in the orthographic condition (lIFG: p<0.0001, lMFG: p<0.0001).
Explicit vs Implicit activation
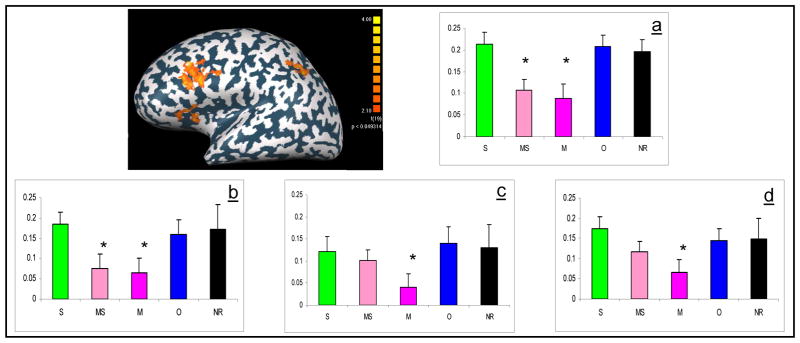
In order to compare activation during explicit morphological processing (Bick et al., 2008) to the findings of the present study during implicit morphological processing, we overlaid the maps from the two separate studies and used the areas identified in the explicit task as ROIs for the present implicit task (figure 3). In the lMFG, there was a clear and strong overlap between the area activated during the explicit task and the area showing morphologically related decrease in activation in the implicit task. Furthermore, in the ROI, defined by the explicit task, there was significant signal reduction during the morphological conditions relative to the other conditions (M vs O+S+NR: p<0.001; MS vs O+S+NR: p<0.031, using the second and third deconvolution components corresponding to the peek of activation). Although both tasks activated areas in the parietal lobe, overlaying the maps shows that these areas do not overlap. This is reflected in the ROI analysis as well: no significant morphologically related modulation could be found in this area (M vs O+S+NR: t = 1.43 n.s.; MS vs O+S+NR: t =−0.12 n.s).
Figure 3.
relation between explicit and implicit morphological processing. a) regions activated by explicit (red) and implicit (yellow) morphological processing. The regions activated by the explicit tasks were used as ROIs for the implicit task. b) activation in the lIPS c) activation in the lMFG
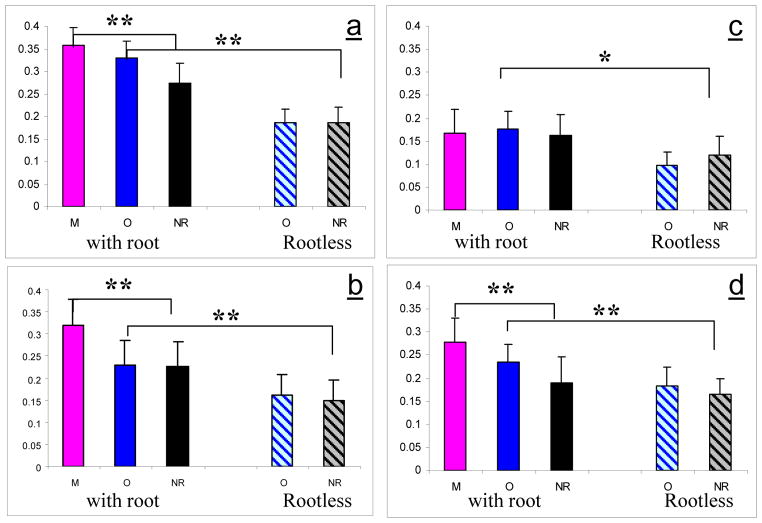
Analysis of non-words
We used the network involved in morphology (previously defined for words) as ROIs for the non-word data, revealing a strong morphological effect for non-words in these areas as well. In all of these regions, activation was significantly larger when non-words were constructed from a legal root (lIFG: p<0.0001; lMFG: p<0.0001, lIPS: p<0.002, lAG: p<0.02), suggesting the involvement of these areas in morphological decomposition aimed at extracting the root morpheme. Additionally, in the frontal areas and lIPS, activation was largest when non-words were primed by non-words that shared the same root: in the lIFG (morphology - orthography : p<0.016; morphology - control: p<0.005; orthography - control: t=0.73 n.s) in the lMFG (morphology - orthography : p<0.005; morphology - control: p<0.001; orthography - control: t=1.04 n.s) and in the lIPS: (morphology - orthography t = 1.862 n.s; morphology - control: p<0.005; orthography - control: t=0.907 n.s) while no significant orthographic priming effect was found in these regions. Note that while for words the morphological relation between prime and target caused a reduction in activation (i.e. adaptation), the morphological effect for non-words caused an increase in activation.
Relation between behavioral results and imaging results
Unlike previous studies (Deutsch et al., 1998; Frost et al., 2000a; Frost et al., 2000b; Frost et al., 1997), we did not obtain the typical root prime facilitation for words. Note, however, that the number of subjects in our study is too small for obtaining reliable behavioral effects. Moreover, the conditions in the magnet are different than those in the laboratory and the overall response latencies are not only significantly longer but also involve greater error variance. We should thus exert extreme caution in discussing the significance of the behavioral data. A subject-by-subject analysis of behavior performance revealed that half of the subjects showed a morphological priming effect (i.e. a reduction in reaction time in both morphological conditions relative to the other three conditions, regardless of semantics), and half did not. Therefore, we divided the subjects into two groups: one with a behavioral morphological effect and the other without. To statistically verify the validity of this division, we conducted a two-way ANOVA of behavioral data with prime condition and subject-group as fixed factors. The ANOVA indeed demonstrated that the interaction of group and condition was significant (F = 4.744, p<0.001), with one group showing, as expected, a significant morphological priming effect (F = 11.579, p<0.005), with no significant effects of semantics or orthography. Our aim in this latter analysis was to understand the relationship between behavioral and imaging effects. We therefore compared the imaging results of the two groups. The striking finding is that activation maps for both groups were similar and no significant difference was found in morphologically related areas between the groups (lIFG: t = 0.271 n.s.; lMFG: t = 1.348 n.s.; lIPS: t = −0.759 n.s.; lAG: t = −0.091 n.s.). This finding suggests that the morphologically related decrease in activation may be independent of behavioral effects and perhaps more directly reflects morphological processing. We will refer to this, at length, in the discussion below.
The greater activation for non-words created from existing roots was correlated with an increase in response latencies in these conditions. Since for the word stimuli these areas were not tuned to task difficulty, it is unlikely that task difficulty can explain the observed effect. Nevertheless, to eliminate this possibility, we calculated, for each subject, the correlations between response latency and activation in the defined ROIs. No significant correlation was found in any of the ROIs for non-words with roots (average correlations- MFG: r=0, IFG: r=−0.1, AG: r=−0.1, IPS: r=−0.2) and without roots (MFG: r=0., IFG: −0.1, AG: −0.2, IPS: r=0). This analysis clearly shows that to the extent that reaction time reflects task difficulty, it cannot explain the morphological effect we obtained for nonwords.
Discussion
In this paper we demonstrate the independent role of morphological processing by revealing morphologically-specific brain activation during Hebrew reading. The unique feature of Hebrew morphology is that most words are morphologically complex, and that morphological and semantic relatedness can be manipulated orthogonally. More importantly, behavioral studies have consistently shown that visual word recognition in Hebrew routinely involves processes of morphological decomposition. Similar to Bick et al. (2008), we identified a network corresponding to morphological activity that is distinct from semantic and orthographic activity. However, while previously, we focused on explicit and perhaps late morphological activation, in the present study we extend our findings to implicit morphological processing in very early time courses of activation.
Implicit and explicit processes involve networks that partially overlap. The differences and similarities of these networks have been demonstrated for a wide range of cognitive processes (Scheuerecker et al., 2007; Voss & Paller, 2008). In the language domain, explicit and implicit processing have been investigated in studies that focused on semantic (Noppeney & Price, 2002; Ruff et al., 2008) and on syntactic processing (Suzuki & Sakai, 2003).
We have found that implicit morphological processing involved bilateral fronto-parietal networks although stronger and more robust effects were observed in the left hemisphere (for involvement of the right hemisphere in morphological processing see: Marangolo et al., 2003). The lMFG area was shown to be involved in both explicit and implicit tasks. In this area, priming by morphologically related primes resulted in significant decrease in activation. Thus, activation in the morphological conditions was significantly lower than activation of targets primed by semantic, orthographic, or unrelated primes. These morphological effects were consistent and systematic regardless of the semantic overlap between primes and targets, suggesting that decrease in activation resulted from the common morphological properties and not from the similarity in semantic features. Consequently, and in line with our previous results, we suggest that the lMFG area is involved in the processing of morphology during reading. This conclusion is in accordance with findings showing that the lMFG area is involved in differential activation to verbs and nouns (Shapiro et al., 2006), in grammar-related tasks (Forkstam et al., 2006; Tyler et al., 2005) and in morphological derivation tasks (Marangolo et al., 2006). Therefore, the involvement of the lMFG area in morphological processing seems robust and is not task specific.
The lIFG area showed similar effects of a decrease in activation in response to morphological priming. This finding is consistent with Bozic et al. (2007), who showed morphologically related adaptation using delayed repetition priming in this area. Stronger activation in the lIFG was also found for inflected Finnish words compared with morphologically simple words (Laine et al., 1999; Lehtonen et al., 2006), and for inflected English verbs compared with inflected nouns (Longe et al., 2007; Tyler et al., 2004). Similarly, the lIFG area was differentially activated for regular vs. irregular verbs, although the direction of the effect was inconsistent (Beretta et al., 2003; Tyler et al., 2005). The involvement of the lIFG in morpho-syntactic processing is also revealed in studies of grammatical gender representation showing that when morphology was used to retrieve gender information, the superior and posterior portion of BA 44 was activated, as well as BA 45/47. In contrast, when morphology did not aid grammatical gender judgment, the activation was focused in the inferior tip of BA 44 (Fiebach et al., 2003; Heim et al., 2005; Hernandez et al., 2004; Longoni et al., 2005; Miceli et al., 2002). These imaging results are consistent with neuropsychological investigations (Shapiro & Caramazza, 2003) and TMS suppression (Shapiro et al., 2001) studies of grammatical category. But note that this area was not identified in our previous explicit task. Although it was activated during explicit morphological processing, this activation was not significantly different than that found in other linguistic tasks.
Based on these findings, we suggest that the lIFG area may be involved in early and automatic morphological processing. Such processes, being automatic, take place during reading regardless of task, and therefore were not modulated significantly by the different explicit judgments, which were required from the subjects in the Bick et al. (2008) study. That is, since all conditions reported by Bick et al. included real words, and since morphological decomposition occurred automatically for any Hebrew word in the lIFG, some morphological activation was probably present for all experimental conditions, and therefore no reliable differences were found between the tasks.
Confusing results were obtained for the left parietal lobe. First, the locus of explicit activation did not overlap with the present implicit activation. More importantly, the morphological effect obtained in the lIPL was significant only for words that were morphologically related and semantically unrelated, whereas only a non-significant trend was revealed for words that were both morphologically and semantically related. At present, we cannot ascertain whether this outcome should be attributed to problems of statistical power in the M+S+ condition, or whether it reflects the masking of two contrasting effects: an increase of activation due to semantic priming, and a decrease in activation due to morphological priming. One possible explanation may emerge from the nature of the lIPL. The lIPL is task sensitive, and it has been specifically shown, using TMS (Nakamura et al., 2006), that the lIPL was not involved in priming when the task was lexical decision but was essential when the task required word naming. The lIPL area in known to be involved in other demanding language tasks such as complex interactions between semantics and phonology (Frost et al., 2005b), or grammar-related tasks (Forkstam et al., 2006; Tyler et al., 2005) and is known to be sensitive to sentence complexity (Constable et al., 2004). This pattern may explain the strong involvement in explicit morphological processes, and to a less extent in implicit morphological processing. Additional studies will be required to understand the involvement of the parietal regions in morphology.
Considering our results for both implicit and explicit tasks, we propose the following fronto-parietal network involved in morphological processing during Hebrew reading. The lIFG is involved in early and automatic morphological decomposition. This area is activated in reading and its involvement is task independent. The lMFG is not constrained to early and automatic processing and, therefore, is involved in more general aspects of morphological processing. This activation exists during reading as demonstrated by the implicit task, but is modulated by explicit morphological judgments as demonstrated in the explicit morphological experiment. The parietal lobe seems to be involved in morphological processing as well, but this involvement depends on the task and its role so far is unclear.
Supporting evidence for our proposed network for morphological processing is provided by the non-word data. Areas involved in morphological processing were more strongly activated when non-words were composed of legal roots, while the strongest activation was revealed when both primes and targets shared a root. Note that since the ROIs for the non-word analyses were defined using only the words; the non-word analysis was not biased in terms of the definition of the regions. As non-words are not represented in the lexicon, the priming effect for non-words resulted in an increase in activation – the opposite effect than that observed for words. While information regarding the root of a word aids its recognition and can often create a facilitatory effect, information regarding the root of a non-word supplies false and misleading information – creating an increase in activation and making it harder to reject the non-word (as expressed in behavioral results). The non-word data support previous behavioral results in Hebrew showing that roots are automatically extracted even for non-words, at least when it comes to verbs (Deutsch et al., 1998). Previous cognitive studies have argued that root extraction is the initial process of reading in Hebrew and that roots serve as an organizing principle in the mental lexicon (Deutsch et al., 2003; Frost, in press; Frost et al., 2005a). The stronger activation we found for non-words derived from legal roots suggests that a root was indeed recognized and extracted from these printed stimuli. Furthermore, these results show that the root morpheme is indeed represented in the lexicon as previously suggested (Frost et al., 1997).
Overall, our results demonstrate that morphological processing is an independent process in visual word recognition. Hebrew speakers seem to activate the morphological network not only when explicitly requested to attend to morphological properties of words, but also when the relation of primes and targets is not consciously perceived. These brain activation results coincide with Frost et al.’s (2005a) previous behavioral research showing that while reading Hebrew, the root morpheme is extracted and processed, and this processing is early and automatic. This processing occurs for non-words as well as for words, exhibiting the important part that the root plays in the organization of the Hebrew lexicon.
Is this network language-dependent? Hebrew is perfect for studying morphological processing due to its systematic and complex morphological system. As previously described, several studies demonstrated the involvement of the lIGF in morphological processing in different Indo-European languages (i.e. Beretta et al., 2003; Lehtonen et al., 2006), and this involvement was shown to be independent of orthographic and semantic processing (Bozic et al., 2007). However, since this effect was not found or replicated in a parallel study (Gold & Rastle, 2007), further research is required to unravel the processing of morphology in languages with more impoverished morphology. In the lMFG and lIPL, evidence for morphological processing was found in Indo-European languages as well, as in Hebrew, but in Indo-European languages it is not evident that this activation is a result of morphological processing and not of other linguistic process such as semantics and orthography. Therefore, the results from Hebrew provide, for the first time, a systematic description of the areas involved in morphology, and future research is necessary to understand the relationship between morphological processing in the brain and the morphological structure of a given language.
Morphologically related decrease in activation was found both for subjects showing a behavioral effect and for subjects showing no clear consistent behavioral morphological effect. Similarly, for non-words, morphological priming caused an increase in activation, although no behavioral effect was found. These results demonstrate the advantage of imaging techniques that can identify processes that are not reflected by behavioral measures of reaction time. Behavioral measures reflect the sum of all processes taking place, and other processes may mask the facilitation created by morphological priming. The advantage of imaging is that it enables focusing on the areas involved in morphological processing alone, thus separating morphological processing from other parallel processes taking place in the human brain. Furthermore, this result shows that the observed effect reflects the differences in processing of the words induced by the different prime conditions rather than behavioral differences having to do with response execution.
In conclusion, converging evidence from explicit and implicit morphological processing clearly demonstrates that morphological processing is distinct from semantic and orthographic processing, at least in a language such as Hebrew. Our results show that morphological decomposition is early and automatic, demonstrating the central role morphology plays in reading Hebrew. The fronto-parietal network we identified is activated by morphology regardless of the semantic properties of words, and its activation is modulated by root information even for non-words. Additional research will be necessary to fully describe the network and its dynamics.
Figure 4.
Activation for non-word conditions in ROIs defined for word conditions. a) lIFG; b) lMFG; c) lAG; d) lIPS. In all regions activation for non-words created from an existing root was significantly higher than for rootless non-words. Furthermore, in all regions except lAG, when the prime was morphologically related, activation was significantly increased.
Table 2.
examples of non-words
| target | Orth. Trans. | prime | Orth. Trans. | Shared letters | Shared letters in same position | ||
|---|---|---|---|---|---|---|---|
| Non-words with roots | M |
|
nwgbn |
|
ngwbt | 3.73±0.7 | 1.68±1.15 |
| O |
|
nwgbn |
|
nwbxh | 3.32±0.67 | 1.5±1.07 | |
| NR |
|
nwgbn |
|
tkswt | 1.2±1.04 | 0.28±0.52 | |
| Rootless no n-words | O |
|
tlyrh |
|
lgr?h | 3.05±0.32 | 1.4±1.32 |
| NR |
|
tlyrh |
|
xwnzg | 0.47±0.64 | 0.1±0.3 |
Table 4.
Activations modulated by priming (p<0.05, corrected).
| a: Orthographic | Brodmann Area | Mean x | Mean y | Mean z | Z at peak voxel | Cluster size (mm) |
|---|---|---|---|---|---|---|
| lMFG and some lIFG ** | 9 | 46 | 14 | 30 | 4.57 | 1459 |
| L precentral (extending to lIFG) * | 44, 45, 6 | 53 | 9 | 7 | 4.15 | 923 |
| L cingulate, L superior frontal gyrus (lSFG), bilateral MFG | 32, 6, 8 | 1 | 19 | 46 | 3.75 | 491 |
| lIPL, L superior parietal lobe (lSPL), precuneus | 40, 7 | 34 | −49 | 47 | 3.42 | 696 |
| lIPL | 40 | 40 | −42 | 49 | 3.24 | 309 |
| lIFG lMFG | 46, 10 | 39 | 34 | 11 | 4.51 | 318 |
| L postcentral, L precentral * | 44, 9, 3, 45, 6, 4 | 54 | −4 | 36 | 3.98 | 1122 |
| L postcentral gyrus, L precentral gyrus | 2,3, 4 | 58 | −20 | 37 | 3.4 | 507 |
| R precuneus | 7 | −21 | −64 | 39 | 3.95 | 269 |
| R cuneus, precuneus | 7, 19 | −23 | −76 | 30 | 3.72 | 386 |
| b: Semantic – decrease in activation | BA | Mean x | Mean y | Mean z | Z at peak voxel | Cluster size (mm) |
|---|---|---|---|---|---|---|
| L claustrum, L insula | 13 | 31 | 17 | 10 | −4.03 | 289 |
| Semantic – increase in activation | ||||||
| L superior temporal gyrus (lSTG) | 22 | 56 | 2 | 4 | 3.34 | 302 |
| L postcentral, L precentral | 43 | 60 | −6 | 13 | 3.81 | 276 |
| L precentral, L postcentral | 3, 4, 43 | 55 | −10 | 24 | 4.31 | 323 |
| L postcentral, L precentral* | 3, 4 | 45 | −18 | 40 | 4.35 | 1161 |
| rMTG | 21, 22 | −51 | −31 | 2 | 4.21 | 361 |
| lMTG* | 21,22 | 58 | −42 | 4 | 4.45 | 447 |
| cerebellum | −2 | −44 | −12 | 3.76 | 463 | |
| lIPL, L supramarginal gyrus | 40 | 47 | −45 | 36 | 3.66 | 512 |
| L cingulate, L precuneus | 31, 7 | 12 | −44 | 46 | 2.98 | 272 |
| cuneus | 17,18 | 4 | −78 | 15 | 4.2 | 668 |
| lSPL | 7 | 31 | −64 | 49 | 3.12 | 298 |
| L middle occipital gyrus | 18 | 28 | −84 | 5 | 3.29 | 414 |
| R cuneus** | 18 | −12 | −76 | 24 | 5.22 | 442 |
| R cuneus | 19 | −23 | −83 | 32 | 3.57 | 325 |
| c: Morphological Task | BA | Mean x | Mean y | Mean z | Z at peak voxel | Cluster size (mm) |
|---|---|---|---|---|---|---|
| lIFG, claustrum, lMFG** | 45, 13, 46 | 37 | 24 | 11 | 5.26 | 1810 |
| lMFG, lIFG** | 9 | 45 | 13 | 30 | 4.23 | 2065 |
| L cingulate | 24, 31 | 12 | −3 | 46 | 4.59 | 289 |
| lIPS | 40 | 38 | −45 | 43 | 3.22 | 358 |
| lAG* | 39 | 32 | −54 | 34 | 3.98 | 503 |
| L precuneus | 31, 7 | 19 | −62 | 29 | 3.95 | 395 |
| R precuneus, lSTG, lSPL* | 39, 7, 19 | −26 | −60 | 33 | 4.86 | 832 |
| R precuneus, rSPL | 7 | −26 | −49 | 51 | 3.24 | 291 |
| rIFG, rMFG, r precentral* | 9, 6 | −38 | 9 | 31 | 4.41 | 1495 |
| R claustrum * | −31 | 4 | −4 | 4.03 | 301 |
voxels in cluster are significant at p<0.02
voxels in cluster are significant at p<0.01
Acknowledgments
We are indebted to Einar Mencl and Steve Frost for their contribution in conducting this research.This work was supported by the Center for Complexity Science, Israel, the Israel Science Foundation (grant award #1334/08), and by the National Institute of Child Health and Human Development (Grant HD-01994 awarded to Haskins Laboratories).
References
- Bentin S, Feldman LB. The contribution of morphological and semantic relatedness to repetition priming at short and long lags: evidence from Hebrew. Q J Exp Psychol A. 1990;42:693–711. doi: 10.1080/14640749008401245. [DOI] [PubMed] [Google Scholar]
- Beretta A, Campbell C, Carr TH, Huang J, Schmitt LM, Christianson K, Cao Y. An ER-fMRI investigation of morphological inflection in German reveals that the brain makes a distinction between regular and irregular forms. Brain Lang. 2003;85:67–92. doi: 10.1016/s0093-934x(02)00560-6. [DOI] [PubMed] [Google Scholar]
- Bick A, Goelman G, Frost R. Neural correlates of morphological processes in Hebrew. J Cogn Neurosci. 2008;20:406–420. doi: 10.1162/jocn.2008.20028. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozic M, Marslen-Wilson WD, Stamatakis EA, Davis MH, Tyler LK. Differentiating morphology, form, and meaning: neural correlates of morphological complexity. J Cogn Neurosci. 2007;19:1464–1475. doi: 10.1162/jocn.2007.19.9.1464. [DOI] [PubMed] [Google Scholar]
- Constable RT, Pugh KR, Berroya E, Mencl WE, Westerveld M, Ni W, Shankweiler D. Sentence complexity and input modality effects in sentence comprehension: an fMRI study. Neuroimage. 2004;22:11–21. doi: 10.1016/j.neuroimage.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Copland DA, de Zubicaray GI, McMahon K, Wilson SJ, Eastburn M, Chenery HJ. Brain activity during automatic semantic priming revealed by event-related functional magnetic resonance imaging. Neuroimage. 2003;20:302–310. doi: 10.1016/s1053-8119(03)00279-9. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch A, Frost R, Forster KI. Verbs and nouns are organized and accessed differently in the mental lexicon: evidence from Hebrew. J Exp Psychol Learn Mem Cogn. 1998;24:1238–1255. doi: 10.1037//0278-7393.24.5.1238. [DOI] [PubMed] [Google Scholar]
- Deutsch A, Frost R, Pelleg S, Pollatsek A, Rayner K. Early morphological effects in reading: evidence from parafoveal preview benefit in Hebrew. Psychon Bull Rev. 2003;10:415–422. doi: 10.3758/bf03196500. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Matthews PM, Gonnerman LM. Morphology and the internal structure of words. Proc Natl Acad Sci U S A. 2004;101:14984–14988. doi: 10.1073/pnas.0403766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman LB, Frost R, Pnini T. Decomposing words into their constituent morphemes: evidence from English and Hebrew. J Exp Psychol Learn Mem Cogn. 1995;21:947–960. doi: 10.1037//0278-7393.21.4.947. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY, Hernandez AE. Distinct brain representations for early and late learned words. Neuroimage. 2003;19:1627–1637. doi: 10.1016/s1053-8119(03)00227-1. [DOI] [PubMed] [Google Scholar]
- Forkstam C, Hagoort P, Fernandez G, Ingvar M, Petersson KM. Neural correlates of artificial syntactic structure classification. Neuroimage. 2006;32:956–967. doi: 10.1016/j.neuroimage.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Forster KI, Davis C. Repetition priming and frequency attenuation in lexical access. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1984;10:680–698. [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Frost R. Reading in Hebrew vs. Reading in English: Is there a qualitative difference? In: Pugh KMP, editor. How Children Learn To Read: Current Issues and New Directions in the Integration of Cognition, Neurobiology and Genetics of Reading and Dyslexia Research and Practice. Psychology Press; (in press) [Google Scholar]
- Frost R, Deutsch A, Forster KI. Decomposing morphologically complex words in a nonlinear morphology. J Exp Psychol Learn Mem Cogn. 2000a;26:751–765. doi: 10.1037//0278-7393.26.3.751. [DOI] [PubMed] [Google Scholar]
- Frost R, Deutsch A, Gilboa O, Tannenbaum M, Marslen-Wilson W. Morphological priming: dissociation of phonological, semantic, and morphological factors. Mem Cognit. 2000b;28:1277–1288. doi: 10.3758/bf03211828. [DOI] [PubMed] [Google Scholar]
- Frost R, Forster KI, Deutsch A. What can we learn from the morphology of Hebrew? A masked-priming investigation of morphological representation. J Exp Psychol Learn Mem Cogn. 1997;23:829–856. doi: 10.1037//0278-7393.23.4.829. [DOI] [PubMed] [Google Scholar]
- Frost R, Kugler T, Deutsch A, Forster KI. Orthographic structure versus morphological structure: principles of lexical organization in a given language. J Exp Psychol Learn Mem Cogn. 2005a;31:1293–1326. doi: 10.1037/0278-7393.31.6.1293. [DOI] [PubMed] [Google Scholar]
- Frost SJ, Mencl WE, Sandak R, Moore DL, Rueckl JG, Katz L, Fulbright RK, Pugh KR. A functional magnetic resonance imaging study of the tradeoff between semantics and phonology in reading aloud. Neuroreport. 2005b;16:621–624. doi: 10.1097/00001756-200504250-00021. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Rastle K. Neural correlates of morphological decomposition during visual word recognition. J Cogn Neurosci. 2007;19:1983–1993. doi: 10.1162/jocn.2007.19.12.1983. [DOI] [PubMed] [Google Scholar]
- Heim S, Alter K, Friederici AD. A dual-route account for access to grammatical gender: evidence from functional MRI. Anat Embryol (Berl) 2005;210:473–483. doi: 10.1007/s00429-005-0032-6. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Kotz SA, Hofmann J, Valentin VV, Dapretto M, Bookheimer SY. The neural correlates of grammatical gender decisions in Spanish. Neuroreport. 2004;15:863–866. doi: 10.1097/00001756-200404090-00026. [DOI] [PubMed] [Google Scholar]
- Horner AJ, Henson RN. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46:1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TW, Gauthier I. Repetition-induced changes in BOLD response reflect accumulation of neural activity. Hum Brain Mapp. 2006;27:37–46. doi: 10.1002/hbm.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester D, Schiller NO. Morphological priming in overt language production: Electrophysiological evidence from Dutch. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.06.043. [DOI] [PubMed] [Google Scholar]
- Laine M, Rinne JO, Krause BJ, Teras M, Sipila H. Left hemisphere activation during processing of morphologically complex word forms in adults. Neurosci Lett. 1999;271:85–88. doi: 10.1016/s0304-3940(99)00527-3. [DOI] [PubMed] [Google Scholar]
- Lavric A, Clapp A, Rastle K. ERP evidence of morphological analysis from orthography: a masked priming study. J Cogn Neurosci. 2007;19:866–877. doi: 10.1162/jocn.2007.19.5.866. [DOI] [PubMed] [Google Scholar]
- Lehtonen M, Vorobyev VA, Hugdahl K, Tuokkola T, Laine M. Neural correlates of morphological decomposition in a morphologically rich language: An fMRI study. Brain Lang. 2006 doi: 10.1016/j.bandl.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Longe O, Randall B, Stamatakis EA, Tyler LK. Grammatical categories in the brain: the role of morphological structure. Cereb Cortex. 2007;17:1812–1820. doi: 10.1093/cercor/bhl099. [DOI] [PubMed] [Google Scholar]
- Longoni F, Grande M, Hendrich V, Kastrau F, Huber W. An fMRI study on conceptual, grammatical, and morpho-phonological processing. Brain Cogn. 2005;57:131–134. doi: 10.1016/j.bandc.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Marangolo P, Incoccia C, Pizzamiglio L, Sabatini U, Castriota-Scanderbeg A, Burani C. The right hemisphere involvement in the processing of morphologically derived words. J Cogn Neurosci. 2003;15:364–371. doi: 10.1162/089892903321593090. [DOI] [PubMed] [Google Scholar]
- Marangolo P, Piras F, Galati G, Burani C. Functional anatomy of derivational morphology. Cortex. 2006;42:1093–1106. doi: 10.1016/s0010-9452(08)70221-1. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK. Morphology, language and the brain: the decompositional substrate for language comprehension. Philos Trans R Soc Lond B Biol Sci. 2007;362:823–836. doi: 10.1098/rstb.2007.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK, Waksler R, Older L. Morphology and meaning in the English mental lexicon. Psychological Review. 1994;101:3–33. [Google Scholar]
- Miceli G, Turriziani P, Caltagirone C, Capasso R, Tomaiuolo F, Caramazza A. The neural correlates of grammatical gender: an fMRI investigation. J Cogn Neurosci. 2002;14:618–628. doi: 10.1162/08989290260045855. [DOI] [PubMed] [Google Scholar]
- Morris J, Frank T, Grainger J, Holcomb PJ. Semantic transparency and masked morphological priming: an ERP investigation. Psychophysiology. 2007;44:506–521. doi: 10.1111/j.1469-8986.2007.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Dehaene S, Jobert A, Le Bihan D, Kouider S. Task-specific change of unconscious neural priming in the cerebral language network. Proc Natl Acad Sci U S A. 2007;104:19643–19648. doi: 10.1073/pnas.0704487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Hara N, Kouider S, Takayama Y, Hanajima R, Sakai K, Ugawa Y. Task-guided selection of the dual neural pathways for reading. Neuron. 2006;52:557–564. doi: 10.1016/j.neuron.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. A PET study of stimulus- and task-induced semantic processing. Neuroimage. 2002;15:927–935. doi: 10.1006/nimg.2001.1015. [DOI] [PubMed] [Google Scholar]
- Plaut D, Gonnerman LM. Are non-semantic morphological effects incompatible with a distributed connectionist approach to lexical processing? Language & Cognitive Processes. 2000;15:445–485. [Google Scholar]
- Race EA, Shanker S, Wagner AD. Neural Priming in Human Frontal Cortex: Multiple Forms of Learning Reduce Demands on the Prefrontal Executive System. J Cogn Neurosci. 2008 doi: 10.1162/jocn.2009.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, Moss HE, Stamatakis EA, Tyler LK. Repetition suppression and semantic enhancement: an investigation of the neural correlates of priming. Neuropsychologia. 2006;44:2284–2295. doi: 10.1016/j.neuropsychologia.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Rastle K, Davis MH. Reading morphologically complex words: Some thoughts from masked priming. In: Kinoshita A, Lupker SJ, editors. Masked priming: The state of the art. New York: Psychology Press; 2003. pp. 279–308. [Google Scholar]
- Rueckl JG, Mikolonski M, Raveh M, Miner C, Mars F. Morphological priming, fragment completion, and connectionist networks. Journal of Memory and Language. 1997;36:382–405. [Google Scholar]
- Ruff I, Blumstein SE, Myers EB, Hutchison E. Recruitment of anterior and posterior structures in lexical-semantic processing: an fMRI study comparing implicit and explicit tasks. Brain Lang. 2008;105:41–49. doi: 10.1016/j.bandl.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuerecker J, Frodl T, Koutsouleris N, Zetzsche T, Wiesmann M, Kleemann AM, Bruckmann H, Schmitt G, Moller HJ, Meisenzahl EM. Cerebral differences in explicit and implicit emotional processing--an fMRI study. Neuropsychobiology. 2007;56:32–39. doi: 10.1159/000110726. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Ryan L, Trouard T, Forster K. Masked word repetition results in increased fMRI signal: a framework for understanding signal changes in priming. Neuroreport. 2002;13:281–284. doi: 10.1097/00001756-200203040-00007. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Zimine I, Mayer E, Michel CM, Khateb A. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp. 2004;23:140–155. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychol Rev. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Caramazza A. The representation of grammatical categories in the brain. Trends Cogn Sci. 2003;7:201–206. doi: 10.1016/s1364-6613(03)00060-3. [DOI] [PubMed] [Google Scholar]
- Shapiro KA, Moo LR, Caramazza A. Cortical signatures of noun and verb production. Proc Natl Acad Sci U S A. 2006;103:1644–1649. doi: 10.1073/pnas.0504142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro KA, Pascual-Leone A, Mottaghy FM, Gangitano M, Caramazza A. Grammatical distinctions in the left frontal cortex. J Cogn Neurosci. 2001;13:713–720. doi: 10.1162/08989290152541386. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sakai KL. An event-related fMRI study of explicit syntactic processing of normal/anomalous sentences in contrast to implicit syntactic processing. Cereb Cortex. 2003;13:517–526. doi: 10.1093/cercor/13.5.517. [DOI] [PubMed] [Google Scholar]
- Taft M. Interactive-activation as a framework for understanding morphological processing. Language and Cognitive Processes. 1994;9:271–294. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tyler LK, Bright P, Fletcher P, Stamatakis EA. Neural processing of nouns and verbs: the role of inflectional morphology. Neuropsychologia. 2004;42:512–523. doi: 10.1016/j.neuropsychologia.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Post B, Randall B, Marslen-Wilson W. Temporal and frontal systems in speech comprehension: an fMRI study of past tense processing. Neuropsychologia. 2005;43:1963–1974. doi: 10.1016/j.neuropsychologia.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Velan H, Frost R. Cambridge University versus Hebrew University: the impact of letter transposition on reading English and Hebrew. Psychon Bull Rev. 2007;14:913–918. doi: 10.3758/bf03194121. [DOI] [PubMed] [Google Scholar]
- Velan H, Frost R, Deutsch A, Plaut DC. The processing of root morphemes in Hebrew: Contrasting localist and distributed accounts. Language and Cognitive Processes. 2005;20:169–206. [Google Scholar]
- Voss JL, Paller KA. Brain substrates of implicit and explicit memory: The importance of concurrently acquired neural signals of both memory types. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. J Neurophysiol. 2009;101:2632–2648. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]