Abstract
African swine fever virus (ASFV) multigene family 360 and 530 (MGF360/530) genes affect viral growth in macrophage cell cultures and virulence in pigs (L. Zsak, Z. Lu, T. G. Burrage, J. G. Neilan, G. F. Kutish, D. M. Moore, and D. L. Rock, J. Virol. 75:3066-3076, 2001). The mechanism by which these novel genes affect virus-host interactions is unknown. To define MGF360/530 gene function, we compared macrophage transcriptional responses following infection with parental ASFV (Pr4) and an MGF360/530 deletion mutant (Pr4Δ35). A swine cDNA microarray containing 7,712 macrophage cDNA clones was used to compare the transcriptional profiles of swine macrophages infected with Pr4 and Pr4Δ35 at 3 and 6 h postinfection (hpi). While at 3 hpi most (7,564) of the genes had similar expression levels in cells infected with either virus, 38 genes had significantly increased (>2.0-fold, P < 0.05) mRNA levels in Pr4Δ35-infected macrophages. Similar up-regulation of these genes was observed at 6 hpi. Viral infection was required for this induced transcriptional response. Most Pr4Δ35 up-regulated genes were part of a type I interferon (IFN) response or were genes that are normally induced by double-stranded RNA and/or viral infection. These included monocyte chemoattractant protein, transmembrane protein 3, tetratricopeptide repeat protein 1, a ubiquitin-like 17-kDa protein, ubiquitin-specific protease ISG43, an RNA helicase DEAD box protein, GTP-binding MX protein, the cytokine IP-10, and the PKR activator PACT. Differential expression of IFN early-response genes in Pr4Δ35 relative to Pr4 was confirmed by Northern blot analysis and real-time PCR. Analysis of IFN-α mRNA and secreted IFN-α levels at 3, 8, and 24 hpi revealed undetectable IFN-α in mock- and Pr4-infected macrophages but significant IFN-α levels at 24 hpi in Pr4Δ35-infected macrophages. The absence of IFN-α in Pr4-infected macrophages suggests that MGF360/530 genes either directly or indirectly suppress a type I IFN response. An inability to suppress host type I IFN responses may account for the growth defect of Pr4Δ35 in macrophages and its attenuation in swine.
African swine fever is a significant disease of domestic swine, with mortality rates approaching 100%. No vaccine is currently available, making quarantine and slaughter the only effective control strategy (44).
African swine fever virus (ASFV), the causative agent of African swine fever, is a unique and complex DNA virus that infects cells of the mononuclear-phagocytic system, including fixed-tissue macrophages and specific lineages of reticular cells. Affected tissues show extensive necrosis following infection with highly virulent viral strains (13, 36, 38). Moderately virulent ASFV strains also appear to infect these cell types, but the degree of tissue involvement and the resulting tissue damage are much less severe (13, 36, 38). The abilities of ASFV to replicate and efficiently induce marked cytopathology in monocytes-macrophages in vivo appear to be critical factors for ASFV virulence.
ASFV is the sole member of the family Asfarviridae and the only known DNA arbovirus (14, 38). ASFV is a large, icosahedral virus that contains a linear double-stranded DNA genome (170 to 190 kbp) encoding approximately 165 genes (50; C. A. Balinsky et al., unpublished data). The availability of complete ASFV genome sequences has revealed that, similar to poxviruses, ASFVs encode proteins with functions essential for viral replication, including those involving structure and assembly of the virion and those responsible for biogenesis of mRNA and DNA. A large number of ASFV genes are of unknown function and may be involved in aspects of viral virulence and host range (46, 50; Balinsky et al., unpublished).
Pathogenic ASFV genomes contain 11 to 15 multigene family 360 (MGF360) genes and either 9 or 10 multigene family 530 (MGF530) genes (Balinsky et al., unpublished). Recently, we have identified MGF360 and MGF530 genes as novel macrophage host range determinants necessary for efficient growth in macrophages (54). Infection of macrophage cell cultures with MGF360-MGF530 (MGF360/530) gene deletion mutant Pr4Δ35 (six MGF360 and two MGF530 genes deleted) resulted in a 2- to 3-log reduction in virus titers and early cell death, suggesting a direct or indirect role for these genes in some aspect of infected-cell survival (54) (L. Zsak, unpublished data). In addition, a swine virulence determinant (VAD) containing MGF360/530 genes was mapped by using in vivo marker rescue to the left variable region of the ASFV genome (37).
The mode of action of the ASFV MGF360/530 genes is unknown. Homology searches reveal no homology to other known genes. MGF360/530 genes have a conserved motif of 100 amino acids (28% amino acid identity) at the amino terminus in common (21, 51). Among MGF members, amino acid similarity ranges from 23 to 88% for MGF360 proteins and from 46 to 74% for MGF530 proteins (54). To investigate MGF360/530 function, we have analyzed and compared macrophage transcriptional responses following infection with Pr4 and the Pr4 MGF360/530 deletion mutant Pr4Δ35.
MATERIALS AND METHODS
Construction of swine macrophage cDNA libraries.
cDNA libraries were generated from mRNA isolated from ASFV-infected and uninfected macrophage cell cultures (60 75-cm2 flasks) prepared from swine peripheral blood mononuclear cells (19). Macrophages were infected with pathogenic ASFV isolate Pr4 at a high multiplicity of infection (MOI) of 20, and at 3, 6, and 18 h postinfection (hpi), infected cells were harvested and lysed. RNA was extracted with acid phenol-chloroform and precipitated with isopropanol. Total RNA was purified by LiCl precipitation, and poly(A) RNA transcripts were enriched by two successive rounds of oligo(dT) affinity chromatography (28). Five micrograms of mRNA was used to generate directional cDNA libraries with commercially available cDNA synthesis and cloning kits (Superscript II system; Life Technologies). Approximately 15,000 cDNA clones were sequenced and characterized by comparison to genetic databases (J. Neilan, unpublished data). Seven thousand seven hundred twelve cDNA clones from two cDNA libraries (2,925 from an ASFV-infected macrophage library and 4,787 from a noninfected macrophage library) were selected by Blast analysis with a cutoff value of 200 (2) and used to construct a cDNA microarray.
Microarray construction.
Microarrays were manufactured by using PCR-amplified swine macrophage cDNAs as previously described (26). Amplicons were generated with M13 primers and Platinum Taq DNA polymerase (Invitrogen) in 96-well plates. PCR products were purified with 96-well filter plates (Millipore), transferred into 384-well plates, vacuum dried, resuspended in 50% dimethyl sulfoxide, and printed onto CMT-GAPII aminopropyl silane-coated glass slides (Corning) with a MicroGrid II (Biorobotics) array printer. Printed slides were baked at 80°C for 4 h and stored desiccated in the dark until used.
Viruses, macrophage cell cultures, infection, mRNA labeling, and hybridization.
ASFV Pr4 was isolated from ticks obtained from Kruger National Park, Republic of South Africa, in 1996 (South Africa, Pretoriuskop/96/4) and has had limited passages in swine macrophage cell cultures (1). Pr4 deletion mutant Pr4Δ35, lacking six MGF360 and two MGF530 genes, was constructed as previously described (54). Macrophage cell cultures were prepared as previously described (19). Cultures were infected with the Pr4 and Pr4Δ35 viruses at an MOI of 10. A virus-depleted, Pr4Δ35-infected culture supernatant was prepared to determine if viral infection is required for induction of the macrophage transcriptional response (MOI of 0.0004). Virus was removed by filtration through a 0.4-μm-pore-size filter, followed by a 0.1-μm-pore-size low-protein-binding filter (Millipore) (prefiltration titer, 1.5 × 108; postfiltration titer, 2.9 × 102). At 3 and 6 hpi, RNA was isolated with Trizol (Invitrogen), followed by LiCl precipitation, and approximately 10 μg of total RNA was labeled with Cy3 and Cy5 with an aminoallyl cDNA labeling kit (Ambion). Slides were prehybridized for 30 min in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate (SDS)-1% bovine serum albumin at 55°C, followed by two 5-min washes at room temperature (RT) in 2× SSC and 0.2× SSC, respectively. cDNAs were hybridized to the cDNA microarrays for 72 h at 45°C in CMT hybridization chambers (Corning) with 40% formamide-4× SSC-1% SDS-2× Denhardt's solution-80 ng of poly(A) (Pharmacia) per μl (26, 43) and washed successively in 0.2% SDS at 55°C (15 min), 2× SSC at RT (15 min), and 0.2× SSC at RT (15 min).
Microarray data collection and analysis.
Differential expression measurements based on simultaneous two-color hybridizations were performed with a GenePix 4000A scanner and the GenePix Pro 4.0 data acquisition and analysis software (Axon Instruments). Unrelated DNA fragments (spikes) were used as positive controls (Stratagene), and dimethyl sulfoxide was used as a negative control. GeneSpring 5.0 computer software (Silicon Genetics) was used for all normalization and statistical analysis of the GenePix output. A minimum of five to eight slides from three independent experiments were analyzed at each time point. The intensity ratio of expression for each gene was calculated by dividing the measured Pr4Δ35 values (test channel) by the intensity of the Pr4 samples (control channel). All outputs with control channel values of less than 100.0 were not considered. Intensity-dependent normalization was used to reduce the ratios to the residual of the Lowess fit of the intensity-versus-ratio curve. Per-chip normalization was done by creating an artificial positive control for each sample and with the 50th percentile of all measurements across the entire chip. Individual-sample t tests were calculated as the average of replicate normalized values for a single gene − 1/standard error. This analysis produces low P values when the average of replicate normalized values (fold changes) is very different from 1 and the standard error is small.
Northern blot assays.
Ten micrograms of total RNA was separated on denaturing gels, transferred to nylon membranes, and baked for 2 h at 80°C, as suggested by the manufacturer (Ambion). 32P-labeled antisense RNAs were used as probes. Antisense RNA probes were obtained by in vitro transcription (Ambion) of purified plasmid DNA (Qiagen) containing the selected genes. Membranes were prehybridized in Ultrahyb solution (Ambion) for 1 h at 64°C, hybridized overnight with 5 × 106 cpm of labeled probe, and washed four times in 0.1× SSC-0.1% SDS (30 min each time) at 68°C.
Real-time PCR.
Real-time PCR of selected genes was used to confirm array data. Levels of mRNA from mock-, Pr4-, and Pr4Δ35-infected macrophages were analyzed with the swine glyceraldehyde-3-phosphate dehydrogenase gene as an endogenous control. Primers designed with Primer Express R software (Applied Biosystems) were 5′-GCATAGATGTAGATTTGATTGAAGTTGTG-3′ and 5′-AACAAGCAAAAACCCATTCTCC-3′ for interleukin-8 (IL-8), 5′-CCAGAGTGGTTGCATTCAGAGA-3′ and 5′-TGTCTGTTGCCATGAAGAAAGAA-3′ for the alpha/beta interferon (IFN-α/β) receptor, 5′-CAGACGATAGGAAAGGTGAGGAA-3′ and 5′-CTGAGGTGCTACATTCTTAGTGAATGT-3′ for IP-10, 5′-CCTTTCCTTCCTCCAGCAGG-3′ and 5′-CAACCAAGAGGGAAATCGCA-3′ for Mx, 5′-TCCCACTCTCCTCCCCTTCT-3′ and 5′-GGAAGGAAACGAAAGGAACAGA-3′ for the unknown-function gene with accession number CB469595, and 5′-TTCTGCACCCAGGTCCTTG-3′ and 5′-GGATGCGCTTCAAAGGGAA-3′ for monocyte chemoattractant protein 1 (MCP-1). Total RNA was diluted at 25 ng/μl, reverse transcribed, PCR amplified with a TaqMan EZ reverse transcription-PCR kit (Applied Biosystems), and analyzed on an ABI prism 7700 (Applied Biosystems). Fold increases in expression were calculated by the comparative ΔΔCT method as described elsewhere (http://www.pebiodocs.com/pebiodocs/04303859.pdf).
IFN biological assays.
IFN antiviral activity was assayed biologically with vesicular stomatitis virus (VSV) in Madin-Darby bovine kidney cells (MDBK) cells as previously described (6, 7). Briefly, supernatants of noninfected and ASFV-infected macrophages were collected, clarified by high-speed centrifugation (35,000 × g, 1 h), acidified at pH 2, and neutralized with NaOH. MDBK cells were then incubated overnight with supernatants obtained from noninfected and ASFV (Pr4 and Pr4Δ35)-infected macrophages and subsequently infected with VSV strain New Jersey. Infected cells were overlaid with medium containing carboxymethyl cellulose and incubated for 24 h. Plates were stained with crystal violet and VSV plaques were counted. For antibody inhibition of IFN activity, an anti-IFN-α antibody (PBL Biomedical Laboratories) was added to the supernatants at a 1/100 dilution 2 h before incubation with the MDBK cells.
RESULTS
IFN response genes are up-regulated in Pr4Δ35-infected macrophage cell cultures.
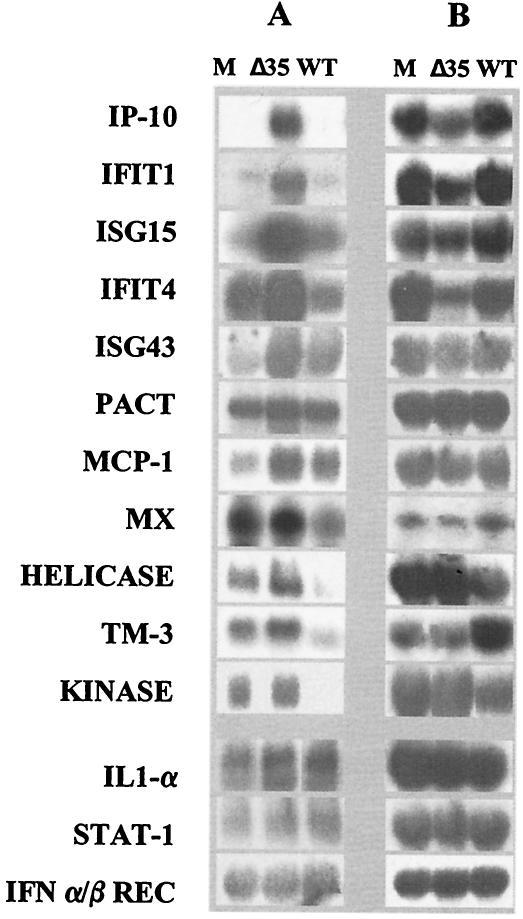
Microarray comparison of mRNA levels of macrophages infected with Pr4 and Pr4Δ35 revealed that transcription of 98% of the host genes examined did not change significantly at 3 hpi. Expression levels of 7,541 cDNA clones varied by less than twofold (data not shown). In Pr4Δ35-infected macrophages, 38 genes had increased and 133 genes had decreased (>2.0-fold, P < 0.05) mRNA levels, respectively (Table 1 and data not shown). Up-regulated genes included a tetratricopeptide repeat-containing protein (IFT1), a ubiquitin-like 17-kDa protein (ISG15), GTP-binding-protein MXB, the cytokine IP-10, MCP-1, IFN-induced transmembrane protein 3 (TM-3), ubiquitin-specific protease 18 (ISG43), and an RNA helicase DEAD box protein (Table 1). These genes are known IFN response genes that are induced solely by IFN, by the combined action of IFN and viral infection, or by double-stranded RNA (dsRNA) (12, 18, 45). Notably, the induced transcriptional response required virus infection since no induction was observed with virus-depleted, Pr4Δ35-infected culture supernatant (MOI of 0.0004) (Table 1). Although not as marked, up-regulation of most of these 38 genes was evident at 6 hpi in the absence (Table 1) or in the presence of cytosine arabinoside, an inhibitor of viral DNA replication (data not shown). Northern blot analysis and real-time PCR, which also included a comparison with mock-infected controls, confirmed microarray results demonstrating increased mRNA levels for these selected genes in Pr4Δ35-infected macrophages (Table 1 and Fig. 1). Expression of these genes did not change significantly in Pr4-infected cells compared to that in mock-infected cells, suggesting that Pr4 MGF360/530 may be responsible for suppression of the IFN response (Table 1). With the exception of p58, which also had a 3.8-fold increase in Pr4, and IL-1β (2.6-fold increase), other genes did not exceed the threshold of a twofold increase over their expression in mock-infected cells (Table 1). Transcription of STAT1 (a transcription factor that induces IFN responses following dimerization and translocation to the nucleus) and IFN-α/β receptor genes were not up-regulated during infection with either virus (Table 1 and Fig. 1). Changes in the expression of the CXC-type proinflammatory cytokine IL-8, IL-1α, and IL-1β (Table 1 and Fig. 1) genes, genes normally involved in macrophage activation and responsible for important inflammatory responses, were not observed.
TABLE 1.
Microarray analysis of gene expression in ASFV-infected macrophage cell cultures
| Gene name or function | cDNA accession no. | Best-hit accession no. | Pr4Δ35-Pr4 fold increase at 3 hpi | t-test P value | Avg real-time PCR fold increase at 3 hpi (SD) | Pr4Δ35 supernatant-Pr4 fold increase at 3 hpia | Pr4Δ35-Pr4 fold increase at 6 hpi | Pr4-mock infection fold increase at 3 hpi |
|---|---|---|---|---|---|---|---|---|
| Upregulated >2-fold | ||||||||
| Cytokine IP-10 | CB469489 | AB099892 | 6.89 | 0.001627 | 11.55 (9.47-14.08) | 1.25 | 2.21 | 1.60 |
| Unknown | CB469321 | 5.45 | 3.75 × 10−4 | 1.16 | 4.01 | 1.04 | ||
| Tetratricopeptide repeats, IFIT1 | CB469191 | P09914 | 4.84 | 2.95 × 10−4 | 1.58 | 1.89 | 1.06 | |
| Ubiquitin-like 17-kDa protein | CB471836 | P05161 | 4.46 | 0.001364 | 1.72 | 5.76 | 1.41 | |
| Unknown | CB469595 | 4.06 | 7.56 × 10−4 | 5.88 (5.32-6.49) | 1.23 | 2.45 | 1.20 | |
| Unknown | CB468971 | U34605 | 3.03 | 0.007793 | 1.24 | 2.19 | 0.80 | |
| Unknown | CB481699 | 2.77 | 0.002065 | 1.08 | 2.45 | 1.15 | ||
| Ubiquitin ligase like | CB473195 | AC020571 | 2.74 | 0.001847 | 1.45 | 1.94 | 1.17 | |
| ISG43, ubiquitin-specific protease 18 | CB470082 | AF134195 | 2.62 | 0.002544 | 1.13 | 1.45 | 1.08 | |
| Unknown | CB479057 | 2.54 | 0.008026 | 1.43 | 2.04 | 1.10 | ||
| LINE1 reverse trancriptase | CB476326 | S80119 | 2.51 | 0.040625 | 1.21 | 1.59 | 1.24 | |
| Unknown | CB478726 | 2.49 | 0.025735 | 1.13 | 2.45 | 0.83 | ||
| PACT, p53-associated protein | CB476189 | AF352051 | 2.38 | 0.026206 | 1.31 | 1.73 | 1.06 | |
| Unknown | CB479458 | BF078260 | 2.34 | 0.011609 | 1.14 | 1.58 | 1.00 | |
| Unknown | CB482476 | BI338737 | 2.32 | 0.008283 | 0.99 | 1.70 | 1.15 | |
| Unknown | CB480284 | BG732648 | 2.30 | 0.019733 | 1.25 | 1.47 | 1.19 | |
| Unknown | CB470560 | BI404223 | 2.28 | 0.002683 | 1.37 | 1.95 | 1.11 | |
| Unknown | CB482594 | BF442191 | 2.27 | 0.003725 | 1.26 | 2.39 | 1.15 | |
| Unknown | CB480019 | BE030527 | 2.23 | 0.004962 | 1.63 | 1.60 | 1.10 | |
| MCP-1 | CB482611 | Z48479 | 2.23 | 0.039477 | 2.81 (2.18-3.62) | 1.57 | 2.21 | 1.53 |
| MXB, GTP binding | CB472512 | P20592 | 2.19 | 0.001413 | 2.85 (2.37-3.42) | 0.82 | 1.44 | 1.66 |
| Unknown | CB470650 | 2.17 | 5.39 × 10−4 | 1.24 | 1.95 | 1.41 | ||
| Unknown | CB480279 | AC104718 | 2.16 | 0.007469 | 1.56 | 1.61 | 1.2 | |
| N-Acetylglucosamine kinase | CB482694 | AJ242910 | 2.15 | 0.069 | 0.94 | 2.16 | 1.00 | |
| Unknown | CB473300 | 2.15 | 0.006824 | 1.47 | 1.45 | 1.12 | ||
| Unknown | CB480200 | BF442147 | 2.14 | 0.027229 | 1.91 | 1.63 | 1.16 | |
| Unknown | CB480314 | AW619120 | 2.12 | 0.004569 | 1.23 | 1.67 | 1.32 | |
| RNA helicase DEAD box protein | CB469189 | BC014949 | 2.11 | 0.006565 | 1.40 | 1.75 | 1.07 | |
| Unknown | CB480289 | AW436409 | 2.08 | 0.010568 | 1.04 | 1.85 | 1.21 | |
| Tyrosine phosphatase motif | CB476178 | NM_080922 | 2.04 | 0.044404 | 1.42 | 1.32 | 1.19 | |
| IFN-induced TM-3 | CB476806 | AF227948 | 2.03 | 1.68 × 10−4 | 1.30 | 2.01 | 1.11 | |
| Sprouty 2 | CB474265 | NP_005833 | 2.03 | 1.42 × 10−4 | 1.31 | 1.59 | 1.09 | |
| Unknown | CB480567 | 2.03 | 0.01083 | 0.99 | 1.78 | 1.53 | ||
| Unknown | CB481133 | BI097843 | 2.02 | 0.038255 | 1.29 | 2.45 | 1.05 | |
| Unknown | CB475887 | 2.01 | 0.007619 | 1.31 | 1.24 | 0.91 | ||
| Unknown | CB481843 | BM724068 | 2.01 | 0.024038 | 1.00 | 1.90 | 1.12 | |
| Unknown | CB479033 | AW415288 | 2.01 | 0.04682195 | 1.40 | 1.97 | 1.13 | |
| Unknown | CB471021 | BF078276 | 2.00 | 0.00444 | 1.61 | 1.32 | 1.10 | |
| No change or down-regulated | ||||||||
| ISGF-3 91-kDa component, STAT1 | CB474498 | P42224 | 1.74 | 0.003545 | 1.12 | 1.73 | 0.99 | |
| IFN-α/β receptor B chain | CB472490 | Q95141 | 0.92 | 0.072824 | 1.40 (1.35-1.46) | 0.52 | 0.83 | 1.16 |
| p58 inhibitor of PKR | CB472968 | U28424 | 0.46 | 7.24 × 10−6 | 0.43 | 0.35 | 3.87 | |
| IL-8 | AB057440 | No data | No data | 0.703 (0.39-1.07) | No data | No data | No data | |
| IL-1α | CB474300 | P18430 | 0.83 | 0.03737 | No data | 1.07 | 0.99 | |
| IL-1β | CB473056 | M86725 | 1.29 | 0.0488191 | No data | 0.87 | 2.63 |
Virus-depleted supernatant was prepared by membrane filtration (MOI of 0.00004).
FIG. 1.
Northern blot analysis of selected cellular genes in ASFV-infected macrophage cell cultures at 3 hpi. Ten micrograms of total RNA was separated on denaturing gels, transferred to nylon membranes, and hybridized to 32P-labeled antisense RNA for selected macrophage genes (A) or to β-actin as a control (B). M, Δ35, and WT represent mock-infected and Pr4Δ35- and Pr4-infected swine macrophages, respectively. Abbreviations: IP-10, IFN-induced cytokine IP-10; IFIT1, IFN-induced tetratricopeptide repeat-containing protein 1; ISG15, ubiquitin-like 17-kDa IFN-induced protein; IFIT4, IFN-induced tetratricopeptide repeat-containing protein 4; ISG43, ubiquitin-specific protease 18; PACT, p53-associated protein; MX, IFN-induced protein Mx; HELICASE, IFN-induced RNA helicase; TM3, IFN-induced TM-3; KINASE, N-acetylglucosamine kinase; STAT-1, ISGF-3 91-kDa component; REC, receptor.
Evidence of an IFN response in Pr4Δ35-infected macrophages was supported by data indicating a possible role for the protein kinase PKR in the mutant phenotype. PKR is an IFN-induced serine-threonine protein kinase with potent antiviral activity (9). PKR becomes autophosphorylated and activated in response to dsRNA (9). Although PKR was not represented in our swine microarray, differential expression of p58 and PACT, two genes involved in regulating PKR activity, suggested involvement of PKR in the response to Pr4Δ35 infection. p58, a member of the tetratricopeptide repeat family of proteins and a cellular inhibitor of PKR that is activated in response to viral infection, was up-regulated in both Pr4- and Pr4Δ35-infected cells in comparison to mock-infected cells (Table 1) (29). In Pr4Δ35, up-regulation of p58 was not as pronounced and RNA expression levels were twofold lower than in Pr4 (0.456 [range, 0.277 to 0.698]; t test, P = 4.27 × 10−6). The PACT gene (activator of PKR) was expressed at higher levels in Pr4Δ35- than in Pr4-infected cells (Table 1). The lower levels of p58 and increased levels of PACT in Pr4Δ35 suggest that PKR antiviral activity might be present during Pr4Δ35 infection.
ASFV MGF360/530 genes affect IFN-α production in macrophage cell cultures.
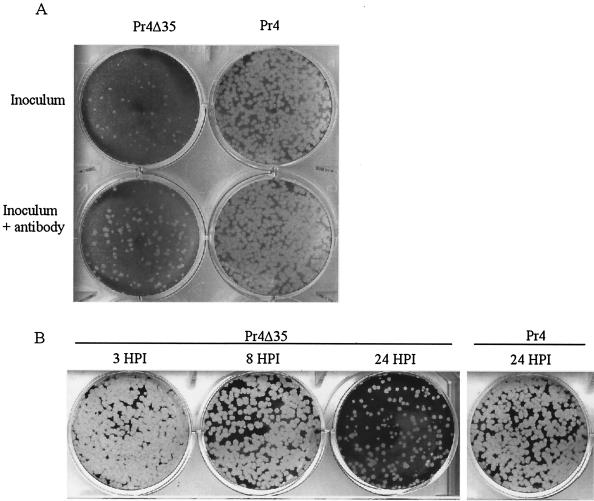
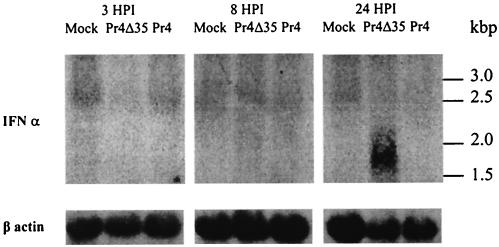
The altered transcriptional pattern observed for Pr4Δ35-infected cells was characteristic of a type 1 IFN response or a combined response to IFN, dsRNA, and/or viral infection (12, 18, 23, 41, 45). Pr4Δ35-infected culture supernatants were assayed for the presence of IFN with a biological VSV plaque reduction assay (7). In three independent experiments, an 80 to 90% reduction in the number of plaques, along with a considerable decrease in plaque size, was observed for Pr4Δ35-infected culture supernatant (Fig. 2A), indicating the presence of an acid-stable soluble factor(s) capable of inhibiting the growth of VSV. This inhibition was partially reversed (115% increase in plaque number) by addition of anti-IFN-α antibodies to the infected culture supernatant (Fig. 2A), indicating a role for IFN-α in VSV growth inhibition. The kinetics of IFN-α and IFN mRNA accumulation were determined at 3, 8, and 24 hpi for Pr4- and Pr4Δ35-infected macrophages (MOI of 10) with the VSV plaque reduction assay and Northern analysis. IFN-α activity was not detectable in Pr4-infected culture supernatant at any time point or in Pr4Δ35-infected supernatants at 3 and 8 hpi; however, a clear reduction (50 to 70% decrease) in the number of VSV plaques was observed for Pr4Δ35-infected culture supernatant at 24 hpi (Fig. 2B). Inhibition was partially reversed (42% increase in plaque number; P = 0.0023) by prior incubation of these infected culture supernatants with anti-IFN-α antibody (data not shown). Northern blot analysis was performed to determine if IFN-α was synthesized de novo during infection with Pr4Δ35 (Fig. 3). RNAs were obtained from noninfected and Pr4- and Pr4Δ35-infected macrophages at 3, 8, and 24 hpi and hybridized to a radiolabeled swine IFN-α cDNA probe. In Pr4Δ35-infected macrophages, a 1.7-kbp transcript corresponding to IFN-α was detected at 24 hpi, but not at earlier times. IFN-α transcription was not detected in mock- or Pr4-infected macrophages at any time point examined, indicating that IFN-α was produced only in Pr4Δ35-infected macrophages.
FIG. 2.
Supernatants from Pr4Δ35-infected macrophage cell cultures contain IFN-α. MDBK cells were preincubated with supernatants from Pr4Δ35- and Pr4-infected macrophages and subsequently infected with VSV as described in Materials and Methods. (A) Pr4Δ35 and Pr4 inocula. Identical supernatants were preincubated for 2 h with antibodies to IFN-α to inhibit IFN activity and to determine the specificity of the antiviral effect (lower row). (B) ASFV-infected supernatants at 3, 8, and 24 hpi.
FIG. 3.
Northern blot analysis of IFN-α expression in swine ASFV-infected macrophage cell cultures infected with mock, Pr4Δ35, and Pr4 at 3, 8, and 24 hpi. Ten micrograms of total RNA was run on denaturing gels, blotted onto nylon membranes, and hybridized to 32P-labeled antisense RNA corresponding to IFN-α (top) and β-actin (bottom).
DISCUSSION
Overall, our data indicate that the ASFV MGF360/530 genes directly or indirectly suppress IFN responses. An infection-dependent type I IFN transcriptional response and the presence of IFN-α were observed in Pr4Δ35-infected macrophage cell cultures but not in parental Pr4-infected cultures. Thus, factors associated with active Pr4Δ35 infection, potentially including dsRNA, viral replicative intermediates, and viral proteins, were necessary for the induced transcriptional response at 3 hpi. Although detectable levels of IFN-α were present in Pr4Δ35-infected culture supernatant, IFN-α was not sufficient by itself for induction of the response (Table 1).
These results support and extend prior observations for IFN responses to ASFV infection (39, 47, 48). As is the case here, Powell et al. were unable to detect IFN-α in mock- or ASFV-infected, phorbol myristic acid-stimulated alveolar macrophage cell culture supernatants (39). IFN-α transcription was suppressed by ASFV at 4 hpi, producing no detectable IFN-α at 16 hpi. UV-inactivated virus was unable to inhibit phorbol myristic acid-induced IFN-α expression, suggesting that viral functions are required for inhibition (39). Additionally, Vallee et al. demonstrated that major histocompatibility complex class I antigens induced in porcine aortal endothelial cells by treatment with IFN-α prior to ASFV infection were rapidly down-regulated following ASFV infection (4 pi), indicating the presence of a viral mechanism for actively suppressing the IFN response (47). The early suppression of IFN responses by MGF360/530 genes described here may account for these observations. However, it is likely that ASFV, like other large DNA viruses, encodes multiple complementary and perhaps redundant mechanisms for controlling host IFN responses (31, 42, 49).
Given the novelty of ASFV MGF360/530 genes, it is difficult to predict how they might function in suppressing host IFN responses. These genes lack sequence similarity to known genes and protein motifs, and consistent with an early host range function, MGF360/530 genes are expressed at 2 to 3 hpi (21, 51). Conceivably, they could be directly or indirectly involved in suppressing early activation of members of the IFN regulatory factor (IRF) family (4). These genes, which are involved in virus-induced activation of type I IFN and of IFN-stimulated genes (ISG) such as Mx, PKR, and ISG-15, participate in both innate and adaptive immune responses to viral infection (8). Possible target candidates include IRF-3, IRF-5 and IRF-7, which are critical transcription factors required for virus-induced IFN production (4, 5). IRF-3 is phosphorylated and activated in response to virus infection and dsRNA (40). Modulation of IRF function to prevent IFN responses occurs in different viral systems. For example, paramyxovirus simian virus 5 V protein sequesters IRF-3 in the cytoplasm, preventing its translocation to the nucleus and the subsequent production of IFN-β (25). The Kaposi's sarcoma herpesvirus encodes a cluster of four genes with homology to cellular IRFs, some of which can inhibit induction of IFN genes and ISG in infected cells (53). Hepatitis C virus encodes a serine protease that blocks the phosphorylation and effector action of IRF-3 (17).
Alternatively, MGF360/530 may prevent amplification of IFN responses. IFN-α acts in an autocrine manner to stimulate its own expression and expression of multiple members of the IFN-α gene family (33). Type I IFN biological activity is initiated by the binding of IFN-α or -β to its unique receptor, with subsequent activation of the JAK/STAT pathway (41). Transcription of IFN-α/β-activated genes starts within minutes of cells coming into contact with these cytokines and often does not require new protein synthesis (41). Viruses are known to block the JAK/STAT pathway with diverse strategies that include sequestering IRF activators, targeting STAT1 or STAT2 proteins for degradation, and inactivating JAK (22). A role of MGF360/530 in suppressing amplification of IFN responses could explain differences in IFN-α mRNA levels and the presence of IFN-α in Pr4Δ35-infected culture supernatant only at late time points (24 hpi). Amplification of IFN-α responses leads to expression of multiple IFN-α gene family members (32). Structural or antigenic differences among these family members may account for the incomplete neutralization of infected culture supernatant inhibitory activity by antibodies to IFN-α, as seen in the IFN biological assay (Fig. 2).
The inability of Pr4Δ35 to suppress an IFN response in infected cells may account for its growth defect in macrophage cell cultures. Pr4Δ35 exhibits a 100- to 1,000-fold growth defect in macrophage cell cultures that is characterized by early apoptotic cell death (54) (Zsak, unpublished). IFN-α has been shown to inhibit ASFV replication in monocytes and alveolar macrophages (16). IFN-induced antiviral cellular responses, together with the potential for increased PKR activity in Pr4Δ35-infected cells, may in part be responsible for the growth defect and the apoptotic response. Increased apoptosis of Pr4Δ35-infected cells may involve PKR or the IFN-induced CARD domain-containing helicase gene. Increased expression of the PKR activator gene PACT and decreased expression of the inhibitor of PKR (P58) suggest that Pr4Δ35 infection may affect PKR activity. PKR has a proapoptotic effect mediating dsRNA- and virus-induced programmed cell death and renders cells extremely susceptible to virus-induced apoptosis (20). The helicase gene induced in Pr4Δ35-infected cells resembles cellular Mda-5, an early-response gene responding primarily to IFN-β and tumor necrosis factor α. It functions as a dsRNA-dependent ATPase that contains a caspase recruitment motif (CARD) (27). Direct involvement of Mda-5 in the prevention or induction of apoptosis has yet to be clearly demonstrated; however, caspases are critical activators of apoptosis, and Mda-5 expression inhibits colony formation in melanoma cells (27).
Pr4Δ35 exhibits a growth defect in vivo and is attenuated in pigs (54) (Zsak et al., unpublished). The inability of Pr4Δ35 to suppress an IFN response may be a significant factor in the attenuated phenotype. Pr4Δ35 infection induces expression of two known chemokines: MCP-1, a C-C-type chemokine that attracts monocytes and promotes mast cell activation, and IP-10, a CXC chemokine that attracts monocytes, T lymphocytes, and NK cells and has the capacity to control viral replication (15, 30, 35). Other Pr4Δ35-induced genes with a potential impact on viral virulence and host range are the Mx protein and IFN-induced TM-3. Mx proteins are IFN-regulated GTP-binding proteins of the dynamin family that block influenza virus mRNA synthesis in the nucleus (3, 24). TM-3 is a protein highly inducible by IFN-α, -β, and -γ (11). Pr4Δ35 infection induces expression of the proteolysis-related genes ISG15 and ISG43. ISG15 induction is the earliest known response to type I IFNs, and ISG15 is one of the most strongly induced proteins after IFN treatment or after viral infection (10). ISG15 is a 17-kDa ubiquitin-like protein that functions by conjugating to other proteins. It has been suggested that it is involved in cell-to-cell signaling, possibly by inducing IFN-γ secretion by monocytes and macrophages (10). ISG15 is secreted from monocytes and lymphocytes, and recently it has been shown that viruses can inhibit ISG15 function, suggesting a possible antiviral function for it (52). ISG43 (ubiquitin-processing protease 43) is an ISG15-specific processing protease whose function is to specifically remove ISG15 from conjugated proteins (34).
Given the novelty of ASFV MGF360/530 genes and their biological significance for viral virulence and macrophage host range, it will be important to define their functions in suppressing an IFN response.
Acknowledgments
We thank E. Tulman for many helpful comments and suggestions and A. Lakowitz, J. Bier, O. Bennet, and A. Zsak for excellent technical assistance.
REFERENCES
- 1.Afonso, C. L., L. Zsak, C. Carrillo, M. V. Borca, and D. L. Rock. 1998. African swine fever virus NL gene is not required for virus virulence. J. Gen. Virol. 79:2543-2547. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Asano, A., J. H. Ko, T. Morozumi, N. Hamashima, and T. Watanabe. 2002. Polymorphisms and the antiviral property of porcine mx1 protein. J. Vet. Med. Sci. 64:1085-1089. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, B., B. Lubyova, and P. M. Pitha. 2002. On the role of IRF in host defense. J. Interferon Cytokine Res. 22:59-71. [DOI] [PubMed] [Google Scholar]
- 5.Barnes, B. J., P. A. Moore, and P. M. Pitha. 2001. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J. Biol. Chem. 276:23382-23390. [DOI] [PubMed] [Google Scholar]
- 6.Chinsangaram, J., M. Koster, and M. J. Grubman. 2001. Inhibition of L-deleted foot-and-mouth disease virus replication by alpha/beta interferon involves double-stranded RNA-dependent protein kinase. J. Virol. 75:5498-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinsangaram, J., M. E. Piccone, and M. J. Grubman. 1999. Ability of foot-and-mouth disease virus to form plaques in cell culture is associated with suppression of alpha/beta interferon. J. Virol. 73:9891-9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Civas, A., M. L. Island, P. Genin, P. Morin, and S. Navarro. 2002. Regulation of virus-induced interferon-A genes. Biochimie 84:643-654. [DOI] [PubMed] [Google Scholar]
- 9.Clemens, M. J. 1997. PKR-a protein kinase regulated by double-stranded RNA. Int. J. Biochem. Cell. Biol. 29:945-949. [DOI] [PubMed] [Google Scholar]
- 10.D'Cunha, J., S. Ramanujam, R. J. Wagner, P. L. Witt, E. Knight, Jr., and E. C. Borden. 1996. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J. Immunol. 157:4100-4108. [PubMed] [Google Scholar]
- 11.Deblandre, G. A., O. P. Marinx, S. S. Evans, S. Majjaj, O. Leo, D. Caput, G. A. Huez, and M. G. Wathelet. 1995. Expression cloning of an interferon-inducible 17-kDa membrane protein implicated in the control of cell growth. J. Biol. Chem. 270:23860-23866. [DOI] [PubMed] [Google Scholar]
- 12.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeTray, D. E. 1963. African swine fever. Adv. Vet. Sci. 8:299-333. [PubMed] [Google Scholar]
- 14.Dixon, L. K., J. V. Costa, J. M. Escribano, D. L. Rock, E. Vinuela, and P. J. Wilkinson. 2000. Family Asfarviridae, p. 159-165. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, and S. M. Lemon (ed.), Virus taxonomy. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 15.Dufour, J. H., M. Dziejman, M. T. Liu, J. H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195-3204. [DOI] [PubMed] [Google Scholar]
- 16.Esparza, I., J. C. Gonzalez, and E. Viñuela. 1988. Effect of interferon-alpha, interferon-gamma and tumour necrosis factor on African swine fever virus replication in porcine monocytes and macrophages. J. Gen. Virol. 69:2973-2980. [DOI] [PubMed] [Google Scholar]
- 17.Foy, E., K. Li, C. Wang, R. Sumpter, Jr., M. Ikeda, S. M. Lemon, and M. Gale, Jr. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300:1145-1148. [DOI] [PubMed] [Google Scholar]
- 18.Geiss, G., G. Jin, J. Guo, R. Bumgarner, M. G. Katze, and G. C. Sen. 2001. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J. Biol. Chem. 276:30178-30182. [DOI] [PubMed] [Google Scholar]
- 19.Genovesi, E. V., F. Villinger, D. J. Gerstner, T. C. Whyard, and R. C. Knudsen. 1990. Effect of macrophage-specific colony-stimulating factor (CSF-1) on swine monocyte/macrophage susceptibility to in vitro infection by African swine fever virus. Vet. Microbiol. 25:153-176. [DOI] [PubMed] [Google Scholar]
- 20.Gil, J., and M. Esteban. 2000. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis 5:107-114. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez, A., V. Calvo, F. Almazan, J. M. Almendral, J. C. Ramirez, I. de la Vega, R. Blasco, and E. Vinuela. 1990. Multigene families in African swine fever virus: family 360. J. Virol. 64:2073-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 23.Guo, J., K. L. Peters, and G. C. Sen. 2000. Induction of the human protein P56 by interferon, double-stranded RNA, or virus infection. Virology 267:209-219. [DOI] [PubMed] [Google Scholar]
- 24.Haller, O., and G. Kochs. 2002. Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic 3:710-717. [DOI] [PubMed] [Google Scholar]
- 25.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 303:15-32. [DOI] [PubMed] [Google Scholar]
- 26.Hegde, P., R. Qi, K. Abernathy, C. Gay, S. Dharap, R. Gaspard, J. E. Hughes, E. Snesrud, N. Lee, and J. Quackenbush. 2000. A concise guide to cDNA microarray analysis. BioTechniques 29:548-550, 552-554, 556. [DOI] [PubMed] [Google Scholar]
- 27.Kang, D. C., R. V. Gopalkrishnan, Q. Wu, E. Jankowsky, A. M. Pyle, and P. B. Fisher. 2002. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 99:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimmel, A. R., and S. L. Berger. 1987. Preparation of cDNA and the generation of cDNA libraries: overview. Methods Enzymol. 152:307-316. [DOI] [PubMed] [Google Scholar]
- 29.Lee, T. G., N. Tang, S. Thompson, J. Miller, and M. G. Katze. 1994. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol. Cell. Biol. 14:2331-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Goffic, R., T. Mouchel, F. Aubry, J. J. Patard, A. Ruffault, B. Jegou, and M. Samson. 2002. Production of the chemokines monocyte chemotactic protein-1, regulated on activation normal T cell expressed and secreted protein, growth-related oncogene, and interferon-gamma-inducible protein-10 is induced by the Sendai virus in human and rat testicular cells. Endocrinology 143:1434-1440. [DOI] [PubMed] [Google Scholar]
- 31.Leib, D. A. 2002. Counteraction of interferon-induced antiviral responses by herpes simplex viruses. Curr. Top. Microbiol. Immunol. 269:171-185. [DOI] [PubMed] [Google Scholar]
- 32.Levy, D. E. 2002. Whence interferon? Variety in the production of interferon in response to viral infection. J. Exp. Med. 195:F15-F18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy, D. E., I. Marie, and A. Prakash. 2003. Ringing the interferon alarm: differential regulation of gene expression at the interface between innate and adaptive immunity. Curr. Opin. Immunol. 15:52-58. [DOI] [PubMed] [Google Scholar]
- 34.Li, X. L., J. A. Blackford, C. S. Judge, M. Liu, W. Xiao, D. V. Kalvakolanu, and B. A. Hassel. 2000. RNase-L-dependent destabilization of interferon-induced mRNAs. A role for the 2-5A system in attenuation of the interferon response. J. Biol. Chem. 275:8880-8888. [DOI] [PubMed] [Google Scholar]
- 35.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, T. A. Hamilton, D. A. Armstrong, and T. E. Lane. 2001. The CXC chemokines IP-10 and Mig are essential in host defense following infection with a neurotropic coronavirus. Adv. Exp. Med. Biol. 494:323-327. [DOI] [PubMed] [Google Scholar]
- 36.Mebus, C. A. 1988. African swine fever. Adv. Virus Res. 35:251-269. [DOI] [PubMed] [Google Scholar]
- 37.Neilan, J. G., L. Zsak, Z. Lu, G. F. Kutish, C. L. Afonso, and D. L. Rock. 2002. Novel swine virulence determinant in the left variable region of the African swine fever virus genome. J. Virol. 76:3095-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plowright, W., G. R. Thomson, and J. A. Neser. 1994. African swine fever, p. 568-599. In J. A. W. Coetzer, G. R. Thomson, and R. C. Tustin (ed.), Infectious diseases in livestock with special reference to South Africa, vol. 1. Oxford University Press, Oxford, United Kingdom.
- 39.Powell, P. P., L. K. Dixon, and R. M. E. Parkhouse. 1996. An IκB homolog encoded by African swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J. Virol. 70:8527-8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75:8909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 42.Smith, S. A., and G. J. Kotwal. 2002. Immune response to poxvirus infections in various animals. Crit. Rev. Microbiol. 28:149-185. [DOI] [PubMed] [Google Scholar]
- 43.Stears, R. L., R. C. Getts, and S. R. Gullans. 2000. A novel, sensitive detection system for high-density microarrays using dendrimer technology. Physiol. Genomics 3:93-99. [DOI] [PubMed] [Google Scholar]
- 44.Thomson, G. R. 1985. The epidemiology of African swine fever: the role of free-living hosts in Africa. Onderstepoort J. Vet. Res. 52:201-209. [PubMed] [Google Scholar]
- 45.Tiwari, R. K., J. Kusari, and G. C. Sen. 1987. Functional equivalents of interferon-mediated signals needed for induction of an mRNA can be generated by double-stranded RNA and growth factors. EMBO J. 6:3373-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tulman, E. R., and D. L. Rock. 2001. Novel virulence and host range genes of African swine fever virus. Curr. Opin. Microbiol. 4:456-461. [DOI] [PubMed] [Google Scholar]
- 47.Vallee, I., S. W. Tait, and P. P. Powell. 2001. African swine fever virus infection of porcine aortic endothelial cells leads to inhibition of inflammatory responses, activation of the thrombotic state, and apoptosis. J. Virol. 75:10372-10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whittall, J. T., and R. M. Parkhouse. 1997. Changes in swine macrophage phenotype after infection with African swine fever virus: cytokine production and responsiveness to interferon-gamma and lipopolysaccharide. Immunology 91:444-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiang, Y., R. C. Condit, S. Vijaysri, B. Jacobs, B. R. Williams, and R. H. Silverman. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 76:5251-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yáñez, R. J., J. M. Rodríguez, M. L. Nogal, L. Yuste, C. Enríquez, J. F. Rodríguez, and E. Viñuela. 1995. Analysis of the complete nucleotide sequence of African swine fever virus. Virology 208:249-278. [DOI] [PubMed] [Google Scholar]
- 51.Yozawa, T., G. F. Kutish, C. L. Afonso, Z. Lu, and D. L. Rock. 1994. Two novel multigene families, 530 and 300, in the terminal variable regions of African swine fever virus genome. Virology 202:997-1002. [DOI] [PubMed] [Google Scholar]
- 52.Yuan, W., and R. M. Krug. 2001. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J. 20:362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, F. X., S. M. King, E. J. Smith, D. E. Levy, and Y. Yuan. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 99:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zsak, L., Z. Lu, T. G. Burrage, J. G. Neilan, G. F. Kutish, D. M. Moore, and D. L. Rock. 2001. African swine fever virus multigene family 360 and 530 genes are novel macrophage host range determinants. J. Virol. 75:3066-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]