Abstract
Background
Animal data suggest that natriuretic peptides play an important role in energy metabolism, but prospective studies evaluating a relationship between these peptides and type 2 diabetes mellitus (T2DM) in humans are few and results are conflicting.
Methods
We used a prospective case-cohort approach (n=491 T2DM cases, n=561 reference subcohort) within the Women's Health Study to evaluate baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations and the risk of incident T2DM. We also tested for associations between 4 common variants in the natriuretic peptide A and B genes (NPPA-NPPB) and NT-proBNP concentrations (n=458) and incident type 2 diabetes (n=1372 cases among 22607 women).
Results
Case subjects had higher median baseline body mass index (29.4 vs. 25.0 kg/m2, P<0.001) and lower baseline median (IQR) NT-proBNP concentrations [46.8 ng/L (26.1, 83.2) vs 66.7 ng/L (39.3, 124.7), P<0.001]. In proportional hazards models adjusting for established diabetes risk factors, women in the highest quartile of baseline NT-proBNP (≥117.4 ng/L) had a 49% reduction in risk of T2DM (HR 0.51, 0.30–0.86, P=0.01) relative to those in the lowest quartile. Two of the 4 tested variants in NPPA-NPPB (rs632793, rs198389) associated with increased NT-proBNP concentrations and reduced risk of T2DM. For example, each copy of the minor allele of rs632793 was associated with increased NT-proBNP (β (SE)=0.201 (0.063), P<0.01) and decreased T2DM risk (HR 0.91, 0.84–0.989, P=0.026).
Conclusions
NT-proBNP concentrations that are high, but still within the reference interval, associate with reduced risk of incident diabetes in women and support a favorable role for natriuretic peptides in the development of T2DM.
Keywords: Natriuretic peptides, type 2 diabetes, women, epidemiology, prevention
Introduction
The roles of natriuretic peptides in promoting vasodilation and natriuresis are well known,(1, 2) and assays for both the active form of B-type natriuretic peptide (BNP) and the inactive amino-terminal fragment of the peptide (NT-proBNP) have gained widespread acceptance as tools for the diagnosis of heart failure in the acute-care setting.(3, 4) B-type natriuretic peptides also play an important role in regulating lipolysis in adipocytes,(5, 6) and several cross-sectional epidemiological studies have reported inverse associations between BNP concentrations and body mass index, glucose concentrations, measures of insulin resistance, and the number of components of the metabolic syndrome.(7–9)
However, prospective studies of the relationship between BNP concentrations and incident type 2 diabetes mellitus (T2DM) are scarce and inconsistent.(10–12) To better understand the possible metabolic effects of the natriuretic peptide system, we first measured baseline concentrations of NT-proBNP and several other plasma markers of metabolism and inflammation in healthy middle aged and older women enrolled in the Women's Health Study (WHS).(13) We then examined correlations between these biomarkers and NT-proBNP concentrations and tested for evidence of association between NT-proBNP concentrations and incident T2DM in a prospective case-cohort study of women from the WHS. We also conducted a series of analyses to determine if common genetic variants previously reported to associate with NT-proBNP concentrations and T2DM demonstrated similar evidence of association in our cohort of women.
Materials and Methods
Study Participants
Study participants were enrolled in the Women's Health Study (WHS), a completed randomized, double-blind, placebo-controlled 2×2 factorial trial of aspirin and vitamin E in the prevention of cardiovascular disease and cancer among 39,876 female health professionals aged 45 years and older.(13) Enrollment began in 1992, participants were free of cardiovascular disease at baseline, and a total of 28,345 women provided a blood sample at baseline. Among these, 19,871 (70.1%) were fasting at time of sample collection. These samples were centrifuged and then stored in liquid nitrogen until laboratory analysis. The randomized portion of the study was completed in March 2004, and women were invited to participate in continued observational follow up. Participants have been followed continuously for incident cardiovascular disease and T2DM. Three different subgroups of WHS participants were identified for the analyses of NT-proBNP and incident T2DM, common genetic variation and NT-proBNP concentrations, and common genetic variation and incident T2DM components of this study.
Prospective Association of NT-proBNP and Type 2 Diabetes
For the prospective analysis of NT-proBNP concentrations and incident T2DM, all subjects were selected from among women without baseline-diagnosed diabetes (absent self report of T2DM and HbA1c < 6.5%) and with adequate baseline fasting blood specimens. We designed a case-cohort study by first selecting a random sample of incident T2DM cases (n=491) from among the entire WHS cohort. Cases were defined as non-diabetic WHS participants who provided baseline blood specimens and subsequently developed newly diagnosed T2DM. A randomly selected subcohort of women with available blood specimens was then chosen as a reference risk set (n=561). The sampling technique excluded women with baseline T2DM, but women who developed T2DM during follow up were eligible for inclusion in the reference subcohort. By chance, 50 such women were selected for the reference subcohort, so the case-cohort set included 491 incident T2DM cases and 511 non-cases. The selection of the reference subcohort was stratified by race/ethnicity (White vs. non-White) as well as age (in 5-year strata) to approximately match the distribution among cases of T2DM (included in this study) as well as a separate group of cases of incident cardiovascular disease (not included in this study).
Common Variants in NPPA-NPPB and NT-proBNP Concentrations
To validate previously reported associations between the common genetic variants in the natriuretic peptide precursor A (NPPA) and natriuretic peptide precursor B (NPPB) genes and NT-proBNP, we used data from women selected for the reference subcohort who also had genotyping performed as part of the larger Women's Genome Health Study, which is a subset of the women enrolled in the WHS. The Women's Genome Health Study includes more than 25,000 women with available genotyping data. The study is described in detail elsewhere.(14) Of the 561 women selected for inclusion in the reference subcohort for this study, 458 were of European ancestry and had both genotyping information and available NT-proBNP concentrations.
Common Variants in NPPA-NPPB and Incident Type 2 Diabetes Mellitus
To test whether common variation in NPPA and NPPB was associated with incident T2DM in the WHS and Women's Genome Health Study, we identified all women of European ancestry with genotyping information who had an HbA1c < 6.5% and did not have a clinical diagnosis of T2DM at baseline. In total, there were 22607 women in this cohort with 1372 cases of incident T2DM.
Type 2 Diabetes Ascertainment
Incident T2DM was identified in an identical fashion for all participants in the WHS and in this study. Possible cases of incident T2DM were initially identified by self-report on annual questionnaires that asked if and when the participant had been diagnosed with T2DM since baseline. Self-reported cases were then confirmed by physician-administered telephone interviews using the American Diabetes Association diagnostic criteria (15) or a self-administered supplemental questionnaire. In a validation study, both interview-based and supplemental questionnaire-based confirmation yielded positive predictive values >90% in comparison to medical record review.(16) In particular, the positive predictive value of the supplemental questionnaire was 99% (95% CI 97–100%). Overall, in 95% of all-self-reported T2DM events, sufficient information for confirmation or disconfirmation of the endpoint was obtained, and only confirmed cases were used in this analysis. Because the prevalence of undiagnosed T2DM among middle-aged US adults is so high, and because this analysis was designed to investigate the role of NT-proBNP as a determinant of future disease, we limited our sample to individuals with a baseline HbA1c less than 6.5%.
Laboratory Analysis
Plasma amino-terminal B-type natriuretic peptide (NT-proBNP) was measured using an electrochemiluminescent immunoassay assay provided by Roche Diagnostics. The assay has day-to-day variability at concentrations of 175, 434 and 6781 ng/L of 3.2, 2.4 and 2.2%, respectively. In addition to NT-proBNP, plasma samples were measured for insulin, leptin, resistin, interleukin-6, free fatty acids, oxidized low-density lipoprotein cholesterol, creatinine, hemoglobin A1c, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, high-sensitivity C-reactive protein, and soluble intercellular adhesion molecule-1 in a core laboratory certified by the National, Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization Program. Details of the assays used and measures of day-to-day variation are available upon request.
DNA samples were genotyped with the Infinium II technology from Illumina (Human HAP300 panel) as previously described.(14) All samples were required to have successful genotyping using the BeadStudio v. 3.3 software (Illumina) for at least 98% of the single nucleotide polymorphisms (SNPs). SNPs with call rates <90% and samples with percentage of missing genotypes higher than 2% or that deviated from Hardy-Weinberg equilibrium were removed from the analysis. We identified 4 single nucleotide polymorphisms (rs198358, rs5068, rs632793, and rs198389) that had been associated with NT-proBNP concentrations, atrial natriuretic peptides concentrations, or aspects of the metabolic syndrome and/or T2DM in previously published literature.(10, 17–20) rs198358 was directly genotyped with 99.25% success. The estimated allele dose at rs5068, rs632793, and rs198389 was imputed using the MACH 1.0.16 program (http://www.sph.umich.edu/csg/abecasis/mach/index.html) and data from HapMap.(21–23) Imputation quality scores ranged from 0.962 (rs632793) to 0.998 (rs5068).
Statistical Analysis
Prospective Association of NT-proBNP and Type 2 Diabetes
Data for this component of the study were analyzed as a case-cohort study using appropriate weighting of the observations.(24, 25) Since sampling was stratified by race/ethnicity and age, we used stratum-specific weighting.(26) Overall population characteristics were estimated using weights equal to the inverse of the inclusion probabilities in the subcohort sample using the Horvitz-Thompson weighting approach in PROC SURVEYMEANS in SAS 9.2.(24, 27) Crude frequencies and median (IQR) values of baseline clinical and biochemical characteristics were compared using chi-square and Wilcoxon rank-sum tests. Estimated population frequencies of categorical variables were compared using the Rao-Scott Chi-Square Test in PROC SURVEYFREQ in SAS 9.2. Estimated population means for continuous variables in the cases and subcohort were compared using weighted linear regression in PROC SURVEYREG in SAS 9.2. Associations between NT-proBNP and other serum metabolic markers, including glycated hemoglobin (HbA1c), creatinine, estimated glomerular filtration rate (eGFR), insulin, ghrelin, and adiponectin were computed in the subcohort using Spearman correlation coefficients.
NT-proBNP concentrations were divided into increasing quartiles based on the distribution in the reference subcohort of non-diabetic women. Cox proportional hazards models adjusted for the weighted sample size(28) were used to calculate adjusted hazard ratios (HR) and 95% confidence intervals (CIs) for incident T2DM according to the increasing quartile of NT-proBNP, with the lowest quartile chosen as the referent category. Tests for trend across quartiles of NT-proBNP were calculated using an indicator variable set to the median value of each quartile of NT-proBNP. We also assessed the association between NT-proBNP and incident diabetes modeled per one standard deviation increment on the natural logarithm-transformed scale. Women without complete information for all covariables included in multivariable models were excluded from those analyses. A maximum number of 27 women were excluded from the primary analysis for missing covariate information.
Because the results of the primary analysis described above suggested the possibility of a threshold effect, we conducted an exploratory analysis in which we analyzed several NT-proBNP concentrations as possible dichotomous cutpoints for association with T2DM. The primary analysis suggested the 75th percentile (117.4 ng/L) would be a possible cutpoint; we then selected the NT-proBNP value corresponding to the 50th, 55th, 60th, 65th, 70th, 80th, 85th, and 90th percentile of the NT-proBNP distribution in the reference subcohort. The risk of T2DM for women with values at or above each threshold were compared to a reference group comprised of women with NT-proBNP values less than the 50th percentile using adjusted Cox proportional hazards models as described for the primary analysis. We also performed sensitivity analyses that censored women at the time when they developed cardiovascular disease (N=14) or congestive heart failure (N=8) prior to either developing diabetes or the close of follow up. All analyses were performed using SAS 9.1 or 9.2 for UNIX.
Common Variants in NPPA-NPPB and NT-proBNP Concentrations
Linear regression models adjusted for age, body mass index, systolic blood pressure, and subject selection criteria (prevalent T2DM, incident T2DM, incident cardiovascular disease, both incident cardiovascular disease and T2DM, or subcohort) were used to test for evidence of association between rs198358, rs5068, rs632793, and rs198389 and natural logarithm-transformed NT-proBNP concentrations in the reference subcohort. Each SNP was tested for evidence of association with body mass index, systolic blood pressure, and fasting insulin concentration. Evidence for deviation of an additive mode of inheritance was evaluated by including a term to test for nonlinearity in the linear regression model.
Common Variants in NPPA-NPPB and Incident Type 2 Diabetes Mellitus
Cox proportional hazards models were constructed to test for evidence of association between rs198358, rs5068, rs632793, and rs198389 and incident T2DM. Models were adjusted for age, BMI, BMI squared, hormone therapy use, family history of diabetes, exercise at least once per week, and alcohol use at least 1 drink per day. Each SNP was tested for evidence of association with body mass index, hormone therapy use, family history of diabetes, exercise, and alcohol use. Evidence for deviation of an additive mode of inheritance was evaluated by including a term to test for nonlinearity in the proportional hazards regression model.
The funding entities played no role in the conduct, data collection, data management, data analysis, or preparation of the manuscript.
Results
Prospective Association of NT-proBNP and Type 2 Diabetes
The baseline characteristics of the women who subsequently developed T2DM and the women selected for inclusion in the reference subcohort, both in crude analyses and after reweighting to reflect sampling from the larger Women's Health Study population, are displayed in Table 1. As anticipated, in both crude and reweighted analyses, women who developed T2DM had higher BMI and blood pressure and were more likely to have a family history of diabetes, drank alcohol and exercised less frequently, and had higher fasting insulin and hsCRP concentrations (each P<0.0001). In both the crude and reweighted analyses, NT-proBNP concentrations were lower among the women who subsequently developed T2DM (each P<0.001). The median (IQR) duration of follow up for all women included in the case-cohort sample was 13.4 (7.7, 11.6) years.
Table 1.
Comparisons of baseline characteristics of type 2 diabetes cases and the random subcohort, in the sample and reweighted to the total Women's Health Study cohort
| Crude | Reweighted to Population | |||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | Diabetes Cases N=491 | Reference Subcohort n=561 | P value | Diabetes Cases N=1228 | Reference Subcohort n=19277 | P-value |
| Age, years | 53.3 (49.0, 59.8) | 56.7 (50.9, 64.2) | <0.0001 | 53.6 (49.3, 59.7) | 53.5 (49.2, 59.3) | 0.53 |
| White race, N (%) | 450 (92) | 516 (92) | 0.37 | 1134 (92.4) | 18468 (95.8) | |
| Body mass index, kg/m2 | 29.4 (25.8, 33.9) | 25.6 (23.0, 28.3) | <0.0001 | 29.6 (26.0, 33.9) | 25.6 (22.9, 28.3) | <0.0001 |
| Blood pressure, mm Hg | 135 (125, 145) | 125 (115, 135) | <0.0001 | 135 (125, 145) | 125 (115, 135) | <0.0001 |
| Current smoking, N (%) | 63 (12.9) | 56 (10.0) | 0.18 | 159 (12.9) | 1999 (10.4) | 0.29 |
| Family history of diabetes, N (%) | 208 (42.4) | 158 (28.2) | <0.0001 | 520 (42.3) | 5178 (26.9) | <0.0001 |
| Alcohol use ≥1 drink per day, N (%) | 27 (5.5) | 45 (8.0) | 0.06 | 361 (29.4) | 7749 (40.2) | 0.0001 |
| Exercise ≥1 times per week, N (%) | 159 (32.4) | 259 (46.2) | <0.0001 | 399 (32.5) | 8991 (46.6) | <0.0001 |
| Hormone therapy use, N (%) | 209 (42.7) | 242 (43.3) | 0.60 | 533 (43.5) | 8379 (43.6) | 0.80 |
| Randomized aspirin use, N (%) | 248 (50.5) | 275 (49.0) | 0.66 | 617 (50.2) | 9312 (48.3) | 0.41 |
| eGFR, ml/min/1.73 m2 | 112.5 (92.4, 141.7) | 92.6 (7584, 110.6) | <0.0001 | 112.7 (92.6, 141.7) | 96.1 (80.5, 115.7) | <0.0001 |
| HbA1c, % | 5.3 (5.1, 5.5) | 5.0 (4.9, 5.2) | <0.0001 | 5.3 (5.1, 5.5) | 5.0 (4.8, 5.2) | <0.0001 |
| Fasting insulin, μIU/mL | 12.5 (8.4, 18.9) | 6.8 (4.4, 10.7) | <0.0001 | 12.5 (8.4, 18.8) | 6.9 (4.3, 10.7) | <0.0001 |
| hsCRP, mg/L | 4.0 (2.2, 7.0) | 2.2 (0.9, 4.6) | <0.0001 | 4.1 (2.3, 7.0) | 2.1 (0.9,4.5) | <0.0001 |
| NT-proBNP, ng/L | 46.8 (26.1, 83.2) | 65.2 (37.8, 117.4) | <0.0001 | 46.9 (26.4, 83.1) | 58.7 (35.2, 103.5) | <0.0001 |
| Ln(NT-proBNP) | 3.9 (3.3, 4.4) | 4.2 (3.7, 4.8) | <0.0001 | 3.9 (3.3, 4.4) | 4.1 (3.6, 4.6) | <0.001 |
Abbreviations: eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; hsCRP, high sensitivity C-reactive protein; NT-proBNP, amino-terminal pro B-type natriuretic peptide.
All values for continuous variables are median (interquartile range).
Correlations between baseline concentrations of NT-proBNP and baseline anthropometric, metabolic, lipid, and inflammatory biomarkers in the reference subcohort are displayed in Table 2. In the unadjusted analysis, age (0.37), insulin (−0.25) and leptin (−0.18) appeared to be at least as closely correlated with NT-proBNP concentrations as other known correlates, including body mass index (−0.17), blood pressure (0.06), and eGFR (−0.20). After adjusting for age, and for age and BMI, correlations between NT-proBNP and insulin persisted (−0.25 and −0.18, respectively). Neither HbA1c nor any of the inflammatory biomarkers showed any important correlations with NT-proBNP.
Table 2.
Spearman correlations between NT-proBNP and other metabolic biomarkers in the reference subcohort, a representative subsample of the Women's Health Study.
| Unadjusted | Age adjusted | Age and BMI adjusted | ||||
|---|---|---|---|---|---|---|
| Characteristic or Biomarker | Rho | P-value | Rho | P-value | Rho | P-value |
| Age, years | 0.371 | <0.0001 | - | - | - | - |
| Body mass index, kg/m2 | −0.165 | <0.0001 | −0.185 | <0.0001 | - | - |
| Systolic blood pressure, mm Hg | 0.059 | 0.17 | −0.044 | 0.30 | 0.025 | 0.57 |
| Metabolic biomarkers | ||||||
| Insulin, μIU/mL | −0.245 | <0.0001 | −0.248 | <0.0001 | −0.180 | <0.0001 |
| Hemoglobin A1c, % | −0.039 | 0.36 | −0.106 | 0.01 | −0.069 | 0.10 |
| Ghrelin, pg/mL | 0.051 | 0.23 | 0.051 | 0.23 | −0.015 | 0.72 |
| Leptin, ng/mL | −0.177 | <0.0001 | −0.178 | <0.0001 | −0.070 | 0.10 |
| Resistin, ng/mL | −0.033 | 0.43 | −0.073 | 0.08 | −0.051 | 0.23 |
| Creatinine, mg/dL | −0.014 | 0.74 | −0.054 | 0.20 | −0.052 | 0.22 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | −0.195 | <0.0001 | −0.048 | 0.26 | 0.070 | 0.10 |
| Lipid biomarkers | ||||||
| Total cholesterol, mg/dL | −0.130 | 0.002 | −0.209 | <0.0001 | −0.196 | <0.0001 |
| Low density lipoprotein cholesterol, mg/dL | −0.151 | 0.0004 | −0.228 | <0.0001 | −0.202 | <0.0001 |
| Oxidized low density lipoprotein cholesterol | −0.027 | 0.52 | −0.087 | 0.04 | −0.054 | 0.21 |
| High density lipoprotein cholesterol, mg/dL | 0.143 | 0.0007 | 0.132 | 0.002 | 0.070 | 0.10 |
| Triglycerides, mg/dL | −0.110 | 0.009 | −0.148 | 0.0005 | −0.098 | 0.02 |
| Free fatty acids mEq/L | 0.031 | 0.46 | −0.048 | 0.26 | −0.022 | 0.61 |
| Inflammatory biomarkers | ||||||
| High-sensitivity C-reactive protein, mg/L | 0.016 | 0.70 | −0.026 | 0.54 | 0.050 | 0.24 |
| Interleukin (IL)-6, pg/mL | 0.076 | 0.07 | −0.009 | 0.83 | 0.047 | 0.27 |
| Soluble intracellular adhesion molecule-1, ng/mL | 0.006 | 0.89 | −0.090 | 0.03 | −0.061 | 0.15 |
The risk of incident T2DM according to increasing quartiles of NT-proBNP concentrations is displayed in Table 3. In models adjusted for age and race, increasing concentrations of NT-proBNP were associated with a reduced risk of incident T2DM for all 3 quartiles compared to the first quartile. After adjusting for BMI, the observed reductions in the risk of T2DM were attenuated in quartiles 2, 3 and 4, but remained statistically significant for the highest as compared to the lowest category of NT-proBNP (HR 0.54, 95% CI 0.33–0.89, P=0.015). This relationship persisted after adjustment for eGFR, hormone therapy use, family history of diabetes, physical activity, and alcohol consumption (HR 0.51, 95% CI 0.30–0.86, P=0.011) and was attenuated by further adjusting for baseline insulin concentrations (HR 0.58, 95% CI 0.35–0.97, P=0.039) We observed a 20% reduction in the risk of incident T2DM per 1 standard deviation unit increase in natural logarithm-transformed NT-proBNP concentrations in fully adjusted models (Table 3 Model 4; HR 0.80, 95% CI 0.67–0.96, P=0.016). This point estimate did not change substantially when HbA1c was added to the model (HR 0.80, 95% CI 0.65–0.99, P=0.044). No significant change in the risk estimates was noted after further adjusting for high sensitivity C-reactive protein or after substituting alternative measures of adiposity (waist circumference, hip circumference, or waist to hip ratio) for BMI in adjusted models (data not shown).
Table 3.
Risk of incident diabetes according to baseline concentration of NT-proBNP.
| Adjusted Risk of Incident Type 2 Diabetes | ||||||
|---|---|---|---|---|---|---|
| Model | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend | Per 1 unit SD* |
| NT-proBNP range (ng/L) | <37.4 | 37.4 to < 64.2 | 64.2 to < 117.0 | ≥117.4 | ||
| Model 1 | 1.0 | 0.56 (0.40–0.78) | 0.61 (0.43–0.85) | 0.40 (0.27–0.60) | <0.001 | 0.68 (0.58–0.79) |
| P-value | 0.0006 | 0.004 | <0.0001 | <0.001 | ||
| Model 2 | 1.0 | 0.84 (0.56–1.26) | 0.93 (0.61–1.42) | 0.54 (0.33–0.89) | 0.047 | 0.79 (0.67–0.94) |
| P-value | 0.40 | 0.75 | 0.015 | 0.007 | ||
| Model 3 | 1.0 | 0.88 (0.58–1.34) | 0.97 (0.62–1.51) | 0.51 (0.30–0.86) | 0.041 | 0.78 (0.65–0.93) |
| P-value | 0.56 | 0.89 | 0.011 | 0.006 | ||
| Model 4 | 1.0 | 0.96 (0.63–1.47) | 0.99 (0.63–1.56) | 0.58 (0.35–0.97) | 0.10 | 0.80 (0.67–0.96) |
| P-value | 0.86 | 0.97 | 0.039 | 0.016 | ||
Model 1: Age and race adjusted
Model 2: Model 1 plus BMI (kg/m2) and BMI squared
Model 3: Model 2 plus eGFR, hormone therapy use, family history of diabetes, exercise at least once per week, alcohol use at least 1 drink per day.
Model 4: Model 3 plus insulin (μIU/mL)
Abbreviations: SD, standard deviation
Per 1 unit standard deviation (SD) of the natural logarithm-transformed NT-proBNP value.
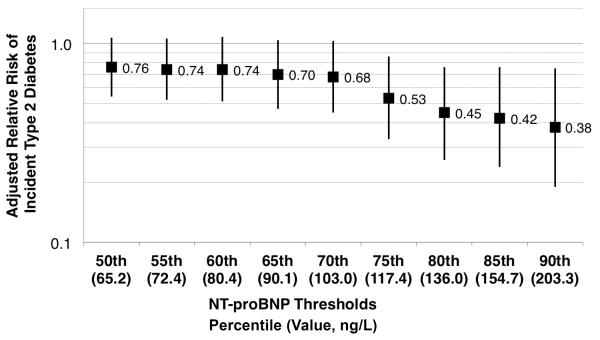
The results of the categorical analysis suggested the possibility of a threshold effect, in which only women with the highest NT-proBNP concentrations were at reduced risk of T2DM. In an exploratory analysis, we examined the risk of incident T2DM for women with NT-proBNP concentrations at or above the 55th through the 90th percentiles, using women with NT-proBNP concentrations below the 50th percentile (<65.2 ng/L) as the referent group (Figure 1). As can be seen, the association with incident T2DM is statistically significant for NT-proBNP cutpoints of the 75th percentile and higher. For example, women with NT-proBNP concentrations ≥75thth percentile (117.4 ng/L) were at 47% lower risk of T2DM (HR 0.53, 95% CI 0.33–0.86, P=0.009) than those with NT-proBNP concentrations below the median after adjustment for all confounders but insulin. Further adjustment for insulin did not substantially alter the results (HR 0.58, 95% CI 0.36–0.93, P=0.024).
Figure 1.
Adjusted relative risk of incident type 2 diabetes calculated for a range of possible cutpoints for NT-proBNP. Possible cutpoints were chosen based on the distribution of NT-proBNP concentrations in the reference subcohort. The risk of incident diabetes is calculated for women with NT-proBNP concentrations at or above the indicated value compared to women with NT-proBNP concentrations less than the 50th percentile (65.2 ng/L). Hazard ratios and 95% confidence intervals are adjusted for age, race, body mass index (kg/m2), estimated glomerular filtration rate, hormone therapy use, family history of diabetes, exercise at least once per week, and alcohol use of 1 drink or more per day. Further adjustment for baseline insulin concentrations did not alter the results substantially (data not shown).
We did not observe any evidence of statistical interaction between NT-proBNP concentrations above or below this threshold and World Health Organization category of BMI (<25, 25 to < 30, 30+kg/m2), a family history of diabetes, systolic blood pressure, or age above or below 53 (the median in the study population). In a sensitivity analysis, we constructed separate proportional hazards models that censored women when they developed either incident cardiovascular disease or congestive heart failure. Our results remained unchanged.
Common Variants in NPPA-NPPB and NT-proBNP Concentrations
The results for the test of association between SNPs rs198358, rs5068, rs632793, and rs198389 are displayed in Table 4. As can be seen, we observed statistically significant evidence of association between natural logarithm transformed baseline NT-proBNP concentrations and rs632792 and rs198389. Each copy of the minor allele at each SNP increased natural-log transformed NT-proBNP concentrations by 0.2012 and 0.1860 standard deviation units, respectively. No statistically significant evidence of deviation from an additive mode of inheritance was detected for any of the SNPs. While none of the SNPs was significantly related to blood pressure, we did detect a statistically significant association between BMI and rs632792 (beta (standard error) per 1 kg/m2 increase in BMI = −0.69682 (0.33556), P=0.038) and rs198389 (−0.66137 (0.32973), P=0.046). Including an interaction term between each SNP and BMI in linear regression models of natural logarithm transformed NT-proBNP concentrations did not improve model fit.
Table 4.
Association of common variants in the NPPA-NPPB region with natural logarithm-transformed NT-proBNP concentrations. For each of the SNPs, the smallest P-value was seen when an additive (rather than dominant) mode of inheritance was assumed.
| Per-allele association with 1 SD change in natural log-transformed NT-proBNP concentrations* | |||||||
|---|---|---|---|---|---|---|---|
| SNP | Chr | Position | Minor/Major Allele | MAF† | Quality Score | Beta (standard error) | P-Value |
| rs198358‡ | 1 | 11826663 | C/T | 0.236 | N/A | 0.0829 (0.0680) | 0.228 |
| rs5068 | 1 | 11828561 | G/A | 0.059 | 0.998 | −0.0035 (0.1255) | 0.978 |
| rs632793 | 1 | 11833264 | G/A | 0.409 | 0.962 | 0.2012 (0.0631) | 0.0015 |
| rs198389 | 1 | 11841858 | G/A | 0.427 | 0.987 | 0.1860 (0.0620) | 0.0029 |
Abbreviations: SNP, single nucleotide polymorphism; Chr, chromosome; MAF, minor allele frequency; SD, standard deviation.
Adjusted for Age, BMI, and systolic blood pressure. The mean (SD) natural logarithm transformed NT-proBNP concentration in the reference subcohort is 4.254 (0.821).
Minor allele frequency in the participants of European descent with available genotyping information and NT-proBNP concentrations. rs198358 was directly genotyped; genotyping information for the remaining SNPs was imputed.
This test of association used directly measured genotypes. The results did not differ when missing genotypes at rs198358 (n=1, or 0.22%) were replaced with the imputed estimated genotype dose.
Common Variants in NPPA-NPPB and Incident Type 2 Diabetes Mellitus
When each of the four SNPs was tested for evidence of association with incident T2DM in adjusted Cox models (n=22607 with 1372 cases of incident T2DM), we observed evidence of association for both rs632793 and rs198389. The per-allele hazard ratio for T2DM was 0.91 (95% CI 0.84–0.989; P=0.026) for rs632793 and 0.92 (95% CI 0.85–0.995; P=0.036) for rs198389. The effect of SNPs at either rs632793 or rs198389on T2DM risk is consistent with the direction of their effect on NT-proBNP concentrations. However, we observed no statistically significant association for either rs198358 (per-allele HR 0.94, 95% CI 0.86–1.03, P=0.18) or rs5068 (per-allele HR 0.98, 95% CI 0.83–1.16, P=0.84). No statistically significant evidence of deviation from an additive mode of inheritance was detected for any of the SNPs, and no significant associations with BMI, family history of diabetes, exercise, hormone therapy use, or alcohol use were noted.
Discussion
In these prospective analyses, we report a statistically robust association between concentrations of NT-proBNP and the risk of incident diabetes in a population of initially healthy women at usual risk for T2DM. We observed a nearly 50% reduction in the risk of type 2 diabetes among women with NT-proBNP concentrations above 117 ng/L (the 75th percentile), a concentration that is both well within the reference interval and routinely observed in clinical practice. This reduced risk cannot be attributed to intervening congestive heart failure or coronary heart disease occurring during follow-up. Furthermore, while we observed the expected associations between NT-proBNP concentrations and age and body mass index, we also observed an inverse relationship between NT-proBNP and fasting insulin that persisted after adjusting for age and body mass index. We also confirmed previously reported associations between common variation in the NPPA-NPPB gene and NT-proBNP concentrations, and an association between two SNPs in NPPA-NPPB and incident T2DM in initially healthy women. The direction of these effects was consistent with data we report here for the association between plasma NT-proBNP and the risk of incident T2DM.
These data, taken together, lend support to the hypothesis that natriuretic peptides are causally involved in the development of insulin resistance and clinical diabetes. The magnitude of risk reduction observed is consistent with that observed by Pfister and colleagues in the EPIC-Norfolk cohort, who reported a 21% reduction in the adjusted risk of incident T2DM per 1 unit SD increase in natural-logarithm transformed NT-proBNP concentrations (HR 0.79, 95% CI 0.64–0.97, P=0.02).(10) In that same report, Pfister and colleagues performed a Mendelian randomization analysis that supports a causal role for natriuretic peptides in the development of T2DM. Our observations support that conclusion, and suggest that alterations in NT-proBNP concentrations precede the clinical diagnosis of diabetes by many years. Other prospective studies have reported similar results, with one study reporting significant associations between B-type natriuretic peptide concentrations, NT-proBNP concentrations, and mid-regional atrial natriuretic peptide concentrations and incident T2DM(11) and another reporting a significant association for mid-regional atrial natriuretic peptide but not NT-proBNP.(12) Cross-sectional studies have also reported inverse associations between natriuretic peptides and features of the metabolic syndrome.(7–9)
Our prospective study adds to this previously published work by closely examining the relationships between NT-proBNP and hormones with important metabolic functions, including insulin, leptin, and ghrelin. We observed a modest inverse correlation between NT-proBNP and fasting insulin concentrations that was independent of BMI and appeared to be stronger than its correlation with blood pressure, a traditionally accepted determinant of natriuretic peptide concentrations. In our study, the relationship between NT-proBNP and incident T2DM is independent of both BMI and fasting insulin concentrations, supporting the hypothesis that alterations in NT-proBNP concentrations contribute to T2DM risk via a different biological mechanism or with different timing than alterations in insulin sensitivity or obesity.
We were also able to replicate the results of several genetic studies that reported significant associations between common variation in the NPPA-NPPB gene and NT-proBNP concentrations, blood pressure, and prevalent T2DM.(17–20) Our results are largely consistent with those previously published findings, as 3 of the 4 SNPs previously reported to associate with NT-proBNP concentrations were also associated with NT-proBNP concentrations in our cohort. The fourth, rs5068, has shown less consistent association with B-type natriuretic peptide concentrations.(19, 20) We also replicated previously published cross-sectional associations between variation in NPPA-NPPB and prevalent T2DM.(17, 18) In our prospective analysis of 1372 cases of incident T2DM, we observed a statistically significant association between two closely linked SNPs (rs632793 and rs198389, r2 = 0.874 and D' =1.00 in the Caucasian population of 1000 Genomes(29)) in the NPPA-NPPB gene and T2DM.
A possible causal link between natriuretic peptides and T2DM is supported by evidence from experimental mouse models of altered natriuretic peptide expression. In recent work by Miyashita and colleagues, transgenic mice that expressed BNP at levels >100 times normal were leaner and were resistant to the effects of a high fat diet on weight gain and insulin resistance, and had lower total body fat and increased oxygen consumption.(30) These effects were mediated by increased mitochondrial biogenesis through a natriuretic peptide activated cyclic GMP signaling pathway. Furthermore, natriuretic peptide receptors are expressed in pancreatic beta-cells and modulate glucose-stimulated insulin secretion.(31, 32)
Although our study is prospective, the possibility of reverse causality cannot be excluded. In particular, we observed a modest inverse correlation between NT-proBNP concentrations and fasting insulin concentrations at baseline, and physiologic evidence of insulin resistance can precede the clinical signs by 3–6 years T2DM.(33) However, the median time to clinical diabetes in our cohort was 8.2 years, and the association between NT-proBNP and incident T2DM was independent of insulin concentrations, suggesting that the two hormones may mark different pathophysiologic processes on the path to T2DM. We also observed an inverse correlation between BMI and NT-proBNP concentrations, as have others.(9) Our prospective data raise the possibility that the relationship between natriuretic peptide concentrations and altered metabolism is not completely mediated by obesity, although overlapping biologic pathways are difficult to exclude. Finally, we observed no attenuation of the relationship between NT-proBNP and T2DM after adjusting for subclinical inflammation, which is an important risk marker for T2DM, and suggests that natriuretic peptides act to alter T2DM risk by an alternative mechanism.(34)
In conclusion, we observed a more than 40% reduction in the risk of incident type 2 diabetes among initially healthy women with NT-proBNP concentrations that were higher than 117 ng/L. This association was independent of other traditional risk factors for diabetes, including body mass index, kidney function, age, and fasting insulin concentrations. We also replicate published reports of associations between common variation in the NPPA-NPPB genes and both NT-proBNP concentrations and the risk of T2DM. These prospective data offer strong support for the hypothesis that the natriuretic peptide system plays an important role in glucose and fatty acid metabolism and in the development of type 2 diabetes.
Acknowledgements
This study was supported by grants HL-043851, HL-080467, and HL-099355 from the National Heart, Lung, and Blood Institute and CA-047988 from the National Cancer Institute, as well as the Donald W. Reynolds Foundation (Las Vegas, NV), the Leducq Foundation (Paris, France), and the Doris Duke Charitable Foundation (New York, NY). Support for the N-terminal pro-B-type natriuretic peptide assays was provided through an investigator-initiated grant to Dr. Everett from Roche Diagnostics and by a Discovery Award from the Brigham and Women's Hospital Cardiovascular Leadership Council. Dr. Everett is also supported by the American Heart Association Scientist Development Grant (0835304N). Support for the construction of the case-cohort study was provided through an investigator-initiated grant to Dr. Ridker from Roche Pharma. Genotyping was provided by Amgen, Inc (Cambridge, MA).
Abbreviations
- BMI
body mass index
- eGFR
estimated glomerular filtration rate
- HbA1c
hemoglobin A1c
- HR
hazard ratio
- hsCRP
high-sensitivity C-reactive protein
- IQR
interquartile range
- NT-proBNP
amino-terminal pro B-type natriuretic peptide
- SNP
single nucleotide polymorphism
- T2DM
type 2 diabetes mellitus
- WHS
Women's Health Study
Footnotes
Human Genes: NPPA – natriuretic peptide A; NPPB – natriuretic peptide B
Publisher's Disclaimer: This is an un-copyedited authored manuscript copyrighted by the American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of 'Fair Use of Copyrighted Materials' (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article will be made available at http://www.clinchem.org 12 months after its publication in Clinical Chemistry.
References
- 1.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. New Engl J Med. 1998;339:321–8. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 3.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of b-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 4.Januzzi JL, Jr., Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, et al. The n-terminal pro-bnp investigation of dyspnea in the emergency department (pride) study. Am J Cardiol. 2005;95:948–54. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: A new lipolytic pathway in human adipocytes. Faseb J. 2000;14:1345–51. [PubMed] [Google Scholar]
- 6.Lafontan M, Moro C, Sengenes C, Galitzky J, Crampes F, Berlan M. An unsuspected metabolic role for atrial natriuretic peptides: The control of lipolysis, lipid mobilization, and systemic nonesterified fatty acids levels in humans. Arterioscler Thromb Vasc Biol. 2005;25:2032–42. doi: 10.1161/01.ATV.0000183728.14712.d8. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 8.Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, et al. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension. 2005;46:660–6. doi: 10.1161/01.HYP.0000179575.13739.72. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–53. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 10.Pfister R, Sharp S, Luben R, Welsh P, Barroso I, Salomaa V, et al. Mendelian randomization study of b-type natriuretic peptide and type 2 diabetes: Evidence of causal association from population studies. PLoS Med. 2011;8:e1001112. doi: 10.1371/journal.pmed.1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salomaa V, Havulinna A, Saarela O, Zeller T, Jousilahti P, Jula A, et al. Thirty-one novel biomarkers as predictors for clinically incident diabetes. PLoS One. 2010;5:e10100. doi: 10.1371/journal.pone.0010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnusson M, Jujic A, Hedblad B, Engstrom G, Persson M, Struck J, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: The prospective malmo diet and cancer study. J Clin Endocrinol Metab. 2012;97:638–45. doi: 10.1210/jc.2011-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, et al. Rationale, design, and methodology of the women's genome health study: A genome-wide association study of more than 25,000 initially healthy american women. Clinical Chemistry. 2008;54:249–55. doi: 10.1373/clinchem.2007.099366. [DOI] [PubMed] [Google Scholar]
- 15.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 16.Ding EL, Song Y, Manson JE, Pradhan AD, Buring JE, Liu S. Accuracy of administrative coding for type 2 diabetes in children, adolescents, and young adults. Diabetes Care. 2007;30:e98. doi: 10.2337/dc07-0903. author reply e9. [DOI] [PubMed] [Google Scholar]
- 17.Meirhaeghe A, Sandhu MS, McCarthy MI, de Groote P, Cottel D, Arveiler D, et al. Association between the t-381c polymorphism of the brain natriuretic peptide gene and risk of type 2 diabetes in human populations. Hum Mol Genet. 2007;16:1343–50. doi: 10.1093/hmg/ddm084. [DOI] [PubMed] [Google Scholar]
- 18.Choquet H, Cavalcanti-Proenca C, Lecoeur C, Dina C, Cauchi S, Vaxillaire M, et al. The t-381c snp in bnp gene may be modestly associated with type 2 diabetes: An updated meta-analysis in 49 279 subjects. Hum Mol Genet. 2009;18:2495–501. doi: 10.1093/hmg/ddp169. [DOI] [PubMed] [Google Scholar]
- 19.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, et al. Association of common variants in nppa and nppb with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–53. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannone V, Boerrigter G, Cataliotti A, Costello-Boerrigter LC, Olson TM, McKie PM, et al. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. Journal of the American College of Cardiology. 2011;58:629–36. doi: 10.1016/j.jacc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International HapMap C. Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, et al. A second generation human haplotype map of over 3.1 million snps. Nature. 2007;449:851–61. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pei YF, Li J, Zhang L, Papasian CJ, Deng HW. Analyses and comparison of accuracy of different genotype imputation methods. PloS One. 2008;3:e3551. doi: 10.1371/journal.pone.0003551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Ding J, Abecasis GR. Mach 1.0: Rapid haplotype reconstruction and missing genotype inference. American Journal of Human Genetics. 2006;S79:2290. [Google Scholar]
- 24.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 25.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50:1064–72. [PubMed] [Google Scholar]
- 26.Borgan O, Langholz B, Samuelsen SO, Goldstein L, Pogoda J. Exposure stratified case-cohort designs. Lifetime Data Anal. 2000;6:39–58. doi: 10.1023/a:1009661900674. [DOI] [PubMed] [Google Scholar]
- 27.Horvitz DG, Thompson DJ. A generalization of sampling without replacement from a finite universe. Journal of the American Statistical Association. 1952;47:663–85. [Google Scholar]
- 28.Langholz B, Jiao J. Computational methods for case-cohort studies. Comp Stats Data Analysis. 2007;51:3737–48. [Google Scholar]
- 29.A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, et al. Natriuretic peptides/cgmp/cgmp-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–92. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soriano S, Ropero AB, Alonso-Magdalena P, Ripoll C, Quesada I, Gassner B, et al. Rapid regulation of k(atp) channel activity by 17{beta}-estradiol in pancreatic {beta}-cells involves the estrogen receptor {beta} and the atrial natriuretic peptide receptor. Mol Endocrinol. 2009;23:1973–82. doi: 10.1210/me.2009-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ropero AB, Soriano S, Tuduri E, Marroqui L, Tellez N, Gassner B, et al. The atrial natriuretic peptide and guanylyl cyclase-a system modulates pancreatic beta-cell function. Endocrinology. 2010;151:3665–74. doi: 10.1210/en.2010-0119. [DOI] [PubMed] [Google Scholar]
- 33.Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: An analysis from the whitehall ii study. Lancet. 2009;373:2215–21. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA : the Journal of the American Medical Association. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]