Abstract
Background
The goal of this study was to determine, in lung transplant patients, if laparoscopic antireflux surgery (LARS) is an effective means to prevent aspiration as defined by the presence of pepsin in the bronchoalveolar lavage fluid (BALF).
Methods
Between September 2009 and November 2010, we collected BALF from 64 lung transplant patients at multiple routine surveillance assessments for acute cellular rejection, or when clinically indicated for diagnostic purposes. The BALF was tested for pepsin by enzyme-linked immunosorbent assay (ELISA). We then compared pepsin concentrations in the BALF of healthy controls (n = 11) and lung transplant patients with and without gastroesophageal reflux disease (GERD) on pH-monitoring (n = 8 and n = 12, respectively), and after treatment of GERD by LARS (n = 19). Time to the development of bronchiolitis obliterans syndrome was contrasted between groups based on GERD status or the presence of pepsin in the BALF.
Results
We found that lung transplant patients with GERD had more pepsin in their BALF than lung transplant patients who underwent LARS (P = .029), and that pepsin was undetectable in the BALF of controls. Moreover, those with more pepsin had quicker progression to BOS and more acute rejection episodes.
Conclusion
This study compared pepsin in the BALF from lung transplant patients with and without LARS. Our data show that: (1) the detection of pepsin in the BALF proves aspiration because it is not present in healthy volunteers, and (2) LARS appears effective as a measure to prevent the aspiration of gastroesophageal refluxate in the lung transplant population. We believe that these findings provide a mechanism for those studies suggesting that LARS may prevent nonallogenic injury to the transplanted lungs from aspiration of gastroesophageal contents.
Long-term morbidity and mortality after lung transplantation are largely attributable to bronchiolitis obliterans syndrome (BOS), a form of chronic rejection.1,2 In turn, BOS and rejection have been associated with gastroesophageal reflux disease (GERD), although direct causality has not been established.3,4 The mechanism by which GERD may cause or exacerbate BOS is not known; however, it is thought that aspiration of gastroesophageal contents may trigger a nonallogenic injury to the transplanted lungs. The investigation of the causal relationship between GERD, aspiration, and BOS is important because GERD can be treated before lung function deteriorates.4–6 In fact, studies have shown that operative control of GERD may stabilize or improve lung function in some patients with BOS, especially when a laparoscopic fundoplication is performed early after lung transplantation.7–10 Control of reflux is key, because GERD may be a modifiable risk factor for the progression of BOS; GERD and aspiration of gastroduodenal substances, like pepsin, might be stopped by laparoscopic antireflux surgery (LARS). Nevertheless, the role and the indications for LARS in the management of patients with BOS or rejection is less clear because it is not known if aspiration can be prevented by LARS, or if aspiration of gastroesophageal refluxate indeed represents a nonallogenic injury to the transplanted lungs.
The aim of this study was to determine if LARS after lung transplantation could represent an effective means to prevent aspiration as defined by the presence of pepsin in the bronchoalveolar lavage fluid (BALF), and if aspiration of gastroesophageal refluxate is associated with a worse clinical outcome than patients without evidence of aspiration. We hypothesized that recovery of pepsin in the BALF confirms aspiration and that LARS could be protective against aspiration of pepsin.
PATIENTS AND METHODS
From September 2009 to November 2010, 168 BALF samples were prospectively collected from 64 lung transplant patients. Patients were enrolled in this study at the time of surveillance bronchoscopy. Data included basic demographics, clinical parameters, and pathology reports. Bronchoscopy with bronchoalveolar lavage and transbronchial biopsy (TBBx) were performed for surveillance of acute cellular rejection at 3, 6, 9, and 12 months post-transplant or when clinically indicated for diagnostic purposes. BALF was collected routinely from the right middle lobe for unilateral right and bilateral lung transplants, and from the lingula for unilateral left lung transplants. The BALF was then centrifuged at 1500 rpm for 10 min, aliquoted, and snap frozen at −80°C for analysis of pepsin levels.11 Similarly, TBBx were obtained from the right upper and lower lobes for bilateral lung transplants, and the upper and lower lobes in unilateral lung transplants. The TBBx were assessed for acute cellular rejection (ACR) and airway inflammation according to the Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection.12 Evidence of aspiration was also recorded and based on the review of the TBBx specimen by the pathologist who categorized aspiration by assessing the presence of exogenous material with foreign-body giant-cell reaction, large lipid droplets, and/or macrophages with large vacuoles.
Of the 64 lung transplant patients from whom BALF fluid was collected, 39 underwent physiologic testing for GERD at the discretion of our pulmonologists based on symptoms, objective findings of aspiration at surveillance bronchoscopy or on transbrochial biopsy, or an unexplained decrease in pulmonary function after lung transplantation. Patients with GERD were then evaluated for LARS and their candidacy for the operation was determined by the results of ambulatory pH monitoring. Evidence of aspiration at the time of bronchoscopy in those with positive pH findings played a substantial role in proceeding with LARS. To analyze our results, a convenient 6-month post-transplant cutoff was established retrospectively to stratify patients in an “early” or “late” fundoplication group given the findings of others that “early” fundoplication for GERD may prevent chronic pulmonary allograft dysfunction.6,7 In summary, 39 patients were either confirmed by ambulatory pH testing as GERD (−) (n = 12) or GERD (+) (n = 8), or had been treated by LARS for GERD (n = 19). BALF pepsin concentrations were subsequently compared between these groups and 11 healthy controls.
The BOS status (0–3) of the lung transplant recipients was established according to the International Society of Heart and Lung Transplantation guidelines (Table I).13 The time from lung transplantation to the development of BOS was compared subsequently between groups based on GERD status. Furthermore, time to development of BOS was also compared between patients based on their average BALF pepsin level, and grouped as having no detectable pepsin (n = 17) or any detectable pepsin (n = 45). Two patients received their transplants from another institution; therefore, as their FEV1 data were incomplete, they were excluded from this analysis. Finally, pepsin levels were compared between groups based on the presence and severity of ACR, evidence of aspiration on TBBx, and pulmonary infection status (all determined prospectively).
Table I.
Classification and grading of BOS in lung transplant recipients
| BOS 0 | FEV1 > 90% of baseline and FEF25–75 > 75% of baseline |
| BOS 0-p | FEV1 81–90% of baseline or FEF25–75≤ 75% of baseline |
| BOS 1 | FEV1 66–80% of baseline |
| BOS 2 | FEV1 51–65% of baseline |
| BOS 3 | FEV1 < 50% of baseline |
BOS, Bronchiolitis obliterans syndrome; BOS-p, potential bronchiolitis obliterans syndrome; FEV1, forced expiratory volume in 1 second; FEF25–75, forced expiratory flow from 25% to 75% of vital capacity.
This study was approved by the Loyola University Medical Center Institutional Review Board and informed consent was obtained from all study subjects.
Pepsin ELISA
Pepsin levels in the BALF were measured by a locally developed ELISA in our laboratories at the Burn and Shock Trauma Institute at Loyola University Medical Center using a monospecific antibody to porcine pepsin (Calbio-chem/EMD4Biosciences, Gibbstown, NJ). In brief, microtiter plates were coated with the capture antibody (Meridian Life Science, Cincinnati, OH) diluted in phosphate buffer saline at pH 7.4 and incubated overnight at 4°C. After coating, plates were blocked with phosphate buffer saline containing fish plasma and the BAL samples were added. This step was followed by incubation with the secondary antibody and the substrate, 3,3′ diaminobenzidine tetrahydrochloride. Plates were read on an ELISA plate reader (SpectraMax Plus384, Molecular Devices, Sunnyvale, CA) using Softmax Pro 3.1.2 software. The assay has a lower limit of detection of 1 ng/ml.
Ambulatory pH-monitoring
Proton pump inhibitors were stopped for 14 days and histamine H2-receptor antagonists were stopped for 3 days before pH-monitoring. A pH catheter (Sleuth system with BioVIEW software; Sandhill Scientific, Denver, CO) was passed through the nose and the pH sensor was positioned 5 cm from the manometrically determined upper border of the lower esophageal sphincter. The DeMeester score was calculated for the distal pH recordings. A score >14.7 was considered abnormal.14 Esophageal manometry was performed according to our previously published technique.15
Technique of fundoplication
All patients who underwent LARS received a 360° Nissen fundoplication according to our standardized technique.16
Statistical analysis
Statistical analyses were calculated with SAS Version 9.1 (SAS Institute Inc., Cary, NC) and GraphPad Prism 5 for Windows (GraphPad Software, La Jolla, CA). Data were analyzed using nonparametric statistical methods after assessing for Gaussian distribution with the D’Agostino and Pearson omnibus normality test. The Kruskal-Wallis and Mann-Whitney U tests were then applied, where appropriate. Cox-Mantel log-rank test was used to compare the distributions of freedom from BOS over time among groups. Results were reported as percentages for categorical variables and as median (with interquartile range) for scaled variables. Correlation between variables was assessed by calculating the Spearman rank correlation coefficient.
RESULTS
Our final cohort comprised 64 lung transplant patients from whom 168 BALF samples were collected at the time of surveillance or diagnostic bronchoscopy (median time of collection was 9 months after lung transplantation). Of the total cohort, 34 were female, 15 had a right single lung transplant, 20 had a left single lung transplant, 25 had a bilateral lung transplant, and 4 were re-transplanted, with the following distribution of end-stage lung diseases: cystic fibrosis (n = 10), chronic obstructive pulmonary disease (n = 27), idiopathic pulmonary fibrosis (n = 13), sarcoidosis (n = 3), alpha-1 antitrypsin deficiency (n = 4), pulmonary veno-occlusive disease (n = 1), bronchiolitis obliterans organizing pneumonia (n = 1), lymphangioleiomyomatosis (n = 1), and pulmonary fibrosis from rheumatoid arthritis, polymyositis, pneumoconiosis, or Jo-1 syndrome (n = 4). These patients were transplanted from February 15, 1996 to April 9, 2010; the mean time after transplantation to study enrollment was 21 months (range, 1–168). All patients were maintained on a standard protocol of maintenance immunosuppressive regimen that consisted of prednisone, a calcineurin inhibitor, and a cell-cycle inhibitor. Moreover, because all patients were enrolled in this study at the time of surveillance bronchoscopy the prevalence of symptoms of GERD prior to transplantation has not been presented.
Thirty-nine patients had been tested for GERD by ambulatory pH monitoring or had undergone LARS for GERD. Therefore, the prevalence of GERD in this study was 69%. Pulmonary function records were available with a median follow-up of 17 months. A total of 17 patients were diagnosed with BOS 1 or greater (median time to BOS 16 months), of which only 4 progressed to BOS during their first year after transplantation.
Table II shows the patient characteristics by GERD status and timing of LARS. Patients who were GERD (+) without antireflux surgery tended to be older than GERD (−) patients and those after LARS, which might be explained by the distribution of transplant indication, though these differences were not statistically significant. Likewise, the groups were generally comparable in terms of sex, type of lung transplant, time of follow-up, and prevalence of BOS at 1 year.
Table II.
Patient characteristics by GERD status and timing of LARS
| GERD (−) | GERD (+) No LARS | Early LARS | Late LARS | P value | |
|---|---|---|---|---|---|
| Number of patients | 12 | 8 | 9 | 10 | – |
| Age (years) | 59 (53–63) | 62 (54–66) | 55 (37–59) | 48 (32–55) | .018 |
| Female gender (%) | 8 (67) | 4 (50) | 5 (56) | 3 (30) | .276 |
| Transplant indication (%) | .322 | ||||
| CF | 1 | 1 | 2 | 3 | |
| COPD | 8 | 4 | 2 | 2 | |
| IPF | 3 | 1 | 1 | 4 | |
| AATD | 0 | 1 | 2 | 1 | |
| BOOP | 0 | 0 | 1 | 0 | |
| Jo-1 Syndrome | 0 | 1 | 0 | 0 | |
| PF from Pneumoconiosis | 0 | 0 | 1 | 0 | |
| Transplant type (%) | .538 | ||||
| Right single | 3 | 1 | 2 | 1 | |
| Left single | 5 | 2 | 2 | 1 | |
| Bilateral | 3 | 4 | 2 | 8 | |
| Re-transplant | 1 | 1 | 3 | 0 | |
| Follow-up (months) | 18 (16–24) | 17 (11–23) | 14 (12–22) | 22 (17–47) | .185 |
| BOS ≥1 at 1 yr (%) | 1 (33) | 1 (33) | 1 (33) | 0 | .651 |
Data presented as median (interquartile range).
AATD, Alpha-1 Antitrypsin deficiency; BOOP, bronchiolitis obliterans organizing pneumonia; BOS, bronchiolitis obliterans syndrome; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; IPF, idiopathic pulmonary fibrosis; LARS, laparoscopic antireflux surgery; PF, pulmonary fibrosis.
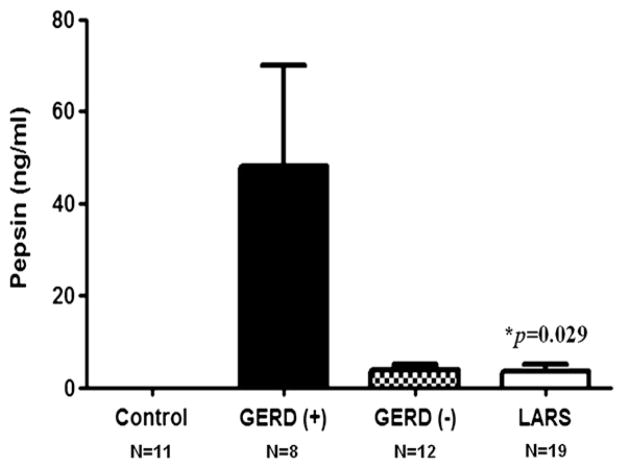
Figure 1 shows our comparison of the pepsin levels in the BALF of those with GERD who did not undergo LARS (n = 8), those with GERD who underwent LARS (n = 19), those without GERD (n = 12), and controls (n = 11). We found that pepsin was not detectable in the BALF of controls. Conversely, pepsin levels were increased in the BALF of lung transplant patients regardless of their reflux status. Most importantly, pepsin levels were less in patients after operative correction of reflux than in patients with GERD who did not undergo LARS (P = .029). Although we found no difference between actuarial curves comparing the time to BOS of lung transplant recipients based on their presence or absence of GERD or treatment by early or late LARS (P = .818) (Fig 2), we did find that those patients demonstrating any detectable pepsin had a quicker progression to BOS than patients without detectable pepsin levels (P = .058) (Fig 3).
Fig 1.
Comparison of the pepsin levels in the BALF among patients who are GERD (+), GERD (−), patients who underwent LARS, and controls shows that pepsin was not detectable in the BALF of controls, and that pepsin levels were less after LARS than in those GERD (+) patients who did not undergo LARS (P = .029). GERD, Gastroesophageal reflux disease; LARS, laparoscopic antireflux surgery.
Fig 2.
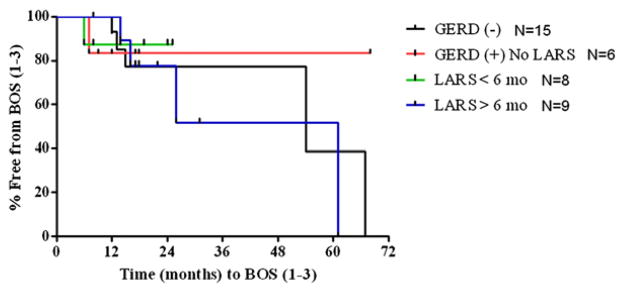
The actuarial curves comparing recipients based on GERD status or treatment by early or late LARS shows that the percentage of patients who did not develop BOS over time after their transplant was similar among those with or without GERD or who had early or late LARS (P = .818). Complete FEV1 data to generate actuarial curves were available in 38 patients. BOS, Bronchiolitis obliterans syndrome; GERD, gastroesophageal reflux disease; LARS, laparoscopic antireflux surgery.
Fig 3.
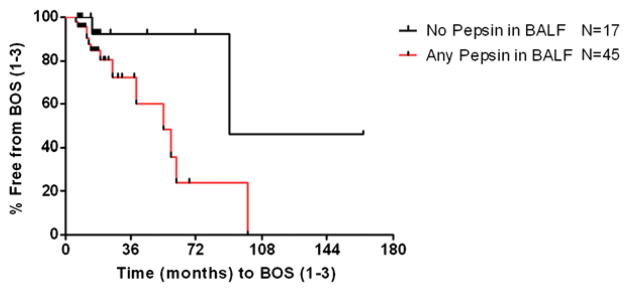
The actuarial curves show a decreased time since lung transplant to development of BOS in patients with any detectable pepsin than in those without pepsin (P = .058). Two patients whose FEV1 data were incomplete were excluded from the analysis. BOS, Bronchiolitis obliterans syndrome.
Given that increased pepsin levels in the BALF have been shown to be associated with an earlier progression to BOS, we were interested in determining whether there was a similar association with ACR events. Of the 168 BALF samples collected, 151 matching TBBx were identified as being sufficient for evaluation of ACR. Indeed, we found that with worse severity of episodes of ACR (≥A2) there was an increasingly greater level of pepsin in the BALF compared to that present in BALF samples of patients without ACR (A0) (P = .023). Subsequently, we evaluated whether pepsin in the BALF was associated with evidence of aspiration seen on TBBx. Among 153 BALF samples matched with TBBx, we found that BALF samples from patients with evidence of aspiration on TBBx had tended to have greater levels of pepsin (P = .111). Likewise, a similar trend was noted among 160 BALF samples from patients in whom the presence or absence of pulmonary infection (fungal, bacterial, or viral) was noted at the time of bronchoscopy (P = .127).
Last, we found no correlation between the DeMeester Score and pepsin concentrations in GERD (+) patients (Spearman’s rank correlation coefficient, R = 0.09).
DISCUSSION
Long-term morbidity and mortality after lung transplantation are largely attributable to BOS, a form of chronic rejection.1,2 It has been proposed that GERD, which is highly prevalent in this patient population, may trigger a nonallogenic mechanism of rejection by causing a chronic injury from continuously aspirating gastric contents. The 69% prevalence of reflux in this study population is consistent with other studies that have shown that nearly two thirds of lung transplant patient are affected by GERD.4,17 This increased prevalence of GERD after transplantation is thought to be secondary to disruption of vagal innervation during the transplant, negative effects of immunosuppressant drugs cyclosporine and tacrolimus on the motility of the gastrointestinal tract, and disruption of protective mechanisms against reflux and aspiration, such as cough reflexes and mucociliary clearance.18–20 This may also explain why patients in our control group had no pepsin in their BALF, because the absence of pepsin in their BALF suggests that they lack risk factors for GERD and have intact protective mechanisms that prevent aspiration.
Although the precise pathophysiology behind such a chronic injury is still unknown, evidence suggests that aspiration might be a contributing factor.21,22 Should aspiration be a link between GERD and BOS, one would also expect that increased levels of pepsin would be associated with a worse outcome, and that operative treatment of aspiration would preserve, if not improve, pulmonary allograft function. Thus far, no studies have elucidated the role of LARS as an effective means to prevent aspiration in lung transplant patients as defined by objective determination of pepsin in a large population. Pepsin seems suitable as a marker of aspiration because pepsin is produced only in the gastrointestinal tract, its secretion is not affected by proton pump inhibitors, and its concentration in the stomach does not seem to be influenced by physiologic responses after lung transplantation or immunosuppressive medications.23
Our results support our hypothesis that LARS is protective against aspiration of pepsin. We showed that pepsin levels were increased in the BALF of lung transplant patients regardless of their reflux status, and that pepsin levels were less in patients after operative correction of reflux than in patients with GERD who did not undergo LARS. These findings could finally validate LARS as a therapeutic option for lung transplant patients. In fact, the results of our study add strength to the literature that supports a role for operatively controlling GERD in this patient population. Indeed, operative control of GERD has been shown already to control BOS as after antireflux surgery lung function was improved, the proportion of patients affected by BOS was decreased, or the overall actuarial survival was better.4,5,7 In addition, when antireflux surgery was performed within 1 month of transplant, the incidence of BOS approached zero at 1 and 3 years after lung transplantation.6 In contrast, freedom from BOS in those without fundoplication was 96% and 60% at 1 and 3 years, respectively.6 While these studies only support a strong therapeutic potential of LARS in treating GERD and BOS, our study, which focuses on the direct detection of aspiration, could confirm the role of LARS in the management of these patients by providing a pathogenic basis of its mechanism of action. Our results imply the superiority of LARS in treating aspiration, rather than medical therapy with acid blocking agents, which only decrease acid reflux but do not affect nonacid reflux or aspiration.24,25 A prospective, multicenter, randomized trial between LARS and medical therapy being conducted currently by the group at Duke will set the future standards and indications of each therapy.
Our results also seem to point to the direct responsibility of aspiration in the pathogenesis of pulmonary allograft injury, as we have shown that aspiration is a pathologic event (eg, absent in healthy volunteers) and proved our hypothesis that aspiration of pepsin is associated with a worse clinical outcome than those patients who do not show evidence of aspiration. Our findings confirm those of Ward et al26 who found pepsin in the BALF of all 13 patients after lung transplantation and no pepsin in the BALF of controls. Ward et al also showed that the greatest levels of pepsin were found in recipients with acute rejection grade ≥A2. We also found that patients with greater levels of pepsin experienced worse episodes of rejection, but, in addition, we have also shown that those with measurable pepsin have quicker progression to BOS. This finding is relevant, because multiple episodes of acute rejection of all severities have been linked with the development of BOS.27 Finally, though others have found a relationship between evidence of aspiration and the presence of infection, we were unable to replicate their findings with our measurements of BALF pepsin levels.28 We also did not find a statistical difference between those patients with increased BALF pepsin and evidence of aspiration on TBBx, and suspect that a larger sample size might yield statistically significant differences for these latter comparisons.
Altogether, this evidence seems to support the causal role of aspiration of pepsin in the development of lung transplant failure; however, we found no difference in progression to BOS based on GERD status alone. The reasons of this finding might be explained by limitations in study design, referral bias, or poor reliability of ambulatory pH monitoring. Another reason could be that pepsin is only associated with aspiration and not the responsible substance in the refluxate that would cause lung damage. For instance, Blondeau et al25 showed that pepsin levels in BALF were similar among patients with BOS ≥ 1 and stable patients, and that there was no correlation between pepsin in BALF and FEV1. These authors concluded that the presence of pepsin represented a marker of aspiration of gastric contents rather than a marker of aspiration-induced BOS, (likely represented by aspiration of bile acids, according to the authors). The reasons for the discrepancy of our results with those of Blondeau et al25 remain elusive, although we believe that the correlation between BOS and reflux did not achieve significance because of their study design. In fact, in the study of Blondeau et al,25 pepsin was measured only once, which may have missed the importance of serial BALF assessment. Nonetheless, our hypothesis remains valid, because we did find that those patients demonstrating any detectable pepsin had a quicker progression to BOS than patients without detectable pepsin levels. This finding would still support a pathogenic role of pepsin in aspiration-induced lung failure. Another larger study that is currently undergoing in our institution may soon clarify this discrepancy, as well as determine the role of the detection of pepsin and bile acids against that of pH monitoring, as detection of pepsin or bile acids might be a better test to diagnose aspiration and its likelihood to predict lung damage than simple measurement of GERD as its surrogate.
Finally, this study was not designed specifically to determine longitudinally the difference in pepsin levels before and after LARS. Only a true prospective evaluation of these patients will give more strength to these preliminary results and clarify the definitive role of LARS as a protective mechanism against GERD and aspiration. Yet, our findings do support our hypothesis that LARS can be protective of aspiration, because they prove that pepsin in the BALF: (1) is highly present in patients with GERD; and almost absent in those with LARS and without GERD, (2) is not present in healthy volunteers; and (3) is associated with a quicker progression to BOS and more acute rejection episodes.
Acknowledgments
Supported by the Dr. Ralph and Marian C. Falk Medical Research Trust and by funding from Loyola University Medical Center. Accepted for oral presentation to the annual meeting of the Central Surgical Association, March 17–19, 2011.
The authors would like to thank Marcia Halerz, RN, BSN, MBA, for her assistance with IRB preparation and submission; Kristin Wojtulewicz for her administrative assistance with grant funding; James Gagermeier, MD, and Daniel Dilling, MD, from the Division of Pulmonary and Critical Care Medicine; Dr. Razan Wafai from the Department of Pathology; and the nurses and technicians (Brian Borger, Diane Duffe, Mary Gibbons, Yash Giri, Fredia Jordan-Turner, and Kim Rice) in the bronchoscopy suite of Loyola University Medical Center without whom this study could not have been possible.
References
- 1.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, et al. Registry for the International Society for Heart and Lung Transplantation: twenty-fifth Official adult lung and heart/lung transplantation report–2008. J Heart Lung Transplant. 2008;27:957–69. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 3.D’Ovidio F, Mura M, Tsang M, Waddell TK, Hutcheon MA, Singer LG, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1144–52. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Hadjiliadis D, Duane Davis R, Steele MP, Messier RH, Lau CL, Eubanks SS, et al. Gastroesophageal reflux disease in lung transplant recipients. Clin Transplant. 2003;17:363–8. doi: 10.1034/j.1399-0012.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 5.Lau CL, Palmer SM, Howell DN, McMahon R, Hadjiliadis D, Gaca J, et al. Laparoscopic antireflux surgery in the lung transplant population. Surg Endosc. 2002;16:1674–8. doi: 10.1007/s00464-001-8251-2. [DOI] [PubMed] [Google Scholar]
- 6.Cantu E, III, Appel JZ, III, Hartwig MG, Woreta H, Green C, Messier R, et al. Maxwell Chamberlain Memorial Paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78:1142–51. doi: 10.1016/j.athoracsur.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Davis RD, Jr, Lau CL, Eubanks S, Messier RH, Hadjiliadis D, Steele MP, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125:533–42. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]
- 8.King BJ, Iyer H, Leidi AA, Carby MR. Gastroesophageal reflux in bronchiolitis obliterans syndrome: a new perspective. J Heart Lung Transplant. 2009;28:870–5. doi: 10.1016/j.healun.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 9.Palmer SM, Miralles AP, Howell DN, Brazer SR, Tapson VF, Davis RD. Gastroesophageal reflux as a reversible cause of allograft dysfunction after lung transplantation. Chest. 2000;118:1214–7. doi: 10.1378/chest.118.4.1214. [DOI] [PubMed] [Google Scholar]
- 10.Parada MT, Alba A, Sepúlveda C. Bronchiolitis obliterans syndrome development in lung transplantation patients. Transplant Proc. 2010;42:331–2. doi: 10.1016/j.transproceed.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 11.Haslam PL, Baughman RP. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur Respir J. 1999;14:245–8. doi: 10.1034/j.1399-3003.1999.14b01.x. [DOI] [PubMed] [Google Scholar]
- 12.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 14.Streets CG, DeMeester TR. Ambulatory 24-hour esophageal pH monitoring: why, when, and what to do. J Clin Gastroenterol. 2003;37:14–22. doi: 10.1097/00004836-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Davis CS, Shankaran V, Kovacs EJ, Gagermeier J, Dilling D, Alex CG, et al. Gastroesophageal reflux disease after lung transplantation: pathophysiology and implications for treatment. Surgery. 2010;148:737–44. doi: 10.1016/j.surg.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis CS, Jellish WS, Fisichella PM. Laparoscopic fundoplication with and without pyloroplasty for gastroesophageal reflux disease in the lung transplant population: how I do it. J Gastrointest Surg. 2010;14:1434–41. doi: 10.1007/s11605-010-1233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young LR, Hadjiliadis D, Davis RD, Palmer SM. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124:1689–93. doi: 10.1378/chest.124.5.1689. [DOI] [PubMed] [Google Scholar]
- 18.Bodet-Milin C, Querellou S, Oudoux A, Haloun A, Horeau-Llanglard D, Carlier T, et al. Delayed gastric emptying scintigraphy in cystic fibrosis patients before and after lung transplantation. J Heart Lung Transplant. 2006;25:1077–83. doi: 10.1016/j.healun.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Maes BD, Vanwalleghem J, Kuypers D, Ghoos Y, Rutgeerts PJ, Vanrenterghem YF. Differences in gastric motor activity in renal transplant recipients treated with FK-506 versus cyclosporine. Transplantation. 1999;68:1482–5. doi: 10.1097/00007890-199911270-00009. [DOI] [PubMed] [Google Scholar]
- 20.Herve P, Silbert D, Cerrina J, Simonneau G, Dartevelle P. Impairment of bronchial mucociliary clearance in long-term survivors of heart/lung and double-lung transplantation. The Paris-Sud Lung Transplant Group. Chest. 1993;103:59–63. doi: 10.1378/chest.103.1.59. [DOI] [PubMed] [Google Scholar]
- 21.Hartwig MG, Appel JZ, Li B, Hsieh CC, Yoon YH, Lin SS, et al. Chronic aspiration of gastric fluid accelerates pulmonary allograft dysfunction in a rat model of lung transplantation. J Thorac Cardiovasc Surg. 2006;131:209–17. doi: 10.1016/j.jtcvs.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Hartwig MG, Appel JZ, Bush EL, Balsara KR, Holzknecht ZE, et al. Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants. Am J Transplant. 2008;8:1614–21. doi: 10.1111/j.1600-6143.2008.02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howden CW, Forrest JA, Reid JL. Effects of single and repeated doses of omeprazole on gastric acid and pepsin secretion in man. Gut. 1984;25:707–10. doi: 10.1136/gut.25.7.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamhankar AP, Peters JH, Portale G, Hsieh CC, Hagen JA, Bremner CG, et al. Omeprazole does not reduce gastroesophageal reflux: new insights using multichannel intraluminal impedance technology. J Gastrointest Surg. 2004;8:890–7. doi: 10.1016/j.gassur.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Blondeau K, Mertens V, Vanaudenaerde BA, Verleden GM, Van Raemdonck DE, Sifrim D, et al. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31:707–13. doi: 10.1183/09031936.00064807. [DOI] [PubMed] [Google Scholar]
- 26.Ward C, Forrest IA, Brownlee IA, Johnson GE, Murphy DM, Pearson JP, et al. Pepsin like activity in bronchoalveolar lavage fluid is suggestive of gastric aspiration in lung allografts. Thorax. 2005;60:872–4. doi: 10.1136/thx.2004.036426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girgis RE, Tu I, Berry GJ, Reichenspurner H, Valentine VG, Conte JV, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 1996;15:1200–8. [PubMed] [Google Scholar]
- 28.Vos R, Blondeau K, Vanaudenaerde BM, Mertens V, Van Raemdonck DE, Sifrim D, et al. Airway colonization and gastric aspiration after lung transplantation: do birds of a feather flock together? J Heart Lung Transplant. 2008;27:843–9. doi: 10.1016/j.healun.2008.05.022. [DOI] [PubMed] [Google Scholar]