Abstract
We present the neonatal complications of two premature newborn infants whose placentas demonstrated placental thrombosis in the fetal circulation. Both mothers presented with a 3-day history of decreased fetal movements before delivery. The first infant presented with thrombocytopenia and disseminated intravascular coagulation. The second infant had extended bilateral extended hemorrhagic venous infarctions. Severe fetal placental vascular lesions seem to be a predisposing factor for some adverse neonatal outcomes. We present these two cases with a brief review of the literature.
Keywords: Newborn, placenta, fetal thrombotic vasculopathy, brain infarct
Most descriptions of placental disease have concentrated on structural abnormalities that affect the “maternal vascular supply line” (uteroplacental insufficiency).1 Only recently has attention been focused on lesions affecting the fetal vascular supply.2,3 Four major pathological processes have been described that affect large fetal placental vessels: fetal thrombotic vasculopathy, villitis of unknown origin with obliterative fetal vasculopathy, chorioamnionitis with severe fetal vasculitis, and meconium-associated vascular necrosis.3 All of these may contribute to perinatal brain injury occurring well before birth. Severe fetal placental vascular lesions are especially highly correlated with neurological impairment and cerebral palsy.3-5 These antenatal processes might lower the threshold for injury by impairing reserve, altering fetal physiological condition, and generating potentially neurotoxic mediators.5,6 They could act by a variety of mechanisms, including impaired fetoplacental vascular regulation, decreased gas and metabolite exchange, activation of platelets and leukocytes, generation of cytokines and other thromboinflammatory mediators, release of heat shock proteins from ischemic placental tissue, and embolism of placental thrombi to other fetal vascular beds.7
We present two cases of newborn infants with neonatal diseases associated with fetal placental thrombosis, along with a brief review of the literature about the evidence of a possible correlation between these thromboses and adverse neonatal outcome.
CASE REPORTS
Patient 1
A male infant with a birth weight of 1630 g was born at 33 weeks’ gestation to a 38-year-old G3, P0 mother. Prenatal history was significant for maternal uterine fibroids and a prior child with trisomy 21. Prenatal screens were normal and the prenatal course was uneventful. The mother presented with a 3-day history of decreased fetal movements. Membranes were intact and there were no historical risk factors for infection or recent trauma, but the fetal heart rate tracing was nonreassuring, prompting emergent cesarean delivery. The infant was initially noted to be apneic and limp, with a heart rate of <100/min. Positive-pressure ventilation was given, with improvement of heart rate and color, but still without respiratory effort. He was intubated by 3 to 4 minutes of life, with overall improvement. Apgar scores were 2 and 6, respectively at 1 and 5 minutes. He was transferred to the neonatal intensive care unit for further observation.
The neonatal course was remarkable for severe respiratory distress syndrome complicated by persistent pulmonary hypertension, requiring three doses of surfactant, mechanical ventilation for 7 days, followed by continuous positive airway pressure (CPAP) and then minimal supplemental oxygen by day 10. There was persistent thrombocytopenia and abnormal coagulation tests that were corrected with three platelets transfusions and three fresh frozen plasma transfusions. Evidence of bleeding was limited to some bloody tracheal secretions in the first days of life. There was initial hypoglycemia that responded to therapy. Because of these clinical factors, the patient was treated with a 7-day course of antibiotics. All blood cultures remained negative.
Following some initial hypotonia, the overall neurological status normalized within a few days. The cranial sonogram was normal at days 3 and 10 of life. In addition, an aortic ultrasound was normal; no thrombi were seen.
Examination of the placenta showed a 210-g singleton placenta, which is small for gestational age. A normal-appearing umbilical cord with three vessels and with normal insertion was noted. It also revealed classical findings of fetal thrombotic vasculopathy (Fig. 1), including a multifocal organizing and recent thrombosis in chorionic plate and stem villous vessels, with terminal chorionic villi demonstrating villous stromal karyorrhexis, and completely avascular terminal chorionic villi. There was initially an increased number of nucleated red blood cells (NRBCs) in the baby’s peripheral blood (maximum to 18,838/mm3 on day of life 3; Fig. 2), suggesting significant fetal hypoxia in the hours and days before delivery due to placental vascular lesions. The mother was discovered to be heterozygous for 2 methylenetetrahydrofolate reductase (MTHFR) mutations (one copy of the MTHFR C677T mutation and one copy of the MTHFR A1298C mutation), which lead to elevated levels of homocysteine.8,9 However, the baby was heterozygous for only one of these mutations (one copy of the MTHFR C677T mutation, but negative for the MTHFR A 1298C mutation), known to lead to normal levels of homocysteine.8,9
Figure 1.
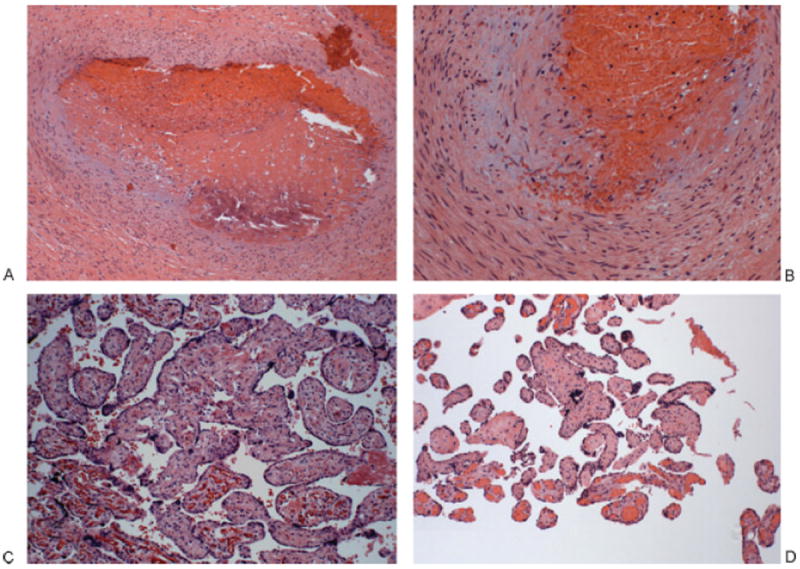
Placenta of patient 1. (A, B) Thrombosed chorionic plate vessel: The vessel lumen is obliterated, filled with an organized, partially calcified thrombus (left); higher power shows a damaged vessel wall with fibrin and extravasated red blood cells; the endothelium is unrecognizable. (A) 100×. (B) 200×. (C) Terminal chorionic villi with villous stromal karyorrhexis. The villi on lower left panel are normal with intact fetal vessels. The remainder shows partially obliterated fetal vessels and a fibrotic stroma with extravasated red cells and karyorrhectic debris. 200×. (D) Terminal chorionic villi, completely avascular. The villi at the bottom are normal with intact fetal vessels; the remainders are completely avascular, with fibrosis. 100×.
Figure 2.
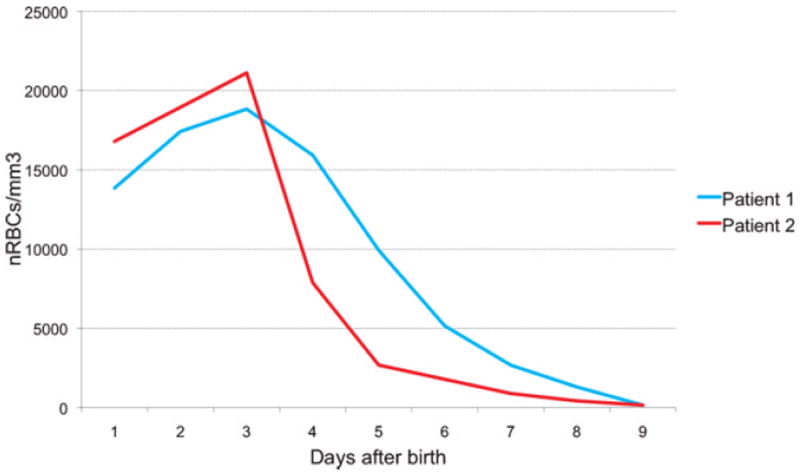
Both patients presented early increased numbers of nucleated red blood cells (nRBCs), suggesting significant fetal hypoxia in the hours and days before delivery due to placental vascular lesions.
Patient 2
An 890-g female infant was born at 27 weeks’ gestation to a 33-year-old G2, P0 mother. The mother had a prior history of ectopic pregnancy. Prenatal screens were normal and the pregnancy was uneventful. On the day of delivery, the mother presented with a 3-day history of decreased fetal movements. Membranes were intact. There was no maternal fever, evidence for infection, or recent trauma, but the fetal heart rate monitoring was nonreassuring, leading to delivery by emergent cesarean section. The baby emerged with a weak cry and poor respiratory effort. Positive-pressure ventilation was given, and then the patient was intubated with improvement of heart rate and color. Apgar scores were 4 and 6, respectively at 1 and 5 minutes. She was transferred to the neonatal intensive care unit for further observation.
The neonatal course was remarkable for moderately severe respiratory distress syndrome, requiring treatment with one dose of surfactant, followed by extubation to CPAP by the second day. There was a patent ductus arteriosus, which was treated with ibuprofen. Hypoglycemia was initially present and treated.
Relative thrombocytopenia (minimal values of 119,000/μL) and coagulation abnormalities were present in the first days of life, which were treated with one infusion of fresh frozen plasma, although there was no evidence of bleeding. The patient was treated with a 7-day course of antibiotics for presumed infection, but all blood cultures remained negative.
Her initial neurological exam was normal. A screening cranial sonogram on day 3 of life (Fig. 3) revealed bilateral germinal matrix hemorrhages, a clot in the left lateral ventricle, and moderately dilated lateral ventricles, but also bilateral extensive parenchymal hyperechogenicities with cystic changes inside. The pattern was atypical because these hyperechogenicities were discontinuous with the lateral ventricles. Acute cerebral venous thrombosis was ruled out, but a brain magnetic resonance imaging done on day 6 of life (Fig. 4) demonstrated large bifrontal parenchymal hematomas with associated subpial hemorrhages and surrounding areas of restricted diffusion, consistent with bilateral hemorrhagic venous infarctions. Bilateral intraventricular, subependymal, and germinal matrix hemorrhages were noted along with a left cerebellar hemorrhage and posterior fossa extra-axial hematoma.
Figure 3.
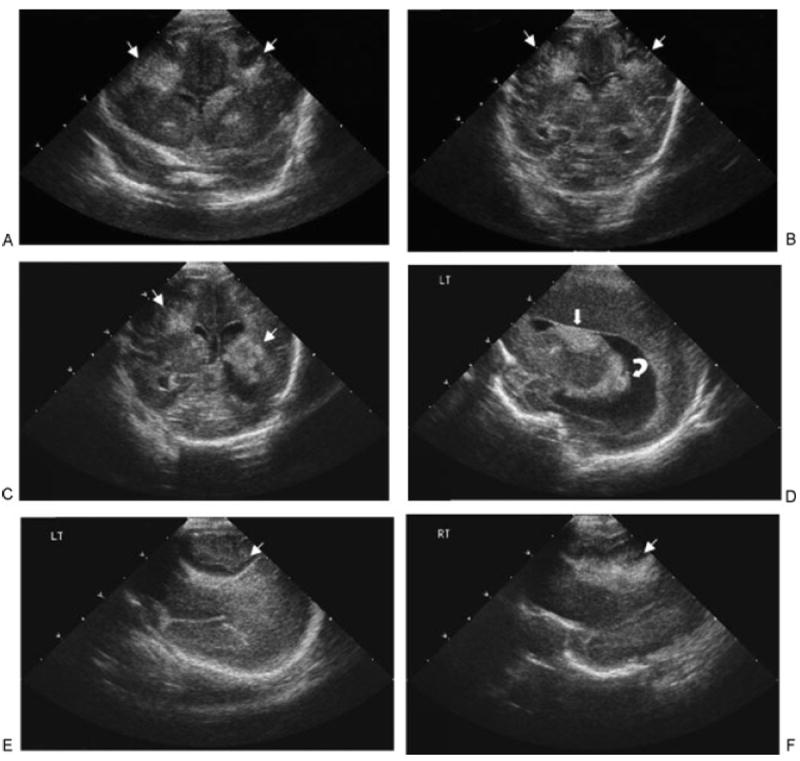
Head ultrasound of patient 2, performed at day 3 of life ( weeks of corrected age). (A–C) Coronal sonograms showed bilateral extensive parenchymal hyperechogenicities with cystic changes inside (thin arrows). These hyperechogenicities seemed to have no contact with the lateral ventricles. (D) Sagittal sonogram revealed left germinal matrix hemorrhage (thick arrow), with clot in left lateral ventricle (curved arrow) and moderately dilated lateral ventricle. (E, F) Sagittal left (LT) (E) and right (RT) (F) showed bilateral extensive parenchymal hyperechogenicities with cystic changes inside (thin arrows).
Figure 4.
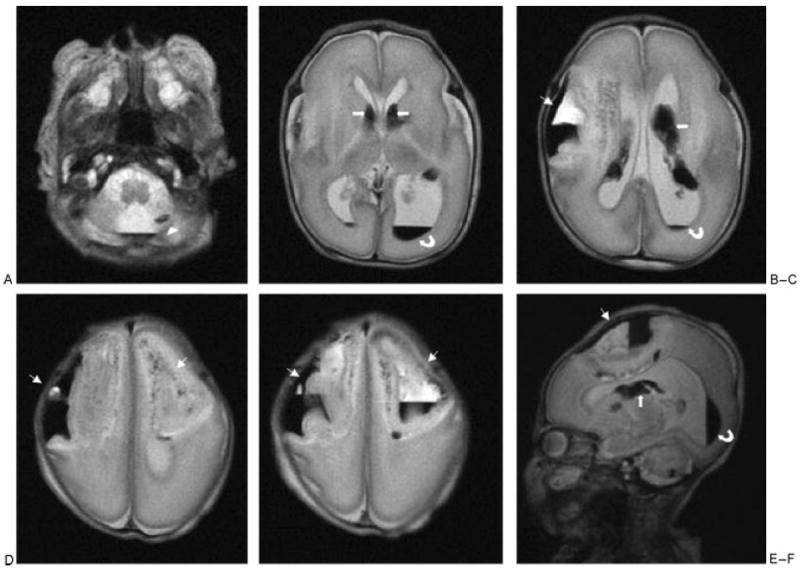
Cerebral magnetic resonance imaging of patient 2, performed at day 6 of life ( weeks of corrected age). (A–E) Axial T2-weighted images showed these large bifrontal parenchymal hematomas (thin arrows), the left cerebellar hemorrhage (arrowheads), and bilateral intraventricular (curved arrows) and germinal matrix (thick arrows) hemorrhages. (F) Left sagittal T2-weighted image showed the left large frontal parenchymal hematomas (thin arrow), the left cerebellar hemorrhage, and left intraventricular (curved arrow) and germinal matrix (thick arrow) hemorrhages.
Examination of the placenta showed a 330-g singleton placenta. A normal-appearing umbilical cord with three vessels and with normal insertion was noted. It also demonstrated organizing vascular thrombosis in several chorionic plate and stem villous vessels, but no avascular villi and no villous stromal karyorrhexis (Fig. 5). There was an increased number of NRBCs in the initial blood counts (maximum to 21,120/mm3 on day of life 3; Fig. 2), suggesting significant fetal hypoxia in the hours and days before delivery due to placental vascular lesions. The evaluation of the mother for MTHFR and other mutations associated with coagulation disorders was normal.
Figure 5.
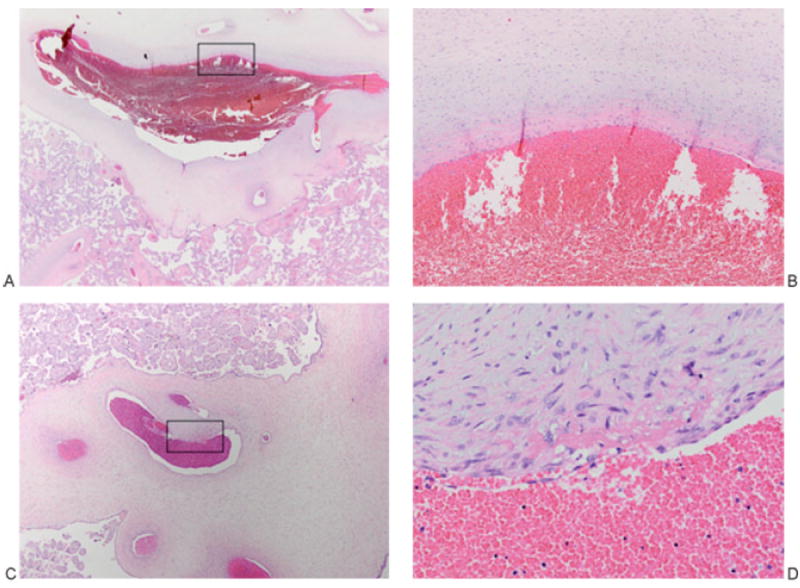
Placenta of patient 2. (A, B) Organizing thrombus, chorionic plate vein: The vessel lumen is ectasic (left), and higher power shows it layered superiorly by a fibrin thrombus (B, represents boxed area in A). (A) 12.5×. (B) 200×. (C, D) Organizing thrombus, stem villous vessel: An ectasic lumen (left) at higher power shows a fibrin thrombus with early incorporation into the vessel wall (D, represents boxed area in C). (C) 40×. (D) 400×.
DISCUSSION
In the literature, fetal thrombotic vasculopathy is defined by the presence of thrombosis in chorionic plate and stem villous vessels leading to degeneration and eventual loss of capillaries in more than 15 affected villi per section.6 Most observations of fetal thrombotic vasculopathy have been in term newborns.7 Some authors4 have studied placentas associated with intrauterine fetal death and have noticed the concomitant presence of clustered groups of recent avascular villi and older completely fibrotic avascular villi. They have hypothesized that thrombi noted in fetal thrombotic vasculopathy probably occurred repeatedly at different times during pregnancy.
We describe the neonatal complications in two premature newborn infants whose placentas demonstrated thrombosis in the fetal circulation. Our two patients presented several common features that have been described in newborn infants in association with fetal thrombotic vasculopathy and have been suggested to be systemic sequelae of placental thrombo-occlusive disease.7,10,11 Both mothers presented with a 3 day-history of decreased fetal movements. Both patients revealed early increased numbers of NRBCs, suggesting significant fetal hypoxia in the hours and days before delivery.10,11 They also presented with thrombocytopenia7 and hypoglycemia in the first days of life.10 These neonatal complications, especially the brain lesions in the second patient, could not be completely attributed to prematurity or to the treatment in the neonatal intensive care unit. The brain lesions did not correspond to the typical intraventricular hemorrhage with associated periventricular hemorrhagic infarction encountered in premature infants, because they did not extend down to the germinal matrix region. Their epicenter was more peripheral, which was consistent with bilateral venous infarctions. With the exception of the placental lesions, there was no other evidence of thrombosis in these two infants. Although the correlation between these placental lesions and neonatal complications cannot be made with certainty, these lesions may have been a predisposing factor. In the literature, placental fetal thrombotic vasculopathy has been associated with stillbirth and intrapartum or neonatal death.4 It has been linked to cerebral palsy, fetal and neonatal thromboembolic disease, perinatal liver disease, and discordant twin growth.4,12-14
The first patient and his mother were found to have a coagulation factor abnormality. The mother was discovered to be heterozygote for two MTHFR mutations, leading to elevated levels of homocysteine.8,9 However, the baby was heterozygote for only one of these mutations, known to lead to normal levels of homocysteine.8,9 The second patient and her mother did not demonstrate any procoagulation disorders. In the literature, inherited fetal coagulation disorders have been shown to result in placental thrombotic vasculopathy in occasional cases.15 However, larger studies have failed to show a significant association between these two entities.2,14,16 Redline,7 in his discussion about the origin of fetal thrombotic vasculopathy, concluded that a fetal hypercoagulable state constitutes a predisposing factor for the placental lesions, but a second antenatal event, such as fetal placental vascular stasis related to chronic partial or intermittent umbilical cord occlusion, is required to lead to the formation of the placental lesions.
In conclusion, severe fetal placental vascular lesions appear to be a predisposing factor for some of the adverse neonatal outcomes. These cases illustrate why the placenta of preterm neonates should always be examined, especially in those patients who present with unexplained thrombocytopenia or thromboembolic events of unknown origin, especially in the brain. A systematic review of such cases may reveal a gestational age continuum between fetal placental thrombosis and fetal thrombotic vasculopathy.
Acknowledgments
The authors thank Ruth Blomquist for her thorough review of the manuscript.
References
- 1.Gruenwald P. Fetal deprivation and placental pathology: concepts and relationships. Perspect Pediatr Pathol. 1975;2:101–149. [PubMed] [Google Scholar]
- 2.Ariel I, Anteby E, Hamani Y, Redline RW. Placental pathology in fetal thrombophilia. Hum Pathol. 2004;35:729–733. doi: 10.1016/j.humpath.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452–457. doi: 10.1016/j.ajog.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies, and cerebral palsy. Hum Pathol. 1999;30:759–769. doi: 10.1016/s0046-8177(99)90136-3. [DOI] [PubMed] [Google Scholar]
- 5.McDonald DG, Kelehan P, McMenamin JB, et al. Placental fetal thrombotic vasculopathy is associated with neonatal encephalopathy. Hum Pathol. 2004;35:875–880. doi: 10.1016/j.humpath.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta. 2008;29(suppl A):S86–S91. doi: 10.1016/j.placenta.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Redline RW. Clinical and pathological umbilical cord abnormalities in fetal thrombotic vasculopathy. Hum Pathol. 2004;35:1494–1498. doi: 10.1016/j.humpath.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Dikmen M, Ozbabalik D, Gunes HV, et al. Acute stroke in relation to homocysteine and methylenetetrahydrofolate reductase gene polymorphisms. Acta Neurol Scand. 2006;113:307–314. doi: 10.1111/j.1600-0404.2005.00556.x. [DOI] [PubMed] [Google Scholar]
- 9.Trabetti E. Homocysteine, MTHFR gene polymorphisms, and cardio-cerebrovascular risk. J Appl Genet. 2008;49:267–282. doi: 10.1007/BF03195624. [DOI] [PubMed] [Google Scholar]
- 10.Redline RW, Pappin A. Fetal thrombotic vasculopathy: the clinical significance of extensive avascular villi. Hum Pathol. 1995;26:80–85. doi: 10.1016/0046-8177(95)90118-3. [DOI] [PubMed] [Google Scholar]
- 11.Redline RW. Elevated circulating fetal nucleated red blood cells and placental pathology in term infants who develop cerebral palsy. Hum Pathol. 2008;39:1378–1384. doi: 10.1016/j.humpath.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Dahms BB, Boyd T, Redline RW. Severe perinatal liver disease associated with fetal thrombotic vasculopathy. Pediatr Dev Pathol. 2002;5:80–85. doi: 10.1007/s10024-001-0111-5. [DOI] [PubMed] [Google Scholar]
- 13.Redline RW, Shah D, Sakar H, Schluchter M, Salvator A. Placental lesions associated with abnormal growth in twins. Pediatr Dev Pathol. 2001;4:473–481. doi: 10.1007/s10024001-0044-z. [DOI] [PubMed] [Google Scholar]
- 14.Leistra-Leistra MJ, Timmer A, van Spronsen FJ, Geven WB, van der Meer J, Erwich JJ. Fetal thrombotic vasculopathy in the placenta: a thrombophilic connection between pregnancy complications and neonatal thrombosis? Placenta. 2004;25(suppl A):S102–S105. doi: 10.1016/j.placenta.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Khong TY, Hague WM. Biparental contribution to fetal thrombophilia in discordant twin intrauterine growth restriction. Am J Obstet Gynecol. 2001;185:244–245. doi: 10.1067/mob.2001.113320. [DOI] [PubMed] [Google Scholar]
- 16.Vern TZ, Alles AJ, Kowal-Vern A, Longtine J, Roberts DJ. Frequency of factor V(Leiden) and prothrombin G20210A in placentas and their relationship with placental lesions. Hum Pathol. 2000;31:1036–1043. doi: 10.1053/hupa.2000.16281. [DOI] [PubMed] [Google Scholar]


