Abstract
Purpose
To retrospectively determine if pretreatment endorectal magnetic resonance (MR) imaging findings are predictive of outcome in patients who undergo external-beam radiation therapy for prostate cancer.
Materials and Methods
Committee on Human Research approval, with waiver of the requirement for informed consent, was obtained for this HIPAA-compliant study. Eighty men with biopsy-proved prostate cancer (mean age, 59 years; range, 47–75 years) who underwent endorectal MR imaging of the prostate prior to external-beam radiation therapy were retrospectively identified; details of baseline tumor characteristics, treatment, and outcome were recorded. Two experienced readers independently reviewed all MR imaging studies and recorded tumor T stage and the radial diameter of extracapsular extension (if present). Univariate and multivariate stepwise Cox regression analyses were used to investigate the relationship between baseline imaging and clinical predictive variables and the end point of metastatic failure.
Results
At MR imaging, readers 1 and 2, respectively, considered 50 and 60 patients to have T1 or T2 disease (ie, organ-confined disease) and 30 and 20 patients to have T3 disease. After a mean follow-up of 43 months, four patients developed metastases. Univariate Cox analysis revealed that baseline serum prostate-specific antigen level, presence of extracapsular extension at MR imaging (according to either reader), and degree of extracapsular extension (according to either reader) were all significantly (P < .05) related to the development of metastases. Multivariate Cox analysis revealed that the sole independent predictive variable was mean diameter of extracapsular extension (relative hazard ratio, 2.06; 95% confidence interval: 1.22, 3.48; P = .007). In particular, three of five patients with extracapsular extension of more than 5 mm at pretreatment MR imaging developed metastases 24, 43, and 63 months after therapy.
Conclusion
The presence and degree of extracapsular extension at MR imaging prior to external-beam radiation therapy are important predictors of posttreatment metastatic recurrence.
Over the past 2 decades, endorectal magnetic resonance (MR) imaging has emerged as a relatively accurate method of evaluating the local extent and aggressiveness of prostate cancer (1-5), although wider implementation of this technology has been limited by concerns about false-positive and false-negative results and interobserver variability (6-8). It is important to note that many of the less promising studies have used surgical pathologic examination as the standard of reference. This method, which superficially seems the most objective and scientific approach, has several consequences that may lead to an underestimation of the true benefit of imaging. Inclusion of only surgical patients introduces a large selection bias, because patients with high-risk disease are more likely to select nonsurgical treatment. Anecdotally, it has been our impression that the largest and most locally advanced prostate cancers are seen in patients at MR imaging prior to radiation therapy. Such patients would never be entered into a study that demanded step-section histopathologic comparison. Furthermore, the emphasis on comparison with histopathologic stage ignores what really matters for the patient, which is clinical outcome. It is conceivable that two tumors of the same histopathologic stage might have quite different MR imaging features, such as size or extent, that are predictive of outcome. A histopathologically based study would miss such differences, which might be critical in improving patient-specific treatment and in tailoring adjuvant therapy to those at highest risk of recurrence.
Results of one study (9) in which the relationship between the performance of MR imaging prior to radiation therapy and patient outcome was examined suggested that MR findings positively influenced radiation treatment planning, both with respect to the distribution of implanted radioactive seeds and the decision to add external-beam radiation therapy, but this study did not examine the prognostic value of specific MR findings. Therefore, we undertook our study to retrospectively determine if pretreatment endorectal MR imaging findings are predictive of outcome in patients who undergo external-beam radiation therapy for prostate cancer.
Materials and Methods
Subjects
This was a retrospective single-institution study that was approved by our Committee on Human Research, with waiver of the requirement for informed consent. The study was compliant with requirements of the Health Insurance Portability and Accountability Act. We performed a computerized search of our radiology and hospital information systems for the period from March 1998 to December 2003 to identify patients who underwent baseline endorectal MR imaging at our institution prior to external-beam radiation therapy for biopsy-proved prostate cancer and who underwent clinical follow-up at our institution (n = 101). Patients were excluded from the study if motion artifacts precluded MR image interpretation (n = 14). We also excluded patients with incomplete or missing follow-up data (n = 7). The final study group consisted of 80 men with a mean age of 59 years (range, 47–75 years), a mean pretreatment serum prostate-specific antigen (PSA) level of 7.8 ng/mL (range, 1.7–24.0 ng/mL), and a median Gleason score of 7 (range, 5–9).
MR Imaging Technique
MR imaging studies were performed with a 1.5-T whole-body MR imaging unit (Signa; GE Medical Systems, Milwaukee, Wis). Patients were imaged in the supine position by using the body coil for excitation and a pelvic phased-array coil (GE Medical Systems) in combination with a commercially available balloon-covered expandable endorectal coil (Medrad, Pittsburgh, Pa) for signal reception. MR imaging included the acquisition of thin-section high-spatial-resolution transverse and coronal T2-weighted fast spin-echo images of the prostate and seminal vesicles with the following parameters: repetition time msec/effective echo time msec, 5000/96; echo train length, 16; section thickness, 3 mm; intersection gap, 0 mm; field of view, 14 cm; matrix, 256 × 192; anteroposterior frequency encoding (to prevent obscuration of the prostate by endorectal coil motion artifact); and number of signals acquired, three.
MR Image Interpretation
Two readers (A.C.W. [reader 1] and F.V.C. [reader 2], with 3 and 10 years of experience, respectively, in the interpretation of endorectal MR images of the prostate) retrospectively reviewed all MR images independently at a picture archiving and communication system workstation (Impax; Agfa, Mortsel, Belgium). Readers knew patients had biopsy-proved prostate cancer that was subsequently treated with external-beam radiation therapy but were unaware of all other clinical and histopathologic findings. They assessed the presence and extent of malignancy on the basis of expert judgment and previously established imaging criteria (10,11) and staged tumors as organ confined or locally advanced according to the American Joint Committee on Cancer guidelines (12). In particular, T1 tumors are not apparent at MR imaging and MR spectroscopic imaging, T2 tumors are visible at MR imaging and MR spectroscopic imaging but are organ confined, and T3 tumors extend outside the capsule. It should be noted that strict objective criteria for prostate cancer identification have not been reported, and reader diagnosis was therefore necessarily somewhat subjective, but in general, tumors were identified as ovoid masslike or crescentic subcapsular foci of reduced T2 signal intensity. When seen, extracapsular extension was quantified by measuring the largest radial diameter of extraprostatic tumor, defined as the perpendicular distance of tumor beyond the expected location of the outer capsular margin (13), on the transverse T2-weighted images.
Patient Treatment and Outcome
One of the authors (D.A.M.) who was not one of the MR imaging readers reviewed all available clinical, radiologic, and laboratory results to establish the details of patient treatment and outcome. All patients underwent definitive external-beam radiation therapy after MR imaging at a mean interval of 6 months (range, 0–69 months). Patients who underwent external-beam radiation therapy alone (n = 40) received a mean dose of 74.2 Gy (range, 70.0–79.0 Gy). Nine patients underwent external-beam radiation therapy combined with radioactive seed implantation and received a mean dose of 121.5 Gy (range, 108.0–135.0 Gy). Two patients underwent external-beam radiation therapy combined with high-dose-rate brachytherapy and received doses of 82 and 83 Gy. We could not establish the radiation dose delivered to the remaining 29 patients because radiation therapy was performed at an outside institution. Neoadjuvant or adjuvant hormonal therapy was administered to 49 patients. Supplementary hormonal therapy was given to 49 patients. Thirty-five patients received neoadjuvant hormonal therapy for a mean duration of 11.7 months (range, 1–19 months). Eleven patients received adjuvant hormonal therapy for a mean duration of 6.6 months (range, 1–19 months). Three patients received both neoadjuvant and adjuvant hormonal therapy for a mean duration of 20.3 months (range, 11–26 months). In two patients, the details of supplementary hormonal therapy were unavailable.
With respect to outcome, both metastatic and biochemical recurrences were recorded. Metastatic recurrence was considered present when this diagnosis was documented in the clinical record and there was appropriately supportive evidence, such as histopathologic confirmation or development of a new lesion with characteristics consistent with metastasis at bone scintigraphy or cross-sectional imaging. Biochemical recurrence (also known as biochemical or PSA failure) was defined as three consecutive increases in serum PSA level after a nadir level had been reached or a serum PSA level 2 ng/mL or more above the nadir level (14).
Statistical Analysis
Descriptive statistics of mean and range were used to summarize the patient cohort with respect to clinical, MR imaging, and outcome variables. Interobserver agreement was evaluated with κ statistics for T-stage assessment and with the intraclass correlation coefficient for the degree of extracapsular extension. For the κ statistic, the level of agreement was interpreted as follows: κ = 0.00–0.20 was considered to indicate poor agreement; κ = 0.21–0.40, fair agreement; κ = 0.41–0.60, moderate agreement; κ = 0.61–0.80, good agreement; and κ = 0.81–1.00, very good agreement (15). The development of metastases was used as the primary outcome measure, in view of the known limitations of biochemical failure as an indicator of patient outcome (16); biochemical control is well recognized as an imperfect surrogate end point for true clinical outcome, and definitions of biochemical failure continue to be debated.
The relationship between the T stage assigned at MR imaging prior to external-beam radiation therapy by both readers and subsequent patient outcome was evaluated by using the log-rank test statistics from the Kaplan-Meier actuarial survival estimates for the freedom from metastatic progression. Cox proportional hazards models were used to analyze the continuous variables, such as patient age, hormonal treatment, baseline serum PSA level, and degree of extracapsular extension at MR imaging as measured by each reader and according to the average measurement of both readers. To determine if MR imaging provided incremental predictive value over these standard clinical variables, patient age, hormonal treatment, baseline serum PSA level, and pathologic findings were routinely adjusted in Cox models of MR imaging variables. Multivariate stepwise Cox regression analysis was used to identify the independently predictive variables. Relative hazard ratios and corresponding 95% confidence intervals were reported. All statistical analyses were performed by using software (SAS, version 9.1; SAS Institute, Cary, NC). A significance level of 5% was used to define statistical significance.
Results
MR Image Interpretation
At MR imaging, readers 1 and 2, respectively, considered 50 and 60 patients to have T1 or T2 disease (ie, organ-confined disease) and 30 and 20 patients to have T3 disease. The mean degree of extracapsular extension recorded by readers 1 and 2 was 7 mm (range, 2–22 mm) and 4 mm (range, 1–21 mm), respectively. Figure 1, a representative example image in a patient with extracapsular extension, illustrates how the degree of extracapsular extension was measured. There was moderate interobserver agreement, with a κ value of 0.49 (95% confidence interval: 0.34, 0.64) for staging at MR imaging and an intraclass correlation coefficient of 0.60 (95% confidence interval: 0.44, 0.73) for the radial diameter of extracapsular extension.
Figure 1.
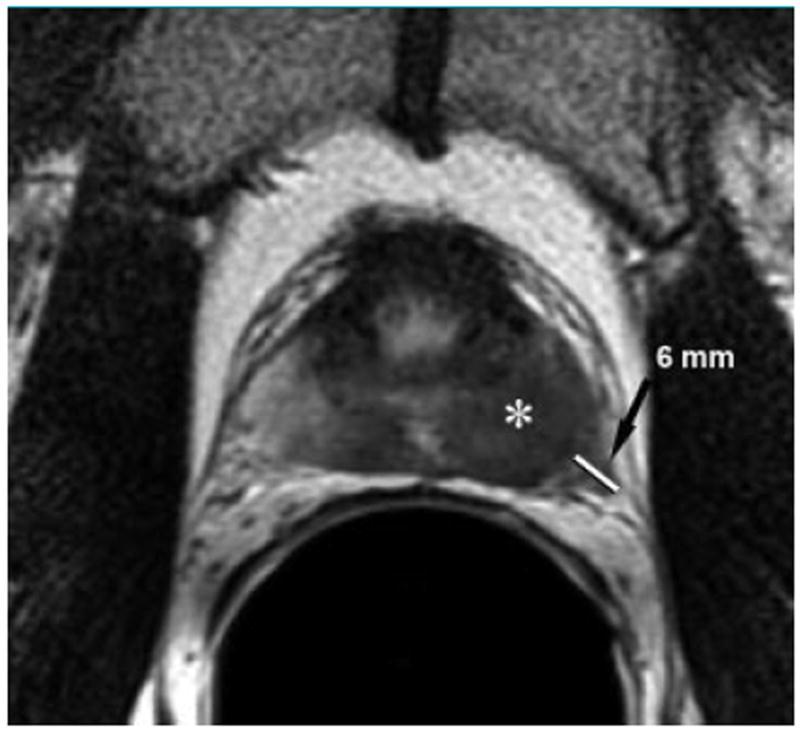
Transverse T2-weighted endorectal MRimage (5000/96) in 58-year-old man with PSA level of 7.9 ng/mL and positive transrectal biopsy results shows Gleason 7 cancer in multiple cores. An ill-defined mass (*) of low signal intensity in the left peripheral zone is associated with extracapsular extension (arrow) that measures 6mmin radial diameter when measured from the expected location of the capsule to the outermost part of the tumor. The patient developed metastases 41 months after external-beam radiation therapy.
Predictors of Outcome
After a mean follow-up of 43 months (range, 3–84 months), 20 patients had biochemical recurrence and four patients had developed metastases (three developed bone metastases and one developed lung metastases). Univariate Cox regression analysis revealed that the presence of extracapsular extension at MR imaging (stage T3) was a significant predictor of the development of metastases for both readers (P = .018 and .001 for readers 1 and 2, respectively). Kaplan-Meier curves for patient outcome as related to MR imaging findings are shown in Figures 2 and 3. Univariate Cox regression analysis revealed that baseline serum PSA level (relative hazard ratio, 1.12; 95% confidence interval: 1.02, 1.23; P = .02) and degree of extracapsular extension (relative hazard ratio, 2.72; 95% confidence interval: 1.23, 5.99; P = 0.01) were also significant predictors for the development of metastases. In multivariate Cox analysis, extracapsular extension was the only significant predictive variable (P < .05) for both readers, with all other variables being dropped from the stepwise analysis. Accordingly, and in the interest of simplicity, we examined the predictive value of the mean diameter of extracapsular extension. In the resulting analysis, the sole independent predictive variable was the mean diameter of extracapsular extension (relative hazard ratio, 2.06; 95% confidence interval: 1.22, 3.48; P = .007). In particular, three of five patients with extracapsular extension of more than 5 mm at MR imaging before radiation therapy developed metastases 24, 43, and 63 months after therapy.
Figure 2.
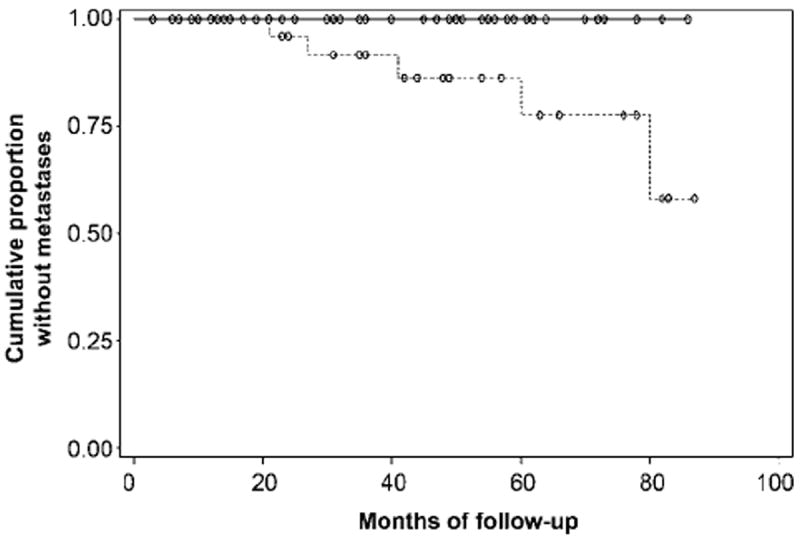
Graph shows cumulative proportion of patients without metastatic recurrence of prostate cancer after external-beam radiation therapy, stratified by the stage assigned by reader 1 at MR imaging performed prior to treatment. Kaplan-Meier analysis revealed a significant difference (P = .02) between patients with organ-confined (T1 or T2) tumor (n = 50, solid line) and those with locally advanced (T3) disease (n = 30, dashed line).
Figure 3.
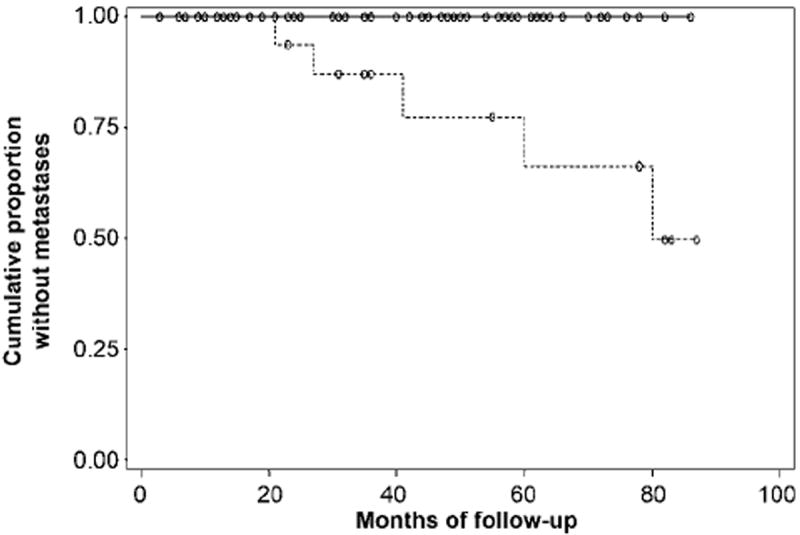
Graph shows cumulative proportion of patients without metastatic recurrence of prostate cancer after external-beam radiation therapy, stratified by the stage assigned by reader 2 at MR imaging performed prior to treatment. Kaplan-Meier analysis revealed a significant difference (P = .01) between patients with organ-confined (T1 or T2) tumor (n = 60, solid line) and those with locally advanced (T3) disease (n = 20, dashed line).
Discussion
Our finding that the presence and degree of extracapsular extension at MR imaging of prostate cancer prior to external-beam radiation therapy is a strong predictor of metastatic recurrence (in both univariate and multivariate analyses) has several important implications. First, it suggests that MR imaging findings may be crucial tumor characteristics that should be incorporated into any system of evaluation intended to predict the risk of metastatic recurrence. In particular, the finding that three of five patients with extracapsular extension of more than 5 mm developed metastases within 63 months of therapy indicates that this subgroup of patients at markedly elevated risk may be candidates for more aggressive therapy such as radiation dose escalation or extended androgen deprivation (17). It should be noted that such strategies may improve local control, but may not affect regional or distant metastatic disease.
Second, our study highlights an aspect of prostate MR imaging that has received little attention; namely, that extracapsular extension is not just a binary variable to be assessed as present or absent but is also quantitative and may be measured objectively. This observation is supported by results of a prior study (18) in which only one of seven patients with extracapsular extension of less than 1 mm was correctly identified at MR imaging, compared with five of seven patients with extracapsular extension of 1 mm or more.
Third, the degree of extracapsular extension seen in our study population was often extensive, with both readers recording radial diameters of more than 2 cm. Such gross extracapsular tumor extension is rarely, if ever, seen in patients selected for radical prostatectomy, because such large and locally advanced tumors are generally not considered appropriate for surgery. Surgical series inevitably include patients with lower-risk tumors as compared with the tumors of patients undergoing radiation therapy and may not fully reflect the potential predictive value of MR imaging findings. Studies that include a wider spectrum of disease and use patient outcome as an end point may allow elucidation of the true benefit of MR imaging in prostate cancer. This is borne out by results of other studies (19,20) that have shownMR imaging to be more useful in patients with medium- or high-risk tumors.
A noteworthy finding in our study was that the degree of extracapsular extension seen at endorectal MR imaging prior to external-beam radiation therapy was associated with the development of posttreatment metastatic recurrence. It is unlikely that this finding was due simply to upstaging to T3 disease; it more likely reflects greater inherent tumor aggressiveness or a longer period of untreated tumor presence that in turn is associated with a greater likelihood of microscopic metastatic spread. Alternatively, it is possible that tumors that have extended far beyond the prostate capsule are not adequately treated with standard external-beam radiation therapy. It is known that poor treatment coverage at radiation therapy is associated with increased risk of local and probably distant recurrence (21,22). The radial distance of extraprostatic extension of prostate cancer influences radiation treatment planning (13), because the dose fall-off at the periphery of the field may result in incomplete treatment, although wide treatment margins increase the risk of radiation injuries such as rectal ulceration and fistula formation (23). Arguably, MR imaging results could allow more patient-specific treatment planning, particularly in patients with marked extracapsular extension.
The idea that pretreatment MR imaging may assist treatment planning and prediction of prognosis has been reported. For example, one study (9) examined the relationship between the performance of MR imaging prior to brachytherapy and patient outcome, and the results suggested that MR imaging findings positively influenced radiation treatment planning, although this study did not examine the prognostic value of specific MR imaging findings. D’Amico et al (19) demonstrated that MR imaging staging was incrementally useful in predicting prognosis in patients prior to radical prostatectomy and also showed, in multivariate analysis, that MR imaging findings were better predictors of extracapsular extension, seminal vesicle invasion, and positive surgical margins than Gleason score or serum PSA level (24). The same group also showed that although a serum PSA level greater than 20 ng/mL was the most accurate predictor of biochemical failure after radical prostatectomy, MR imaging findings of extracapsular extension could also predict biochemical failure (25). MR imaging findings were shown to significantly improve surgical planning with respect to the decision to preserve or resect the neurovascular bundle in a Memorial Sloan-Kettering Cancer Center study of 135 patients prior to radical prostatectomy (20). The general theme in such studies, as in our study, is that pretreatment imaging can assist treatment planning and prediction of prognosis, which is clearly beneficial to both clinicians and patients.
Our study had limitations. First, this was a retrospective study performed at a single institution. Second, we could be criticized for lack of histopathologic comparison for our findings related to the presence or degree of extracapsular extension, although outcome is really the ultimate standard of reference, and the fact that imaging findings were important outcome predictors would seem to validate our results, irrespective of histopathologic findings. Third, our study population demonstrated substantial treatment heterogeneity with respect to radiation dose, administration of external-beam radiation alone or in combination with brachytherapy, and the use of adjuvant or neoadjuvant hormonal therapy. On a related note, the mean follow-up of 43 months in our study is relatively short given the long natural history of prostate cancer. However, our finding that the presence and degree of extracapsular extension at preradiation MR imaging are important predictors of outcome is arguably even more compelling given that the results arose from a heterogeneous population that was followed up for a short time. Nonetheless, our results remain preliminary and will require further investigation and validation in larger prospective studies—particularly the suggestion that patients with high-risk tumors on the basis of MR findings may be candidates for more aggressive treatment; there is no certainty that such an approach would improve systemic control or long-term patient outcome.
In conclusion, the presence and degree of extracapsular extension at MR imaging prior to external-beam radiation therapy are important predictors of posttreatment metastatic recurrence; in particular, patients with extracapsular extension of more than 5 mm may be potential candidates for more aggressive therapy such as radiation dose escalation or extended androgen deprivation.
Advances in Knowledge
-
■
In univariate analysis, baseline serum prostate-specific antigen level and the presence and degree of extracapsular extension at pretreatment MR imaging were all significant (P < .05) predictors of the development of metastases in patients undergoing external-beam radiation therapy for prostate cancer.
-
■
In multivariate analysis, the sole independent predictive variable for the development of metastases in patients undergoing external-beam radiation therapy for prostate cancer was the mean diameter of extracapsular extension at pretreatment MR imaging (measured as the radial diameter of tumor outside the expected location of the prostatic capsule).
-
■
The finding of extracapsular extension of more than 5 mm at MR imaging before radiation therapy seems to be a particularly poor prognostic finding, with three of five patients with this finding in this study developing metastases 24, 43, and 63 months after therapy.
Implications for Patient Care
-
■
MR imaging findings may be important tumor characteristics to be considered in an evaluation intended to predict the risk of metastatic recurrence in patients undergoing external-beam radiation therapy for prostate cancer.
-
■
Patients with a substantial degree of extracapsular extension may be candidates for more aggressive therapy such as radiation dose escalation or extended androgen deprivation.
Abbreviation
- PSA
prostate-specific antigen
Footnotes
Author contributions:
Guarantors of integrity of entire study, D.A.M., F.V.C.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, D.A.M.; clinical studies, D.A.M., A.C.W., B.P., M.R.; and manuscript editing, all authors
Authors stated no financial relationship to disclose.
References
- 1.Huzjan R, Sala E, Hricak H. Magnetic resonance imaging and magnetic resonance spectroscopic imaging of prostate cancer. Nat Clin Pract Urol. 2005;2(9):434–442. doi: 10.1038/ncpuro0296. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Hricak H, Kattan MW, Chen HN, Scardino PT, Kuroiwa K. Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology. 2006;238(2):597–603. doi: 10.1148/radiol.2382041905. [DOI] [PubMed] [Google Scholar]
- 3.Scheidler J, Hricak H, Vigneron DB, et al. Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging—clinicopathologic study. Radiology. 1999;213(2):473–480. doi: 10.1148/radiology.213.2.r99nv23473. [DOI] [PubMed] [Google Scholar]
- 4.Yu KK, Scheidler J, Hricak H, et al. Prostate cancer: prediction of extracapsular extension with endorectal MR imaging and three-dimensional proton MR spectroscopic imaging. Radiology. 1999;213(2):481–488. doi: 10.1148/radiology.213.2.r99nv26481. [DOI] [PubMed] [Google Scholar]
- 5.Coakley FV, Qayyum A, Kurhanewicz J. Magnetic resonance imaging and spectroscopic imaging of prostate cancer. J Urol. 2003;170(6 pt 2):S69–S75. doi: 10.1097/01.ju.0000094958.23276.c4. [DOI] [PubMed] [Google Scholar]
- 6.Coakley FV, Kurhanewicz J, Lu Y, et al. Prostate cancer tumor volume: measurement with endorectal MR and MR spectroscopic imaging. Radiology. 2002;223(1):91–97. doi: 10.1148/radiol.2231010575. [DOI] [PubMed] [Google Scholar]
- 7.Dhingsa R, Qayyum A, Coakley FV, et al. Prostate cancer localization with endorectal MR imaging and MR spectroscopic imaging: effect of clinical data on reader accuracy. Radiology. 2004;230(1):215–220. doi: 10.1148/radiol.2301021562. [DOI] [PubMed] [Google Scholar]
- 8.Schiebler ML, Yankaskas BC, Tempany C, et al. MR imaging in adenocarcinoma of the prostate: interobserver variation and efficacy for determining stage C disease. AJR Am J Roentgenol. 1992;158(3):559–562. doi: 10.2214/ajr.158.3.1738994. [DOI] [PubMed] [Google Scholar]
- 9.Clarke DH, Banks SJ, Wiederhorn AR, et al. The role of endorectal coil MRI in patient selection and treatment planning for prostate seed implants. Int J Radiat Oncol Biol Phys. 2002;52(4):903–910. doi: 10.1016/s0360-3016(01)02736-5. [DOI] [PubMed] [Google Scholar]
- 10.Yu KK, Hricak H, Alagappan R, Chernoff DM, Bacchetti P, Zaloudek CJ. Detection of extracapsular extension of prostate carcinoma with endorectal and phased-array coil MR imaging: multivariate feature analysis. Radiology. 1997;202(3):697–702. doi: 10.1148/radiology.202.3.9051019. [DOI] [PubMed] [Google Scholar]
- 11.Fütterer JJ, Engelbrecht MR, Huisman HJ, et al. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology. 2005;237(2):541–549. doi: 10.1148/radiol.2372041724. [DOI] [PubMed] [Google Scholar]
- 12.American Joint Committee on Cancer. Prostate. In: Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6. New York, NY: Springer; 2002. pp. 309–316. [Google Scholar]
- 13.Davis BJ, Pisansky TM, Wilson TM, et al. The radial distance of extraprostatic extension of prostate carcinoma. Cancer. 1999;85(12):2630–2637. [PubMed] [Google Scholar]
- 14.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 16.Kuban DA, Thames HD, Shipley WU. Defining recurrence after radiation for prostate cancer. J Urol. 2005;173(6):1871–1878. doi: 10.1097/01.ju.0000157682.40869.65. [DOI] [PubMed] [Google Scholar]
- 17.Pickles T, Pollack A. The case for dose escalation versus adjuvant androgen deprivation therapy for intermediate risk prostate cancer. Can J Urol. 2006;13(suppl 2):68–71. [PubMed] [Google Scholar]
- 18.Jager GJ, Ruijter ET, van de Kaa CA, et al. Local staging of prostate cancer with endorectal MR imaging: correlation with histopathology. AJR Am J Roentgenol. 1996;166(4):845–852. doi: 10.2214/ajr.166.4.8610561. [DOI] [PubMed] [Google Scholar]
- 19.D’Amico AV, Whittington R, Malkowicz B, et al. Endorectal magnetic resonance imaging as a predictor of biochemical outcome after radical prostatectomy in men with clinically localized prostate cancer. J Urol. 2000;164(3 pt 1):759–763. doi: 10.1097/00005392-200009010-00032. [DOI] [PubMed] [Google Scholar]
- 20.Hricak H, Wang L, Wei DC, et al. The role of preoperative endorectal magnetic resonance imaging in the decision regarding whether to preserve or resect neurovascular bundles during radical retropubic prostatectomy. Cancer. 2004;100(12):2655–2663. doi: 10.1002/cncr.20319. [DOI] [PubMed] [Google Scholar]
- 21.Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: longterm results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys. 1991;21(3):537–547. doi: 10.1016/0360-3016(91)90668-t. [DOI] [PubMed] [Google Scholar]
- 22.Yorke ED, Fuks Z, Norton L, Whitmore W, Ling CC. Modeling the development of metastases from primary and locally recurrent tumours: comparison with a clinical data base for prostatic cancer. Cancer Res. 1993;53(13):2987–2993. [PubMed] [Google Scholar]
- 23.Stromberg J, Martinez A, Benson R, et al. Improved local control and survival for surgically staged patients with locally advanced prostate cancer treatment with up-front low dose rate iridium-192 prostate implantation and external beam irradiation. Int J Radiat Oncol Biol Phys. 1994;28(1):67–75. doi: 10.1016/0360-3016(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 24.D’Amico AV, Whittington R, Malkowicz SB, et al. Critical analysis of the ability of the endorectal coil magnetic resonance imaging scan to predict pathologic stage, margin status and postoperative prostate-specific antigen failure in patients with clinically organ-confined prostate cancer. J Clin Oncol. 1996;14(6):1770–1777. doi: 10.1200/JCO.1996.14.6.1770. [DOI] [PubMed] [Google Scholar]
- 25.D’Amico AV, Whittington R, Malkowicz SB, et al. A multivariate analysis of clinical and pathological factors that predict for prostate specific antigen failure after radical prostatectomy for prostate cancer. J Urol. 1995;154(1):131–138. [PubMed] [Google Scholar]


