Abstract
High-dose intravenous immunoglobulin is a widely used therapeutic preparation of highly purified immunoglobulin G (IgG) antibodies. It is administered at high doses (1–2 grams per kilogram) for the suppression of autoantibody-triggered inflammation in a variety of clinical settings1. This anti-inflammatory activity of intravenous immunoglobulin is triggered by a minor population of IgG crystallizable fragments (Fcs), with glycans terminating in α2,6 sialic acids (sFc) that target myeloid regulatory cells expressing the lectin dendritic-cell-specific ICAM-3 grabbing non-integrin (DC-SIGN; also known as CD209)2–4. Here, to characterize this response in detail, we generated humanized DC-SIGN mice (hDC-SIGN), and demonstrate that the anti-inflammatory activity of intravenous immunoglobulin can be recapitulated by the transfer of bone-marrow-derived sFc-treated hDC-SIGN+ macrophages or dendritic cells into naive recipients. Furthermore, sFc administration results in the production of IL-33, which, in turn, induces expansion of IL-4-producing basophils that promote increased expression of the inhibitory Fc receptor FcγRIIB on effector macrophages. Systemic administration of the TH2 cytokines IL-33 or IL-4 upregulates FcγRIIB on macrophages, and suppresses serum-induced arthritis. Consistent with these results, transfer of IL-33-treated basophils suppressed induced arthritic inflammation. This novel DC-SIGN–TH2 pathway initiated by an endogenous ligand, sFc, pro-vides an intrinsic mechanism for maintaining immune homeostasis that could be manipulated to provide therapeutic benefit in auto-immune diseases.
Binding of intravenous immunoglobulin (IVIG) or sFc to specific ICAM-3 grabbing non-integrin-related 1 (SIGN-R1) on splenic marginal zone macrophages suppresses autoantibody-mediated inflammation4. Although the human orthologue of SIGN-R1, DC-SIGN, showed similar binding specificity for sFc as mouse SIGN-R1, its expression pattern is broader, as it is detected systemically on myeloid-derived cells, including dendritic cells, macrophages and some monocytes5,6. DC-SIGN recognizes high-mannose glycans from a variety of pathogens, and acts as a pattern recognition receptor bridging innate and adaptive immunity7. Ligation of DC-SIGN by bacteria-derived mannosylated glycans can induce their internalization, and also synergize with other innate receptor pathways promoting inflammation and resistance to infection. In contrast, binding of sFc to DC-SIGN requires both carbohydrate and protein determinants, and results in an anti-inflammatory response2,4. The immunosuppressive potential of DC-SIGN has been documented following ligation by HIV-derived gp120 or anti-DC-SIGN antibody, which promotes the development of tolerogenic, IL-10-producing dendritic cells, and interferes with Toll-like receptor (TLR) signalling8,9.
To study human DC-SIGN in the context of IVIG anti-inflammatory activity, we expressed hDC-SIGN—driven by its endogenous promoter to reproduce the characteristically broad in vivo expression pattern of hDC-SIGN—in a mouse. Human bacterial artificial chromosome (BAC) clones encoding the DC-SIGN gene and its regulatory regions were introduced as a transgene into mice (Fig. 1a). Transgenic mice showed surface expression of this human lectin on dendritic cells, macrophages, and monocytes, in the peripheral blood, bone marrow and spleen, resembling the human expression pattern of DC-SIGN (Supplementary Fig. 2a–c), although a higher percentage of murine monocytes were found to express DC-SIGN.
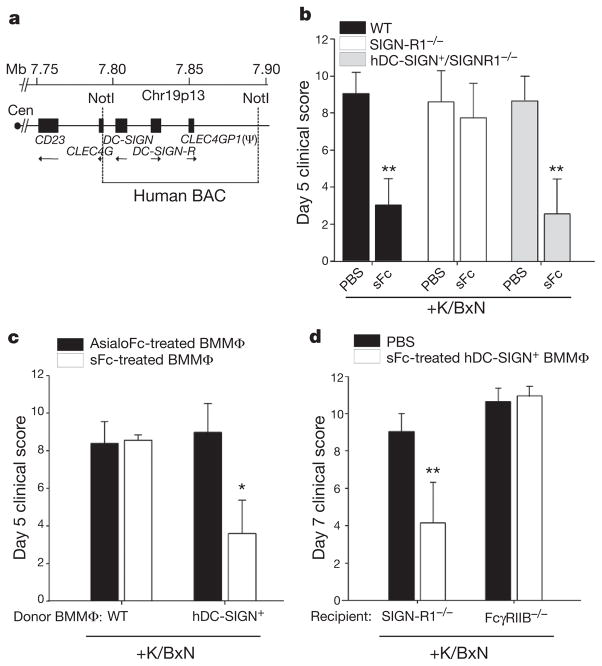
Figure 1. Human DC-SIGN conveys sFc anti-inflammatory activity.
a, A map of the human chromosome 19 BAC clone containing DC-SIGN and DC-SIGN-R genes. Cen, centromere. b, Wild-type (WT; black bars), SIGN-R1−/− (white bars), or hDC-SIGN+/SIGN-R1−/−(grey bars) mice were administered K/BxN sera and sFc. **P >0.001 determined by a Fisher least significant difference (LSD) post-hoc test. c, AsialoFc- (black bars) or sFc- (white bars) treated wild-type and hDC-SIGN+ bone-marrow derived macrophages (BMMΦ) were administered to K/BxN-sera-treated wild-type recipients. *P >0.05 determined by Tukey’s post-hoc test. d, PBS (black bars) or sFc-treated hDC-SIGN+ bone-marrow-derived macrophages (white bars) were administered to SIGN-R1−/− and FccRIIB−/− recipients. Means and standard deviations are plotted; **P >0.001 determined by Tukey’s post-hoc test.
To determine if hDC-SIGN could substitute for SIGN-R1 in mediating IVIG protection, hDC-SIGN+ mice were crossed to SIGN-R1-deficient animals (hDC-SIGN+/SIGN-R1−/−) and challenged with arthritogenic K/BxN serum10. Both induction of arthritis and responsiveness to IVIG and sFc were similar in wild-type mice and hDC-SIGN+/SIGN-R1−/− mice (Fig. 1b and Supplementary Fig. 3a). In contrast, induced arthritis was not suppressed by IVIG or sFc in SIGN-R1−/−mice. Thus, hDC-SIGN expression was sufficient to trigger the IVIG and sFc anti-inflammatory response.
A related lectin, DC-SIGN-R, is linked to DC-SIGN on the BAC transgene (Fig. 1a). hDC-SIGN-R has reduced affinity to sFc as compared to hDC-SIGN (Supplementary Fig. 3b). To define the contribution of DC-SIGN-R to sFc anti-inflammatory activity, mice that express hDC-SIGN alone as a transgene11 were crossed with SIGN-R1−/− mice (CD11c-DC-SIGN/SIGN-R1−/−). These mice were protected from inflammatory arthritis by IVIG (Supplementary Fig. 3c). Further, selective blockade of hDC-SIGN in transgenic hDC-SIGN+/ SIGN-R1−/− mice expressing both hDC-SIGN and hDC-SIGN-R resulted in a loss of IVIG protection in vivo (Supplementary Fig. 3d). These results support a requirement for hDC-SIGN but not hDC-SIGN-R in this anti-inflammatory response triggered by sFc.
Next, we sought to determine if stimulation of hDC-SIGN+ cells matured from bone marrow with sFc was sufficient to induce an anti-inflammatory response. Bone-marrow-derived macrophages and dendritic cells cultured from hDC-SIGN+ transgenic animals expressed hDC-SIGN, but not hDC-SIGN-R or SIGN-R1 (Supplementary Fig. 4a, b, c). Bone-marrow-derived cells cultured from hDC-SIGN+ transgenic or wild-type mice were pulsed for 30 min with sFc or asialylated Fcs (asialoFc) at a concentration representative of in vivo treatments. The treated cells were collected, washed and administered to wild-type mice, which were then challenged with K/BxN serum (Supplementary Fig. 4d). Mice receiving hDC-SIGN+ bone-marrow-derived macrophages or dendritic cells pulsed with sFc or IVIG showed reduced joint inflammation as compared to recipient mice that received wild-type cells, or hDC-SIGN+ cells pulsed with asialoFc (Fig. 1c and Supplementary Fig. 4e, f.). The anti-inflammatory response triggered by transferred sFc-stimulated hDC-SIGN+ bone-marrow-derived macrophages required the expression of the inhibitory Fc receptor (FcR) FcγRIIB, as FcγRIIB−/− recipient mice were not protected from inflammation induced by K/BxN serum (Fig. 1d). Collectively, these results were consistent with the in vivo requirements for IVIG protection previously defined1–4, and demonstrated that ligation of hDC-SIGN by sFc on bone-marrow-derived myeloid cells is sufficient to induce an anti-inflammatory cellular response.
DC-SIGN engagement has been reported to result in dendritic cell production of IL-10 (refs 7–8), making this anti-inflammatory cyto-kine an appealing candidate responsible for mediating IVIG anti-inflammatory activity. However, IL-10−/− mice were protected from induced arthritis by IVIG similarly to wild-type controls (Supplementary Fig. 5). We next addressed other cytokines that could be responsible for this response. The TH2 cytokine IL-4 has been shown to upregulate FcγRIIB surface expression on peripheral monocytes12, and increase the threshold for activation by pathogenic immune complexes, consistent with the FcγRIIB requirement of IVIG1,13,14. Therefore, sFc-treated DC-SIGN+ bone-marrow-derived macrophages were administered to naive wild-type mice or IL-4−/− mice, and the recipient mice challenged with K/BxN serum. Wild-type recipients were protected from induced arthritis, whereas IL-4−/− recipients were not (Fig. 2a).
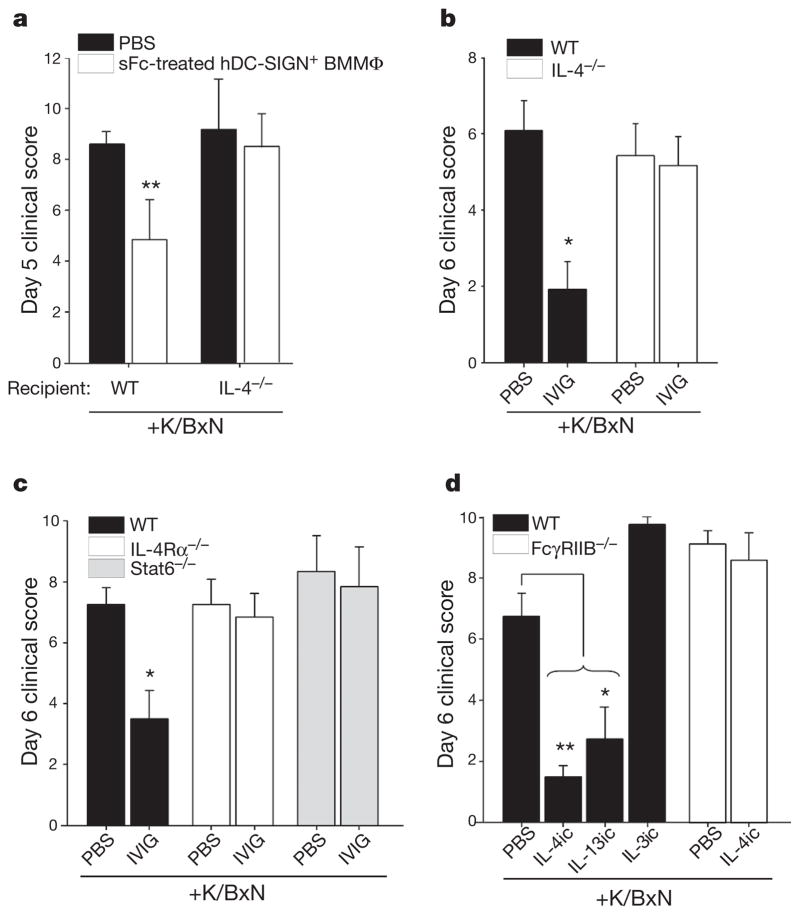
Figure 2. IL-4 requirements of sFc anti-inflammatory activity.
a, sFc-treated hDC-SIGN+bone-marrow-derived macrophages (BMMΦ; white bars) or PBS was administered to K/BxN-treated wild-type or IL-4−/− recipient mice. **P >0.002 determined by Fisher LSD post-hoc test. b, Wild-type (black bars) and IL-4−/− (white bars) mice were treated with K/BxN sera and IVIG. *P >0.01 determined by Mann–Whitney’s U test. c, Wild-type (black bars), IL-4Rα−/− (white bars), or Stat6−/− mice (grey bars) were given K/BxN sera and IVIG. *P >0.01 determined by Tukey’s post-hoc test. d, Wild-type (black bars) and FccRIIB−/− mice (white bars) were administered cytokine immune complexes (IL-4ic, IL-3ic, IL-13ic) and K/BxN sera. Means and standard deviations are plotted; *P >0.01, **P >0.001 determined by Mann–Whitney’s U test.
These results led us to predict that IVIG anti-inflammatory activity would require IL-4 signalling. Indeed, mice deficient in IL-4 (IL-4−/−; Fig. 2b), the IL-4 receptor (IL-4Rα−/−; Fig. 2c), or the IL-4R signalling adaptor (Stat6−/−; Fig. 2c), were not protected from K/BxN-induced inflammation by IVIG or sFc. Further, monocytes in the peripheral blood and bone marrow of wild-type mice, but not IL-4Rα−/− mice, upregulated FcγRIIB after sFc administration (Supplementary Fig. 6). Next we examined whether exogenous TH2 cytokines could also suppress autoantibody-induced inflammation. Mice were treated with cytokine immune complexes (ic)15 of TH2 cytokines IL-4 (IL-4ic), and IL-13 (IL-13ic), or a non-TH2 cytokine complex of IL-3 (IL-3ic), and challenged with K/BxN serum. Inflammation was significantly attenuated after single administration of IL-4ic or IL-13ic, but not after IL-3ic treatment (Fig. 2d). However, IL-4ic treatment did not attenuate inflammation in FcγRIIB−/− mice, consistent with IL-4ic also requiring the FcγRIIB to suppress inflammation (Fig. 2d).
We next examined whether TH2 cytokines were induced after IVIG or sFc administration. No changes in Il4 messenger RNA levels were observed (Fig. 3a and Supplementary Fig. 7a–c), so we surveyed cyto-kines known to induce Il4 expression, including IL-33 (refs 16–18), IL-25 (refs 18–20), and thymic stromal lymphopoietin (TSLP)18,21,22. Interestingly, Il33 mRNA was upregulated in wild-type mice after IVIG and sFc administration, but remained unchanged SIGN-R1−/− mice. We then administered IL-33, IL-25, or TSLP to mice challenged with K/BxN serum. Exogenous IL-33 fully suppressed K/BxN arthri-togenic activity and induced IL-4 production in vivo, whereas IL-25 promoted only modest protection, and TSLP provided no protection (Fig. 3b), all of which correlated with systemic IL-4 and IL-13 levels (Fig. 3b and Supplementary Fig. 7d, e). Further, exogenous IL-33 and IL-4ic were unable to ameliorate serum-induced arthritis in IL-4Rα−/− mice (Fig. 3c and Supplementary Fig. 7e), indicating that IL-4Rα acts downstream of IL-33 in this pathway.
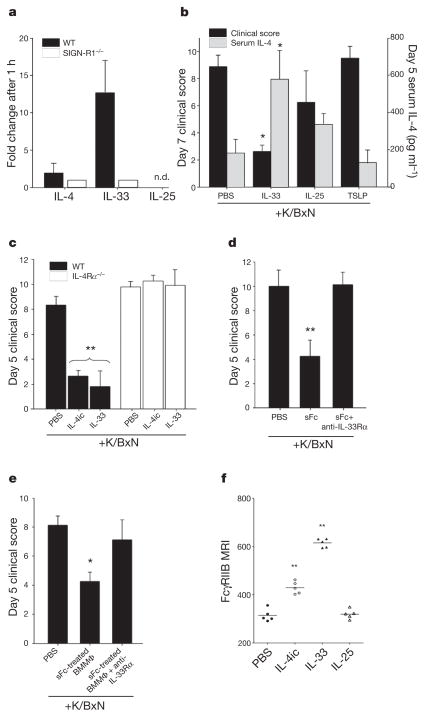
Figure 3. IL-33 triggers IL-4 anti-inflammatory activity.
a, Cytokine expression 1 h after IVIG administration in wild-type (black bars) or SIGN-R1−/− mice (white bars) determined by quantitative polymerase chain reaction (qPCR). n.d., not detected. b, K/BxN-treated wild-type mice received PBS, IL-33, IL-25, or TSLP. *P >0.05 determined by Tukey’s test. c, K/BxN-treated wild-type (black bars) or IL-4Rα−/− (white bars) mice received PBS, IL-4ic, or IL-33. **P >0.001 determined by Tukey’s test. d, hDC-SIGN+/SIGN-R1−/− mice received K/BxN sera, sFc and anti-IL-33Rα. **P >0.001 determined by Fisher LSD test. e, sFc-treated hDC-SIGN+ bone-marrow-derived macrophages were administered to wild-type mice, K/BxN- and anti-IL-33Rα-treated wild-type mice. Means and standard deviations are plotted; *P >0.05 determined by Tukey’s test. f, Individual mean fluorescence intensities (MFI) of bone marrow monocyte (CD11b+ Ly6G−) FcγRIIB surface expression 24 h after PBS, IL-4, IL-33, or IL-25 treatment by FACS. **P >0.01 determined by Tukey’s test.
Our results support an anti-inflammatory cascade where DC-SIGN ligation by sFc promotes IL-33 production, IL-33 induces IL-4 expression, culminating in FcγRIIB upregulation on monocytes and macrophages (Supplementary Fig. 1). To confirm this, hDC-SIGN+/ SIGN-R1−/− mice were treated with arthritogenic sera and sFc, in combination with a blocking antibody to the IL-33 receptor (anti-IL-33Rα). This intervention ablated the ability of sFc to protect hDC-SIGN+/SIGN-R1−/− mice (Fig. 3d and Supplementary Fig. 7f). Protection of transferred sFc-treated hDC-SIGN+ bone-marrow-derived macrophages was also diminished by anti-IL-33Rα treatment (Fig. 3e). Further, administration of exogenous IL-4ic or IL-33 increased FcγRIIB surface expression on monocytes, whereas IL-25 had no effect (Fig. 3f and Supplementary Fig. 8). IL-4 treatment down-regulated FcγRIIB expression on B cells (Supplementary Fig. 8), consistent with the diverse effects of this cytokine on different leukocyte types.
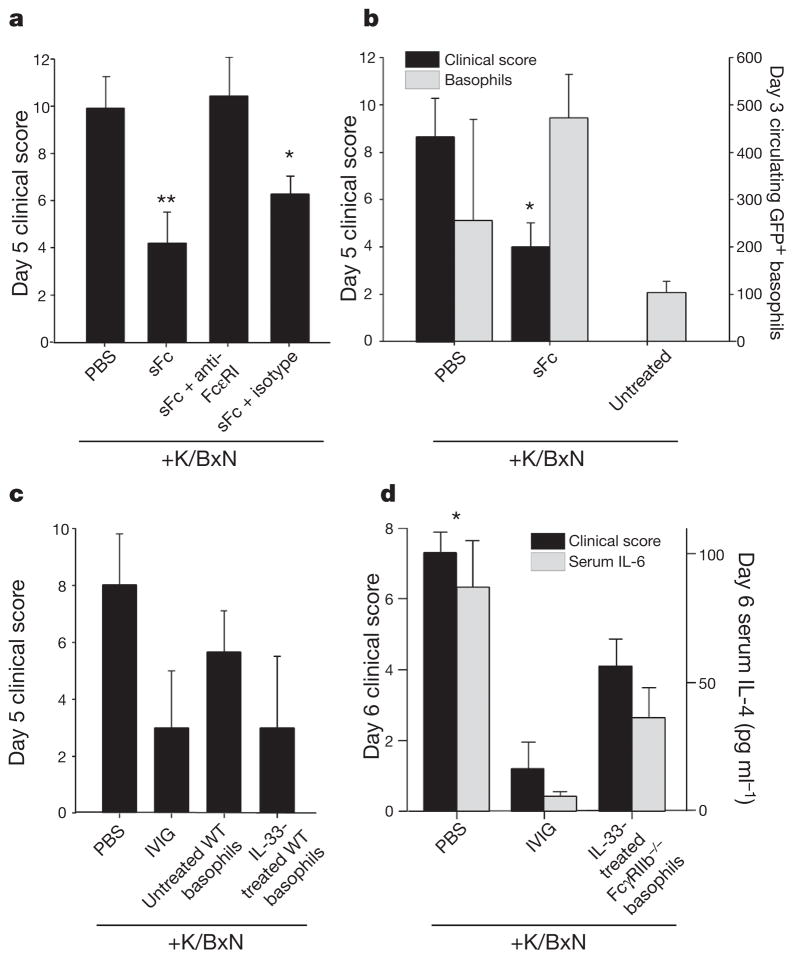
IL-4 can be produced by T cells and several innate immune cell populations18, including basophils23,24, mast cells25,26, eosinophils27 and progenitor cells19. IVIG activity is T-cell independent4, thus eliminating these cells as a source of sFc-induced IL-4. To determine whether baso-phils were involved in this response, we selectively depleted these cells in vivo28 (Fig. 4a and Supplementary Figs 9–11). Arthritis could be induced in basophil-depleted hDC-SIGN+/SIGN-R1−/− mice (Supplementary Fig. 9a) but the protective capacity of sFc and IVIG was lost (Fig. 4a and Supplementary Fig. 11), indicating that these cells have a pivotal role. We next administered sFc and K/BxN sera to IL-4–GFP reporter mice (4get29), and found a twofold increase in GFP+ basophils (DX5+FcεRI+c-Kit−) in the circulation of protected sFc-treated mice, indicating that basophils produced IL-4 in response to sFc (Fig. 4b). To determine whether basophils were ultimately responsible for the anti-inflammatory activity induced by sFc through IL-33, we transferred PBS- or IL-33-treated basophils to K/BxN-treated recipient mice (Fig. 4c, d and Supplementary Fig. 12a–c). IL-33-treated basophils derived from wild-type or FcγRIIB−/− mice were equally effective at suppressing arthritic inflammation, reducing serum IL-6 levels, and curbing leukocyte infiltration to arthritic paws (Fig. 4c, d and Supplementary Fig. 12d, e). These results confirm the anti-inflammatory potential of these cells, and support a model whereby IL-4 produced by basophils increases FcγRIIB expression on inflammatory macrophages (Supplementary Fig. 1).
Figure 4. Anti-inflammatory activity mediated by basophils.
a, hDC-SIGN+/SIGN-R1−/− mice were administered K/BxN sera, sFc and anti-FcεRI or an isotype control. **P >0.001, *P >0.05 determined by Fisher LSD test. b, 4get mice were administered K/BxN sera and sFc. Circulating IL-4+ basophils (grey bars, DX5+ FcεRI+ GFP+) and clinical scores (black bars) are plotted. *P >0.05 determined by Tukey’s test. c, PBS or IL-33-treated basophils (DX5+ FcεRI+ c-Kit−) were administered to K/BxN-treated wild-type mice. Control mice received PBS or IVIG. d, Basophils from IL-33-treated FcγRIIB−/− mice were administered to K/BxN-treated wild-type recipients. Clinical scores (black) and serum IL-6 levels (grey) are plotted. Means and standard deviations are plotted;*P >0.05 determined by Mann–Whitney’s U test.
Analysing the anti-inflammatory activity of IVIG led to identification of an endogenous, innate pathway in which sialylated IgG, a minor component of serum IgG antibodies, binds DC-SIGN, promoting production of IL-33, which expands IL-4+ basophils. These cytokines are capable of suppressing autoantibody-mediated inflammation by modulating FcγRIIB expression on effector cells (Supplementary Fig. 1). IL-4 and IL-33 have pleiotropic activities, and mediate TH2 responses to helminth parasites and allergens18, as well as enhance inflammatory arthritis30,31, in addition to their activities reported here. Cytokine concentration, cellular environment, and differential responses of individual cell types are likely to explain these distinct effector functions.
Various stimuli have been reported to modulate the level of IgG Fc sialylation, and could regulate this intrinsic pathway. Antigenic stimulation results in the production of pro-inflammatory, antigen-specific, asialylated IgG antibodies2. Pathogenic autoantibodies, such as those produced during rheumatoid arthritis that recognize citrullinated peptides, similarly show reduced sialic acid as compared to other serum antibodies32. Conversely, increases in sialylated IgG antibodies occur during pregnancy33, which could contribute to the remission in arthritis seen in pregnant women. Therefore, affecting the sialylation of IgG antibodies could provide an intrinsic mechanism for regulating TH2 cytokine production by innate myeloid cells in a DC-SIGN-dependent manner, provide a means for maintaining homeostasis, and is an attractive therapeutic approach to suppressing inflammation in autoimmune diseases.
METHODS SUMMARY
hDC-SIGN BAC transgenic mice were created using the BAC clone CTD2102F19 (Invitrogen) containing the human DC-SIGN gene. The BAC was linearized by the NotI restriction endonuclease. The human DC-SIGN gene fragment was purified and injected into one-day-old C57BL/6 embryos via pronuclear microinjection. The embryos were then implanted into ICR surrogate females and the resulting progeny were screened by PCR for the presence of the human DC-SIGN transgene. hDC-SIGN+ mice were crossed to SIGN-R1−/− mice to generate hDC-SIGN+/ SIGN-R1−/− lines.
METHODS
Mice
Eight-to-twelve-week old, sex and age matched mice were used for all experiments in compliance with federal laws, institutional guidelines and have been approved by the Rockefeller University. Wild-type C57BL/6, wild-type BALB/c, NOD, IL-4−/−, IL-4Rα−/−, Stat6−/−, IL-10−/−, 4get (IL-4–GFP reporter) mice were purchased from Jackson Laboratories, and maintained at the Rockefeller University animal facility. FcγRIIB−/− mice34 were generated previously in the laboratory. SIGN-R1−/− mice35 were provided by A. McKenzie. CD11c-hDCSIGN+ mice11 were provided by T. Sparwasser. KRN T-cell receptor transgenic mice on a C57BL/6 background (K/B) were gifts from D. Mathis and C. Benoist and were bred to NOD mice to generate K/BxN mice10. K/BxN serum was prepared as described previously13. Briefly, serum was separated from blood collected from the K/BxN mice (6–12-weeks old). Several weeks of serum collection were pooled together and frozen in aliquots to be used in the experiments described here. One intravenous injection of 200 μl K/BxN serum was used to induce arthritis. Severity of arthritis was scored by clinical examination by adding the index of all four paws, where 0 is unaffected, 1 is swelling of one joint, 2 is swelling of more than one joint, and 3 is severe swelling of the entire paw. All experiments shown yielded similar results at least 3 times with treatment groups of 4–5 mice, and means and standard deviations are plotted in bar graphs.
hDC-SIGN BAC transgenic mice were created using the BAC clone CTD2102F19 (Invitrogen) containing the human DC-SIGN gene. The BAC was linearized by the NotI restriction endonuclease. The human DC-SIGN gene fragment was purified and injected into one-day-old C57BL/6 embryos via pronuclear microinjection. The embryos were then implanted into ICR surrogate females and the resulting progeny were screened by PCR for the presence of the human DC-SIGN transgene. hDC-SIGN+mice were crossed to SIGN-R1−/− mice to generate hDC-SIGN+/SIGN-R1−/− lines.
Reagents and treatments
IVIG (Octagam, Octapharma) or IVIG-derived Fcs was enriched for terminal sialic acid using SNA-agarose2 (Vector Laboratories) or hypersialyated in vitro as previously described3 to generate sFc. AsialoFc was generated by treating Fcs with neuraminidase (NEB) as per the manufacturer’s directions. Sialic acid content was verified by lectin blotting with SNA-biotin (Vector Laboratories). IVIG and IVIG derivations were administered intravenously (i.v.) at 1 g kg−1, SNA-enriched IVIG at 0.1 g kg−1, and sFc at 0.03 g kg−1 one hour before K/BxN sera administration.
Mice receiving cytokine:immune complexes (ic) with prolonged half-life were treated with a single i.v. injection 2.5 μg of cytokine (IL-3, IL-4, IL-13; Peprotech) and 12.5 μg of neutralizing antibody at day 0. Neutralizing antibodies used were anti-IL-3 (MP2-8F8, Biolegend), anti-IL4 (11B11, BD Biosciences), and anti-IL-13 (eBio1316H, eBioscience). Other cytokine treatments included intraperitoneal (i.p.) administration of 400 ng (or 800 ng) of IL-25 (R&D), 400 ng of IL-33 (R&D), or 1 μg of TSLP (R&D) on days 0, 1, 2, and 3. Basophils were depleted as described28 by daily i.p. injection with 10 μg of anti-FcεRI (MAR-1, eBioscience) or hamster IgG isotype control (eBioscience) on days 0–5. Alternatively, mice received a single i.v. injection of 30 μg anti-CD200RL3 (ref. 36) (Ba103, Hycult Biotech) or rat IgG isotype control (BD Biosciences). IL-33Rα was blocked by i.v. injection of 80 μg of anti-IL-33Rα (DT8, MD Biosciences) or rat IgG1 isotype control (BD Biosciences) on day 0. hDC-SIGN was blocked in vivo by administration of 125 μg E9E A8 (ref. 37) or isotype control mouse IgG2a (BioLegend).
IL-6 was measured in serum by ELISA as suggested by the manufacturer (BioLegend). Serum IL-4 and IL-13 was measured using an in vivo cytokine capture assay as described38. Briefly, 10 μg biotinylated anti-IL-4 antibody (clone BVD4-1D11, BioLegend) or biotinylated anti-IL-13 (eBio1316HA, eBioscience) was injected i.v. into treated mice, and sera were collected 24 h later. Cytokine levels were quantified by ELISA assay using anti-IL-4 (BVD6-24G1, BioLegend) or anti-IL-13 (eBio13A, eBioscience) as capture antibodies.
Splenic RNA was purified using RNeasy Mini Kits (Qiagen) and reverse-transcribed using Verso cDNA synthesis kit (Thermo Scientific). Quantitative PCR (qPCR) was conducted in 7300 Real-time PCR System (Life Technologies) with primer-probe sets for mouse IL-4, IL-13, IL-33, IL-25, or rRNA (Life Technologies), and gene expression levels were determined by normalization to rRNA levels.
Saturation binding experiments were performed as previously described4, comparing CHO and CHO-hDC-SIGN cells or Hep-CD81 and Hep-hDC-SIGN-R cells.
Flow cytometry
Single cell suspensions were prepared from peripheral blood, spleen, bone marrow, or paws from mice. After red blood cell lysis, cells were stained with the indicated monoclonal antibodies, and subjected to analysis using a FACSCalibur or LSR-II cytometer (BD Biosciences). Human leukocytes were obtained from peripheral blood samples (New York Blood Center) after density gradient centrifugation (Ficoll-Paque, GE Healthcare). Antibodies used for murine cell staining were as follows: anti-CD19 (1D3), anti-B220 (RA3-6B2), anti-CD3ε (145-2C11), anti-CD11b (M1/70), anti-Ly6G (1A8), anti-CD11c (HL3), anti-I-Ab (AF6-120.1), anti-CD49b (DX5 and HMa2), anti-c-Kit (2B8), anti-CD45.2 (104) from BD Biosciences, anti-NKp46 (29A1.4), anti-SIGN-R1 (22D1), anti-CD123 (5B11) from eBioscience, anti-hDC-SIGN (9E9A8), anti-FceR1 (MAR-1) from Biolegend, anti-FcγRIIB (K9.361), anti-hDC-SIGNR (120604) from R&D systems. Antibodies used for human cell staining were: anti-CD14 (M5E2), anti-CD16 (B73.1), anti-CD3 (UCHT1), anti-CD56 (B159), anti-CD19 (SJ25C1), anti-CD11c (B-Ly6), anti-HLA-DR (L243 (G46-6)), anti-hDC-SIGN (AZND1) from BD Biosciences, and anti-hDC-SIGN (9E9A8, Biolegend). AccuCheck Counting Beads (Invitrogen) were used to quantify cells.
Bone-marrow macrophage and dendritic cell cultures and transfers
Bone-marrow-derivedmacrophages were cultured as described previously39. Briefly, marrow was recovered from tibias and femurs of mice, and seeded in non-tissue culture treated 10-cm plates with DMEM supplemented with 10% fetal bovine serum, 2% penicillin/streptomicin (Invitrogen), 1% glutamine 200 mM (Invitrogen), 0.1% μ-mercaptoethanol, IL-3 (5 ng ml−1, Peprotech) and M-CSF (5 ng ml−1, Peprotech) overnight at 37 °C, 5% CO2. The next day, non-adherent cells were recovered and plated in 10-cm non-tissue culture treated plates in supplemented DMEM with cytokines, and cultured for 5–7 days. Once the cultured cells were mature macrophages (>90% CD11b+F4/80+ by FACS), the cells were detached and 2 × 106 macrophages were plated per well in 6-well plates, and allowed to attach overnight. The next day, the cells were pulsed with IVIG (15 mg ml−1), BSA (15 mg ml−1, Sigma), sFc (0.5 mg ml−1), or asialoFc (0.5 mg ml−1) for 30 min at 37 °C. The cells were recovered, washed thoroughly in cold PBS, and 1 ×106 macrophages were administered i.v. into naive recipients. One hour later, the recipient mice were treated with K/BxN sera. Dendritic cells were cultured from mouse tibia and femur bone marrow cells as described40. Briefly, 1 ×106 cells ml−1 were plated in 24-well plates with DMEM supplemented with 10% FBS and 10 ng ml−1 mouse granulocyte macrophage colony stimulating factor (GM-CSF, Peprotech). On day 6, loosely adherent cells were collected by gentle pipetting, and were subjected to flow cytometric analysis or bone marrow cell transfer experiments as described above.
Histology
Human lymph node samples were from ISL Bio, and M. Pack provided human spleen samples. Spleens or lymph nodes embedded in O.C.T. compound (Sakura Finetek) were fixed in ice-cold acetone for 10 min, and stained with anti-SIGN-R1, anti-hDC-SIGN or anti-hDC-SIGN-R for 1 h at 25 °C in combination with antibodies for macrophages or B cells. Antibodies used included anti-F4/80 (BM8, Invitrogen) for mouse red pulp macrophages, anti-B220 for mouse B cells, anti-hCD20 (2H7, Biolegend) for human B cells, and anti-CD68 (Y1/82A, Biolegend) for human macrophages. Sections were visualized by wide-field fluorescence microscope (Zeiss).
Basophil adoptive transfers
Basophils were expanded by administering IL-3ic41, as described above, to wild-type or FcγRIIB−/− mice. Five days later, IL-3ic-treated mice were administered PBS or IL-33 (400 ng) i.p. The next day, basophils (DX5+FcRI+cKit−) were sorted using a FACSAria II (BD Biosciences). Sorted basophils were washed in cold PBS, and 0.7 ×106 basophils were administered to naive recipient mice subsequently administered K/BxN sera. A Wright–Giemsa stain of sorted, cytospun basophils was performed as suggested by the manufacturer (Sigma).
Supplementary Material
Acknowledgments
The authors thank P. Smith, M. Kibe, J. Brown and K. Velinzon for technical support, K. L. Jeffrey, C. Cheong, R. Steinman and J. J. Lee for discussions, M. Pack for providing human spleen sections, A. McKenzie for providing SIGN-R1−/− mice, T. Sparwasser for providing CD11c-hDC-SIGN mice, C. G. Park for providing hDC-SIGN and hDC-SIGN-R expressing cell lines, and H. Watarai for providing anti-IL-25R antibodies. R.M.A. is an Irvington Institute fellow of the Cancer Research Institute. F.W. is supported by the Wenner-Gren Foundations, Sweden. This work was performed with support from Virdante Pharmaceuticals and NIH grants to J.V.R.
Footnotes
Reprints and permissions information is available at www.nature.com/reprints. The authors declare competing financial interests: details accompany the full-text HTML version of the paper at www.nature.com/nature. Readers are welcome to comment on the online version of this article at www.nature.com/nature.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions R.M.A., T.K., F.W. and J.V.R. designed the experiments and interpreted the results. R.M.A., T.K. and F.W. performed the experiments, and R.M.A. and J.V.R. wrote the manuscript.
References
- 1.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 2.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 3.Anthony RM, et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granelli-Piperno A, et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol. 2005;175:4265–4273. doi: 10.4049/jimmunol.175.7.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soilleux EJ, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 7.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nature Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gringhuis SI, et al. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Hodges A, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nature Immunol. 2007;8:569–577. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- 10.Korganow AS, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer M, et al. Decreased pathology and prolonged survival of human DC-SIGN transgenic mice during mycobacterial infection. J Immunol. 2008;180:6836–6845. doi: 10.4049/jimmunol.180.10.6836. [DOI] [PubMed] [Google Scholar]
- 12.Pricop L, et al. Differential modulation of stimulatory and inhibitory Fcγ receptors on human monocytes by Th1 and Th2 cytokines. J Immunol. 2001;166:531–537. doi: 10.4049/jimmunol.166.1.531. [DOI] [PubMed] [Google Scholar]
- 13.Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18:573–581. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 14.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 15.Finkelman FD, et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1244. [PubMed] [Google Scholar]
- 16.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul WE, Zhu J. How are TH2-type immune responses initiated and amplified? Nature Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saenz SA, et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fort MM, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 21.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 22.Wang YH, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2memorycells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seder RA, et al. Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc Natl Acad Sci USA. 1991;88:2835–2839. doi: 10.1073/pnas.88.7.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seder RA, et al. Production of interleukin-4 and other cytokines following stimulation of mast cell lines and in vivo mast cells/basophils. Int Arch Allergy Appl Immunol. 1991;94:137–140. doi: 10.1159/000235345. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, Saxon A, Diaz-Sanchez D. Early IL-4 production driving Th2 differentiation in a human in vivo allergic model is mast cell derived. Clin Immunol. 1999;90:47–54. doi: 10.1006/clim.1998.4628. [DOI] [PubMed] [Google Scholar]
- 27.Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 28.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nature Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohrs M, Shinkai K, Mohrs K, Locksely RM. Analysis oftype 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 30.Ohmura K, et al. Interleukin-4 can be a key positive regulator of inflammatory arthritis. Arthritis Rheum. 2005;52:1866–1875. doi: 10.1002/art.21104. [DOI] [PubMed] [Google Scholar]
- 31.Xu D, et al. IL-33 exacerbates autoantibody-induced arthritis. J Immunol. 2010;184:2620–2626. doi: 10.4049/jimmunol.0902685. [DOI] [PubMed] [Google Scholar]
- 32.Scherer HU, et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62:1620–1629. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 33.van de Geijn FE, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther. 2009;11:R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 35.Lanoue A, et al. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J Exp Med. 2004;200:1383–1393. doi: 10.1084/jem.20040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obata K, et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 37.Cheong C, et al. New monoclonal anti-mouse DC-SIGN antibodies reactive with acetone-fixed cells. J Immunol Methods. 2010;360:66–75. doi: 10.1016/j.jim.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finkelman F, Morris S, Orekhova T, Sehy D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. Curr Protoc Immunol. 2003:6.28.1–6.28.10. doi: 10.1002/0471142735.im0628s54. [DOI] [PubMed] [Google Scholar]
- 39.Jeffrey KL, et al. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nature Immunol. 2006;7:274–283. doi: 10.1038/ni1310. [DOI] [PubMed] [Google Scholar]
- 40.Inaba K, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohmori K, et al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J Immunol. 2009;182:2835–2841. doi: 10.4049/jimmunol.0802870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.