Abstract
Experiments were designed to determine if the vasodilatory peptides maxadilan and pituitary adenylate cyclase-activating peptide (PACAP-38) may cause plasma leakage through activation of leukocytes and to what extent these effects could be due to PAC1 and CXCR1/2 receptor stimulation. Intravital microscopy of hamster cheek pouches utilizing FITC-dextran and rhodamine, respectively, as plasma and leukocyte markers was used to measure arteriolar diameter, plasma leakage and leukocyte accumulation in a selected area (5 mm2) representative of the hamster cheek pouch microcirculation. Our studies showed that the sand fly vasodilator maxadilan and PACAP-38 induced arteriolar dilation, leukocyte accumulation and plasma leakage in postcapillary venules. The recombinant mutant of maxadilan M65 and an antagonist of CXCR1/2 receptors, reparixin, and an inhibitor of CD11b/CD18 up-regulation, ropivacaine, inhibited all these effects as induced by maxadilan. Dextran sulfate, a complement inhibitor with heparin-like anti-inflammatory effects, inhibited plasma leakage and leukocyte accumulation but not arteriolar dilation as induced by maxadilan and PACAP-38. In vitro studies with isolated human neutrophils showed that maxadilan is a potent stimulator of neutrophil migration comparable with fMLP and leukotriene B4 and that M65 and reparixin inhibited such migration. The data suggest that leukocyte accumulation and plasma leakage induced by maxadilan involves a mechanism related to PAC1- and CXCR1/2-receptors on leukocytes and endothelial cells.
Introduction
The sand fly Lutzomyia longipalpis is the vector of Leishmaniasis disease and its saliva functions as a vehicle for the Leishmania chagasi promastigotes. Several studies have shown that salivary gland lysates, or its most active vasodilator component, maxadilan, may enhance infectivity of several Leishmania species possibly through an up-regulation of IL-4 and IL-10 (Lima and Titus, 1996, Mbow et al., 1998, Morris et al., 2001, Norsworthy et al., 2004). In spite of the emphasis placed on the immunomodulatory effects of sandfly saliva, the mechanisms underlying inflammation are not well characterized as yet. In model studies, it has been shown that Lu. longipalpis salivary gland homogenates (SGH), maxadilan as well as PACAP caused long-lasting vasodilation and plasma leakage when applied topically to the microcirculation of the hamster cheek pouch, Svensjö et al. (2009). However, it is unclear whether this enhancement is due to vasodilatory effects or to some additional properties of proteins or peptides in the saliva, e.g. activation of leukocytes and formation of chemokines that may result in an enhancement of plasma leakage in postcapillary venules. Among the repertoire of Lu. longipalpis salivary proteins, maxadilan has been characterized and cloned (Lerner and Shoemaker, 1992, Lerner et al., 1991, Moro and Lerner, 1997). It has been shown that maxadilan primary sequence has no homology to that of pituitary adenylate cyclase-activating peptide (PACAP), despite the fact that maxadilan is an agonist of the PAC1 receptor, one of the three receptors of PACAP (Lerner et al., 2007, Pereira et al., 2002). PACAP may act as an activator of human neutrophils and monocytes (El Zein et al., 2008, Harfi and Sariban, 2006, Harfi et al., 2004). Evidence for an important role of neutrophils at Leishmania infection has been provided by Peters et al. (2008). Using dynamic intravital microscopy, a rapid and sustained neutrophilic infiltration could be observed at localized sand fly bite sites. Invading neutrophils efficiently captured L. major parasites early after sand fly transmission or needle inoculation (Peters et al., 2008). The activation of neutrophils by a sand fly bite appears to be independent of the presence of L. major parasites in the injected saliva (Peters and Sacks, 2009, Teixeira et al., 2005). In order to characterize the role played by saliva components for the successful Leishmania parasite infection we have specifically studied microvascular effects induced by maxadilan in the hamster cheek pouch and complemented this analysis by examining the effects of this classical vector-borne vasodilator on neutrophil migration in vitro. We found that maxadilan induced plasma leakage and leukocyte accumulation were both inhibited by (i) the maxadilan mutant M65, (ii) the CXCR1/ 2-antagonist reparixin, (iii) dextran sulfate and (iv) ropivacaine. The functional implications of these findings will be discussed.
Material and methods
Intravital digital microscopy of the hamster cheek pouch
Hamsters were maintained and anesthetized according to regulations given by the local ethical committee (IBCCF, protocol-014, 23/02/ 2008). Altogether 60 hamsters with a mean body weight of 117±15 g (Anilab, São Paulo, Brazil), were used. Anesthesia was induced by intra-peritoneal injection of sodium pentobarbital that was supplemented with i.v. α-chloralose (2.5% W/V, solution in saline) through a femoral vein catheter. A tracheal canula (PE 190) was inserted to facilitate spontaneous breathing and the body temperature was maintained at 37 °C by a heating pad monitored with a rectal thermistor. The hamster cheek pouch (HCP) was prepared and used for intravital microscopy as previously reported (Svensjö, 1978), and now modified for measurements of plasma leakage and leukocyte accumulation with a digital camera and analyzed as described (Schmitz et al., 2009, Svensjö et al., 2009). The microcirculation of the HCP was observed using an Axioskop 40 microscope, objective 4x and oculars 10x (Carl Zeiss, Germany) equipped with appropriate filters (490/520 nm, and 540/ 580 nm, rhodamine) for observations of fluorescence in epiluminescence. A digital camera, AxioCam HRc, and a computer with the AxioVision 4.4 Software program (Carl Zeiss, Germany) was used for image analysis of arteriolar diameter and total fluorescence in a representative rectangular area (5 mm2) of the prepared HCP. Fluorescence was recorded for 30 min prior to experimental interventions to secure normal blood flow and unaltered vascular permeability and the fluorescence measured at 30 minute after FITC-dextran (FITC-dextran 150 kDa, 100 mg/kg bodyweight, TdB Consultancy, Uppsala, Sweden) injection was adjusted to 2000 fluorescent units (RFU=Relative Fluorescent Units) for statistical reasons. Leukocytes were labeled in vivo by i.v. injection of rhodamine 100 μg/kg b.w. at 10 min prior to experimental interventions and followed by 10 μg/ kg b.w. every 10 min until 60 min. The recorded fluorescence at 10 min after rhodamine injection in each experiment was adjusted to 3000 fluorescent units (RFU) for statistical reasons. Two images of exactly the same area were recorded at every 5 min interval during the entire experiment out of which one was used to measure plasma leakage and arteriolar diameter (490/520 nm) and the other to measure total fluorescence of rhodamine-labeled leukocytes in circulation, rolling, adherent and migrated (540/580 nm) in the observed area (5 mm2) here defined as leukocyte accumulation. Exposure time was limited to 15 s for each captured image in order to avoid phototoxicity (Harris et al., 2002). Following a 30 min control period after FITC-dextran injection HCPs were topically exposed to 250 μl of either saline (controls) or maxadilan 134 nM in saline for 9 min during interrupted superfusion or M65 8 μM in saline for 4 min followed by maxadilan 134 nM until 9 min. In order to demonstrate morphological and temporal correlation between leukocyte accumulation and plasma leakage, two circular areas (Ø=100 μm, 0.008 mm3, were selected within the 5 mm2-area-image for measurements of FITC-fluorescence (denoted in figures as PCV-FITC and CAP-FITC) and rhodamine-fluorescence (denoted in figures as PCV-RHOD and CAP-RHOD) in one hamster out of six in the maxadilan 134 nM treated group. One area contained only terminal arterioles and capillaries (CAPFITC, CAP-RHOD) and the other area contained postcapillary venules (PCV-FITC, PCV-RHOD).
PACAP-38 was applied in a similar way or 50 nM PACAP-38 in 250 μl of saline.
Ropivacaine (AstraZeneca, Södertälje, Sweden) was added to the superfusion solution at ten min prior to maxadilan application for 9 min resulting in a final concentration of 100 μM in the prepared cheek pouch. Dextran sulfate 500 kDa (TdB Consultancy, Uppsala, Sweden) was given as an i.v. injection of 2–5 mg/Kg b.w. 10 min prior to maxadilan or PACAP-38 application. Recombinant maxadilan and the maxadilan antagonist M65 were obtained as described (Lerner et al., 2007). Reparixin (R(–2-(4-isobutylphenyl)propionyl methansulphonamide) as the L-lysine salt (Souza et al., 2004) was obtained from Dompé Pharma (Italy) and given as an i.v. injection (40 mg/kg). PACAP-38 was from Sigma-Aldrich, St Louis, USA.
Neutrophil isolation and treatment
Human neutrophils were isolated from EDTA (0.5%)-treated peripheral venous blood of healthy volunteers using a four-step discontinuous Percoll (Sigma Chemical Co., St Louis, MO) gradient (Dooley et al., 1982). Erythrocytes were removed by hypotonic lysis. Isolated neutrophils resuspended in RPMI-1640 medium (Sigma), presented 97% purity, as accessed by direct observation under optical microscopy of H&E stained cells, and estimated to be >98% viable by trypan blue exclusion.
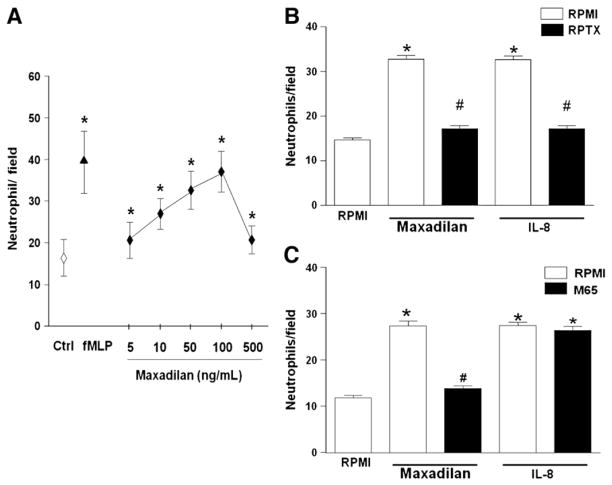
Neutrophil chemotaxis was assayed in a 48-well Boyden chamber (Neuroprobe microchemotaxis system) using a 5 μm PVP-free polycarbonate filter as previously described (Coelho et al., 2001, Saldanha-Gama et al., 2010). For chemotaxis assays, the chemotactic stimuli, N-formyl-methionyl-leucyl-phenylalanine peptide (fMLP-Sigma) (100 nM); IL-8 (30 nM) or Maxadilan (5–500 ng/mL, (0.67–67 nM) diluted in RPMI medium at different concentrations, were added to the bottom wells of the chamber. Cells suspended in RPMI medium (106 cells/ml) were added (50 μl) to the top wells of the Boyden chamber and allowed to migrate for 60 min at 37 °C in a 5% CO2 atmosphere. In some experiments, cells were pre-incubated with M65 (160 nM) or reparixin (1 μM) and for 5 min at 37 °C prior to the chemotactic assay. After that, cells were incubated for 60 min at 37 °C in a 5% CO2 atmosphere and then the filters were removed from the chambers, fixed and stained with a Diff-Quick stain kit (Baxter Travenol Laboratories, ON, Canada). Neutrophils that had migrated through the membrane were counted under light microscopy (100× objective) on at least five random fields. Results, expressed as number of neutrophils per field, were representative of three different experiments performed in triplicate for each sample. PMN migration towards RPMI-1640 medium alone (random chemotaxis) was used as a negative control.
Statistical analysis
Data were analyzed with help of GraphPad Prism 4 for Windows. Statistical significance was tested using ANOVA followed by Bonferroni post-test. P<0.05 was considered statistically significant.
Results
Topical application of maxadilan (134 nM) caused an immediate and long lasting increase in arteriolar dilation, associated with plasma leakage (FITC-dextran fluorescence) and leukocyte accumulation (rhodamine fluorescence), the maximal responses being detected at 35 min and 60 min respectively as shown in four images of the observed area, Figs. 1 and 2. After application of maxadilan the intensity of FITC-dextran and rhodamine fluorescence was higher in areas enriched with postcapillary venules and larger venules, whereas areas dominated by capillaries showed lower levels of fluorescence (Fig. 1). Measurements of fluorescence of the entire observed area (5 mm2) in two groups of hamsters showed that maxadilan increased both FITC-dextran and rhodamine fluorescence as compared with the saline control group, (p<0.05) (Fig. 2A and B). Measurement of rhodamine fluorescence in the cheek pouch will include fluorescence emitted from unbound rhodamine and from rhodamine-labeled leukocytes and platelets in the circulating blood and possibly also endothelial cells in venules. The rhodamine fluorescence is thus a measure of the presence of all labeled cells in the observed area of the micro-circulation regardless of their localization in the circulating blood, rolling or adhering in the venules or during and after migration into the extravascular space. Analysis of the fluorescence measurements in circular areas (r=50 μm or 0.008 mm2) within the entire observed area (5 mm2) of one hamster showed that both FITC-dextran and rhodamine fluorescence increased more in areas with postcapillary venules (PCV-FITC, PCV-RHOD) as compared with similar sized areas with capillaries following the application of maxadilan (CAP-FITC, CAP-RHOD) Fig. 2C and D. This result indicates that the sites of plasma leakage and leukocyte accumulation were overlapping. The increased fluorescence in capillary areas was most probably due to the diffusion of fluorescent molecules from postcapillary areas.
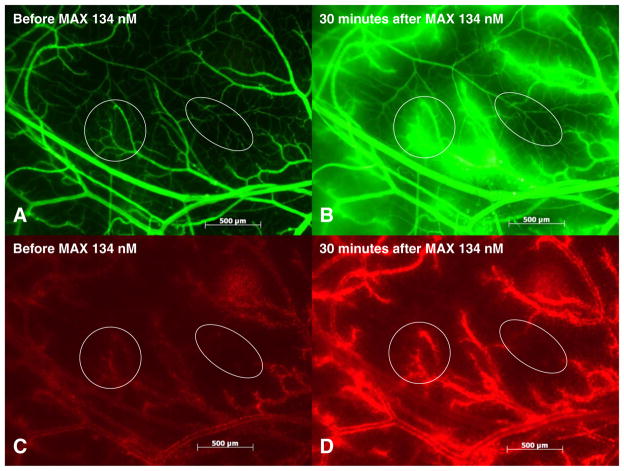
Fig. 1.
Maxadilan induced plasma leakage and leukocyte accumulation. Fluorescent light images of the observed area of 5 mm3 in one hamster cheek pouch preparation showing plasma leakage (a and b) and leukocyte accumulation (c and d) at 30 min after topical application of maxadilan 134 nM. Plasma leakage from postcapillary and larger venules is shown in (b). Leukocyte accumulation in postcapillary and larger venules is shown in (d). Circles include an area with postcapillary venules and ellipses include a different area with terminal arterioles and capillaries.
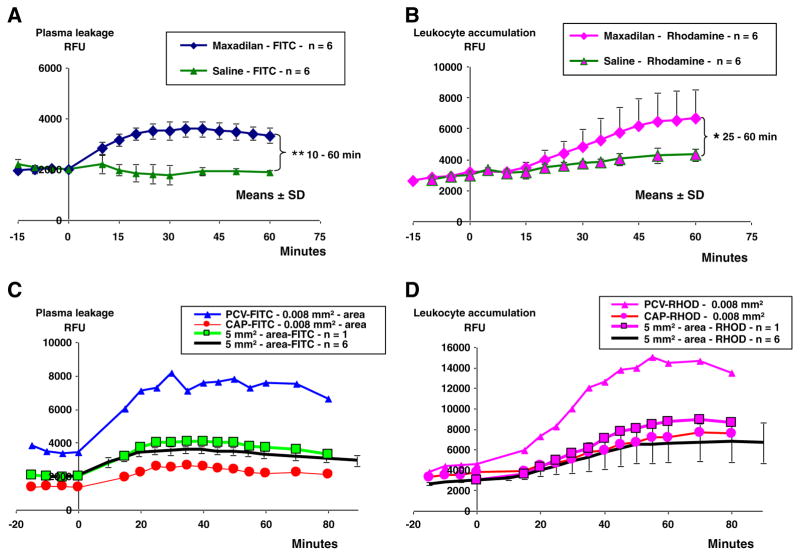
Fig. 2.
Measurements of plasma leakage and leukocyte accumulation in a 5 mm2-area and in smaller areas, 0.008 mm2. Fluorescence (RFU=Relative fluorescence Units, mean±SD) measured at intravital microscopy of cheek pouches in six hamsters subjected to application of 250 μl 134 nM maxadilan and in other six hamsters subjected to application of 250 μl of saline during 9 minutes. All hamsters received an i.v. injection of FITC-dextran 150 kDa at 30 min and of rhodamine (100 μg/kg) at 10 min before maxadilan or saline application at time zero. A. Maxadilan caused a lasting increase in fluorescence of FITC-dextran (plasma leakage) in the 5 mm2-area that was clearly above that induced by saline at 10 min after maxadilan application and onwards (**=p<0.01). B. Maxadilan 134 nM caused an increase in leukocyte accumulation in the 5 mm2-area as indicated by increased fluorescence of rhodamine labeled leukocytes that was greater in maxadilan treated hamsters at 25 min after maxadilan application (*=p<0.05) until end of experiments. C. Measurements of plasma leakage (FITC-fluorescence, RFU) in one out of six hamster cheek pouch preparations of the observed area of 5 mm2 (green squares) and in circular areas with diameters of 100 μm (0.008 mm2) including post-capillary venules (PCV-FITC, blue triangles) and in other circular areas including capillaries (CAP-FITC, red circles). Fluorescence of FITC-dextran increased in post-capillary areas (PCV-FITC) to maximally 239% and increased less in capillary areas (CAP-FITC) to maximally 188% of the zero control value prior to maxadilan 134 nM application. Fluorescence of FITC-dextran of the 5 mm2-area reached a maximal value of 191% of the zero control value which was similar to the mean increase (Black line) in the 5 mm2-area of the six hamsters. D. Measurements of leukocyte accumulation (rhodamine fluorescence, RFU) in one out of six hamster cheek pouch preparations of the 5 mm2–area (Rose squares) and in circular areas with diameters of 100 μm (0.008 mm2) including post-capillary venules (PCV-RHOD, rose triangles) and in other circular areas including capillaries (CAP-RHOD, rose circles). Fluorescence of rhodamine increased in post-capillary areas (PCV-RHOD) to maximally 329% and increased less in capillary areas (CAP-RHOD) to maximally 203% of the zero control value prior to maxadilan 134 nM application. Fluorescence in the 5 mm2-area of one single hamster was maximally 296% and was within the mean value±SD of the 5 mm2-area in six hamsters (Black line).
A statistical analysis of the data presented in Fig. 2A and B showed that there was a significant positive correlation between the increase in leukocyte accumulation and the increase in plasma leakage in maxadilan-stimulated hamsters in the 5 mm2-area during the 0–40 min period after maxadilan application (n=48, r=0.595, p=0.0001) as shown in Fig. 3. In saline control hamsters there was no increase in plasma leakage although there was a slight increase in leukocyte accumulation and there was no correlation (n=64, r=−0.111, p=0.3988) between the analyzed parameters. These data suggest that the increase in vascular permeability was related to the presence of leukocytes. Application of 250 μl of 50 nM PACAP-38 in two hamsters resulted in increased plasma leakage and leukocyte accumulation similar to that seen with maxadilan. Data from these two experiments with PACAP-38 showed a linear correlation between plasma leakage and leukocyte accumulation and were entered in Fig. 3 showing a striking similarity with maxadilan induced effects, (n=24, r=0.800, p=0.0001).
Fig. 3.
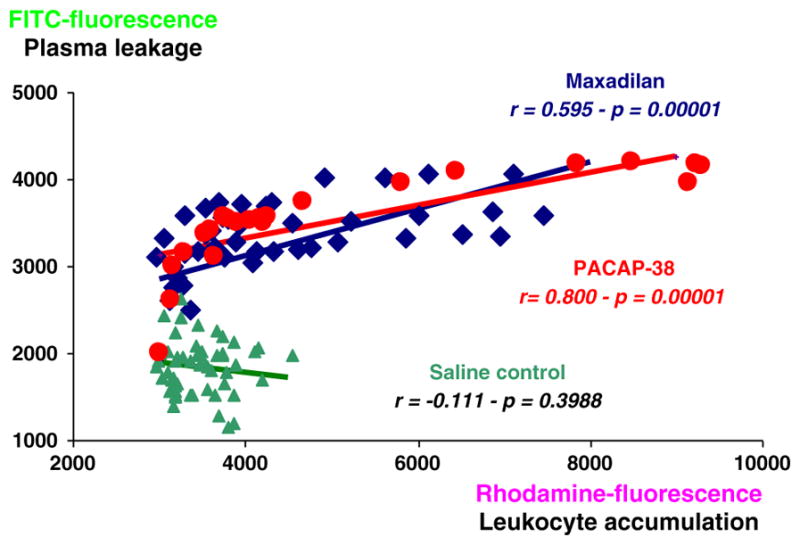
Linear correlation between plasma leakage and leukocyte accumulation. Analysis of linear correlation between measurements of plasma leakage and leukocyte accumulation in 8 saline control hamsters and in 6 maxadilan 134 nM treated hamsters at 0 to 40 min after maxadilan or saline application showing a significant positive correlation in maxadilan treated hamsters (maxadilan, n=48, r=0.595, p=0.0001, black diamonds) and no correlation (saline, n=64, r=−0.111, p=0.3988, filled triangles) between the plasma leakage and leukocyte accumulation until 40 min after maxadilan or saline application. Data from two experiments with PACAP-38 application were used for analysis of linear correlation between plasma leakage and leukocyte accumulation and showed a similarity between maxadilan and PACAP-38 induced effects, (n=24, r=0.800, p=0.0001, filled circles).
Influence of the PAC1 antagonist M65 on maxadilan induced effects
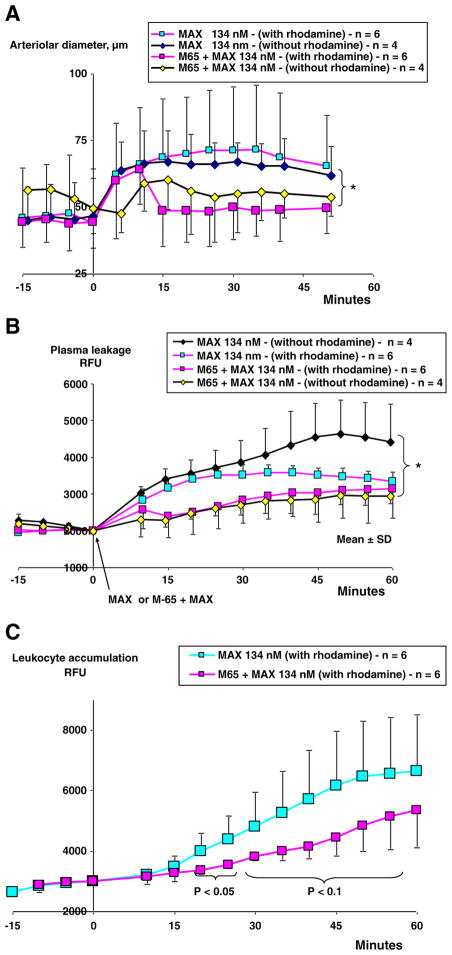
The receptor specificity of responses induced by maxadilan (134 nM) was studied by applying 60 X fold (8 μM) of recombinant M65 peptide (PAC1 antagonist) during 4 min prior to maxadilan application. Under these conditions, we found a statistically significant inhibition of both arteriolar dilation and plasma leakage (Fig. 4A and B). Leukocyte accumulation induced by maxadilan was reduced by M65 until 30 min after maxadilan application (p<0.05). The level of leukocyte accumulation was still 20% below that seen in the maxadilan treated group at 60 min (Fig. 4C).
Fig. 4.
Effects of maxadilan and its antagonist M65 on microvascular parameters. Fluorescence (RFU, mean±SD) measured at intravital microscopy of the cheek pouches in four groups of hamsters (n=4–6). Two groups were subjected to application of 250 μl saline from 0 to 4 min followed by 250 μl of 134 nM maxadilan during 4–9 min. Two groups of hamsters (n=4–6) were subjected to application of 250 μl of 8 μM maxadilan antagonist M65 during 4 min prior to application of 250 μl maxadilan 134 nM during 4–9 min. All groups received an i.v. injection of FITC-dextran 150 kDa (100 mg/kg) at 30 min before saline, M65 and maxadilan application and two groups of hamsters (n=6) also received rhodamine (100 μg/kg) 10 min before saline, M65 and maxadilan application. A. Maxadilan 134 nM caused arteriolar dilation that was maximally 32% above that induced in the presence of M65 (* =p <0.05). B. Maxadilan 134 nM caused an increase in plasma leakage (fluorescence of FITC-dextran, RFU) that was maximally 35% above that induced in the presence of M65 (* =p <0.05). Presence of rhodamine had no significant influence on the inhibition exerted by M65 of maxadilan induced effects but rhodamine inhibited the continued increase in plasma leakage as compared with the maxadilan group without presence of rhodamine. C. Maxadilan caused leukocyte accumulation that was inhibited by M65 between 20 to 30 min (p<0.05) and 35 to 50 min (p<0.1).
To evaluate whether rhodamine had non-specific effects in the microvasculature, two groups of hamsters (n=4) were studied without rhodamine. Presence of rhodamine did not influence the inhibition which M65 exerted on maxadilan induced effects. However, rhodamine inhibited the continued increase in plasma leakage induced by maxadilan as compared with the maxadilan group that was not exposed to rhodamine.
Influence of reparixin on maxadilan induced effects
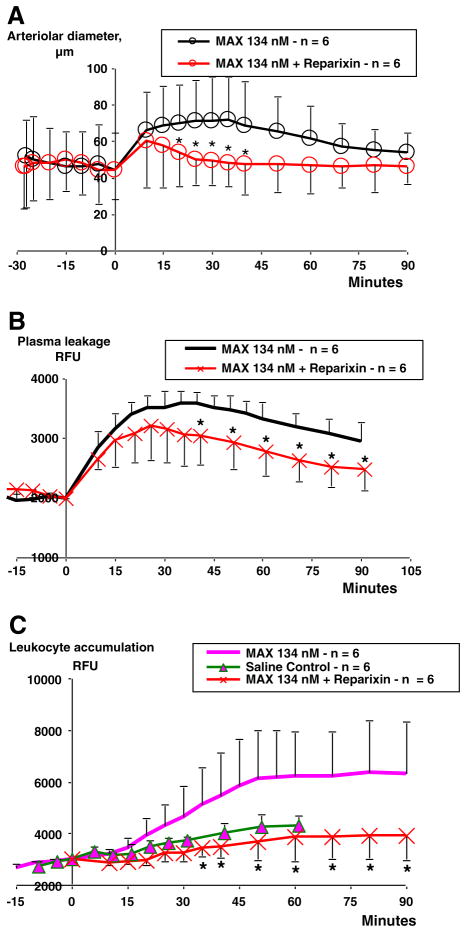
In order to test the role of CXCR2 chemokine receptors on maxadilan-induced migration of neutrophils, we administered (i.v.) the CXCR2 antagonist reparixin. Treatment with the CXCR2-antagonist 10 min prior to maxadilan potently reduced the vasodilator effect, which was significant at 15 min after maxadilan application and was maintained for 20 min (Fig. 5A). Reparixin also reduced plasma leakage, but only at 40 min after maxadilan, and this effect remained until the end of the experiment (Fig. 5B). Importantly, reparixin inhibited leukocyte accumulation to a level below that seen in saline control hamsters, (Fig. 5C).
Fig. 5.
Modulation of maxadilan induced effects by a CXCR1/2-antagonist. Effects of reparixin (40 mg/kg b.w., i.v.) injected 10 min prior to topical application of maxadilan 134 nM at time zero. Results of maxadilan induced arteriolar dilation, plasma leakage and leukocyte accumulation were inserted for comparison. A. Repertaxin inhibited the maxadilan induced dilation at 20 to 40 minutes after injection; B. Repertaxin inhibited maxadilan induced plasma leakage at 40–90 min; and C. Repertaxin inhibited maxadilan induced leukocyte accumulation at 40–90 min (*=p <0.05).
In vitro studies: maxadilan induces human neutrophil chemotaxis
In order to investigate a possible direct effect of maxadilan on leukocytes, isolated human neutrophils were allowed to migrate towards maxadilan. Fig. 6A shows that maxadilan is a chemoattractant for human neutrophils, depicting a characteristic dose-dependent, bell-shape curve for chemotaxis. The chemotactic effect of maxadilan at 100 nM is comparable to that of fMLP (100 nM) and IL-8 (30 nM) Reparixin inhibited almost completely neutrophil migration induced by maxadilan and IL-8 (Fig. 6B), whereas M65 inhibited migration induced by maxadilan but not by IL-8 (Fig. 6C).
Fig. 6.
Maxadilan induces human neutrophil migration. A. Maxadilan induces human neutrophil migration in a dose dependent fashion: freshly isolated cells (5×105) were assayed for chemotaxis in response to maxadilan at the indicated concentrations. Medium alone (random migration) was used as control (Ctrl) and fMLP (100 nM) were used for comparison. Data represent mean±SD of the number of neutrophils per 1000× field counted under optical microscopy. B. M65 inhibited neutrophil migration induced by maxadilan but not by IL-8: Neutrophils were treated for 60 min with M65 (160 nM), after washing, cells were assayed for chemotaxis in response to IL-8 (30 nM) Data represent mean±S.E.M of the number of neutrophils per 1,000x field counted under optical microscopy. Data derived from three independent experiments. (*) p<0.001 compared with Ctrl; (#) p<0.05 compared with maxadilan alone. C. Reparixin inhibited neutrophil migration induced by maxadilan and IL-8: Neutrophils were treated for 15 min with reparixin 1 μM (RPTX), after washing; cells were assayed for chemotaxis in response to maxadilan (100 ng/mL). Data represent mean±S.E.M of the number of neutrophils per 1,000x field counted under optical microscopy. Data derived from three independent experiments. (*) p<0.001 compared with Ctrl; (#) p<0.05 compared with maxadilan alone.
Influence of dextran sulfate on maxadilan and PACAP-38 induced effects
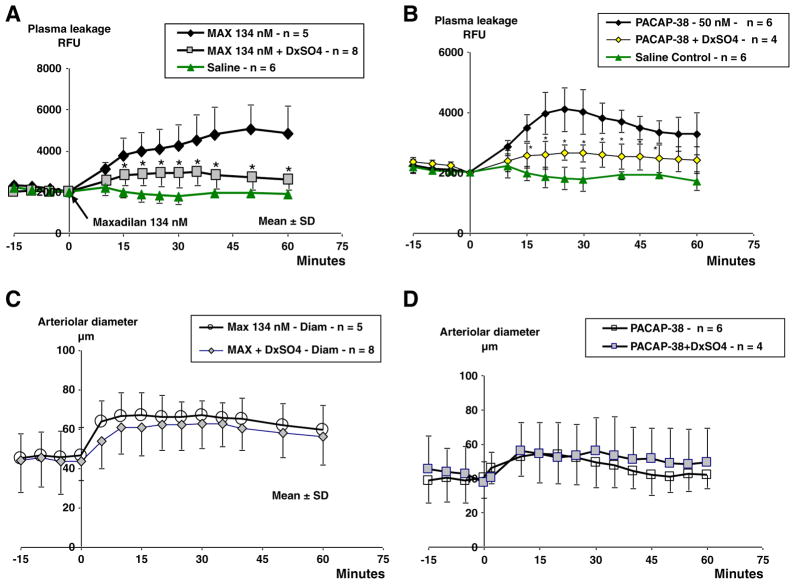
In a previous study we observed that maxadilan and PACAP-38 have similar effects on arteriolar dilation and plasma leakage (Svensjö et al., 2009). As an extension of these findings, here we asked whether if the proinflammatory effects induced by PACAP-38 and maxadilan translated into leukocyte accumulation. Using 50 nM of PACAP-38, a concentration that evokes plasma leakage, we confirmed in two hamsters that rhodamine-labeled leukocytes accumulated in the microvasculature at a magnitude comparable to that observed for maxadilan. Further analysis of these two sets of experimental data revealed a linear correlation (n=24, r=0.800, p=0.0001) between plasma leakage and leukocyte accumulation (Fig. 3).
Previous studies have shown that LTB4-induced plasma leakage in the hamster is neutrophil-dependent (Björk et al. 1982) the microvascular reaction being inhibited by dextran sulfate 500 kDa given i.v. prior to LTB4-challenge (Rosengren et al., 1989). Here we show that dextran sulfate significantly inhibited plasma leakage induced by either maxadilan or PACAP-38 (Fig. 7A and B). In contrast, arteriolar dimensions were not altered in the presence of dextran sulfate. Combined, these results suggest that dextran sulfate does not interfere with the PAC1-receptor function in arteriolar smooth muscles, although it may neutralize cationic proteins released from the activated leukocytes and/or act as an inhibitor of complement (Fig. 7C and D). In three out of eight hamsters treated with dextran sulfate prior to maxadilan the accumulation of rhodamine labeled leukocytes was reduced to levels seen in M65 treated hamsters (data not shown).
Fig. 7.
Comparison of maxadilan and PACAP-38 induced effects. Changes in plasma leakage and arteriolar diameter measured at intravital microscopy of cheek pouches in four groups with 5 – 8 hamsters each subjected to local application of 134 nM maxadilan or 50 nM PACAP-38. Control groups received 0.2 ml of normal saline and the other groups dextran sulfate (500 kDa, 4 mg/kg i.v.) at 10 min before application of maxadilan or PACAP-38. Dextran sulfate given prior to maxadilan and PACAP-38 inhibited the induced plasma leakage after application of Maxadilan (A) and after PACAP-38 (B) application. There were no differences in arteriolar response between control groups and those treated with dextran sulfate prior to maxadilan (C) or PACAP-38 (D), P>0.05.
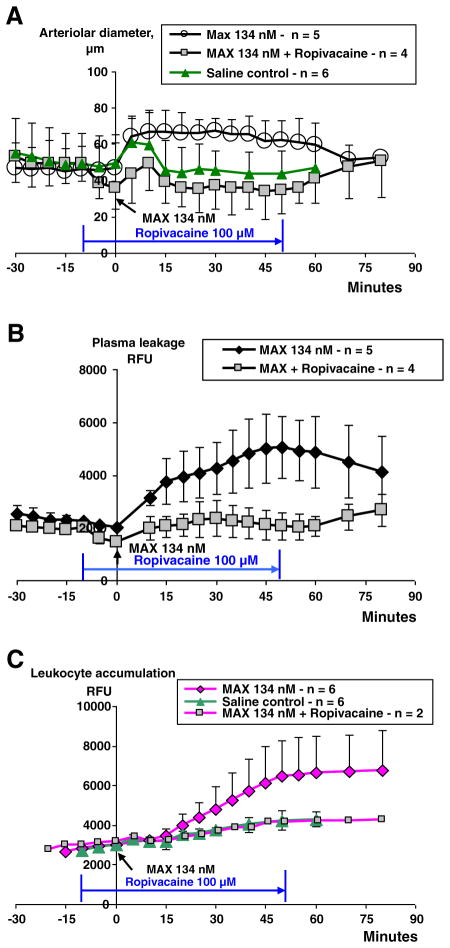
Ropivacaine effects
Local anesthetics such as lidocaine and ropivacaine are known to interfere with leukocyte function and therefore we tested ropivacaine based on our experience in a previous study (Martinsson et al. 1997). Ropivacaine applied 10 min prior to maxadilan caused arteriolar constriction that was counteracted by maxadilan during 10 min after which point in time arterioles regained their initial diameter (Fig. 8A). Maxadilan-induced plasma leakage was almost completely inhibited until the interruption of ropivacaine administration, at which time a tendency of plasma leakage increase occurred (Fig. 8B). Rhodamine was given to two hamsters and it could be seen that ropivacaine also inhibited leukocyte accumulation (Fig. 8C).
Fig. 8.
Inhibition of maxadilan induced effects by ropivacaine. Effects of locally applied ropivacaine (100 μM) at 10 min prior to maxadilan induced arteriolar dilation, plasma leakage and leukocyte accumulation (RFU, means±SD). Results of maxadilan (MAX) induced arteriolar dilation and plasma leakage (n=5) were inserted for comparison. A. Ropivacaine caused a constriction that resulted in arteriolar diameters that were significantly less (p<0.05) as compared with the MAX-group from 5 min until 60 min after MAX application. B. Ropivacaine inhibited the maxadilan induced plasma leakage during its continuous application (p<0.05). C. In two out of four ropivacaine treated hamsters rhodamine was used to show inhibition of MAX induced leukocyte accumulation. Results of MAX and saline induced leukocyte accumulation were inserted for comparison.
Discussion
In a previous study we have shown that homogenates of salivary glands from sand-flies (Lu. longipalpis) and maxadilan, a vasoactive peptide present in intact salivary glands, are both able to induce plasma leakage in postcapillary venules as indicated by FITC-dextran (Svensjö et al., 2009) Using M65, the recombinant mutant of maxadilan that serves as a high-affinity antagonist of the PAC1-receptor (Lerner et al., 2007), we have now shown that PAC1-receptor is involved in maxadilan-induced plasma leakage as well as in leukocyte accumulation in postcapillary and larger venules. Simultaneous measurements of FITC- and rhodamine-fluorescence made it possible to demonstrate a statistically significant linear correlation between plasma leakage and leukocyte accumulation when we integrated the entire image information of maxadilan and PACAP-38 treated hamsters but not in saline treated hamsters. Analysis of FITC- and rhodamine-fluorescence in smaller and circular areas (r=50 μm) that contained only terminal arterioles and capillaries and in other areas containing only postcapillary venules showed that plasma leakage overlapped with sites where leukocytes accumulated in postcapillary venules and surrounding tissue. This observation implied that vascular permeability for large molecules was enhanced at endothelial sites that accumulated higher numbers of leukocytes. Although a positive test of linear correlation cannot prove the proposed relation between leukocyte accumulation and plasma leakage we think that the more detailed analysis of smaller areas of the microcirculation provides evidence for a relation between plasma leakage and leukocyte accumulation during the first 30–40 min after maxadilan stimulation. The striking similarity between the slope of the maxadilan and the PACAP-38 induced correlation curves is supported by previous studies showing that equal concentrations of maxadilan and PACAP-38 have almost identical effects in terms of plasma leakage and arteriolar dilation and exert their effects via PAC1-receptor stimulation [6]. Neutrophil-induced increase in plasma leakage was first shown by Wedmore and Williams (1981). Since then several studies have shown this effect of neutrophils as reviewed by DiStasi and Ley (2009). Additional evidence supporting that maxadilan induced leukocyte accumulation and activation caused plasma leakage was achieved by the use of four inhibitors with different mechanisms of action, M65, reparixin, dextran sulfate and ropivacaine, all of which inhibited plasma leakage and leukocyte accumulation. The in vivo effects of M65 and reparixin were consistent with in vitro evidence indicating that maxadilan induced neutrophil migration was inhibited by M65 or by reparixin, a selective CXCR1/2-antagonist (Souza et al., 2004, Villa et al., 2007). Furthermore, we showed that IL-8 induced neutrophil migration was also inhibited by reparixin but not by M65. Taken together these results suggest that maxadilan may upregulate IL-8 in leukocytes leading to IL-8 dependent activation of endothelial cells and, further downstream, induce plasma leakage. Studies have shown that reparixin is a potent inhibitor of neutrophil induced effects in consequence of transient brain ischemia in rats (Garau et al., 2005). Another study showed that acute lung injury in mice induced by lipopolysaccharide or intratracheal injection of hydrochloric acid was effectively counteracted by reparixin that reduced neutrophil migration and vascular permeability (Zarbock et al., 2008).
Among several recombinant mutants of maxadilan M65 was found to be the most potent inhibitor of maxadilan binding to the PAC1-receptor (Lerner et al., 2007). Here we have demonstrated that M65 inhibited leukocyte accumulation in the microcirculation and that exposure of human neutrophils to M65 in vitro prior to addition of maxadilan resulted in a 70–80% inhibition of neutrophil migration. Reparixin also inhibited maxadilan as well as IL-8 induced neutrophil migration in vitro and appeared slightly more effective in reducing leukocyte accumulation in vivo as compared with M65. The lack of M65-inhibition of IL-8 induced chemotaxis confirms that M65 is a selective blocker of maxadilan (Lerner et al., 2007).
Our finding that dextran sulfate could inhibit neutrophil activation in the hamster cheek pouch has been shown in previous studies using LTB4 as a stimulant and it was suggested that such neutrophil induced vascular leakage was suppressed by dextran sulfate due to its ability to neutralize cationic proteins (Rosengren et al., 1989). The microvascular activity elicited by dextran sulfate may inhibit rolling and adhesion of neutrophils (Ley et al., 1989). Heparin has been shown to have anti-inflammatory properties and it was reported that heparin as well as a 10 kDa dextran sulfate inhibited neutrophil activation, neutrophil accumulation and plasma leakage in the rabbit skin after i.v. injection (Brown et al., 2003, Jones et al., 2002). TNF-induced neutrophil accumulation and subsequent pulmonary edema was inhibited by an 8 kDa dextran sulfate and to a greater extent by dextran sulfate of 500 kDa (Höcking et al., 1991). In a review of recent studies it was postulated that dextran sulfate 5 kDa is an inhibitor of complement (Spirig et al., 2008). An alternative mechanism could be that dextran sulfate through activation of the contact system and subsequent formation of bradykinin (Morimoto et al., 1996, Siebeck et al., 1994) might induce down-regulation of bradykinin receptors along with other GPCRs. Their pleiotropic effects could reduce the sensitivity of endothelium to various inflammatory mediators compatible with a preconditioning effect of bradykinin.
Inhibitory effects of leukocyte function by local anesthetics such as lidocaine have been known for a long time (Giddon and Lindhe, 1972). Lidocaine reduces leukocyte adherence in injured venules and counteracts the endothelial damage induced by sticking leukocytes (Stewart et al., 1974) and inhibits granulocyte recruitment to sites of inflammation (MacGregor et al., 1980). Anti-inflammatory effects of local anesthetics have been documented in several studies as reviewed by Cassuto et al. (2006). One of these studies showed that LTB4-induced leukocyte rolling and adhesion in venules and postcapillary venules as well as plasma leakage was inhibited by ropivacaine, a local anesthetic with a chemical structure close to that of lidocaine (Martinsson et al., 1997). It was also shown that ropivacaine attenuated CD11b/CD18 up-regulation a result that has recently been confirmed (Zhu et al., 2010). Since we have used the same concentration of ropivacaine, our data might imply that a similar mechanism may underlie ropivacaine dependent inhibition of maxadilan and LTB4-induced plasma leakage. However, considering that bradykinin induced vasodilation and plasma leakage may occur without activation of leukocytes and may be inhibited by ropivacaine (Dias et al., 2008, Svensjö, unpublished results) it is possible that ropivacaine inhibition could be exerted at more than one site, on the leukocytes and on the endothelium of postcapillary venules.
Conclusion
We have confirmed that the vasoactive peptides maxadilan and PACAP-38 increase plasma leakage through stimulation of PAC1-receptors and showed a positive correlation between plasma leakage and activation of leukocytes in postcapillary venules. Reparixin blocked maxadilan and IL-8 induced neutrophil migration in vitro and reduced plasma leakage and leukocyte accumulation supporting a role for CXCR1/2 in the microvascular effects induced by the insect derived salivary gland molecule.
Acknowledgments
This research was supported by funds from CNPq and FAPERJ; PRONEX (26/110.562/2010), FAPERJ; (E-26/100.938/2007, E-26/ 110.529/2011) and Projeto PensaRio Leishmaniose.
Abbreviations
- fMLP
(N-formyl-methionine-leucine-phenylalanine)
- MAX
maxadilan
- M65
recombinant mutant of MAX and antagonist of MAX
- PACAP-38
pituitary adenylate cyclase-activating peptide
- LTB4
Leucotriene B4
- PCV
post-capillary venule
- CAP
capillary
- RFU
relative fluorescence units
- HCP
hamster cheek pouch
- CAP-FITC
FITC-fluorescence (RFU) in a circular area (Ø=100 μm) of the 5 mm2-area
- PCV-FITC
FITC-fluorescence (RFU) in a circular area (Ø=100 μm) of the 5 mm2-area
- CAP-RHOD
Rhodamine fluorescence (RFU) in a circular area (Ø=100 μm) of the 5 mm2-area
- PCV-RHOD
Rhodamine fluorescence (RFU) in a circular area (Ø =100 μm) of the 5 mm2-area
Contributor Information
Erik Svensjö, Email: erik.svensjo@gmail.com.
Elvira M. Saraiva, Email: esaraiva@micro.ufrj.br.
Rafael Silveira Amendola, Email: rafael.silveira.amendola@gmail.com.
Christina Barja-Fidalgo, Email: barja-fidalgo@uerj.br.
Marcelo T. Bozza, Email: mbozza@micro.ufrj.br.
Ethan A. Lerner, Email: ethan.lerner@cbrc2.mgh.harvard.edu.
Mauro M. Teixeira, Email: mmtex.ufmg@gmail.com.
Julio Scharfstein, Email: jscharf2@gmail.com.
References
- Björk J, Hedqvist P, Arfors KE. Increase in vascular permeability induced by leukotriene B4 and the role of polymorphonuclear leukocytes. Inflammation. 1982;6:189–200. doi: 10.1007/BF00916243. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lever R, Jones NA, Page CP. Effects of heparin and related molecules upon neutrophil aggregation and elastase release in vitro. Br J Pharmacol. 2003;139:845–853. doi: 10.1038/sj.bjp.0705291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto J, Sinclair R, Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand. 2006;50:265–282. doi: 10.1111/j.1399-6576.2006.00936.x. Review. [DOI] [PubMed] [Google Scholar]
- Coelho AL, DeFreitas MS, Mariano-Oliveira A, Oliveira-Carvalho AL, Zingali RB, Barja-Fidalgo C. Interaction of disintegrins with human neutrophils induces cytoskeleton reorganization, focal adhesion kinase activation, and extracellular-regulated kinase-2 nuclear translocation, interfering with the chemotactic function. FASEB J. 2001;15:1643–1645. doi: 10.1096/fj.00-0812fje. [DOI] [PubMed] [Google Scholar]
- Dias MP, Newton DJ, McLeod GA, Khan F, Belch JJ. The inhibitory effects of local anaesthetics on the vascular flare responses to bradykinin and substance P in human skin. Anaesthesia. 2008;63:151–155. doi: 10.1111/j.1365-2044.2007.05324.x. [DOI] [PubMed] [Google Scholar]
- DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30:547–556. doi: 10.1016/j.it.2009.07.012. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley DC, Simpson JF, Merryman HT. Isolation of large numbers of fully viable human neutrophils: a preparative technique using Percoll density gradient centrifugation. Exp Hematol. 1982;10:591–599. [PubMed] [Google Scholar]
- El Zein N, Badran B, Sariban E. The neuropeptide pituitary adenylate cyclase activating polypeptide modulates Ca2+ and pro-inflammatory functions in human monocytes through the G protein-coupled receptors VPAC-1 and formyl peptide receptor-like 1. Cell Calcium. 2008;43:270–284. doi: 10.1016/j.ceca.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Garau A, Bertini R, Colotta F, Casilli F, Bigini P, Cagnotto A, Mennini T, Ghezzi P, Villa P. Neuroprotection with the CXCL8 inhibitor repertaxin in transient brain ischemia. Cytokine. 2005;30:125–131. doi: 10.1016/j.cyto.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Giddon DB, Lindhe J. In vivo quantitation of local anesthetic suppression of leukocyte adherence. Am J Pathol. 1972;68:327–338. [PMC free article] [PubMed] [Google Scholar]
- Harfi I, Sariban E. Mechanisms and modulation of pituitary adenylate cyclase-activating protein-induced calcium mobilization in human neutrophils. Ann N Y Acad Sci. 2006;1070:322–329. doi: 10.1196/annals.1317.037. [DOI] [PubMed] [Google Scholar]
- Harfi I, D’Hondt S, Corazza F, Sariban E. Regulation of human polymorphonuclear leukocytes functions by the neuropeptide pituitary adenylate cyclase-activating poly-peptide after activation of MAPKs. J Immunol. 2004;173:4154–4163. doi: 10.4049/jimmunol.173.6.4154. [DOI] [PubMed] [Google Scholar]
- Harris AG, Sinitsina I, Messmer K. Intravital fluorescence microscopy and phototoxicity: effects on leukocytes. Eur J Med Res. 2002;7:117–124. [PubMed] [Google Scholar]
- Höcking DC, Ferro TJ, Johnson A. Dextran sulfate inhibits PMN-dependent hydrostatic pulmonary edema induced by tumor necrosis factor. J Appl Physiol. 1991;70:1121–1128. doi: 10.1152/jappl.1991.70.3.1121. [DOI] [PubMed] [Google Scholar]
- Jones H, Paul W, Page CP. The effects of heparin and related molecules on vascular permeability and neutrophil accumulation in rabbit skin. Br J Pharmacol. 2002;135:469–479. doi: 10.1038/sj.bjp.0704505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner EA, Shoemaker CB. Maxadilan: cloning and functional expression of the gene encoding this potent vasodilator peptide. J Biol Chem. 1992;267:1062–1066. [PubMed] [Google Scholar]
- Lerner EA, Ribeiro JM, Nelson RJ, Lerner MR. Isolation of maxadilan, a potent vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. J Biol Chem. 1991;266:11234–11236. [PubMed] [Google Scholar]
- Lerner EA, Iuga AO, Reddy VB. Maxadilan, a PAC1 receptor agonist from sand flies. Peptides. 2007;28:1651–1654. doi: 10.1016/j.peptides.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Lundgren E, Berger E, Arfors KE. Shear-dependent inhibition of granulocyte adhesion to cultured endothelium by dextran sulfate. Blood. 1989;73:1324–1330. [PubMed] [Google Scholar]
- Lima HC, Titus RG. Effects of sand fly vector saliva on development of cutaneous lesions and the immune response to Leishmania braziliensis in BALB/c mice. Infect Immun. 1996;64:5442–5445. doi: 10.1128/iai.64.12.5442-5445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor RR, Thorner RE, Wright DM. Lidocaine inhibits granulocyte adherence and prevents granulocyte delivery to inflammatory sites. Blood. 1980;56:203–209. [PubMed] [Google Scholar]
- Martinsson T, Oda T, Fernvik E, Roempke K, Dalsgaard CJ, Svensjö E. Ropivacaine inhibits leukocyte rolling, adhesion and CD11b/CD18 expression. J Pharmacol Exp Ther. 1997;283:59–65. [PubMed] [Google Scholar]
- Mbow ML, Bleyenberg JA, Hall LR, Titus RG. Phlebotomus papatasi sand fly salivary gland lysate down-regulates a Th1, but up-regulates a Th2, response in mice infected with Leishmania major. J Immunol. 1998;161:5571–5577. [PubMed] [Google Scholar]
- Morimoto H, Yamashita M, Matsuda A, Ohori M, Miyake H, Fujii T. Characterization of dextran sulfate-induced guinea pig tracheal plasma extravasation. Jpn J Pharmacol. 1996;72:217–221. doi: 10.1254/jjp.72.217. [DOI] [PubMed] [Google Scholar]
- Moro O, Lerner EA. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type 1 agonist. J Biol Chem. 1997;272:966–970. doi: 10.1074/jbc.272.2.966. [DOI] [PubMed] [Google Scholar]
- Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2001;167:5226–5230. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- Norsworthy NB, Sun J, Elnaiem D, Lanzaro G, Soong L. Sand fly saliva enhances Leishmania amazonensis infection by modulating interleukin-10 production. Infect Immun. 2004;72:1240–1247. doi: 10.1128/IAI.72.3.1240-1247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira P, Reddy VB, Kounga K, Bello Y, Lerner E. Maxadilan activates PAC1 receptors expressed in Xenopus laevis melanophores. Pigment Cell Res. 2002;15:461–466. doi: 10.1034/j.1600-0749.2002.02077.x. [DOI] [PubMed] [Google Scholar]
- Peters NC, Sacks DL. The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis. Cell Microbiol. 2009;11:1290–1296. doi: 10.1111/j.1462-5822.2009.01348.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren S, Ley K, Arfors KE. Dextran sulfate prevents LTB4-induced permeability increase, but not neutrophil emigration, in the hamster cheek pouch. Microvasc Res. 1989;38:243–254. doi: 10.1016/0026-2862(89)90003-4. [DOI] [PubMed] [Google Scholar]
- Saldanha-Gama R, Moraes JA, Mariano-Oliveira A, Coelho AL, Walsh EM, Marcinkiewicz C, Barja-Fidalgo C. Alpha (9) beta (1) integrin engagement inhibits neutrophil spontaneous apoptosis: involvement of Bcl-2 family members. Biochim Biophys Acta. 2010;1803:848–857. doi: 10.1016/j.bbamcr.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Schmitz V, Svensjö E, Serra RR, Teixeira MM, Scharfstein J. Proteolytic generation of kinins in tissues infected by Trypanosoma cruzi depends on CXC-chemokine secretion by macrophages activated via Toll-like 2 receptors. J Leukocyte Biol. 2009;85:1005–1014. doi: 10.1189/jlb.1108693. [DOI] [PubMed] [Google Scholar]
- Siebeck M, Cheronis JC, Fink E, Kohl J, Spies B, Spannagl M, Jochum M, Fritz H. Dextran sulfate activates contact system and mediates arterial hypotension via B2 kinin receptors. J Appl Physiol. 1994;77:2675–2680. doi: 10.1152/jappl.1994.77.6.2675. [DOI] [PubMed] [Google Scholar]
- Souza DG, Bertini R, Vieira AT, Cunha FQ, Poole S, Allegretti M, Colotta F, Teixeira MM. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;143:132–142. doi: 10.1038/sj.bjp.0705862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirig R, Gajanayake T, Korsgren O, Nilsson B, Rieben R. Low molecular weight dextran sulfate as complement inhibitor and cytoprotectant in solid organ and islet transplantation. Mol Immunol. 2008;45:4084–4094. doi: 10.1016/j.molimm.2008.07.024. Review. [DOI] [PubMed] [Google Scholar]
- Stewart GJ, Ritchie WG, Lynch PR. Venous endothelial damage produced by massive sticking and emigration of leukocytes. Am J Pathol. 1974;74:507–532. [PMC free article] [PubMed] [Google Scholar]
- Svensjö E. Bradykinin and prostaglandin E1, E2 and F2α-induced macromolecular leakage in the hamster cheek pouch. Prostaglandins Med. 1978;1:397–410. doi: 10.1016/0161-4630(78)90126-x. [DOI] [PubMed] [Google Scholar]
- Svensjö E, Saraiva EM, Bozza MT, Oliveira SMP, Lerner EA, Scharfstein J. Salivary gland homogenates of Lutzomyia longipalpis and its vasodilatory peptide maxadilan cause plasma leakage through activation of PAC1 receptor. J Vasc Res. 2009;46:435–446. doi: 10.1159/000197866. [DOI] [PubMed] [Google Scholar]
- Teixeira CR, Teixeira MJ, Gomes RB, Santos CS, Andrade BB, Raffaele-Netto I, Silva JS, Guglielmotti A, Miranda JC, Barral A, Brodskyn C, Barral-Netto M. Saliva from Lutzomyia longipalpis induces CC chemokine ligand 2/monocyte chemoattractant protein-1 expression and macrophage recruitment. J Immunol. 2005;175:8346–8353. doi: 10.4049/jimmunol.175.12.8346. [DOI] [PubMed] [Google Scholar]
- Villa P, Triulzi S, Cavalieri B, Di Bitondo R, Bertini R, Barbera S, Bigini P, Mennini T, Gelosa P, Tremoli E, Sironi L, Ghezzi P. The interleukin-8 (IL-8/CXCL8) receptor inhibitor reparixin improves neurological deficits and reduces long-term inflammation in permanent and transient cerebral ischemia in rats. Mol Med. 2007;13:125–133. doi: 10.2119/2007-00008.Villa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedmore CV, Williams TJ. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981;289:646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Zarbock A, Allegretti M, Ley K. Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung injury in mice. Brit J Pharmacol. 2008;155:357–364. doi: 10.1038/bjp.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Tan Z, Chen J, Zhu M, Xu Y. Effects of ropivacaine on adhesion molecule CD11b expression and function in human neutrophils. Int Immunopharmacol. 2010;10:662–667. doi: 10.1016/j.intimp.2010.03.009. [DOI] [PubMed] [Google Scholar]