Abstract
Background and Purpose
Brain injury caused by stroke is a frequent cause of perinatal morbidity and mortality with limited therapeutic options. Mesenchymal stem cells (MSC) have been shown to improve outcome after neonatal hypoxic-ischemic brain injury mainly by secretion of growth factors stimulating repair processes. We investigated whether MSC-treatment improves recovery after neonatal stroke and whether MSC overexpressing brain-derived neurotrophic factor (MSC-BDNF) further enhances recovery.
Methods
We performed 1.5-hour transient middle cerebral artery occlusion (MCAO) in 10-day-old rats. Three days after reperfusion, pups with evidence of injury by diffusion-weighted MRI were treated intranasally with MSC, MSC-BDNF or vehicle. To determine the effect of MSC-treatment, brain damage, sensorimotor function and cerebral cell proliferation were analysed.
Results
Intranasal delivery of MSC- and MSC-BDNF significantly reduced infarct size and grey matter loss in comparison to vehicle-treated rats without any significant difference between MSC- and MSC-BDNF-treatment. Treatment with MSC-BDNF significantly reduced white matter loss with no significant difference between MSC- and MSC-BDNF-treatment. Motor deficits were also improved by MSC-treatment when compared to vehicle treated rats. MSC-BDNF-treatment resulted in an additional significant improvement of motor deficits 14 days post-MCAO, but there was no significant difference between MSC or MSC-BDNF 28 days post-MCAO. Furthermore, treatment with either MSC or MSC-BDNF induced long lasting cell proliferation in the ischemic hemisphere.
Conclusions
Intranasal administration of MSC after neonatal stroke is a promising therapy for treatment of neonatal stroke. In this experimental paradigm MSC and BNDF-hypersecreting-MSC are equally effective in reducing ischemic brain damage.
Keywords: neonatal stroke, cerebral ischemia, postnatal, cell transplantation, mesenchymal stem cells
Introduction
Neonatal stroke occurs in approximately 1 in 4,000 live births and is associated with significant morbidity and mortality.1 Neonates suffering from perinatal stroke often develop long term disabilities including motor deficits, cognitive dysfunction and epilepsy.2 Currently there are no accepted treatment options for this vulnerable group of infants. Therefore, development of new treatment strategies is urgently needed.
Over the past decades a large number of studies have evaluated potential therapies to prevent progression of injury via pharmacological neuroprotection. More recently, means to enhance repair of the damaged immature brain are being investigated. Several studies, using different types of brain injury including adult stroke models of middle cerebral artery occlusion (MCAO) and neonatal hypoxic-ischemic brain injury, have shown that administration of MSC promotes functional neurological recovery.3,4 This beneficial effect of MSC-transplantation might involve replacement of damaged cells by the transplanted cells. However, data in the literature suggest that it is more likely that transplanted MSC induce repair by stimulating secretion of growth and differentiation factors thereby providing an environment that stimulates repair processes like neurogenesis and angiogenesis.5 We and others have shown that in response to the growth factor environment in the damaged brain, MSC secrete several factors which have the potential to stimulate repair processes in the brain.5–7
In this study we investigated whether MSC transplantation has beneficial effects on functional outcome and lesion volume in a rat model of neonatal stroke. Furthermore, we determined if the therapeutic potential of MSC could be enhanced by genetically modifying the MSC to secrete more brain-derived neurotrophic factor (BDNF).
Methods
Adenoviral vector
Adenoviral vector (pAd-HM41-K7; Alphagen) carrying the gene for polylysine-mutated fiberknot was constructed as described previously.8 Mouse brain-derived neurotrophic factor (BDNF) cDNA was cloned using RT-PCR with total RNA isolated from brain as template. BDNF sequence was confirmed by sequencing and comparison to GenBank sequence NM_007540. Mouse BDNF-primer sequences were Forward 5’-TCTAGACACCCCACCATGACCATCCTTTTCCTT-3’, Reverse 5’-TCTTCCCCTTTTAATGGTCAGT-3’. BDNF cDNA was coupled to an IRES-eGFP sequence to allow labeling of infected cells. The BDNF-IRES-eGFP sequence was inserted into the pShuttle2 vector between the XbaI and AflII sites resulting in the pShuttle2-BDNF-IE plasmid. pAd-HM41-K7-BDNF-IE was constructed by ligation of I-CeuI/PI-SceI-digested pShuttle2-BDNF-IE with I-CeuI/PI-SceI-digested pAd-HM41-K7. An empty vector control was generated and consisted only of the IRES-eGFP sequence insert (pAd-HM410K7-IE).
Virus particles were generated by transfection of PacI-digested pAd-HM41-K7-BDNF-IE into 293 cells with Lipofectamine2000 (Invitrogen). Before being used, virus titer was determined and stocks were examined for potential contamination with replication-competent viruses.
MSC
Rat Sprague-Dawley MSC (GIBCO) were cultured according to manufacturer’s instructions. Cells were negative for myeloid and haematopoietic cell lineage specific antigens and positive for CD29, CD44, CD90 and CD106. MSC were seeded at a density of 3×106 cells per 25cm2 flask, exposed to pAd-HM41-K7-BDNF-IE or pAd-HM41-K7-IE virus particles in 7.5ml DMEM for 6 hours after which cultures were washed 3 times with DMEM and re-cultured with normal medium. Following infection, MSC were cultured for an additional 24 hours after which transplantation was performed.
Animals
All animal research was approved by the University of California San Francisco Institutional Animal Care and Use Committee and performed in accordance with the Guide for Care and Use of Laboratory Animals (U.S. Department of Health and Human Services, Publication Number 85-23, 1985).
Transient 1.5-hour right MCAO was performed in 10d-old Sprague-Dawley rats as described previously.9 Briefly, surgery was performed on spontaneously breathing pups anesthetized with 1.75% isoflurane in a mixture of 70% N2O and 30% O2. The internal carotid artery (ICA) was dissected and a temporary ligature was applied at its origin using a 6-0 silk suture. A second suture was looped around the ICA, just above the pterygopalatine artery, and retracted laterally to prevent retrograde blood flow. A small arteriotomy was made in the proximal isolated ICA segment, a coated 6-0 Dermalon filament was inserted and advanced 7.5–8.5mm depending on the animal’s weight and secured with a temporary suture. The filament and both sutures around the ICA were removed 1.5 hours later, reestablishing blood flow.
Spin-echo planar diffusion-weighted MRI (DWI) was performed using a 7T magnet 3 days after MCAO to identify injured animals; only animals with injury extending throughout the middle cerebral artery territory on DWI were used. Injury volume was determined in 6 consecutive 2mm thick coronal sections. A total of 36 pups met inclusion criteria based on DWI after MCAO (85%). Overall survival of injured animals was 87%.
At 3 days post-MCAO, MSC, BDNF-secreting-MSC (MSC-BDNF) or vehicle was delivered intranasally to awake rats. 30 Minutes prior to MSC or vehicle administration two doses of 5µl hyaluronidase (Sigma-Aldrich) in PBS were applied to each nostril and spontaneously inhaled.10 Subsequently, a total of 1×106 MSC in 20µl PBS or vehicle were administered as two doses of 5µl applied to each nostril.
To evaluate cell proliferation/survival, rats received ethynyldeoxyuridine (EdU; 50mg/kg, i.p.; Invitrogen) at days 3 to 5 post-MCAO and bromodeoxyuridine (BrdU; 50mg/kg, i.p.; Sigma-Aldrich) at days 21 to 23 post-MCAO. Animals were sacrificed at 28 days post-MCAO and perfused with 4% paraformaldehyde in PBS.
Functional outcome
The cylinder-rearing test was used to assess forelimb use asymmetry.11 The weight-bearing forepaw(s) to contact the wall during a full rear was recorded as left (impaired), right (non-impaired) or both. Paw preference was calculated as [(nonimpaired–impaired)/(nonimpaired forepaw+impaired forepaw+both)]×100. Adhesive removal test was performed at 28 days after MCAO. Stickers (tough-spots, Diversified Biotech, Boston MA) were placed on left and right forepaw and the latency to removal was recorded. The mean time until complete removal of three stickers per forepaw was recorded. Sticker placement on left and right forepaw was alternated between and within animals.11
Histology and Immunohistochemistry
Coronal paraffin sections (10µm) were incubated with mouse-anti-MBP (Sternberger Monoclonals) or mouse-anti-MAP2 (Sigma-Aldrich) and binding was visualized with a Vectastain ABC kit (Vector Laboratories). Volumetric injury analysis was performed on a series of six MAP2, MBP and cresyl violet stained sections (ImageJ, NIH). Injury volume was expressed as ratio ipsi- to contralateral hemisphere volume.4,12
For cell proliferation analysis, sections were incubated with biotinylated sheep-anti-BrdU and rabbit-anti-Ki67 (Abcam). Visualization was done with AlexaFluor-594 conjugated streptavidin and donkey-anti-rabbit AlexaFluor488 (Molecular Probes). EdU incorporation was detected by incubating sections in 100mM Tris containing 0.5mM CuSO4, 50mM ascorbic acid and 10µM AlexaFluor-594-azide (Molecular Probes). EdU- and BrdU-positive cells were counted in three high magnification fields in the striatum of the damaged hemisphere in three sections per brain. Ki67-positive cells were counted in SVZ in three sections per brain.
Statistical analysis
All data are expressed as mean±SEM. Functional outcome measured with cylinder rearing test and adhesive removal test were analysed using two-way ANOVA with Fisher’s LSD post-tests. Histological measures were analyzed using one-way ANOVA with Bonferroni post-tests. p<0.05 was considered statistically significant.
Results
MSC treatment following neonatal stroke reduces lesion volume
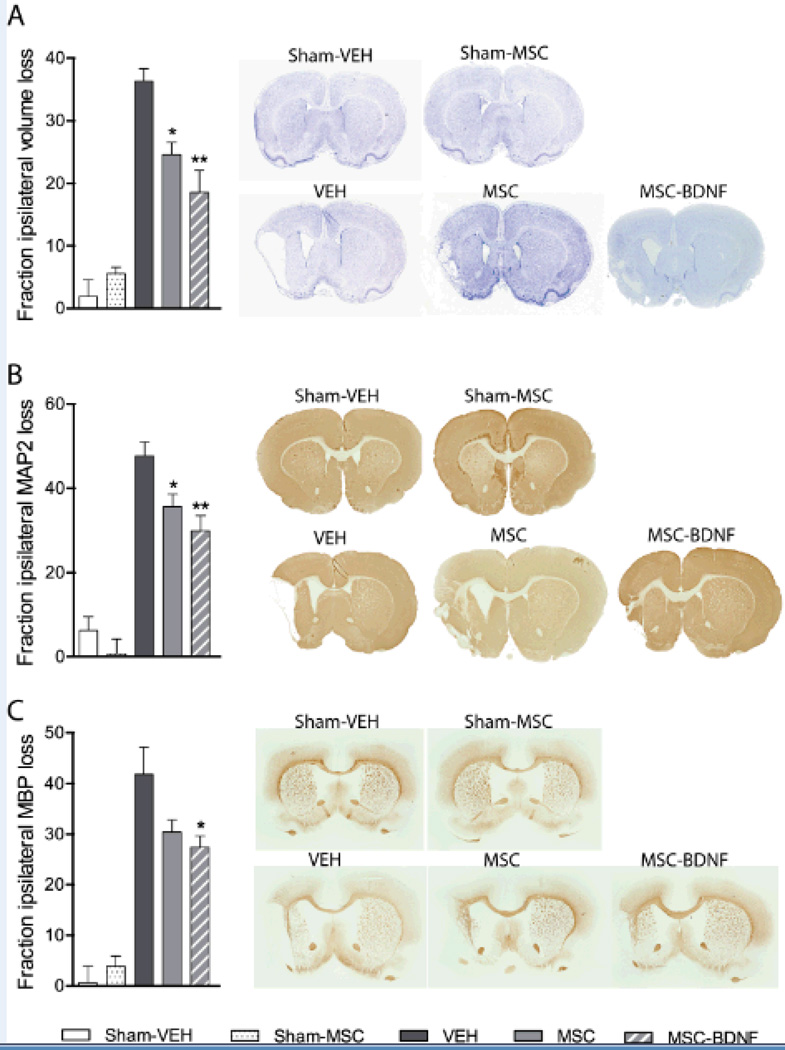
Rats underwent MCAO at p10 and were treated with MSC, MSC-BDNF or vehicle at 3 days after the insult. Animals that underwent MCAO had significant brain tissue loss in the ipsilateral hemisphere at 28 days post-MCAO (Fig. 1A). Following treatment with MSC there was a significant reduction in ipsilateral tissue loss in comparison to vehicle-treated rats (p<0.05). Treatment with MSC-BDNF resulted in a reduction of tissue loss when compared to (p<0.01 vs. vehicle), but not significantly different from MSC-treated rats.
Figure 1. Effect of MSC transplantation on lesion size.
Quantification of damage using Nissl staining (A), MAP2-positive area loss (B), MBP-positive area loss (C) and representative sections. Data represent mean±SEM. Sham controls n=5, VEH n=8, MSC n=9, MSC-BDNF n=8. *p<0.05 vs. VEH, **p<0.01 vs. VEH.
We also analyzed the effect of MCAO and subsequent MSC treatment on grey and white matter loss. At 28 days post-MCAO MAP2-positive area loss, as a measure of grey matter injury, was significantly lower after treatment with MSC or MSC-BDNF in comparison to vehicle treated rats (p<0.05 and p<0.01 respectively). Treatment with MSC-BDNF did not result in significantly lower MAP-2 loss than treatment with MSC (Fig. 1B).
Injury to white matter was analyzed by measuring MBP-positive area. MCAO resulted in significant loss of MBP-positive tissue in vehicle-treated rats (Fig. 1C). Treatment with MSC did not significantly decrease MBP area loss when compared to vehicle-treated rats. Treatment with MSC-BDNF, however, did cause a significant reduction in MBP-positive area loss (p<0.05) with no significant difference between MSC and MSC-BDNF treatment.
MSC transplantation after neonatal stroke reduces motor deficits
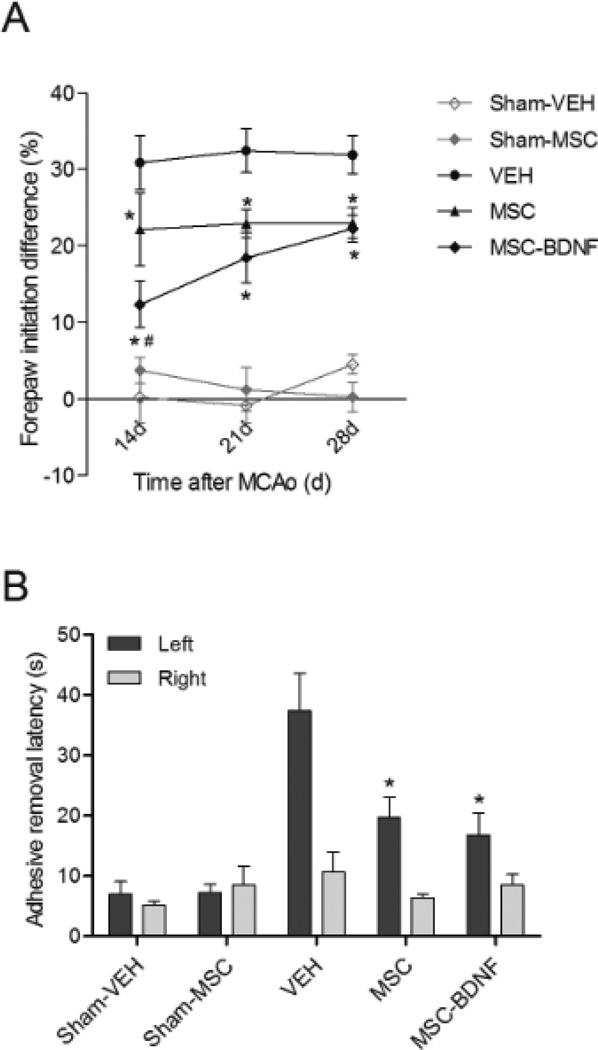
To determine the extent of lateralizing motor deficits caused by our stroke model, the cylinder rearing test was performed at 14, 21 and 28 days after MCAO. In this test, sham-operated rats did not show any paw preference (Fig. 2A). MCAO caused lateralization shown by a ~30% preference to use the right unimpaired forepaw in vehicle-treated rats. Following treatment with MSC, performance in the cylinder rearing test significantly improved at all three time points measured in comparison to vehicle-treated rats. At 14 days post-MCAO forepaw impairment in MSC-BDNF treated rats was significantly lower than in MSC-treated rats (p<0.05). However, the effect of MSC-BDNF-treatment was only temporary as there was no difference between MSC-BDNF and MSC-treated rats at 28 days post-MCAO.
Figure 2. Effect of MSC transplantation on functional outcome.
Rats underwent MCAO at p10 and were treated with vehicle or MSC at 3 days post-MCAo. Lateralizing motor deficits were measured in the cylinder rearing test at 14, 21 and 28 days post-MCAo (A) and using the adhesive removal test at 28 days post-MCAo (B). Data represent mean±SEM. Sham-controls n=5, VEH n=8, MSC n=9, MSC-BDNF n=8. *p<0.05 vs. VEH, #p<0.05 MSC-BDNF vs. MSC.
At 28 days post-MCAO all rats were subjected to the adhesive removal test. This test evaluates sensory and motor deficits related to the paw. Sham-operated controls did not show a difference in adhesive removal latency between left and right forepaw (Fig. 2B). After MCAO all rats were impaired in the adhesive removal test meaning that the latency to remove the sticker from the impaired forepaw was significantly higher than removing the sticker from the unimpaired forepaw. More importantly, MSC- and MSC-BDNF-treated rats showed a reduced latency to remove the adhesive from the impaired forepaw when compared to vehicle treated rats. There was no difference in adhesive removal latency between rats treated with MSC or MSC-BDNF.
MSC treatment following neonatal stroke induces long lasting cell proliferation in the ipsilesional striatum and subventricular zone (SVZ)
To determine the effect of MSC transplantation and modified-MSC transplantation on cell proliferation in the brain after neonatal stroke, rats were injected with cell proliferation markers EdU on days 3 to 5 post-MCAO and, to check for late proliferation, with BrdU on days 21 to 23 post-MCAO. At 28 days post-MCAO the number of EdU- and BrdU-labelled cells and expression of the cell proliferation marker Ki67 were analysed.
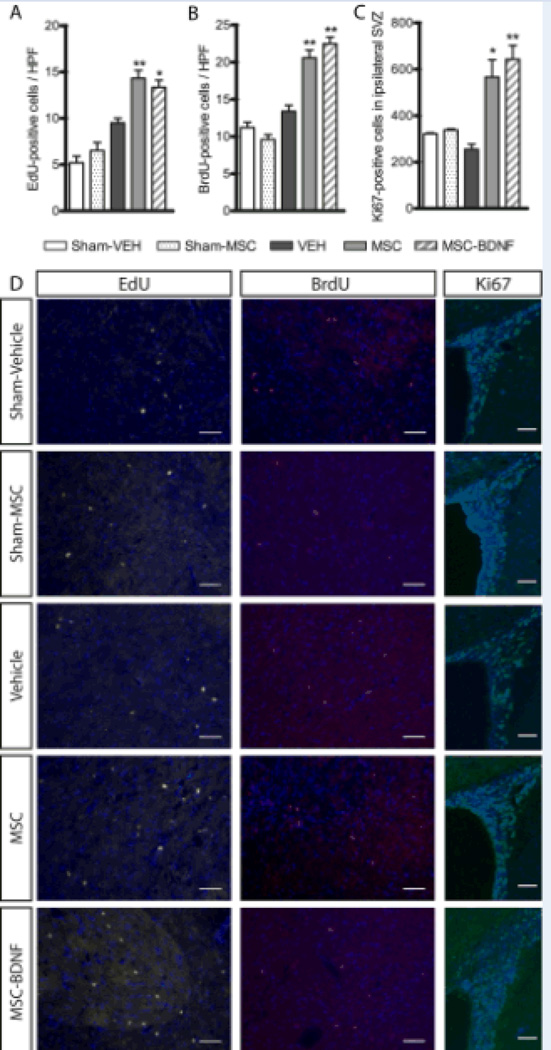
At 28 days after HI only some EdU- and BrdU-positive cells could be detected in the subventricular zone. However, in the injured striatum and near the ischemic boundary zone a significant number of both EdU- and BrdU-positive cells were visible. No increase in EdU-positive cell number in the striatum was detected at 28 days post-MCAO in vehicle-treated rats in comparison to sham-operated controls. Treatment with either MSC or MSC-BDNF significantly increased EdU-positive cells number in the ipsilateral striatum. There was no difference in the number of EdU-positive cells between MSC- and MSC-BDNF treated rats (Fig. 3A and D), indicating that there was no difference in cell proliferation induced by either treatment with MSC or MSC-BDNF.
Figure 3. Effect of MSC transplantation on cell proliferation in ipsilateral striatum and subventricular zone.
The number of EdU-positive cells (A) and BrdU-positive cells (B) was measured in the ipsilateral striatum. Cell proliferation in the SVZ at 28 days post-MCAO was measured using Ki67 (C). Panel D shows representative photomicrographs of EdU-, BrdU- and Ki67-labelled cells. Scale bar represents 50µm. Data represent mean±SEM. Sham controls n=5, VEH n=8, MSC n=9, MSC-BDNF n=8. *p<0.05 vs. VEH, **p<0.01 vs. VEH.
Measurement of late cell proliferation by analysis of BrdU-positive cell number, showed that MCAO with vehicle-treatment had no effect on cell proliferation at 21–23 days post MCAO. Following treatment with MSC and MSC-BDNF the number of BrdU-positive cells increased when compared to vehicle treated rats. However, there was no additional effect of MSC-BDNF when compared to MSC treatment (Fig. 3B and D).
At 28 days post-MCAO cell proliferation in the MSC-treated rats, proliferation was still increased as compared to vehicle-treated MCAO rats as witnessed by increased Ki67-positive cell number in the subventricular zone. Treatment with MSC-BDNF did significantly increase the number of Ki67-positive cells in the SVZ when compared to vehicle treated rats, but was not different from treatment with MSC alone (Fig. 3C and D).
Discussion
This study, for the first time, shows that intranasal application of MSC or BDNF hypersecreting MSC following neonatal stroke in rats effectively reduces long-term functional impairment and infarct volume and increases cell proliferation in the ischemic hemisphere. Improved outcome after transplantation of MSC has been attributed to inhibition of inflammation, neuroprotection, direct replacement of lost tissue by MSC and stimulation of endogenous repair processes.13,14 MSCs secrete a variety of factors, including neurotrophins like BDNF and NGF, growth factors like VEGF and IGF and interleukins.6,7 Recently Kim et al.15 showed that intraventricular administration of human umbilical cord blood-derived MSC 6 hours after onset of neonatal stroke in a non-reperfusion model reduced ipsilesional tissue loss. This was accompanied by a dampened inflammatory response and decreased cell death. It has been suggested that the beneficial effects of MSC are mediated by the immuno-modulatory effect of MSC as well as by delivery of trophic factors.13,14 We observed a decrease in lesion volume after MSC treatment. However, iIn the adult MCAO model, treatment with MSC has been shown to improve neurological function without reducing lesion volume.13,16 All of these results could either be interpreted as MSC-induced endogenous repair or MSC-induced neuroprotection. In some studies in juvenile animals there is evidence for significant late neuronal loss.17 However, we did not observe a deterioration of neuronal function between day 3 and 10 post-HI when using the hypoxia-ischemia model in p9 mice.4 Therefore we proposed that MSC transplantation in the neonatal hypoxia-ischemia model may well promote endogenous neuronal repair. In the model of neonatal stroke currently described we do not know at present whether late neuronal loss develops, so we cannot conclude whether MSC mainly function by protecting the brain against late neurodegeneration and/or by endogenous repair mechanisms. The fact that treatment with MSC increase cell proliferation in the SVZ lasting for at least 28 days post-MCAO may indicate that endogenous repair processes are contributing to the therapeutic effects of MSC in our neonatal stroke model.
Recent results from our group showed that MSC-transplantation 10 days after induction of neonatal HI induces various changes in the cytokine and growth factor environment.5,7 Following transplantation of MSC after neonatal HI the gene expression profile in the brain shifts towards a growth promoting environment. In vivo this is visible as increased cell proliferation, formation of new neurons and decreased gliosis suggesting decreased inflammation following MSC treatment of ischemic brain injury.4 In the present study, transplantation of both MSC and MSC-BDNF resulted in long-lasting cell proliferation in the SVZ. The SVZ contains neural stem cells, which under normal circumstances differentiate into neurons and migrate towards the olfactory bulb.18 Local injury can stimulate cell proliferation in the SVZ upon which cells migrate towards the injured area and differentiate into neurons, oligodendrocytes and asrocytes.19 Our results in this study show that MSC treatment increases cell proliferation for at least 28 days post-MCAO. Cell proliferation markers EdU and BrdU were injected, directly after administration of the MSC and 2 weeks later respectively, to determine cell proliferation during the course of the experiment. At 28 days after HI only some EdU- and BrdU-positive cells could be detected in the SVZ. However, in the injured striatum and near the ischemic boundary zone a significant number of both EdU- and BrdU-positive cells was visible. These EdU- or BrdU-labelled cells probably originate from the subventricular zone and migrated towards the injured area where they potentially contribute to repair of the injured tissue and decreased lesion volume that was observed after MSC transplantation.
Administration of MSC following neonatal stroke significantly reduces lateralizing motor deficits. Besides the effect of MSC-transplantation following neonatal stroke on motor and somatosensory function, it is likely that also cognitive function is improved in rats following treatment with MSC. Recent studies have shown that neurorestorative treatments like MSC-transplantation or erythropoietin can indeed improve cognitive function following ischemic brain damage.20,21 A study on cognitive effects may be of importance in view of repair of white matter structures induced by treatment with MSC or MSC-BDNF (see Fig 2).
Neurotrophic factors play an important role in repair of ischemic brain damage. BDNF, in particular, is an important neurotrophic factor promoting neurogenesis and angiogenesis, it offers neuroprotection, modulates inflammation and can improve synaptic plasticity after ischemic brain injury.22–24 Furthermore, it has been shown that intracranial infusion of BDNF using an osmotic pup can reduce infarct volume after stroke.
Our present study shows that the effect of MSC-BDNF administration seems to be temporary. Treatment with MSC-BDNF causes less lateralizing motor deficits measured at one week after administration of the stem cells after which motor deficits get more pronounced and at 4 weeks reach the level of rats treated with MSC alone. However, at 4 weeks post-stroke BDNF-MSC are not more effective in reducing gross infarct size, grey and white matter loss and induction of cell proliferation than treatment with MSC alone. It is possible that the additive effect of BDNF on transplantation of MSC transplantation of MSC observed in the early measurement in the cylinder rearing test (day 14) is a direct effect of BDNF on neuronal activity. BDNF plays a role in several stages of the development of neural circuits, including neural stem cell survival and differentiation, axonal growth and refinement of developing circuits.25 The effect of BDNF-MSC might be targeted towards the refinement of neural circuits that are developing. Addition of BDNF in this period of development could lead to selective stabilization of some connections and elimination of others more effectively than MSC alone does.
In this study we used a non-integrating adenoviral construct to overexpress BDNF in MSC, meaning that BDNF expression subsides over time as the vector is removed from the cell. The temporary expression of BDNF might have been too short to induce long lasting effects. Therefore additional administrations of BDNF secreting MSC at later time points may be necessary to produce a more sustained effect. However, there is a time window of administration for MSC to be effective. We have recently shown, using the murine hypoxia-ischemia model, that a second dose of MSC at 17 days post-HI has no additive effect on top of a first dose at 10 days post-HI while two doses at 3 and 10 days post-HI effectively reduce HI brain damage.20
Apart from the fact that efficacy of (modified) MSC transplantation is of great importance, safety is a key issue in light of future clinical application of transplantation of (modified) MSC to babies with brain damage.26 Allogeneic MSC have been used for many years now in the treatment of hematopoietic diseases which means that protocols regarding isolation, administration and safety are already established. The use of an adenovirus to modify MSC instead of lenti- or retrovirus reduces the risk of developing neoplasms, since adenoviruses do not integrate into the host genome. However, fate of transplanted cells and possible adverse behavioural effects must be monitored closely in a phase II trial applying (modified) MSC transplantation in newborn babies with brain damage.
In summary, this study shows that intranasal application of BDNF-secreting MSC is equally effective in reducing grey and white matter loss and motor deficits and inducing cell proliferation after neonatal MCAO as use of MSC. Thus intranasal treatment with MSC may be a promising therapy for neonatal ischemic brain damage.
The signals responsible for orchestrating repair processes such as neurogenesis, gliogenesis and angiogenesis are complex and depend on an intricate balance of various intra- and extracellular molecules.27 It may well be possible that MSC are equipped well enough to react to the needs of the ischemic environment to stimulate repair processes in the neonatal ischemic brain.
Acknowledgments
Sources of Funding
This study was funded by the European Union (HEALTH-F2-2009-241778, NEUROBID); Zon-MW Project (no 116002003) and NIH (grant NS35902)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 2.Kirton A, deVeber G. Advances in perinatal ischemic stroke. Pediatr Neurol. 2009;40:205–214. doi: 10.1016/j.pediatrneurol.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 4.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun. 2010;24:387–393. doi: 10.1016/j.bbi.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 5.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav Immun. 2011;25:1342–1348. doi: 10.1016/j.bbi.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27:355–363. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J Neurosci. 2010;30:9603–9611. doi: 10.1523/JNEUROSCI.1835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuguchi H, Sasaki T, Kawabata K, Sakurai F, Hayakawa T. Fiber-modified adenovirus vectors mediate efficient gene transfer into undifferentiated and adipogenic-differentiated human mesenchymal stem cells. Biochem Biophys Res Commun. 2005;332:1101–1106. doi: 10.1016/j.bbrc.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 9.Derugin N, Ferriero DM, Vexler ZS. Neonatal reversible focal cerebral ischemia: a new model. Neurosci Res. 1998;32:349–353. doi: 10.1016/s0168-0102(98)00096-0. [DOI] [PubMed] [Google Scholar]
- 10.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res. 2010;68:419–422. doi: 10.1203/PDR.0b013e3181f1c289. [DOI] [PubMed] [Google Scholar]
- 11.Nijboer CH, van der Kooij MA, van Bel F, Ohl F, Heijnen CJ, Kavelaars A. Inhibition of the JNK/AP-1 pathway reduces neuronal death and improves behavioral outcome after neonatal hypoxic-ischemic brain injury. Brain Behav Immun. 2010;24:812–821. doi: 10.1016/j.bbi.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torrente Y, Polli E. Mesenchymal stem cell transplantation for neurodegenerative diseases. Cell Transplant. 2008;17:1103–1113. doi: 10.3727/096368908787236576. [DOI] [PubMed] [Google Scholar]
- 15.Kim ES, Ahn SY, Im GH, Sung DK, Park YR, Choi SH, et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr Res. 2012;72:277–284. doi: 10.1038/pr.2012.71. [DOI] [PubMed] [Google Scholar]
- 16.Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geddes R, Vannucci RC, Vannucci SJ. Delayed cerebral atrophy following moderate hypoxia-ischemia in the immature rat. Dev Neurosci. 2001;23:180–185. doi: 10.1159/000046140. [DOI] [PubMed] [Google Scholar]
- 18.Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Covey MV, Bitel CL, Ni L, Jonakait GM, Levison SW. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann Neurol. 2007;61:199–208. doi: 10.1002/ana.21068. [DOI] [PubMed] [Google Scholar]
- 20.Donega V, van Velthoven CT, Nijboer CH, van Bel F, Kas MJ, Kavelaars A, et al. Intranasal mesenchymal stem cell treatment for neonatal brain damage: long-term cognitive and sensorimotor improvement. PLoS One. 2013;8:e51253. doi: 10.1371/journal.pone.0051253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31:403–411. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 2011;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 23.Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 24.Ploughman M, Windle V, MacLellan CL, White N, Dore JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 25.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2012;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 26.Borlongan CV, Weiss MD. Baby STEPS: a giant leap for cell therapy in neonatal brain injury. Pediatr Res. 2011;70:3–9. doi: 10.1203/PDR.0b013e31821d0d00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]