Abstract
The receptor tyrosine kinase, c-kit, and its ligand, stem cell factor (SCF), function in a diverse range of biological functions. The role of c-kit in the maintenance and survival of hematopoietic stem cells and of mast cells is well recognized. c-kit also plays an important role in melanogenesis, erythropoiesis and spermatogenesis. Recent work from our laboratory highlights an important role of c-kit in the regulation of expression of two molecules in dendritic cells (DCs), interleukin-6 (IL-6) and Jagged-2 (a ligand of Notch), which are known to regulate T helper cell differentiation. Our study shows that induction of c-kit expression and its signaling in DCs promotes Th2 and Th17 responses but not Th1 response. c-kit inhibition by imatinib mesylate (Gleevec) in DCs was previously shown to promote natural killer cell activation which may be due to dampening of IL-6 production by the DCs. Since dysregulation of c-kit function has been associated with various disease states including cancer, in this perspective we have focused on known and novel functions of c-kit to include molecules such as IL-6 and Notch that were not previously recognized to be within the purview of c-kit biology. We have also reviewed the differential expression pattern of SCF and c-kit on various cell types and its variation during development or pathology. The recognition of previously unappreciated roles for c-kit will provide better insights into its function within and beyond the immune system and pave the way for developing better therapeutic strategies.
Keywords: c-kit, SCF, interleukin-6, jagged-2, dendritic cells, T cells, differentiation
c-kit and Stem Cell Factor
c-kit is a type III tyrosine kinase receptor that was cloned soon after the identification of the v-kit oncogene as the transforming gene in the Hardy-Zuckerman 4 feline virus.1–3 It shares strong homology and function to platelet-derived growth factor receptor, and macrophage colony stimulating factor receptor.4 All type III receptors are characterized by the five immunogloblulin-like domains in the extracellular region, followed by a 70–100 amino acids long intracellular kinase domain. Similar to most tyrosine kinase receptors, the extracellular domain facilitates the binding of the ligand and the cytoplasmic domain serves to transduce the signal.2,3,5,6 Alternate splicing of murine c-kit mRNA results in two isoforms characterized by the presence or absence of a GNNK (glycine-asparagine-asparagine-lysine; residues 510–513) tetrapeptide in the juxtamembrane region of the extracellular domain.7,8 In humans, the expression of similar splice variants has also been documented. These isoforms of c-kit are expressed in different ratios in various cell types and also differ in their signaling capabilities.9,10
Stem Cell Factor, the ligand of c-kit is encoded by the Steel (Sl) locus on chromosome 12 in humans and chromosome 10 in mice.11,12 Like c-kit, SCF also exhibits two distinct isoforms that arise from alternative splicing of exon 6 of the mRNA.13,14 The primary translation product of 248 amino acids contains a proteolytic cleavage site encoded by exon 6 and post-translational processing at this site results in the soluble form of SCF comprising 165 amino acid residues.13–15 In contrast, the membrane-bound SCF, which is 220 amino acid residues long, results from an alternatively spliced mRNA that lacks the proteolytic cleavage site encoded by exon 6, resulting in anchoring of the protein to the membrane. The membrane-bound form may also produce a soluble form by proteolytic cleavage.16,17 Membrane-bound SCF has signaling properties, distinct from that of the soluble form and this results in varied biological functions mediated by the two isoforms.18,19
The binding of SCF induces the homodimerization of the c-kit receptor resulting in the phosphorylation of selective tyrosine residues in c-kit, thereby unmasking docking sites for the Src-homology2 (SH2)-containing signal transducers.20 Site-specific mutagenesis studies have revealed a hierarchical importance in the phosphorylation of tyrosine residues. Some mutations can completely abrogate c-kit signaling, while others only significantly dampen the overall signaling.21,22
The discovery of the c-kit proto-oncogene marked an important milestone in understanding the biology of this receptor that is widely expressed in hematopoietic stem cells (HSC), myeloid progenitor cells, dendritic cells (DCs), mast cell and pro-B and pro-T cells.2,3 In many cell types, like the B and T cells, the expression of c-kit is lost upon cell differentiation. However, mast cells, natural killer (NK) cells and DCs of the immune system retain their expression of c-kit suggesting an important role for this molecule in these cell types.11,23 c-kit plays a crucial role in mast cell development, survival and function through interactions with its ligand, SCF.24–26 Other cell types that express c-kit include melanocytes, germ cells, and interstitial cells of Cajal.27
Certain lineages of cells that express c-kit also produce its ligand, SCF, indicating a self-regulated23 feedback to enhance receptor expression. c-kit signaling has profound effects in various biological functions such as spermatogenesis, melanin formation and erythropoiesis.23,28 Mutations in c-kit results in the development of various tumors due to aberrant signaling of the receptor, which necessitates a complete understanding of c-kit structure, the initiation of signaling events2,28 as well as characterization of downstream targets of the receptor.2,29
c-kit Signaling Pathway
The involvement of PI3-kinase in c-kit signaling has been extensively characterized. A combination of molecular mutagenesis studies and biochemical analyses has shed light on the relationship between c-kit and PI3-kinase. The p85 regulatory subunit of PI3-kinase specifically associates with phoshorylated tyrosine residue 719 of c-kit, resulting in the recruitment and phosphorylation of protein kinase B (AKT).30 Transgenic mice harboring a point mutation in the tyrosine residue have revealed the physiological importance of this residue.31 Substitution of tyrosine with phenyalanine resulted in both reduced spermatogenesis and impaired follicular development. Phosphorylated-AKT enhances the survival and proliferation of the primordial cells and specifically mediates several downstream functions through NFκB signaling as well as phosphorylation of the pro-apoptotic molecule BAD.20 Phosphorylation of BAD inhibits the pro-apoptotic function of the molecule, which is one of the reasons why impaired c-kit signaling results in reduced proliferation and survival of several cell types. The c-kit gene maps to the dominant white spotting (W) locus in mice.32 Mutations in the W locus cause deficiency in melanocytes25,29,31,33,34 as well as reduced PI3-kinase activity.35 It is interesting to note that extracellular c-kit mutations result in hyperactivation of c-kit, marked by prolonged PI3-kinase activation and enhanced cell survival and proliferation.34 In addition to PI3-kinase activation, binding of SCF has been shown to induce activation of multiple additional pathways, including phospholipase C (PLC)-gamma, Src kinase, Janus kinase (JAK)/Signal Transducers and Activators of Transcription (STAT) and mitogen activated protein (MAP) kinase pathways.23 In a recent study, distinct roles of the Src kinase and the PI3-kinase pathways were noted in regards to c-kit function. Mutation in the Src kinase docking site blocked pro B and pro T cell development while that in the PI3-kinase binding site affected spermatogenesis.36
There are mechanisms in place in cells that downregulate c-kit signaling to prevent chronic activation of the receptor, which can promote cancer. Protein kinase C, a known negative regulator of PI3 kinase pathways, phosphorylates residues S741 and S746 and downregulates c-kit signaling.20,37 This is evident from mutational studies, where substitution of these serine residues to alanine resulted in prolonged c-kit signaling. SOCS1, SOCS6 and SOCS8 bind to c-kit and dampen downstream signaling of the receptor.33,38,39 Additional negative regulators of c-kit signaling include SHIP40,41 and the GTPase activating protein neurofibromin-1 (NF-1).42 Furthermore, ubiquitination of c-kit via Cbl, a ubiquitin ligase, and subsequent proteasomal degradation may be also involved in c-kit downregulation.43 While chronic activation of c-kit may promote tumorigenesis, loss of function of c-kit can be also deleterious since studies suggest that loss of kit may allow melanoma cells to escape SCF-mediated apoptosis thus allowing tumor growth and metastasis.44 Thus, deregulation of activation or inhibitory pathways of c-kit has adverse effects, often resulting in tumor formation.
Differential Expression of c-kit and SCF Regulates Biological Functions
The regulation of c-kit signaling is also fine-tuned by whether SCF is expressed in membrane-bound or soluble form. Association of c-kit with soluble SCF results in transient activation of the receptor whereas membrane-bound SCF prevents the internalization of the receptor and promotes sustained downstream activation.45,46 The expression of membrane-bound form of SCF brings into play cell-cell interactions that underlie many of the biological functions of c-kit. For example, the expression of c-kit but not SCF is found on a significant population of HSCs and the renewal of these cells is promoted by fibroblasts, which express SCF but not the receptor.47–49 Additionally, the fact that the expression of both ligand and receptor is altered during injury, infection and inflammation reinforces the concept that selective expression of the ligand or the receptor is key in maintaining homeostasis. The brain produces high levels of soluble SCF.15 However, during brain injury, the level of membrane-bound SCF is elevated which has been shown to be important not only for recruiting neural stem cells to the site of injury, but for also activating c-kit expressed on the stem cells which contributes to the repair process.50 Our recent study has shown that the expression of c-kit on lung DCs from naïve mice is low, but the expression of both the membrane-bound ligand and receptor is significantly elevated in response to certain allergens causing persistent signaling downstream of c-kit due to cell-cell interactions.51 Given that both receptor and ligand can be expressed by the same cell type under specific conditions, it is critical that such expression patterns are prevented under homeostatic conditions to minimize inadvertent activation of the receptor. By the same token, such interactions are an integral part of development and repair after tissue injury and must be stringently regulated to prevent adverse effects such as oncogenesis. In the setting of cancers, alteration of c-kit function and signaling has been studied extensively in association with gastrointestinal stromal tumors (GIST).52 In GIST, the gain-of function mutation in exon 11 leads to constitutive activation of the receptor even in the absence of SCF.52 However, recent studies have demonstrated increased tyrosine phosphorylation of the receptor even in the absence of gain-of function mutations in c-kit in GIST patients, which has also been associated with the presence of membrane-bound SCF expressed by tumor cells.53 Several other cancers have also shown altered or increased expression of the receptor and ligand suggesting a paracrine or autocrine mechanism that induces an oncogenic signaling cascade in cells.54
Recent studies have demonstrated a role for the intracellular second messenger, cyclic AMP (cAMP), in the expression of both c-kit and SCF in different cell types. In several cancer cell lines, an elevated level of cAMP induced by agents such as forskolin has been associated with increased expression of c-kit.55–58 However, the elevation in c-kit expression mediated by cAMP is not solely restricted to tumor cells as was revealed in our study of allergen-induced c-kit expression in DCs. In DCs, the increase in c-kit expression in response to both mucosal adjuvant cholera toxin (CT) and the allergen house dust mite (HDM) was inhibited by a cAMP antagonist.51 Similarly, treatment with forskolin or CT promoted c-kit expression in human ovarian carcinoma cell lines, which constitutively express SCF.58 In keeping with the antiproliferative effects of cAMP, the increase in intracellular cAMP level in these cells inhibited cell proliferation, which was not dependent on c-kit expression. Interestingly, cAMP has been also shown to directly activate the SCF promoter in Sertoli cells. An unidentified cAMP-induced factor has been shown to bind SCF promoter resulting in increased expression of the gene.59 Since c-kit plays an important role in the development of various cell types, cAMP may play a dual role in upregulating c-kit/SCF expression and promoting cell differentiation.
c-kit and IL-6
c-kit regulates various biological functions and studies including our own show that c-kit is an important regulator of interleukin-6 (IL-6) production.51,60,61 This suggests that the biological effects attributed to c-kit may be partly mediated by the pleiotropic cytokine IL-6 that possesses diverse pro- and anti-inflammatory properties.62 Studies involving mast cells have defined a role for c-kit in regulating IL-6 production. Bone marrow mast cells from c-kit mutant mice displayed reduced IL-6 levels and conversely mice lacking RabGEF1, a negative regulator of c-kit signaling, showed enhanced IL-6 production and sustained phosphorylation of AKT and ERK in mast cells.60,61 Recently, we have shown that co-expression of c-kit and membrane-bound SCF promotes IL-6 production in dendritic cells (DCs) mediated by the PI3-kinase pathway.51 Impaired c-kit signaling or AKT activation resulted in a decrease in IL-6 production in DCs in response to allergen or the mucosal adjuvant cholera toxin (CT). When stimulated with CT, DCs from mice expressing a catalytically inactive form of the p110δ subunit of PI3-kinase were substantially impaired in IL-6 production.51 The residual IL-6 production in these DCs was probably due to functional MAP kinase pathway, which can also activate NFκB resulting in IL-6 production.63
A well-documented role for IL-6 is its ability to inhibit IL-12 production.64–66 Several lines of studies have now established that IL-12−/− mice are prone to formation of several tumors and administration of IL-12 mitigates tumor growth.67,68 Hence, the ratio of the signaling molecules downstream of IL-6 and IL-12, STAT-3 and STAT-4 respectively, in tumor cells during their genesis can influence tumor progression.69 It is also interesting to note that while the PI3 kinase pathway promotes IL-6 production, it negatively regulates IL-12 gene expression.70 In summary, dysregulated c-kit signaling in tumors resulting in continuous activation of the AKT or MAP kinase pathway could contribute to the high levels of IL-6 observed in these cancers. Also, IL-6, via prolonged activation of STAT-3 and concomitant suppression of IL-12, could further accelerate tumor growth.
Several adapter proteins have been associated with PI3-kinase signaling and a significant body of research has focused on adapter molecule, Gab2. Gab2 is an important activator of PI3-kinase and Gab2-deficient mice show reduced airway inflammation, decreased IL-6 production and a reduction in mast cells.71 This study highlighted the role of c-kit-mediated signaling via PI3-kinase and Gab-2 in IL-6 production. Gab-2 is also a limiting signaling component in the MAP kinase pathway72 underscoring the importance of this molecule in the fine-tuning of IL-6 gene expression.
In the area of stem cells, a relationship between c-kit and IL-6 has not been adequately explored, even though pivotal roles for these two molecules in regulating stem cell renewal has been documented in several studies. In a model of myocardial infarction, c-kit+ stem cells from the bone marrow were recruited to the heart to repair myocardial injury.73 In addition, c-kit receptor mutant mice were found to be less efficient in the mobilization of these stem cells.73 Similarly, IL-6 also acts as a trigger in recruiting myocardial stem cells to the heart, and a recent study profiling early gene activation following myocardial infarction showed a 420-fold increase in IL-6 expression that was maintained for 28 days following the procedure. This strengthens the role of IL-6 as a cytokine involved in stem cell renewal and proliferation, but not necessarily as an inflammatory cytokine.74 Although STAT-3 is activated by IL-6, the transcription factor, hypoxia inducible factor-1α (HIF-1α), can be also activated via IL-6.75 Studies have documented HIF-1α to be responsible for the recruitment and stabilization of neural stem cells.76 It is tempting to speculate that the deficient mobilization of neural and myocardial stem cells to the site of injury in c-kit mutant mice is a result of impaired IL-6 production and, in turn, reduced HIF-1α activation. Intriguingly, a recent study has suggested that HIF-1α can activate the SCF promoter and SCF production in breast cancer cells was found to increase significantly upon overexpression of HIF-1α.77 Collectively, these studies suggest that the convergence of signaling events triggered upon c-kit activation influences the balance between cell division and cell death.
c-kit and Notch in Hematopoietic Stem Cells and Progenitors
Kit-mediated signal transduction is critical for the normal development and survival of haematopoietic progenitor cells.78,79 However, expression of c-kit is generally lost during hematopoietic cell differentiation.24 Similar to c-kit, the protein Notch is also expressed by HSCs.80,81 Notch is a transmembrane protein and has 4 members, Notch 1–4 that interact with the ligands, Jagged-1, Jagged-2, Delta-like 1, 3 and 4 that are also expressed on the cell surface.80,81 Thus, cell-cell interaction is also involved in Notch activation, which leads to a stepwise proteolytic processing of Notch to Notch intracellular domain (NICD).80,81 NICD then translocates to the nucleus where it interacts with the DNA-binding protein RBP-Jk to induce target gene transcription via recruitment of histone acetylases and transcriptional coactivators such as Mastermind.80,81 During fetal and adult development, expression of Notch continues in the proliferative layers of several mature tissues. There is considerable interest in the events that trigger Notch-Notch ligand interactions but relative concentrations appear to play an important role in deciding whether interactions occur inter- or intracellularly.80,81 More importantly, Notch ligands have been associated with both activation and inhibition of Notch signaling. Notch is involved in cell fate decisions. Somite formation in vertebrates provides an excellent example of Notch behaving as a transcriptional oscillator.82,83 Thus, repetitive cycles of Notch activation and inactivation cause specific pattern formation and segmental boundary in the presomitic mesoderm. High levels of ligand have been shown to inhibit Notch signaling while lower levels promote Notch activation. This mechanism was initially appreciated in Drosophila during wing development.84,85 In higher eukaryotes, Delta-like 3 in the Xenopus appears to only exert inhibitory activity.86 In humans, it has been suggested that high levels of Delta expression during keratinocyte differentiation could act as an inhibitory mechanism of Notch signaling to maintain stem cell population.87 The detailed mechanisms responsible for the inhibitory effects are unclear although intercellular ligand-ligand interactions as well as intracellular receptor titration by ligand have been proposed.88
Recent studies suggest functional interaction between c-kit and Notch pathways. Using a Pax-5-deficient pro-B cell line blocked in its B cell potential, as well as a bone-marrow-derived lymphoid and myeloid progenitor, it was shown that Notch signaling rapidly upregulates c-kit expression which was required for T cell development.89 However, the development of non-T cells (NK or myeloid) was found to be c-kit-independent.89 In tissue culture, Lin-Sca-1+c-kit+ murine HSCs stimulated by Flt3 ligand, interleukin-7 and Delta-like 1-expressing OP-9 fibroblasts undergo de novo T cell development.90 In subsequent studies, however, conditional deletion of Delta-like 1 was found to block the development of marginal zone B cells but did not impair T cell development.91 In the developing nervous system, c-kit signaling is involved in survival, migration, proliferation and differentiation of neural crest precursors.92,93 In the ciliary epithelium, which is derived from the central nervous system (CNS), c-kit signaling upregulated Notch expression and cooperation between c-kit and Notch was required for the maintenance of neural stem cells.94
c-kit and Notch Connection Beyond Hematopoietic Stem Cells
It is clear that the role of c-kit in the regulation of the immune system extends beyond its well-established function in mast cells.26 A role for c-kit in the function of DCs, the key antigen-presenting cells of the immune system, was largely unappreciated prior to our study investigating mechanisms underlying production of IL-6, a cytokine that promotes Th17 and Th2 development.51 The HDM and the mucosal adjuvant CT, both of which promote Th2 and Th17 responses, upregulated c-kit expression in DCs causing increased IL-6 production by the cells.51 In another study, c-kit signaling in DCs was shown to inhibit NK cell activation, which was alleviated by Gleevec resulting in antitumor effects.95 Gleevec (imatinib mesylate/STI571) is an allosteric inhibitor of c-kit, which is being effectively used in the treatment of different cancers like GIST and chronic myeloid leukemia. Gleevec’s mode of action involves inhibition of tyrosine kinase activity of specific receptors including that of c-kit.96 Although the NK-activating effect of Gleevec mediated by DCs was found to be independent of IL-12,95 it is possible that inhibition of c-kit by Gleevec resulting in downmodulation of IL-6 production contributed to NK activation since IL-6 has been shown to cause anergy and NK cell dysfunction.97 In our study linking c-kit to increased IL-6 production, basal Jagged-2 expression was blunted in DCs isolated from c-kit mutant mice which, unlike in DCs isolated from wild-type mice, could not be upregulated by CT.51 Unlike CT, the Th1-promoting adjuvant, CpG oligodeoxy-nucleotide, inhibited Jagged-2 expression but promoted expression of Delta-like 4, which inhibits Th2 and promotes Th1 development. The expression of Delta-like 4 or Jagged-1 was not affected by lack of functional c-kit in the DCs. This study showed that both basal and induced Jagged-2 expression is dependent on c-kit in DCs. In additional studies, Jagged-2 expression has been shown to be upregulated under Th2-inducing conditions.98,99
The relationship between c-kit and Jagged-2 and increased IL-6 production revealed in our study was similar to observations of Jagged-2-promoted IL-6 secretion from malignant plasma cells from multiple myeloma (MM) patients that may involve paracrine or autocrine mechanisms.100,101 MM is a plasma cell malignancy associated with increased accumulation of monoclonal plasma cells in the bone marrow. Interestingly, while MM is characterized by increased IL-6 secretion which functions as an autocrine growth factor for these cells, ~30% of MM patients also express c-kit. c-kit is not expressed by plasma cells present in healthy individuals. Although expression of c-kit was associated with better prognosis in MM in one study,102 a different study has shown that MM cells with expression of the c-kit isoform lacking the GNNK tetrapeptide in its juxtamembrane domain are more resistant to the anti-myeloma drugs, bortezomin and melphalan.103 In both GNNK+ and GNNK− MM cells, SCF promoted Akt phosphorylation although the kinetics and duration of phosphorylation were different between cells expressing the 2 isoforms.103 Activation of ERK1/2 was low in response to SCF in both types of cells and explained the weak mitogenic effect of SCF on MM cells in general.103 Another Phase II clinical trial with Gleevec found limited therapeutic effect of this drug in MM.104 These studies suggest that the function of c-kit in different cell types may depend on the specific isoform of c-kit expressed by the cell. It will be interesting to determine whether c-kit+ plasma cells in MM patients exhibit increased Jagged-2 expression and also whether Jagged-2 expression is related to a specific isoform of c-kit. Thus, the success of therapies targeting c-kit in specific malignancies may depend on which isoform of c-kit is expressed in an individual. In GIST, where Gleevec has met with better success, the GNNK form of c-kit may be more prevalent.
While a role for c-kit in Jagged-2 expression was not recognized until recently, co-expression of c-kit and Jagged-2 was noted in other contexts in previous studies. For example, hematopoietic progenitors were shown to express c-kit and Jagged-2,105 and the latter promoted survival and proliferation of the progenitors and their development into NK cells.106 It is interesting that c-kit can promote both development and activation of NK cells. Jagged-2 is expressed in multiple tissues in adult mice and homozygous Jagged-2 mutant mice display limb and craniofacial deformities along with altered T cell receptor (TCR) αβ/γδ ratios.107 Jagged-2-expressing mobilized Lin-Sca-1+c-kit+ hematopoietic progenitor cells, unlike HSCs, can directly promote expansion of CD4+CD25+Foxp3+ T regulatory (Treg) cells and this was found to be associated with Notch signaling.108 In an earlier study, Jagged-1 overexpressing antigen-presenting cells when adoptively transferred into mice induced tolerance in the recipients.109 Collectively, these reports beg the question as to why c-kit and Jagged-2 in one context promotes Th2/Th17 but induces Tregs/immunesuppression in another. Although the reason is unclear at the present time, based on prior literature it may be speculated that the level of Jagged-2 expression in a DC or hematopoietic progenitor cell dictates immune activation versus suppression. It will be also important to determine why c-kit specifically influences Jagged-2 expression. One possible reason is to utilize Jagged-2 in cell survival and maintenance of a specific phenotype based on recent findings.103,106
Perspective
Among tyrosine kinase receptors, c-kit ranks high as a regulator of a broad spectrum of biological functions. Recent discoveries involving c-kit continue to provide insights into the diverse roles of this receptor.51,95 The cardinal rule for tyrosine kinase receptor activation is that phosphorylation and dephosphorylation should be stringently regulated. Dysregulation in either of these key events leads to altered function of the receptor. While c-kit function has been best studied in the context of mast cell biology, recent studies including our own highlight additional functions of this receptor in the immune system particularly in DC function.51,95 Harnessing the potential of these DCs in immunotherapy could lead to treatments for many tumors as well as to combat viral infections, both of which require significantly high levels of IFNγ to activate the cytolytic CD8 T cells. In future studies, it will be also important to evaluate the proliferative and differentiation potential of HSCs under conditions of constitutive c-kit expression. Stem cells isolated from patients with persistent c-kit signaling may have high self-renewal potential given that mice with specific c-kit mutations resulting in reduced c-kit activation have been shown to be fertile and viable, but with significantly reduced numbers of HSCs.110 Stem cells endowed with increased self-renewal capacity arising from specific c-kit mutations could be identified, selected and potentially utilized in the treatment of neurodegenerative diseases like Alzheimer’s and Parkinson’s. One of the areas in c-kit biology that has not been adequately explored is the function of the different splice forms of c-kit and SCF in different biological contexts. Broadening our understanding of the generation of these splice forms and their signaling abilities will provide better insights into c-kit function and aid in the design of more efficient therapeutic targets.
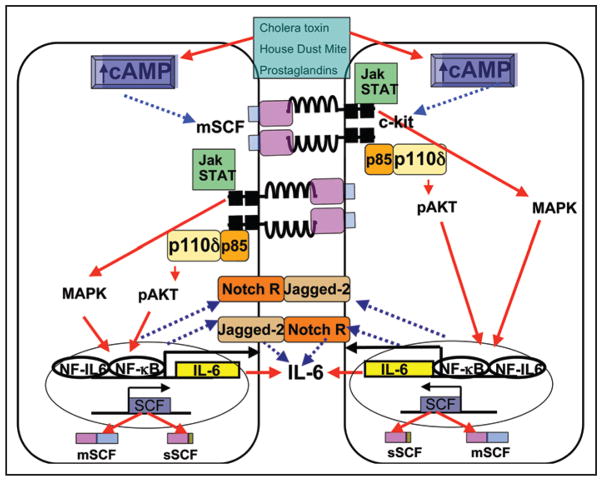
Figure 1.
Dual upregulation of c-kit and membrane-bound SCF (mSCF) on dendritic cells by allergens and allergy-inducing adjuvants promotes IL-6 and Jagged-2 expression. Dendritic cells stimulated with CT, house dust mite or prostaglandins upregulate c-kit and mSCF expression that is mediated by cAMP.51 c-kit activation by mSCF via cell-cell interactions, triggers sustained downstream activation of the PI3 kinase/AKT pathway51 and possibly MAPK and JAK-STAT pathways23 resulting in increased production of IL-6, whose transcription is known to be dependent on NFκB and NF-IL6,111 and may also involve Notch/Notch ligand. Activation of c-kit also upregulates expression of Jagged-2,51 and Notch receptors (NotchR).94 The red arrows show known mechanisms of activation while the broken blue arrows indicate activation pathways for which the mechanisms have yet to be determined.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants HL 060207 and HL 069810 (to P.R.), HL 077430 and AI 048927 (to A.R.) and HL 084932 (to P.R. and A.R.).
Abbreviations
- SCF
stem cell factor
- DC
dendritic cells
- HSC
hematopoietic stem cells
- CT
cholera toxin
- MM
multiple myeloma
References
- 1.Besmer P, Murphy JE, George PC, Qiu FH, Bergold PJ, Lederman L, Snyder HW, Jr, Brodeur D, Zuckerman EE, Hardy WD. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986;320:415–21. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- 2.Qiu FH, Ray P, Brown K, Barker PE, Jhanwar S, Ruddle FH, Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family—oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988;7:1003–11. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–51. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fantl WJ, Johnson DE, Williams LT. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–81. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- 5.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 6.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi S, Kunisada T, Ogawa M, Yamaguchi K, Nishikawa S. Exon skipping by mutation of an authentic splice site of c-kit gene in W/W mouse. Nucleic acids res. 1991;19:1267–71. doi: 10.1093/nar/19.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reith AD, Ellis C, Lyman SD, Anderson DM, Williams DE, Bernstein A, Pawson T. Signal transduction by normal isoforms and W mutant variants of the Kit receptor tyrosine kinase. EMBO J. 1991;10:2451–9. doi: 10.1002/j.1460-2075.1991.tb07784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosier PS, Ricciardi ST, Hall LR, Vitas MR, Clark SC, Crosier KE. Expression of isoforms of the human receptor tyrosine kinase c-kit in leukemic cell lines and acute myeloid leukemia. Blood. 1993;82:1151–8. [PubMed] [Google Scholar]
- 10.Caruana G, Cambareri AC, Ashman LK. Isoforms of c-KIT differ in activation of signalling pathways and transformation of NIH3T3 fibroblasts. Oncogene. 1999;18:5573–81. doi: 10.1038/sj.onc.1202939. [DOI] [PubMed] [Google Scholar]
- 11.Besmer P. The kit ligand encoded at the murine Steel locus: a pleiotropic growth and differentiation factor. Curr Opin Cell Biol. 1991;3:939–46. doi: 10.1016/0955-0674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 12.Geissler EN, Liao M, Brook JD, Martin FH, Zsebo KM, Housman DE, Galli SJ. Stem cell factor (SCF), a novel hematopoietic growth factor and ligand for c-kit tyrosine kinase receptor, maps on human chromosome 12 between 12q14. 3 and 12qter. Somat Cell Mol Genet. 1991;17:207–14. doi: 10.1007/BF01232978. [DOI] [PubMed] [Google Scholar]
- 13.Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991;64:1025–35. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 14.Toksoz D, Zsebo KM, Smith KA, Hu S, Brankow D, Suggs SV, Martin FH, Williams DA. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci USA. 1992;89:7350–4. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang EJ, Nocka KH, Buck J, Besmer P. Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell. 1992;3:349–62. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumdar MK, Feng L, Medlock E, Toksoz D, Williams DA. Identification and mutation of primary and secondary proteolytic cleavage sites in murine stem cell factor cDNA yields biologically active, cell-associated protein. J Biol Chem. 1994;269:1237–42. [PubMed] [Google Scholar]
- 17.Longley BJ, Tyrrell L, Ma Y, Williams DA, Halaban R, Langley K, Lu HS, Schechter NM. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc Natl Acad Sci USA. 1997;94:9017–21. doi: 10.1073/pnas.94.17.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brannan CI, Lyman SD, Williams DE, Eisenman J, Anderson DM, Cosman D, Bedell MA, Jenkins NA, Copeland NG. Steel-Dickie mutation encodes a c-kit ligand lacking transmembrane and cytoplasmic domains. Proc Natl Acad Sci USA. 1991;88:4671–4. doi: 10.1073/pnas.88.11.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massague J, Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–41. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- 20.Blume-Jensen P, Janknecht R, Hunter T. The kit receptor promotes cell survival via activation of PI3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr Biol. 1998;8:779–82. doi: 10.1016/s0960-9822(98)70302-1. [DOI] [PubMed] [Google Scholar]
- 21.Nocka K, Majumder S, Chabot B, Ray P, Cervone M, Bernstein A, Besmer P. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice—evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989;3:816–26. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- 22.Tan JC, Nocka K, Ray P, Traktman P, Besmer P. The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science. 1990;247:209–12. doi: 10.1126/science.1688471. [DOI] [PubMed] [Google Scholar]
- 23.Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 2006;533:327–40. doi: 10.1016/j.ejphar.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 24.Orfao A, Garcia-Montero AC, Sanchez L, Escribano L. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12–30. doi: 10.1111/j.1365-2141.2007.06619.x. [DOI] [PubMed] [Google Scholar]
- 25.Ray P, Higgins KM, Tan JC, Chu TY, Yee NS, Nguyen H, Lacy E, Besmer P. Ectopic expression of a c-kitW42 minigene in transgenic mice: recapitulation of W phenotypes and evidence for c-kit function in melanoblast progenitors. Genes Dev. 1991;5:2265–73. doi: 10.1101/gad.5.12a.2265. [DOI] [PubMed] [Google Scholar]
- 26.Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–28. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–20. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 28.Ali S, Ali S. Role of c-kit/SCF in cause and treatment of gastrointestinal stromal tumors (GIST) Gene. 2007;401:38–45. doi: 10.1016/j.gene.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein A, Chabot B, Dubreuil P, Reith A, Nocka K, Majumder S, Ray P, Besmer P. The mouse W/c-kit locus. Ciba Found Symp. 1990;148:158–66. [PubMed] [Google Scholar]
- 30.Serve H, Hsu YC, Besmer P. Tyrosine residue 719 of the c-kit receptor is essential for binding of the p85 subunit of phosphatidylinositol (PI)3-kinase and for c-kit-associated PI3-kinase activity in COS-1 cells. J Biol Chem. 1994;269:6026–30. [PubMed] [Google Scholar]
- 31.Hashimoto K, Matsumura I, Tsujimura T, Kim DK, Ogihara H, Ikeda H, Ueda S, Mizuki M, Sugahara H, Shibayama H, Kitamura Y, Kanakura Y. Necessity of tyrosine 719 and phosphatidylinositol 3′-kinase-mediated signal pathway in constitutive activation and oncogenic potential of c-kit receptor tyrosine kinase with the Asp814Val mutation. Blood. 2003;101:1094–102. doi: 10.1182/blood-2002-01-0177. [DOI] [PubMed] [Google Scholar]
- 32.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–48. doi: 10.1007/s00018-004-4189-6. [DOI] [PubMed] [Google Scholar]
- 33.Pello OM, del Moreno-Ortiz MC, Rodriguez-Frade JM, Martinez-Munoz L, Lucas D, Gomez L, Lucas P, Samper E, Aracil M, Martinez C, Bernad A, Mellado M. SOCS upregulation mobilizes autologous stem cells through CXCR4 blockade. Blood. 2006;108:3928–37. doi: 10.1182/blood-2006-02-006353. [DOI] [PubMed] [Google Scholar]
- 34.Kohl TM, Schnittger S, Ellwart JW, Hiddemann W, Spiekermann K. KIT exon 8 mutations associated with core-binding factor (CBF)-acute myeloid leukemia (AML) cause hyperactivation of the receptor in response to stem cell factor. Blood. 2005;105:3319–21. doi: 10.1182/blood-2004-06-2068. [DOI] [PubMed] [Google Scholar]
- 35.Timokhina I, Kissel H, Stella G, Besmer P. Kit signaling through PI3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 1998;17:6250–62. doi: 10.1093/emboj/17.21.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agosti V, Corbacioglu S, Ehlers I, Waskow C, Sommer G, Berrozpe G, Kissel H, Tucker CM, Manova K, Moore MA, Rodewald HR, Besmer P. Critical role for Kit-mediated Src kinase but not PI3-kinase signaling in pro T and pro B cell development. J Exp Med. 2004;199:867–78. doi: 10.1084/jem.20031983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibbons SJ, Rich A, Distad MA, Miller SM, Schmalz PF, Szurszewski JH, Sha L, Blume-Jensen P, Farrugia G. Kit/stem cell factor receptor-induced phosphatidylinositol 3′-kinase signalling is not required for normal development and function of interstitial cells of Cajal in mouse gastrointestinal tract. Neurogastroenterol Motil. 2003;15:643–53. doi: 10.1046/j.1350-1925.2003.00448.x. [DOI] [PubMed] [Google Scholar]
- 38.Rottapel R, Ilangumaran S, Neale C, La Rose J, Ho JM, Nguyen MH, Barber D, Dubreuil P, de Sepulveda P. The tumor suppressor activity of SOCS-1. Oncogene. 2002;21:4351–62. doi: 10.1038/sj.onc.1205537. [DOI] [PubMed] [Google Scholar]
- 39.Bayle J, Letard S, Frank R, Dubreuil P, De Sepulveda P. Suppressor of cytokine signaling 6 associates with KIT and regulates KIT receptor signaling. J Biol Chem. 2004;279:12249–59. doi: 10.1074/jbc.M313381200. [DOI] [PubMed] [Google Scholar]
- 40.Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, Jirik F, Krystal G, Humphries RK. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–20. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber M, Helgason CD, Scheid MP, Duronio V, Humphries RK, Krystal G. Targeted disruption of SHIP leads to Steel factor-induced degranulation of mast cells. EMBO J. 1998;17:7311–9. doi: 10.1093/emboj/17.24.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang YY, Vik TA, Ryder JW, Srour EF, Jacks T, Shannon K, Clapp DW. Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J Exp Med. 1998;187:1893–902. doi: 10.1084/jem.187.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sattler M, Salgia R, Shrikhande G, Verma S, Pisick E, Prasad KV, Griffin JD. Steel factor induces tyrosine phosphorylation of CRKL and binding of CRKL to a complex containing c-kit, phosphatidylinositol 3-kinase, and p120(CBL) J Biol Chem. 1997;272:10248–53. doi: 10.1074/jbc.272.15.10248. [DOI] [PubMed] [Google Scholar]
- 44.Huang S, Luca M, Gutman M, McConkey DJ, Langley KE, Lyman SD, Bar-Eli M. Enforced c-KIT expression renders highly metastatic human melanoma cells susceptible to stem cell factor-induced apoptosis and inhibits their tumorigenic and metastatic potential. Oncogene. 1996;13:2339–47. [PubMed] [Google Scholar]
- 45.Kurosawa K, Miyazawa K, Gotoh A, Katagiri T, Nishimaki J, Ashman LK, Toyama K. Immobilized anti-KIT monoclonal antibody induces ligand-independent dimerization and activation of Steel factor receptor: biologic similarity with membrane-bound form of Steel factor rather than its soluble form. Blood. 1996;87:2235–43. [PubMed] [Google Scholar]
- 46.Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–9. [PubMed] [Google Scholar]
- 47.Nocka K, Buck J, Levi E, Besmer P. Candidate ligand for the c-kit transmembrane kinase receptor: KL, a fibroblast derived growth factor stimulates mast cells and erythroid progenitors. EMBO J. 1990;9:3287–94. doi: 10.1002/j.1460-2075.1990.tb07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89:1502–6. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowie MB, Kent DG, Copley MR, Eaves CJ. Steel factor responsiveness regulates the high self-renewal phenotype of fetal hematopoietic stem cells. Blood. 2007;109:5043–8. doi: 10.1182/blood-2006-08-037770. [DOI] [PubMed] [Google Scholar]
- 50.Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest. 2004;113:1364–74. doi: 10.1172/JCI20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krishnamoorthy N, Oriss TB, Paglia M, Fei M, Yarlagadda M, Vanhaesebroeck B, Ray A, Ray P. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med. 2008;14:565–73. doi: 10.1038/nm1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corless CL, McGreevey L, Town A, Schroeder A, Bainbridge T, Harrell P, Fletcher JA, Heinrich MC. KIT gene deletions at the intron 10-exon 11 boundary in GI stromal tumors. J Mol Diagn. 2004;6:366–70. doi: 10.1016/S1525-1578(10)60533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theou-Anton N, Tabone S, Brouty-Boye D, Saffroy R, Ronnstrand L, Lemoine A, Emile JF. Co expression of SCF and KIT in gastrointestinal stromal tumours (GISTs) suggests an autocrine/paracrine mechanism. Br J Cancer. 2006;94:1180–5. doi: 10.1038/sj.bjc.6603063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lennartsson J, Ronnstrand L. The stem cell factor receptor/c-Kit as a drug target in cancer. Curr Cancer Drug Targets. 2006;6:65–75. doi: 10.2174/156800906775471725. [DOI] [PubMed] [Google Scholar]
- 55.Hou L, Panthier JJ, Arnheiter H. Signaling and transcriptional regulation in the neural crest-derived melanocyte lineage: interactions between KIT and MITF. Development. 2000;127:5379–89. doi: 10.1242/dev.127.24.5379. [DOI] [PubMed] [Google Scholar]
- 56.Leslie MC, Bar-Eli M. Regulation of gene expression in melanoma: new approaches for treatment. J Cell Biochem. 2005;94:25–38. doi: 10.1002/jcb.20296. [DOI] [PubMed] [Google Scholar]
- 57.Poole DP, Van Nguyen T, Kawai M, Furness JB. Protein kinases expressed by interstitial cells of Cajal. Histochem Cell Biol. 2004;121:21–30. doi: 10.1007/s00418-003-0602-8. [DOI] [PubMed] [Google Scholar]
- 58.Shaw TJ, Keszthelyi EJ, Tonary AM, Cada M, Vanderhyden BC. Cyclic AMP in ovarian cancer cells both inhibits proliferation and increases c-KIT expression. Exp Cell Res. 2002;273:95–106. doi: 10.1006/excr.2001.5426. [DOI] [PubMed] [Google Scholar]
- 59.Grimaldi P, Capolunghi F, Geremia R, Rossi P. Cyclic adenosine monophosphate (cAMP) stimulation of the kit ligand promoter in sertoli cells requires an Sp1-binding region, a canonical TATA box, and a cAMP-induced factor binding to an immediately downstream GC-rich element. Biol Reprod. 2003;69:1979–88. doi: 10.1095/biolreprod.103.019471. [DOI] [PubMed] [Google Scholar]
- 60.Gagari E, Tsai M, Lantz CS, Fox LG, Galli SJ. Differential release of mast cell interleukin-6 via c-kit. Blood. 1997;89:2654–63. [PubMed] [Google Scholar]
- 61.Tam SY, Tsai M, Snouwaert JN, Kalesnikoff J, Scherrer D, Nakae S, Chatterjea D, Bouley DM, Galli SJ. RabGEF1 is a negative regulator of mast cell activation and skin inflammation. Nat Immunol. 2004;5:844–52. doi: 10.1038/ni1093. [DOI] [PubMed] [Google Scholar]
- 62.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nature clin pract Rheumatol. 2006;2:619–26. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 63.Kishimoto T. Interleukin-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 64.Eisen-Vandervelde AL, Waggoner SN, Yao ZQ, Cale EM, Hahn CS, Hahn YS. Hepatitis C virus core selectively suppresses interleukin-12 synthesis in human macrophages by interfering with AP-1 activation. J Biol Chem. 2004;279:43479–86. doi: 10.1074/jbc.M407640200. [DOI] [PubMed] [Google Scholar]
- 65.La Flamme AC, MacDonald AS, Pearce EJ. Role of IL-6 in directing the initial immune response to schistosome eggs. J Immunol. 2000;164:2419–26. doi: 10.4049/jimmunol.164.5.2419. [DOI] [PubMed] [Google Scholar]
- 66.Liu J, Chen M, Wang X. Calcitonin gene-related peptide inhibits lipopolysaccharide-induced interleukin-12 release from mouse peritoneal macrophages, mediated by the cAMP pathway. Immunology. 2000;101:61–7. doi: 10.1046/j.1365-2567.2000.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meeran SM, Punathil T, Katiyar SK. IL-12 Deficiency Exacerbates Inflammatory Responses in UV-Irradiated Skin and Skin Tumors. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lesinski GB, Badgwell B, Zimmerer J, Crespin T, Hu Y, Abood G, Carson WE., 3rd IL-12 pretreatments enhance IFNalpha-induced Janus kinase-STAT signaling and potentiate the antitumor effects of IFNalpha in a murine model of malignant melanoma. J Immunol. 2004;172:7368–76. doi: 10.4049/jimmunol.172.12.7368. [DOI] [PubMed] [Google Scholar]
- 69.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–69. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 70.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–81. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 71.Gu H, Saito K, Klaman LD, Shen J, Fleming T, Wang Y, Pratt JC, Lin G, Lim B, Kinet JP, Neel BG. Essential role for Gab2 in the allergic response. Nature. 2001;412:186–90. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- 72.Dorsey JF, Cunnick JM, Mane SM, Wu J. Regulation of the Erk2-Elk1 signaling pathway and megakaryocytic differentiation of Bcr-Abl(+) K562 leukemic cells by Gab2. Blood. 2002;99:1388–97. doi: 10.1182/blood.v99.4.1388. [DOI] [PubMed] [Google Scholar]
- 73.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–77. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vandervelde S, van Luyn MJ, Rozenbaum MH, Petersen AH, Tio RA, Harmsen MC. Stem cell-related cardiac gene expression early after murine myocardial infarction. Cardiovasc Res. 2007;73:783–93. doi: 10.1016/j.cardiores.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 75.Jeong HJ, Hong SH, Park RK, Shin T, An NH, Kim HM. Hypoxia-induced IL-6 production is associated with activation of MAP kinase, HIF-1 and NFkappaB on HEI-OC1 cells. Hear Res. 2005;207:59–67. doi: 10.1016/j.heares.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Chu K, Jung KH, Kim SJ, Lee ST, Kim J, Park HK, Song EC, Kim SU, Kim M, Lee SK, Roh JK. Transplantation of human neural stem cells protect against ischemia in a preventive mode via hypoxia-inducible factor-1alpha stabilization in the host brain. Brain Res. 2008;1207:182–92. doi: 10.1016/j.brainres.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 77.Han ZB, Ren H, Zhao H, Chi Y, Chen K, Zhou B, Liu YJ, Zhang L, Xu B, Liu B, Yang R, Han ZC. Hypoxia-inducible factor (HIF)-1{alpha} directly enhances the transcriptional activity of stem cell factor (SCF) in response to hypoxia and epidermal growth factor (EGF) Carcinogenesis. 2008 doi: 10.1093/carcin/bgn066. [Epub] [DOI] [PubMed] [Google Scholar]
- 78.Lennartsson J, Jelacic T, Linnekin D, Shivakrupa R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem cells (Dayton, Ohio) 2005;23:16–43. doi: 10.1634/stemcells.2004-0117. [DOI] [PubMed] [Google Scholar]
- 79.Kent D, Copley M, Benz C, Dykstra B, Bowie M, Eaves C. Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin Cancer Res. 2008;14:1926–30. doi: 10.1158/1078-0432.CCR-07-5134. [DOI] [PubMed] [Google Scholar]
- 80.Fiuza UM, Arias AM. Cell and molecular biology of Notch. J Endocrinol. 2007;194:459–74. doi: 10.1677/JOE-07-0242. [DOI] [PubMed] [Google Scholar]
- 81.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–74. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 82.Giudicelli F, Lewis J. The vertebrate segmentation clock. Curr Opin Genet Dev. 2004;14:407–14. doi: 10.1016/j.gde.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 83.Palmeirim I, Henrique D, Ish-Horowicz D, Pourquie O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–48. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- 84.Klein T, Brennan K, Arias AM. An intrinsic dominant negative activity of serrate that is modulated during wing development in Drosophila. Dev Biol. 1997;189:123–34. doi: 10.1006/dbio.1997.8564. [DOI] [PubMed] [Google Scholar]
- 85.Micchelli CA, Rulifson EJ, Blair SS. The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development (Cambridge, England) 1997;124:1485–95. doi: 10.1242/dev.124.8.1485. [DOI] [PubMed] [Google Scholar]
- 86.Ladi E, Nichols JT, Ge W, Miyamoto A, Yao C, Yang LT, Boulter J, Sun YE, Kintner C, Weinmaster G. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J Cell Biol. 2005;170:983–92. doi: 10.1083/jcb.200503113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lowell S, Jones P, Le Roux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- 88.Sakamoto K, Ohara O, Takagi M, Takeda S, Katsube K. Intracellular cell-autonomous association of Notch and its ligands: a novel mechanism of Notch signal modification. Dev Biol. 2002;241:313–26. doi: 10.1006/dbio.2001.0517. [DOI] [PubMed] [Google Scholar]
- 89.Massa S, Balciunaite G, Ceredig R, Rolink AG. Critical role for c-kit (CD117) in T cell lineage commitment and early thymocyte development in vitro. Eur J Immunol. 2006;36:526–32. doi: 10.1002/eji.200535760. [DOI] [PubMed] [Google Scholar]
- 90.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–56. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 91.Hozumi K, Negishi N, Suzuki D, Abe N, Sotomaru Y, Tamaoki N, Mailhos C, Ish-Horowicz D, Habu S, Owen MJ. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5:638–44. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- 92.Carnahan JF, Patel DR, Miller JA. Stem cell factor is a neurotrophic factor for neural crest-derived chick sensory neurons. J Neurosci. 1994;14:1433–40. doi: 10.1523/JNEUROSCI.14-03-01433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin K, Mao XO, Sun Y, Xie L, Greenberg DA. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest. 2002;110:311–9. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Das AV, James J, Zhao X, Rahnenfuhrer J, Ahmad I. Identification of c-Kit receptor as a regulator of adult neural stem cells in the mammalian eye: interactions with Notch signaling. Dev Biol. 2004;273:87–105. doi: 10.1016/j.ydbio.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 95.Borg C, Terme M, Taieb J, Menard C, Flament C, Robert C, Maruyama K, Wakasugi H, Angevin E, Thielemans K, Le Cesne A, Chung-Scott V, Lazar V, Tchou I, Crepineau F, Lemoine F, Bernard J, Fletcher JA, Turhan A, Blay JY, Spatz A, Emile JF, Heinrich MC, Mecheri S, Tursz T, Zitvogel L. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004;114:379–88. doi: 10.1172/JCI21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Druker BJ. STI571 (Gleevec) as a paradigm for cancer therapy. Trends Mol Med. 2002;8:14–8. doi: 10.1016/s1471-4914(02)02305-5. [DOI] [PubMed] [Google Scholar]
- 97.Vredevoe DL, Widawski M, Fonarow GC, Hamilton M, Martinez-Maza O, Gage JR. Interleukin-6 (IL-6) expression and natural killer (NK) cell dysfunction and anergy in heart failure. Am J Cardiol. 2004;93:1007–11. doi: 10.1016/j.amjcard.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 98.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 99.Blazquez AB, Berin MC. Gastrointestinal dendritic cells promote Th2 skewing via OX40L. J Immunol. 2008;180:4441–50. doi: 10.4049/jimmunol.180.7.4441. [DOI] [PubMed] [Google Scholar]
- 100.Houde C, Li Y, Song L, Barton K, Zhang Q, Godwin J, Nand S, Toor A, Alkan S, Smadja NV, Avet-Loiseau H, Lima CS, Miele L, Coignet LJ. Overexpression of the NOTCH ligand JAG2 in malignant plasma cells from multiple myeloma patients and cell lines. Blood. 2004;104:3697–704. doi: 10.1182/blood-2003-12-4114. [DOI] [PubMed] [Google Scholar]
- 101.Takeuchi T, Adachi Y, Ohtsuki Y. Skeletrophin, a novel ubiquitin ligase to the intracellular region of Jagged-2, is aberrantly expressed in multiple myeloma. The Am J Pathol. 2005;166:1817–26. doi: 10.1016/S0002-9440(10)62491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bataille R, Pellat-Deceunynck C, Robillard N, Avet-Loiseau H, Harousseau JL, Moreau P. CD117 (c-kit) is aberrantly expressed in a subset of MGUS and multiple myeloma with unexpectedly good prognosis. Leuk Res. 2008;32:379–82. doi: 10.1016/j.leukres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 103.Montero JC, Lopez-Perez R, San Miguel JF, Pandiella A. Expression of c-Kit isoforms in multiple myeloma: differences in signaling and drug sensitivity. Haematologica. 2008;93:851–9. doi: 10.3324/haematol.12171. [DOI] [PubMed] [Google Scholar]
- 104.Dispenzieri A, Gertz MA, Lacy MQ, Geyer SM, Greipp PR, Rajkumar SV, Kimlinger T, Lust JA, Fonseca R, Allred J, Witzig TE. A phase II trial of imatinib in patients with refractory/relapsed myeloma. Leuk Lymphoma. 2006;47:39–42. doi: 10.1080/10428190500271269. [DOI] [PubMed] [Google Scholar]
- 105.Tsai S, Fero J, Bartelmez S. Mouse Jagged2 is differentially expressed in hematopoietic progenitors and endothelial cells and promotes the survival and proliferation of hematopoietic progenitors by direct cell-to-cell contact. Blood. 2000;96:950–7. [PubMed] [Google Scholar]
- 106.DeHart SL, Heikens MJ, Tsai S. Jagged2 promotes the development of natural killer cells and the establishment of functional natural killer cell lines. Blood. 2005;105:3521–7. doi: 10.1182/blood-2004-11-4237. [DOI] [PubMed] [Google Scholar]
- 107.Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, Weinmaster G, Gridley T. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev. 1998;12:1046–57. doi: 10.1101/gad.12.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kared H, Adle-Biassette H, Fois E, Masson A, Bach JF, Chatenoud L, Schneider E, Zavala F. Jagged2-expressing hematopoietic progenitors promote regulatory T cell expansion in the periphery through notch signaling. Immunity. 2006;25:823–34. doi: 10.1016/j.immuni.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 109.Hoyne GF, Le Roux I, Corsin-Jimenez M, Tan K, Dunne J, Forsyth LM, Dallman MJ, Owen MJ, Ish-Horowicz D, Lamb JR. Serrate1-induced notch signalling regulates the decision between immunity and tolerance made by peripheral CD4(+) T cells. Int Immunol. 2000;12:177–85. doi: 10.1093/intimm/12.2.177. [DOI] [PubMed] [Google Scholar]
- 110.Thoren LA, Liuba K, Bryder D, Nygren JM, Jensen CT, Qian H, Antonchuk J, Jacobsen SE. Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol. 2008;180:2045–53. doi: 10.4049/jimmunol.180.4.2045. [DOI] [PubMed] [Google Scholar]
- 111.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NFkappaB and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1994;91:752–6. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]