Abstract
Myeloid-derived suppressor cells (MDSC) are a heterogeneous group of myeloid cells that play a major role in the regulation of immune responses in many pathological conditions. These cells have a common myeloid origin, relatively immature state, common genetic and biochemical profiles, and, most importantly, the ability to inhibit immune responses. Although initial studies of MDSC were almost exclusively performed in tumor-bearing mice or cancer patients, in recent years, it became clear that MDSC play a critical role in the regulation of different types of inflammation which are not directly associated with cancer. This review discusses the nature of the complex relationship between MDSC and the different populations of CD4+ T cells.
Myeloid-derived suppressor cells (MDSC) play a major role in the regulation of immune responses in cancer and many pathological conditions, associated with chronic inflammation. These cells have a common myeloid origin, relatively immature state, common genetic and biochemical features, and most importantly, the ability to inhibit immune responses. MDSCs consist of two main subsets: polymorphonuclear cells (PMN-MDSCs) and monocytic (M-MDSCs) cells (1, 2). The phenotype of these populations is now well defined in mice and recently, these cells were also defined in cancer patients, as well (3). PMN-MDSC consist of relatively immature and pathologically activated neutrophils (4), whereas M-MDSC - pathologically activated inflammatory monocytes. A small proportion of MDSCs is represented by precursors of myeloid cells, with the ability to form colonies in semi-solid medium. It appears that, at least in cancer, M-MDSC may play a central role in the development of immune suppressive myeloid cells. In tumor site, they differentiate to tumor-associated macrophages (MΨ) with potent immune suppressive activity and, in the periphery, may give rise to PMN-MDSC (5, 6). The MDSC phenotype, mechanisms of expansion and the specific mechanisms, by which MDSC exert their suppressive effects, are described in many reviews (3, 7–10). Initial studies of MDSC were, almost exclusively, performed in tumor-bearing mice or cancer patients. Cancer still remains the main focus of MDSC research. However, in recent years, it became increasingly clear that MDSC play a critical role in the regulation of different types of inflammation, not directly associated with cancer. It also became clear that the interaction of MDSC with different populations of CD4+ T cells is not a one-way street and goes beyond the simple direct immune suppressive activity of MDSC on T cells. These issues will be discussed in this review.
Suppressive activity of MDSC on T cells in pathologic conditions not associated with cancer
Ample evidence favors the important functional role of MDSCs in various pathologic conditions associated with non-cancerous inflammation. The priming of mice, with complete Fruend’s adjuvant, resulted in an expansion of MDSCs. These cells could, subsequently, be stimulated by activated T cells to produce reactive oxygen species (ROS) and nitric oxide (NO) (11). M. bovis bacillus Calmette-Guerin (BCG) vaccination recruited NO producing MDSCs. These cells were unable to kill BCG or the non-pathogenic M. smegmatis, and impaired T cell priming in the draining lymph node. The elimination of MDSCs, by all-trans retinoid acid (ATRA), increased the number of IFN-γ-producing CD4+ T cells, after vaccination with BCG (12). In individuals who received Hepatitis B virus (HBV) vaccine, GM-CSF augmented the preservation of peripheral blood MDSCs, which could contribute to the lack of improved vaccine responses (13). Most chronic infections cause an expansion of M-MDSCs. Oscar Goni et al. found that, during T. cruzi infection, suppression was mediated through IFN-γ-dependent NO secretion by MDSCs (14). In lupus-prone MRL-Fas(lpr) mice, MDSCs had a suppressive effect on CD4+ T-cell proliferation, which was restored by an Arginase 1(Arg1) inhibitor (15). The MyD88-dependent expansion of MDSCs induced T-cell suppression and Th2 polarization in sepsis (16). The administration of cerulean, which induces gallbladder contraction and the release of insulin, to MyD88−/− mice resulted in severe pancreatitis; whereas, this effect was much smaller in MyD88+/+ mice. The number of IL-10-expressing MDSCs, in cerulean treated MyD88−/− mice, was significantly smaller than in the control MyD88+/+ mice, which was associated with a reciprocal increase in the infiltration of CD4+ T cells (17).
In an inflammatory bowel disease (IBD) model, the repeated transfer of antigen-specific T cells led to an increase in the frequency of nitric oxide synthase 2 (nos2) and arg1-expressing MDSCs in spleen and intestine. The co-transfer of MDSCs, with specific CD8+ T cells, into mice ameliorated enterocolitis and suggested a direct immune regulatory effect of MDSCs on the induction of IBD by antigen-specific T cells (18). In IBD, induced by resveratrol, MDSCs also attenuated T cell proliferation and reduced the IFN-γ and GM-CSF production by Lamina propria derived T cells (19).
Multiple sclerosis is a demyelinating disease, associated with an inflammatory immune response in the central nervous system (CNS). In a Theiler’s murine encephalomyelitis virus (TMEV) mouse model of multiple sclerosis, the depletion of M-MDSCs increased the virus-specific CD4+ and CD8+ T cell responses, during the early virus infection, associated with an increased expression of IFN-γ and IL-17 and a decreased expression of IL-10 in the CNS (20). The in vivo transfer of MDSCs ameliorated the experimental autoimmune encephalomyelitis, significantly decreased demyelination, and delayed disease onset through the inhibition of encephalitogenic Th1 and Th17 immune responses (21).
MDSCs were shown to counter pro-inflammatory immune cells in the liver and adipose tissue, during obesity. In obese mice, MDSCs suppressed the proliferation, induced apoptosis of CD8+ T cells and skewed the differentiation of macrophages into insulin-sensitizing, alternatively activated M2 macrophages (22). Lysosomal acid lipase (LAL) cleaves cholesteryl esters and triglycerides to generate free fatty acids and cholesterol in lysosomes. LAL deficiency causes an expansion of MDSCs, the loss of T cells, and an impairment of T cell function (23). MDSCs were essential for the IL-6-mediated protection of liver injury, caused by an anti-CD137 antibody, via inhibition of CD8+ T cells proliferation, and IFN-γ expression (24).
MDSC were implicated in the regulation of immune response, during organ transplantation and graft versus host diseases (GVHD). The data suggested that the expansion of MDSC, together with regulatory T cells (Tregs), may be an important factor in the survival of cardiac allografts (25). The administration of recombinant G-CSF or IL-2, in mice, resulted in the accumulation of MDSCs and Tregs in the peripheral lymphoid organs. This treatment significantly delayed MHC class II disparate allogeneic donor skin rejection (26). GVHD is the significant cause of morbidity and mortality, after allogeneic bone marrow transplantation (BMT). It was shown that, in minor histocompatibility, mismatched BMT is associated with the accumulation of MDSC in blood, which peaked at week 3 and returned to the physiological level at week 12 (27). MDSC, generated in vitro or in vivo, alleviated GVHD in murine allogeneic BMT models (28–30). The addition of functional MDSCs to the donor graft alleviated GVHD; whereas, removal of MDSCs in vivo exacerbated GVHD. MDSC accumulation positively correlated with the severity of GVHD (31).
Recent reports have also implicated MDSC in viral diseases. Patients with chronic Hepatitis C virus (HCV) showed a significant correlation between the MDSC levels, disease progression, and the patients’ response to antiviral therapy. MDSCs suppressed T cell function in an Arg1-dependent manner (32). Human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections induced a population, phenotypically similar to M-MDSC, that expressed higher levels of STAT3 and NOS2, and a suppressed expansion of CD8+ T cells (33). In a large study of HIV-1-seropositive subjects, compared with healthy controls, the presence of M-MDSCs, in peripheral blood, correlated with prognostic HIV-1 disease markers, including the HIV-1 load and CD4+ T cell loss. M-MDSCs, from HIV-1+ subjects, suppressed T cell responses in both HIV-1-specific and antigen-nonspecific manners (34). In a recent study, infections, with an acute Armstrong (ARM) or a chronic Clone 13 (C13) strain of the lymphocytic choriomeningitis virus, led to two distinct phases of innate immune response. Seven days after infection, there was an increase in the immune suppressive M-MDSC and PMN-MDSC in lymphoid organs and blood. This expansion was sustained only in the chronic C13 infection; whereas, it occurred only transiently in acute ARM infection (35).
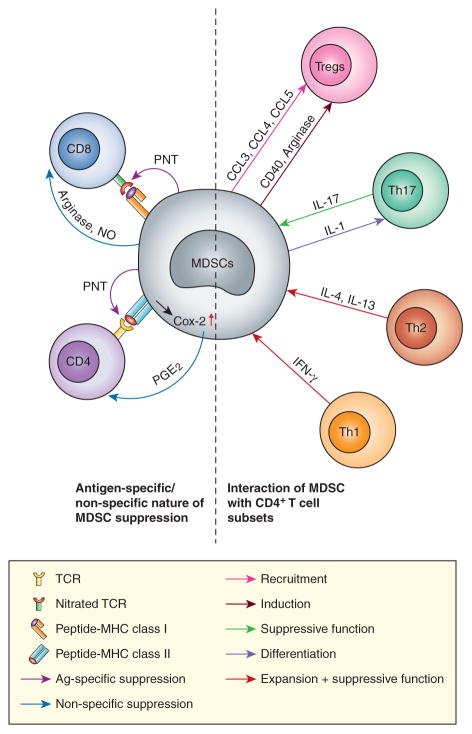
Thus, the role of MDSC, as an important negative regulator of immune responses, is extended beyond cancer and observed in many pathological conditions. Although the immune suppressive activity of MDSC is the most prominent feature of these cells, ample evidence points to their role in the regulation of different populations of CD4+ T cells. Importantly, it appears that T cells can, in turn, regulate MDSC expansion and activity as well (Figure). Herein, we discuss the interaction between MDSC and specific subsets of CD4+ T cells.
Figure. Complex interaction between MDSC and different populations of T cells.
See description in the text.
Interaction between MDSC and Th1/Th2 CD4+ T cells
In an early study, Terabe et al. demonstrated that MDSC can be activated to produce TGFβ, in response to IL-13 in tumor-bearing mice (36). More recently, in a mammary adenocarcinoma model, IL-4-expressing CD4+ Th2 cells promoted expansion of MDSCs and tumor associated macrophages (TAM). This enhanced pulmonary metastasis, through activation of the epidermal growth factor receptor signaling in malignant mammary epithelial cells (37). Immune-mediated liver injury in hepatitis is caused by activated IFN-γ producing Th1 cells. The accumulation of Th1 cells in liver was associated with the accumulation of MDSCs and suppression of T cell proliferation. TGFβ1 deficient mice acutely develop liver inflammation caused by Th1 cells. The rapid accumulation of MDSC in TGFβ1 deficient liver was abrogated when mice were either depleted of CD4+ T cells or rendered unable to produce IFN-γ, demonstrating that Th1 cells can induce MDSCs accumulation (38). In humans, lipopolysaccharide (LPS) has been associated with protection from allergic diseases such as asthma. However, in mouse models of allergic asthma, a low dose of LPS promoted Th2 responses and allergic disease; whereas, a high dose has been associated with suppression of allergic airway inflammation. The adoptive transfer of LPS-induced CD11b+Gr1intF4/80+ cells suppressed allergen-induced airway inflammation, suggesting that these cells may have regulatory functions in asthma. These cells were found to blunt the ability of the lung dendritic cells to upregulate GATA-3 or to promote STAT5 activation in primed Th2 cells (39). TLR7 was shown to modulate the accumulation of MDSCs during influenza A virus infections in mice. A lack of TLR7 signaling led to a Th2-biased response and an accumulation of MDSC in the lungs (40). Trauma induced STAT6 dependent MDSC accumulation in spleens. This process was dependent on Th2 type cytokine release (41). Taken together, these data suggest that in contrast to Th1 cells, Th2 cells are directly involved in the expansion and activation of MDSC, apparently via STAT6 (Figure). The exact role of the specific cytokines (IL-4, IL-13 or others) and the molecular pathways, responsible for this phenomenon, remains to be elucidated.
Interaction between MDSC and regulatory T cells
The interaction between MDSC and Tregs in cancer is well documented. In an initial study, Huang et al showed that Gr-1+CD115+F4/80+ MDSCs induced the expansion of Foxp3+CD25+ Tregs in vitro (42). In addition, the adoptive transfer of CD115+Gr-1+ MDSCs induced IL-10 and IFN-γ dependent Foxp3+CD25+ Tregs in vivo and suppressed the antitumor response in a mouse colon carcinoma model (42). Another study, from the same group, demonstrated that CD40 expression by MDSCs was required for MDSCs-mediated Treg induction and tolerance (43). The Lewis lung cancer model showed increased MDSCs and Foxp3+ Tregs accumulation in the tumor tissue. After in vivo depletion of MDSCs, the number of tumor infiltrating Tregs was significantly decreased and this reduced the tumor growth and prolonged survival of tumor-bearing mice (44). The Arg1-dependent induction of Tregs, by MDSCs, was found in a B cell lymphoma model (45). MDSCs may attract Tregs via various chemokines. Tumor-infiltrating M-MDSCs had significantly higher levels of CCL3, CCL4, and CCL5, as compared to the other subsets of MDSCs in lymphoma-bearing mice. Tregs, from CCR5 knockout mice, had a diminished ability to migrate toward chemokines secreted by M-MDSCs (46). It was suggested that infiltration of tumors, by Tregs, could be coordinated by mast cell and MDSCs. Study showed that mast cells could mobilize MDSCs to tumor and induce the production of IL-17 by MDSCs. IL-17 increased the level of CCL18 and CCL22 in tumor microenvironment, which attracted Tregs to tumor (47). Conversely, Tregs depletion down-regulated the production of IL-10 and the expression of PD-L1 in MDSCs, from melanoma-bearing mice, and promoted the MDSCs conversion into a less immunosuppressive phenotype (48). The depletion of CD4+CD25+ Tregs abrogated the suppression activity of CD80+Gr-1+ MDSCs from mice bearing ovarian carcinoma (49).
There are some data suggesting interaction between MDSC and Tregs in cancer patients. CD14+ HLA-DR−/low MDSCs, from hepatocellular carcinoma patients, induce functional CD4+CD25+Foxp3+ Tregs when co-cultured with autologous T cells. The induction of Tregs was cell contact dependent and was abrogated when MDSCs and T cells were separated (50).
There are now some indications that MDSC and Tregs can interact in conditions other than cancer. M-MDSCs, accumulated in lungs of mice with evolving experimental allergic airway inflammation, were able to down-regulate T-cell activation, recruit Tregs, and dramatically decrease antigen-induced airway hyper-responsiveness (51). The MDSCs-mediated expansion of Tregs and T-cell suppression required MHC-dependent antigen presentation in a murine type 1 diabetes model, in which the animals received CD4-HA-TCR transgenic T cells. A significant reduction in the incidence of diabetes was observed in recipients receiving MDSCs plus influenza hemagglutinin (HA), but not ovalbumin (OVA) peptide. The protective effects of MDSCs required an induction of anergy in autoreactive T cells and the development of Tregs (52). The administration of MDSCs in mice, with pancreatic islet transplants, was associated with attenuation of CD8+ T cells in grafts and a marked expansion of Tregs in a B7-H1 dependent manner (53).
Interaction between MDSC and Th17 cells
The exact contribution of Th17 cells to tumor progression is not clear. Th17 were implicated in both tumorigenesis and in the eradication of established tumors. For instance, Th17 cells elicited neovascularization and promoted angiogenesis and tumor growth (54). Increased Th17 cell density, within the tumors in patients with hepatocellular carcinoma, correlated with microvessel density and poor prognosis (55). In contrast, it was reported that tumor-specific Th17 cells could mediate the destruction of advanced B16 melanoma (56). It appears that Th17 cells may play opposite roles depending on the stage of cancer. It has been shown that MDSCs could induce Th17 cell polarization from naïve CD4+ T cells. The generation of Th17 cells, by MDSCs, was independent on MDSCs-T cell contact, but dependent on the cytokines secreted by MDSCs (57). Novitskiy et al found that the incubation of MDSCs, with IL-17, increased the suppressive activity of MDSCs through the up-regulation of Arg1, indoleamine 2,3-dioxygenase (IDO), and cyclooxygenase (COX)-2 (58). Consistent with that report, another study showed that MDSCs, from IL-17R−/− tumor-bearing mice, expressed lower levels of Arg1, matrix metalloproteinases 9 (MMP9), and S100A8/A9, than from wild type tumor-bearing mice, and did not have an inhibitory effect on T cell proliferation (59). One study demonstrated rather different results. MDSC reduced Th17 responses in an HLA-G+ xenotumor model. HLA-G induced the expansion of MDSC and formation of the Th2-type cytokine environment rather than Th1 or Th17. However, no data were provided indicating whether those MDSC were directly involved in the Th17 cytokine profile in the HLA-G+ tumor model (60).
MDSCs could drive a Th17 response that consequently contributes to the pathogenesis of experimental autoimmune encephalomyelitis (EAE). MDSCs, from mice with EAE, promoted Th17 cell differentiation under Th17-polarizing conditions. Th17 cell differentiation was mediated by IL-1 from MDSCs and required an IL-1 receptor on T cells. The depletion of MDSCs, by gemcitabine, reduced the frequency of Th17 cells in vivo and ameliorated EAE (61). Flagellin-induced MDSCs efficiently suppressed polyclonal T cell proliferation in a dose-dependent manner, and substantially dampened released IL-17 protein by Th17 cells (62). However, in a clinical study, a negative correlation between increased circulating of MDSCs and Th17 cells was found in the peripheral blood of patients with rheumatoid arthritis (RA). Compared with healthy controls (HC), both the prevalence of circulating MDSCs and plasma Arg1 increased significantly in RA patients. However, no significant difference was observed in the mRNA level of NOS2 between RA patients and HC. The frequency of Th17 cells in RA patients was significantly higher than in HC, but correlated negatively with the frequency of MDSCs and plasma Arg1 (63).
Antigen-specific vs. non-specific suppression of T-cell responses by MDSC
The complex nature of interaction between MDSC and T cells contributed to the controversy associated with the role of antigens in the MDSC mediated suppression of T-cell responses. The fact that MDSC can inhibit different types of T-cell responses is widely accepted. It was demonstrated that MDSC can inhibit antigen-specific CD8+ or CD4+ T-cell responses (64–66). The suppression of MDSC was mediated by cell-to-cell contact between MDSC and T cells (65). Peroxynitrite (PNT) production by MDSCs, during direct contact with T cells, resulted in the nitration of the T-cell receptor and CD8 molecules, which induced conformational changes in these molecules and a loss of binding of the T cells. Ultimately, T cells are rendered non-responsive to antigen-specific stimulation (67). PNT scavenger completely eliminated the MDSC-induced T-cell tolerance, suggesting that ROS, and peroxynitrite in particular, could be responsible for MDSC mediated CD8+ T-cell tolerance. MDSCs are also reported to inhibit non-specific immune responses. MDSCs, from BM or spleen from tumor-bearing mice, significantly suppressed the CD3/CD28-induced T cell proliferation (68–70). Human prostatic adenocarcinomas were reported to be infiltrated by terminally differentiated unresponsive cytotoxic T lymphocytes (71). A higher presence of nitrotyrosine, in prostatic tumor-infiltrating lymphocytes, suggested a local production of PNT. Thus, local PNT production could represent one of the important mechanisms by which tumor escape immune response.
The antigen-specific nature of MDSC mediated immune suppression could be regulated by several factors: the type of MDSC involved; the local microenvironment; the state of T cell activation, and the retrograde signaling provided to MDSC from T cells.
Type of MDSC may influence the nature of immune suppression
There is now enough evidence demonstrating that PMN-MDSC and M-MDSC use different mechanisms of immune suppression (72). The immune suppressive activity of M-MDSC is largely dependent on a high level of production of NO and different immune suppressive cytokines and intermediates. There is a large body of literature indicating that these cells exert their suppressive activity in antigen-independent manner (73–76). In contrast, PMN-MDSC are largely dependent on ROS, which requires closer and more prolonged cell-cell contact, which is be better provided during antigen-specific interaction (1, 2, 77, 78). This may explain the fact that PMN-MDSCs, in contrast to M-MDSCs, were implicated in antigen-specific T-cell suppression. However, the type of MDSCs cannot fully explain the nature of immune suppression since several reports demonstrated that PMN-MDSC could also inhibit the antigen non-specific immune responses (79–81).
Local microenvironment may define the nature of immune suppression by MDSC
Several recent reports have demonstrated that MDSCs may exhibit different activities in peripheral lymphoid organs and in tumor tissues. We found that splenic MDSCs suppress only antigen-specific T cell response; whereas, tumor MDSCs exerted a profound suppressive effect on both antigen-specific and non-specific T cell responses. Splenic MDSCs displayed a significantly higher level of ROS than tumor MDSCs; whereas, tumor MDSCs had much higher levels of NO and Arg1 than splenic MDSCs (6). A similar phenomenon exists in the peripheral blood and tumor MDSCs from patients with head and neck cancer. The data suggested that the tumor microenvironment converted MDSCs into non-specific suppressor cells by up-regulating Arg1 activity or NO production via HIF-1α (6). Recently Lesokhin et al also demonstrated that CD11b+ MDSC (mainly CCR2+CD11b+ M-MDSCs) from tumor tissues, but not from the spleens, were able to suppress the antigen non-specific proliferation of CD8+ T cells, induced by CD3/CD28 antibodies in mouse melanoma model (75).
Activated T cells could be more sensitive to antigen-specific suppression
It was suggested that the state of T-cell activation may determine the antigen-specific nature of immune suppression mediated by MDSC (7). In most of the studies that investigated the nature of CD8+ T-cell tolerance induced by MDSC, T cells were activated by specific peptides. Therefore, this hypothesis needs to be formally tested. However, in recent study, the non-specific activation of CD4+ T cells did not affect the antigen-specific suppression of these cells by MDSC (82).
T cells may change the nature of MDSC-mediated immune suppression
CD8+ T-cell tolerance, caused by MDSC was mediated via MHC class I (83). MDSC could induce antigen-specific CD4+ T-cell tolerance via MHC class II (82). Since, in most mouse tumor models, expression of MHC class II on MDSC was low (82), this mechanism, apparently, is operational only in few experimental systems. Similar variability in MHC class II expression was described in some human studies (84–87). This may explain some of the contradictory data regarding the effect of MDSC on CD4+ T-cell function. Antigen-specific CD4+ T cells (but not CD8+ T cells) could dramatically enhance the immune suppressive activity of MDSC, by converting them into powerful non-specific suppressor cells. This effect was mediated through cross-linking of MHC class II on MDSC with subsequent up-regulation of Cox-2 expression and prostaglandin E2 production by MDSC (82), which were previously implicated in MDSC mediated immune suppression (88–90). We suggest that activated antigen-specific CD4+ T cells may enhance the immune suppressive activity of MDSC and convert these cells into non-specific suppressors, a mechanism that normally might serve as a negative feedback loop to control hyperactivated immune responses (Figure). In cancer, this mechanism is hijacked by tumor cells and contributes to heightened immune suppression associated with tumor progression.
Conclusions
Recent years have brought understanding that MDSC may play a critical role in regulation of immune responses, not only in cancer but also in many other pathologic conditions. It is clear that the interaction of MDSC with T cells is not a one-way street, where MDSC inhibit T cell proliferation, cytokine production or tumor cell killing. T cells can affect MDSC function in a major way by promoting their expansion and suppressive activity. Many questions regarding the molecular mechanisms of the complex interaction between MDSC and T cells have remained unanswered. Understanding of the nature of this interaction may help to develop more precise targeted therapy for many diseases.
Acknowledgments
This work was supported by NIH grants CA084488 and CA100062 to DIG and NIH 1P30HL101265-01 to SN.
References
- 1.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T-cell suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 2.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youn JI, Collazo M, Shalova I, Biswas S, Gabrilovich D. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, Sarnaik A, Horna P, Sotomayor E, Gabrilovich DI. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14:211–220. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solito S, Bronte V, Mandruzzato S. Antigen specificity of immune suppression by myeloid-derived suppressor cells. J Leukoc Biol. 2010 doi: 10.1189/jlb.0111021. [DOI] [PubMed] [Google Scholar]
- 8.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raber P, Ochoa AC, Rodriguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest. 2012;41:614–634. doi: 10.3109/08820139.2012.680634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monu NR, Frey AB. Myeloid-derived suppressor cells and anti-tumor T cells: a complex relationship. Immunol Invest. 2012;41:595–613. doi: 10.3109/08820139.2012.673191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietlin TA, Hofman FM, Lund BT, Gilmore W, Stohlman SA, van der Veen RC. Mycobacteria-induced Gr-1+ subsets from distinct myeloid lineages have opposite effects on T cell expansion. J Leukoc Biol. 2007;81:1205–1212. doi: 10.1189/jlb.1006640. [DOI] [PubMed] [Google Scholar]
- 12.Martino A, Badell E, Abadie V, Balloy V, Chignard M, Mistou MY, Combadiere B, Combadiere C, Winter N. Mycobacterium bovis bacillus Calmette-Guerin vaccination mobilizes innate myeloid-derived suppressor cells restraining in vivo T cell priming via IL-1R-dependent nitric oxide production. J Immunol. 2010;184:2038–2047. doi: 10.4049/jimmunol.0903348. [DOI] [PubMed] [Google Scholar]
- 13.Anthony DD, Umbleja T, Aberg JA, Kang M, Medvik K, Lederman MM, Peters MG, Koziel MJ, Overton ET. Lower peripheral blood CD14+ monocyte frequency and higher CD34+ progenitor cell frequency are associated with HBV vaccine induced response in HIV infected individuals. Vaccine. 2011;29:3558–3563. doi: 10.1016/j.vaccine.2011.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goni O, Alcaide P, Fresno M. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+) immature myeloid suppressor cells. Int Immunol. 2002;14:1125–1134. doi: 10.1093/intimm/dxf076. [DOI] [PubMed] [Google Scholar]
- 15.Iwata Y, Furuichi K, Kitagawa K, Hara A, Okumura T, Kokubo S, Shimizu K, Sakai N, Sagara A, Kurokawa Y, Ueha S, Matsushima K, Kaneko S, Wada T. Involvement of CD11b+ GR-1 low cells in autoimmune disorder in MRL-Fas lpr mouse. Clin Exp Nephrol. 2010;14:411–417. doi: 10.1007/s10157-010-0309-9. [DOI] [PubMed] [Google Scholar]
- 16.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koike Y, Kanai T, Saeki K, Nakamura Y, Nakano M, Mikami Y, Yamagishi Y, Nakamoto N, Ebinuma H, Hibi T. MyD88-dependent interleukin-10 production from regulatory CD11b(+)Gr-1(high) cells suppresses development of acute cerulein pancreatitis in mice. Immunol Lett. 2012;148:172–177. doi: 10.1016/j.imlet.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, Greten TF. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. 881 e871–875. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Singh UP, Singh NP, Singh B, Hofseth LJ, Taub DD, Price RL, Nagarkatti M, Nagarkatti PS. Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(−/−) mice. Brain Behav Immun. 2012;26:72–82. doi: 10.1016/j.bbi.2011.07.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowen JL, Olson JK. Innate immune CD11b+Gr-1+ cells, suppressor cells, affect the immune response during Theiler’s virus-induced demyelinating disease. J Immunol. 2009;183:6971–6980. doi: 10.4049/jimmunol.0902193. [DOI] [PubMed] [Google Scholar]
- 21.Ioannou M, Alissafi T, Boon L, Boumpas D, Verginis P. In Vivo Ablation of Plasmacytoid Dendritic Cells Inhibits Autoimmunity through Expansion of Myeloid-Derived Suppressor Cells. J Immunol. 2013;190:2631–2640. doi: 10.4049/jimmunol.1201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286:23591–23599. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu P, Yan C, Blum JS, Kapur R, Du H. Myeloid-specific expression of human lysosomal acid lipase corrects malformation and malfunction of myeloid-derived suppressor cells in lal−/− mice. J Immunol. 2011;187:3854–3866. doi: 10.4049/jimmunol.1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng L, Wang J, Li X, Xing Q, Du P, Su L, Wang S. Interleukin-6 induces Gr-1+CD11b+ myeloid cells to suppress CD8+ T cell-mediated liver injury in mice. PLoS One. 2011;6:e17631. doi: 10.1371/journal.pone.0017631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Stolz DB, Zheng XX, Demetris AJ, Liew FY, Wood KJ, Thomson AW. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol. 2011;187:4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adeegbe D, Serafini P, Bronte V, Zoso A, Ricordi C, Inverardi L. In vivo induction of myeloid suppressor cells and CD4(+)Foxp3(+) T regulatory cells prolongs skin allograft survival in mice. Cell transplantation. 2011;20:941–954. doi: 10.3727/096368910X540621. [DOI] [PubMed] [Google Scholar]
- 27.Billiau AD, Fevery S, Rutgeerts O, Landuyt W, Waer M. Transient expansion of Mac1+Ly6-G+Ly6-C+ early myeloid cells with suppressor activity in spleens of murine radiation marrow chimeras: possible implications for the graft-versus-host and graft-versus-leukemia reactivity of donor lymphocyte infusions. Blood. 2003;102:740–748. doi: 10.1182/blood-2002-06-1833. [DOI] [PubMed] [Google Scholar]
- 28.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, Tolar J, Ochoa AC, Blazar BR. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joo YD, Lee SM, Lee SW, Lee WS, Lee SM, Park JK, Choi IW, Park SG, Choi I, Seo SK. Granulocyte colony-stimulating factor-induced immature myeloid cells inhibit acute graft-versus-host disease lethality through an indoleamine dioxygenase-independent mechanism. Immunology. 2009;128:e632–640. doi: 10.1111/j.1365-2567.2009.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Z, French DL, Ma G, Eisenstein S, Chen Y, Divino CM, Keller G, Chen SH, Pan PY. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010;28:620–632. doi: 10.1002/stem.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Yu Y, Haarberg K, Fu J, Kaosaard K, Nagaraj S, Anasetti C, Gabrilovich D, Yu XZ. Dynamic Change and Impact of Myeloid Derived Suppressor Cells in Allogeneic Bone Marrow Transplantation in Mice. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai W, Qin A, Guo P, Yan D, Hu F, Yang Q, Xu M, Fu Y, Zhou J, Tang X. Clinical Significance and Functional Studies of Myeloid-Derived Suppressor Cells in Chronic Hepatitis C Patients. J Clin Immunol. 2013 doi: 10.1007/s10875-012-9861-2. in press. [DOI] [PubMed] [Google Scholar]
- 33.Gama L, Shirk EN, Russell JN, Carvalho KI, Li M, Queen SE, Kalil J, Zink MC, Clements JE, Kallas EG. Expansion of a subset of CD14highCD16negCCR2low/neg monocytes functionally similar to myeloid-derived suppressor cells during SIV and HIV infection. Journal of leukocyte biology. 2012;91:803–816. doi: 10.1189/jlb.1111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, Liu Y, Yan D, Hu F, Guo P, Chen X, Chen L, Zhang H, Tang X, Zhou J. Expansion of Monocytic Myeloid-Derived Suppressor Cells Dampens T Cell Function in HIV-1-Seropositive Individuals. J Virol. 2013;87:1477–1490. doi: 10.1128/JVI.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norris BA, Uebelhoer LS, Nakaya HI, Price AA, Grakoui A, Pulendran B. Chronic but Not Acute Virus Infection Induces Sustained Expansion of Myeloid Suppressor Cell Numbers that Inhibit Viral-Specific T Cell Immunity. Immunity. 2013;38:309–321. doi: 10.1016/j.immuni.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cripps JG, Wang J, Maria A, Blumenthal I, Gorham JD. Type 1 T helper cells induce the accumulation of myeloid-derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology. 2010;52:1350–1359. doi: 10.1002/hep.23841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora M, Poe SL, Ray A, Ray P. LPS-induced CD11b+Gr1(int)F4/80+ regulatory myeloid cells suppress allergen-induced airway inflammation. Int Immunopharmacol. 2011;11:827–832. doi: 10.1016/j.intimp.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeisy-Scott V, Davis WG, Patel JR, Bowzard JB, Shieh WJ, Zaki SR, Katz JM, Sambhara S. Increased MDSC accumulation and Th2 biased response to influenza A virus infection in the absence of TLR7 in mice. PloS one. 2011;6:e25242. doi: 10.1371/journal.pone.0025242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munera V, Popovic PJ, Bryk J, Pribis J, Caba D, Matta BM, Zenati M, Ochoa JB. Stat 6-dependent induction of myeloid derived suppressor cells after physical injury regulates nitric oxide response to endotoxin. Ann Surg. 2010;251:120–126. doi: 10.1097/SLA.0b013e3181bfda1c. [DOI] [PubMed] [Google Scholar]
- 42.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer research. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 43.Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, Divino CM, Chen SH. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer research. 2010;70:99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Liu Q, Zhang M, Yu Y, Liu X, Cao X. Fas signal promotes lung cancer growth by recruiting myeloid-derived suppressor cells via cancer cell-derived PGE2. J Immunol. 2009;182:3801–3808. doi: 10.4049/jimmunol.0801548. [DOI] [PubMed] [Google Scholar]
- 45.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer research. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189:5602–5611. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 47.Yang Z, Zhang B, Li D, Lv M, Huang C, Shen GX, Huang B. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS One. 2010;5:e8922. doi: 10.1371/journal.pone.0008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T cells stimulate B7-H1 expression in myeloid-derived suppressor cells in ret melanomas. J Invest Dermatol. 2012;132:1239–1246. doi: 10.1038/jid.2011.416. [DOI] [PubMed] [Google Scholar]
- 49.Yang R, Cai Z, Zhang Y, HtYutzy W, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer research. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 50.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 51.Deshane J, Zmijewski JW, Luther R, Gaggar A, Deshane R, Lai JF, Xu X, Spell M, Estell K, Weaver CT, Abraham E, Schwiebert LM, Chaplin DD. Free radical-producing myeloid-derived regulatory cells: potent activators and suppressors of lung inflammation and airway hyperresponsiveness. Mucosal immunology. 2011;4:503–518. doi: 10.1038/mi.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin B, Ma G, Yen CY, Zhou Z, Wang GX, Divino CM, Casares S, Chen SH, Yang WC, Pan PY. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol. 2010;185:5828–5834. doi: 10.4049/jimmunol.0903636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chou HS, Hsieh CC, Charles R, Wang L, Wagner T, Fung JJ, Qian S, Lu LL. Myeloid-derived suppressor cells protect islet transplants by B7-H1 mediated enhancement of T regulatory cells. Transplantation. 2012;93:272–282. doi: 10.1097/TP.0b013e31823ffd39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 55.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 56.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatterjee S, Das S, Chakraborty P, Manna A, Chatterjee M, Choudhuri SK. Myeloid derived suppressor cells (MDSCs) can induce the generation of Th17 response from naive CD4(+) T cells. Immunobiology. 2012 doi: 10.1016/j.imbio.2012.08.271. [DOI] [PubMed] [Google Scholar]
- 58.Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M, Wu H, Shyr Y, Moses HL. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011;1:430–441. doi: 10.1158/2159-8290.CD-11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agaugue S, Carosella ED, Rouas-Freiss N. Role of HLA-G in tumor escape through expansion of myeloid-derived suppressor cells and cytokinic balance in favor of Th2 versus Th1/Th17. Blood. 2011;117:7021–7031. doi: 10.1182/blood-2010-07-294389. [DOI] [PubMed] [Google Scholar]
- 61.Yi H, Guo C, Yu X, Zuo D, Wang XY. Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. 2012;189:4295–4304. doi: 10.4049/jimmunol.1200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rieber N, Brand A, Hector A, Graepler-Mainka U, Ost M, Schafer I, Wecker I, Neri D, Wirth A, Mays L, Zundel S, Fuchs J, Handgretinger R, Stern M, Hogardt M, Doring G, Riethmuller J, Kormann M, Hartl D. Flagellin Induces Myeloid-Derived Suppressor Cells: Implications for Pseudomonas aeruginosa Infection in Cystic Fibrosis Lung Disease. J Immunol. 2013;190:1276–1284. doi: 10.4049/jimmunol.1202144. [DOI] [PubMed] [Google Scholar]
- 63.Jiao Z, Hua S, Wang W, Wang H, Gao J, Wang X. Increased circulating myeloid-derived suppressor cells correlated negatively with Th17 cells in patients with rheumatoid arthritis. Scandinavian journal of rheumatology. 2013;42:85–90. doi: 10.3109/03009742.2012.716450. [DOI] [PubMed] [Google Scholar]
- 64.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 65.Gabrilovich DI, Velders M, Sotomayor E, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 66.Sinha P, V, Clements K, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–11751. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 67.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kusmartsev S, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–785. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181:3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 70.Connolly MK, Mallen-St Clair J, Bedrosian AS, Malhotra A, Vera V, Ibrahim J, Henning J, Pachter HL, Bar-Sagi D, Frey AB, Miller G. Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor. J Leukoc Biol. 2010;87:713–725. doi: 10.1189/jlb.0909607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bronte V, Casic T, Gri G, Gallana K, Borsellino G, Marrigo I, Battistini L, Iafrate M, Prayer-Galletti U, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nausch N, I, Galani E, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cuervo H, Guerrero NA, Carbajosa S, Beschin A, De Baetselier P, Girones N, Fresno M. Myeloid-derived suppressor cells infiltrate the heart in acute Trypanosoma cruzi infection. J Immunol. 2011;187:2656–2665. doi: 10.4049/jimmunol.1002928. [DOI] [PubMed] [Google Scholar]
- 75.Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN, Merghoub T, Wolchok JD. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–886. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, Liu Y, Yan D, Hu F, Guo P, Chen X, Chen L, Zhang H, Tang X, Zhou J. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol. 2013;87:1477–1490. doi: 10.1128/JVI.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi J, Suh B, Ahn YO, Kim TM, Lee JO, Lee SH, Heo DS. CD15+/CD16low human granulocytes from terminal cancer patients: granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour Biol. 2012;33:121–129. doi: 10.1007/s13277-011-0254-6. [DOI] [PubMed] [Google Scholar]
- 79.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 80.Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591–599. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ioannou M, Alissafi T, Lazaridis I, Deraos G, Matsoukas J, Gravanis A, Mastorodemos V, Plaitakis A, Sharpe A, Boumpas D, Verginis P. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol. 2012;188:1136–1146. doi: 10.4049/jimmunol.1101816. [DOI] [PubMed] [Google Scholar]
- 82.Nagaraj S, Nelson A, Youn JI, Cheng P, Quiceno D, Gabrilovich DI. Antigen-specific CD4(+) T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling. Cancer Research. 2012;72:928–938. doi: 10.1158/0008-5472.CAN-11-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber D, Schneck J, Gabrilovich D. Altered recognition of antigen is a novel mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 85.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 86.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, Garrett-Mayer E, Montero AJ, Bronte V, Mandruzzato S. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 88.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, Grizzle WE, Mobley J, Zhang HG. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Donkor MK, Lahue E, Hoke TA, Shafer LR, Coskun U, Solheim JC, Gulen D, Bishay J, Talmadge JE. Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int Immunopharmacol. 2009;9:937–948. doi: 10.1016/j.intimp.2009.03.021. [DOI] [PubMed] [Google Scholar]