Abstract
Serum levels of the tumor marker CA19-9 have been reported to be elevated in patients with hepatocellular carcinoma (HCC), but its clinicopathologic significance is still unknown. A cohort of 304 patients undergoing surgical resection for HCC and having preoperative CA19-9 data was enrolled in this study. Serum CA19-9 levels were correlated with clinicopathologic factors. Univariate and multivariate analyses were performed to determine the predictors of patient survival. On receiver operating characteristic curve analysis, the cut off value of CA19-9 was determined to be 27 U/mL. One hundred and six patients had preoperative CA19-9 values >27 U/mL. High serum CA19-9 levels did not correlate with patient age, sex, viral status, α-fetoprotein level, tumor size, tumor grade, tumor stage, multiplicity, and vascular invasion. Patients with elevated preoperative CA19-9 levels had lower 10-year survival than those without CA19-9 elevation. Multivariate analysis revealed that CA19-9 level, tumor grade, and tumor size are independent prognostic factors for long-term survival. In conclusion, a preoperative CA19-9 value >27 U/mL is associated with poor prognosis after resection for HCC.
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third most common cause of cancer deaths globally. It is one of the most common fatal malignancies in Taiwan and many other countries in Asia and Africa [1]. The major risk factors are hepatitis B and hepatitis C infections, cirrhosis of any etiology, and aflatoxin exposure [2]. Despite improved treatment, most patients with HCC die soon after diagnosis because most of them are discovered at late stage, and no effective systemic chemotherapy is available for surgically unresectable tumors. Molecular approaches have revealed the involvement of p53 and β-catenin mutations in hepatocarcinogenesis [3, 4]. The mutations account for approximately 50% of HCCs. Somatic mutations of other known oncogenes and tumor suppressor genes in HCC are rare. Hence, the molecular mechanisms of HCC remain largely unclear.
CA19-9, also known as sialyl Lewis-a (sLea), is a tumor-associated antigen originally isolated from a human colorectal cancer cell line by Koprowski et al. [5]. It is expressed on the surface of cancer cells as a glycolipid and as an O-linked glycoprotein [6]. CA19-9 is now used as a tumor marker for patients with gastrointestinal cancers, including colorectal, gastric, biliary, and pancreatic cancers [7–10]. Serum level of CA19-9 is elevated in about 75% of cholangiocarcinoma patients [10]. Serum CA19-9 level is also frequently elevated in patients with combined HCC-cholangiocarcinoma, which has two distinct HCC and cholangiocarcinoma components that coexist within a tumor nodule [11]. CA19-9 serum level is also reported to be elevated in a small proportion of HCC patients, and expression of CA19-9 can be detected in tumor cells by immunohistochemistry, but the clinicopathologic significance of its expression is still unknown [12–14].
In this study, we retrospectively analyzed the serum levels of CA19-9 in patients with HCC and studied the clinicopathologic significance and prognostic implication of elevated CA19-9 in HCC patients.
2. Patients and Methods
2.1. Patients and Samples
From 2000 to 2008, 2182 patients underwent surgical resection for HCC at National Taiwan University Hospital. Of them, 304 patients had preoperative CA19-9 values and were enrolled in this study. The study was conducted according to the regulation of the ethics committee of National Taiwan University Hospital, and the data were analyzed in a blinded manner. After surgery, all patients received laboratory examinations such as serum α-fetoprotein (AFP) at 1- to 6-month intervals and ultrasonography of liver at 3–12 month intervals. 94 patients died during the follow-up periods. The median follow-up months of the survivors were 61 months (range: 1 to 132 months).
2.2. Histology Study and Tumor Staging
Surgically resected specimens were formalin fixed and paraffin embedded. Histologic sections cut at 5-μm thickness were stained with hematoxylin and eosin and reviewed by one of the authors (Y.-M. Jeng) to determine the tumor grade and stage and exclude mixed hepatocellular and cholangiocarcinoma. The tumor grade was based on the criteria proposed by Edmonson and Steiner [15]. The tumors were staged according to American Joint Committee on Cancer (AJCC) system [16].
2.3. CA19-9 Analysis
All serum CA19-9 values were measured using a radioimmunoassay kit manufactured by Abbott Laboratories (Chicago, IL, USA). The recommended upper limit of the normal range for CA19-9 is 37 U/mL. The measurement of CA19-9 was performed within 3 months before operation. If multiple values were available, the maximal values before operation were used for statistical analysis.
2.4. Statistical Analysis
Data analyses were carried out using MedCalc statistical software (version 11.4.2.0; MedCalc, Mariakerke, Belgium). A receiver operating characteristic (ROC) curve was constructed to estimate the optimal cut off value of preoperative CA19-9 as the predictor for patient death within three years after operation. Correlation between CA19-9 serum levels and clinicopathologic parameters was evaluated by using the χ 2 test. Survival rates were calculated by using the Kaplan-Meier method, and difference in survival curves was analyzed by using the log-rank test. The variables with P < 0.05 by univariate analysis were subjected to multivariate logistic regression analysis. Multivariate analysis of time to death was analyzed by Cox's proportional hazards models. Two-tailed P < 0.05 was considered statistically significant.
3. Results
3.1. Clinical Features
The patients included 240 man and 64 women with a mean age of 57.7 years (range 24–89 years). Serum hepatitis B surface antigen (HBsAg) was detected in 183 cases and anti-HCV antibody in 85, including 12 positive for both. The overall 1-, 3-, and 5-year survival rates were 86.5%, 74.7%, and 68.5%, respectively. The age, sex, virus status, and stage distribution of the study group are similar to those without CA19-9 values (data not shown).
3.2. Serum CA19-9 Levels in HCC Patients
Preoperative serum levels of CA19-9 in these patients were determined by radioimmunoassay. An ROC curve demonstrated that a CA19-9 value of 27 U/mL was the optimal cut off point for patient death within three years after operation (Figure 1). The area under the curve (AUC) was 0.586. A total of 106 patients had preoperative CA19-9 values >27 U/mL, among whom 32 (30.2%) patients died within three years. In contrast, only 38 of the 198 patients (19.2%) with CA19-9 values ≤27 U/mL died within three years. In the 106 patients with preoperative CA19-9 values >27 U/mL, 60 patients had CA19-9 values between 27 and 50 U/mL. Twenty-five patients had CA19-9 values between 50 and 100 U/mL. Twenty-one patients had CA19-9 values higher than 100 U/mL.
Figure 1.
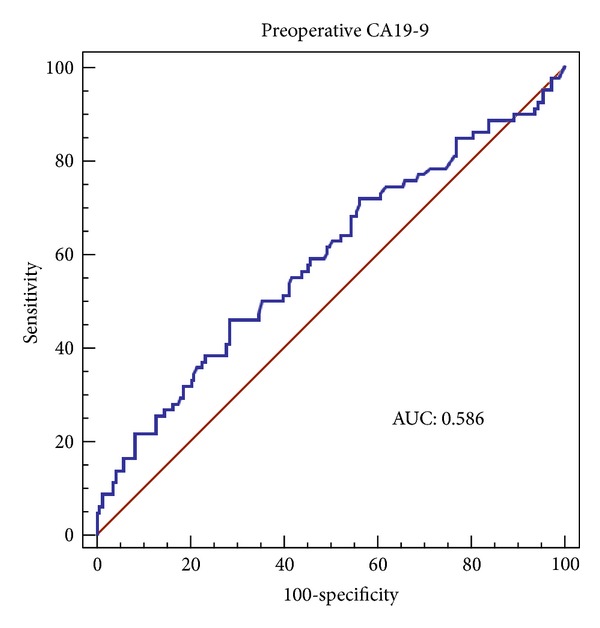
The ROC curve for CA19-9 values and patient death within three years in HCC patients who underwent partial hepatectomy.
3.3. Correlation of Serum CA19-9 Level and Clinicopathological Features
We correlated CA19-9 serum levels with a variety of clinicopathological features. As shown in Table 1, elevated preoperative serum CA19-9 level did not correlate with patient age, sex, viral status, α-fetoprotein level, tumor size, tumor grade, tumor stage, multiplicity, and vascular invasion. However, patients with elevated preoperative CA19-9 levels had a lower 10-year survival rate than those without CA19-9 elevation (P = 0.0020) (Figure 2(a)). The effect of CA19-9 level on patient survival was most obvious in patients with stage I HCC (P = 0.0039) (Figure 2(b)). In patients with stage II and III HCCs, those with high CA19-9 levels had slightly lower survival rate than those with low CA19-9 levels, but the difference did not reach statistical significance (P = 0.1238) (Figure 2(c)). Most importantly, elevated CA19-9 values in stage I HCC patients indicated similar prognosis with stages II and III patients, whereas patients with low CA19-9 values and stage I tumors had better prognosis than those with high stage tumor or high CA19-9 values (P = 0.0011) (Figure 2(d)).
Table 1.
Univariate analysis of CA19-9 serum level with various clinicopathological features in 304 patients with surgically removed hepatocellular carcinoma.
| Variables | CA19-9 serum level (U/mL) | P value | |
|---|---|---|---|
| >27 | ≤27 | ||
| Age | 0.2560 | ||
| >55 | 72 | 120 | |
| ≤55 | 34 | 78 | |
| Gender | 0.8097 | ||
| Male | 85 | 155 | |
| Female | 21 | 43 | |
| HBsAg | 0.4840 | ||
| Negative | 42 | 70 | |
| Positive | 60 | 123 | |
| Anti-HCV | 0.2552 | ||
| Negative | 68 | 143 | |
| Positive | 34 | 51 | |
| α-fetoprotein (ng/mL) | 0.8732 | ||
| ≤200 | 73 | 134 | |
| >200 | 25 | 50 | |
| Tumor size (cm) | 0.6927 | ||
| <5 | 61 | 78 | |
| ≥5 | 45 | 120 | |
| Grade | 0.7288 | ||
| 1-2 | 40 | 70 | |
| 3~4 | 65 | 128 | |
| Vascular invasion | 0.9864 | ||
| No | 68 | 128 | |
| Yes | 38 | 69 | |
| Multiple | 0.5695 | ||
| No | 74 | 145 | |
| Yes | 32 | 52 | |
| Stage | 0.7495 | ||
| I | 55 | 97 | |
| II~III | 51 | 199 | |
Figure 2.
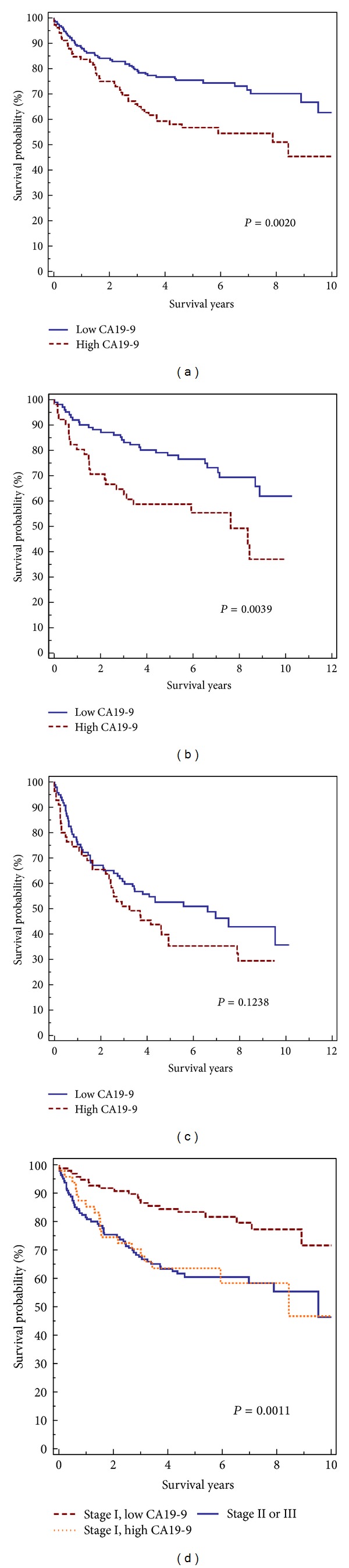
Postoperative survivals calculated by the Kaplan-Meier method. (a) Patients with high CA19-9 serum levels had a significantly lower overall survival than patients with low serum level. (b) Stage I patients with high CA19-9 serum levels had a significantly lower overall survival than patients with low serum level. (c) The survival of patients with high CA19-9 serum levels and stage II and III HCCs was slightly lower than patients with low CA19-9 serum levels, but the difference was not statistically significant. (d) Stage I HCC patients with high CA19-9 levels had similar prognosis with stage II and III patients. Patients with low CA19-9 values and stage I tumors had better prognosis than those with high stage tumor or high CA19-9 values.
3.4. Identification of Independent Factors for Predicting Long-Term Survival of HCC Patients
Univariate analysis found that α-fetoprotein level, CA19-9 level, tumor grade, tumor size, vascular invasion, multiplicity, and tumor stage were significant predictors of long-term overall survival (Table 2). To identify independent prognostic factors, we used these parameters for multivariate analysis. As shown in Table 3, only CA19-9 level, tumor grade, and tumor size emerged as independent prognostic factors.
Table 2.
Univariate analysis of overall survival for clinicopathological parameters.
| Clinicopathological features | Hazard ratio (HR) | 95% CI for HR | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (<55 versus ≥55) | 0.84 | 0.54 | 1.27 | 0.3943 |
| Gender (Male versus Female) | 1.18 | 0.70 | 1.94 | 0.5317 |
| HBV infection ((−) versus (+)) | 1.14 | 0.75 | 1.75 | 0.5403 |
| HCV infection ((−) versus (+)) | 0.89 | 0.57 | 1.41 | 0.6468 |
| AFP (<200 ng/mL versus >200 ng/mL) | 1.93 | 1.16 | 3.20 | 0.0031 |
| CA19-9 (≤27 U/mL versus >27 U/mL) | 1.87 | 1.21 | 2.90 | 0.0020 |
| Grade (1, 2 versus 3, 4) | 2.16 | 1.39 | 3.34 | 0.0001 |
| Size (<5 cm versus ≥5 cm) | 2.04 | 1.33 | 3.13 | 0.0004 |
| Vascular invasion ((−) versus (+)) | 1.70 | 1.09 | 2.66 | 0.0094 |
| Multiple ((−) versus (+)) | 1.79 | 1.11 | 2.87 | 0.0060 |
| Stage (I versus II, III) | 1.76 | 1.17 | 2.65 | 0.0057 |
Table 3.
Multivariate analysis of overall survival for clinicopathological parameters.
| Clinicopathological features | b | SE | Exp(b) | 95% CI of Exp(b) | P value |
|---|---|---|---|---|---|
| AFP (<200 ng/mL versus >200 ng/mL) | 0.35 | 0.24 | 1.42 | 0.89–2.27 | 0.1444 |
| CA19-9 (≤27 U/mL versus >27 U/mL) | 0.55 | 0.22 | 1.74 | 1.13–2.68 | 0.0125 |
| Grade (1, 2 versus 3, 4) | 0.49 | 0.23 | 1.62 | 1.04–2.55 | 0.0342 |
| Size (<5 cm versus ≥5 cm) | 0.58 | 0.23 | 1.78 | 1.14–2.79 | 0.0123 |
| Vascular invasion ((−) versus (+)) | 0.53 | 0.40 | 1.69 | 0.77–3.69 | 0.1895 |
| Multiple ((−) versus (+)) | 0.61 | 0.34 | 1.85 | 0.94–3.61 | 0.0742 |
| Stage (I versus II, III) | −0.35 | 0.48 | 0.71 | 0.28–1.81 | 0.4732 |
4. Discussion
Many molecular markers were identified to be prognostic factors for HCC [17–19]. Most of these markers are expressed in the tumor tissue, so they can be used only when tissue specimen is available. Few serum markers are available for predicting prognosis of HCC patients. One of the serum markers is α-fetoprotein. A high α-fetoprotein level correlates with high stage, early recurrence, and poor prognosis of HCC [20]. Serum levels of glypican-3, the absence of vitamin K or antagonist-II (PIVKA-II), manganese superoxide dismutase, and vascular endothelial growth factor have been reported to have prognostic values for HCC patients [21–24]. However, these novel markers are not readily available in most hospitals.
In this report, we identify CA19-9 as a novel serum marker for predicting survival for patients with HCC. The CA19-9 assay detects a mucin containing a pentasaccharide epitope (fucopentaose II) considered to be a tumor marker in pancreatic adenocarcinoma [25]. Abnormal CA19-9 serum levels were also found in patients with cholangiocarcinoma [26], colorectal cancer [27], gastric cancer [28], and a wide range of benign conditions, such as liver diseases, ascending cholangitis, and pancreatitis [29]. The preoperative CA19-9 level has been reported to be a prognostic factor for pancreatic cancer [30] and cholangiocarcinoma [31]. CA19-9 serum level has been reported to be elevated in HCC patients, but the prognostic implication is still unknown.
In this study, we found that an elevated CA19-9 level is a predictor of shorter long-term survival for HCC patients. The effect of CA19-9 serum levels is most obvious in patients with stage I HCC. Multivariate analysis also identified that CA19-9 is an independent prognostic factor for HCC patients. In contrast with other molecular markers, CA19-9 is a routine cancer marker in daily practice and is available in most hospitals. So it can be applied in clinical practice without difficulty.
The source of CA19-9 in HCC is still unknown. An elevated CA19-9 serum level is frequently seen in biliary obstruction [32]. CA19-9 is synthesized by normal biliary epithelium. A high CA19-9 level was detected in normal bile [33]. The healthy biliary tract is a secretory pathway for CA19-9. Local compression of the biliary tree by the tumor mass may cause obstruction of small bile ducts and hence produce an increase in the serum level of CA19-9. A subset of hepatocellular carcinoma exhibits partial cholangiocytic differentiation which is characterized by expression of biliary cytokeratin [12]. An elevated CA19-9 serum level strongly associated with this dual-phenotype HCC group [12]. These dual-phenotype HCCs tend to have more aggressive behaviors than pure HCCs [12, 22]. These observations may partially explain the prognostic implication of serum CA19-9 level.
We use 27 U/mL as the cut off point for elevated CA19-9 level in our study, which is lower than the upper limit of the normal range recommended by the manufacturer. Measurement of CA19-9 is usually used in preoperative evaluation and postoperative followup of patients with gastrointestinal adenocarcinomas, which usually produce high levels of CA19-9 by tumor cells. Using a higher cut off can prevent false positive results. In HCC patients, the serum levels of CA19-9 are usually lower than those of gastrointestinal cancer patients. We do not recommend using CA19-9 in screening or followup of HCC patients, but CA19-9 measurement can be used for prognostication in HCC patients, and 27 U/mL may be the optimal cut off for this purpose.
5. Conclusion
In conclusion, our study demonstrates that the preoperative serum CA19-9 level is a associated with poor prognosis in HCC patients. This assay is most useful in stratifying stage I HCC patients into different prognostic groups.
Acknowledgments
The serum CA19-9 value data were provided by the Department of Laboratory Medicine, National Taiwan University Hospital. The authors also thank the Department of Oncology and Cancer Registry Office, National Taiwan University Hospital for providing the follow-up information.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Yu MC, Yuan J-M, Govindarajan S, Ross RK. Epidemiology of hepatocellular carcinoma. Canadian Journal of Gastroenterology. 2000;14(8):703–709. doi: 10.1155/2000/371801. [DOI] [PubMed] [Google Scholar]
- 3.Hsu H-C, Jeng Y-M, Mao T-L, Chu J-S, Lai P-L, Peng S-Y. β-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. American Journal of Pathology. 2000;157(3):763–770. doi: 10.1016/s0002-9440(10)64590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu H, Huang A-M, Lai PO-L, Chien W, Peng S, Lin S. Genetic alterations at the splice junction of p53 gene in human hepatocellular carcinoma. Hepatology. 1994;19(1):122–128. [PubMed] [Google Scholar]
- 5.Koprowski H, Steplewski Z, Mitchell K. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genetics. 1979;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 6.Kannagi R. Carbohydrate antigen sialyl Lewis a—its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Medical Journal. 2007;30(3):189–209. [PubMed] [Google Scholar]
- 7.Yakabe T, Nakafusa Y, Sumi K, et al. Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Annals of Surgical Oncology. 2010;17(9):2349–2356. doi: 10.1245/s10434-010-1004-5. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura T, Uesaka K, Kanemoto H, et al. Serum CA19-9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. Journal of Gastrointestinal Surgery. 2012:1–9. doi: 10.1007/s11605-012-1859-9. [DOI] [PubMed] [Google Scholar]
- 9.Kodera Y, Yamamura Y, Torii A, et al. The prognostic value of preoperative serum levels of CEA and CA19-9 in patients with gastric cancer. American Journal of Gastroenterology. 1996;91(1):49–53. [PubMed] [Google Scholar]
- 10.Qin X, Wang Z, Shi J, Lu M, Wang L, He Q. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World Journal of Gastroenterology. 2004;10(3):427–432. doi: 10.3748/wjg.v10.i3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chantajitr S, Wilasrusmee C, Lertsitichai P, Phromsopha N. Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. Journal of Hepato-Biliary-Pancreatic Surgery. 2006;13(6):537–542. doi: 10.1007/s00534-006-1117-1. [DOI] [PubMed] [Google Scholar]
- 12.Lu X, Xi T, Lau W, et al. Hepatocellular carcinoma expressing cholangiocyte phenotype is a novel subtype with highly aggressive behavior. Annals of Surgical Oncology. 2011;18(8):2210–2217. doi: 10.1245/s10434-011-1585-7. [DOI] [PubMed] [Google Scholar]
- 13.Tao L, Cai L, He X, Liu W, Qu Q. Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. American Surgeon. 2010;76(11):1210–1213. [PubMed] [Google Scholar]
- 14.Maestranzi S, Przemioslo R, Mitchell H, Sherwood RA. The effect of benign and malignant liver disease on the tumour markers CA19-9 and CEA. Annals of Clinical Biochemistry. 1998;35(1):99–103. doi: 10.1177/000456329803500113. [DOI] [PubMed] [Google Scholar]
- 15.Edmonson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900. Cancer. 1954;7(3):462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Tortti A, editors. AJCC Cancer Staging Manualed. 7th edition. New York, NY, USA: Springer; 2010. [Google Scholar]
- 17.Jeng Y, Chang C, Hu F, et al. RNA-binding protein insulin-like growth factor II mRNA-binding protein 3 expression promotes tumor invasion and predicts early recurrence and poor prognosis in hepatocellular carcinoma. Hepatology. 2008;48(4):1118–1127. doi: 10.1002/hep.22459. [DOI] [PubMed] [Google Scholar]
- 18.Yuan R, Jeng Y, Pan H, et al. Overexpression of KIAA0101 predicts high stage, early tumor recurrence, and poor prognosis of hepatocellular carcinoma. Clinical Cancer Research. 2007;13(18):5368–5376. doi: 10.1158/1078-0432.CCR-07-1113. [DOI] [PubMed] [Google Scholar]
- 19.Jeng Y, Peng S, Lin C, Hsu H. Overexpression and amplification of aurora-a in hepatocellular carcinoma. Clinical Cancer Research. 2004;10(6):2065–2071. doi: 10.1158/1078-0432.ccr-1057-03. [DOI] [PubMed] [Google Scholar]
- 20.Peng S, Chen WJ, Lai P, Jeng Y, Sheu J, Hsu H. High α-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and β-catenin mutations. International Journal of Cancer. 2004;112(1):44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 21.Özkan H, Erdal H, Koçak E, et al. Diagnostic and prognostic role of serum glypican 3 in patients with hepatocellular carcinoma. Journal of Clinical Laboratory Analysis. 2011;25(5):350–353. doi: 10.1002/jcla.20484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang SH, Kim DY, Jeon SM, et al. Clinical characteristics and prognosis of hepatocellular carcinoma with different sets of serum AFP and PIVKA-II levels. European Journal of Gastroenterology and Hepatolog. 2012;24:849–856. doi: 10.1097/MEG.0b013e3283535c34. [DOI] [PubMed] [Google Scholar]
- 23.Tamai T, Uto H, Takami Y, et al. Serum manganese superoxide dismutase and thioredoxin are potential prognostic markers for hepatitis C virus-related hepatocellular carcinoma. World Journal of Gastroenterology. 2011;17(44):4890–4898. doi: 10.3748/wjg.v17.i44.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong C, Wei W, Su X, Li H, Xu F, Guo R. Serum and tissue vascular endothelial growth factor predicts prognosis in hepatocellular carcinoma patients after partial liver resection. Hepato-Gastroenterology. 2012;59(113):93–97. doi: 10.5754/hge10638. [DOI] [PubMed] [Google Scholar]
- 25.Duffy MJ, Sturgeon C, Lamerz R, et al. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Annals of Oncology. 2009;21(3):441–447. doi: 10.1093/annonc/mdp332.mdp332 [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Tang S, Sreenarasimhaiah J, Lara LF, Siddiqui A. The clinical utility and limitations of serum carbohydrate antigen (CA19-9) as a diagnostic tool for pancreatic cancer and cholangiocarcinoma. Digestive Diseases and Sciences. 2011;56(8):2491–2496. doi: 10.1007/s10620-011-1709-8. [DOI] [PubMed] [Google Scholar]
- 27.Novis BH, Gluck E, Thomas P. Serial levels of CA 19-9 and CEA in colonic cancer. Journal of Clinical Oncology. 1986;4(6):987–993. doi: 10.1200/JCO.1986.4.6.987. [DOI] [PubMed] [Google Scholar]
- 28.Mennini G, Silecchia G, Zanna C. Clinical value of CA 19-9 (carbohydrate antigen) in gastrointestinal adenocarcinoma. Italian Journal of Surgical Sciences. 1985;15(1):37–43. [PubMed] [Google Scholar]
- 29.Akdoğan M, Şaşmaz N, Kayhan B, Biyikoğlu I, Dişibeyaz S, Şahin B. Extraordinarily elevated CA19-9 in benign conditions: a case report and review of the literature. Tumori. 2001;87(5):337–339. doi: 10.1177/030089160108700513. [DOI] [PubMed] [Google Scholar]
- 30.Hallemeier CL, Botros M, Corsini MM, Haddock MG, Gunderson LL, Miller RC. Preoperative CA 19-9 level is an important prognostic factor in patients with pancreatic adenocarcinoma treated with surgical resection and adjuvant concurrent chemoradiotherapy. American Journal of Clinical Oncology. 2011;34(6):567–572. doi: 10.1097/COC.0b013e3181f946fc. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Song Z, Hu Q, et al. Serum carbohydrate antigen (CA) 19-9 as a prognostic factor in cholangiocarcinoma: a meta-analysis. Frontiers of Medicine in China. 2010;4(4):457–462. doi: 10.1007/s11684-010-0240-1. [DOI] [PubMed] [Google Scholar]
- 32.Strom BL, Iliopoulos D, Atkinson B, et al. Pathophysiology of tumor progression in human gallbladder: flow cytometry, CEA, and CA 19-9 levels in bile and serum in different stages of gallbladder disease. Journal of the National Cancer Institute. 1989;81(20):1575–1580. doi: 10.1093/jnci/81.20.1575. [DOI] [PubMed] [Google Scholar]
- 33.Yuan RH, Jeng Y, Hu R, et al. Role of p53 and β-catenin mutations in conjunction with CK19 expression on early tumor recurrence and prognosis of hepatocellular carcinoma. Journal of Gastrointestinal Surgery. 2011;15(2):321–329. doi: 10.1007/s11605-010-1373-x. [DOI] [PubMed] [Google Scholar]


