SUMMARY
Reactive oxygen species (ROS) play complex roles in aging, having both damaging effects and signaling functions. Transiently elevating mitochondrial stress, including mitochondrial ROS (mtROS), elicits beneficial responses that extend lifespan. However, these adaptive, longevity-signaling pathways remain poorly understood. We show here that Tel1p and Rad53p, homologs of the mammalian DNA-damage-response kinases ATM and Chk2, mediate a hormetic mtROS longevity signal that extends yeast chronological lifespan. This pathway senses mtROS in a manner distinct from the nuclear DNA-damage response and ultimately imparts longevity by inactivating the histone demethylase Rph1p specifically at subtelomeric heterochromatin, enhancing binding of the silencing protein Sir3p, and repressing subtelomeric transcription. These results demonstrate the existence of conserved mitochondria-to-nucleus stress-signaling pathways that regulate aging through epigenetic modulation of nuclear gene expression.
INTRODUCTION
As multi-functional organelles, mitochondria influence aging, lifespan and health span by a variety of mechanisms. Perhaps the most recognized is the production of reactive oxygen species (ROS), byproducts of mitochondrial respiration that damage macromolecules to promote cell dysfunction, apoptosis and aging (Balaban et al., 2005). However, ROS also function as signaling molecules that regulate physiological processes (Finkel, 2011; Sena and Chandel, 2012) and participate in conserved signal transduction pathways, including the Target of Rapamycin (TOR) and insulin-sensing pathways that regulate longevity (Longo et al., 2012; Pan et al., 2011; Raimundo et al., 2012; Zarse et al., 2012). Additionally, studies in model organisms show that increasing ROS levels can positively influence health and lifespan (Lee et al., 2010; Pan et al., 2011; Schulz et al., 2007; Yang and Hekimi, 2010; Zarse et al., 2012).
The chronological lifespan (CLS) of the budding yeast, Saccharomyces cerevisiae, is measured by assaying the amount of time that cells survive in the late post-diauxic and stationary phases of culturing that follow exponential growth (Longo et al., 2012). Yeast CLS studies, which model post-mitotic cellular aging in higher eukaryotes, have led to the identification of conserved pathways and processes involved in lifespan regulation (Fabrizio and Longo, 2007; Kaeberlein, 2010; Longo et al., 2012). We recently demonstrated that elevating mitochondrial ROS (mtROS) during yeast exponential growth elicits an adaptive response that reduces ROS in post-diauxic and stationary phases and extends CLS (Pan et al., 2011). Interventions that elevate mtROS in C. elegans also extend lifespan (Lee et al., 2010; Schulz et al., 2007; Yang and Hekimi, 2010; Zarse et al., 2012), and exercise in mammals is believed to improve health via increasing mtROS (Ristow et al., 2009). These examples indicate that hormetic adaptation to ROS is an emerging paradigm in lifespan and health span regulation (Gems and Partridge, 2008; Ristow and Schmeisser, 2011; Tapia, 2006). However, the signaling pathways that sense ROS and coordinate hormetic changes in cellular function to curtail aging and promote longevity remain largely unknown.
Hormetic ROS might extend lifespan by increasing stress resistance or decreasing ROS levels late in life (Gems and Partridge, 2008). However, other cellular processes may be targets of mtROS signaling. In particular, aspects of telomere function determine replicative capacity and impact lifespan (Blasco, 2005). In yeast, telomeres consist of a repetitive TG1-3 sequence bound by several highly conserved proteins that regulate telomere length, transcription, and packaging (Grunstein, 1997). The Silent Information Regulator (Sir) proteins maintain telomeric heterochromatin to repress transcription (Rusche et al., 2003). Histone modifications adjacent to telomeres regulate the spread of silencing, termed Telomere Position Effect (TPE), by enhancing or preventing Sir complex binding (Altaf et al., 2007; Dang et al., 2009; Kozak et al., 2010; Park and Lustig, 2000). Yeast impaired in specific histone modifications that support TPE have a shorter replicative life span (Dang et al., 2009), and decreased heterochromatin formation at telomeres and subtelomeres in mammals induces telomere dysfunction to trigger apoptosis or senescence (Blasco, 2007). However, the signaling pathways that regulate histone modifications to support subtelomeric silencing are not fully understood. Additionally, although substantial progress has been made towards understanding the relationships between telomere dysfunction and replicative lifespan, how telomere function might influence post-mitotic aging remains unknown.
Herein, we focused on the process of hormetic mtROS signaling and adaptation in yeast, reasoning that our studies would reveal conserved mechanisms of ROS sensing and longevity regulation. We show that mtROS signal via Tel1p and Rad53p, homologs of mammalian ATM and Chk2, uncovering a function for the DNA-damage response in communicating mitochondria functional status to the nucleus. This signaling pathway inhibits the histone demethylase Rph1p specifically at subtelomeric regions, which enhances transcriptional silencing to extend CLS. In yeast and mammals, telomere dysfunction signals changes in mitochondrial biogenesis and function (Nautiyal et al., 2002; Sahin et al., 2011). Thus, our results demonstrate that two-way communication pathways exist between mitochondria and telomeres that regulate aging and longevity.
RESULTS
The H3K36 Demethylase Rph1p Mediates Hormetic Responses to mtROS
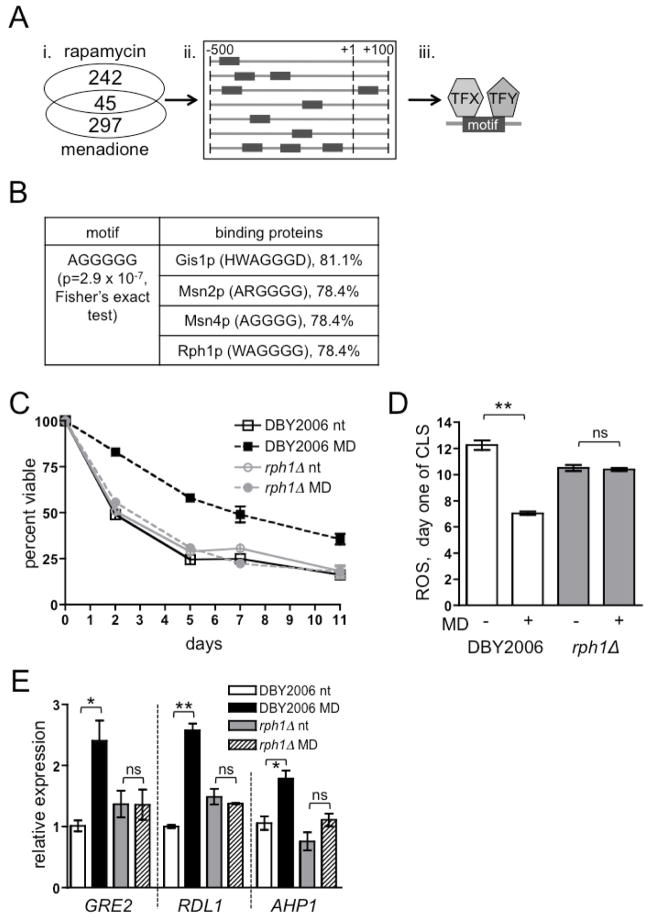
Reduced TOR Complex 1 (TORC1) signaling extends lifespan in many model organisms (Kenyon, 2005), and we showed previously that mtROS hormesis contributes to this mode of CLS extension in yeast (Pan et al., 2011). Furthermore, transient treatment with a sub-lethal dose of the redox-cycling compound menadione during exponential growth generates mtROS (Castro et al., 2008) and extends yeast CLS (Pan et al., 2011) (Fig. S1A). Menadione treatment and reduced TORC1 signaling decrease cellular ROS levels in post-diauxic and stationary phases, supporting that hormetic adaptation to ROS occurs under these conditions (Bonawitz et al., 2007; Pan et al., 2011). To identify factors that mediate mtROS longevity signaling, we crossed results of two published microarrays from conditions that increase mtROS in yeast (1 mM menadione and 100 nm rapamycin, a TORC1 inhibitor) and identified the commonly induced genes (Fig. 1Ai and Table S1) (Gasch et al., 2000; Hardwick et al., 1999). Rather than systematically analyzing all identified genes, which may not show individual robust contributions to mtROS adaptation, we searched first for transcriptional regulators that globally coordinate these gene expression responses and second for signaling events that determine their activity (Fig. 1Aii-iii and Fig. S1B). We identified a promoter motif, AG5, enriched in these genes (Fig. 1B) (Romer et al., 2007) and transcriptional regulators that bind motifs similar to AG5 (Abdulrehman et al., 2011). Candidate transcriptional regulators included Gis1p and Msn2/4p, which have known roles in oxidative stress resistance, nutrient sensing, and CLS (Wei et al., 2008). Strains lacking Msn2p or Msn4p demonstrated a moderately reduced adaptive CLS response relative to the wild-type strain (72% and 81% of the menadione-dependent CLS extension observed in wild-type; Fig. S1C–D), indicating non-redundant contributions of each transcription factor. Combined deletion of MSN2 and MSN4 abrogated menadione-induced CLS extension to 53% of that observed in the wild-type strain (Fig. S1E). This additive effect suggests that Msn2p and Msn4p have partially redundant functions in mtROS adaptation, consistent with their overlapping regulation of gene expression in response to stress and downstream of TORC1 signaling (Gasch et al., 2000; Wei et al., 2008; Wei et al., 2009). Deletion of GIS1 reduced menadione-dependent CLS extension to 47% of the response seen in the wild-type (Fig. S1F), indicating it also participates in mtROS adaptation. Gis1p and Msn2/4p cooperatively contribute to yeast CLS extension under conditions of reduced TORC1 signaling and caloric restriction (Wei et al., 2008). However, a gis1Δ/msn2Δ/msn4Δ mutant strain retained an adaptive CLS response that was 47% of wild-type (Fig. S1G), identical to that of the gis1Δ single mutant. This epistatic relationship indicates that Gis1p, Msn2p and Msn4p partially contribute to the mtROS adaptive response in the same genetic pathway. Only deletion of the histone 3 lysine 36 (H3K36) demethylase Rph1p completely abrogated CLS extension (Fig. 1C), eliminated the hormetic decrease in ROS at day one of CLS (Fig. 1D), and impaired expression of mtROS-responsive genes (Fig. 1E). Rph1p also contributed to the CLS extension observed in a tor1Δ strain (Fig. S1H), consistent with Rph1p participating in TORC1-regulated mtROS hormesis (Pan et al., 2011). Intrigued by the notion that mtROS adaptation might involve epigenetic changes in nuclear gene expression, Rph1p became our primary focus.
Figure 1. The Histone Demethylase Rph1p Mediates Hormetic mtROS Longevity Signaling.
(A) Strategy to identify factors that regulate the global transcriptional response to hormetic mtROS signaling. i. Identification of genes induced under conditions of elevated mtROS. ii. Promoter sequences of induced genes were analyzed to identify enriched motifs. iii. Known transcription factor binding sequences were scanned for the presence of motifs similar to those enriched in mtROS-induced gene promoters.
(B) Results of promoter analysis. The AG5 motif is significantly enriched in promoters of genes induced by mtROS, and is similar to the DNA sequences recognized by the proteins listed. The percentages of mtROS-induced genes containing the exact motifs recognized by the candidate proteins are indicated to the right of the motif. See also Table S1.
(C) CLS of wild-type (DBY2006) and rph1Δ strains treated with 50 μM menadione (MD) or vehicle (not treated, nt) during logarithmic growth. In all CLS curves, data points represent the mean of three biological replicates inoculated from single colonies ±SEM.
(D) ROS levels in wild-type and rph1Δ strains treated with menadione (MD+) or vehicle (−) during exponential growth. Samples were analyzed at day one of CLS. In all graphs, data points represent the mean of three biological replicates ±SEM unless otherwise indicated. “ns” designates p>0.05, * designates p≤0.05, and ** designates p≤0.01.
(E) RT-PCR of transcripts induced by mtROS. Wild type and rph1Δ were treated with 50 μM menadione (MD) or vehicle (nt) for two hours in exponential phase, and the expression of three genes selected from the list of 45 mtROS-responsive transcripts (Fig. 1A) was analyzed. See also Figure S1.
Hormetic menadione treatment induces mitochondria superoxide to extend CLS
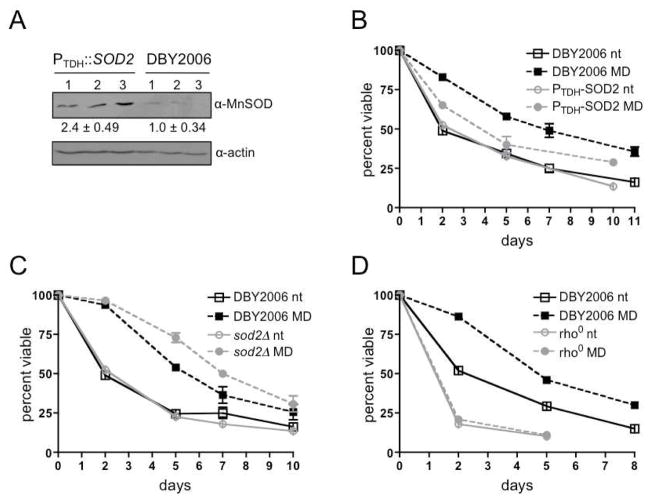
Overexpression of SOD2, which encodes the mitochondria matrix superoxide dismutase, curtails CLS extension in tor1Δ yeast, supporting that elevated mtROS initiates an adaptive signal (Pan et al., 2011). To determine if hormetic menadione treatment elicits adaptive responses via a similar mechanism, we measured CLS in strains with different mtROS detoxification capacities. We utilized a strain in which the strong TDH3 promoter (Kotter et al., 2009) drives SOD2 expression (PTDH-SOD2) (Fig. 2A) and a sod2Δ strain, with increased or decreased mtROS detoxification capabilities, respectively. The PTDH-SOD2 strain demonstrated reduced CLS extension relative to the wild-type strain (Fig. 2B). In contrast, sod2Δ amplified CLS extension (Fig. 2C). Menadione treatment also failed to extend CLS in a rho0 strain, which lacks mtDNA and hence is unable to respire (Fig. 2D). We therefore conclude that menadione is affecting CLS via alteration of mtROS levels (i.e. superoxide).
Figure 2. A Mitochondrial Superoxide Signal Extends CLS.
(A) Western blot analysis of Sod2p (MnSOD) in PTDH::SOD2 and wild-type (DBY2006). Samples were collected at an OD600 of 1.0. MnSOD band intensity was normalized to actin, and the DBY2006 protein level was set to one. Values under the blot represent the mean ±SEM for three biological replicates.
(B) CLS in wild-type and PTDH::SOD2 strains following exponential phase treatment with menadione (MD) or ethanol (nt).
(C) CLS in wild-type and sod2Δ strains as in (B).
(D) CLS in wild-type and rho0 as in (B).
Tel1p and Rad53p, Components of the Nuclear DNA-Damage Response, Contribute to mtROS Hormesis
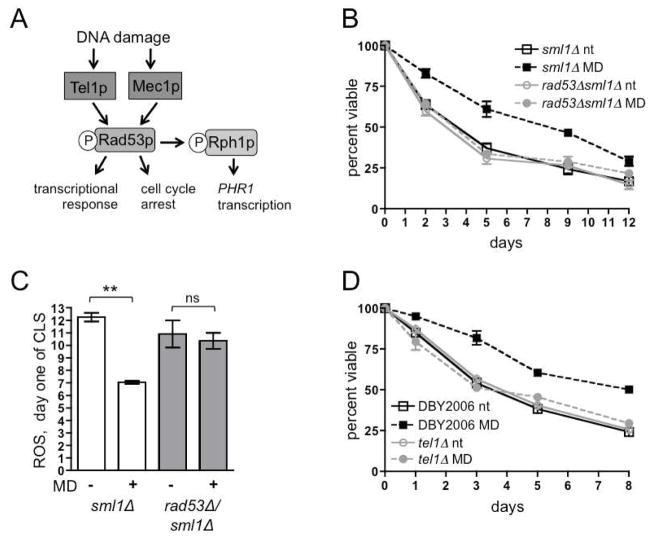
The DNA-damage-checkpoint kinase Rad53p (Chk2 in mammals) phosphorylates Rph1p in response to DNA damage (Kim et al., 2002) (Fig. 3A). Subsequent Rph1p-mediated chromatin modifications permit transcription of the DNA repair enzyme PHR1 (Liang et al., 2011). Mec1 (ATR in mammals) activates and phosphorylates Rad53p (Pellicioli et al., 1999) and phosphorylation of Rph1p in response to DNA damage is Mec1p-dependent (Kim et al., 2002); therefore, we investigated the requirement for the Rad53p and Mec1p kinases in mtROS signaling. Deletion of SML1 suppresses the lethality of rad53Δ and mec1Δ and allowed us to examine their role in mtROS signaling and adaptation (Zhao et al., 1998). Although Rad53p was required for mtROS-mediated CLS extension (Fig. 3B) and hormetic reduction of ROS at day one of CLS (Fig. 3C), Mec1p was not (Fig. S2A). Therefore, we investigated the involvement of the related kinase Tel1p, which has partially overlapping functions with Mec1p (Fig. 3A), but unique roles in telomere maintenance (Harrison and Haber, 2006). Additionally, the mammalian homolog of Tel1p, ATM, senses ROS (Guo et al., 2010), making Tel1p an attractive candidate to mediate mtROS signaling in yeast. Menadione treatment did not extend CLS in a tel1Δ strain (Fig. 3D), implicating Tel1p as an upstream kinase in the adaptive mtROS pathway. The lack of adaptation to mtROS in rph1Δ, rad53Δ, and tel1Δ strains is not due to endogenous differences in ROS levels or an inability of these strains to elevate mtROS upon menadione treatment during exponential growth (Fig. S2B). Thus far, these results indicate that Tel1p, Rad53p, and Rph1p together mediate mtROS signaling and adaptation to extend yeast CLS.
Figure 3. Hormetic mtROS Longevity Signaling Requires the Tel1p and Rad53p DNA-damage Response Kinases.
(A) Schematic of DNA-damage signaling in Saccharomyces cerevisiae.
(B) CLS of sml1Δ and sml1Δ/rad53Δ strains treated with 50 μM menadione (MD) or vehicle (nt) during exponential growth.
(C) ROS levels in sml1Δ and sml1Δ/rad53Δ strains treated with menadione (+) or vehicle (−) during exponential growth as in Figure 1D. Samples were collected at day one of CLS.
(D) CLS of wild-type (DBY2006) and tel1Δ strains as in (B). See also Figure S2.
Hormetic Menadione Treatment Activates DNA Damage Responsive Kinases in the Absence of Nuclear DNA Damage
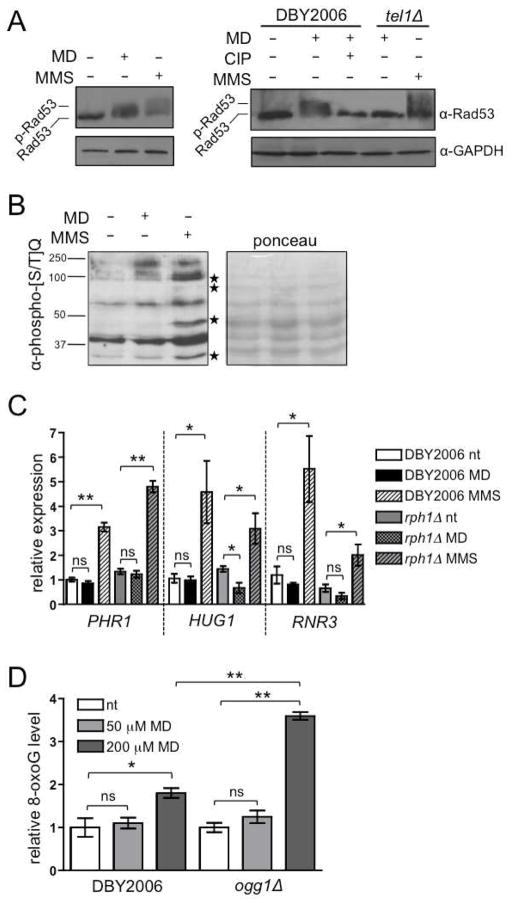
Tel1p phosphorylates Rad53p upon telomere dysfunction or DNA damage (Sanchez et al., 1996). Hormetic menadione treatment caused an upward mobility shift in Rad53p, but this shift was intermediate to that observed following treatment with a comparable dose of methylmethane sulfate (MMS), a direct DNA-damaging agent (Fig. 4A, left, and Fig. S3A–C). The menadione-induced mobility shift was abolished by phosphatase treatment and did not occur in a tel1Δ strain, yet MMS-induced Rad53p phosphorylation was not affected by tel1Δ (Fig. 4A, right). Rad53p contains multiple Tel1p/Mec1p target phosphorylation sites, characterized by an [S/T]Q motif (Kim et al., 1999; Lee et al., 2003). Analysis of [S/T]Q phosphoprotein profiles revealed that hormetic menadione treatment induced phosphorylation of a specific subset of [S/T]Q-containing targets compared to an MMS treatment that had similar effects on cell growth and toxicity (Fig. 4B). This distinct profile, combined with partial phosphorylation of Rad53p in response to menadione, indicated to us that hormetic mtROS signaling via Tel1p and Rad53p is distinct from canonical Mec1p-mediated DNA-damage signaling. We additionally demonstrated that the transcriptional outcomes of hormetic mtROS signaling are distinct from a DNA damage transcriptional signature (DDS), which corresponds to genes similarly altered by 0.02% MMS and 170 Gray ionizing radiation (Gasch et al., 2001) (Fig. S3D and Table S2). Reverse-transcription PCR (RT-PCR) analysis of three well-characterized DNA damage-induced transcripts confirmed that they are not induced by hormetic menadione treatment (Fig. 4C). Instead, further characterization of menadione-induced genes identified several involved in oxidative-stress responses that were elevated in wild-type but not rph1Δ strains (AHP1, CTA1, CUP1-1, LOT6, MXR1, TRX1/2/3, TSA1, YPR1). Collectively, these likely explain the hormetic decrease in ROS observed at day one of CLS (Fig. S3E and Table S2). Furthermore, although menadione treatment extends CLS via modulating mtROS levels (Fig. 2), hormetic menadione treatment did not induce oxidative DNA damage in a wild-type strain or an ogg1Δ strain that lacks the oxidized purine repair enzyme and is therefore hypersensitive to oxidative DNA damage (Karahalil et al., 1998) (Fig. 4D). Finally, mtROS adaptation does not require DNA double-strand break detection or signaling mediated by Mre11, a component of the MRN/MRX complex (Nakada et al., 2004) (Fig. S3F). Together, these data support that mtROS produced during hormetic menadione treatment activates specific DNA-damage-response kinases without inducing oxidative nuclear DNA damage or a canonical nuclear DNA damage response.
Figure 4. mtROS Activate a Non-canonical, Tel1p-dependent DNA Damage Response.
(A) Left: western blot analysis of Rad53p phosphorylation in the wild-type treated with 50 μM menadione (MD+), 0.01% methylmethane sulfate (MMS+) or ethanol (−) for 30 minutes. Right: wild-type and tel1Δ strains treated with menadione, MMS, ethanol and/or calf intestinal phosphatase (CIP).
(B) Protein phosphorylation at [S/T]Q sites in response to 30 minute treatment with 50 μM menadione or 0.01% MMS. Molecular weights (kDa) are indicated on the left. Starred bands indicate proteins that are phosphorylated in response to MMS treatment but not menadione treatment.
(C) RT-PCR of transcripts induced by DNA damage. Wild-type and rph1Δ were treated with 50 μM menadione (MD), 0.01% MMS, or vehicle (nt) for 30 minutes in exponential phase. See also Table S2.
(D) Quantification of 8-hydroxyguanosine (8-oxoG) formation in wild-type and ogg1Δ. Cells were treated for 30 minutes prior to DNA extraction. Values were normalized to untreated wild-type, which was set to one. Data points represent the mean of three biological replicates inoculated from single colonies ±SEM. “ns” designates p>0.05, * designates p≤0.05, ** designates p≤0.01. See also Figure S3.
Hormetic mtROS signaling Inactivates Rph1p to Alter Subtelomeric Chromatin
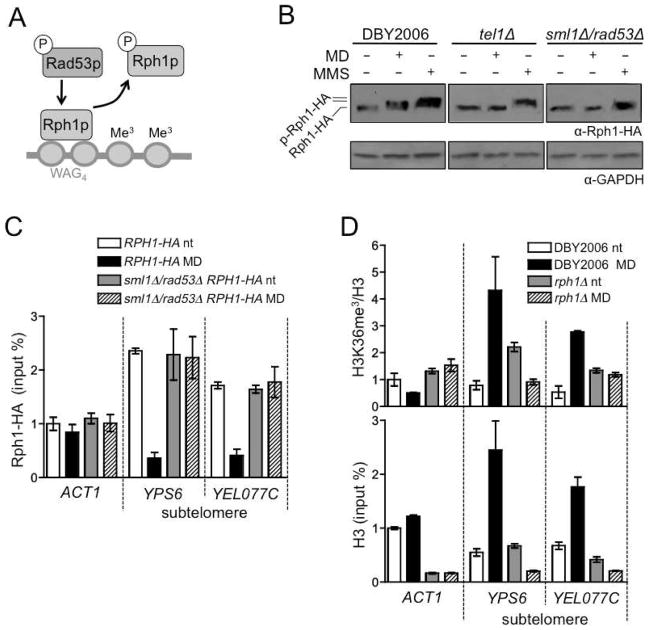
Rad53p-dependent phosphorylation of Rph1p causes it to dissociate from chromatin, elevating H3K36me3 levels to regulate transcription (Fig. 5A) (Liang et al., 2011). We examined Rph1p phosphorylation in response to mtROS and found that hormetic menadione treatment induces a partial Rph1p upward mobility shift relative to treatment with MMS (Fig. 5B, left and Fig. S4A), mirroring the partial activation of Rad53p under these conditions (Fig. 4A). Rph1p phosphorylation in response to menadione, but not MMS, also required Tel1p and Rad53p (Fig. 5B). These data show that mtROS signaling via Tel1p and Rad53p induces phosphorylation of Rph1p, which may subsequently alter the chromatin-binding capacity of Rph1p and H3K36me3 levels.
Figure 5. mtROS Regulate Rph1p to Modify Subtelomeric Heterochromatin.
(A) Schematic of Rad53p-dependent regulation of Rph1p chromatin binding and histone demethylase activity.
(B) Western blot analysis of Rph1-HA phosphorylation in wild-type, tel1Δ and sml1Δ/rad53Δ strains treated with 50 μM menadione or 0.01% MMS. Samples for western blots were collected after 30 minutes of treatment during exponential growth.
(C) ChIP of Rph1-HA at a control region (ACT1) and two subtelomeric genes (YPS6 and YEL077C) in a wild-type or sml1Δ/rad53Δ background at day one of CLS. Data points represent the mean of two independent ChIP experiments, error bars represent the range. Values were normalized to the wild-type untreated ACT1 sample, which was set to one.
(D) ChIP using antibodies against trimethyl H3K36 or total H3 in wild-type or rph1Δ as in (C). See also Figure S4.
In yeast, histone methylation and acetylation contribute to heterochromatin formation at the rDNA gene cluster, mating loci and subtelomeric regions, defined as within 25 kb of telomeric repeat DNA (Barton and Kaback, 2006; Dang et al., 2009; Millar and Grunstein, 2006; Santos-Rosa et al., 2004). Yeast deficient in heterochromatin formation at these silenced loci exhibit decreased replicative lifespan (Dang et al., 2009; Kaeberlein et al., 1999), yet it is unknown how silencing at these regions influences chronological aging. H3K36me3 is implicated in both transcriptional repression and activation within euchromatic regions (Wagner and Carpenter, 2012), but the role of this modification at silenced loci has not been described. Since Rph1p phosphorylation regulates its chromatin-binding capacity (Liang et al., 2011) and Rph1p is phosphorylated in response to mtROS (Fig. 5B), we investigated whether mtROS signaling altered the association of Rph1p with silenced regions of the yeast genome. Chromatin immunoprecipitation (ChIP) of Rph1-HA at day one of CLS (when adaptive response to menadione would be evident, Figure S4B) revealed that, in the absence of hormetic menadione treatment, Rph1p binding at all examined silenced genes was enhanced relative to ACT1 (Fig. 5C and S4C). However, Rph1p binding at subtelomeric regions was specifically altered in response to hormetic mtROS signaling in a manner dependent on Rad53p (Fig. 5C and Fig. S4C). Consistent with loss of Rph1p, H3K36me3 levels increased at subtelomeric genes in an mtROS and RPH1-dependent manner, suggesting that mtROS signaling regulates the H3K36me3 demethylase activity of Rph1p (Fig. 5D and Fig. S4D). The requirement for H3K36 demethylation in adaptive CLS extension is specific to Rph1p, as deletion of Jhd2p, which also demethylates di- and tri-methyl H3K36 (Tu et al., 2007), did not affect mtROS adaptation (Fig. S4E). These data suggest that, although Rph1p may function at several silenced regions in chronologically aged yeast, hormetic mtROS signaling via Rad53p specifically targets Rph1p activity at subtelomeric regions, eliciting epigenetic changes that promote lifespan extension.
Signaling by mtROS Represses Transcription of Subtelomeric Genes
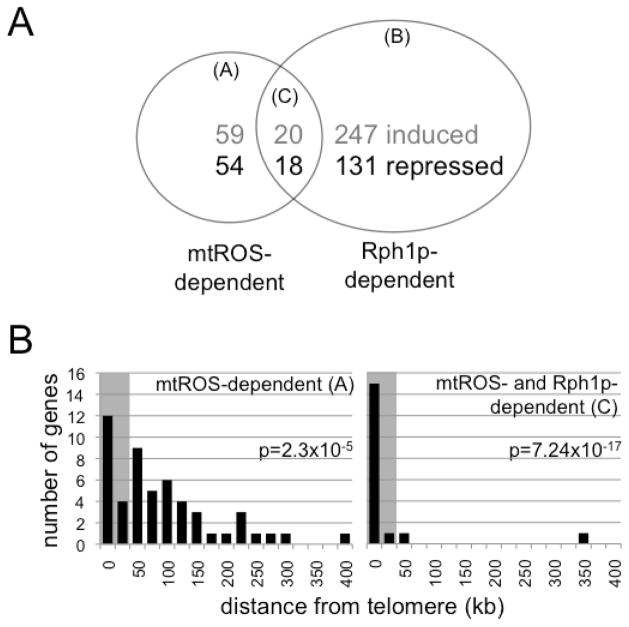
We next investigated the adaptive transcriptional consequences of Rph1p-mediated epigenetic changes in response to mtROS signaling. We performed gene expression microarrays of wild-type and rph1Δ yeast at day one of CLS that had been treated with menadione or a vehicle control during exponential growth. To focus the analysis on Rph1p-dependent transcriptional changes, we excluded transcripts that were altered in an rph1Δ strain treated with menadione. Additionally, we compared the remaining transcripts with those altered in an untreated rph1Δ strain. Since mtROS signaling to Rad53p essentially inactivates Rph1p demethylase activity, we reasoned that, although Rph1p has mtROS-independent functions (Orzechowski Westholm et al., 2012), a null strain might reveal a subset of the transcriptional outcomes relevant to mtROS-mediated CLS extension. Gene list comparisons (Table S3) are summarized in Figure 6A. We observed three fold enrichment for the Rph1p binding motif (WAG4) among transcripts induced by both mtROS signaling and Rph1p deletion (Fig. S5A, sector C), consistent with the involvement of Rph1p in regulating gene induction following hormetic mtROS signaling. mtROS signaling specifically affects Rph1p binding at subtelomeric regions (Fig. 5C), and subtelomeric transcripts were enriched only among genes repressed by mtROS signaling and Rph1 deletion (sectors A and C, Fig. 6B, Fig. S5B–C and Table S4). Using a subtelomeric URA3 reporter and RT-PCR, we confirmed that adaptive mtROS signaling represses transcription from subtelomeric regions (Fig. S5D–E), but not at other silenced regions (Fig. S5F and Table S5). Together, these results indicate that hormetic mtROS signaling to Rph1p selectively silences subtelomeric transcription.
Figure 6. Hormetic mtROS Signaling to Rph1p Promotes Subtelomeric Silencing.
(A) Gene expression changes induced by exponential phase menadione treatment in a wild-type strain (mtROS-dependent, sector A), rph1Δ strain (Rph1p-dependent, sector B), or mtROS-dependent inactivation of Rph1p (sector C) at day one of CLS. Gray font indicates the number of induced genes and black the number of repressed genes. See Table S3 for full gene lists.
(B) Repressed genes located the indicated distance (in kilobases, kb) from telomeres. Subtelomeric genes are within 25 kb (gray region) of telomeres and are listed in Table S4. See also Figure S5.
Hormetic mtROS Signaling Modifies Higher-order Chromatin Structure to Extend CLS
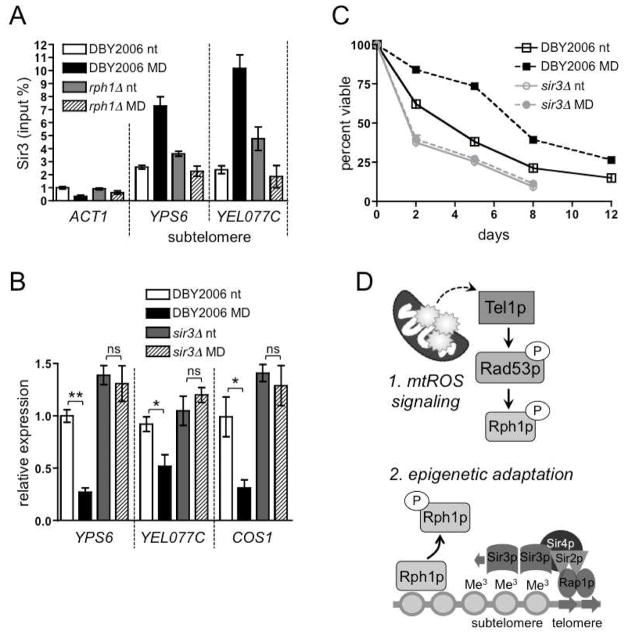
Histone modifications at subtelomeric regions can influence higher-order chromatin formation, which determines gene expression (Schoeftner and Blasco, 2009). The Sir complex is initially recruited to silenced regions via Sir2-mediated histone deacetylation (Rusche et al., 2003). This enhances the binding of Sir3p, which recruits additional Sir2p to nucleate heterochromatin spreading (Norris and Boeke, 2010). Histone methylation can enhance Sir3p binding, thereby influencing transcriptional silencing (Ng et al., 2002). ChIP experiments revealed that treatment with menadione during exponential growth strongly enhanced the association of Sir3p with subtelomeric regions in a wild-type strain, but not in an rph1Δ strain, at day one of CLS (Fig. 7A). Sir3p binding was not similarly affected at other silenced loci (Fig. S6A). Additionally, Sir3p was required for subtelomeric silencing following mtROS signaling (Fig. 7B), but at other silenced loci, menadione-induced transcriptional changes were mainly Sir3p-independent (Fig. S6B). Finally, hormetic mtROS signaling failed to extend CLS of a sir3Δ strain (Fig. 7C and Fig. S6C). These findings demonstrate that Rph1p-regulated subtelomeric silencing, mediated by Sir3p, is a key outcome of hormetic mtROS signaling that extends lifespan.
Figure 7. mtROS Signaling via Rph1p Promotes Sir3p Binding at Subtelomeres to Repress Gene Expression and Extend CLS.
(A) ChIP of Sir3p in wild type (DBY2006) and rph1Δ at a control region (ACT1) and two subtelomeric genes (YPS6 and YEL077C). Cells were analyzed at day one of CLS following treatment with 50 μM menadione or vehicle during exponential growth.
(B) Quantitative RT-PCR of subtelomeric transcripts relative to ACT1 at day one of CLS in wild-type or sir3Δ yeast following 50 μM menadione or vehicle during exponential growth.
(C) CLS of wild-type and sir3Δ treated with 50 μM menadione (MD) or vehicle (nt) during logarithmic growth.
(D) Model for mtROS signaling via DNA damage response kinases and Rph1p to regulate subtelomeric heterochromatin formation and CLS.
DISCUSSION
Mitochondria, epigenetics, and telomere function have all been independently implicated in lifespan regulation. Our investigation of mitochondrial-stress signaling in yeast demonstrates coordination among these processes to promote lifespan extension. Specifically, we have elucidated a mitochondria-to-nucleus signaling pathway (Fig. 7D) in which the DNA-damage kinase Tel1p transduces an mtROS signal to activate Rad53p, which in turn regulates the ability of the histone demethylase Rph1p to bind subtelomeric chromatin. The loss of Rph1p from subtelomeric chromatin elevates H3K36me3 and enhances binding of the silencing protein Sir3p to repress subtelomeric transcription. Sir3p-mediated subtelomeric silencing is an essential outcome of mtROS signaling and adaptation that ultimately extends CLS. Although heterochromatin maintenance at several loci influences yeast lifespan (Dang et al., 2009; Kaeberlein et al., 1999), mtROS signaling specifically targets subtelomeric heterochromatin. We propose that this specificity is a key component of the mtROS hormetic response, and corroborates previous studies in which Sir proteins target distinct genomic regions under different conditions, with unique consequences for chronological and replicative aging (Ruault et al., 2011; Salvi et al., 2013). For example, sir2Δ enhances CLS extension under caloric restriction or in sch9Δ strains by inducing stress response and metabolic genes that promote longevity (Fabrizio et al., 2005), but shortens RLS due to a failure to maintain heterochromatin at the rDNA cluster (Kaeberlein et al., 1999).
Subtelomeric and telomeric heterochromatin maintenance preserves telomere length and genomic stability in yeast and mammals (Schoeftner and Blasco, 2009). Telomere function is intimately connected with health and lifespan, but is most often associated with replicative senescence. Our finding that altered subtelomeric silencing supports CLS extension implies that telomere function regulates post-mitotic cellular health and aging. At this point, the consequences of subtelomeric silencing that promote CLS are not clear. Transcription of subtelomeric coding or non-coding genes may limit lifespan by altering metabolism, telomere length, or general transcription (Pryde and Louis, 1997; Schoeftner and Blasco, 2009; Smith et al., 2011; Yamada et al., 1998). Alternatively, increased telomere packaging would both repress transcription and protect telomeres from DNA damage-induced attrition, which can lead to gross chromosomal rearrangements that destabilize the nuclear genome (Davoli and de Lange, 2011). We propose that hormetic mtROS stimulate pre-emptive telomere and subtelomere heterochromatin formation early in life, which minimizes telomeric DNA damage and maintains genomic stability during aging.
Although epigenetic outcomes are essential to the adaptive response, our data also indicate that transcriptional changes mediated by Msn2/4p, Gis1p, and Rph1p support mtROS adaptation. Menadione treatment induces a number of oxidative stress response genes in a manner dependent on Rph1p, which may account for the hormetic decrease in ROS during post-diauxic and stationary phases (Fig. S3E and Table S2D). All of the candidate mtROS-responsive proteins we investigated bind a similar DNA motif (Fig. 1B), and since all proteins are at least partially required for mtROS adaptation (Fig. S1), we speculate that Rph1p-dependent chromatin remodeling might facilitate Msn2/4p and/or Gis1p-mediated transcription at mtROS responsive genes. Future studies to explore the relationship between Rph1p, Msn2/4p, and Gis1p in transcriptional regulation and mtROS hormesis may uncover additional cooperation among these proteins in lifespan regulation.
Our results also indicate that Tel1p propagates an mtROS signal in the absence of nuclear DNA damage (Figures 4C–D and S3E–F), which suggests that certain DNA damage response kinases function as both ROS and DNA damage sensors, and can regulate distinct cellular processes in a stimulus-specific manner. Recent studies show that ATM (the mammalian homolog of Tel1p) acts as a redox-sensor (Guo et al., 2010). Our finding that Tel1p mediates the hormetic mtROS response in yeast suggests that this function is evolutionarily conserved.
Hormetic mtROS signaling ultimately inhibits the chromatin binding capacity of the histone demethylase Rph1p at subtelomeres, thereby increasing H3K36me3 at these regions (Figures 5C–D). Although the role of histone acetylation in transcriptional silencing at subtelomeres is well-established (Dang et al., 2009; Kozak et al., 2010; Santos-Rosa et al., 2004), it is increasingly evident that histone methylation can also contribute to this process in both yeast and mammals (Blasco, 2007; Caslini et al., 2009; Dang et al., 2009; Kozak et al., 2010; Santos-Rosa et al., 2004). Thus, it will important to determine which methyltransferases and demethylases function at subtelomeres during mammalian aging. Our study would support members of the jumonji-domain demethylases related to yeast Rph1p as prime candidates.
In conclusion, our results provide insight into multiple aspects of aging by integrating mitochondrial ROS, DNA-damage signaling, telomeres and epigenetics into a pro-longevity pathway. The mtROS signaling pathway identified herein connects mitochondria and telomere functional status and supports recent findings that these processes do not likely regulate aging in isolation (Sahin et al., 2011). The hormetic mtROS signaling pathway we describe should provide a platform for more integrated network theories of aging and lifespan regulation.
EXPERIMENTAL PROCEDURES
Yeast Strains
All strains used in this study are derivatives of DBY2006 (MATa his3-Δ200 leu2-3,-112 ura3-52 trp1-Δ1 ade2-1) and are listed in Table S7. Deletion strains were generated via gene replacement with URA3, KanMX6, or hphMX3 cassettes and transformed using the lithium acetate method (Gietz and Schiestl, 2007). Endogenous RPH1 was tagged at the C-terminus with a HA-KanMX6 cassette (Longtine et al., 1998). To generate the SOD2 overexpression strain (Fig. S3D), the endogenous SOD2 promoter (−600 to −1) was replaced with the TDH3 promoter (Kotter et al., 2009). All strains were verified by PCR.
Chronological Life Span Measurement
Yeast were grown in minimal dextrose medium supplemented with essential amino acids (Sherman, 1991). For lifespan assays, saturated cultures from single colonies were diluted to an OD600 of 0.01 in 50 mL fresh media and grown at 30°, 200 RPM until the OD reached 0.5. Either menadione (50 μM final concentration, Sigma) or an equivalent volume of ethanol (no treatment control) was added, and cultures were grown until the OD reached 2.0. At this point, cells were pelleted and resuspended in media from an untreated parallel culture. CLS was determined by trypan blue staining as described (Bonawitz et al., 2006). Measurements were initiated at day two of CLS. All strains and treatments were analyzed in triplicate. To quantitatively compare CLS extension between different strain backgrounds, the area under the viability curve was calculated in GraphPad Prism. The difference between the untreated and menadione treated wild-type areas was set to one, and differences within strain backgrounds are expressed as percent of wild-type CLS extension.
Chemical Toxicity
Wild-type yeast were grown to an OD600 of 0.5 and treated with 50 μM menadione, 200 μM menadione, 0.01% MMS, 0.02% MMS, or ethanol (vehicle control). Cell growth was monitored for 24 hours of treatment, and viability was assessed via trypan blue staining.
Flow Cytometry
Measurement of cellular superoxide using dihydroethidium (DHE) was performed as described (Bonawitz et al., 2007; Pan et al., 2011).
Immunoblotting
Yeast whole-cell extracts were prepared from cells in the exponential growth phase treated with menadione, MMS (0.01% final concentration) or ethanol for 30 minutes (Keogh et al., 2006). Proteins were separated in 6% SDS-PAGE gels (to resolve Rad53p and Rph1-HA phosphorylation) or 12% SDS-PAGE gels and transferred to PVDF membranes (Millipore Immobilon). Blocking and antibody incubations were carried out in TBST (10 mM Tris [pH 8.0], 150 mM NaCl, 0.05% Tween 20) containing 5% nonfat dry milk. Membranes were incubated with anti-Rad53 (Santa Cruz, 1:300), anti-phospho-[S/T]Q (Cell Signaling, 1:1000), anti-MnSOD (Assay Designs, 1:500), anti-HA (Covance, 1:1000), anti-actin (1:1000, Chemicon) or anti-GAPDH-HRP (1:1000, Abcam). Immunoblotting was performed on protein isolated from at least two independent experiments. Band density traces and quantification were determined using ImageJ (National Institutes of Health).
8-hydroxyguanosine Quantification
Yeast cultures in exponential growth phase were treated with ethanol, 50 μM menadione, or 200 μM menadione for 30 minutes prior to DNA extraction (Hoffman, 2001). Total DNA was treated with RNase A and proteinase K (Sigma), and then precipitated with phenol:chloroform and ethanol. DNA (5 μg per sample) was blotted onto a nitrocellulose membrane. The membrane was dried for one hour at 85°C, blocked in TBST with 5% bovine serum albumin (BSA, Sigma), and incubated overnight in anti-8-hydroxyguanosine (Chemicon) diluted 1:500 in BSA-TBST. After washing in TBST, the membrane was incubated in HRP-conjugated anti-goat secondary at a 1:2000 dilution for one hour and visualized with Supersignal West Pico Chemiluminescent Substrate. To normalize dot intensity to total DNA, the membrane was incubated in 2.5 μg DAPI and visualized on a Versadoc imager (Bio-Rad). Antibody and DAPI dot intensity from triplicate independent treatments were quantified using ImageJ.
RNA Preparation and Microarray Analysis
RNA extraction and purification were performed as described (Bonawitz et al., 2008) from wild-type DBY2006 and rph1Δ cultures grown for 24 hours after addition of 50 μM menadione or ethanol. RNA quality bioanalysis, cDNA synthesis, and hybridization to a Roche/NimbleGen 12x135K array were performed in collaboration with the Yale University W.M. Keck Foundation Biotechnology Resource Laboratory according to the manufacturer’s protocol. Two independent biological replicates were prepared for each sample, and hybridization was performed in duplicate to generate technical replicates. Raw data were RMA normalized (Irizarry et al., 2003) using Nimblescan software. Probe expression values were used to determine fold-change and filtered to significantly induced and repressed probe lists (p≤0.01 as determined by student’s t- test). Gene lists were generated based on the occurrence of at least two probes for a particular gene present exclusively in induced or repressed probe lists. To map the chromosomal location of genes in sectors A, B, and C, the distance from the transcription start site to the nearest telomere was determined. Raw microarray data were deposited in the Gene Expression Omnibus repository (accession # GSE45383). Gene lists are described in the Supplemental Excel File.
Normalized expression data were downloaded from the Gene Expression Omnibus at NCBI (GSM1102, record GDS108 [Gasch et al., 2000]) and from the supplemental online material associated with Hardwick et al, 1999. Gene lists were ranked according to fold change, and the top 5% induced genes were selected for further analysis to identify genes induced in both datasets. The co-induced genes were further analyzed using Webmotifs (Romer et al., 2007) and Yeastract (Abdulrehman et al., 2011) to generate the data shown in Figure 1C.
Quantitative Reverse Transcription PCR
RNA was isolated as described above, and converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (Agilent) according to the manufacturer’s instructions. cDNA was diluted 1:10, and each 20 μL reaction contained 10 μL diluted cDNA, 0.4 μL each primer (from a 25 μM stock), and 9.2 μL BioRad iQ Sybr Mix. PCR conditions were as described (Cotney et al., 2007).
Chromatin Immunoprecipitation
Yeast cultures were treated with 50 μM menadione or ethanol from OD 0.5 to OD 2.0. Twenty-four hours after the initiation of menadione treatment at OD 0.5, cells were processed for ChIP analysis, which was performed essentially as described (Aparicio et al., 2004) with the following modifications. DNA was sheared in a Bioruptor UCD-200 sonicator at 4°C five times for 20 seconds each. Chromatin was pre-cleared by incubating with Protein A agarose beads (Millipore) for 30 minutes at room temperature. Antibodies used were anti-HA (Covance), anti-trimethyl H3K36 (Epigentek), anti-H3 (Abcam), and anti-Sir3 (Santa Cruz). Quantitative PCR of three technical replicates per sample was performed using the same conditions described for RT-PCR, except the reaction proceeded for 40 cycles. Primers are listed in Table S8.
Statistical Analysis
For CLS, FACS, 8-hydroxyguanosine, and RT-PCR analysis, all data points represent the mean of biological triplicate samples inoculated from isolated single colonies. Error bars represent the standard error of the mean (SEM). P values were determined using student’s unpaired t-test, and values for relevant comparisons are indicated within figures. Statistical analysis for enrichment of subtelomeric transcripts was determined using a hypergeometric probability distribution (Martin et al., 2004). For ChIP analysis, data points represent the mean of two biological replicate samples inoculated from isolated single colonies. Error bars represent the range.
Supplementary Material
HIGHLIGHTS.
The H3K36 demethylase Rph1p is required for mitochondrial stress-induced longevity
Conserved DNA-damage response kinases transduce mitochondrial ROS signals
Mitochondria ROS signals reduce Rph1p binding at subtelomeres and increase H3K36me3
Subtelomeric silencing is essential for mitochondrial ROS-mediated longevity
Acknowledgments
This work was supported by grant W911NF-11-1-0376 from the Army Research Office to G.S.S. E.A.S. was supported by NIH Genetics Training Grant T32 GM07499 and currently by NIH pre-doctoral fellowship F31 AG043242. N.R. was supported by a postdoctoral fellowship from the United Mitochondrial Disease Foundation. We thank Drs. Megan King, Patrick Lusk, and David Stern for reagents used in the study and insightful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Abdulrehman D, Monteiro PT, Teixeira MC, Mira NP, Lourenco AB, dos Santos SC, Cabrito TR, Francisco AP, Madeira SC, Aires RS, et al. YEASTRACT: providing a programmatic access to curated transcriptional regulatory associations in Saccharomyces cerevisiae through a web services interface. Nucleic Acids Res. 2011;39:D136–140. doi: 10.1093/nar/gkq964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaf M, Utley RT, Lacoste N, Tan S, Briggs SD, Cote J. Interplay of chromatin modifiers on a short basic patch of histone H4 tail defines the boundary of telomeric heterochromatin. Mol Cell. 2007;28:1002–1014. doi: 10.1016/j.molcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio O, Geisberg JV, Struhl K. Chromatin immunoprecipitation for determining the association of proteins with specific genomic sequences in vivo. Curr Protoc Cell Biol. 2004;Chapter 17(Unit 17):17. doi: 10.1002/0471143030.cb1707s23. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Barton AB, Kaback DB. Telomeric silencing of an open reading frame in Saccharomyces cerevisiae. Genetics. 2006;173:1169–1173. doi: 10.1534/genetics.106.058420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- Blasco MA. The epigenetic regulation of mammalian telomeres. Nat Rev Genet. 2007;8:299–309. doi: 10.1038/nrg2047. [DOI] [PubMed] [Google Scholar]
- Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonawitz ND, Chatenay-Lapointe M, Wearn CM, Shadel GS. Expression of the rDNA-encoded mitochondrial protein Tar1p is stringently controlled and responds differentially to mitochondrial respiratory demand and dysfunction. Curr Genet. 2008;54:83–94. doi: 10.1007/s00294-008-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonawitz ND, Rodeheffer MS, Shadel GS. Defective mitochondrial gene expression results in reactive oxygen species-mediated inhibition of respiration and reduction of yeast life span. Mol Cell Biol. 2006;26:4818–4829. doi: 10.1128/MCB.02360-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caslini C, Connelly JA, Serna A, Broccoli D, Hess JL. MLL associates with telomeres and regulates telomeric repeat-containing RNA transcription. Mol Cell Biol. 2009;29:4519–4526. doi: 10.1128/MCB.00195-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro FA, Mariani D, Panek AD, Eleutherio EC, Pereira MD. Cytotoxicity mechanism of two naphthoquinones (menadione and plumbagin) in Saccharomyces cerevisiae. PLoS One. 2008;3:e3999. doi: 10.1371/journal.pone.0003999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotney J, Wang Z, Shadel GS. Relative abundance of the human mitochondrial transcription system and distinct roles for h-mtTFB1 and h-mtTFB2 in mitochondrial biogenesis and gene expression. Nucleic Acids Res. 2007;35:4042–4054. doi: 10.1093/nar/gkm424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T, de Lange T. The causes and consequences of polyploidy in normal development and cancer. Annu Rev Cell Dev Biol. 2011;27:585–610. doi: 10.1146/annurev-cellbio-092910-154234. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Methods Mol Biol. 2007;371:89–95. doi: 10.1007/978-1-59745-361-5_8. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Huang M, Metzner S, Botstein D, Elledge SJ, Brown PO. Genomic expression responses to DNA-damaging agents and the regulatory role of the yeast ATR homolog Mec1p. Mol Biol Cell. 2001;12:2987–3003. doi: 10.1091/mbc.12.10.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007;2:31–34. doi: 10.1038/nprot.2007.13. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- Hardwick JS, Kuruvilla FG, Tong JK, Shamji AF, Schreiber SL. Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc Natl Acad Sci U S A. 1999;96:14866–14870. doi: 10.1073/pnas.96.26.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JC, Haber JE. Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet. 2006;40:209–235. doi: 10.1146/annurev.genet.40.051206.105231. [DOI] [PubMed] [Google Scholar]
- Hoffman CS. Preparation of yeast DNA. Curr Protoc Mol Biol. 2001;Chapter 13(Unit13):11. doi: 10.1002/0471142727.mb1311s39. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M. Lessons on longevity from budding yeast. Nature. 2010;464:513–519. doi: 10.1038/nature08981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahalil B, Girard PM, Boiteux S, Dizdaroglu M. Substrate specificity of the Ogg1 protein of Saccharomyces cerevisiae: excision of guanine lesions produced in DNA by ionizing radiation- or hydrogen peroxide/metal ion-generated free radicals. Nucleic Acids Res. 1998;26:1228–1233. doi: 10.1093/nar/26.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Keogh MC, Kim JA, Downey M, Fillingham J, Chowdhury D, Harrison JC, Onishi M, Datta N, Galicia S, Emili A, et al. A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature. 2006;439:497–501. doi: 10.1038/nature04384. [DOI] [PubMed] [Google Scholar]
- Kim EM, Jang YK, Park SD. Phosphorylation of Rph1, a damage-responsive repressor of PHR1 in Saccharomyces cerevisiae, is dependent upon Rad53 kinase. Nucleic Acids Res. 2002;30:643–648. doi: 10.1093/nar/30.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Lim DS, Canman CE, Kastan MB. Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem. 1999;274:37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- Kotter P, Weigand JE, Meyer B, Entian KD, Suess B. A fast and efficient translational control system for conditional expression of yeast genes. Nucleic Acids Res. 2009;37:e120. doi: 10.1093/nar/gkp578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak ML, Chavez A, Dang W, Berger SL, Ashok A, Guo X, Johnson FB. Inactivation of the Sas2 histone acetyltransferase delays senescence driven by telomere dysfunction. EMBO J. 2010;29:158–170. doi: 10.1038/emboj.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Schwartz MF, Duong JK, Stern DF. Rad53 phosphorylation site clusters are important for Rad53 regulation and signaling. Mol Cell Biol. 2003;23:6300–6314. doi: 10.1128/MCB.23.17.6300-6314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CY, Hsu PH, Chou DF, Pan CY, Wang LC, Huang WC, Tsai MD, Lo WS. The histone H3K36 demethylase Rph1/KDM4 regulates the expression of the photoreactivation gene PHR1. Nucleic Acids Res. 2011;39:4151–4165. doi: 10.1093/nar/gkr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Shadel GS, Kaeberlein M, Kennedy B. Replicative and Chronological Aging in Saccharomyces cerevisiae. Cell Metab. 2012;16:18–31. doi: 10.1016/j.cmet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Millar CB, Grunstein M. Genome-wide patterns of histone modifications in yeast. Nat Rev Mol Cell Biol. 2006;7:657–666. doi: 10.1038/nrm1986. [DOI] [PubMed] [Google Scholar]
- Nakada D, Hirano Y, Sugimoto K. Requirement of the Mre11 complex and exonuclease 1 for activation of the Mec1 signaling pathway. Mol Cell Biol. 2004;24:10016–10025. doi: 10.1128/MCB.24.22.10016-10025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal S, DeRisi JL, Blackburn EH. The genome-wide expression response to telomerase deletion in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2002;99:9316–9321. doi: 10.1073/pnas.142162499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HH, Feng Q, Wang H, Erdjument-Bromage H, Tempst P, Zhang Y, Struhl K. Lysine methylation within the globular domain of histone H3 by Dot1 is important for telomeric silencing and Sir protein association. Genes Dev. 2002;16:1518–1527. doi: 10.1101/gad.1001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris A, Boeke JD. Silent information regulator 3: the Goldilocks of the silencing complex. Genes Dev. 2010;24:115–122. doi: 10.1101/gad.1865510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzechowski Westholm J, Tronnersjo S, Nordberg N, Olsson I, Komorowski J, Ronne H. Gis1 and rph1 regulate glycerol and acetate metabolism in glucose depleted yeast cells. PLoS One. 2012;7:e31577. doi: 10.1371/journal.pone.0031577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011;13:668–678. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Lustig AJ. Telomere structure regulates the heritability of repressed subtelomeric chromatin in Saccharomyces cerevisiae. Genetics. 2000;154:587–598. doi: 10.1093/genetics/154.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicioli A, Lucca C, Liberi G, Marini F, Lopes M, Plevani P, Romano A, Di Fiore PP, Foiani M. Activation of Rad53 kinase in response to DNA damage and its effect in modulating phosphorylation of the lagging strand DNA polymerase. EMBO J. 1999;18:6561–6572. doi: 10.1093/emboj/18.22.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryde FE, Louis EJ. Saccharomyces cerevisiae telomeres. A review. Biochemistry (Mosc) 1997;62:1232–1241. [PubMed] [Google Scholar]
- Raimundo N, Song L, Shutt TE, McKay SE, Cotney J, Guan MX, Gilliland TC, Hohuan D, Santos-Sacchi J, Shadel GS. Mitochondrial Stress Engages E2F1 Apoptotic Signaling to Cause Deafness. Cell. 2012;148:716–726. doi: 10.1016/j.cell.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer KA, Kayombya GR, Fraenkel E. WebMOTIFS: automated discovery, filtering and scoring of DNA sequence motifs using multiple programs and Bayesian approaches. Nucleic Acids Res. 2007;35:W217–220. doi: 10.1093/nar/gkm376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruault M, De Meyer A, Loiodice I, Taddei A. Clustering heterochromatin: Sir3 promotes telomere clustering independently of silencing in yeast. J Cell Biol. 2011;192:417–431. doi: 10.1083/jcb.201008007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche LN, Kirchmaier AL, Rine J. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu Rev Biochem. 2003;72:481–516. doi: 10.1146/annurev.biochem.72.121801.161547. [DOI] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi JS, Chan JN, Pettigrew C, Liu TT, Wu JD, Mekhail K. Enforcement of a lifespan-sustaining distribution of Sir2 between telomeres, mating-type loci, and rDNA repeats by Rif1. Aging Cell. 2013;12:67–75. doi: 10.1111/acel.12020. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Desany BA, Jones WJ, Liu Q, Wang B, Elledge SJ. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science. 1996;271:357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Bannister AJ, Dehe PM, Geli V, Kouzarides T. Methylation of H3 lysine 4 at euchromatin promotes Sir3p association with heterochromatin. J Biol Chem. 2004;279:47506–47512. doi: 10.1074/jbc.M407949200. [DOI] [PubMed] [Google Scholar]
- Schoeftner S, Blasco MA. A ‘higher order’ of telomere regulation: telomere heterochromatin and telomeric RNAs. EMBO J. 2009;28:2323–2336. doi: 10.1038/emboj.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Miller LR, Kreisberg R, Vazquez L, Wan Y, Aitchison JD. Environment-responsive transcription factors bind subtelomeric elements and regulate gene silencing. Mol Syst Biol. 2011;7:455. doi: 10.1038/msb.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “Mitohormesis” for health and vitality. Med Hypotheses. 2006;66:832–843. doi: 10.1016/j.mehy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Tu S, Bulloch EM, Yang L, Ren C, Huang WC, Hsu PH, Chen CH, Liao CL, Yu HM, Lo WS, et al. Identification of histone demethylases in Saccharomyces cerevisiae. J Biol Chem. 2007;282:14262–14271. doi: 10.1074/jbc.M609900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol. 2012;13:115–126. doi: 10.1038/nrm3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Madia F, Hu J, Ge H, Li LM, Longo VD. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet. 2009;5:e1000467. doi: 10.1371/journal.pgen.1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Hayatsu N, Matsuura A, Ishikawa F. Y’-Help1, a DNA helicase encoded by the yeast subtelomeric Y’ element, is induced in survivors defective for telomerase. J Biol Chem. 1998;273:33360–33366. doi: 10.1074/jbc.273.50.33360. [DOI] [PubMed] [Google Scholar]
- Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, Ristow M. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Muller EG, Rothstein R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell. 1998;2:329–340. doi: 10.1016/s1097-2765(00)80277-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.