Abstract
Ke, Tao, Jiye Wang, Erik R. Swenson, Xiangnan Zhang, Yunlong Hu, Yaoming Chen, Mingchao Liu, Wenbin Zhang, Feng Zhao, Xuefeng Shen, Qun Yang, Jingyuan Chen, and Wenjing Luo. Effect of acetazolamide and gingko biloba on the human pulmonary vascular response to an acute altitude ascent. High Alt Med Biol 14:162–167, 2013.—Acetazolamide and gingko biloba are the two most investigated drugs for the prevention of acute mountain sickness (AMS). Evidence suggests that they may also reduce pulmonary artery systolic pressure (PASP). To investigate whether these two drugs for AMS prevention also reduce PASP with rapid airlift ascent to high altitude, a randomized controlled trial was conducted on 28 healthy young men with acetazolamide (125 mg bid), gingko biloba (120 mg bid), or placebo for 3 days prior to airlift ascent (397 m) and for the first 3 days at high altitude (3658 m). PASP, AMS, arterial oxygen saturation (Sao2), mean arterial pressure (MAP), heart rate (HR), forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and peak expiratory flow (PEF) were assessed both at 397 m and 3658 m. HR, PEF, and PASP increased with altitude exposure (p<0.05), and SaO2 decreased (p<0.05). PASP with acetazolamide (mean at 3658 m, 26.2 mm Hg; incremental change, 4.7 mm Hg, 95% CI., 2.6–6.9 mm Hg) was lower than that with ginkgo biloba (mean at 3658 m, 33.7 mm Hg, p=0.001; incremental change, 13.1 mm Hg, 95%CI., 9.6–16.5 mm Hg, p=0.002), and with placebo (mean at 3658 m, 34.7 mm Hg, p<0.001; 14.4 mm Hg, 95% CI., 8.8–20.0 mm Hg, p=0.001). The data show that a low prophylactic dosage of acetazolamide, but not gingko biloba, mitigates the early increase of PASP in a quick ascent profile.
Key words: acetazolamide, gingko Biloba, pulmonary artery systolic pressure, acute mountain sickness, randomized controlled trial
Introduction
High-altitude pulmonary edema (HAPE) is a life-threatening noncardiogenic form of pulmonary edema that usually occurs after acute ascent to altitudes above 2500 m (Maggiorini, 2006). The pathogenic mechanism of HAPE involves an excessive elevation of pulmonary artery pressure (PAP), as measured directly by pulmonary artery catheterization or indirectly by echocardiography (Allemann et al., 2000; Maggiorini et al., 2006). As a significant feature of HAPE susceptibility, there is an exaggerated rise of PAP in hypoxia (Kawashima et al., 1992) and/or exercise (Grünig et al., 2000; Bärtsch et al., 2005). Upon exposure to altitude, PAP elevation is due to hypoxic pulmonary vasoconstriction (HPV). Uneven HPV (i.e., heterogeneity of regional HPV) causes overperfusion of the lesser constricted regional vessels, with greater transmission of the high PAP to the microvasculature and capillaries, resulting in alveolar–capillary barrier leakage (Swenson et al., 2002; Hopkins et al., 2005). Prevention and treatment of HAPE by reducing the PAP in humans (Richalet et al., 2005; Maggiorini et al., 2006) and experimental animal models (Berg et al., 2004) support the concept that high PAP plays a pivotal role in the development of HAPE.
Acetazolamide and ginkgo biloba extract are two drugs that have been mostly investigated for prevention of acute mountain sickness (AMS). An inhibitory effect of acetazolamide on HPV was first observed as early as 1977 (Emery et al., 1977), but it was not confirmed until more comprehensive studies in isolated perfused lungs and live animals (Deem et al., 2000; Hohne et al., 2004). It was observed that both acetazolamide and ginkgo biloba extract could prevent early HAPE in rats (Berg, 2004; Berg et al., 2004). Further evidence showed that ginkgo biloba administration increased production of NO and decreased endothelin-1 and intracellular free Ca2+ of vascular endothelial cells (Ren et al., 2002; Wu et al., 2008), both of which were important mediators of pulmonary vasoconstriction. Although the two drugs hold promise for the prevention of pulmonary artery systolic pressure (PASP), there is no trial evaluating the effect of acetazolamide or ginkgo biloba extract on PASP in humans in the context of a quick ascent altitude exposure, such as travelers ascending rapidly from lowland elevations.
An acute exposure from low land to high altitude by air is an ideal protocol for the assessment of vascular responses to hypoxia, since the initial acclimatization time that may vary with different ascent profiles in other studies is well controlled. It also has direct clinical relevance for the many people nowadays who fly to high altitude regions for recreation or work. In the present study, we took advantage of an airlift ascent to evaluate the effect of acetazolamide and gingko biloba on PASP in a group of young men who were flown by airplane from Xi'an (397 m) to Lhasa (3658 m) in a randomized, double-blind and placebo-controlled clinical trial.
Methods
Participants
The trial was performed according to a prospective, double-blind, placebo-controlled design. Twenty-eight healthy lowland young men (17–22 years old) with no altitude experiences (>2500 m) in the preceding 2 years were enrolled into the trial on July 17–20, 2011. The highest altitudes they had ever climbed to were 1560 m to 2300 m, and none of them had ever been to the Tibet plateau. They were flown by a commercial airplane from Xi'an to Lhasa nonstop in 3 hours. All participants were fully aware of the study procedures and provided informed written consent. The Chinese Ethics Committee of Registering Clinical Trials (approval number, ChiECRCT-2011046) approved the study, which was conducted in accordance with the Declaration of Helsinki.
Treatment
The participants were randomized into three groups according to random numbers generated by using a software package (SPSS version 16.0, SPSS Inc, Chicago, Il), with nine in the acetazolamide group (125 mg bid), ten in the gingko biloba group (120 mg bid), and nine in the placebo group. Acetazolamide (Diamox; Wyeth Pharmaceuticals; USA), gingko biloba (Ginaton; Dr. Willmar Schwabe Pharmaceuticals; Germany), and placebo (provided by the Institute of Pharmaceuticals of the Fourth Military Medical University) were packaged in visually identical capsules at the Institute of Pharmaceuticals of the Fourth Military Medical University. Drug administration started 3 days before the ascent (in Xi'an, 397 m) and was continued for the first day in Lhasa (3658 m): a 3-day pretreatment and 1-day treatment duration. The compliance of each participant was followed and supervised by three investigators.
Outcomes
The primary outcome was PASP to hypoxia on the first day in the three groups. PASP on the second day and the third day were also observed as follow-up data. Secondary outcomes included AMS, arterial oxygen saturation (Sao2), mean artery pressure (MAP), heart rate (HR), and spirometry parameters (FVC, FEV1%, PEF) to hypoxia. PASP was estimated by the tricuspid gradient (TR) method using FLYING Diagnostic Ultrasound System equipped with a 2.5 MHz transthoracic probe (Neusoft, China) (Peacock et al., 1990; Yock and Popp, 1984). Recordings were read by two independent cardiologists who were blinded to the treatment assignment, and any disagreements were resolved by consensus. Intra- and interobserver variation of peak velocity of TR was tested with repeated analysis of ten randomly selected tracings by the two cardiologists with an interexamination time of 1 week. The coefficient of variation (CV) was 6.0% for intraobserver variability and 8.3% for interobserver variability, respectively. AMS was measured by the Lake Louise Acute Mountain Sickness Scoring System, a well-validated standard for field evaluation of AMS (Roach et al., 1993). AMS is quantified on the Lake Louise questionnaire as the presence of headache and at least one of the symptoms of nausea or vomiting, fatigue, dizziness, or difficulty sleeping, and a total score of at least 3. Spirometry variables were obtained using a portable spirometer (MasterScope, Jaeger, Germany). The blood pressure was measured by sphygmomanometry, and MAP was estimated by the equation of “MAP=2/3 diastolic pressure plus 1/3 systolic pressure”. A three-lead ECG was used to measure HR. Sao2 was measured by polygraph (BIOPAC systems, MP150, USA) with a peripheral blood oxygen sensor applied to a finger, which was tested and calibrated following the manufacturer's recommendations. Baseline measurements were conducted in the Fourth Military Medical University in Xi'an. Altitude measurements were conducted in Fukang Hospital in Lhasa. The instruments and examination performers were consistent in the two settings. The participants were instructed to avoid exercise within 12 h prior to the experimental testing and were forbidden to do physical activity at least 30 min before examination.
Statistical methods
PASP, Sao2, MAP, HR, FVC, FEV1, and PEF values in the three arms were compared at lowland and at high altitude using one-way ANOVA. The changes in PASP, Sao2, MAP, HR, FVC, FEV1, and PEF from lowland to high altitude in each arm were compared using paired samples t test. The average LLS were contrasted using Kruskal-Wallis H test, and followed by the multiple comparisons if p<0.05. Group differences of PASP over the study period were analyzed using a mixed linear model with one randomized group (placebo, acetazolamide, gingko biloba) and a repeated measure (PASP at sea level, altitude day 1, 2, and 3). The linear functions of PASP and Sao2 of the three groups were compared by using F test. Analysis for efficacy was performed on the intention to treat samples. All statistical analyses were conducted using SPSS version 16.0 (SPSS Inc, Chicago, Illinois).
Results
Baseline characteristics of the 28 participants are summarized in Table 1. The acetazolamide, ginkgo biloba, and placebo groups were well matched with no statistically significant differences in age, weight, height, and BMI. All subjects completed the treatment and follow up as planned.
Table 1.
Baseline Characteristics of the Study Groups
| Characteristics | Acetazolamide | Ginkgo biloba | Placebo | P value* |
|---|---|---|---|---|
| Age, y | 19.2 (1.5) | 19.4 (1.5) | 19.2 (1.7) | 0.962 |
| Weight, kg | 61.0 (4.5) | 64.9 (8.0) | 64.7 (5.8) | 0.357 |
| Height, cm | 170.4 (5.9) | 173.6 (5.9) | 174.2 (3.7) | 0.291 |
| BMI, kg/m2 | 21.0 (1.8) | 21.4 (1.8) | 21.2 (1.3) | 0.863 |
One-way ANOVA, data are shown as means±SD.
Sao2 was decreased on the first day at high altitude, and the HR and PEF were increased significantly. The Sao2 in the acetazolamide group, but not the ginkgo biloba group, was higher than that in the placebo group (Table 2). The Lake Louise score in the acetazolamide group was lower than that in the ginkgo biloba group and the placebo group (Kruskal-Wallis Test, p=0.016).
Table 2.
Heart Rate, Mean Arterial Pressure, Arterial Oxygen Saturation, and Spirometry Parameters of the Three Groups at 397 m and 3658 m (The First Day)
| |
Acetazolamide |
Ginkgo biloba |
Placebo |
|||
|---|---|---|---|---|---|---|
| Variables | 397 m | 3658 m | 397 m | 3658 m | 397 m | 3658 m |
| HR | 76 (68, 84) | 85 (73, 90)* | 76 (72, 81) | 88 (79, 96)* | 72 (63, 81) | 79 (68, 91)* |
| MAP | 85 (76, 90) | 88 (83, 94) | 84 (77, 90) | 88 (83, 93) | 84 (80, 89) | 88 (84, 92) |
| SaO2% | 97.6 (97.2, 97.9) | 93.7 (92.4, 95.0)†‡ | 97.6 (97.2, 97.9) | 90.0 (88.3, 91.7)† | 97.5 (97.0, 98.0) | 91.2 (89.0, 93.5)† |
| LLS§ | 0 (0–1) | 1 (0–7) | 0 (0–1) | 5 (0–9) | 0 (0–2) | 3 (2–9) |
| FVC | 4.7 (4.4, 4.9) | 4.7 (4.3, 5.1) | 4.7 (4.5, 5.0) | 4.7 (4.3, 5.1) | 4.8 (4.5, 5.2) | 4.8 (4.4, 5.2) |
| FEV1% | 85.2 (79.5, 90.8) | 85.8 (79.6, 92.0) | 85.8 (80.6, 90.9) | 85.7 (80.9, 90.4) | 88.1 (84.5, 91.6) | 89.7 (86.5, 92.9) |
| PEF | 8.2 (7.1, 9.3) | 9.9 (9.2, 10.5)† | 8.6 (7.5, 9.6) | 10.0 (8.7, 11.2)† | 9.0 (8.1, 9.8) | 10.4 (9.1, 11.8)† |
FEV1%, forced expiratory volume in one second/forced vital capacity; FVC, forced vital capacity, liters; HR, heart rate, beats/minute; LLS, Lake Louise score; MAP, mean arterial pressure, mm Hg; PEF, peak expiratory flow, liters/minute. SaO2%, arterial oxygen saturation. Data are shown as mean and 95% CI.
p<0.05, †p<0.01: Paired Samples t Test, comparing the measurement at 3658 m with the measurement at 397 m; ‡p<0.05, one-way ANOVA with Bonferroni multi-comparison test, comparing acetazolamide or ginkgo biloba with the placebo group. §Data are shown as median and range.
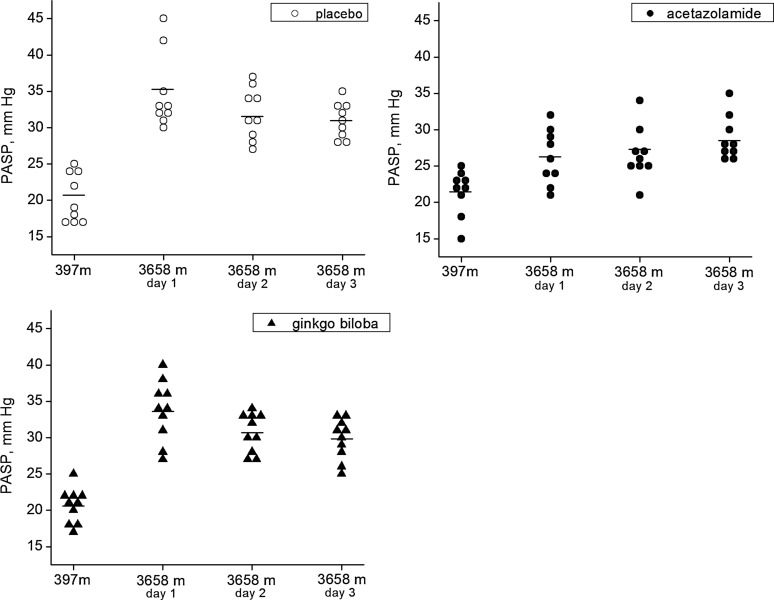
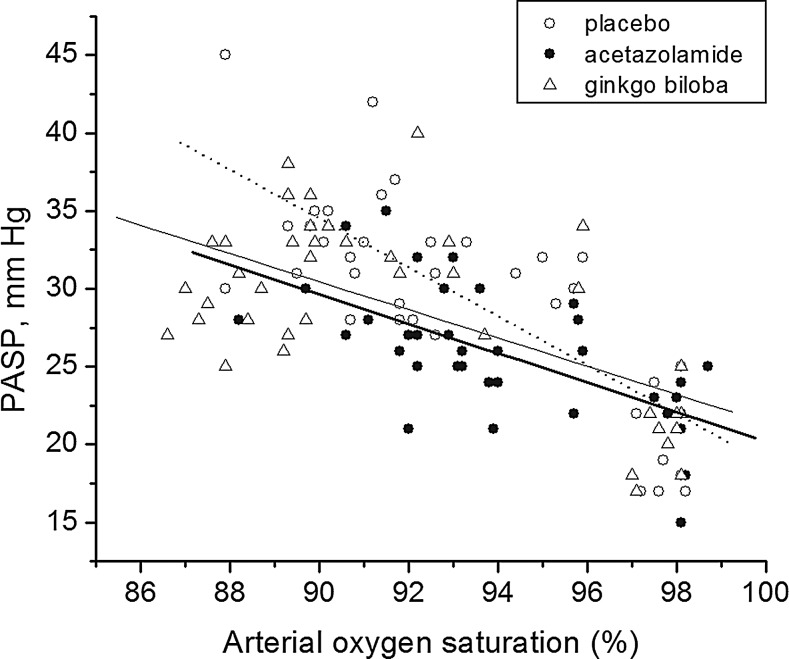
The data of PASP of the four observational days are shown in Figure 1. PASP in the three arms at 397 m were not significantly different (range, 15–25 mm Hg), but they were all increased on the first day after ascending from 397 m to 3658 m (p<0.01). PASP in the acetazolamide group (mean at 3658 m, 26.2 mm Hg) was significantly lower than that in the ginkgo biloba group (mean at 3658 m, 33.7 mm Hg, p=0.001) and the placebo group (mean at 3658m, 34.7 mm Hg, p<0.001). The incremental change in PASP in the acetazolamide group was 4.7 mm Hg (95% CI., 2.6–6.9 mm Hg), which was much lower than that in the ginkgo biloba group (13.1 mm Hg, 95% CI., 9.6–16.5 mm Hg, p=0.002) and the placebo group (14.4 mm Hg, 95% CI., 8.8–20.0 mm Hg, p=0.001). In the mixed linear model, the mean PASP of the 4 observational days in the acetazolamide group (25.7 mm Hg) was lower than that in the placebo group (29.5 mm Hg, p=0.009, multiple comparison using Bonferroni correction). After including Sao2 as a covariate in the mixed model, the differences of PASP in the three groups were still statistically significant (p=0.045), and the PASP in the acetazolamide group was lower than that in the placebo group (p<0.001, multiple comparison using Bonferroni correction). The slopes of the relation of PASP rise to Sao2 fall with transport to altitude were not significantly different between the three groups (Fig. 2).
FIG. 1.
Pulmonary artery systemic pressure (PASP) of the three groups (acetazolamide, ginkgo biloba, and placebo) at 397 m and on the first 3 days at 3658 m. The individual data of PASP in the three groups were plotted. PASP was estimated from the maximum velocity of continuous Doppler tricuspid regurgitation with an estimated value of 5 mm Hg of right atrium pressure (RAP). Bar represents mean PASP.
FIG. 2.
Arterial oxygen saturation (Sao2) versus pulmonary artery systemic pressure (PASP) plots in baseline and the first 3 days in high altitude. The slope of linear regression of Sao2 and PASP of the placebo group (dot line, y=175.80–1.57x) was not statistically different from the acetazolamide group (thick solid line, y=114.68–0.94x, p>0.05) or the ginkgo biloba group (thin solid line, y=111.82–0.90x, p>0.05).
During the trial, no subject experienced disorientation or incapacitating fatigue as assessed by clinical assessment of LLS, and no case of HAPE or high altitude cerebral edema (HACE) was diagnosed in any group. Adverse reactions in AZ group were limited to one subject with polyuria, and three subjects with numbness and tingling of the fingers and toes. There were no reported adverse reactions in either the gingko biloba or placebo groups, beyond the typical symptoms of AMS.
Discussion
The main finding of our trial was that a 3-day pretreatment with acetazolamide at a dosage of 125 mg bid and a further day of administration at high altitude can effectively reduce altitude-related pulmonary hypertension in young male adults. A similar prophylactic effect was not found for gingko biloba, despite published animal work suggesting a possible utility (Ren et al., 2002; Berg, 2004; Wu et al., 2008). Acetazolamide was also able to reduce severity of AMS in an airlift ascent altitude exposure profile, which gives further support for the use of a lower dose of acetazolamide for the prevention of AMS as shown by others (Basnyat et al., 2003; 2006; Tissot van Patot et al., 2008).
The effect of acetazolamide on the pulmonary vasculature has received much attention for its use not only in preventing AMS, but possibly in preventing HAPE. A recent human study using the dynamic end-tidal forcing technique showed that 750 mg/day of acetazolamide (250 mg tid started 1 day before an acute normobaric hypoxic exposure) was effective in inhibiting pulmonary vascular resistance (Teppema et al., 2007) with 12% O2, but the result was obtained in a simulated altitude. Basynat et al. (2008) found that acetazolamide fails to decrease PASP at high altitude in already partially acclimatized humans. It was reasoned that the dosage of 500 mg acetazolamide, which was effective in preventing AMS, was insufficient to alter hypoxic pulmonary pressure (PASP) after partial or full altitude acclimatization, similar to the findings by Faoro et al. (2007) after 2 weeks at high altitude in Bolivia. However, we show by using an airlift altitude exposure to minimize the confounding effect of partial or complete acclimatization, that 125 mg bid acetazolamide is effective in reducing PASP, as well as preventing AMS.
As far as we know, this is the first trial to test the efficacy of low-dose acetazolamide and gingko biloba on PASP at altitude. The strength of the present study was that acclimatization time was well controlled in an airlift ascent profile. As quick ascent rate is a risk factor for HAPE (Basnyat and Murdoch, 2003), the airlift model used in the present study may well reflect the real context under which many persons may use drug prophylaxis for quick ascent. The increase in PASP on the first day was reduced by acetazolamide, and the effect persisted on the following 2 days. Although the duration of the pharmacologic effect of a single administration of acetazolamide usually exceeds its serum half-life (6–8 h) (Moviat et al., 2006), the carryover effect of acetazolamide on the PASP at altitude was apparently diminishing and likely would have been sustained had the subjects continued to take acetazolamide.
Although the relation of change with altitude of PASP to Sao2 was not statistically different in the three groups and the effect of treatment was independent of Sao2 in the mixed model, a physiologic effect of Sao2 could not be excluded. Because acetazolamide stimulates breathing at high altitude as indirectly shown by the 2% higher Sao2 of the acetazolamide group versus placebo and gingko biloba groups, acetazolamide may also lower PASP by reducing the main stimulus for HPV, namely a lower alveolar Po2 (Hohne et al., 2004). Other aspects arising from the systematic effect of carbonic anhydrase inhibition, such as a decrease in end-tidal Pco2 (Balanos et al., 2003) with hyperventilation can be invoked because respiratory alkalosis does reduce HPV. We cannot discriminate the underlying pathway of PASP inhibition by acetazolamide in the current study, but studies in dogs (Hohne et al., 2004) where alveolar Po2 and ventilation was held constant demonstrated that acetazolamide is acting directly on the vasculature and not indirectly by increasing ventilation and altering alveolar Po2. It has been demonstrated that the inhibitory effect of acetazolamide on HPV is unrelated to carbonic anhydrase inhibition (Hohne et al., 2007; Shimoda et al., 2007), but rather by reducing the magnitude of intracellular Ca2+ rise with hypoxia in pulmonary vascular smooth muscle (Shimoda et al., 2007).
Because increased PAP is considered a key causal factor in HAPE, the present study has significance for the future evaluation of the effect of this lower dose of acetazolamide on HAPE prevention in HAPE-susceptible subjects. As treatment of chronic mountain sickness by acetazolamide is recommended (Richalet et al., 2005), it will be worth testing the effect of acetazolamide on chronic hypoxic pulmonary hypertension, as well as on the amelioration of polycythemia in chronic mountain sickness cases. Although the current study holds promise for a lower dose of acetazolamide in preventing HAPE, future studies of larger samples including both males and females are warranted. The minor but significant effect of acetazolamide on PASP that may be more prominent in higher risk ascent or in exercise and hypoxia needs further investigations.
In summary, our data suggest that a low dosage of acetazolamide (125 mg bid for several days before altitude ascent), but not gingko biloba, can mitigate early increase of PASP in a situation of rapid ascent to high altitude.
Acknowledgments
The authors thank Bin Wang, Dali Xi, Shenming Zhang, and Pengchong Cao for the help during the physical measurement in Lhasa, and Ruian Wang for providing medical support during the trial. Special thanks to the research participants who generously contributed with their time and energy during the trial.
Author Disclosure Statement
No authors have conflicts of interest.
This work was supported by the National Key Technology R&D Program (Grant 2009BAI85B04); National Nature Science Foundation of China (Grant 81172621); and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, IRT1112).
References
- Allemann Y. Sartori C. Lepori M. Pierre S. Mélot C. Naeije R. Scherrer U. Maggiorini M. Echocardiographic and invasive measurements of pulmonary artery pressure correlate closely at high altitude. Am J Physiol Heart Circ Physiol. 2000;279:H2013–2016. doi: 10.1152/ajpheart.2000.279.4.H2013. [DOI] [PubMed] [Google Scholar]
- Balanos GM. Talbot NP. Dorrington KL. Robbins PA. Human pulmonary vascular response to 4 h of hypercapnia and hypocapnia measured using Doppler echocardiography. J Appl Physiol. 2003;94:1543–1551. doi: 10.1152/japplphysiol.00890.2002. [DOI] [PubMed] [Google Scholar]
- Bärtsch P. Mairbäurl H. Maggiorini M. Swenson ER. Physiological aspects of high-altitude pulmonary edema. J Appl Physiol. 2005;98:1101–1110. doi: 10.1152/japplphysiol.01167.2004. [DOI] [PubMed] [Google Scholar]
- Basnyat B. Gertsch JH. Holck PS. Johnson EW. Luks AM. Donham BP. Fleischman RJ. Gowder DW. Hawksworth JS. Jensen BT. Kleiman RJ. Loveridge AH. Lundeen EB. Newman SL. Noboa JA. Miegs DP. O'Beirne KA. Philpot KB. Schultz MN. Valente MC. Wiebers MR. Swenson ER. Acetazolamide 125 mg BD is not significantly different from 375 mg BD in the prevention of acute mountain sickness: The prophylactic acetazolamide dosage comparison for efficacy (PACE) trial. High Alt Med Biol. 2006;7:17–27. doi: 10.1089/ham.2006.7.17. [DOI] [PubMed] [Google Scholar]
- Basnyat B. Gertsch JH. Johnson EW. Castro-Marin F. Inoue Y. Yeh C. Efficacy of low-dose acetazolamide (125 mg BID) for the prophylaxis of acute mountain sickness: A prospective, double-blind, randomized, placebo-controlled trial. High Alt Med Biol. 2003;4:45–52. doi: 10.1089/152702903321488979. [DOI] [PubMed] [Google Scholar]
- Basnyat B. Hargrove J. Holck PS. Srivastav S. Alekh K. Ghimire LV. Pandey K. Griffiths A. Shankar R. Kaul K. Paudyal A. Stasiuk D. Basnyat R. Davis C. Southard A. Robinson C. Shandley T. Johnson DW. Zafren K. Williams S. Weiss EA. Farrar JJ. Swenson ER. Acetazolamide fails to decrease pulmonary artery pressure at high altitude in partially acclimatized humans. High Alt Med Biol. 2008;9:209–216. doi: 10.1089/ham.2007.1073. [DOI] [PubMed] [Google Scholar]
- Basnyat B. Murdoch DR. High-altitude illness. Lancet. 2003;361:1967–1974. doi: 10.1016/S0140-6736(03)13591-X. [DOI] [PubMed] [Google Scholar]
- Berg JT. Ginkgo biloba extract prevents high altitude pulmonary edema in rats. High Alt Med Biol. 2004;5:429–434. doi: 10.1089/ham.2004.5.429. [DOI] [PubMed] [Google Scholar]
- Berg JT. Ramanathan S. Swenson ER. Inhibitors of hypoxic pulmonary vasoconstriction prevent high-altitude pulmonary edema in rats. Wilderness Environ Med. 2004;15:32–37. doi: 10.1580/1080-6032(2004)015[0032:iohpvp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Deem S. Hedges RG. Kerr ME. Swenson ER. Acetazolamide reduces hypoxic pulmonary vasoconstriction in isolated perfused rabbit lungs. Respir Physiol. 2000;123:109–119. doi: 10.1016/s0034-5687(00)00148-1. [DOI] [PubMed] [Google Scholar]
- Emery CJ. Sloan PJ. Mohammed FH. Barer GR. The action of hypercapnia during hypoxia on pulmonary vessels. Bull Eur Physiopathol Respir. 1977;13:763–776. [PubMed] [Google Scholar]
- Faoro V. Huez S. Giltaire S. Pavelescu A. van Osta A. Moraine JJ. Guenard H. Martinot JB. Naeije R. Effects of acetazolamide on aerobic exercise capacity and pulmonary hemodynamics at high altitudes. J Appl Physiol. 2007;103:1161–1165. doi: 10.1152/japplphysiol.00180.2007. [DOI] [PubMed] [Google Scholar]
- Grünig E. Mereles D. Hildebrandt W. Swenson ER. Kübler W. Kuecherer H. Bärtsch P. Stress Doppler echocardiography for identification of susceptibility to high altitude pulmonary edema. J Am Coll Cardiol. 2000;35:980–987. doi: 10.1016/s0735-1097(99)00633-6. [DOI] [PubMed] [Google Scholar]
- Hohne C. Krebs MO. Seiferheld M. Boemke W. Kaczmarczyk G. Swenson ER. Acetazolamide prevents hypoxic pulmonary vasoconstriction in conscious dogs. J Appl Physiol. 2004;97:515–521. doi: 10.1152/japplphysiol.01217.2003. [DOI] [PubMed] [Google Scholar]
- Hohne C. Pickerodt PA. Francis RC. Boemke W. Swenson ER. Pulmonary vasodilation by acetazolamide during hypoxia is unrelated to carbonic anhydrase inhibition. Am J Physiol Lung Cell Mol Physiol. 2007;292:L178–184. doi: 10.1152/ajplung.00205.2006. [DOI] [PubMed] [Google Scholar]
- Hopkins SR. Garg J. Bolar DS. Balouch J. Levin DL. Pulmonary blood flow heterogeneity during hypoxia and high-altitude pulmonary edema. Am J Respir Crit Care Med. 2005;171:83–87. doi: 10.1164/rccm.200406-707OC. [DOI] [PubMed] [Google Scholar]
- Kawashima A. Kubo K. Matsuzawa Y. Kobayashi T. Sekiguchi M. Hypoxia-induced ANP secretion in subjects susceptible to high-altitude pulmonary edema. Respir Physiol. 1992;89:309–317. doi: 10.1016/0034-5687(92)90089-f. [DOI] [PubMed] [Google Scholar]
- Maggiorini M. High altitude-induced pulmonary oedema. Cardiovasc Res. 2006;72:41–50. doi: 10.1016/j.cardiores.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Maggiorini M. Brunner-La Rocca HP. Peth S. Fischler M. Böhm T. Bernheim A. Kiencke S. Bloch KE. Dehnert C. Naeije R. Lehmann T. Bärtsch P. Mairbäurl H. Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema: a randomized trial. Ann Intern Med. 2006;145:497–506. doi: 10.7326/0003-4819-145-7-200610030-00007. [DOI] [PubMed] [Google Scholar]
- Maggiorini M. Mélot C. Pierre S. Pfeiffer F. Greve I. Sartori C. Lepori M. Hauser M. Scherrer U. Naeije R. High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation. 2001;103:2078–2083. doi: 10.1161/01.cir.103.16.2078. [DOI] [PubMed] [Google Scholar]
- Moviat M. Pickkers P. van der Voort PH. van der Hoeven JG. Acetazolamide-mediated decrease in strong ion difference accounts for the correction of metabolic alkalosis in critically ill patients. Crit Care. 2006;10:R14. doi: 10.1186/cc3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock AJ. Challenor V. Sutherland G. Estimation of pulmonary artery pressure by Doppler echocardiography in normal subjects made hypoxic. Respir Med. 1990;84:335–337. doi: 10.1016/s0954-6111(08)80063-7. [DOI] [PubMed] [Google Scholar]
- Ren DC. Du GH. Zhang JT. Protective effect of ginkgo biloba extract on endothelial cell against damage induced by oxidative stress. J Cardiovasc Pharmacol. 2002;40:809–814. doi: 10.1097/00005344-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Richalet JP. Gratadour P. Robach P. Pham I. Déchaux M. Joncquiert-Latarjet A. Mollard P. Brugniaux J. Cornolo J. Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:275–281. doi: 10.1164/rccm.200406-804OC. [DOI] [PubMed] [Google Scholar]
- Richalet JP. Rivera M. Bouchet P. Chirinos E. Onnen I. Petitjean O. Bienvenu A. Lasne F. Moutereau S. León-Velarde F. Acetazolamide: a treatment for chronic mountain sickness. Am J Respir Crit Care Med. 2005;172:1427–1433. doi: 10.1164/rccm.200505-807OC. [DOI] [PubMed] [Google Scholar]
- Roach RC. BP Hackett PH. Oelz O. The Lake Louise Acute Mountain Sickness Scoring System. In: Sutton JR, editor; Coates G, editor; Houston CS, editor. Hypoxia and Molecular Medicine: Proceedings of the 8th International Hypoxia Symposium; Lake Louise, Alberta, Canada. Burlington, Vt: Queen City Printers; 1993. [Google Scholar]
- Shimoda LA. Luke T. Sylvester JT. Shih HW. Jain A. Swenson ER. Inhibition of hypoxia-induced calcium responses in pulmonary arterial smooth muscle by acetazolamide is independent of carbonic anhydrase inhibition. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1002–1012. doi: 10.1152/ajplung.00161.2006. [DOI] [PubMed] [Google Scholar]
- Swenson ER. Maggiorini M. Mongovin S. Gibbs JS. Greve I. Mairbäurl H. Bärtsch P. Pathogenesis of high-altitude pulmonary edema: Inflammation is not an etiologic factor. JAMA. 2002;287:2228–2235. doi: 10.1001/jama.287.17.2228. [DOI] [PubMed] [Google Scholar]
- Teppema LJ. Balanos GM. Steinback CD. Brown AD. Foster GE. Duff HJ. Leigh R. Poulin MJ. Effects of acetazolamide on ventilatory, cerebrovascular, and pulmonary vascular responses to hypoxia. Am J Respir Crit Care Med. 2007;175:277–281. doi: 10.1164/rccm.200608-1199OC. [DOI] [PubMed] [Google Scholar]
- Tissot van Patot MC. Leadbetter G., 3rd Keyes LE. Maakestad KM. Olson S. Hackett PH. Prophylactic low-dose acetazolamide reduces the incidence and severity of acute mountain sickness. High Alt Med Biol. 2008;9:289–293. doi: 10.1089/ham.2008.1029. [DOI] [PubMed] [Google Scholar]
- Wu YZ. Li SQ. Zu XG. Du J. Wang FF. Ginkgo biloba extract improves coronary artery circulation in patients with coronary artery disease: Contribution of plasma nitric oxide and endothelin-1. Phytother Res. 2008;22:734–739. doi: 10.1002/ptr.2335. [DOI] [PubMed] [Google Scholar]
- Yock PG. Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]