Abstract
Background
There is risk for disordered eating behaviors in type 1 diabetes, especially related to insulin manipulation. Implementation of insulin pump therapy may encourage either normalization of eating behaviors or a greater focus on food intake due to renewed emphasis on carbohydrate counting. There is need for prospective studies to assess disordered eating behaviors upon implementation of pump therapy using diabetes-specific measurement tools.
Subjects and Methods
In a multicenter pilot study, 43 youth with type 1 diabetes, 10–17 years old, were assessed prior to pump initiation and after 1 and 6 months of pump therapy. Youth completed the Diabetes-specific Eating Problems Survey-Revised (DEPS-R), a validated measure of risk for both diabetes-specific and general disordered eating behaviors.
Results
Youth (45% female), 13.3 years old with diabetes for 2.1 years, had a mean hemoglobin A1c of 8.3±1.3% (68±14.5 mmol/mol) at baseline. DEPS-R scores decreased over time (P=0.01). Overall rate of high risk for eating disorders was low. Overweight/obese youth endorsed more disordered eating behaviors than normal-weight participants. DEPS-R scores were correlated with z-score for body mass index at all three time points and with hemoglobin A1c after 1 and 6 months. Hemoglobin A1c did not change significantly over the 6 months and was higher in overweight/obese compared with normal-weight participants.
Conclusions
Initiation of insulin pump therapy was associated with diminished endorsement of disordered eating behaviors in youth with type 1 diabetes. Longer follow-up studies are needed to assess the impact of insulin pump therapy on glycemic control, weight status, and disordered eating behaviors in this vulnerable population.
Background
Disordered eating behaviors are more common in youth with type 1 diabetes (T1D) than in a normative sample of youth.1–3 In addition to more typical disordered eating behaviors, youth with T1D may use a unique disordered eating behavior, namely, insulin restriction or omission, which is seen in up to 30% of youth/young adults with T1D.4,5 Although there is a body of research on disordered eating and T1D, there are few prospective studies in general and few studies using diabetes-specific assessment tools.
Conventional insulin regimens demand that eating patterns match times of peak insulin action, rather than follow physiologic hunger signals. Modern, physiologic insulin replacement programs using either basal-bolus injection programs or pump therapy may allow for the reestablishment of appetite as well as flexible eating patterns. Insulin pump therapy, in particular, is an increasingly popular treatment option for managing diabetes in youth,6 and because hunger can be a symptom of hypoglycemia, patients who have difficulty distinguishing between hunger and hypoglycemia may benefit from pump therapy because insulin can be titrated at small doses, which can help avoid hypoglycemia. In addition, pump therapy not only may allow greater awareness of hypoglycemia, but also provides flexibility around eating and exercise so youth can feel more like their peer group. Although the majority of research on disordered eating and T1D does not account for insulin regimen, one study showed no difference in prevalence of disordered eating behaviors between adolescent females treated with pump therapy and those treated with multiple daily injections, using a general measure of disordered eating behaviors.7 Another study of teenage females with T1D and preexisting eating disorders demonstrated that those treated by pump had better glycemic control than those on multiple daily injections.8 The transition to insulin pump therapy may have an impact on eating behaviors, providing an opportunity either to normalize behaviors or to intensify a focus on eating behaviors. Additional prospective research in this area is needed as no studies to date have examined the relationship among disordered eating, initiation of pump therapy, and glycemic control, particularly using a diabetes-specific measure of disordered eating behaviors.
To better understand the development of disordered eating behaviors in youth with T1D, the effects of insulin regimen needs to be examined. One approach is to assess disordered eating behaviors prior to and following initiation of insulin pump therapy, which is a time when patients direct more attention to their diabetes management in general and to their eating behaviors in particular, with a rigorous focus on carbohydrate counting for pump bolus management. Thus, the start of insulin pump therapy could be considered a “resetting” of diabetes treatment and the patient's relationship with food.
The majority of studies examining disordered eating and T1D use general measures of disordered eating.9 These general measures may be both oversensitive to eating behaviors that are normative in T1D, including a focus on food, and undersensitive to disordered eating behaviors specific to T1D, such as insulin underdosing or omission. In this multisite pilot study of 43 patients, we prospectively examined disordered eating behaviors, assessed by a diabetes-specific measure, the 16-item Diabetes-specific Eating Problem Survey-Revised (DEPS-R), in youth with T1D prior to starting insulin pump therapy and again 1 and 6 months later. Of note is that this survey measures behaviors related to disordered eating, not clinically diagnosed eating disorders. We hypothesized that self-reported symptoms of disordered eating would diminish over time following initiation and ongoing use of insulin pump therapy, while glycemic control, measured as hemoglobin A1c (A1c), would also improve.
Subjects and Methods
Participants were youth with T1D, 10–17 years old, recruited from three diabetes care centers: Emory University (Atlanta, GA), Georgia Health Sciences University (Augusta, GA), and the Joslin Diabetes Center (Boston, MA). All participants had established T1D treated with injection-based therapy and plans to initiate insulin pump therapy. Exclusion criteria included unstable medical/psychiatric conditions affecting weight or interfering with diabetes management. At each site, eligible participants were approached at an outpatient medical visit.
Youth participants were assessed prospectively using standardized and diabetes-specific questionnaires at baseline (prior to insulin pump start), after 1 month, and after 6 months of insulin pump therapy. Data collected by medical chart review included age, duration of T1D, height, weight, A1c, and insulin regimen prior to pump-start. Age- and sex-adjusted z-score for body mass index (zBMI) was calculated10; overweight/obese was defined as ≥85th percentile.
All participants/families underwent site-directed training and preparation for insulin pump therapy, which included understanding pump principles, carbohydrate counting, insulin dosing, and sick day management.
Institutional review boards at participating centers approved all study procedures. Parents/guardians and youth provided written informed consent and assent, as appropriate.
Measures
DEPS-R.11
Youth completed the DEPS-R, a 16-item self-report questionnaire to measure general and diabetes-specific disordered eating behaviors. The DEPS-R has good internal consistency (Cronbach's α=0.86) and is validated for use in the pediatric population. The six response items include the possible answers never, rarely, sometimes, usually, often, or always. Endorsement was defined as answering with any of the following: “sometimes,” “often,” “usually,” or “always.” Higher scores indicate greater disordered eating behaviors, with a score of ≥20 indicating high risk for disordered eating.
Glycemic control
Blood samples, to measure A1c, were obtained at diabetes visits most proximal to the three study visits. All sites used their own A1c methods with assays standardized to Diabetes Control and Complications Trial values (reference range, 4.0–6.0% [20–42 mmol/mol]).
zBMI and weight categories
Age- and sex-adjusted zBMI was calculated based on growth charts from the Centers for Disease Control and Prevention.10 Overweight/obese was defined as a BMI ≥85th percentile; underweight was defined as <5th percentile.
Statistical analysis
Analyses used SAS software (version 9.2 for Windows; SAS Institute, Cary, NC). All data are presented as mean±SD values, median (range) values, or percentages as indicated. DEPS-R scores are presented as median (25th and 75th quartiles) because of the right skewed distribution. Statistics included Pearson's and Spearman's correlations, paired and unpaired t tests, χ2 tests, Wilcoxon rank sum, mixed effects modeling, and general linear models. A P value of <0.05 conveyed statistical significance.
Results
Participant characteristics at baseline and follow-up
The pilot study consisted of 43 youth (45% female) with T1D, although the analyses include only the 37 participants who completed the DEPS-R at all three time points. Of the six remaining participants, one completed the DEPS-R at baseline only, one at only 1 month and 6 months of follow-up, and four at only baseline and 6-months of follow-up. Characteristics of participants appear in Table 1. Prior to insulin pump start, patients had a mean age of 13.3±1.9 years and median duration of T1D of 2.1 years (25th–75th percentile, 1.2–6.3). In addition, 84% of patients were on a basal-bolus regimen, and 16% received NPH as part of their insulin treatment.
Table 1.
Participant Characteristics at Baseline, Hemoglobin A1c Values, and Diabetes-specific Eating Problems Survey-Revised Scores Displayed from Baseline, 1-Month, and 6-Month Assessments
| Characteristic | Value |
|---|---|
| Number of participants | 37 |
| Age (years) (mean±SD) | 13.3±1.9 |
| Sex (% female) | 45% |
| Diabetes duration (years) [median (25th–75th percentiles)] | 2.1 (1.2–6.3) |
| Race/ethnicity (% minority) | 8% |
| Family structure (% married) | 83% |
| Parent education (% college/graduate degree) | 56% |
| Blood glucose monitoring (times/day) (mean±SD) | 5.6±2.0 |
| zBMI (SDS) (mean±SD) | 0.7±0.9 |
| Overweight/obese (%) (≥85th percentile) | |
| Baseline | 41% |
| 1 month | 44% |
| 6 months | 46% |
| A1c (mean±SD) [% (mmol/mol)] | |
| Baseline | 8.3±1.3 (68±14.5) |
| 1 month | 8.0±0.7 (64±7.8) |
| 6 months | 8.2±1.0 (66±10.9) |
| DEPS-R score [median (25th–75th percentiles)] | |
| Baseline | 8.0 (4.0–13.0) |
| 1 month | 5.0 (2.0–11.0) |
| 6 months | 6.0 (2.0–11.0) |
A1c, hemoglobin A1c; DEPS-R, Diabetes-specific Eating Problems Survey-Revised; SDS, SD score; zBMI, z-score for body mass index.
Mean baseline A1c was 8.3±1.3% (68±14.5 mmol/mol). Although mean A1c did not change over the 6-month study, approximately one-third of the participants had a decrease in A1c of 0.5% (5 mmol/mol), one-third increased by ≥0.5% (5 mmol/mol), and one-third remained within 0.5% (5 mmol/mol) of the baseline A1c value. Mean zBMI was 0.7±0.9; no participant was underweight. The proportion of overweight/obese participants increased nonsignificantly over time from 41% at baseline to 44% at 1 month and 46% at 6 months (P=0.64). A1c did not differ by weight status at baseline or at 1 month; however, at 6 months of follow-up, A1c was significantly higher in overweight/obese participants compared with normal-weight participants (8.7% [72 mmol/mol] vs. 7.8% [62 mmol/mol]; P=0.005).
DEPS-R scores at baseline and follow-up
Median DEPS-R score at baseline was 8.0 (25th–75th percentile, 4.0–13.0); only two patients at baseline attained a score that indicates high risk for disordered eating behavior (score of ≥20). There was a significant difference in baseline median DEPS-R scores between females and males, with females scoring higher (females, 11.0 [25th–75th percentile, 8.0–15.0]; males, 5.0 [25th–75th percentile, 2.5–11.2]; P=0.04). Similarly, sex differences in DEPS-R scores persisted at the 1- and 6-month assessments. At all three assessments, participants who were overweight/obese scored significantly higher on the DEPS-R than those who were normal weight (median scores for overweight/obese vs. normal-weight participants, respectively: baseline, 12.0 vs. 4.5; 1-month follow-up, 10.5 vs. 3.0; 6-month follow-up, 10.0 vs. 4.0; all P<0.02).
Compared with baseline score, DEPS-R scores for the sample were significantly reduced at 1 month to 5.0 (25th–75th percentile, 2.0–11.0; P=0.01) and at 6 months to 6.0 (25th–75th percentile, 2.0–11.0; P=0.02). During follow-up, an additional three patients scored ≥20 on the DEPS-R. Mixed effects modeling showed a trend toward a difference in DEPS-R scores over the three time points (P=0.06). Change in DEPS-R scores over time in the subgroups according to sex and weight status showed similar downward trends, but the numbers were too small to attain significance.
Correlations between DEPS-R scores with zBMI and A1c over time
The associations between participant characteristics and DEPS-R scores varied over time (Table 2). Diabetes duration was positively correlated with DEPS-R scores at baseline and 6 months, whereas age showed no correlation with DEPS-R at any time. zBMI had the strongest correlations with DEPS-R scores over time (all r≥0.48, P≤0.003). With respect to glycemic control, A1c levels were positively correlated with DEPS-R scores at 1 month and 6 months of follow-up, although not at baseline. In a subset of 18 patients, 12-month A1c and DEPS-R data were available and revealed a similar association (r=0.47, P=0.05). In order to account for multiple comparisons, a more vigorous P value of <0.01 retained significance only between zBMI and DEPS-R scores at each time point. Nonetheless, in a general linear model controlling for diabetes duration, sex, and baseline zBMI, change in DEPS-R scores from baseline to 6 months predicted change in A1c (P=0.01); as DEPS-R scores decreased, A1c improved over time following initiation of pump therapy.
Table 2.
Correlations with Diabetes-specific Eating Problems Survey-Revised Scores at Baseline, 1 Month, and 6 Months
| |
DEPS-R score |
|||||
|---|---|---|---|---|---|---|
| |
Baseline |
1 month |
6 months |
|||
| r | P | r | P | r | P | |
| Age | 0.06 | 0.74 | −0.13 | 0.44 | −0.05 | 0.79 |
| Diabetes duration | 0.35a | 0.03a | 0.18 | 0.29 | 0.38a | 0.02a |
| zBMI | ||||||
| Baseline | 0.51a | 0.001a | 0.54a | 0.0004a | 0.48a | 0.003a |
| 1 month | 0.51a | 0.001a | 0.49a | 0.002a | ||
| 6 months | 0.43a | 0.007a | ||||
| A1c | ||||||
| Baseline | 0.23 | 0.17 | 0.40a | 0.01a | 0.25 | 0.13 |
| 1 month | 0.34a | 0.04a | 0.03 | 0.86 | ||
| 6 months | 0.38a | 0.02a | ||||
Significant at the P≤0.05 level.
A1c, hemoglobin A1c; DEPS-R, Diabetes-specific Eating Problems Survey-Revised; zBMI, z-score for body mass index.
Changing endorsement of DEPS-R items over time
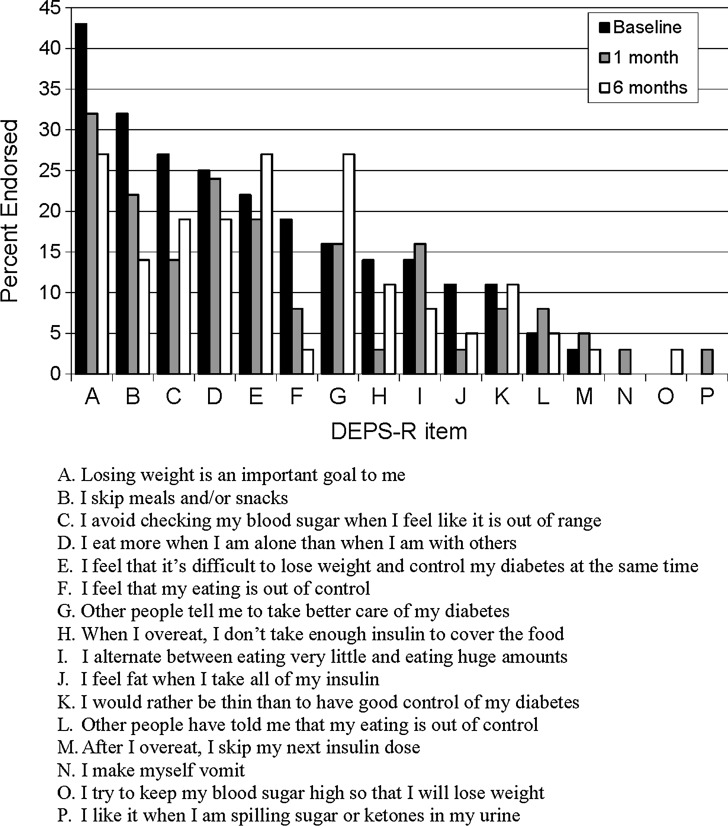
At baseline, four of the 16 items were endorsed by ≥25% of the participants (Fig. 1). At 1-month follow-up, only one item was endorsed by ≥25% of participants, and at 6-month follow-up, three items were endorsed by ≥25% of participants. Of the 16 items on the DEPS-R, only five were endorsed more frequently from baseline to 1-month follow-up, and only three were endorsed more frequently from baseline to 6-month follow-up.
FIG. 1.
Frequency of Diabetes-specific Eating Problems Survey-Revised (DEPS-R) endorsed at baseline, 1 month, and 6 months after pump start.
Discussion
For patients with T1D, the transition from injection-based insulin therapy to pump-based management provides an opportunity for families to reassess their approaches to diabetes care and potentially to modify their eating behaviors. As a result, the transition to insulin pump therapy may have an impact on disordered eating behaviors, either as an opportunity to normalize eating behaviors consequent to reestablishment of appetite and eating flexibility or with the potential to intensify disordered eating behaviors because of a renewed focus on diet in general and carbohydrate counting in particular. This prospective study of disordered eating behaviors used the diabetes-specific measure, the DEPS-R, and followed patients from initiation of insulin pump therapy through 6 months of follow-up. Our findings demonstrated a decrease in diabetes-specific disordered eating behaviors by 1 month, with maintenance of the decrease over 6 months.
The improvement in disordered eating behaviors associated with initiation of pump therapy appears to derive from greater flexibility in eating patterns afforded by pump therapy, noted by less endorsement of DEPS-R items A–D (Fig. 1). The reduction in disordered eating behaviors occurred despite a greater focus by families on diabetes management, likely with the goal of improving glycemic control, as noted by greater endorsement of DEPS-R item G, “Other people tell me to take better care of my diabetes.”
Body mass index percentile was significantly associated with DEPS-R score at all three assessment points, even using the rigorous P value of <0.01, and participants who were overweight or obese scored higher on the DEPS-R than those who were normal weight. Similarly, previous research in adolescent females with T1D has found that self-report of a history of being overweight was significantly associated with disordered eating behaviors using a general measure rather than a diabetes-specific tool of disordered eating.12
Mean A1c did not change from baseline to 6 months, with approximately one-third of participants each demonstrating increases and decreases of 0.5% (5 mmol/mol). Although previous research in this area varies, some studies have suggested an improvement in A1c following insulin pump initiation.13,14 This may reflect our small sample size, the diversity of the sample, or potentially a need for longer follow-up. It is interesting that a higher A1c level at both baseline and 1 month was related to a higher DEPS-R score at 1 month; the 6-month A1c level was similarly associated with the DEPS-R score at 6 months. All of these associations between A1c level and DEPS-R score were modest to moderate (r>0.3), likely because of the small sample size. Nonetheless, it does appear that there are interrelationships between A1c and endorsement of disordered eating behaviors, supporting the clinical impression that youth with elevated blood glucose levels may experience symptoms such as out of control eating behaviors associated with disease process, or that youth exhibiting disordered eating behaviors are less concerned with glycemic control than with efforts at weight control. Expansion of our prospective study will help to elucidate these relationships.
Overall, our sample did not endorse high rates of diabetes-specific disordered eating behaviors (i.e., did not score particularly high on the DEPS-R); across all three time points, five participants endorsed increased risk for disordered eating (DEPS-R score ≥20). One participant scored ≥20 at baseline, 1 month, and 6 months; the remainder endorsed risk at only one time point (one at baseline, two at 1 month, and one at 6 months). Of the five participants endorsing risk, three were female, and two were male. The majority of the items on the DEPS-R were endorsed less frequently from baseline to the 1-month and 6-month follow-up assessments. Compared with a previous11 sample of youth with T1D, 13–19 years old, baseline DEPS-R scores in this sample were slightly lower (median, 9.8 vs. 8.0), although the range was wider (range, 4.0–13.0 vs. 0–52.0). This may be due to the wider age range of the current sample (10–17 years) and their shorter diabetes duration compared with the earlier group. In addition, in the current sample, there were fewer patients who scored in the high-risk range (≥20) than in a previous sample (5% vs. 20%, respectively). This may be due to the fact that participants in the current sample were all initiating insulin pump therapy and may have less pathology than a more general sample.
There are several limitations to our study. In particular, our sample size is small, at times resulting in modest significance levels. However, to our knowledge, this is the first longitudinal, multicenter study to assess changes in disordered eating behaviors using a diabetes-specific measurement in youth with T1D. The DEPS-R is a clinically relevant measure that accounts for diabetes treatment and diabetes-specific disordered eating behaviors. We also did not use a central A1c assay across all three study sites. However, all A1c assays were standardized to the Diabetes Control and Complications Trial reference range,15 and we conducted within-person comparisons over time, for which avoidance of variation in assays across sites would not be required. Finally, longer-term follow-up of study participants would be informative, tracking changes as pump therapy becomes normalized for patients, supporting the need for additional, larger studies.
In summary, our data indicate that youth with T1D of longer duration and who have heavier body weights and higher A1c levels appear to endorse more disordered eating behaviors. Implementation of insulin pump therapy appears to mitigate endorsement of disordered eating behaviors, possibly because of the greater flexibility in eating behavior and insulin administration afforded to patients by pump therapy as well as a renewed focus on diabetes management. Although T1D in general is associated with an increased occurrence of disordered eating behaviors and eating disorders,1–3 insulin pump therapy may normalize eating patterns, in turn reducing risk for the development of eating disorders. For families of youth with T1D, the transition to pump therapy likely resensitizes families to the demands of diabetes management and the need for attention to diet in a manner similar to those experienced by families at the time of onset of T1D in youth. Pump therapy offers greater flexibility for lifestyle and eating behaviors and may help to normalize appetite, especially if glycemic control is not poor. Future prospective studies are needed, using diabetes-specific measurement tools to assess disordered eating behaviors in youth with T1D from onset of disease as well as from the transition to insulin pump therapy. Long-term follow-up will help in the understanding of the determinants of eating disorders in this population and, subsequently, to inform the design of effective interventions aimed at prevention.
Acknowledgments
This work was funded by an American Diabetes Association Clinical Research Award (number 7-08-CR-66), a K23 award from the National Institute of Diabetes and Digestive and Kidney Diseases (number 1K23DK092335-01A1), the Eleanor Chesterman Beatson Fund, the Katherine Adler Astrove Youth Education Fund, and the Maria Griffin Drury Pediatric Fund.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Nielsen S. Eating disorders in females with type 1 diabetes: an update of a meta-analysis. Eur Eat Disord Rev. 2002;10:241–254. [Google Scholar]
- 2.Jones JM. Lawson ML. Daneman D. Olmsted MP. Rodin G. Eating disorders in adolescent females with and without type 1 diabetes: cross sectional study. BMJ. 2000;320:1563–1566. [PMC free article] [PubMed] [Google Scholar]
- 3.Colton P. Olmsted M. Daneman D. Rydall A. Rodin G. Disturbed eating behavior and eating disorders in preteen and early teenage girls with type 1 diabetes: a case-controlled study. Diabetes Care. 2004;27:1654–1659. doi: 10.2337/diacare.27.7.1654. [DOI] [PubMed] [Google Scholar]
- 4.Goebel-Fabbri AE. Fikkan J. Franko DL. Pearson K. Anderson BJ. Weinger K. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care. 2008;31:415–419. doi: 10.2337/dc07-2026. [DOI] [PubMed] [Google Scholar]
- 5.Polonsky WH. Anderson BJ. Lohrer PA. Aponte JE. Jacobson AM. Cole CF. Insulin omission in women with IDDM. Diabetes Care. 1994;17:1178–1185. doi: 10.2337/diacare.17.10.1178. [DOI] [PubMed] [Google Scholar]
- 6.Paris CA. Imperatore G. Klingensmith G. Petitti D. Rodriguez B. Anderson AM. Schwartz ID. Standiford DA. Pihoker C. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009;155:183–189. doi: 10.1016/j.jpeds.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 7.Battaglia MR. Alemzadeh R. Katte H. Hall PL. Perlmuter LC. Brief report: disordered eating and psychosocial factors in adolescent females with type 1 diabetes mellitus. J Pediatr Psychol. 2006;31:552–556. doi: 10.1093/jpepsy/jsj047. [DOI] [PubMed] [Google Scholar]
- 8.Pinhas-Hamiel O. Graph-Barel C. Boyko V. Tzadok M. Lerner-Geva L. Reichman B. Long-term insulin pump treatment in girls with type 1 diabetes and eating disorders—is it feasible? Diabetes Technol Ther. 2010;12:873–878. doi: 10.1089/dia.2010.0049. [DOI] [PubMed] [Google Scholar]
- 9.Young-Hyman DL. Davis CL. Disordered eating behavior in individuals with diabetes: importance of context, evaluation, and classification. Diabetes Care. 2010;33:683–689. doi: 10.2337/dc08-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuczmarski RJ. Ogden CL. Guo SS. Grummer-Strawn LM. Flegal KM. Mei Z. Wei R. Curtin LR. Roche AF. Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 11.Markowitz JT. Butler DA. Volkening LK. Antisdel JE. Anderson BJ. Laffel LM. Brief screening tool for disordered eating in diabetes: internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care. 2010;33:495–500. doi: 10.2337/dc09-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markowitz JT. Lowe MR. Volkening LK. Laffel LM. Self-reported history of overweight and its relationship to disordered eating in adolescent girls with type 1 diabetes. Diabet Med. 2009;26:1165–1171. doi: 10.1111/j.1464-5491.2009.02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plotnick LP. Clark LM. Brancati FL. Erlinger T. Safety and effectiveness of insulin pump therapy in children and adolescents with type 1 diabetes. Diabetes Care. 2003;26:1142–1146. doi: 10.2337/diacare.26.4.1142. [DOI] [PubMed] [Google Scholar]
- 14.Yeh HC. Brown TT. Maruthur N. Ranasinghe P. Berger Z. Suh YD. Wilson LM. Haberl EB. Brick J. Bass EB. Golden SH. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157:336–347. doi: 10.7326/0003-4819-157-5-201209040-00508. [DOI] [PubMed] [Google Scholar]
- 15.Diabetes Research in Children Network (DirecNet) Study Group: Performance of the DCA2000 for measurement of HbA1c levels in children with T1DM in a DirecNet outpatient clinical trial. Pediatr Diabetes. 2005;6:13–16. doi: 10.1111/j.1399-543X.2005.00088.x. [DOI] [PubMed] [Google Scholar]