Abstract
Mesenchymal stem cells (MSCs) are being explored extensively as a promising treatment for autoimmune diseases. We have recently reported that MSCs could ameliorate experimental autoimmune uveoretinitis (EAU) in rats. In this study, we examined further the effects of MSCs on the dynamics of T cell subsets in both eye and spleen and their cytokine production during the course of EAU. We focused on when and where the MSCs had inhibitory effects on T helper type 1 (Th1) and Th17 cells and how long the inhibitory effect lasted, in order to provide more mechanistic evidence for MSCs on the treatment of uveitis. Compared to the control group, administration of MSCs decreased the production of Th1 and Th17 cytokines significantly, while the production of Th2 and regulatory T cell (Treg) cytokines [interleukin (IL)-10 and transforming growth factor (TGF)-β] was elevated during the entire course of EAU. Correspondingly, the dynamic levels of IL-17 in the aqueous humour (AqH) were reduced in MSC-treated rats. Moreover, the ratio of Th17/Treg cells in both spleen and eye was decreased. These results provide powerful evidence that MSCs can regulate negatively both Th1 and Th17 responses and restore the balance of Th17/Tregs in the whole course of EAU, which is important for the regression of the disease.
Keywords: experimental autoimmune uveoretinitis, mesenchymal stem cells, Th1, Th17, Treg
Introduction
Autoimmune uveitis is one of the major causes of visual loss worldwide. A wide range of systemic and ocular complications associated with long-term usage of corticosteroids and immunosuppressive medications has pushed the development of new therapies to decrease the burden of these traditional immunosuppressive agents on patients with refractive uveitis. Biologics, also known as cytokine inhibitors, targeting specific mediators of the immune-inflammatory system potentially offer a safer profile and faster response than traditional immunosuppressive agents 1. However, as human uveitis is highly heterogeneous, with a wide spectrum of disorders, targeting one specific mediator [such as tumour necrosis factor (TNF) or interleukin (IL)-1] does not necessarily apply to all cases. In addition, it is still difficult to make an evidence-based decision for which agent is best for a given clinical entity. Hence, the development of specific and effective therapeutic approaches is important and urgent.
Mesenchymal stem cells (MSCs) are the current focus of research for their potential therapeutic usage in the clinic. As well as features such as the capacities of multi-lineage differentiation and prompting tissue repair, anti-inflammatory and neurotrophic effect, ease of isolation and expansion and poor immunogenicity 2, MSCs can exert profound immunosuppression by inhibiting proliferation and functions of a number of cell types including T lymphocytes, natural killer (NK) cells and dendritic cells (DCs), and up-regulating regulatory T cells (Treg) cells 3,4. The mechanisms underlying these effects are largely unknown, but are likely to be mediated by soluble factors 4,5. These immunosuppressive properties have been exploited extensively in a number of experimental autoimmune diseases and translated recently to the clinical setting in several diseases, such as systemic lupus erythematosus (SLE) 6, Crohn's disease 7, multiple sclerosis (MS) 8, rheumatoid arthritis 9 and Sjögren's syndrome 10. In autoimmune diseases, MSCs can inhibit both T helper type 1 (Th1) and Th17 responses and modulate the Th17/Treg balance 11,12. Contrary to corticosteroid and immunosuppressive agents, no tissue toxicity of MSCs has been found so far, and MSCs could even enhance the ability of the host immune cells on bacterial clearance 13. Together with their neurotrophic effect in nervous system 14, MSCs could therefore be an ideal approach for treating uveitis.
We reported recently that MSCs could ameliorate experimental autoimmune uveoretinitis (EAU) in rats 15. The therapeutic role of MSCs on EAU might be due to the inhibition of both Th1 and Th17 responses and an up-regulation of Th2 response and Tregs. Similar results have also been demonstrated in the mouse model of uveitis, in which MSCs inhibited EAU by inducing antigen-specific Treg 16. In this study, we examined further the effects of MSCs on the dynamics of T cell subsets in both eye and spleen and their cytokine production during the course of EAU, focusing on when and where the inhibitory effects of MSCs develop on Th1 and Th17 cells and how long the effects last, in order to provide more mechanistic evidence for MSCs on the treatment of uveitis.
Materials and methods
Animals
Male Lewis and Wistar rats (6–8 weeks old), purchased from Vital River (Beijing, China), were housed under pathogen-free conditions. All procedures involving rats were approved by the Laboratory Animal Care and Use Committee of the Tianjin Medical University and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Reagents
Interphotoreceptor retinoid-binding protein (IRBP) peptide 1169-1191(R16) was synthesized by Sangon (Shanghai, China). Complete Freund's adjuvant (CFA) was purchased from Sigma (St Louis, MO, USA). Mycobacterium tuberculosis strain H37RA was obtained from Difco (Detroit, MI, USA). Enzyme-linked immunosorbent assay (ELISA) kits of IL-2, IL-4, IL-6, IL-10, IL-17A, interferon (IFN)-γ and TGF-β were obtained from Xinbo Sheng (Shenzhen, China) and antibodies used for flow cytometry from eBioscience (San Diego, CA, USA).
Isolation and characterization of MSCs
Bone marrow MSCs were isolated from Wistar rats, as described previously 15. Briefly, the tibiae and femurs were removed aseptically from Wistar rats, and the bone marrow (BM) was flushed and suspended in phosphate-buffered saline (PBS). MSCs were isolated using a Percoll gradient (d = 1·073 g/ml; Sigma) and incubated in low-glucose Dulbecco's modified Eagle's medium (L-DMEM) (HyClone, Logan UT, USA) containing 10% fetal bovine serum (FBS) (HyClone), 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in 5% CO2 for at least 24 h. Non-adherent cells were discarded and the remaining adherent cells were then incubated for 10–14 days in L-DMEM with 10% FBS until they reached approximately 80% confluence. MSCs from passages three to five were used in the subsequent experiments.
Mature MSCs were defined by their capacity, when cultured under appropriate in-vitro conditions, to differentiate into adipocytes, endothelial cells and osteocytes. Further characterization was based on the expression of CD90 and CD73 and the lack of haematopoietic markers CD45 and CD34 on their surfaces, as described previously 17
Induction of EAU and treatment protocols
Lewis rats were immunized subcutaneously in one rear footpad with 200 μl emulsion containing 30 μg R16 and 500 μg M. tuberculosis H37Ra in CFA. To investigate both preventive and therapeutic effects of MSCs on EAU, the immunized rats were treated intravenously with 5 × 106 MSCs for 3 consecutive days starting either on day 0 (preventive group) or day 12 (therapeutic group) post-R16 immunization. Control groups received an equal volume of PBS. Each group comprised 10 rats.
Clinical and histological assessment of EAU
The immunized rats were examined daily for clinical signs of uveitis by slit-lamp biomicroscope starting on day 4 post-immunization. The incidence and severity of EAU were scored in a masked fashion on a scale of 0–4, according to the criteria reported by Caspi's group 18. For histology, animals were killed on days 6, 9, 12, 15 and 20 in the preventive group and on days 15 and 20 in the therapeutic group. Eyes were collected and immersed for 1 h in 4% glutaraldehyde/PBS and transferred into 10% glutaraldehyde/PBS for 24 h until further processing. Fixed and dehydrated tissues were embedded in paraffin wax and 4 μm sections were stained with standard haematoxylin and eosin. The presence of disease was evaluated in a double-blinded fashion by examining four sections at different levels in each eye. Severity of EAU was scored on a scale of 0–4, according to Caspi's criteria 18.
Cytokine production
Mononuclear cells (MNCs) enriched from the spleens and lymph nodes of either healthy or EAU-induced rats by Ficoll gradient (Roche, Mannheim, Germany) were cultured at a density of 2 × 105 cells/well with 30 μg/ml of R16 peptide in a final volume of 200 μL RPMI-1640 medium containing 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% FBS (Gibco, Carlsbad, CA, USA). After 72 h incubation at 37°C in 5% CO2, supernatants were collected for cytokine detection. Cytokine levels of IL-2, IL-4, IL-6, IL-10, IL-17, TGF-β and IFN-γ were detected by ELISA kits according to the manufacturer's instructions.
Flow cytometry analysis
MNCs from spleens and lymph nodes were prepared and stimulated as described above, and then incubated with 50 ng/ml phorbol myristate acetate (PMA) (Sigma), 1 μg/ml ionomycin (Sigma) and 10 μg/ml monensin (Sigma) for an additional 5 h. Aliquots of 1 × 106 cells were two-colour-stained with combinations of phycoerythrin cyanin 5 (PECy5)-conjugated anti-CD4 monoclonal antibody (mAb) and PE-conjugated anti-IL-17 mAb and three-colour-stained with combinations of PECy5-, PE- or fluorescein isothiocyanate (FITC)-conjugated mAbs against rat CD4, CD25 and forkhead box protein 3 (FoxP3). The cells were first incubated for 30 min at room temperature (RT) with anti-CD4, anti-CD25 or isotype control antibodies, fixed overnight at 4°C with 1 ml FoxP3 staining buffer set (Fix and Perm Cell Permeabilization Kit; eBioscience, San Diego, CA, USA). The cells were then incubated for 30 min at RT with anti-rat IL-17 or FoxP3, fixed with 2% paraformaldehyde and analysed by flow cytometer (FACSCalibur; BD Biosciences). The ratios of CD4+IL-17+ (Th17) cells/CD4+T cells and CD4+CD25+FoxP3+ (Treg) cells/CD4+T cells were calculated in each group.
Aqueous humour collection
Aqueous humour (AqH) was collected from both eyes by an anterior chamber puncture using a 30-gauge needle under a surgical microscope on the days indicated above and stored immediately in a −80°C freezer until use. The concentrations of IL-17 and IFN-γ in the AqH were measured by ELISA.
Isolation of eye-infiltrating cells
Uveitic eyes were collected from five rats per group after PBS perfusion 19. External tissues were trimmed, and the eyes were dissected carefully along the limbus for lens removal. The remaining tissue was transferred into six-well plates containing 3 ml RPMI-1640, 10% FCS, collagenase D (1 mg/ml) and DNase (100 μg/ml), minced with scissors and incubated for 10 min in 37°C. Cell suspension was passed through the cotton column, dispersed by pipetting several times, resuspended in staining buffer (PBS containing 3% FCS and 0·1% sodium azide) and stained with fluorescent mAb to identify inflammatory cells by flow cytometry.
Statistical analysis
Data were expressed as mean ± standard deviation (s.d.). sas version 9·2 software was used for statistical analysis. EAU clinical scores and histopathological scores were assessed by repeated-measures analysis of variance (anova) using mixed models. Data were tested using the Kolmogorov–Smirnov test for normal distribution and the Levene test for homogeneity of variance. Concentrations of cytokines released from T cells in the mice with or without MSC treatment were evaluated by a two-factorial design analysis of quantitative variance. The correlation analysis of cytokine expression and histopathological scores were analysed by Spearman's rank correlation test. A value of P < 0·05 was considered significant.
Result
MSCs ameliorate EAU
MSCs have been proven effective in treatment of autoimmune diseases. To determine whether MSCs had an effect on R16-induced uveitis, R16-immunized Lewis rats received three consecutive i.v. injections of MSC (5 × 106/injection) starting on the same day (Fig. 1a) of R16 immunization (day 0), while control rats received PBS. The results showed that, although all control animals developed full disease, MSC-treated rats developed mild disease with a delayed onset, and the differences between MSC-treated and vehicle-treated conditions were statistically significant from day 6 (Fig. 1a). It was then determined whether or not delayed injection of MSCs had a similar protective effect. As shown in Fig. 1b, three i.v. injections starting on day 12 (i.e. at disease peak) also had a significant effect on EAU development, indicating that MSC treatment not only prevents EAU, but also suppresses EAU when given at the peak of the disease. Histological results confirmed further the efficacy of MSC on EAU. Eyes collected at the indicated days after R16 immunization revealed that MSC-treated eyes displayed less inflammation and tissue damage than those of PBS-treated eyes (Fig. 1c,d).
Figure 1.
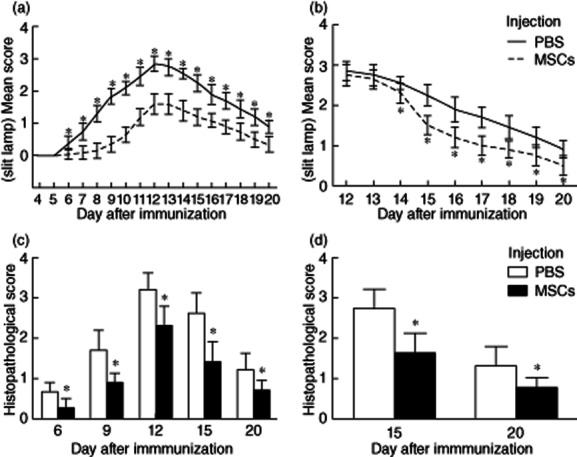
Effects of mesenchymal stem cells (MSCs) on the clinical development and histological changes of autoimmune uveoretinitis (EAU). In the group treated simultaneously with immunization the rats developed mild disease with a delayed onset, and the differences between MSC-treated and vehicle-treated conditions were statistically significant from day 6 (a). The group treated on day 12 after immunization also showed significant improvement, which started from day 14 (b). Histological examinations revealed that MSC-treated eyes displayed less inflammation and tissue damage than those of phosphate-buffered saline (PBS)-treated (control) eyes at all time-points (Fig. 1c,d).
Dynamic effects of MSCs on cytokine expression of Th1/Th17/Th2/Treg cells
To identify the role of MSCs on R16-specific T cell subsets, we collected kinetically the splenic cells from both MSC- and PBS-treated rats, stimulated them with immunized R16 peptides and measured the cytokines in their culture supernatants. In the control group, the expression levels of Th1 and Th17 cytokines such as IL-2, IFN-γ, IL-6 and IL-17 began to increase on day 6, reached a peak on day 12 and decreased gradually on days 15 and 20. The dynamic change of these cytokines presented the same trend as the EAU course. Histopathological scores of EAU had a strong positive correlation with Th1 and Th17 cytokine levels, especially with levels of IL-2 (r = 0·837, P < 0·05), IFN-γ (r = 0·839, P < 0·05), IL-6 (r = 0·736, P < 0·05) and IL-17 (r = 0·906, P < 0·05) (Fig. 2). Moreover, the expression levels of Th2 and Treg cytokines (IL-4, IL-10 and TGF-β) were at a higher level on day 6, decreased at the peak of disease and then increased again when inflammation started to resolve on days 15 and 20. The dynamic change of these cytokines presented the opposite trend with EAU course. Histopathological scores of EAU had a strong negative correlation with Th2 and Treg cytokine levels, especially with levels of IL-4 (r = −0·965, P < 0·05), IL-10 (r = −0·878, P < 0·05) and TGF-β (r = −0·893, P < 0·05) (Fig. 2).
Figure 2.
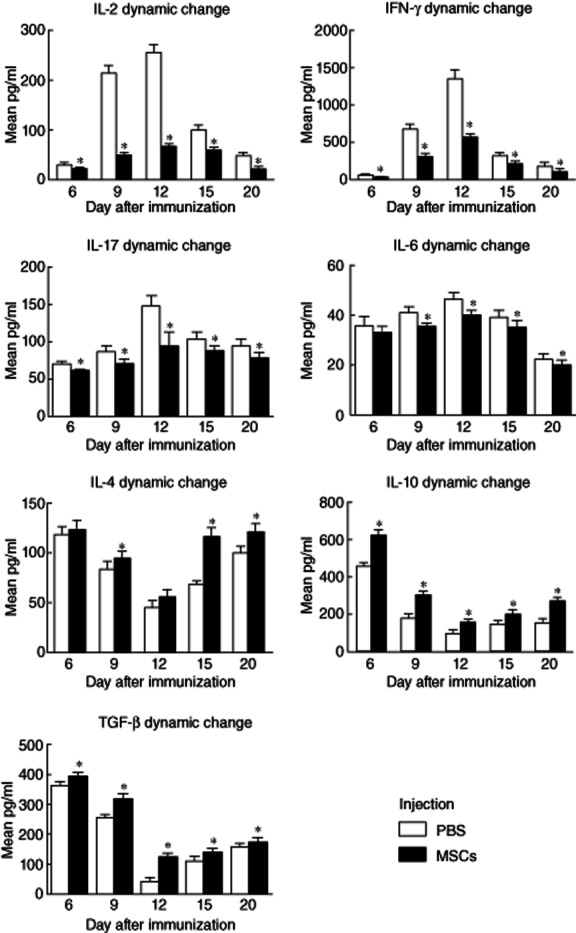
Dynamic effects of mesenchymal stem cells (MSCs) on cytokine expression in the group treated simultaneously with immunization. In the control group, the expression levels of T helper type 1 (Th1) and Th17 cytokines [interleukin (IL)-2, interferon (IFN)-γ, IL-6 and IL-17] began to increase on day 6, reached a peak on day 12 and decreased gradually on days 15 and 20; the expression levels of Th2 and regulatory T cell (Treg) cytokines [IL-4, IL-10 and transforming growth factor (TGF)-β] were at a higher level on day 6, decreased at the peak of disease and then increased again when inflammation started to resolve on days 15 and 20. MSCs reduced significantly the productions of IL-2, IFN-γ, IL-6 and IL-17 compared to the control group at all time-points (P < 0·05).
In contrast, MSCs treatment showed immunosuppressive potential with regard to Th1 and Th17 cytokine secretion. In the group treated simultaneously with R16-immunization, MSCs reduced significantly the production of IL-2, IFN-γ, IL-6 and IL-17 compared to the control group at all time-points (P < 0·05) (Fig. 2). In the group treated on day 12 post-immunization, MSCs reduced significantly the cytokine production released by R16-specific Th1 and Th17 cells when the cells were collected on days 15 and 20 post-immunization (P < 0·05) (Fig. 3). Conversely, MSC treatment increased Th2 and Treg cytokines. In the group treated simultaneously with R16 immunization, MSCs elevated significantly the production of IL-4, IL-10 and TGF-β at all time-points (P < 0·05) (Fig. 2). In the group treated on day 12 (disease peak), MSCs elevated significantly the production of IL-4, IL-10 and TGF-β on days 15 and 20 post-immunization (P < 0·05) (Fig. 3).
Figure 3.
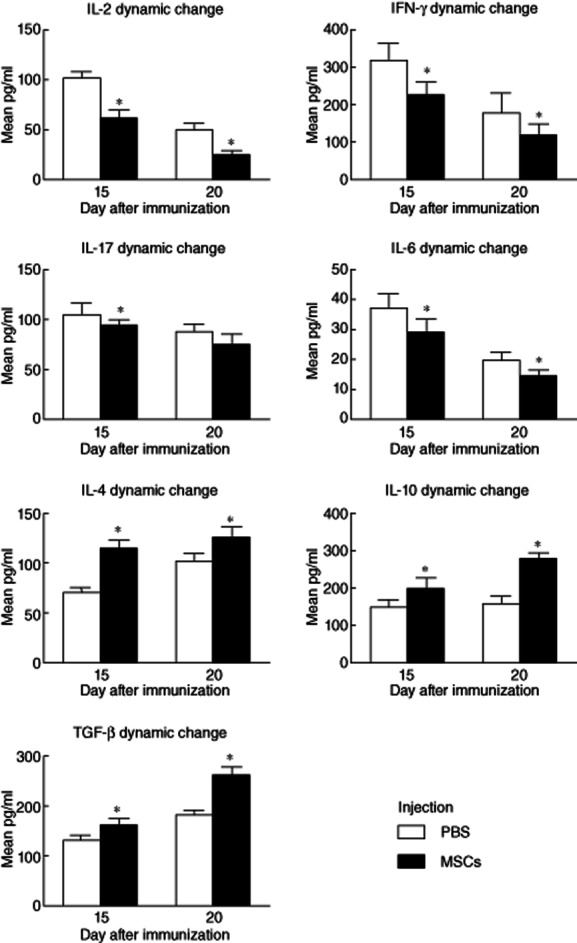
Dynamic effects of mesenchymal stem cells (MSCs) on cytokine expression in the delay-treated group. Therapeutic administration of MSCs at the peak of the disease also reduced significantly the cytokine production released by R16-specific T helper type 1 (Th1) and Th17 cells (P < 0·05), while the production of Th2 and regulatory T cell (Treg) cytokines was elevated on days 15 and 20 post-immunization (P < 0·05).
Reduced IL-17 and IFN-γ and elevated IL-10 levels were detected in the AqH of MSC-treated EAU
To determine whether MSC's effects on R16-specific T cell subsets in the periphery were reflected in the inflamed eye, we measured kinetically the cytokine released by Th1/Th17/Treg in the AqH. As seen in Fig. 4, in the control group the production of IFN-γ and IL-17 in AqH increased from day 6 and reached a peak on day 12, then decreased gradually on days 15 and 20 post-immunization. The levels of IFN-γ and IL-17 were correlated with histopathological scores (r = 0·872, r = 0·903; P < 0·05) (Fig. 4a,b). In contrast, the levels of IFN-γ and IL-17 in the AqH of the MSC-treated group either on the same day or 12 days after R16 immunization were significantly lower than those in the AqH collected from the non-treated (control) group (Fig. 4a,b,d,e). Conversely, IL-10 in AqH was high at the early stage of EAU, but decreased when EAU reached a peak and then increased again when the disease started to resolve. Thus, the level of IL-10 in the AqH was correlated negatively with histopathological scores (r = −0·765, P < 0·05) (Fig. 4c). However, in the groups when MSCs were given at either days 0 or 12 post-immunization, the level of IL-10 in AqH was high in comparison to the control group at all time-points (P < 0·05) (Fig. 4c,f). Hence, the data suggest that the cytokine profile in the eye is similar to that observed in splenic R16-specific T cells.
Figure 4.
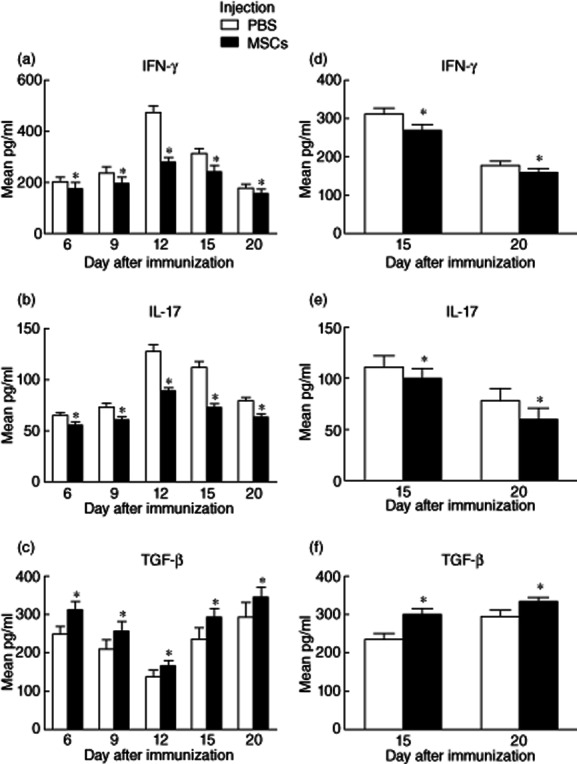
Cytokine release in the aqueous humour (AqH). In the control group, the production of interferon (IFN)-γ and interleukin (IL)-17 in AqH increased from day 6 and reached a peak on day 12, then decreased gradually on days 15 and 20 post-immunization, and both preventive and therapeutic administration of mesenchymal stem cells (MSCs) decreased the levels of IFN-γ and IL-17 in the AqH at all time-points (a,b,d,e). IL-10 in AqH was high at the early stage of autoimmune uveoretinitis (EAU), but decreased when EAU reached a peak and then increased again when the disease started to resolve, and both preventive and therapeutic administration of MSCs elevated the level of IL-10 in AqH at all time-points (P < 0·05) (c,f).
MSCs inhibited Th17, but up-regulated Treg cells in EAU
Further, we examined the ratio of Th17/Treg in the groups with and without MSC treatment, because the cytokines in the environment may direct the conversion of T cell subsets. As shown in Fig 4, the trends of Th17 and Treg cell numbers from the spleen and eye in both groups are parallel with those of Th17 and Treg cytokines. In the control group, Th17 cells from spleen (r = 0·897, P < 0·05, Fig. 5a) and eye (r = 0·933, P < 0·05, Fig. 5c) presented the same trend as the EAU course and were correlated positively with the histopathological scores of EAU. By contrast, MSCs, when given at either days 0 or 12 post-immunization, decreased significantly the number of Th17 cells in both spleen and eye compared with the control group at all time-points (P < 0·05) (Fig. 5a–d). Conversely, Treg numbers in the spleen (r = −0·449, P < 0·05, Fig. 5e) and the eye (r = −0·449, P < 0·05, Fig. 5g) showed the opposite trend to the EAU course and were correlated negatively with the histopathological scores of EAU. MSC treatment either at days 0 or 12 after immunization increased significantly the number of Tregs in both spleen and eye when compared to the control group at all time-points (P < 0·05) (Fig. 5e–h).
Figure 5.
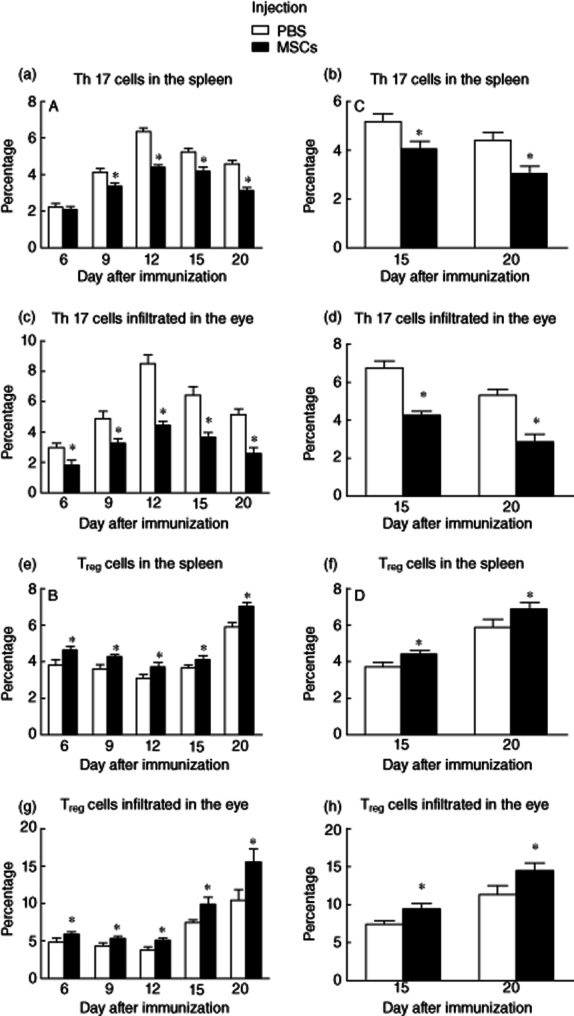
Dynamic effects of mesenchymal stem cells (MSCs) on T helper type 17 (Th17) and regulatory T cell (Treg) cells from spleen and eye. In the control group, Th17 cells from spleen and eye presented the same trend as the autoimmune uveoretinitis (EAU) course (P < 0·05, a–d), while Tregs in the spleen and the eye presented the opposite trend to the EAU course (P < 0·05, e–h). By contrast, MSCs, when given at either days 0 or 12 post-immunization, decreased significantly the number of Th17 cells and increased the number of Tregs in both spleen and eye compared with the control group at all time-points (P < 0·05).
Discussion
Early studies have implicated Th1 cells as the aetiological agent of EAU; however, subsequent studies on human uveitis and its animal models have revealed that the Th17 cell lineage (IL-17-producing CD4+ effector lineage) is another main contributor to autoimmune diseases 20–22. Th1 and Th17 subsets have the capacity to cause autoimmune disease independent of each other. However, cytokines produced by these two subsets can be paradoxical and counter-regulated, as well as co-operated, depending on when and where the cytokines are produced 23–25. Therefore, therapies targeting one subset specifically may prove potentially therapeutic for patients suffering from autoimmune uveitis, but might cause simultaneously an exacerbation of the other, leaving disease progression unaffected or even worsened.
In our study, the dynamic changes of R16-specific Th1 cytokines, such as IL-2 and IFN-γ, and Th17 cytokines, including IL-17A and IL-6, from EAU-inducing rats presented the same trend as the EAU course, and had a strong positive correlation with histopathological scores. Both preventive and therapeutic administration of MSCs decreased significantly the production of these Th1 and Th17 cytokines compared to the control group at all time-points. The levels of IFN-γ and IL-17 in the AqH were also lowered significantly by MSC therapy. These results suggest that MSCs regulate negatively both Th1 and Th17 responses simultaneously in the whole course of EAU, avoiding the potentially adverse effect of targeting one subset specifically.
CD4+CD25+ Tregs are powerful inhibitors that contribute to recovery from autoimmune uveitis 26,27, and decreased frequency and diminished function of Tregs are associated with active uveitis 28. In addition, it is believed that Th2 cells, as counter-regulatory to Th1, would be protective although they also have the ability to induce uveitis under special conditions 25. Tregs can produce abundant anti-inflammatory cytokines (IL-10 and TGF-β), while IL-4 and IL-10 are the main cytokines produced by Th2 cells. We found that the dynamic change of the production of IL-4, IL-10 and TGF-β from T cells isolated from EAU-inducing rats presented the opposite trend to EAU course, and had a strong negative correlation with histopathological scores. Both preventive and therapeutic administration of MSCs elevated significantly the production of these Th2 and Treg cytokines compared to the control group at all time-points. The kinetic levels of IL-10 in the AqH were also significantly higher in the group treated with MSCs. These results suggested that MSC treatment could up-regulate the levels of cytokines released by Th2 cells and Tregs during the whole course of EAU, thereby decreasing the severity of EAU in rats.
Th17 cells and Tregs have a common induced pathway 29–31 and a disturbed Th17/Treg balance has been found in several autoimmune diseases 32–34. TGF-β and IL-6 are the most important cytokines that can orchestrate the differentiation of Tregs and Th17 cells in a concentration-dependent manner. High concentrations of TGF-β favour the development of FoxP3+ Tregs, while in low TGF-β concentrations IL-6 suppresses FoxP3 expression, and the development of Th17 cells prevails 29–31. Restoring the balance of Th17 and Tregs may represent a new therapy to autoimmune diseases such as uveitis.
In this study, we found that Th17 cells from both the spleen and the eye presented the same trend as the EAU course, and was correlated positively with the histopathological scores of EAU, while Tregs presented an opposite trend. As described above, MSC therapy reduced the production of IL-6, whereas it elevated TGF-β secretion from T cells isolated from EAU-inducing rats. Correspondingly, dynamic levels of IL-17 in both AqH and produced from T cells in vitro were decreased by MSC infusion, while IL-10 secretion was prompted. Parallel with the trends of Th17 and Treg cytokines, the number of Th17 cells in both the spleen and the eye was decreased, while the number of Tregs was increased by preventive as well as therapeutic administration of MSCs in comparison to the control group at all time-points. These results provide powerful evidence that MSCs can restore the balance of Th17/Tregs in EAU, which is important for the disease regression.
In this study, MSCs were isolated from the BM of the rats. Nevertheless, in the clinical setting, invasiveness of the BM aspiration procedure and the age-dependent degradation in quantity and quality of the BM–MSCs limit their clinical potential. Alternative sources such as MSCs obtained from Wharton's jelly of the umbilical cords have gained much attention during recent years, as they can be isolated easily and without ethical concerns 35. Wharton's jelly-derived MSCs also represent a more primitive population than their adult counterparts, with an equal or higher degree of self-renewal capacity, multi-differentiation potential and immumodulatory effect, increasing the feasibility for cell-based therapies.
In conclusion, we have demonstrated that a systemic infusion of MSCs ameliorated autoimmune uveoretinitis effectively by inhibiting Th1 and Th17 responses, up-regulating Tregs and modulating the balance of Treg/Th17 cells during the whole course of the disease. These regulatory effects of MSCs occur not only in the systemic lymphoid organs but also in the local target site, suggesting that MSCs is a strong autoimmune inhibitor in both autoreactive T cell priming and effectory phases. In addition, retinal damage is the major cause of vision defect in uveitis and MSCs could repair and restore damaged central nervous system tissues and cells 13. Thus, MSC therapy has the potential to target multiple levels of uvetis pathology, including immunomodulation and photoreceptor cell repair and regeneration, which make it more applicable in the treatment of uveitis.
Acknowledgments
We gratefully acknowledge Ms Changping Li, for assistance with the statistical analysis. This paper was supported by grants from the Tianjin Municipal Science and Technology Commission (07JCYBJC16500, 11JCZDJC19600 and 11JCZDJC18600) and the National Natural Science Foundation of China (30901656)
Disclosure
The authors have no competing interests.
References
- 1.Servat JJ, Mears KA, Black EH, Huang JJ. Biological agents for the treatment of uveitis. Exp Opin Biol Ther. 2012;12:311–328. doi: 10.1517/14712598.2012.658366. [DOI] [PubMed] [Google Scholar]
- 2.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Jiao C, Zhao S. Role of mesenchymal stem cells in immunological rejection of organ transplantation. Stem Cell Rev. 2009;5:402–409. doi: 10.1007/s12015-009-9076-y. [DOI] [PubMed] [Google Scholar]
- 4.De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 5.Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012;67:1–8. doi: 10.1111/j.1600-0897.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Wang D, Liang J, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 7.Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 8.Darlington PJ, Boivin MN, Bar-Or A. Harnessing the therapeutic potential of mesenchymal stem cells in multiple sclerosis. Exp Rev Neurother. 2011;11:1295–1303. doi: 10.1586/ern.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Mu R, Wang S, et al. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R210. doi: 10.1186/ar3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Wang D, Liu D, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren's syndrome. Blood. 2012;120:3142–3151. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2:34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svobodova E, Krulova M, Zajicova A, et al. The role of mouse mesenchymal stem cells in differentiation of naive T-cells into anti-inflammatory regulatory T-cell or proinflammatory helper T-cell 17 population. Stem Cells Dev. 2012;21:901–910. doi: 10.1089/scd.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 14.Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int. 2011;59:347–356. doi: 10.1016/j.neuint.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Ren X, Li G, et al. Mesenchymal stem cells ameliorate experimental autoimmune uveoretinitis by comprehensive modulation of systemic autoimmunity. Invest Ophthalmol Vis Sci. 2011;52:3143–3152. doi: 10.1167/iovs.10-6334. [DOI] [PubMed] [Google Scholar]
- 16.Tasso R, Ilengo C, Quarto R, Cancedda R, Caspi RR, Pennesi G. Mesenchymal stem cells induce functionally active T regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53:786–793. doi: 10.1167/iovs.11-8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Jiao C, Jia Z, Ren X, Li X, Zhao X. Investigation of the role of mesenchymal stem cells on keratoplasty rejection. Chin J Ophthalmol. 2012;48:733–738. [PubMed] [Google Scholar]
- 18.Caspi RR. Experimental autoimmune uveoretinitis in the rat and mouse. Curr Protoc Immunol. 2003 doi: 10.1002/0471142735.im1506s53. Chapter 15: Unit 15–16. [DOI] [PubMed] [Google Scholar]
- 19.Shao H, Van Kaer L, Sun SL, Kaplan HJ, Sun D. Infiltration of the inflamed eye by NKT cells in a rat model of experimental autoimmune uveitis. J Autoimmun. 2003;21:37–45. doi: 10.1016/s0896-8411(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 20.Amadi-Obi A, Yu CR, Liu X, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 21.Chi W, Yang P, Li B, et al. IL-23 promotes CD4+ T cells to produce IL-17 in Vogt-Koyanagi-Harada disease. J Allergy Clin Immunol. 2007;119:1218–1224. doi: 10.1016/j.jaci.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Chi W, Zhu X, Yang P, et al. Upregulated IL-23 and IL-17 in Behçet patients with active uveitis. Invest Ophthalmol Vis Sci. 2008;49:3058–3064. doi: 10.1167/iovs.07-1390. [DOI] [PubMed] [Google Scholar]
- 23.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horai R, Caspi RR. Cytokines in autoimmune uveitis. J Interferon Cytokine Res. 2011;31:733–744. doi: 10.1089/jir.2011.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ke Y, Jiang G, Sun D, Kaplan HJ, Shao H. Ocular regulatory T cells distinguish monophasic from recurrent autoimmune uveitis. Invest Ophthalmol Vis Sci. 2008;49:3999–4007. doi: 10.1167/iovs.07-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun M, Yang P, Du L, Zhou H, Ren X, Kijlstra A. Contribution of CD4+CD25+ T cells to the regression phase of experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2010;51:383–389. doi: 10.1167/iovs.09-3514. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Yang P, Zhou H, et al. Diminished frequency and function of CD4+CD25high regulatory T cells associated with active uveitis in Vogt–Koyanagi–Harada syndrome. Invest Ophthalmol Vis Sci. 2008;49:3475–3482. doi: 10.1167/iovs.08-1793. [DOI] [PubMed] [Google Scholar]
- 29.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korn T, Mitsdoerffer M, Croxford AL, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferraro A, Socci C, Stabilini A, et al. Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes. 2011;60:2903–2913. doi: 10.2337/db11-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu Q, Cai B, Huang ZC, Shi YY, Wang LL. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol Int. 2012;32:2731–2736. doi: 10.1007/s00296-011-1984-x. [DOI] [PubMed] [Google Scholar]
- 34.Khoury SJ. Th17 and Treg balance in systemic sclerosis. Clin Immunol. 2011;139:231–232. doi: 10.1016/j.clim.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Batsali AK, Kastrinaki MC, Papadaki HA, Pontikoglou C. Mesenchymal stem cells derived from Wharton's jelly of the umbilical cord: biological properties and emerging clinical applications. Curr Stem Cell Res Ther. 2013;8:144–155. doi: 10.2174/1574888x11308020005. [DOI] [PubMed] [Google Scholar]


