Abstract
Recent evidence points to the T helper type 17 (Th17) subset as key in the pathogenesis of psoriasis, but cells of this type in lesions remain to be fully characterized. Here we isolated, enumerated, functionally tested and clonotyped the CD4+ Th cell population ex vivo from lesional biopsies and paired peripheral blood samples from psoriasis patients. Th17 cells were over-represented dramatically in lesions from all patients, representing 49–93% of CD4+ Th cells compared with 3–18% in blood. Most lesional Th17 cells produced interleukin (IL)-17A ex vivo without further stimulation and expressed the CD45RO+ phenotype characteristic of activated or memory cells. There was no increase in ‘natural’ [CD25hiforkhead box protein 3 (FoxP3+)] regulatory T cells in lesions versus peripheral blood, but there was enrichment of ‘induced’ IL-10+ regulatory T cell numbers in biopsies from some patients. The lesional Th17 cells exhibited a bias in T cell receptor Vβ chain usage, suggestive of specific expansion by antigen. The therapeutic challenge is to overcome the dominance of overwhelming numbers of such antigen-specific Th17 cells in psoriatic lesions.
Keywords: clonal expansion, IL-10, psoriasis, regulatory T cell subsets, T helper type 17 cell
Introduction
Psoriasis is a common and distressing chronic skin disease. It is widely accepted that the condition is T lymphocyte-driven and autoimmune in origin, with genetic, immunological and therapeutic evidence converging to highlight a central pathogenic role for the T helper type 17 (Th17) CD4+ subset 1,2. Although central to current models of the disease, Th17 cells have yet to be enumerated and characterized directly in lesions.
Th17 cells are characterized by predominant secretion of the inflammatory cytokine interleukin (IL)-17A, and can also produce other mediators such as IL-17F, IL-6, IL-22 and tumour necrosis factor (TNF)-α 3 and express a characteristic combination of chemokine receptors, CCR4 and CCR6 4. Factors that promote human Th17 differentiation from uncommitted CD4+ T cells continue to be identified, but include IL-1β and IL-6, with subsequent expansion enhanced by IL-23 5. It has become apparent that this subset is responsible for inflammation attributed previously to Th1 cells in a number of autoimmune diseases 3, and evidence has accumulated linking Th17 effector pathways with psoriasis 6.
Early clinical observations in psoriasis first suggested a role played by Th17 cells. For example, Ustekinumab and Briakinumab, biologics targeting the p40 subunit shared by IL-12 and IL-23 7–9, have elicited marked clinical improvements in the disease. Th17 cells are equipped to migrate efficiently to the skin, via their chemokine receptors, CCR4 and CCR6, which mediate chemotaxis specifically to CCL17 and CCL20 expressed on cutaneous endothelium and dermal fibroblasts 10,11. Cells capable of producing IL-17 have now been detected in psoriatic lesions 12,13 and may correspond to the Th17 phenotype.
In addition to effector subsets, the CD4+ helper population includes at least two regulatory T (Treg) cell types, defined as ‘natural’ and ‘antigen-induced’ (also known sometimes as ‘adaptive’), which are important in the maintenance of self-tolerance and resolution of immune pathology 14–17. Natural Tregs are committed to a regulatory phenotype in the thymus, constitutively express activation markers such as CD25hi, are characterized by the transcription factor forkhead box protein 3 (FoxP3) and suppress via cell–contact-dependent mechanisms, at least in vitro. In contrast, antigen-induced Tregs differentiate from naive T cells in the periphery and regulate by secreting inhibitory cytokines such as IL-10 and transforming growth factor (TGF)-β1. Antigen-induced Treg can be divided further into subtypes on the basis of cytokine secretion, with T regulatory 1 (Tr1) cells producing IL-10 and little IL-2, IL-4 or TGF-β1, while Th3 cells make high levels of TGF-β1, but only small amounts of IL-10 or IL-4 18. Natural, but not induced, Tregs have been studied in psoriasis, and although present in lesions appear to have a reduced capacity to inhibit effector responses 19. Recently, natural Tregs have been shown to have the capacity to differentiate into IL-17-producing Th17 cells in psoriasis under inflammatory conditions 20.
Despite the evidence accumulating to implicate the Th17 subset in psoriatic pathology 21,22, a number of important questions remain unanswered. These include the numbers of lesional Th17 cells within the CD4+ helper population, their ability to mediate inflammatory functions and their state of activation. Previous studies have relied primarily on assays of IL-17A protein or message to detect Th17 cells, but such approaches can be confounded by the ability of other cell types, including γδT cells, CD8 memory T cells, eosinophils, neutrophils and monocytes to also produce the cytokine 4,23. Moreover, IL-17 release by mast cells and neutrophils may contribute to the pathology of the disease 24. Another limitation has been the dependence on techniques to expand and extract cells from lesions, which preclude the direct enumeration of Th17 cells ex vivo. Furthermore, not all the Th17 in lesions would be detected by IL-17A assays if the cells were inactive or suppressed by the Treg cells that are also reported to be present 19. It is therefore necessary to identify the exact nature of the IL-17A-producing cells in psoriatic lesions. The balances locally between Th17 cells and both natural and induced Treg cells are important potential determinants of whether or not the inflammation perpetuates or resolves. What drives any Th17 expansion in lesions also remains to be established and, in particular, whether this is the result of an antigen-specific response, which would be predicted if the Th17 cells are the primary effectors of an autoimmune pathology.
The current study addressed directly the questions outlined above by characterizing CD4+ lymphocytes isolated directly ex vivo from untreated lesional psoriasis and from paired samples of peripheral blood. The first aims were to enumerate Th17 cells with a panel of characteristic surface markers that are independent of activation state, and then determine their ability to mount IL-17A and other cytokine responses. Any relative expansion of Th17 and natural or induced Treg cells in lesions versus peripheral blood was also established. Finally, it was ascertained whether the Th17 lesional population had undergone clonal expansion as evidenced by biased T cell receptor (TCR) Vβ chain usage.
Materials and methods
Patients
Twenty-two patients aged 20–65 years (median age 50 years, 16 male, six female) were recruited with lesions that had been untreated with systemic agents or phototherapy for 1 month, or with topical therapy for 2 weeks, prior to investigation. Lesional skin was biopsied using a 6-mm punch. Two patients attending clinic for nevus excision were recruited as healthy donors, with the distal portions of the ellipse and peripheral blood collected. Skin samples were disaggregated physically immediately ex vivo for 1 min using 50 μM medicons in the Medimachine (BD Biosciences, San Diego, CA, USA), allowing efficient collection of viable lymphocytes 25. The cell suspension was then prepared for culture or flow cytometry 26. In addition, paired donations of blood from the patient group were collected by venepuncture into heparin. Peripheral blood mononuclear cells (PBMC) were obtained by density gradient centrifugation (Ficoll-Paque; Amersham Biosciences, Piscataway, NJ, USA).
The study was approved by the North of Scotland Research Ethics Committee, and followed the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All patients gave written informed consent.
Flow cytometry
The numbers of cells of each Th subset were determined by flow cytometry. Cell suspensions were analysed after staining with combinations of the following antibodies: anti-CD3-fluorescein isothiocyanate (FITC) (Beckman Coulter, Fullerton, CA, USA), anti-CD4-FITC or anti-CD4-allophycocyanin-cyanine 7 (APC-Cy7), anti-CCR4-phycoerythrin (PE)-Cy7, anti-CCR6-AlexaFluor 647 or anti-CCR6-PE (BD Pharmingen, San Diego, CA, USA), anti-IL-23R-APC or anti-IL-23R-PE (R&D Systems, Abingdon, UK) for Th17 cells 4. Anti-CD45RO-FITC (clone UCHL1, a generous gift from Diaclone, Besançon, France) was used to identify activated or memory T cells 27. Anti-CD25-AlexaFluor 700 (BioLegend, San Diego, CA, USA) or anti-CD25-PE-Cy5 (Beckman Coulter) plus anti-FoxP3-AlexaFluor 647 (BD Pharmingen) and anti-IL-10-Pacific blue (eBioscience, San Diego, CA, USA) allowed respective identification of the natural and induced Treg populations. The production of IL-17A was measured by intracellular staining using anti-IL-17A-AlexaFluor 647 (eBioscience). For intracellular protein staining, cells were fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences), as per the manufacturer's protocol. IFN-γ production was assessed using an anti-IFN-γ-PE antibody (Beckman Coulter). Compensation beads (BD Pharmingen) were used for each sample, providing single positive controls for voltage adjustments and compensation calculations.
Unless stated otherwise, the cytoplasmic staining of cytokines was enhanced by 12 h cell culture with 10 μg/ml Brefeldin A (Sigma-Aldrich, St Louis, MO, USA). In some experiments, cells were activated with 25 ng/ml phorbol 12-myristate 13-acetate (PMA) and 2 μg/ml ionomycin (Sigma-Aldrich) and cultured with Brefeldin A.
The clonality of CD4+ populations was assessed using a commercially available flow cytometry array kit (Beckman Coulter) containing antibodies specific for a panel of human TCR Vβ chains.
Data from each patient were acquired independently on different days using the LSR II (BD Biosciences) and analysed using FCS Express version 3 software (DeNovo Software, London, ON, Canada) and FlowJo version 7 (Tree Star Inc., Ashland, OR, USA). Cell populations were consistently gated and subgated on isotype controls.
Immunohistochemistry
Tissue samples were fresh-frozen, sectioned and fixed with acetone. Sections were incubated with a panel of primary antibodies detailed in the flow cytometry section, followed by a biotinylated anti-mouse secondary antibody (Vector Laboratories, Peterborough, UK). Positive staining was visualized using the avidin–biotin–horseradish peroxidase complex (the Vectastain Elite ABC Kit) (Vector Laboratories). Sections were counterstained with haematoxylin and Scot's tap water, as detailed by the manufacturer. A no-primary antibody (NPA) section was used as a negative control.
Statistics
Statistical analyses were conducted by the non-parametric Kruskal–Wallis test using Minitab® Statistical Software (Minitab Ltd, Coventry, UK). The level of significance was taken as P < 0·05.
Results
Expansion of the Th17 subset in psoriatic lesions
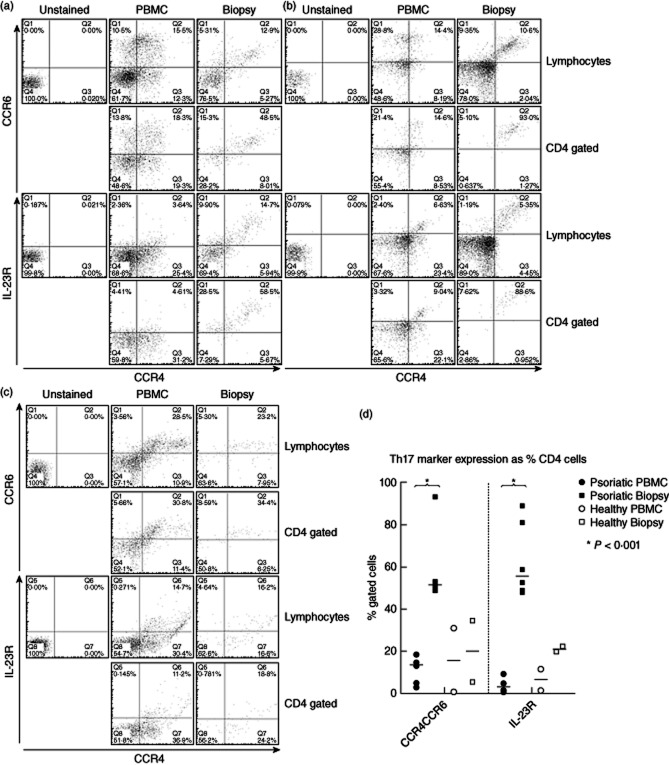
The main purpose of the work was to identify, enumerate and characterize precisely Th17 cells in psoriatic lesions. Initial flow cytometric analyses focused on combinations of surface markers, in particular CCR4+CCR6+ and IL-23R, which can be used to identify human Th17 cells 4 independent of their activation state or current ability to secrete cytokines. IL-23R can also be expressed on γδ T cells and natural killer (NK) cell lines, and at low levels on monocytes, macrophages and dendritic cells 28, but staining in combination with the other markers supports the identification of the Th17 phenotype. Representative data from two psoriatic individuals are illustrated in Fig. 1a,b and one healthy control in Fig. 1c, with the results from the patient group summarized in Fig. 1d. It can be seen that, typically, cells with this Th17 phenotype make up more than 50% of the CD4+ population in lesions (mean 57·6%, n = 6), compared with fewer than 15% in peripheral blood (mean 11·2%, n = 6). The consistent over-representation of the Th17 cells in lesions versus peripheral blood is highly significant (P < 0·001, Kruskal–Wallis test) and striking, in some cases with, for example, the vast majority (> 90%) of lesional CD4+ cells expressing the Th17 phenotype in one individual (Fig. 1b). To corroborate these results, expression the receptor for the Th17 growth factor IL-23 29 was also determined. Figure 1 demonstrates a significantly increased proportion of CD4+ cells with this marker in lesions versus peripheral blood, comparable to that observed for the CCR4+CCR6+ phenotype. Healthy control skin, as expected, contains lower Th cell numbers. The large lesional CD4+ population, including IL-23R+ cells, can be visualized in both the dermis and the epidermis by immunohistochemistry (Fig. 4b).
Figure 1.
CD4+ lymphocytes expressing T helper type 17 (Th17) markers CCR4 and CCR6 or interleukin (IL)-23R are greatly enriched in psoriatic lesions compared with peripheral blood. (a,b) Representative flow cytometric analyses from two patients and (c) from a healthy control. For each set, the expression of CCR4 and CCR6 (upper set) or CCR4 and IL-23R (lower set) are illustrated for paired samples of peripheral blood mononuclear cells (PBMC) (middle) and lesional cells (left), gated on the lymphocyte population (upper pairs), or CD4+CD3+ cells (lower pairs). Th17 cells stain CCR4+CCR6+ or CCR4+IL-23R+, (upper right quadrants). (d) Summarizes data from analyses of CD4+ cells from lesional biopsies and blood of psoriatic patients (n = 6) and healthy controls (n = 2), demonstrating the percentages of CD4+ cells that express Th17 markers (bar indicates median value; *P < 0·001, Kruskal–Wallis test).
Figure 4.
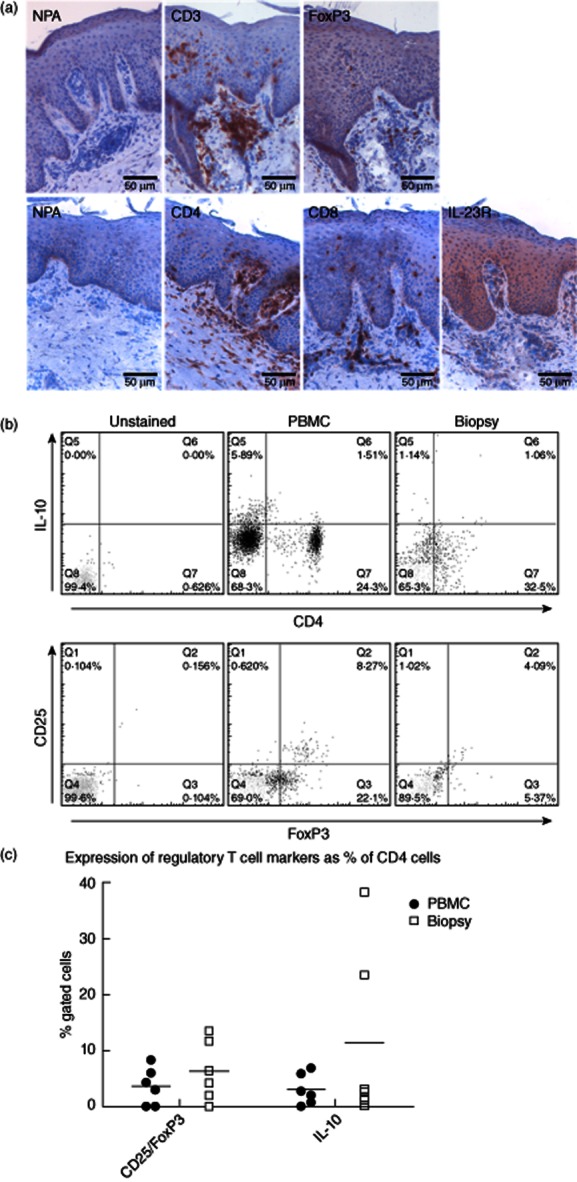
Natural and induced regulatory T cells (Tregs) are present in psoriatic plaques, but while the frequency of the natural type is similar to that in peripheral blood, induced Tregs can be enriched in lesions from some patients. (a) Immunohistochemistry slides showing that CD4+ lymphocytes, forkhead box protein 3 (FoxP3+) cells and interleukin (IL)-23R+ cells are all found in both the dermis and epidermis of psoriatic lesions. (b) A set of representative flow cytometric data from psoriatic patients are shown, demonstrating the numbers of IL-10+-induced T regulatory 1(Tr1) phenotype (upper) and CD25hiFoxP3+ cells within the CD4+ lymphocyte (lower) populations from peripheral blood mononuclear cells (PBMC) (middle) and paired lesional biopsies (right). (c) Summarizes data from analyses of CD4+ cells from lesional biopsies and blood of psoriatic patients (n = 6), demonstrating the percentages of CD4+ cells that express Treg markers (bar indicates median value).
Functional and activation state of Th17 cells in psoriatic lesions
The next experiments were designed to determine the proportions of Th17 cells that are functionally active in producing the signature cytokine IL-17A. Peripheral blood and lesional cells were permeabilized and stained for IL-17A, either directly ex vivo or after brief culture with Brefeldin A, which increases the sensitivity of intracellular cytokine detection by blocking secretion and promoting cytoplasmic accumulation (Fig. 2). The results of flow cytometric analysis demonstrate that only rare peripheral blood CD4+ cells with either the CCR4+CCR6+ or IL-23R+ Th17 phenotypes also stained for IL-17A but by contrast, after brief incubation, 20–42% of lesional Th17 cells were active in producing this cytokine. IL-17A production by lesional cells was strongest after incubation with the potent polyclonal activator PMA/ionomycin and Brefeldin A, but in many cases there was spontaneous production sufficiently robust to be detectable even in the absence of these treatments (Fig. 2b).
Figure 2.
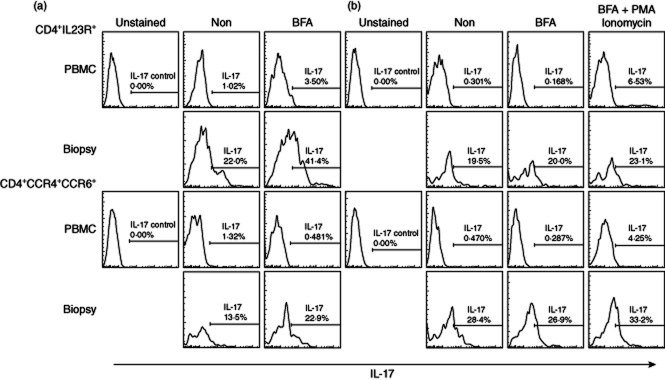
Abundant production of interleukin (IL)-17A by CD4+ T cells expressing the T helper type 17 (Th17) markers CCR4 plus CCR6 or IL-23R from psoriatic lesions but not peripheral blood. (a,b) Representative flow cytometric analyses of peripheral blood mononuclear cells (PBMC) and paired lesional biopsies from two patients demonstrating the percentages of CD4+IL23R+ (upper sets of panels) or CD4+CCR4+CCR6+ (lower sets of panels) T cells that stain for intracellular IL-17A. Cells were stained for spontaneous IL-17A production ex vivo (Non), or after 12 h culture in Brefeldin A (BFA) to enhance cytokine accumulation, or after stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin and culture with BFA. The marked spontaneous production of IL-17A by lesional Th17 phenotype cells indicates that they have been activated to produce cytokine in vivo.
The functionality of the lesional CD4+ population was dissected further by asking whether it also includes cells producing inflammatory cytokines of the Th1 rather than the Th17 type. Peripheral blood and lesional CD4+ T cells were co-stained for intracellular IL-17A and IFN-γ after incubation with Brefeldin A and analysed by flow cytometry. Figure 3a demonstrates that, as expected, few peripheral blood CD4+ cells produced either effector cytokine in the absence of any ex-vivo stimulation, while a large fraction (> 29%) of the lesional population stained for IL-17A only, up to 9% for both IL-17 and IFN-γ and up to 6% for IFN-γ alone, consistent with the mixed phenotypes elicited typically in human Th cells, even in highly polarized responses 30.
Figure 3.
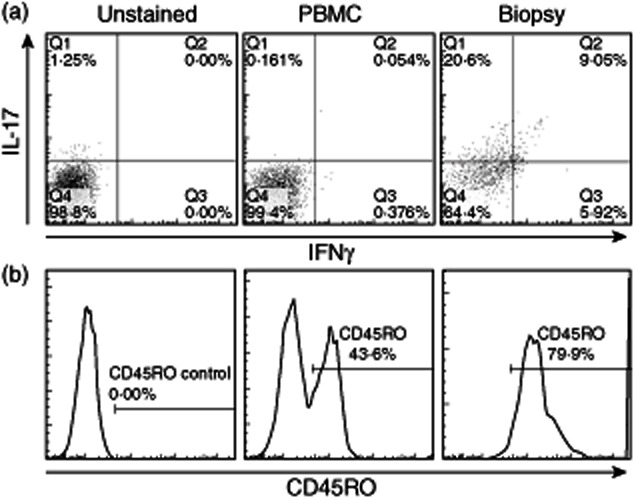
T helper type 17 (Th17) cells from psoriatic lesions can produce the Th1 cytokine interferon (IFN)-γ and are all of the activated CD45RO phenotype. Representative flow cytometric plots of paired peripheral blood mononuclear cells (PBMC) and lesional CD4+ T cells showing staining for interleukin (IL)-17A and IFN-γ (a) after 12-h culture with Brefeldin A, or expression of the activation or memory marker CD45RO by IL-23R+ gated Th17 phenotype cells (b).
The ex-vivo flow cytometric assays for spontaneous cytokine production are consistent with the lesional Th17 cells having been activated in vivo. To confirm this activation status, peripheral blood and lesional CD4+ cells were analysed for co-expression of the Th17 marker IL-23R and CD45RO, which stains recently activated or memory human Th cells 27 (Fig. 3b). Approximately 40–45% of peripheral blood IL-23R+ cells were CD45RO+, consistent with previous studies of CD4+ populations in healthy adults 27 but, by contrast, more than 79% of lesional Th17 cells were of the activated or memory phenotype.
Natural and induced Treg cells in psoriatic lesions
The dramatic expansion of Th17 cells in psoriasis lesions leads to the question of whether or not there is any comparable increase in local Treg cells that might balance their activity. FoxP3 cells can be seen in the dermis and the epidermis by immunohistochemistry (Fig. 4a), and the numbers and phenotype were analysed by flow cytometry. Peripheral blood and lesional CD4+ cells were co-stained with the natural Treg markers CD25 and FoxP3 and the induced regulatory cytokine IL-10. Representative examples are shown in Fig. 4b. The proportion of CD4+ cells with a natural CD25hiFoxP3+ Treg phenotype in blood lies within the anticipated normal adult range (2–10%) 31, but there is no significant difference between the numbers circulating and in the lesions. Across all six patients tested, there is also no significant difference between peripheral blood and lesions in the numbers of CD4+ cells positive for the induced regulatory cytokine IL-10 (Fig. 4c). However, in two patients there was a marked over-representation of IL-10+CD4+ cells in lesions, accounting for up to 20% of the CD4+ population Fig. 4c). Although it cannot be excluded formally that the IL-10 was produced by Th2 rather than Treg cells 32 these results suggest that, overall, CD4+ cells with an induced, but not natural, Treg phenotype can be over-represented in some psoriatic lesions.
Clonality of CD4+ and Th17 cells in psoriatic lesions
If the lesional Th17 cells are the primary effectors of an autoimmune pathology, as is currently widely proposed, then their over-representation would be expected to be the result of a response to specific antigen. To test this, the clonality of CD4+ cells within lesions and peripheral blood was compared by flow cytometric staining of lesional cells from four patients with an array kit of antibodies to a panel of 24 TCR Vβ chain sequences. A summary and representative data are shown in Fig. 5. As expected, the frequencies of CD4+ cells within peripheral blood expressing each TCR Vβ chain were distributed evenly. In contrast, all patients demonstrated marked over-representation of particular Vβ chains in the CD4+ population from lesions. Although there was some overlap between patients in the chains affected, most notably Vβ5·1 in two cases, the patterns of preferential TCR expression varied between individuals.
Figure 5.
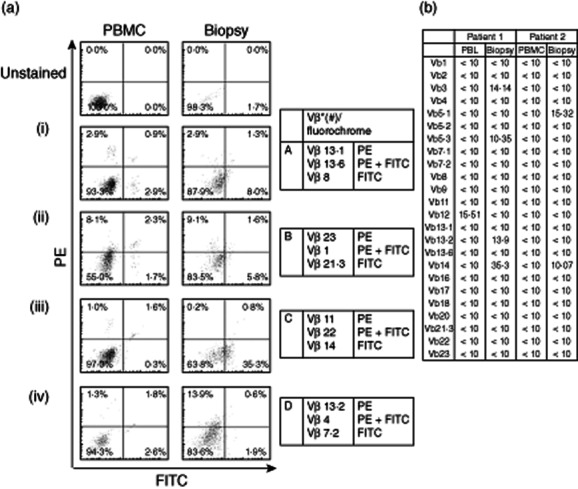
CD4+ T cells from psoriatic lesions exhibit biased T cell receptor (TCR) Vβ chain usage indicative of clonal expansion. (a) Representative flow cytometric plots of peripheral blood mononuclear cells (PBMC) and lesional biopsy samples, after staining with antibodies specific for a panel of TCR Vβ chains. The panels show the proportions of CD4+ T cells expressing each Vβ chain from the paired samples of individual patients. (b) Complete data sets from two patients showing TCR usage as a percentage of CD4+ cells.
Discussion
This is the first study to provide a full quantitation of both active and inactive Th17 cells within psoriatic lesions by flow cytometry. The results demonstrated that cells with the characteristic Th17 surface phenotype 4 are expanded dramatically in sites of psoriatic inflammation compared to peripheral blood, representing the predominant Th subset, and accounts for a median of 51–55% of all lesional CD4+ T cells. The inflammatory activity of this lesional population was confirmed by staining for IL-17A, which revealed that many of the Th17 phenotype cells produced the cytokine ex vivo spontaneously with no further need for stimulation. The status of the minor fraction of the lesional cells with the Th17 surface phenotype that did not produce detectable IL-17A, even after potent non-specific stimulation, is unclear, and may point to a Th22 CD4 cell subtype 33 which shares some of the phenotypic markers as Th17 cells. Another explanation may be that a proportion of T cells in any highly activated population would be expected to exhibit signs of exhaustion or suppression 34.
We identified fewer natural Treg cells in psoriatic lesions than Sugiyama et al. 19, who reported that they were functionally defective, but this discrepancy in numbers may be due to the use of different markers for the population. To isolate Treg cells for functional studies Sugiyama et al. selected them by CD25 expression, which is up-regulated on activated as well as regulatory cells 31. Here, enumeration of natural Treg cells by dual staining for CD25 plus the characteristic transcription factor FoxP3 was more rigorous, excluding the single positive CD25+FoxP3− T cells with an activated, non-regulatory phenotype, which can comprise a relatively large population. This may be a conservative estimate, as some Treg cells in vivo may lose phenotypic markers such as FoxP3 following repetitive TCR stimulation 35. However, irrespective of any differences arising from the use of different markers, Th17 cells are far more common than natural Treg cells in psoriatic lesions, and such a numerical advantage could potentially overwhelm the ability of even fully active Treg cells to suppress 36. Furthermore, the effectiveness of Treg cells could be compromised further by their reported ability to differentiate into Th17 cells in psoriasis 20.
We show that CD4+ T cells producing the suppressive cytokine IL-10, corresponding to the induced T regulatory 1 (Tr1) subset 15, can be present in lesions and, unlike natural Treg cells, their numbers can be enriched markedly relative to peripheral blood in particular patients. There may be therapeutic advantage in boosting local IL-10 responses, as production of the cytokine has been implicated previously in the inhibition of psoriatic keratinocyte and T cell proliferation 37.
Evidence from the current study points to the expansion of Th17 cells in psoriatic lesions being driven by antigen. Analysis of TCR Vβ chain usage revealed a skew towards limited clonality in lesional skin compared to peripheral blood, supporting and extending earlier observations of psoriatic skin T cells 38. Recent sequencing of a panel of T cells cloned from psoriatic plaques indicated extensive sharing of TCR 39, and we confirm that similar over-representation can be found in unselected lesional cells isolated ex vivo. The variation between patients in the clonotypes expanded reflects the heterogeneity of human leucocyte antigen restriction and epitope selection seen in the pathogenic responses in other autoimmune diseases 40–42.
The ratio and activation status of Th17 and Treg cells in psoriatic lesions may be important in locally controlling the pathogenesis of inflammation, and this balance may potentially be shifted therapeutically. For example, manipulating IL-10 levels could shift Th17 to a regulatory phenotype and ameliorate autoimmune or inflammatory diseases in which Th17 cells are involved 43. Specificity of such treatment may be possible by targeting Th cells that recognize particular autoantigens. It may not be necessary to identify the autoantigens definitively, as one lesson from experimental models of autoimmune disease 31 is that reinstatement of self-tolerance to any single involved antigen can induce potent bystander effects on other, co-localized pathogenic responses.
Acknowledgments
We would like to thank the staff in the Dermatology Department at the Aberdeen Royal Infirmary. This project was funded by the Psoriasis Association and Dr Lewis by an unrestricted educational grant from Abbott.
Disclosure
In relation to the subject matter in this paper, Dr A. D. Ormerod has received non-personal, departmental support from Janssen Pharmaceuticals and Abbott and has provided consultancy work for Abbott and Amgen. The remaining authors state no other conflicts of interest.
References
- 1.Blauvelt A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J Invest Dermatol. 2008;128:1064–1067. doi: 10.1038/jid.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 5.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 7.Kimball AB, Gordon KB, Langley RG, Menter A, Chartash EK, Valdes J. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Arch Dermatol. 2008;144:200–207. doi: 10.1001/archdermatol.2007.63. [DOI] [PubMed] [Google Scholar]
- 8.Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 9.Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 10.Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175–2183. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanda N, Shibata S, Tada Y, Nashiro K, Tamaki K, Watanabe S. Prolactin enhances basal and IL-17-induced CCL20 production by human keratinocytes. Eur J Immunol. 2009;39:996–1006. doi: 10.1002/eji.200838852. [DOI] [PubMed] [Google Scholar]
- 12.Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 13.Pene J, Chevalier S, Preisser L, et al. Chronically inflamed human tissues are infiltrated by highly differentiated th17 lymphocytes. J Immunol. 2008;180:7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 14.Crellin NK, Garcia RV, Levings MK. Flow cytometry-based methods for studying signaling in human CD4+CD25+FOXP3+ T regulatory cells. J Immunol Methods. 2007;324:92–104. doi: 10.1016/j.jim.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 16.Roncarolo MG, Levings MK. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr Opin Immunol. 2000;12:676–683. doi: 10.1016/s0952-7915(00)00162-x. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz DA, Zheng SG, Gray JD. The role of the combination of IL-2 and TGF-beta or IL-10 in the generation and function of CD4+CD25+ and CD8+ regulatory T cell subsets. J Leukoc Biol. 2003;74:471–478. doi: 10.1189/jlb.0503228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama H, Gyulai R, Toichi E, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131:1853–1860. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Yang XQ, Cheng J, Hui RS, Gao TW. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol. 2010;135:108–117. doi: 10.1016/j.clim.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 24.Lin AM, Rubin CJ, Khandpur R, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brockhoff G, Fleischmann S, Meier A, Wachs FP, Hofstaedter F, Knuechel R. Use of a mechanical dissociation device to improve standardization of flow cytometric cytokeratin DNA measurements of colon carcinomas. Cytometry. 1999;38:184–191. doi: 10.1002/(sici)1097-0320(19990815)38:4<184::aid-cyto5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich M, Krammig S, Henze M, Docke WD, Sterry W, Asadullah K. Flow cytometric characterization of lesional T cells in psoriasis: intracellular cytokine and surface antigen expression indicates an activated, memory/effector type 1 immunophenotype. Arch Dermatol Res. 2000;292:519–521. doi: 10.1007/s004030000167. [DOI] [PubMed] [Google Scholar]
- 27.Barker RN, Elson CJ. Multiple self epitopes on the Rhesus polypeptides stimulate immunologically ignorant human T cells in vitro. Eur J Immunol. 1994;24:1578–1582. doi: 10.1002/eji.1830240719. [DOI] [PubMed] [Google Scholar]
- 28.Rong C, Hu W, Wu FR, Cao XJ, Chen FH. Interleukin-23 as a potential therapeutic target for rheumatoid arthritis. Mol Cell Biochem. 2012;361:243–248. doi: 10.1007/s11010-011-1109-6. [DOI] [PubMed] [Google Scholar]
- 29.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991;12:A49–53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 33.Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann P, Boeld TJ, Eder R, et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor RA, Taams LS, Anderton SM. Translational mini-review series on Th17 cells: CD4 T helper cells: functional plasticity and differential sensitivity to regulatory T cell-mediated regulation. Clin Exp Immunol. 2010;159:137–147. doi: 10.1111/j.1365-2249.2009.04040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Z, Chen L, Liu YF, et al. Altered keratin 17 peptide ligands inhibit in vitro proliferation of keratinocytes and T cells isolated from patients with psoriasis. J Am Acad Dermatol. 2006;54:992–1002. doi: 10.1016/j.jaad.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 38.Prinz JC, Vollmer S, Boehncke WH, Menssen A, Laisney I, Trommler P. Selection of conserved TCR VDJ rearrangements in chronic psoriatic plaques indicates a common antigen in psoriasis vulgaris. Eur J Immunol. 1999;29:3360–3368. doi: 10.1002/(SICI)1521-4141(199910)29:10<3360::AID-IMMU3360>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.Larsen M, Arnaud L, Hie M, et al. Multiparameter grouping delineates heterogeneous populations of human IL-17 and/or IL-22 T-cell producers that share antigen specificities with other T-cell subsets. Eur J Immunol. 2011;41:2596–2605. doi: 10.1002/eji.201041131. [DOI] [PubMed] [Google Scholar]
- 40.Cairns LS, Phelps RG, Bowie L, et al. The fine specificity and cytokine profile of T-helper cells responsive to the alpha3 chain of type IV collagen in Goodpasture's disease. J Am Soc Nephrol. 2003;14:2801–2812. doi: 10.1097/01.asn.0000091588.80007.0e. [DOI] [PubMed] [Google Scholar]
- 41.Hall AM, Ward FJ, Vickers MA, Stott LM, Urbaniak SJ, Barker RN. Interleukin-10-mediated regulatory T-cell responses to epitopes on a human red blood cell autoantigen. Blood. 2002;100:4529–4536. doi: 10.1182/blood-2002-05-1383. [DOI] [PubMed] [Google Scholar]
- 42.Sukati H, Watson HG, Urbaniak SJ, Barker RN. Mapping helper T-cell epitopes on platelet membrane glycoprotein IIIa in chronic autoimmune thrombocytopenic purpura. Blood. 2007;109:4528–4538. doi: 10.1182/blood-2006-09-044388. [DOI] [PubMed] [Google Scholar]
- 43.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]