Abstract
Ankylosing spondylitis (AS) is a chronic inflammatory disorder characterized by dysregulated T cells. We hypothesized that the aberrant expression of microRNAs (miRNAs) in AS T cells involved in the pathogenesis of AS. The expression profile of 270 miRNAs in T cells from five AS patients and five healthy controls were analysed by real-time polymerase chain reaction (PCR). Thirteen miRNAs were found potentially differential expression. After validation, we confirmed that miR-16, miR-221 and let-7i were over-expressed in AS T cells and the expression of miR-221 and let-7i were correlated positively with the Bath Ankylosing Spondylitis Radiology Index (BASRI) of lumbar spine in AS patients. The protein molecules regulated by miR-16, miR-221 and let-7i were measured by Western blotting. We found that the protein levels of Toll-like receptor-4 (TLR-4), a target of let-7i, in T cells from AS patients were decreased. In addition, the mRNA expression of interferon (IFN)-γ was elevated in AS T cells. Lipopolysaccharide (LPS), a TLR-4 agonist, inhibited IFN-γ secretion by anti-CD3+anti-CD28 antibodies-stimulated normal T cells but not AS T cells. In the transfection studies, we found the increased expression of let-7i enhanced IFN-γ production by anti-CD3+anti-CD28+ lipopolysaccharide (LPS)-stimulated normal T cells. In contrast, the decreased expression of let-7i suppressed IFN-γ production by anti-CD3+anti-CD28+ LPS-stimulated AS T cells. In conclusion, we found that miR-16, miR-221 and let-7i were over-expressed in AS T cells, but only miR-221 and let-7i were associated with BASRI of lumbar spine. In the functional studies, the increased let-7i expression facilitated the T helper type 1 (IFN-γ) immune response in T cells.
Keywords: ankylosing spondylitis, interferon gamma, let-7i, microRNA, T cells, Toll-like receptor-4
Introduction
Ankylosing spondylitis (AS) is a chronic inflammation arthritis that affects both axial and peripheral skeletons and soft tissues. It is conceivable that human leucocyte antigen (HLA)-B27 is the most important risk factor for AS 1, whereas misregulation of T cells could contribute to the inflammatory responses in AS patients 2. The misfolded HLA-B27 heavy chain homodimer in an animal model has supported the importance of HLA-B27 in the pathogenesis of AS 3. Subsequent studies have revealed that the activation of Th17 cells is also critical for sustaining the inflammatory responses in AS patients clinically 4–6. Recently, genetic studies of AS patients have also suggested that T cells might play an essential role in the immunopathogenesis of AS 7. Dysregulated CD4+ and CD8+ T cells were found in peripheral blood 8,9 and inflammatory joints 10,11 of the AS patients. Moreover, increased intracellular nitric oxide (NO) production and delayed calcium responses were observed in T cells from peripheral blood of AS patients 12.
MicroRNAs (miRNAs) are small, non-coding RNA molecules that regulate the expression of multiple target genes at the post-transcriptional level and hence play critical roles in modulating innate and adaptive immune responses. Altered miRNA expression has been implicated in the pathogenesis of different forms of arthritis, including rheumatoid arthritis (RA) and osteoarthritis (OA). Many studies have demonstrated that altered expression of miRNAs in synovia, peripheral blood mononuclear cells (PBMCs) or T cells from patients with RA or OA is associated with innate immunity, inflammation, osteoclastogenesis and cartilage synthesis 13–20. However, the roles of aberrant expressed miRNAs in the pathogenesis of AS remain unclear. We hypothesized that aberrant expression of miRNAs in the T cells of AS patients may alter expression of the downstream target molecules that may contribute to the pathogenesis of AS.
Indeed, our study demonstrated that miR-16, miR-221 and let-7i were over-expressed in AS T cells, and the latter two were associated with radiographic change. Transfection studies suggest that increased expression of let-7i enhanced interferon (IFN)-γ production but suppressed Toll-like receptor-4 (TLR-4) expression in AS T cells.
Material and methods
Patients and controls
Twenty-seven HLA-B27-positive patients fulfilling the 1984 modified New York criteria for the classification of ankylosing spondylitis 21 were recruited for this study. Twenty-three age- and sex-matched healthy volunteers served as a control group. Each participant signed informed consent forms approved by the local institutional review board and ethics committee of Buddhist Dalin Tzu Chi General Hospital, Chia-Yi, Taiwan (no. 09801019). Blood samples were collected at least 12 h after the last dosage of immunosuppressants to minimize the drug effects. The grade of sacroiliitis was identified according to the New York criteria 22 and the lumbar spine involvement was graded by the Bath Ankylosing Spondylitis Radiology Index (BASRI) 23 in AS patients.
Isolation of RNA from T cells
Heparinized venous blood obtained from AS patients and healthy volunteers was mixed with one-fourth volume of 2% dextran solution (MW 464 000 daltons; Sigma-Aldrich, St Louis, MO, USA) and incubated at room temperature for 30 min. Leucocyte-enriched supernatant was collected and layered over a Ficoll-Hypaque density gradient solution (specific gravity 1·077; Pharmacia Biotech, Uppsala, Sweden). After centrifugation at 250 g for 25 min, mononuclear cells were aspirated from the interface. Then, T cells were purified further by anti-human CD3 magnetic beads using IMag Cell Separation System (BD Bioscience, Franklin Lakes, NJ, USA). The T cell concentration was adjusted to 1 × 106/ml in RPMI-1640 containing 10% heat-inactivated fetal bovine serum (FBS), 2 mmol/l L-glutamine, penicillin (100 U/ml) and streptomycin (100 mg/ml) (10% FBS-RPMI) for further analysis. Total RNA including miRNA from the T cells was extracted using the mirVana miRNA isolation kit (Ambion, Austin, TX, USA), according to the manufacturer's protocol. The RNA concentration was quantified using a NanoDrop Spectrophotometer.
Reverse transcription (RT) of miRNAs
We converted all miRNAs into corresponding cDNAs in a one-step RT reaction by the method developed by Chen et al. 24. Briefly, 10 μl reaction mixture containing miRNA-specific stem-loop RT primers (final 2 nM each), 500 μM deoxyribonucleotide (dNTP), 0·5 μl Superscript III (Invitrogen, Carlsbad, CA, USA), and 1 μg total RNA were used for the RT reaction. The pulsed RT reaction was performed in the following conditions: 16°C for 30 min, followed by 50 cycles at 20°C for 30 s, 42°C for 30 s and 50°C for 1 s. After RT the products were diluted 20-fold before further analysis.
Measurement of microRNA (miRNA) expression by real-time PCR
A real-time PCR-based method was used to quantify the expression levels of miRNA in this study using the protocol described previously 25. One microlitre of prepared RT product was used as template for PCR. Then 1 × SYBR Master Mix (Applied Biosystems, Foster City, CA, USA), 200 nM miRNA-specific forward primer and 200 nM universal reverse primer was added for each PCR reaction. All reactions were performed in duplicate on an ABI Prism 7500 Fast real-time PCR system (Applied Biosystems). The condition for quantitative PCR is 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 63°C for 32 s. The expression of the U6 small nuclear RNA was used as endogenous control for data normalization. The threshold cycle (Ct) is defined as the cycle number at which the change of fluorescence intensity crosses the average background level of the fluorescence signal.
First, T cells purified from five AS patients and five healthy controls were analysed for the expression profile of 270 human miRNAs by real-time PCR. We then validated the expression levels of those potentially aberrant expressed miRNAs in T cells from in another 22 AS patients and 18 healthy controls.
Western blotting of cell lysates
T cells were lysed with 1% NP-40 (Sigma-Aldrich) in the presence of a proteinase inhibitor cocktail (Sigma-Aldrich). Seventy micrograms of the cell lysates were electrophoresed and transferred to a polyvinylidene difluoride (PVDF) sheet (Sigma-Aldrich). After blocking, the membranes were incubated with the primary antibodies followed by horseradish peroxidase (HRP)-conjugated secondary antibodies. Mouse monoclonal anti-c-kit, anti-Bcl-2 and anti-TLR-4 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and anti-β-actin was purchased from Sigma-Aldrich as an internal control. Goat anti-rabbit and goat anti-mouse immunoglobulin (Ig)G antibodies as secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The complexes formed were visualized after a chemiluminescence reaction (ECL; GE Healthcare, Little Chalfont, UK). The intensity of the respective band was semi-quantified by Image J (version 1·42; http://rsb.info.nih.gov/ij).
Expression levels of let-7i in T cells from patients with SLE or RA
Eight patients fulfilling the 1982 American College of Rheumatology (ACR) revised criteria for the classification of SLE 26, nine patients fulfilling the 1987 ACR revised classification criteria for RA 27 and 14 healthy volunteers were recruited. The expression level of let-7i in T cells from these patients was measured by the methods described above.
Transfection of miRNA mimics or inhibitors into Jurkat cells
Fresh isolated human T cells or Jurkat cells (1 × 106/ml) purchased from the American Type Culture Collection (Manassas, VA, USA) were electroporated with 1 μg of scrambled oligonucleotides, miRNA mimics (Ambion) or miRNA inhibitors (Ambion) using the Gene Pulser MXcell electroporation system (Bio-Rad Laboratories, Hercules, CA, USA), with the conditions developed by Jordan et al. 28. The expression of miRNA in miRNA-mimic or miRNA inhibitor transfected Jurkat cells was analysed after culturing for 24 h at 37°C in a humidified atmosphere containing 5% CO2. Because the endogenous TLR-4 protein expression in Jurkat cells is minimal, ionomycin (250 ng/ml; Sigma-Aldrich) and 10 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) were added to activated Jurkat cells for another 24 h. These cells were then lysed by Western blotting for analysing the expression of TLR-4.
Measurement of mRNA expression
The expression levels of TLR-4 and IFN-γ mRNA were quantified by real-time PCR using a one-step RT–PCR kit (TaKaRa, Shiga, Japan) on an ABI Prism 7500 Fast real-time PCR system (Applied Biosystems). The primers used for TLR-4 were 5′-CGAGGCTTTTCTGAGTCGTC-3′ (forward) and 5′-TGAGCAGTCGTGCTGGT- ATC-3′ (reverse). The primers used for IFN-γ were forward 5′-CTTTAAAGATGACCA- GACCATCCA-3′ and reverse 5′-ATCTCGTTTCTTTTTGTTGCTATTGA-3′. Conditions for the quantitative PCR were 42°C for 5 min and 95°C for 10 s for RT, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s. Expression of 18S ribosomal RNA was used as endogenous control for data normalization. The normalized mRNA level was defined by the equation: 39 – Ct after normalization by the expression of 18S ribosomal RNA.
Functional analysis of let-7i in human T cells by transfection with let-7i mimic or inhibitor?
T cells isolated from healthy volunteer or AS patients (1 × 106/well) were cultured in the following three conditions: (i) in culture medium only, (ii) in an anti-human CD3 antibody (1 μg; BioLegend, San Diego, CA, USA) precoated plate + 1 μg anti-human CD28 antibody (BioLegend) and (iii) in an anti-human CD3 antibody precoated plate + 1 μg anti-human CD28 antibody + 100 ng/ml lipopolysaccharide (LPS; Sigma-Aldrich) for 24 h at 37°C in a humidified atmosphere containing 5% CO2. Furthermore, let-7i mimic, let-7i inhibitor or scrambled oligonucleotide-transfected T cells (3 × 106/well) were also cultured in an anti-human CD3 antibody precoated plate + 1 μg anti-human CD28 antibody + 100 ng/ml LPS for 24 h at 37°C. Then, the cells were pelleted down by centrifugation at 300 g. The supernatants were collected and stored at −80°C for the measurement of IFN-γ by enzyme-linked immunosorbent assay (ELISA) kits (BD Biosciences), according to the manufacturer's protocol.
Statistical analysis
All data are represented as the mean ± standard deviation (s.d.). Univariate and multivariate linear regression was applied to calculate the correlation coefficient and significance among different parameters using STATA software (StataCorp, College Station, TX, USA). Statistical significance was assessed by Mann–Whitney U-test and a P-value less than 0·05 was considered statistically significant.
Results
Patients and controls
The demographic and clinical data of the AS patients were recorded and are summarized in Table 1.
Table 1.
Demographics and clinical data of the ankylosing spondylitis (AS) patients and healthy volunteers.
| AS patients (n = 22) | Healthy volunteers (n = 18) | P-value | |
|---|---|---|---|
| Age (years, mean ± s.d.) | 42·6 ± 10·2 | 40·1 ± 9·3 | 0·33 |
| Sex (F : M) | 8:14 | 5:13 | 0·74 |
| HLA-B27 (+) | 100% (22/22) | – | |
| C-reactive protein (mg/dl) | 0·50 ± 0·68 | – | |
| BASRI-lumbar spine | 1·9 ± 1·4 | – | |
| Sacroiliitis (grading) | 2·6 ± 1·1 | – | |
| Medication | |||
| NSAID | 91% (20/22) | – | |
| Salazopyrine | 91% (20/22) | – | |
| Anti-TNF therapy | 5% (1/22) | – |
BASRI: Bath Ankylosing Spondylitis Radiology Index; NSAID: non-steroidal anti-inflammatory drugs; TNF: tumour necrosis factor; s.d.: standard deviation; –: not determined.
Identification and verification of differential expression of miRNAs in T cells from AS patients and healthy controls
The expression profile of 270 miRNAs in T cells from five AS patients and five healthy controls is shown in Fig. 1a. Each scatter-spot represents the average of normalized miRNA levels of T cells from five AS patients and normal controls. We noted that the expression of eight microRNAs, including miR-150, miR-16, miR-342-5p, miR-221, let-7i, miR-99b, let-7b and miR-513-5p, were significantly higher and five microRNAs including miR-218, miR-409-3p, miR-30e, miR-199a-5p and miR-215 were significantly lower in AS T cells than in normal T cells (fold change >4·5 and P < 0·05; Fig. 1b). Then, we chose only the five most differentially expressed miRNAs (defined as fold change >6 and P < 0·05), including miR-150, miR-16, miR-342-5p, miR-221 and let-7i for further validation. In the second step, T cells from another 22 AS patients and 18 healthy controls were compared. We confirmed that the expression levels of miR-16, miR-221 and let-7i (fold change: 2·34, 2·38 and 3·17, respectively; all the P values < 0·05) were significantly higher in AS T cells than in normal T cells (Fig. 1c).
Figure 1.
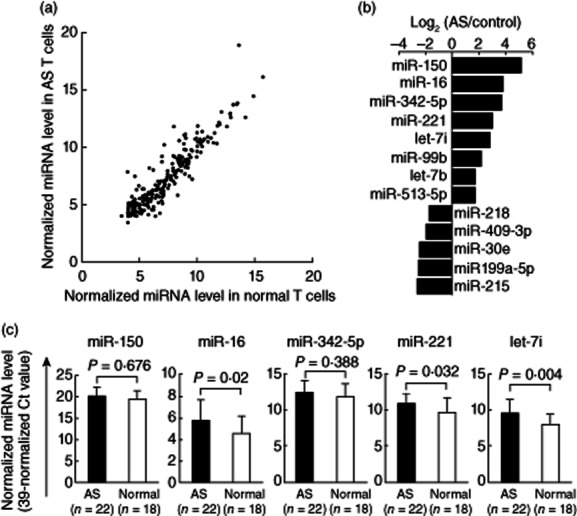
Comparison of microRNAs (miRNAs) expression in T cells from patients with ankylosing spondylitis (AS) and healthy controls. (a) The expression profile of 270 miRNAs measured by real-time polymerase chain reaction (PCR). Each scatter-spot represents the average of normalized miRNA level in T cells from five AS patients and five healthy controls for each miRNA. The threshold cycle (Ct) is defined as the cycle number at which the change of fluorescence intensity crosses the average background level of the fluorescence signal. The normalized miRNA level was defined as [39 – Ct after normalization with the internal control (U6 small nuclear RNA)]. (b) Eight miRNAs were found potentially over-expressed and five mRNAs were under-expressed in AS T cells (fold change >4·5; P-value < 0·05). (c) The five most potentially differentially expressed miRNAs (defined as fold change >6) were validated further by real-time PCR in T cells from another 22 AS patients and 18 compatible healthy controls. Increased expression of miR-16, miR-221 and let-7i in T cells from AS patients was confirmed after validation.
Correlations of miR-16, miR-221 and let-7i expression with the clinical parameters of AS patients
We then intended to correlate different clinical parameters with the expression levels of miR-16, miR-221 and let-7i in AS T cells by univariate and multivariate linear regression analysis. We found that the expression of miR-221 (P = 0·022) and let-7i (P = 0·031) were associated positively with BASRI of lumber spine. The expression of miR-16 (P = 0·086) was associated positively with BASRI of lumbar spine (Fig. 2). After adjusting for age and gender, the expression of miR-221 (fold change = 1·58, P = 0·033) and let-7i (fold change = 1·75, P = 0·029), but not miR-16 (fold change = 1·67, P = 0·059), were still correlated positively with BASRI of lumbar spine, which reflects inflammatory activity in the lumbar spine (Table 2). However, expression of miR-16, miR-221 and let-7i did not correlate with serum C-reactive protein levels or sacroiliitis by radiography in AS patients (Table 2).
Figure 2.
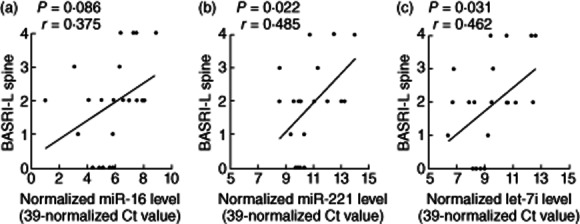
The correlation of the three over-expressed miRNAs in ankylosing spondylitis (AS) T cells with Bath Ankylosing Spondylitis Radiology Index (BASRI) of lumbar spine. (a) miR-16, (b) miR-221 and (c) let-7i.
Table 2.
Univariate and multivariate liner regression models for assessing the correlations among different clinical parameters and miR-16, miR-221 and let-7i expression levels in T cells from 22 patients with ankylosing spondylitis (AS).
| Univariate analysis | Multivariate analysis† | |||||
|---|---|---|---|---|---|---|
| Fold change (95% confidence interval) | Fold change (95% confidence interval) | |||||
| miR-16 | miR-221 | let-7i | miR-16 | miR-221 | let-7i | |
| Age (per 10 years) | 1·09 (0·64–1·85) | 1·18 (0·77–1·80) | 1·13 (0·68–1·89) | 0·75 (0·40–1·42) | 0·85 (0·52–1·40) | 0·77 (0·43–1·38) |
| Sex (male/female) | 0·98 (0·29–3·33) | 1·17 (0·43–3·16) | 1·20 (0·37–3·88) | 0·69 (0·20–2·37) | 0·86 (0·33–2·25) | 0·83 (0·27–2·56) |
| BASRI-L spine (per 1 score) | 1·42* (0·95–2·13) | 1·45** (1·06–1·98) | 1·52** (1·04–2·21) | 1·67 (0·98–2·86) | 1·58 (1·04–2·40) | 1·75 (1·07–2·87) |
| Sacroiliitis (per 1 grade) | 1·07 (0·61–1·88) | 1·03 (0·65–1·62) | 1·08 (0·63–1·85) | – | – | – |
| CRP (per 1 mg/dl) | 1·69 (0·73–3·90) | 1·38 (0·69–2·77) | 1·79 (0·81–3·96) | – | – | – |
P = 0·086;
P <0·05;
After analysis with multivariate linear regression model adjusted for age and gender, only BASRI-L spine was correlated significantly with increased expression of miR-221 and let-7i. The expression of miR-16 was found (P = 0·059) to correlate with BASRI-L spine. BASRI-L spine: Bath Ankylosing Spondylitis Radiology Index of lumbar spine; CRP: C-reactive protein.
Expression of proteins regulated by miR-16, miR-221 and let-7i in T cells from AS patients and healthy controls
Several studies have demonstrated that miR-16, miR-221 and let-7i regulate the protein expression of Bcl-2, c-kit and TLR-4, respectively 29–31. To identify the potential biological/pathological relationships between the increased expression of these miRNAs and their target proteins in AS T cells, we compared the protein expression of Bcl-2, c-kit and TLR-4 in AS patients and healthy controls by Western blotting (Fig. 3). The expression level of TLR-4 (Fig. 3c), but not Bcl-2 (Fig. 3b), was significantly lower in AS T cells than in normal T cells. Although miR-221 was over-expressed in AS T cells and its expression level was correlated significantly with BASRI of lumbar spine in AS patients, the expression of c-kit was undetectable by Western blotting in both normal and AS T cells (data not shown). We speculated that miR-221 may play a physiological role in suppressing the protein expression of c-kit in T cells. Thus, we transfected miR-221 inhibitor or scrambled oligonucleotide into AS T cells. The expression level of miR-221 decreased dramatically (fold change: 0·035, P < 0·05) after miR-221 inhibitor transfection (Fig. 4a). However, the expression of c-kit remained undetectable by Western blotting (Fig. 4b).
Figure 3.
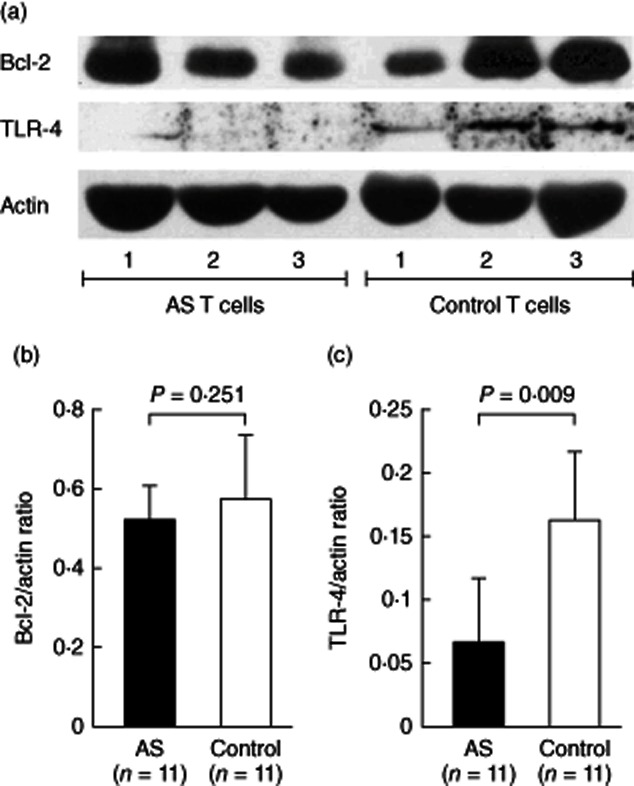
The protein targeted by miR-16 (Bcl-2) and let-7i [Toll-like receptor (TLR)-4] in T cell lysates were detected by Western blotting. (a) Protein expression of Bcl-2, TLR-4 molecules in T cell lysates from three ankylosing spondylitis (AS) patients and three healthy controls were shown as representative. The expression levels of (b) Bcl-2 and (c) TLR-4 in T cell lysates from 11 AS patients and 11 healthy controls normalized to the actin expression are shown.
Figure 4.
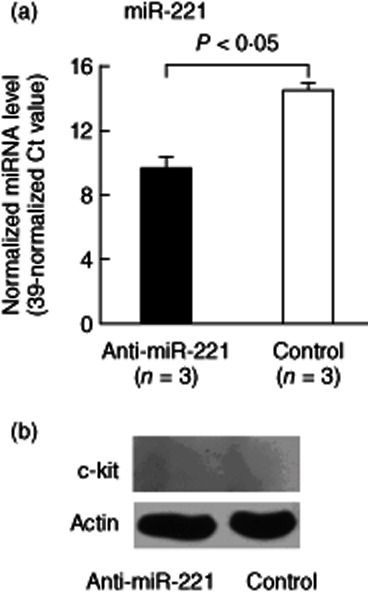
Effect of miR-221 inhibitor transfection on c-kit protein expression in ankylosing spondylitis (AS) T cells. We transfected miR-221 inhibitor into AS T cells via electroporation. (a) The expression level of miR-221 was decreased dramatically after miR-221 inhibitor transfection for 24 h. (b) The protein expression levels of c-kit were undetectable in AS T cells transfected with miR-221 inhibitor or scrambled oligonucleotides.
Expression level of let-7i in T cells from healthy controls, patients with SLE or RA and activated Jurkat cells
The above results suggest that increased expression of let-7i in AS T cells. We then analysed the expression of let-7i in other systemic autoimmune diseases, including patients with SLE and RA, for identifying the specificity in AS T cells. We found that the expression of let-7i was not changed in T cells from these patients compared with controls (Fig. 5a). Another interesting finding is that let-7i expression in Jurkat cells was decreased after activation by ionomycin + PMA (Fig. 5b). This may indicate that activated T cells enhance TLR-4 expression. However, further investigation is required to confirm it.
Figure 5.
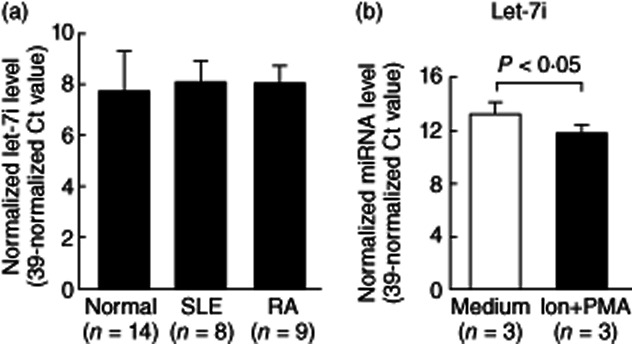
The expression level of let-7i in T cells from healthy controls, patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) and activated Jurkat cells were compared. (a) Comparison of expression levels of let-7i in T cells from 14 controls, eight SLE and nine RA patients. (b) The expression levels of let-7i were decreased significantly in Jurkat cells after stimulation with 250 ng/ml ionomycin and 10 ng/ml phorbol myristate acetate (PMA) for 24 h.
Let-7i mimic transfection suppressed TLR-4 protein expression
For confirming further the roles of let-7i on TLR-4 protein expression, we transfected let-7i mimic or scrambled oligonucleotides into Jurkat cells by eletroporation to detect the effects on TLR-4 mRNA and protein expression. The expression levels of let-7i increased dramatically (fold change: 395·78, P < 0·05) after let-7i mimic transfection (Fig. 6a). Increased let-7i expression did not suppress the mRNA expression of TLR-4 in Jurkat cells (Fig. 6b), whereas the protein expression of TLR-4 in both Jurkat and normal T cells was suppressed significantly by Western blotting, as shown in Fig. 6c,d.
Figure 6.
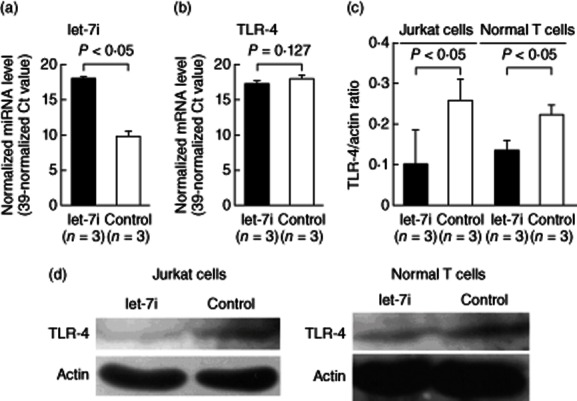
Effect of let-7i mimic transfection on Toll-like receptor (TLR)-4 mRNA and protein expression. Let-7i mimic was transfected into Jurkat cells via electroporation. (a) After let-7i mimic transfection for 24 h, the expression level of let-7i was increased dramatically in the Jurkat cells compared with the scrambled oligonucleotides transfection group. (b) The TLR-4 mRNA expression levels did not change after let-7i transfection. (c) Jurkat cells were cultured with 250 ng/ml ionomycin and 10 ng/ml phorbol myristate acetate (PMA) and normal T cells were cultured with anti-CD3+anti-CD28 antibodies for 24 h after let-7i mimic transfection. The protein expression of TLR-4 was inhibited significantly in Jurkat and normal T cells after let-7i mimic transfection. (d) A representative case showing increased TLR-4 protein expression after let-7i mimic transfection into Jurkat (left) and normal T cells (right).
Let-7i inhibitor transfection enhanced TLR-4 expression
Conversely, we transfected let-7i inhibitor or scrambled oligonucleotides into Jurkat cells. As expected, the expression level of let-7i was decreased dramatically (fold change: 0·006, P < 0·05) after let-7i inhibitor transfection (Fig. 7a). The decreased let-7i expression did not increase TLR-4 mRNA expression significantly in Jurkat cells (Fig. 7b), but enhanced significantly the protein expression of TLR-4 in Jurkat and AS T cells, as shown in Fig. 7c,d by Western blotting. These results confirmed that let-7i inhibited protein translation rather than mRNA degradation of TLR-4 in Jurkat cells.
Figure 7.
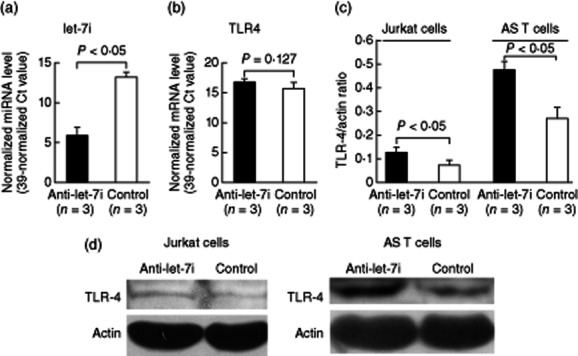
Effect of let-7i inhibitor transfection on Toll-like receptor (TLR)-4 mRNA and protein expression. We transfected let-7i inhibitor into Jurkat cells via electroporation. (a) After let-7i inhibitor transfection for 24 h, the expression level of let-7i was decreased dramatically in the Jurkat cells compared with the scrambled oligonucleotide transfection groups. (b) The TLR-4 mRNA expression levels did not change after let-7i transfection. (c) The protein expression of TLR-4 was increased significantly in Jurkat and ankylosing spondylitis (AS) T cells after let-7i inhibitor transfection. (d) Representative case showing increased TLR-4 protein expression after let-7i inhibitor transfection into Jurkat (left) and AS T cells (right).
Functional studies of let-7i on IFN-γ secretion in anti-CD3+anti-CD28-activated T cells co-culture with LPS
Bacterial LPS, a TLR-4 agonist, has been proved to play a crucial role in AS pathogenesis 32, and José et al. 33 have reported that LPS could inhibit the T cell response through TLR-4 in mice. We deduced that LPS might exert an inhibitory role on the T cell response in humans, which is involved in the immunopathogenesis of AS. In this study, we demonstrated that there was no difference between the IFN-γ secretion in anti-CD3+anti-CD28-activated T cells from healthy controls and AS patients (46·9 ± 12·0 pg/ml versus 58·0 ± 46·0 pg/ml, P = 0·88). The addition of 100 ng/ml LPS could suppress IFN-γ secretion effectively in anti-CD3+anti-CD28- activated normal T cells but not AS T cells (6·5 ± 8·2 pg/ml versus 73·6 ± 38·8 pg/ml, P < 0·05; Fig. 8a). We proposed that the increased expression of let-7i may contribute to the increased production of IFN-γ in AS T cells. Therefore, we transfected let-7i mimic, let-7i inhibitor or scrambled oligonucleotides into normal and AS T cells. In the scrambled oligonucleotide-transfected control groups, we found that IFN-γ production was increased in anti-CD3+anti-CD28+ LPS-stimulated AS T cells compared with normal T cells (87·8 ± 73·1 pg/ml versus 27·9 ± 18·4 pg/ml, P = 0·0283; Fig. 8b). The transfection of let-7i mimic promoted IFN-γ production in anti-CD3+ anti-CD28+ LPS-stimulated normal T cells compared with those transfected with scrambled oligonucleotides (74·9 ± 18·9 pg/ml versus 27·9 ± 18·4 pg/ml, P = 0·009). In contrast, transfection of let-7i inhibitor suppressed IFN-γ production by anti-CD3+anti-CD28+ LPS-stimulated AS T cells compared with those transfected with scrambled oligonucleotides (14·5 ± 26·7 pg/ml versus 87·8 ± 73·1 pg/ml, P = 0·047).
Figure 8.
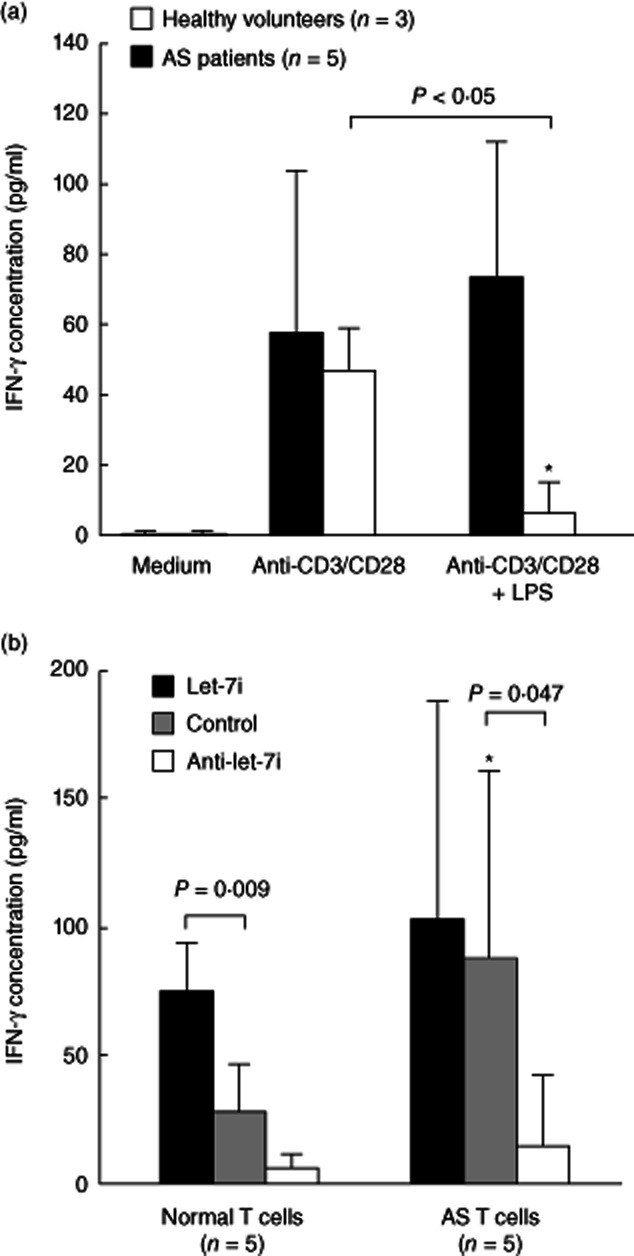
Functional analysis of let-7i in anti-CD3+anti-CD28-activated normal and ankylosing spondylitis (AS) T cells via transfection with let-7i mimic and inhibitor. (a) Lipopolysaccharide (LPS) (100 ng/ml) potently suppress interferon (IFN)-γ production in anti-CD3+anti-CD28 activated T cells from healthy volunteers but not AS patients. *The IFN-γ concentration was lower in anti-CD3+anti-CD28+ LPS-stimulated normal T cells compared with AS T cells. (P < 0·05) (b) The transfection of let-7i mimic increased IFN-γ production in anti-CD3+anti-CD28+ LPS-activated T cells from healthy volunteers, but not AS patients. The transfection of let-7i inhibitor suppressed IFN-γ production by anti-CD3+anti-CD28+ LPS-stimulated AS T cells, but not normal T cells. *In the scrambled oligonucleotides transfected control groups, the IFN-γ concentration was higher in anti-CD3+anti-CD28+ LPS-stimulated AS T cells compared with normal T cells (P < 0·05).
Expression level of IFN-γ mRNA in T cells from AS patients and its correlation with BASRI score of lumbar spine
Because the increased expression of let-7i in anti-CD3+ anti-CD28+ LPS-stimulated T cells could enhance IFN-γ production in vitro (Fig. 8b), we compared the mRNA expression of IFN-γ in non-stimulated T cells from AS patients and controls. Indeed, mRNA expression of IFN-γ is increased significantly in resting T cells from AS patients (Fig. 9a). However, we noted no significant correlation between the expression levels of let-7i or BASRI of lumbar spine with the mRNA expression levels of IFN-γ in AS T cells (Fig. 9b,c). It is possible that the IFN-γ expression can be affected by viral or intracellular pathogen infection other than disease activity per se, and other bone destructive/formation factors such as MMP1 and BMPs, etc. may probably play a role in the syndesmophyte formation in AS spine 34. We conclude that the let-7i expression level did not affect the IFN-γ mRNA expression directly and was not relevant to the BASRI of lumbar spine in AS patients.
Figure 9.
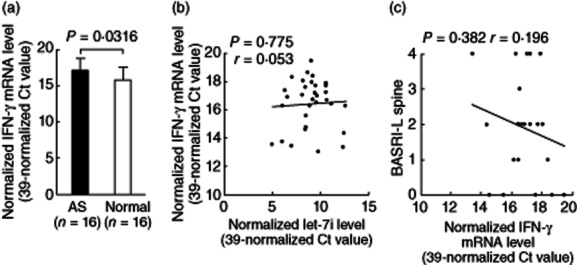
Comparison of interferon (IFN)-γ mRNA expression between ankylosing spondylitis (AS) and normal T cells and correlations among IFN-γ mRNA with let-7i mRNA and BASRI of lumbar spine in AS patients. (a) The mRNA expression levels of IFN-γ in AS T cells were significantly higher than normal T cells. (b)Correlation between expression levels of IFN-γ mRNA and let-7i. (c) Correlation between expression levels of IFN-γ mRNA and BASRI of lumbar spine in AS patients.
Discussion
Our study demonstrated that the expression of three miRNAs (miR-16, miR-221 and let-7i) was increased in T cells from AS patients compared to those from healthy controls. Clinically, the increased expression of the two miRNAs (miR-221 and let-7i) showed an association with BASRI lumbar spine in AS patients. These results provided an alternative view: that misregulated T cells contribute to the pathological changes in patients with AS via aberrant expression of certain miRNAs.
Previous studies have detected increased TLR-4 protein expression in peripheral blood mononuclear cells, especially monocytes from AS patients by flow cytometry and real-time PCR 35–37. In contrast, using Western blotting in this study we found that TLR-4 expression specifically in AS T cells was suppressed by let-7i. As TLR-4 is expressed abundantly on monocytes, we proposed that the decreased expression of TLR-4 in AS T cells could be masked easily by the abundant amount of TLR-4 on monocyte or other cell types from AS patients. Moreover, in the cell transfection studies, we found that there were discrepancies between mRNA and protein expressions of TLR-4 due to the effect of let-7i (Figs 6 and 7). As TLR-4 is the prime cellular pattern recognition sensor for microbial pathogens, TLR-4 activation via LPS leads to production of proinflammatory cytokines in innate immune systems 38. Interestingly, TLR-4 is also expressed on T cells 39, which might have a different immunoregulatory function in the adaptive immune system, as shown in our study. José et al. 33 have reported that LPS signalling through TLR-4 could suppress T cell receptor-dependent extracellular signal-regulated kinase 1/2 (ERK1/2) activation in CD4+ T cells in the murine model. Similar to their findings, in this study we demonstrated that LPS could exert an inhibitory signal on the T cell response in humans. Clinical observations revealed that there was a link between AS development with chronic prostatitis in men or pelvic inflammatory disease in women. It is purposed that the microbe infection is from a source of damage-associated molecular pattern molecules (DAMPs) involved in AS pathogenesis. These DAMPs could activate TLRs to elicit the inflammatory reaction and ectopic enchondral bone formation in AS spine 32. Although bacterial infection such as Chlamydia could cause chronic arthritis 40, it is still premature to conclude that bacterial infection can cause AS 41. Conversely, evidence suggests that AS disease activity became worse, following the different bacterial infections such as Salmonella, Yersinia, Campylobacter and Chlamydia 42–46. Although molecular mimicry between the bacterial components and self-peptides was considered to play a role 47, our results may provide an alternative explanation, that the bacterial LPS could suppress IFN-γ production in activated normal T cells. However, this regulatory mechanism was abrogated by the over-expressed let-7i in AS T cells (Fig. 8a).
IFN-γ is a key proinflammatory cytokine which has been shown to be elevated in serum from AS patients 48. Although we found no correlation between let-7i and the mRNA expression of IFN-γ in AS patients (Fig. 9b), contradictory to the finding that let-7i may regulate IFN-γ production (Fig. 8b) it is possible that various factors, such as viral or bacterial infection, trigger IFN-γ gene expression to confound our results. In addition, we demonstrated that the increased expression of let-7i enhanced IFN-γ production in anti-CD3+anti-CD28+ LPS-stimulated T cells, but the mRNA expression of IFN-γ was compared in non-stimulated T cells from AS patients and controls. However, we still demonstrated that the IFN-γ mRNA expression levels were increased in AS T cells. Therefore, we propose that the increased let-7i expression in AS T cells activate the Th1 immune responses upon LPS stimulation. Although we showed that increased let-7i expression in T cells could suppress TLR-4 expression, it is premature to conclude that decreased TLR-4 expression on AS T cells contributed directly to this phenomenon. Instead, other molecule(s) involving the T cell signalling pathway targeted by let-7i might play an essential role. Selbach et al. demonstrated 49 that one miRNA can translationally repress hundreds of target genes. Nevertheless, the downstream molecular mechanism of increased let-7i expression stimulating a T helper type 1 (Th1) (IFN-γ) immune response requires more detailed studies.
O'Hara et al. 50 demonstrated that let-7i expression was suppressed by nuclear factor-kappa B (NF-κB), and many medications used for AS treatment have the potential to suppress NF-κB activity 51. However, AS is a chronic inflammatory disease; the elevation of NF-κB DNA binding activity in lymphocytes could persist even after several months of adequate therapy 52. In addition, we observed two newly diagnosed AS patients in this study who had not yet been treated with immunosuppressant. Their T cell let-7i expression levels appeared to be no different from those of the treated AS patients. Therefore, we consider that the increased expression of let-7i was irrelevant to treatment with immunosuppressive drugs. Therefore, the increased let-7i expression is a direct effect from AS disease per se and is involved in AS pathogenesis.
In contrast, the expression of Bcl-2 targeted by miR-16 remained unchanged in AS T cells compared with normal T cells (Fig. 3b). This is because other molecules and signalling pathways may compensate Bcl-2 expression that was suppressed by miR-16. In T cell lineage, the expression of c-kit target by miR-221 is limited to the progenitor T cells, and lost gradually upon differentiation 53. Thus the expression of c-kit could not be detected in T cells from peripheral blood in our study (Fig. 4b). In addition to AS T cells, over-expression of miR-16 was also found in peripheral mononuclear cells from RA patients 16 and activated normal T cells 54. It is possible that the increased expression of miR-16 and miR-221 in AS patients may trigger inflammatory reactions. The inter-relationships among these three miRNAs and their respective target molecules require further investigation. Recently, the expression of miRNAs was under the control of epigenetic mechanisms such as DNA methylation. The disruption of this network could cause pathological conditions such as malignant changes in gastrointestinal, urological and haematological systems 55–57. Undoubtedly, investigation of the methylation status of the promoter region in miR-16, miR-221 and let-7i genes is important in elucidating the immunopathogenesis of AS. Conversely, the pathological roles of other altered expressed miRNAs, including miR-99b, let-7b, miR-513-5p, miR-218, miR-409-3p, miR-30e, miR-199a-5p and miR-215 in AS T cells (Fig. 1b), are now under investigation.
In conclusion, we found three highly expressed miRNAs: miR-16, miR-221 and let-7i in T cells from AS patients, among which let-7i and miR-221 were found to be correlated positively with BASRI for lumbar spine. The increased expression of let-7i in AS T cells contributes to the immunopathogenesis of AS via enhancing the Th1 (IFN-γ) inflammatory response.
Acknowledgments
This work was supported by the grant from the National Science Council (NCS 101-2314-B-303-028-MY3) and Buddhist Dalin Tzu-Chi General Hospital (Thematic studies 98-2-1), Taiwan.
Disclosure
None.
References
- 1.McHugh K, Bowness P. The link between HLA-B27 and SpA – new ideas on an old problem. Rheumatology (Oxf) 2012;51:1529–1539. doi: 10.1093/rheumatology/kes061. [DOI] [PubMed] [Google Scholar]
- 2.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 3.Colbert RA, DeLay ML, Klenk EI, Layh-Schmitt G. From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol Rev. 2010;233:181–202. doi: 10.1111/j.0105-2896.2009.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appel H, Maier R, Wu P, et al. Analysis of interleukin-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17 mediated adaptive immune response. Arthritis Res Ther. 2011;13:R95. doi: 10.1186/ar3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowness P, Ridley A, Shaw J, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186:2672–2680. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–2317. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 7.Reveille JD. The genetic basis of spondyloarthritis. Ann Rheum Dis. 2011;70(Suppl. 1):i44–50. doi: 10.1136/ard.2010.140574. [DOI] [PubMed] [Google Scholar]
- 8.Boyle LH, Goodall JC, Opat SS, Gaston JS. The recognition of HLA-B27 by human CD4(+) T lymphocytes. J Immunol. 2001;167:2619–2624. doi: 10.4049/jimmunol.167.5.2619. [DOI] [PubMed] [Google Scholar]
- 9.Atagunduz P, Appel H, Kuon W, et al. HLA-B27-restricted CD8+ T cell response to cartilage-derived self peptides in ankylosing spondylitis. Arthritis Rheum. 2005;52:892–901. doi: 10.1002/art.20948. [DOI] [PubMed] [Google Scholar]
- 10.Baeten D, Kruithof E, Van den Bosch F, et al. Immunomodulatory effects of anti-tumor necrosis factor alpha therapy on synovium in spondylarthropathy: histologic findings in eight patients from an open-label pilot study. Arthritis Rheum. 2001;44:186–195. doi: 10.1002/1529-0131(200101)44:1<186::AID-ANR25>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Bollow M, Fischer T, Reisshauer H, et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis-cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis. 2000;59:135–140. doi: 10.1136/ard.59.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szalay B, Mészáros G, Cseh Á, et al. Adaptive immunity in ankylosing spondylitis: phenotype and functional alterations of T-cells before and during infliximab therapy. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/808724. Article ID 808724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulci V, Scappucci G, Sebastiani GD, et al. miR-223 is overexpressed in T-lymphocytes of patients affected by rheumatoid arthritis. Hum Immunol. 2010;71:206–211. doi: 10.1016/j.humimm.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Jones SW, Watkins G, Le Good N, et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17:464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Li YT, Chen SY, Wang CR, et al. Amelioration of collagen-induced arthritis in mice by lentivirus-mediated silencing of microRNA-223. Arthritis Rheum. 2012;64:3240–3245. doi: 10.1002/art.34550. [DOI] [PubMed] [Google Scholar]
- 16.Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther. 2008;10:R101. doi: 10.1186/ar2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu S, Pan W, Song X, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 18.Miyaki S, Nakasa T, Otsuki S, et al. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60:2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurowska-Stolarska M, Alivernini S, Ballantine LE, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci USA. 2011;108:11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanczyk J, Pedrioli DM, Brentano F, et al. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58:1001–1009. doi: 10.1002/art.23386. [DOI] [PubMed] [Google Scholar]
- 21.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 22.Moll JM, Wright V. New York clinical criteria for ankylosing spondylitis. A statistical evaluation. Ann Rheum Dis. 1973;32:354–363. doi: 10.1136/ard.32.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKay K, Mack C, Brophy S, Calin A. The Bath Ankylosing Spondylitis Radiology Index (BASRI): a new, validated approach to disease assessment. Arthritis Rheum. 1998;41:2263–2270. doi: 10.1002/1529-0131(199812)41:12<2263::AID-ART23>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Ridzon DA, Broomer AJ, et al. Real-time quantification of microRNAs by stem-loop RT–PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen HC, Chen GH, Chen YH, et al. MicroRNA deregulation and pathway alterations in nasopharyngeal carcinoma. Br J Cancer. 2009;100:1002–1011. doi: 10.1038/sj.bjc.6604948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 27.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 28.Jordan ET, Collins M, Terefe J, Ugozzoli L, Rubio T. Optimizing electroporation conditions in primary and other difficult-to-transfect cells. J Biomol Tech. 2008;19:328–334. [PMC free article] [PubMed] [Google Scholar]
- 29.Felli N, Fontana L, Pelosi E, et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci USA. 2005;102:18081–18086. doi: 10.1073/pnas.0506216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen XM, Splinter PL, O'Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem. 2007;282:28929–28938. doi: 10.1074/jbc.M702633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cittelly DM, Das PM, Salvo VA, Fonseca JP, Burow ME, Jones FE. Oncogenic HER2{Delta}16 suppresses miR-15a/16 and deregulates BCL-2 to promote endocrine resistance of breast tumors. Carcinogenesis. 2010;31:2049–2057. doi: 10.1093/carcin/bgq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pöllänen R, Sillat T, Pajarinen J, Levón J, Kaivosoja E, Konttinen YT. Microbial antigens mediate HLA-B27 diseases via TLRs. J Autoimmun. 2009;32:172–177. doi: 10.1016/j.jaut.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 33.González-Navajas JM, Fine S, Law J, et al. TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental colitis in mice. J Clin Invest. 2010;120:570–581. doi: 10.1172/JCI40055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam LS, Gu J, Yu D. Pathogenesis of ankylosing spondylitis. Nat Rev Rheumatol. 2010;6:399–405. doi: 10.1038/nrrheum.2010.79. [DOI] [PubMed] [Google Scholar]
- 35.Yang ZX, Liang Y, Zhu Y, et al. Increased expression of Toll-like receptor 4 in peripheral blood leucocytes and serum levels of some cytokines in patients with ankylosing spondylitis. Clin Exp Immunol. 2007;149:48–55. doi: 10.1111/j.1365-2249.2007.03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Rycke L, Vandooren B, Kruithof E, De Keyser F, Veys EM, Baeten D. Tumor necrosis factor alpha blockade treatment down-modulates the increased systemic and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondylarthropathy. Arthritis Rheum. 2005;52:2146–2158. doi: 10.1002/art.21155. [DOI] [PubMed] [Google Scholar]
- 37.Raffeiner B, Dejaco C, Duftner C, et al. Between adaptive and innate immunity: TLR4-mediated perforin production by CD28null T-helper cells in ankylosing spondylitis. Arthritis Res Ther. 2005;7:R1412–1420. doi: 10.1186/ar1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol. 2011;8:388–403. doi: 10.1038/cmi.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Carter JD, Gérard HC, Espinoza LR, et al. Chlamydiae as etiologic agents in chronic undifferentiated spondylarthritis. Arthritis Rheum. 2009;60:1311–1316. doi: 10.1002/art.24431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inman RD. Mechanisms of disease: infection and spondyloarthritis. Nat Clin Pract Rheumatol. 2006;2:163–169. doi: 10.1038/ncprheum0118. [DOI] [PubMed] [Google Scholar]
- 42.Feng XG, Xu XJ, Ye S, et al. Recent Chlamydia pneumoniae infection is highly associated with active ankylosing spondylitis in a Chinese cohort. Scand J Rheumatol. 2011;40:289–291. doi: 10.3109/03009742.2011.560891. [DOI] [PubMed] [Google Scholar]
- 43.Paul IR, Mitchell ES, Bell AL. Salmonella reactive arthritis in established ankylosing spondylitis. Ulster Med J. 1988;57:215–217. [PMC free article] [PubMed] [Google Scholar]
- 44.Leirisalo-Repo M, Suoranta H. Ten-year follow-up study of patients with Yersinia arthritis. Arthritis Rheum. 1988;31:533–537. doi: 10.1002/art.1780310410. [DOI] [PubMed] [Google Scholar]
- 45.Martínez A, Pacheco-Tena C, Vázquez-Mellado J, Burgos-Vargas R. Relationship between disease activity and infection in patients with spondyloarthropathies. Ann Rheum Dis. 2004;63:1338–1340. doi: 10.1136/ard.2003.011882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rynes RI, Volastro PS, Bartholomew LE. Exacerbation of B27 positive spondyloarthropathy by enteric infections. J Rheumatol. 1984;11:96–97. [PubMed] [Google Scholar]
- 47.Frauendorf E, von Goessel H, May E, Märker-Hermann E. HLA-B27-restricted T cells from patients with ankylosing spondylitis recognize peptides from B*2705 that are similar to bacteria-derived peptides. Clin Exp Immunol. 2003;134:351–359. doi: 10.1046/j.1365-2249.2003.02289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang PT, Kasai H, Zhao LJ, Xiao WG, Tanabe F, Ito M. Increased CCR4 expression on circulating CD4(+) T cells in ankylosing spondylitis, rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Immunol. 2004;138:342–347. doi: 10.1111/j.1365-2249.2004.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 50.O'Hara SP, Splinter PL, Gajdos GB, et al. NFkappaB p50-CCAAT/enhancer-binding protein beta (C/EBPbeta)-mediated transcriptional repression of microRNA let-7i following microbial infection. J Biol Chem. 2010;285:216–225. doi: 10.1074/jbc.M109.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eggert M, Seeck U, Semmler M, et al. An evaluation of anti-TNF-alpha-therapy in patients with ankylosing spondylitis: imbalanced activation of NF kappa B subunits in lymphocytes and modulation of serum cortisol concentration. Rheumatol Int. 2007;27:841–846. doi: 10.1007/s00296-007-0303-z. [DOI] [PubMed] [Google Scholar]
- 53.Moore TA, Zlotnik A. T-cell lineage commitment and cytokine responses of thymic progenitors. Blood. 1995;86:1850–1860. [PubMed] [Google Scholar]
- 54.Grigoryev YA, Kurian SM, Hart T, et al. MicroRNA regulation of molecular networks mapped by global microRNA, mRNA, and protein expression in activated T lymphocytes. J Immunol. 2011;187:2233–2243. doi: 10.4049/jimmunol.1101233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li XQ, Guo YY, De W. DNA methylation and microRNAs in cancer. World J Gastroenterol. 2012;18:882–888. doi: 10.3748/wjg.v18.i9.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong KY, Huang X, Chim CS. DNA methylation of microRNA genes in multiple myeloma. Carcinogenesis. 2012;33:1629–1638. doi: 10.1093/carcin/bgs212. [DOI] [PubMed] [Google Scholar]
- 57.Liep J, Rabien A, Jung K. Feedback networks between microRNAs and epigenetic modifications in urological tumors. Epigenetics. 2012;7:315–325. doi: 10.4161/epi.19464. [DOI] [PubMed] [Google Scholar]


