Abstract
Anthrax is a toxin-mediated disease, the lethal effects of which are initiated by the binding of protective antigen (PA) with one of three reported cell surface toxin receptors (ANTXR). Receptor binding has been shown to influence host susceptibility to the toxins. Despite this crucial role for ANTXR in the outcome of disease, and the reported immunomodulatory consequence of the anthrax toxins during infection, little is known about ANTXR expression on human leucocytes. We characterized the expression levels of ANTXR1 (TEM8) on human leucocytes using flow cytometry. In order to assess the effect of prior toxin exposure on ANTXR1 expression levels, leucocytes from individuals with no known exposure, those exposed to toxin through vaccination and convalescent individuals were analysed. Donors could be defined as either ‘low’ or ‘high’ expressers based on the percentage of ANTXR1-positive monocytes detected. Previous exposure to toxins appears to modulate ANTXR1 expression, exposure through active infection being associated with lower receptor expression. A significant correlation between low receptor expression and high anthrax toxin-specific interferon (IFN)-γ responses was observed in previously infected individuals. We propose that there is an attenuation of ANTXR1 expression post-infection which may be a protective mechanism that has evolved to prevent reinfection.
Keywords: anthrax, ANTXR1, monocyte, TEM 8, toxin
Introduction
Anthrax is a toxin-mediated disease caused by infection with the opportunistic Gram-positive bacterial pathogen, Bacillus anthracis 1. The anthrax toxin is a tripartite A–B toxin, comprising two alternative A-subunits, lethal factor (LF) and oedema factor (EF), and a single receptor-binding B-subunit, consisting of heptamers of protective antigen (PA). PA combines with LF to form lethal toxin (LT) or with EF to form oedema toxin (ET). PA consists of four folding domains 2; domain 1 acts as a binding site for LF or EF, domain 2 forms the transmembrane pore and participates in receptor binding, while domain 3 is involved in heptamerization and domain 4 binds to the host cell receptor 2–4. PA alone is not toxic, and is the principal component of existing licensed vaccines for anthrax in the United Kingdom and United States.
PA binds in a 1:1 ratio 5 with either one of three known cell surface receptors: tumour endothelial marker 8 (TEM8 or ANTXR1), capillary morphogenesis protein 2 (CMG2 or ANTXR2) 6,7; more recently it was reported that beta1-integrin can also function as a receptor 8. Both ANTXR1 and ANTXR2 are expressed highly in epithelial cells lining the sites of entry favoured by B. anthracis – the lungs, skin and intestine 7,9,10. The physiological functions of these receptors are associated with binding to extracellular matrix components and are believed to include regulation of endothelial cell–matrix interactions, adhesion, migration, cell spreading on collagen and angiogenesis 11–13.
The interaction of the anthrax toxins with their receptors has a significant impact on the disease process. A mutant cell line lacking ANTXR1/2 is resistant to the effects of purified toxin 14, while cells that over-express either ANTXR show increased susceptibility to lethal toxin and rapid apoptosis 14,15. These effects are also seen during anthrax infection in vivo: mice supplemented with mutant macrophages lacking ANTXR1/2 expression are able to clear a dose of B. anthracis spores, which is lethal in mice supplemented with wild-type macrophages 16.
Despite the clear role of ANTXR in the disease process 16 and the reported immunomodulatory consequence of anthrax toxins during infection 17, little is known about the expression of these receptors on leucocytes. Recently, it has been demonstrated that PA binds natural killer (NK) T cells preferentially rather than NK cells or T cells 18. Furthermore, in-vitro exposure of macrophages to ET has been shown to up-regulate mRNA expression of both receptor types 19, whereas mRNA levels for ANTXR were down-regulated in the lungs of mice injected intranasally with B. anthracis Sterne strain spores.
We have reported previously the detailed characterization of immune responses to anthrax toxins in cohorts of naturally infected, vaccinated and unexposed individuals 20,21. These cohorts offer a unique opportunity to determine the modulatory impact of previous toxin exposure in vivo in humans in a controlled comparison. Thus, the aim of the research presented here was to carry out the first detailed characterization of the surface expression of ANTXR1 on human leucocytes and, more specifically, to assess the effect of previous toxin exposure by profiling ANTRX1 expression levels in convalescent individuals by comparison with non-toxin-exposed individuals.
Materials and methods
Isolation of peripheral blood mononuclear cells (PBMCs) from whole blood
As described previously 21, blood samples were obtained from each of three cohorts: patients treated for and recovered from cutaneous anthrax (n = 10), volunteers vaccinated routinely every 12 months for a minimum of 4·5 years with the UK Anthrax Vaccine Precipitated vaccine (UK Department of Health) (n = 10) and healthy controls with no known exposure to PA or anthrax toxins (UK, n = 14; Turkey, n = 10). Full informed consent was provided by each subject and ethical approval for the study was granted, respectively, by Ericyes University Ethical Committee, Turkey, Chemical and Biological Defence Independent Ethics Committee for the UK Ministry of Defence and the Research Ethics Committee reference number 08/H0707/173.
PBMCs were prepared from sodium heparinized blood using Accuspin tubes (Sigma-Aldrich, Dorset, UK) and centrifuged at 800 g for 30 min, after which the cells were removed from the interface and washed twice in AIM V serum-free media (Gibco Invitrogen, Carlsbad, CA, USA).
Antibody and protein conjugation
Polyclonal TEM8 (ANTXR1), goat immunoglobulin (Ig)G isotype control (both Santa Cruz Biotechnology, Santa Cruz, CA, USA), recombinant PA (Defence Science and Technology Laboratory, Salisbury, UK) and a control of bovine serum albumin (Sigma, Dorset, UK) were fluorescently labelled using an AlexaFluor 488 protein-labelling kit (Invitrogen, Paisley, UK), following the manufacturer's protocol.
Analysis of ANTXR1 expression and protective antigen binding by flow cytometry
Isolated PBMC were washed twice in fluorescence activated cell sorter (FACS) buffer [phosphate-buffered saline (Invitrogen, UK), 10% fetal bovine serum (Autogen Bioclear, Calne, UK)] by centrifuging at 500 g for 10 min. They were then stained with the following antibodies: CD56 phycoerythrin (PE), CD3 PE-cyanin 5 (Cy5), CD19 PE-Cy5, CD14 PE (all eBioscience, Hatfield, UK), Alex 488 conjugated TEM8 (ANTXR1), IgG isotype control, PA or control bovine serum albumin. All antibodies were used at optimal titrated concentrations as recommended by the manufacturers. Post-staining, the cells were washed with FACS buffer, fixed with 2% paraformaldehyde and stored at 4°C until analysis. Approximately 100 000 events within the lymphocyte gate were acquired using a FACScalibur (BD Bioscience, San Jose, CA, USA) and analysed with FlowJo software (Tree Star, Ashland, OR, USA). The lymphocyte and monocyte gates were identified based on their forward-/side-scatter properties and the cell populations defined further as T cells (CD3+CD56–), NK cells (CD3–CD56+), NK T cells (CD3+CD56+), B cells (CD19+) and monocytes (CD14+). The isotype control antibody or Brefeldin A protein binding control were used to establish levels of non-specific binding and set the gates for positive PA binding or ANTXR1 expression (Fig. 1a).
Figure 1.
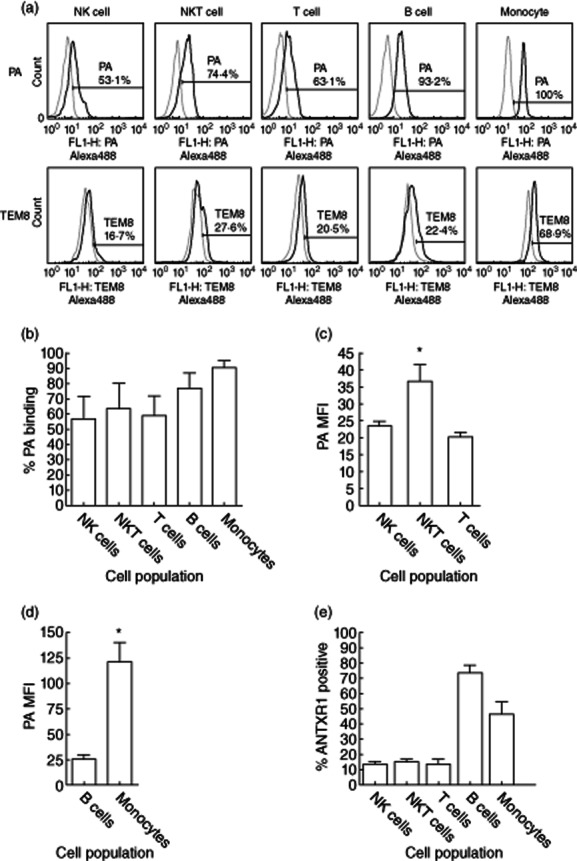
Representative flow cytometry data in a non-exposed control subject are shown depicting the levels of protective antigen (PA) binding (a, top panel) and anthrax toxin receptor 1 (ANTXR1) expression (a, bottom panel) on natural killer (NK) cells (CD3–CD56+), NK T cells (CD3+CD56+), T cells (CD3+CD56–), B cells (CD19+) and monocytes (CD14+). Cells were defined as positive if the levels of Alexa488-conjugated PA or ANTXR1 (TEM8) antibody (black line) were above the non-specific background level of conjugated bovine serum albumin or an immunoglobulin (Ig)G isotype control, respectively (grey line). The percentage of leucocytes binding PA (b) and the median fluorescent intensity (MFI, an indication of levels of binding per cell) of PA binding on NK, NK T and T cell was determined (c). PA binding to NK T is significantly higher than binding to T cells or NK cells (P = 0·008 and P = 0·02, respectively). However, a higher percentage of B cells and monocytes bound PA (b) and monocytes had significantly higher MFI of PA binding (P = 0·002) compared to NKT cells (d). The levels of expression of ANTR1 were also higher on both B cells and monocytes (e).
Statistical analysis
As flow cytometric data are inherently non-parametric, the Kruskal–Wallis test, with Dunn's post-hoc testing, was used to compare the levels of PA binding and ANTXR1 expression level between cell types and cohorts. During analysis, it could be seen that there were distinct groupings of individuals based on the percentage of ANTXR1-positive monocytes. Using the boundaries of these groupings, the populations were categorized as low (≤ 35%) or high (> 35%); a comparison of the number of subjects falling into each of these categories was made using a two-tailed χ2 test. The expression levels of ANTXR1 were logged before linear regression analysis with previously published interferon (IFN)-γ responses to PA detected by enzyme-linked immunospot (ELISPOT) assay 20 from the same individual. Graphpad Prism version 4·0 software (Graphpad, Inc., La Jolla, CA, USA) was used for all analyses.
Results
A detailed characterization of PBMC for ANTXR1 expression and binding of the anthrax toxin component PA was performed (Fig. 1a). Although no significant differences in the percentage of T, NK and NK T cells binding PA were observed (Fig. 1b), the median fluorescent intensity (MFI) was significantly higher on NK T cells compared to both NK cells (P = 0·02) or T cells (P = 0·008) (Fig. 1c). The highest levels of both PA binding and MFI were observed on monocytes (Fig. 1d), mirrored by a high percentage of monocytes expressing ANTXR1, although B cells showed the highest percentage of expression (Fig. 1e).
During the analysis of ANTXR1 expression on monocytes, it was apparent that individuals could be divided into two main groupings termed ‘low’ and ‘high’ expressers (Fig. 2a). As ANTXR expression is known to relate to the susceptibility of a cell to anthrax toxins 1,4,16, it could be hypothesized that the ‘high’ expresser population would be more susceptible to anthrax infection. To examine this further, we examined ANTXR1 expression levels of the monocytes of individuals who had previously been infected naturally with anthrax due to interaction with livestock in an anthrax-endemic region of Turkey 21. Contrary to the predicted results, all previously infected individuals were categorized as low expressers (Fig. 2b, Table 1). In order to ensure that this was not due to a generic local genetic variation in ANTXR1 expression levels, a cohort of local volunteers with no known previous B. anthracis was also examined. There was no difference in the percentage of high and low expressers in the unexposed volunteers from the United Kingdom and Turkey (P = 0·63) (Fig. 2b, Table 1). However, the proportion of individuals defined as low or high expressers was significantly different in the Turkish individuals based on exposure (P = 0·003) (Table 1), with 60% of the unexposed controls defined as high expressers while 100% of the convalescent subjects were low expressers. To establish if the over-representation of low expressers in the previously exposed subjects was as a result of their exposure to the components of the anthrax toxins, a cohort of anthrax vaccine precipitated (AVP)-vaccinated individuals was tested. It was shown that there were significantly more ‘high’ expressers (Fig. 2b, Table 1) compared to the UK healthy control cohort (P = 0·008) and the convalescent individuals (P < 0·0001) (Fig. 2b). Furthermore, in a subset of the samples both the percentage of PA binding and the median fluorescent intensity (MFI) of PA on the positive cells was measured post-exposure (Fig. 3). There was a significant reduction in the convalescent individuals (n = 4) in both the percentage of positive cells and the MFI of PA binding in comparison to the vaccinated subjects (n = 10; P = 0·03 and 0·09, respectively); however, only the percentage of PA binding was reduced in comparison to the unexposed controls (n = 7; P = 0·007).
Figure 2.
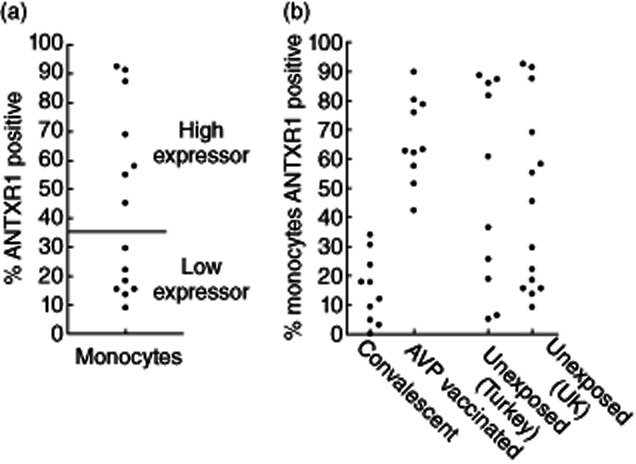
The percentage of anthrax toxin receptor 1 (ANTXR1)-positive monocytes in UK healthy volunteers (a) and in naturally infected convalescent, vaccinated and unexposed (UK and Turkish) individuals (b).
Table 1.
The percentage of subjects within each cohort defined as ‘low’ or ‘high’ expressers of anthrax toxin receptor 1 (ANTXR1) based on the percentage of positive monocytes detected by flow cytometry
| Cohort | Level of ANTXR1 expression | |
|---|---|---|
| Low | High | |
| ≤ 35% | > 35% | |
| Naturally infected | 100 | 0 |
| AVP vaccinated | 0 | 100 |
| Non-exposed (Turkey) | 40 | 60 |
| Non-exposed (UK) | 50 | 50 |
AVP: anthrax vaccine precipitated.
Figure 3.
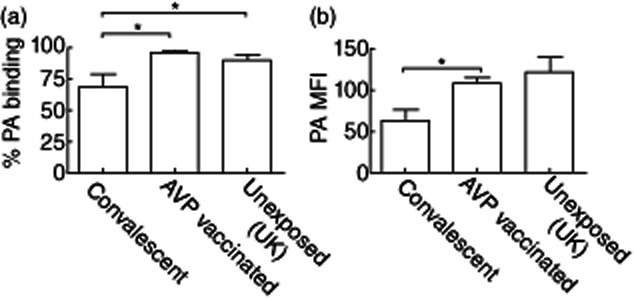
The percentage of monocytes binding protective antigen (PA) (a) and the median fluorescent intensity (MFI) of levels of PA in naturally infected convalescent (n = 4), vaccinated (n = 10) and unexposed (UK) individuals (n = 7) was determined by flow cytometery. Significantly lower levels of PA binding in the convalescent samples compared to both the unexposed controls and the anthrax vaccine precipitated (AVP)-vaccinated individuals (P = 0·03 and 0·007, respectively), while PA MFI was significantly lower compared to the AVP-vaccinated subjects (P = 0·008) but not unexposed controls (P = 0·09).
We have previously published CD4+ T cell production of IFN-γ in response to PA, quantified in a PBMC ELISPOT assay, from these cohorts of naturally infected and vaccinated individuals 21. To assess if the binding of PA to ANTXR on the surface of antigen-presenting cells influences its uptake and presentation to T cells, the IFN-γ response of each individual was correlated with the ANTXR1 expression. No significant correlation was observed (P = 0·87, r2 = 0·003) in AVP-vaccinated individuals (Fig. 4a), even when only those individuals who mount an immune response to PA were considered (P = 0·13, r2 = 0·75). In contrast, there was a significant negative correlation in the naturally infected individuals (P = 0·016, r2 = 0·58) (Fig. 4b).
Figure 4.
While there was no significant relationship between the percentage of monocytes expressing anthrax toxin receptor 1 (ANTXR1) and interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) responses to protective antigen (PA) in anthrax vaccine precipitated (AVP)-vaccinated subjects (a) and naturally infected individuals showed an inverse correlation between ANTRXR1 expression and IFN-γ responses to PA (b), i.e. those individuals with the lowest percentage of ANTXR1-positive monocytes showed the highest levels of IFN-γ production in response to PA stimulation.
Discussion
This study represents the first comprehensive characterization of ANTXR1 expression on human leucocytes. It has been observed previously that NK T cells bind PA preferentially compared with NK or T cells 18. While the results from this study validate this finding, it was observed that PA binding to monocytes was far higher; this was mirrored by a higher percentage of cells expressing ANTXR1. Two distinct groups could be identified among our control blood donors, based on the percentage of ANTXR1-positive monocytes, and these were termed ‘low’ and ‘high’ expressers. These results are substantiated by the recent demonstration that there is a striking diversity in the sensitivity of human lymphoblastoid cell lines to anthrax toxin which results, at least in part, to expression levels of ANTXR2 22. Using parent–child trios Martchenko et al. 22 demonstrated that this variability in expression levels is inherited genetically. It was proposed in that study that lethal B. anthracis clades may have exerted evolutionary selection pressure on the incidence of toxin receptor polymorphisms in human populations. However, we consider it unlikely that anthrax, which is not spread easily between humans, has imposed strong selection pressure during human evolutionary history.
Given that anthrax toxin receptor expression has been correlated with cell susceptibility to the effects of toxin in both in-vitro and animal models 14–16,22, we postulated that ANTXR1 ‘high’ expressers would be more susceptible to either anthrax infection or to the development of severe disease. To test this premise, the expression levels of ANTXR1 were measured on cells from a cohort of individuals who received hospital treatment for cutaneous anthrax infection 21. As the clinical picture of cutaneous anthrax ranges from mild to severe 23 we reasoned that a correlation between expression level and the severity of disease would be observed. However, we found that all the exposed and recovered individuals were low expressers. To rule out that this was due to some form of generic low expression across this population, we also examined local healthy volunteers with no known history of exposure to anthrax, but the grouping of unexposed individuals was comparable in the UK and Turkish cohorts.
All the Turkish samples were collected by the same clinician, processed by a single researcher and analysed under the same flow cytometry conditions, arguing that the striking attenuation of ANTXR1 expression on the monocytes of exposed individuals is not an artefact of sample preparation or processing. The preponderance of low expressers in the exposed cohort could be theorized to result from modulation of receptor expression levels by exposure to the anthrax toxin components. Alternatively, low expressers may be at increased risk of infection. To examine these postulations, we tested a cohort of individuals receiving multiple boosts with AVP vaccine, thus exposed to PA, LF and EF without infection. If exposure to these anthrax proteins is capable of modulating ANTXR1 expression, then these individuals would also have reduced expression of ANTXR1 on their monocytes, while if low expression results in increased risk of infection, the expression profile of the vaccinated individuals should resemble that of the healthy controls. Surprisingly, the AVP-vaccinated cohort were all classified as high expressers. This divergence in response may be due to the concentration and/or ratio in which the infected and vaccinated individuals are exposed to the individual anthrax toxin components. The vaccinees will have been exposed primarily to PA, with low concentrations of LF and EF, therefore a much lower toxin concentration than in infected individuals. Alternatively, down-regulation of ANTXR1 may require PA binding within the context of the inflammatory milieu resulting from infection. It has been demonstrated that PA and EF exposure induces an up-regulation of ANTXR1 in vitro 19, and perhaps these conditions model more accurately the immunological setting of vaccination, as opposed to the highly inflammatory conditions that will be present during a natural infection. The infected individuals also received antibiotic treatment at the time of exposure to the anthrax toxins 21, which the vaccinated group did not. We therefore cannot exclude the possibility that the therapeutics play a role in the reduction of ANTXR1 levels seen in the convalescent cohort. It could be postulated equally that the divergence of response between the infected and vaccinated individuals is due to the kinetics of antigen exposure. There is no correlation in either cohort between the percentage of monocytes expressing TEM8 and the time in days since last exposure (data not shown). We have, however, demonstrated previously a significant correlation between the duration of infection and the induction of a long-lasting T cell memory response to LF, but not in vaccinees 21. Therefore, the reduction of TEM8 expression could also be as a result of a more prolonged exposure to toxin in the infected individuals.
It is well established that anthrax LT induces pyroptosis in macrophages 24; this rapid killing causes LT activation of the Nlrp1 inflammasome, resulting in caspase-1 activation 25,26. It is therefore possible that this mechanism may have eliminated many of the ANTXR1-expressing cells in the cohort of individuals who have been exposed previously. It has been suggested that this LT-mediated activation of Nlrp1b and subsequent lysis of macrophages may be a protective host-mediated innate immune response, as opposed to a virulence mechanism exploited by B. anthracis 26.
Although we have demonstrated that unexposed individuals can be classified as high or low expressers based on the percentage of positive monocytes, universally high levels of PA binding to these cells was observed. This is likely to be due to the co-expression of ANTXR2 and/or beta1-integrin on the monocytes. Unfortunately, due to the logistical complexity in obtaining these invaluable convalescent samples, it is not feasible to go back and reassess the expression of these additional receptors. The rarity of these samples is reflected by the small sample number in which we were able to assess the levels of PA binding. Despite this, we see clearly that the reduction in ANTXR1 expression is associated with a reduction both in the percentage of PA-positive cells and the MFI of PA binding. While ANTXR2 is reported to bind PA with a far greater affinity than ANTXR1 27, the pH at which binding of PA to the receptor occurs also differs, with ANTXR1 binding at the more physiologically relevant pH 6·8 in comparison to ANTXR2, which binds at pH 5·6 28. Furthermore, it has been reported that a mutant form of ANTXR1, L56A, performs similarly if not slightly better than ANTXR2 mutants in both in-vitro and in-vivo toxin protection assays 29. Taken together, this is indicative that the reduction in the percentage of monocytes expressing ANTXR1 could have a biological role in the host response to anthrax toxins.
Individuals in the convalescent cohort were infected at least 1 year previously, and in one case more than 7 years previously 21. This suggests either that infection causes a permanent alteration in the expression of ANTXR1 or that there is ongoing exposure without subsequent reinfection which maintains the depressed expression levels, or that low expressers among that population were more likely to become infected in the first place.
There was no correlation between the levels of ANTXR1 expression observed within this study and the IFN-γ response to PA in the same AVP-vaccinated cohort 21, which suggests that a cellular recall response to PA is not related to ANTXR1 expression per se. However, only a minority of vaccinees responded to PA 21; as only responsive individuals were included in the analysis a much stronger r2 value was observed. This was not statistically significant, but may be more reflective of the small number of responders. In contrast, in naturally infected individuals, a lower percentage of ANTRX1 expression on monocytes correlated significantly with an increased PA T cell-specific IFN-γ response in that individual. Murine models have suggested that IFN-γ responses by CD4 T cells are protective against anthrax spores 30. The findings presented may have implications for vaccine efficacy, although a more extensive study correlating the levels of PA binding, expression of all ANTXRs, T cell responses and antibody titres would need to be carried out to address this. However, given the role played by ANTXRs in determining susceptibility to anthrax toxin 14–16, and the inverse relationship between the percentage of ANTXR1-expressing monocytes and the magnitude of T cell responses, any vaccine or adjuvant able to down-regulate ANTXR expression could only be beneficial.
To our knowledge, this is the first documentation of modulation of the expression of ANTXR1 in humans due to exposure to anthrax toxins. There is a significant correlation between low ANTXR1 expression and high cellular IFN-γ recall responses to PA in individuals exposed to toxin in the context of natural infection, suggesting that the attenuation of ANTXR1 expression is one of the protective mechanisms to prevent reinfection in convalescent cutaneous anthrax patients.
Acknowledgments
This work was supported by funding from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, contract HHSN266200400084C. D.M.A and S.S. are grateful for support from the National Institute for Health Research Biomedical Research funding scheme. The authors would also like to offer their gratitude to the volunteers who participated in this study.
Disclosure
The authors have no financial or commercial conflicts to declare.
References
- 1.Moayeri M, Leppla SH. The roles of anthrax toxin in pathogenesis. Curr Opin Microbiol. 2004;7:19–24. doi: 10.1016/j.mib.2003.12.001. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15036135 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 2.Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385:833–838. doi: 10.1038/385833a0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9039918 (accessed 2 March 2012) [DOI] [PubMed] [Google Scholar]
- 3.Lacy DB, Wigelsworth DJ, Scobie HM, Young JAT, Collier RJ. Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor. Proc Natl Acad Sci USA. 2004;101:6367–6372. doi: 10.1073/pnas.0401506101. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=404051&tool=pmcentrez&rendertype=abstract (accessed 11 April 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santelli E, Bankston LA, Leppla SH, Liddington RC. Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature. 2004;430:905–908. doi: 10.1038/nature02763. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15243628 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 5.Wigelsworth DJ, Krantz BA, Christensen KA, Lacy DB, Juris SJ, Collier RJ. Binding stoichiometry and kinetics of the interaction of a human anthrax toxin receptor, CMG2, with protective antigen. J Biol Chem. 2004;279:23349–23356. doi: 10.1074/jbc.M401292200. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15044490 (accessed 2 April 2012) [DOI] [PubMed] [Google Scholar]
- 6.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11700562 (accessed 4 March 2012) [DOI] [PubMed] [Google Scholar]
- 7.Scobie HM, Rainey GJA, Bradley KA, Young JAT. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci USA. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=154317&tool=pmcentrez&rendertype=abstract (accessed 11 April 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martchenko M, Jeong S-Y, Cohen SN. Heterodimeric integrin complexes containing beta1-integrin promote internalization and lethality of anthrax toxin. Proc Natl Acad Sci USA. 2010;107:15583–15588. doi: 10.1073/pnas.1010145107. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2932583&tool=pmcentrez&rendertype=abstract (accessed 6 November 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonuccelli G, Sotgia F, Frank PG, et al. ATR/TEM8 is highly expressed in epithelial cells lining Bacillus anthracis' three sites of entry: implications for the pathogenesis of anthrax infection. Am J Physiol Cell Physiol. 2005;288:C1402–1410. doi: 10.1152/ajpcell.00582.2004. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15689409 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 10.Xu Q, Hesek ED, Zeng M. Transcriptional stimulation of anthrax toxin receptors by anthrax edema toxin and Bacillus anthracis Sterne spore. Microb Pathog. 2007;43:37–45. doi: 10.1016/j.micpath.2007.03.002. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1973154&tool=pmcentrez&rendertype=abstract (accessed 11 April 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotchkiss KA, Basile CM, Spring SC, Bonuccelli G, Lisanti MP, Terman BI. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell–matrix interactions on collagen. Exp Cell Res. 2005;305:133–144. doi: 10.1016/j.yexcr.2004.12.025. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15777794 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 12.Werner E, Kowalczyk AP, Faundez V. Anthrax toxin receptor 1/tumor endothelium marker 8 mediates cell spreading by coupling extracellular ligands to the actin cytoskeleton. J Biol Chem. 2006;281:23227–23236. doi: 10.1074/jbc.M603676200. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16762926 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 13.Young JJ, Bromberg-White JL, Zylstra C, et al. LRP5 and LRP6 are not required for protective antigen-mediated internalization or lethality of anthrax lethal toxin. PLoS Pathog. 2007;3:e27. doi: 10.1371/journal.ppat.0030027. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?%20artid=1808072&tool=pmcentrez&rendertype=abstract (accessed 11 April 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks DJ, Barnajian M, Maldonado-Arocho FJ, Sanchez AM, Bradley KA. Anthrax toxin receptor 2 mediates Bacillus anthracis killing of macrophages following spore challenge. Cell Microbiol. 2005;7:1173–1185. doi: 10.1111/j.1462-5822.2005.00545.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16008584 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 15.Salles II, Voth DE, Ward SC, et al. Cytotoxic activity of Bacillus anthracis protective antigen observed in a macrophage cell line overexpressing ANTXR1. Cell Microbiol. 2006;8:1272–1281. doi: 10.1111/j.1462-5822.2006.00708.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16882031 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 16.Cote CK, DiMezzo TL, Banks DJ, France B, Bradley KA, Welkos SL. Early interactions between fully virulent Bacillus anthracis and macrophages that influence the balance between spore clearance and development of a lethal infection. Microbes Infect. 2008;10:613–619. doi: 10.1016/j.micinf.2008.02.006. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18467145 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 17.Fukao T. Immune system paralysis by anthrax lethal toxin: the roles of innate and adaptive immunity. Lancet Infect Dis. 2004;4:166–170. doi: 10.1016/S1473-3099(04)00940-5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14998502 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 18.Joshi SK, Lang GA, Larabee JL, et al. Bacillus anthracis lethal toxin disrupts TCR signaling in CD1d-restricted NKT cells leading to functional anergy. PLoS Pathog. 2009;5:e1000588. doi: 10.1371/journal.ppat.1000588. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2742733&tool=pmcentrez&rendertype=abstract (accessed 18 March 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado-Arocho FJ, Fulcher JA, Lee B, Bradley KA. Anthrax oedema toxin induces anthrax toxin receptor expression in monocyte-derived cells. Mol Microbiol. 2006;61:324–337. doi: 10.1111/j.1365-2958.2006.05232.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16856939 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 20.Ingram RJ, Chu KK, Metan G, et al. An epitope of Bacillus anthracis protective antigen that is cryptic in rabbits may be immunodominant in humans. Infect Immun. 2010;78:2353. doi: 10.1128/IAI.00072-10. author reply 2353–4. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2863510&tool=pmcentrez&rendertype=abstract (accessed 6 April 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingram RJ, Metan G, Maillere B, et al. Natural exposure to cutaneous anthrax gives long-lasting T cell immunity encompassing infection-specific epitopes. J Immunol. 2010;184:3814–3821. doi: 10.4049/jimmunol.0901581. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20208010 (accessed 22 March 2012) [DOI] [PubMed] [Google Scholar]
- 22.Martchenko M, Candille SI, Tang H, Cohen SN. Human genetic variation altering anthrax toxin sensitivity. Proc Natl Acad Sci USA. 2012;109:2972–2977. doi: 10.1073/pnas.1121006109. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3286947&tool=pmcentrez&rendertype=abstract (accessed 11 April 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doganay M, Metan G, Alp E. A review of cutaneous anthrax and its outcome. J Infect Public Health. 2010;3:98–105. doi: 10.1016/j.jiph.2010.07.004. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20869669 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]
- 24.Popov SG, Villasmil R, Bernardi J, et al. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem Biophys Res Commun. 2002;293:349–355. doi: 10.1016/S0006-291X(02)00227-9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12054607 (accessed 8 November 2012) [DOI] [PubMed] [Google Scholar]
- 25.Muehlbauer SM, Evering TH, Bonuccelli G, et al. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle. 2007;6:758–766. doi: 10.4161/cc.6.6.3991. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17374996 (accessed 8 November 2012) [DOI] [PubMed] [Google Scholar]
- 26.Terra JK, Cote CK, France B, et al. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol. 2010;184:17–20. doi: 10.4049/jimmunol.0903114. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2811128&tool=pmcentrez&rendertype=abstract (accessed 8 November 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scobie HM, Young JAT. Interactions between anthrax toxin receptors and protective antigen. Curr Opin Microbiol. 2005;8:106–112. doi: 10.1016/j.mib.2004.12.005. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15694864 (accessed 9 November 2012) [DOI] [PubMed] [Google Scholar]
- 28.Fu S, Tong X, Cai C, et al. The structure of tumor endothelial marker 8 (TEM8) extracellular domain and implications for its receptor function for recognizing anthrax toxin. PloS ONE. 2010;5:e11203. doi: 10.1371/journal.pone.0011203. Available at: http://www.plosone.org/article/info:doi/10.1371/journal.pone.0011203#pone.0011203-Rainey1 (accessed 9 November 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai C, Che J, Xu L, et al. Tumor endothelium marker-8 based decoys exhibit superiority over capillary morphogenesis protein-2 based decoys as anthrax toxin inhibitors. PloS ONE. 2011;6:e20646. doi: 10.1371/journal.pone.0020646. Available at: http://dx.plos.org/10.1371/journal.pone.0020646 (accessed 9 November 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glomski IJ, Corre J-P, Mock M, Goossens PL. Cutting edge: IFN-gamma-producing CD4 T lymphocytes mediate spore-induced immunity to capsulated Bacillus anthracis. J Immunol. 2007;178:2646–2650. doi: 10.4049/jimmunol.178.5.2646. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17312104 (accessed 11 April 2012) [DOI] [PubMed] [Google Scholar]


