Abstract
Growing evidence suggests a prominent role of the complement system in the pathogenesis of cardio- and cerebrovascular diseases (CVD). Mannan-binding lectin-associated serine proteases (MASPs) MASP-1 and MASP-2 of the complement lectin pathway contribute to clot formation and may represent an important link between inflammation and thrombosis. MBL-associated protein MAp44 has shown cardioprotective effects in murine models. However, MAp44 has never been measured in patients with CVD and data on MASP levels in CVD are scarce. Our aim was to investigate for the first time plasma levels of MAp44 and MASP-1, -2, -3 concomitantly in patients with CVD. We performed a pilot study in 50 healthy volunteers, in stable coronary artery disease (CAD) patients with one-vessel (n = 51) or three-vessel disease (n = 53) and age-matched controls with normal coronary arteries (n = 53), 49 patients after myocardial infarction (MI) and 66 patients with acute ischaemic stroke. We measured MAp44 and MASP-1 levels by in-house time-resolved immunofluorometric assays. MASP-2 and MASP-3 levels were measured using commercial enzyme-linked immunosorbent assay kits. MASP-1 levels were highest in subacute MI patients and lowest in acute stroke patients. MASP-2 levels were lower in MI and stroke patients compared with controls and CAD patients. MASP-3 and MAp44 levels did not differ between groups. MASP or MAp44 levels were not associated with severity of disease. MASP and MAp44 levels were associated with cardiovascular risk factors including dyslipidaemia, obesity and hypertension. Our results suggest that MASP levels may be altered in vascular diseases. Larger studies are needed to confirm our results and elucidate the underlying mechanisms.
Keywords: complement system, coronary heart disease, ischaemic stroke, MAp44, MASP
Introduction
Chronic inflammation and changes in blood coagulation and fibrinolysis result in a proinflammatory and prothrombotic state that contributes to the development of cardio- and cerebrovascular diseases (CVD) 1,2. The complement system is part of the innate immune system and an important player in inflammation, and there is a substantial body of work clearly indicating a prominent role of complement in the pathogenesis of CVD 3–5 and their complications arising from ischaemia–reperfusion injury 6 by promoting inflammatory processes and by interacting with blood coagulation 7,8.
When the complement system is activated a cascade of enzymatic reactions occur, leading to anti-microbial processes, but if activated it may also lead to the release of a number of potent inflammatory reactions resulting in damage to the host's own tissue 9. Different pathways lead to initiation of the complement system; the classical, the alternative and the lectin pathways. The lectin pathway may be initiated when either one of the recognition molecules mannan-binding lectin (MBL), H-ficolin, L-ficolin M-ficolin or collectin-K1 10 binds to a fitting pattern, e.g. on microorganisms or apoptotic host cells. All five recognition molecules are in complex with so-called MBL-associated serine proteases (MASPs); MASP-1, MASP-2, and MASP-3. Two other proteins, MAp44 and MAp19, are also found in complex with these recognition molecules. These components of the lectin pathway were reviewed recently by Yongqing et al. 11 and Degn et al. 12. MBL or ficolins bind specifically to pathogen surfaces or to altered self-structures via target structures which form a pattern of ligands and fit the geometry of the recognition domains of the recognition molecules. Upon binding, conformational changes lead to autoactivation of MASPs 11. Activated MASP-1 will cleave MASP-2, which subsequently will cleave complement factors C4 and C2 leading to formation of new enzymatic activities, i.e. forming C3 convertases and later C5 convertases 13–15. Apart from this activity, MASP-1 also seems to be a multi-functional protein with roles in blood coagulation (as detailed below). MASP-3 has a function in embryonic development, as mutations in the MASP-3-specific parts of the gene MASP1 (encoding MASP-1, MASP-3 and MAp44) are associated with human malformation syndromes 16,17. Otherwise, the physiological role of MASP-3 is not well understood. Both an inhibitory role in the lectin pathway 18,19 and an accelerating effect on activation of the alternative pathway 20 have been proposed. MAp44 (also known as MAP-1), together with MASP-1 and MASP-3, is one of three alternative splicing products of the MASP1 gene 12. MAp44 shares the first four domains with MASP-1 and MASP-3, but its unique C-terminus lacks a serine protease domain 21. The function of MAp44 is regulation of the lectin pathway, as it has been shown to compete with MASPs for binding to MBL and ficolins, resulting in inhibition of complement activation 21–23.
MASP-1 and MASP-2 have been shown to contribute to in-vitro fibrin clot formation 24–28 and MASP-1 was essential for obstructive thrombosis in a mouse model of arterial injury 29. MASPs may therefore represent an important link between inflammation and thrombosis. Conversely, MAp44 may have a protective effect in CVD, as it preserved cardiac function, decreased infarct size and prevented thrombogenesis in murine models of ischaemia/reperfusion injury and arterial thrombosis 23. However, MAp44 plasma levels have never been measured in patients with CVD, whereas recent data suggest a role of the lectin pathway and MASPs in patients with CVD. In a study in 890 patients with acute MI, mortality within 90 days was significantly lower in patients with a functional deficiency of MBL, defined as serum MBL levels ≤ 100 ng/ml, than in patients with MBL levels > 100 ng/ml 30. Similarly, in 353 patients with acute ischaemic stroke, low MBL levels were associated with smaller cerebral infarct volume and favourable outcome after 3 months 31. MASP-2 levels were measured in a cohort of 397 patients with type 2 diabetes mellitus (T2DM) and suspected myocardial infarction (MI). During a median follow-up period of 2·1 years, 141 patients suffered from cardiovascular events. These patients had significantly lower MASP-2 levels at admission compared with 256 patients without cardiovascular events, but after adjustment for risk factors MASP-2 was not an independent predictor of cardiovascular events 32. In another study, 29 patients with acute MI had significantly lower MASP-2 levels compared with 50 healthy individuals, or 27 patients with coronary artery disease (CAD) but without acute MI 33. Lower MASP-2 levels were also found in 81 patients after cardiac surgery with cardiopulmonary bypass compared with before surgery, and it was suggested that reduction of MASP-2 levels was caused by complement activation during myocardial ischaemia 33. In a study in 135 patients with acute stroke, MASP-2 levels were measured on days 0–4, 7 and 90, but showed neither any significant changes over time, differences between ischaemic (n = 109) and haemorrhagic stroke (n = 26), nor any association with clinical outcome 34. Only very recently has it become possible to test for MASP-1, MASP-3 and MAp44 individually and data on these proteins are only available in very few clinical cohorts.
The aim of our study is to gain more insight into the role of MASPs and MAp44 in CVD. We investigate for the first time plasma levels of MAp44 and all three MASPs, MASP-1, MASP-2 and MASP-3 concomitantly, in patients with coronary artery disease (CAD), myocardial infarction (MI) and acute ischaemic stroke. We also analyse MASP and MAp44 levels with regard to severity of CVD and cardiovascular risk factors. Our pilot study may form the basis for future larger clinical studies on MASP levels in patients with CVD and for studies aiming to elucidate the underlying pathophysiological mechanisms and preparing the basis for putative therapies targeting the complement lectin pathway.
Materials and methods
Patients
Fifty healthy volunteers were recruited at the University Clinic of Haematology, University Hospital Bern between June 2008 and August 2009. All participants gave informed consent.
As described earlier 35, 496 consecutive patients undergoing coronary angiography were recruited at the Department of Cardiology, University Hospital Bern between January and September 2000. The study was approved by the local ethics committee and all patients gave informed consent. Demographic data and cardiovascular risk factors were recorded and routine laboratory parameters were measured upon hospital admission. Venous blood was taken, citrate plasma was prepared and stored frozen in aliquots at −80°C until analysis. Indications for coronary angiography were (i) suspected CAD, (ii) known CAD, (iii) routine angiography before closure of persisting foramen ovale or (iv) routine angiography before valve replacement surgery. Patients with normal coronary vessels were defined as CAD controls; patients with stenoses of ≥ 20% were defined as CAD patients and classified according to the number of affected vessels. For the present study, 53 CAD controls, 51 CAD patients with one-vessel disease and 53 CAD patients with three-vessel disease, all age- and sex-matched, were selected.
Between January 2004 and February 2006, approximately 340 patients were enrolled into a secondary cardiac prevention and rehabilitation programme at the Division of Cardiovascular Prevention, Rehabilitation and Sports Cardiology, Department of Cardiology, University Hospital Bern, only days after having suffered from MI. Venous blood was taken, citrate plasma was prepared and stored frozen in aliquots at −80°C until analysis. Demographic data and cardiovascular risk factors were recorded and routine laboratory parameters were measured upon enrolment and after 1 year. The study was approved by the local ethics committee and all patients gave informed consent. We included 49 of these subacute MI patients in the present study.
Sixty-six patients with acute ischaemic stroke were recruited at the Stroke Unit, Department of Neurology, University Hospital Bern between June 2007 and November 2008, as described earlier 36. The study was approved by the local ethics committee and all patients gave informed consent. The National Institutes of Health Stroke Scale (NIHSS) 37 was used to assess clinical severity of stroke, and aetiology was defined according to ‘Trial of ORG 10172 in Acute Stroke Therapy’ criteria 38. The first blood sample was taken upon hospital admission (day 0) before lysis therapy. Further blood samples were taken on the two following days (unless patients were referred back to local hospitals).
Laboratory analysis
We measured MASP and MAp44 levels in citrate plasma that had been stored frozen in aliquots at −80°C until analysis. MAp44 was measured with a time-resolved immunofluorometric assay (TRIFMA), as described earlier 39. The assay is a sandwich-type assay with a catching antibody and a biotinylated detecting antibody, followed by europium-labelled streptavidin and measurement by time-resolved fluorometry. MASP-1 was measured with a novel competition assay using microtitre wells coated with MASP-1 as testing surface followed by incubation with the sample mixed with a MASP-1 specific antibody (the MASP-1 in the sample competing with the coated MASP-1 for binding of the antibody), as described recently in detail 40. MASP-2 and MASP-3 levels were measured with commercial enzyme-linked immunosorbent assay (ELISA) kits (Hycult Biotech, Uden, the Netherlands). The levels of all three MASPs are quite stable throughout a 1-year period, and acute-phase reactions do not seem to influence levels of MASP 40 or MAp44 39. Routine laboratory diagnostics had been performed upon hospital admission.
Statistical analysis
Statistical analysis was performed with spss version 17.0. Data were tested for normal distribution using Kolmogorov–Smirnov or Shapiro–Wilk tests. Normally distributed data are usually shown as mean with standard deviation (s.d.); non-normally distributed data are usually shown as median with interquartile range (IQR). For better comparability, MASP and Map44 levels are always shown as median (IQR). The appropriate statistical tests were chosen depending upon the type of distribution. Normally distributed data were compared between two groups with t-test, or with analysis of variance (anova) for multiple groups. Data not showing normal distribution were compared between two groups using the Mann–Whitney U-test, or the Kruskal–Wallis test in the case of multiple groups. Wilcoxon's or Friedmann's tests were used to compare two or more, respectively, paired samples. Bivariate correlations were expressed as Pearson's or Spearman's rank correlation coefficients. Categorical data were compared between groups using the χ2 test. A P-value of less than 0·05 was considered statistically significant.
Results
MASP and MAp44 levels in patients with CAD
Characteristics of CAD patients with one- or three-vessel disease and of age- and sex-matched controls with angiographically proven normal coronary vessels are shown in Table 1. MASP and MAp44 levels did not differ between the groups, although a trend towards highest levels in patients with three-vessel disease could be observed (Table 2). When patients with one- and three-vessel disease were taken together, there was again no significant difference in MASP and MAp44 levels between these and CAD controls (data not shown).
Table 1.
Characteristics of coronary artery disease (CAD) patients and controls.
| Normal coronary vessels | One-vessel disease | Three-vessel disease | P-value | |
|---|---|---|---|---|
| n = 53 | n = 51 | n = 53 | ||
| Age (years)† | 63·1 (9·7) | 63·9 (10·4) | 65·3 (11·4) | n.s.§ |
| Male gender (%) | 26 (49) | 26 (51) | 27 (51) | n.s.¶ |
| Positive family history (%) | 15 (28) | 19 (37) | 29 (55) | 0·019¶ |
| Smoker (%) | 8 (15) | 19 (37) | 18 (34) | 0·025¶ |
| Hypertension (%) | 25 (47) | 35 (69) | 41 (77) | 0·004¶ |
| Diabetes (%) | 3 (6) | 14 (27) | 16 (30) | 0·003¶ |
| BMI (kg/m2)† | 26·3 (4·6) | 28·7 (4·2) | 27·6 (4·4) | 0·022§ |
| Total cholesterol (mmol/l)‡ | 5·4 (2·0) | 5·2 (1·7) | 5·8 (1·4) | n.s.** |
| Triglycerides (mmol/l)‡ | 1·5 (1·2) | 1·7 (1·1) | 1·7 (1·1) | n.s.** |
| HDL (mmol/l)‡ | 1·3 (0·4) | 1·2 (0·4) | 1·2 (0·4) | n.s.** |
| Leucocyte count (×103/ul)‡ | 6·7 (2·0) | 7·6 (2·6) | 6·6 (2·3) | 0·002** |
| Fibrinogen (g/l)‡ | 2·7 (1·2) | 3·5 (0·9) | 3·4 (1·0) | 0·007** |
| PAI-1 (ng/ml)‡ | 138·9 (132·8) | 114·2 (114·0) | 152·4 (113·9) | n.s.** |
| No. of plaques†† | 0 | 2 (1–5) | 6 (1–14) | <0·001** |
Mean (standard deviation);
median (interquartile range);
analysis of variance;
χ2 test.
Kruskal–Wallis test.
Median (range); n.s.: not significant; BMI: body mass index; HDL: high-density lipoprotein; PAI-1: plasminogen activator inhibitor-1.
Table 2.
MASP and MAp44 levels in patients with normal coronary vessels, one- and three-vessel disease.
| Normal coronary vessels | One-vessel disease | Three-vessel disease | P-value | |
|---|---|---|---|---|
| n = 53 | n = 51 | n = 53 | ||
| MASP-1 (μg/ml) | 9·12 (3·43) | 9·61 (4·01) | 10·31 (3·14) | n.s. |
| MASP-2 (ng/ml) | 486·7 (573·8) | 457·8 (394·2) | 539·5 (293·4) | n.s. |
| MASP-3 (μg/ml) | 5·52 (2·96) | 5·54 (2·73) | 5·60 (3·45) | n.s. |
| MAp44 (μg/ml) | 1·80 (0·79) | 1·80 (0·71) | 1·94 (0·76) | n.s. |
Data shown as median (interquartile range). Differences between groups tested with Kruskal–Wallis test. MASP: mannan-binding lectin-associated serine protease; n.s.: not significant.
MASP and MAp44 levels in patients after MI
Characteristics of the included patients with subacute MI are shown in Table 3 and MASP and MAp44 levels are shown in Table 4. Again, the highest levels were found in patients with three-vessel disease, but the differences were not significant. MASP and MAp44 plasma levels showed no correlations with cardiac ejection fraction. A follow-up was performed after 1 year in 27 of the 49 MI patients and MASP levels were similar to baseline values (data not shown), whereas MAp44 decreased slightly from median 1·98 μg/ml (IQR 0·53) at admission to 1·88 μg/ml (IQR 0·54) 1 year later (P = 0·022).
Table 3.
Characteristics of myocardial infarction (MI) patients.
| Age (years)† | 57·0 (9·2) |
| Male gender (%) | 39 (80) |
| Positive family history (%) | 20 (41) |
| Smoker (%) | 9 (18) |
| Hypertension (%) | 28 (57) |
| Diabetes (%) | 1 (2) |
| BMI (kg/m2)† | 26·6 (4·1) |
| Total cholesterol (mmol/l)† | 5·5 (1·3) |
| Triglycerides (mmol/l)‡ | 1·5 (1·0) |
| LDL (mmol/l)† | 3·6 (1·2) |
| HDL (mmol/l)‡ | 1·4 (0·6) |
| Fibrinogen (g/l)‡ | 3·4 (1·2) |
| Degree of disease | |
| One-vessel disease (%) | 21 (43) |
| Two-vessel disease (%) | 19 (39) |
| Three-vessel disease (%) | 9 (18) |
Mean (standard deviation).
Median (interquartile range); BMI: body mass index; HDL: high-density lipoprotein; LDL: low-density lipoprotein.
Table 4.
MASP and MAp44 levels in myocardial infarction (MI) patients.
| One-vessel disease | Two-vessel disease | Three-vessel disease | P-value | |
|---|---|---|---|---|
| n = 21 | n = 19 | n = 9 | ||
| MASP-1 (μg/ml) | 12·17 (5·45) | 11·51 (3·41) | 12·30 (5·15) | n.s. |
| MASP-2 (ng/ml) | 376·4 (259·2) | 359·4 (247·3) | 410·4 (224·9) | n.s. |
| MASP-3 (μg/ml) | 5·81 (2·75) | 5·98 (1·52) | 7·04 (2·87) | n.s. |
| MAp44 (μg/ml) | 1·91 (0·61) | 1·90 (0·71) | 2·02 (0·80) | n.s. |
Data shown as median (interquartile range). Differences between groups tested with Kruskal–Wallis test. MASP: mannan-binding lectin-associated serine protease.
MASP and MAp44 levels in patients with acute stroke
Characteristics of the stroke patients are shown in Table 5 and MASP and MAp44 levels are shown in Table 6. When patients who had had all three blood samples taken (admission, days 1 and 2) were compared, MASP-3 and MAp44 showed a significant decrease over the 3 days following acute stroke. Comparison between patients with atherosclerotic and cardioembolic stroke revealed a trend towards higher MASP-2 levels in atherosclerotic [median 405·9 ng/ml (IQR 351·7)] than cardioembolic [298·8 ng/ml (191·5)] stroke (P = 0·062). MASP-1, MASP-3 and MAp44 showed no differences in the same analysis (data not shown). MASP and MAp44 levels showed no correlation with NIHSS and did not differ between patients with mild, moderate or severe stroke as judged by NIHSS criteria (data not shown).
Table 5.
Characteristics of stroke patients.
| Age (years) | 65·4 (15·7) |
| Male gender (%) | 41 (62) |
| Duration between symptom onset and hospital admission (h) | 3·1 (1·4) |
| NIHSS at admission | 11·5 (6·5) |
| Stroke severity according to NIHSS | |
| Mild (0–4) (%) | 8 (12) |
| Moderate (5–12) (%) | 29 (44) |
| Severe (> 12) (%) | 28 (42) |
| Stroke aetiology | |
| Atherosclerotic vessel disease (%) | 14 (21) |
| Cardioembolic (%) | 29 (44) |
| Other (%) | 2 (3) |
| More than one (%) | 2 (3) |
| Unknown (%) | 18 (27) |
| Smoker (%) | 10 (15) |
| Hypertension (%) | 39 (59) |
| Total cholesterol (mmol/l) | 4·8 (1·1) |
| LDL (mmol/l) | 2·8 (0·9) |
| HDL (mmol/l) | 1·3 (0·4) |
| HbA1c | 6·1 (0·8) |
Continuous variables are shown as mean (standard deviation). Categorical data are shown as number (%). HbA1c: haemoglobin A1c; HDL: high-density lipoprotein; LDL: low-density lipoprotein; NIHSS: National Institutes of Health Stroke Scale.
Table 6.
MASP and MAp44 levels in stroke patients.
| Day 0 | Day 1 | Day 2 | |
|---|---|---|---|
| n = 66 | n = 34 | n = 28 | |
| MASP-1 (μg/ml) | 8·61 (2·87) | 7·85 (2·22) | 7·82 (1·54) |
| MASP-2 (ng/ml) | 361·6 (287·0) | 408·0 (255·4) | 398·7 (255·7) |
| MASP-3 (μg/ml) | 5·36 (3·09) | 4·59 (1·83) | 4·42 (2·55) |
| MAp44 (μg/ml) | 1·82 (0·83) | 1·19 (0·70) | 1·38 (0·77) |
| Patients with all three blood samples (n = 22) | |||
|---|---|---|---|
| MASP-1 (μg/ml) | 9·09 (2·50) | 8·19 (2·38) | 7·77 (1·59) |
| MASP-2 (ng/ml) | 444·0 (354·6) | 409·6 (268·4) | 413·1 (289·9) |
| MASP-3 (μg/ml) | 4·97 (2·70)* | 4·30 (1·95)* | 4·12 (2·49)* |
| MAp44 (μg/ml) | 1·61 (0·66)* | 1·19 (0·68)* | 1·19 (0·73)* |
Results are shown as median (interquartile range).
P < 0·001 (Friedman test). MASP: mannan-binding lectin-associated serine protease.
Comparison of MASP and MAp44 levels between all groups
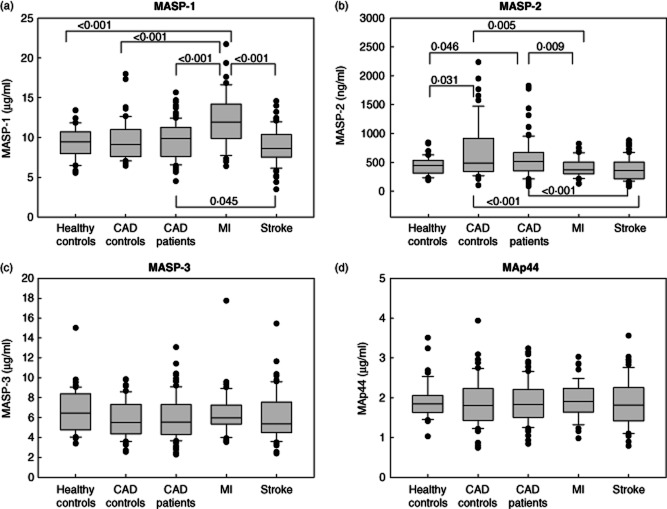
Figure 1 shows the comparison of MASP and MAp44 levels between all groups. Significantly higher MASP-1 levels were observed in MI patients [median 11·93 μg/ml (IQR 4·31)] compared with healthy controls [9·44 μg/ml (2·73), P < 0·001], CAD controls [9·12 μg/ml (3·43), P < 0·001], CAD patients [9·85 μg/ml (3·62), P < 0·001] and stroke patients [8·61 μg/ml (2·87), P < 0·001] (Fig. 1a). Stroke patients had significantly lower MASP-1 levels than CAD patients (P = 0·045) (Fig. 1a).
Figure 1.
Mannan-binding lectin-associated serine protease (MASP) and MAp44 levels in patients with cerebrovascular disease (CVD) and controls. (a) MASP-1 levels were highest in patients who had suffered from myocardial infarction (MI) and lowest in patients with acute ischaemic stroke. (The box represents the 25th to 75th percentiles, the whisker indicates the 10th and 90th percentiles, while the outliers are shown as filled circles.) (b) MASP-2 levels were lower in MI and stroke patients than in controls and coronary artery disease (CAD) patients. (c) MASP-3 levels did not differ between groups. (d) MAp44 levels did not differ between groups.
As shown in Fig. 1b, MASP-2 levels were higher in CAD patients [median 512·0 ng/ml (IQR 318·8), P = 0·046] and CAD controls [486·7 ng/ml (573·8), P = 0·031] than in healthy controls [442·9 ng/ml (219·3)]. Patients with acute stroke [361·6 ng/ml (287·0)] had lower MASP-2 levels than CAD patients (P < 0·001) and CAD controls (P < 0·001). Patients with subacute MI [369·8 ng/ml (207·9)] also had lower MASP-2 levels compared with CAD patients (P = 0·009) and CAD controls (P = 0·005).
MASP-3 (Fig. 1c) and MAp44 (Fig. 1d) showed no significant differences between the groups. Because it was suggested that the relative levels of MASPs and MAp44 may fine-tune the responsiveness of the lectin pathway 21, we also calculated the ratio of MASP-1/MAp44 levels and the ratio of MASP-2/MAp44 levels. However, these ratios mirrored only the differences in MASP-1 and MASP-2 between the groups and were not associated with severity of disease (data not shown).
Associations of MASP and MAp44 levels with cardiovascular risk factors
We tested associations between MASP and MAp44 levels and age, gender, smoking, obesity, lipids and coagulation/inflammatory markers.
MASP-1 levels were related inversely to age in CAD controls (correlation coefficient −0·284, P = 0·039). MASP-1 levels were higher in female CAD patients [median 10·37 μg/ml (IQR 3·17)] than in male CAD patients [9·14 μg/ml (3·92)] (P = 0·029). MASP-1 levels were also higher in CAD patients with hypertension [10·14 μg/ml (3·32)] compared to normotensive CAD patients [8·44 μg/ml (3·08)] (P = 0·049) and higher in CAD patients with diabetes [10·89 μg/ml (3·52)] compared to patients without diabetes [9·38 μg/ml (3·29)] (P = 0·004). Weak positive correlations were observed between MASP-1 and triglyceride levels in CAD patients (0·226, P = 0·023) and in CAD controls (0·328, P = 0·019).
MASP-2 levels were associated with lipid levels in stroke patients, showing positive correlations with total cholesterol (0·374, P = 0·004) and LDL cholesterol (0·300, P = 0·022).
MASP-3 levels were higher in male healthy volunteers [8·04 μg/ml (3·30)] than in females [5·68 μg/ml (2·79)] (P = 0·001). MASP-3 levels showed a weak inverse correlation with age in CAD patients (−0·184, P = 0·021). MASP-3 levels showed positive correlations with body mass index (BMI) in CAD controls (0·314, P = 0·022) and in CAD patients (0·341, P < 0·001), and with triglyceride levels (0·239, P = 0·016) and plasminogen activator inhibitor-1 (PAI-1) (0·308, P = 0·002) in CAD patients. In stroke patients, there was a trend towards higher MASP-3 levels in smokers [5·72 μg/ml (3·09)] compared with non-smokers [4·78 μg/ml (2·52)] (P = 0·055).
In CAD controls, MAp44 levels were slightly higher in women [1·95 μg/ml (0·89)] than in men [1·73 μg/ml (0·58)] (P = 0·032), and higher in non-smokers [1·87 μg/ml (0·75)] than in smokers [1·60 μg/ml (0·43)] (P = 0·037). MAp44 levels showed a positive correlation with BMI in CAD controls (0·364, P = 0·007) and CAD patients (0·197, P = 0·049), and with triglyceride levels in CAD controls (0·308, P = 0·028) and CAD patients (0·202, P = 0·044). In CAD patients, MAp44 levels also correlated with PAI-1 levels (0·274, P = 0·005) and were associated inversely with levels of HDL cholesterol (−0·218, P = 0·029).
We found no correlations between MASP levels and blood pressure levels, glucose and haemoglobin A1c (HbA1c) levels, HDL cholesterol, fibrinogen and high-sensitivity C-reactive protein (hsCRP) levels. No associations with cardiovascular risk factors were observed in MI patients.
Discussion
The complement system, as is the immune system, is also often seen as the defence mechanism against pathogens, but the complement system is also involved in many homeostatic processes and plays a role in a number of pathological conditions 9. Although normally held at bay, the highly potent enzymes that are activated when the complement system is initiated thus have the potential to elicit an attack against the host. There is substantial evidence for a role of the complement system in CVD with implications for new therapeutic possibilities in the future. In two recent animal studies on the effect of the lectin pathway on ischaemia reperfusion injuries, targeting the lectin pathway by MASP-2 knock-out, anti-MASP-2 monoclonal antibodies, MBL knock-out, an MBL binding molecule or an anti-MBL antibody had protective effects in myocardial and cerebral ischaemia 41,42. The development of new therapeutic approaches requires deep understanding of the underlying pathophysiological mechanisms. So far, however, there are only limited or no data available on MASPs and MAp44 of the complement lectin pathway in patients with CVD. In the present study we measured and compared concomitantly plasma levels of MASP-1, MASP-2, MASP-3 and MAp44 in healthy controls, patients with stable CAD, patients with subacute MI and patients with acute stroke. Our aims were to determine MAp44 levels in patients with CVD for the first time, and to increase our knowledge on MASP levels in these chronic vascular diseases with or without acute events and the relationship between MASP or MAp44 levels and cardio- and cerebrovascular risk factors. A strong point of our study is the thorough characterization of patients, including age- and sex-matched CAD controls with angiographically proven normal coronary vessels. A limitation of our study may be its somewhat descriptive character, due to the small sample size of only approximately 50 individuals per group; however, we performed this study as a pilot study which may represent a starting point to future larger clinical studies, and our data may be used as a basis for power calculations for larger studies.
Normal values for MASPs and MAp44 levels in serum or plasma have been established only recently. Normal MASP-1 concentration in serum (and similar in citrate plasma) is 11 μg/ml (range 4–30 μg/ml) 40. For MASP-2, 534 ng/ml (range 170–1196 ng/ml) in plasma 43, 413 ng/ml (range 74–1058 ng/ml) in serum and 416 ng/ml (range 125–1152 ng/ml) in plasma 44 were reported. For MASP-3, 6·4 μg/ml (range 2–12·9 μg/ml) 21 and 5·0 μg/ml (range 1·8–10·6 μg/ml) 39 have been found in serum. MAp44 has been found in EDTA plasma at a mean concentration of 0·80 μg/ml (range 0·14–2·04 μg/ml) 21, and in serum at 1·38 μg/ml (range 0·34–3·00 μg/ml) 21 and 1·7 μg/ml (range 0·8–3·2 μg/ml) 39.
Overall, the MASP and MAp44 levels we measured in the present study were in good agreement with the (normal) values reported in earlier studies. We observed that MASP-1 levels were highest in patients with subacute MI and lowest in stroke patients. MASP-2 levels were elevated in patients with CAD, but also in matched controls with normal coronary vessels compared with younger healthy volunteers, whereas patients with cardiovascular events, i.e. acute stroke or subacute MI, had lower MASP-2 levels. Both MASP-1 and MASP-2 levels were not associated with severity of CVD. These results indicate that an acute cardiovascular event can induce changes in MASP levels, which is in agreement with the observation of Zhang et al. 33 that myocardial ischaemia induces complement activation with consumption of MASP-2. Our findings of lower MASP-2 and MASP-1 levels in patients with acute stroke compared with CAD patients are in line with this explanation. Conversely, we found increased MASP-1 levels in patients who had suffered from MI a few days before blood sampling. It remains unclear whether these increased MASP-1 levels are due to the underlying CAD and clustering of cardiovascular risk factors, or whether an acute-phase reaction or counter-regulation with up-regulation of MASP-1 following the acute MI has led to increased plasma levels. Intriguingly, levels of MASP-3 were unaltered in our study and did not correlate with MASP-1 levels, although they are both expressed from the same gene, MASP1, confirming the distinct regulation and expression of MASP-1 and MASP-3 40. MAp44 showed cardioprotective and anti-atherothrombotic effects in two mouse models 23. In our patients with CVD, we did not see any differences in MAp44 plasma levels and MAp44 was not associated with a lower degree of disease severity. MAp44 even showed positive correlations with some cardiovascular risk factors. If MAp44 plasma levels reflected the observed cardioprotective effects, inverse correlations with risk factors might have been expected. As MAp44 is highly expressed in the heart 21, we therefore assume that the observed cardioprotective effects must be local effects which are not related to circulating MAp44 plasma levels.
We found several, mainly weak, associations between MASP or MAp44 levels and some cardiovascular risk factors. Interestingly, the associations we observed in patients with stable CAD were lost, or at least not present, in patients who were in the subacute phase of MI. This may support the above hypothesis that changes in MASP levels have occurred during the acute event overriding the status before the acute event. Although MASP-3 was not altered by CVD, it showed associations with cardiovascular risk factors such as obesity, triglyceride and PAI-1 levels, which are important components of the metabolic or insulin resistance syndrome 45. Recent in-vitro experiments identified insulin-like growth factor-binding protein 5 (IGFBP-5), a regulator of insulin-like growth factor 1 (IGF-1), as a possible substrate for MASP-3 46, and the IGF-1 axis may have a role in CVD 47,48. Whether there is a (patho-)physiological relationship between MASP-3 and the IGF-1 axis remains to be investigated.
In summary, we present evidence that circulating plasma levels of MASP-1 and MASP-2, but not MASP-3 and MAp44, are altered in CVD, and all three MASPs and MAp44 are associated with some cardiovascular risk factors. Larger studies are needed to confirm our data, to elucidate the underlying pathophysiological mechanisms, and to conclude whether MASPs and MAp44 may be novel targets in the diagnosis, prevention and treatment of CVD.
Acknowledgments
This work was supported by a grant awarded to V.S. by the OPO Foundation, Zurich, Switzerland. S.T. was supported by the Danish Council for Independent Research, Medical Sciences and the Novo Nordisk Foundation.
Disclosure
The authors have no conflict of interest to declare.
References
- 1.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter AM. Inflammation, thrombosis and acute coronary syndromes. Diab Vasc Dis Res. 2005;2:113–121. doi: 10.3132/dvdr.2005.018. [DOI] [PubMed] [Google Scholar]
- 3.Oksjoki R, Kovanen PT, Meri S, et al. Function and regulation of the complement system in cardiovascular diseases. Front Biosci. 2007;12:4696–4708. doi: 10.2741/2419. [DOI] [PubMed] [Google Scholar]
- 4.Speidl WS, Kastl SP, Huber K, et al. Complement in atherosclerosis: friend or foe? J Thromb Haemost. 2011;9:428–440. doi: 10.1111/j.1538-7836.2010.04172.x. [DOI] [PubMed] [Google Scholar]
- 5.Frauenknecht V, Schroeder V. Complement – a phylogenetically old system as a new player in the development of atherosclerosis [in German] Hämostaseologie. 2012;32:276–285. doi: 10.5482/ha-1191. [DOI] [PubMed] [Google Scholar]
- 6.Banz Y, Rieben R. Role of complement and perspectives for intervention in ischemia-reperfusion damage. Ann Med. 2012;44:205–217. doi: 10.3109/07853890.2010.535556. [DOI] [PubMed] [Google Scholar]
- 7.Markiewski MM, Nilsson B, Nilsson Ekdahl K, et al. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Amara U, Flierl MA, Rittirsch D, et al. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricklin D, Hajishengallis G, Yang K, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen S, Selman L, Palaniyar N, et al. Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity. J Immunol. 2010;185:6096–6104. doi: 10.4049/jimmunol.1002185. [DOI] [PubMed] [Google Scholar]
- 11.Yongqing T, Drentin N, Duncan RC, et al. Mannose-binding lectin serine proteases and associated proteins of the lectin pathway of complement: two genes, five proteins and many functions? Biochim Biophys Acta. 2012;1824:253–262. doi: 10.1016/j.bbapap.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Degn SE, Jensenius JC, Thiel S. Disease-causing mutations in genes of the complement system. Am J Hum Genet. 2011;88:689–705. doi: 10.1016/j.ajhg.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidmose RT, Laursen NS, Dobó J, et al. Structural basis for activation of the complement system by C4 cleavage. Proc Natl Acad Sci USA. 2012;109:15425–15430. doi: 10.1073/pnas.1208031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degn SE, Jensen L, Hansen AG, et al. Mannan-binding lectin-associated serine protease (MASP)-1 is crucial for lectin pathway activation in human serum, whereas neither MASP-1 nor MASP-3 is required for alternative pathway function. J Immunol. 2012;189:3957–3969. doi: 10.4049/jimmunol.1201736. [DOI] [PubMed] [Google Scholar]
- 15.Héja D, Kocsis A, Dobó J, et al. Revised mechanism of complement lectin-pathway activation revealing the role of serine protease MASP-1 as the exclusive activator of MASP-2. Proc Natl Acad Sci USA. 2012;109:10498–10503. doi: 10.1073/pnas.1202588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirmaci A, Walsh T, Akay H, et al. MASP1 mutations in patients with facial, umbilical, coccygeal, and auditory findings of Carnevale, Malpuech, OSA, and Michels syndromes. Am J Hum Genet. 2010;87:679–686. doi: 10.1016/j.ajhg.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rooryck C, Diaz-Font A, Osborn DP, et al. Mutations in lectin complement pathway genes COLEC11 and MASP1 cause 3MC syndrome. Nat Genet. 2011;43:197–203. doi: 10.1038/ng.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skjoedt MO, Palarasah Y, Munthe-Fog L, et al. MBL-associated serine protease-3 circulates in high serum concentrations predominantly in complex with ficolin-3 and regulates ficolin-3 mediated complement activation. Immunobiology. 2010;215:921–931. doi: 10.1016/j.imbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Dahl MR, Thiel S, Matsushita M, et al. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity. 2001;15:127–135. doi: 10.1016/s1074-7613(01)00161-3. [DOI] [PubMed] [Google Scholar]
- 20.Iwaki D, Kanno K, Takahashi M, et al. The role of mannose-binding lectin-associated serine protease-3 in activation of the alternative complement pathway. J Immunol. 2011;187:3751–3758. doi: 10.4049/jimmunol.1100280. [DOI] [PubMed] [Google Scholar]
- 21.Degn SE, Hansen AG, Steffensen R, et al. MAp44, a human protein associated with pattern recognition molecules of the complement system and regulating the lectin pathway of complement activation. J Immunol. 2009;183:7371–7378. doi: 10.4049/jimmunol.0902388. [DOI] [PubMed] [Google Scholar]
- 22.Skjoedt MO, Roversi P, Hummelshøj T, et al. Crystal structure and functional characterization of the complement regulator mannose-binding lectin (MBL)/ficolin-associated protein-1 (MAP-1) J Biol Chem. 2012;287:32913–32921. doi: 10.1074/jbc.M112.386680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlov VI, Skjoedt MO, Tan YS, et al. Endogenous and natural complement inhibitor attenuates myocardial injury and arterial thrombogenesis. Circulation. 2012;126:2227–2235. doi: 10.1161/CIRCULATIONAHA.112.123968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajela K, Kojima M, Ambrus G, et al. The biological functions of MBL-associated serine proteases (MASPs) Immunobiology. 2002;205:467–475. doi: 10.1078/0171-2985-00147. [DOI] [PubMed] [Google Scholar]
- 25.Krarup A, Wallis R, Presanis JS, et al. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS ONE. 2007;2:e623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krarup A, Gulla KC, Gál P, et al. The action of MBL-associated serine protease 1 (MASP1) on factor XIII and fibrinogen. Biochim Biophys Acta. 2008;1784:1294–1300. doi: 10.1016/j.bbapap.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Gulla KC, Gupta K, Krarup A, et al. Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot. Immunology. 2010;129:482–495. doi: 10.1111/j.1365-2567.2009.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hess K, Ajjan R, Phoenix F, et al. Effects of MASP-1 of the complement system on activation of coagulation factors and plasma clot formation. PLoS ONE. 2012;7:e35690. doi: 10.1371/journal.pone.0035690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Bonte LR, Pavlov VI, Tan YS, et al. Mannose-binding lectin-associated serine protease-1 is a significant contributor to coagulation in a murine model of occlusive thrombosis. J Immunol. 2012;188:885–891. doi: 10.4049/jimmunol.1102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trendelenburg M, Theroux P, Stebbins A, et al. Influence of functional deficiency of complement mannose-binding lectin on outcome of patients with acute ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J. 2010;31:1181–1187. doi: 10.1093/eurheartj/ehp597. [DOI] [PubMed] [Google Scholar]
- 31.Osthoff M, Katan M, Fluri F, et al. Mannose-binding lectin deficiency is associated with smaller infarction size and favorable outcome in ischemic stroke patients. PLoS ONE. 2011;6:e21338. doi: 10.1371/journal.pone.0021338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellbin LG, Bjerre M, Thiel S, et al. Complement activation and prognosis in patients with type 2 diabetes and myocardical infarction. Diabetes Care. 2012;35:911–917. doi: 10.2337/dc11-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, Hou YJ, Cavusoglu E, et al. MASP-2 activation is involved in ischemia-related necrotic myocardial injury. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.11.032. http://dx.doi.org/10.1016/j.ijcard.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cervera A, Planas AM, Justicia C, et al. Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS ONE. 2010;5:e8433. doi: 10.1371/journal.pone.0008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroeder V, Chatterjee T, Mehta H, et al. Thrombin activatable fibrinolysis inhibitor (TAFI) levels in patients with coronary artery disease investigated by angiography. Thromb Haemost. 2002;88:1020–1025. [PubMed] [Google Scholar]
- 36.Schroeder V, Ortner E, Mono ML, et al. Coagulation factor XIII activation peptide and subunit levels in patients with acute ischemic stroke: a pilot study. Thromb Res. 2010;126:e122–127. doi: 10.1016/j.thromres.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 38.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 39.Degn SE, Jensen L, Gál P, et al. Biological variations of MASP-3 and MAp44, two splice products of the MASP1 gene involved in regulation of the complement system. J Immunol Methods. 2010;361:37–50. doi: 10.1016/j.jim.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Thiel S, Jensen L, Degn SE, et al. Mannan-binding lectin (MBL)-associated serine protease-1 (MASP-1), a serine protease associated with humoral pattern-recognition molecules: normal and acute-phase levels in serum and stoichiometry of lectin pathway components. Clin Exp Immunol. 2012;169:38–48. doi: 10.1111/j.1365-2249.2012.04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwaeble WJ, Lynch NJ, Clark JE, et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2011;108:7523–7528. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orsini F, Villa P, Parrella S, et al. Targeting mannose-binding lectin confers long-lasting protection with a surprisingly wide therapeutic window in cerebral ischemia. Circulation. 2012;126:1484–1494. doi: 10.1161/CIRCULATIONAHA.112.103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Møller-Kristensen M, Jensenius JC, Jensen L, et al. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Methods. 2003;282:159–167. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Ytting H, Christensen IJ, Thiel S, et al. Biological variation in circulating levels of mannan-binding lectin (MBL) and MBL-associated serine protease-2 and the influence of age, gender and physical exercise. Scand J Immunol. 2007;66:458–464. doi: 10.1111/j.1365-3083.2007.01991.x. [DOI] [PubMed] [Google Scholar]
- 45.Kohler HP. Insulin resistance syndrome: interaction with coagulation and fibrinolysis. Swiss Med Wkly. 2002;132:241–252. doi: 10.4414/smw.2002.09856. [DOI] [PubMed] [Google Scholar]
- 46.Cortesio CL, Jian W. Mannan-binding lectin-associated serine protease 3 cleaves synthetic peptides and insulin-like growth factor-binding protein 5. Arch Biochem Biophys. 2006;449:164–170. doi: 10.1016/j.abb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Fischer F, Schulte H, Mohan S, et al. Associations of insulin-like growth factors, insulin-like growth factor binding proteins and acid-labile subunit with coronary heart disease. Clin Endocrinol (Oxf) 2004;61:595–602. doi: 10.1111/j.1365-2265.2004.02136.x. [DOI] [PubMed] [Google Scholar]
- 48.Conti E, Musumeci MB, De Giusti M, et al. IGF-1 and atherothrombosis: relevance to pathophysiology and therapy. Clin Sci. 2011;120:377–402. doi: 10.1042/CS20100400. [DOI] [PubMed] [Google Scholar]