Abstract
The main aim of the present novel reanalysis of archival data was to compare the time to remission during 12 weeks of treatment of chronic depression following antidepressant medication (n = 218), psychotherapy (n = 216), and their combination (n = 222). Cox regression survival analyses revealed that the combination of medication and psychotherapy produced full remission from chronic depression more rapidly than either of the single modality treatments, which did not differ from each other. Receiver operating characteristic curve analysis was used to explore predictors (treatment group, demographic, clinical, and psychosocial) of remission. For those receiving the combination treatment, the most likely to succeed were those with low baseline depression (24-item Hamilton Rating Scale for Depression [HRSD; M. Hamilton, 1967] score < 26) and those with high depression scores but low anxiety (HRSD ≥ 26 and Hamilton Anxiety Rating Scale [M. Hamilton, 1959] <14). Both profiles were associated with at least 40% chance of attaining full remission. The model did not identify predictors for those receiving medication or psychotherapy alone, and it did not distinguish between the 2 monotherapies. The authors conclude that combined antidepressant medications and psychotherapy result in faster full remission of chronic forms of major depressive disorder.
Keywords: chronic depression, remission, psychotherapy, antidepressant medications, combined treatments
Major depressive disorder (MDD) is highly prevalent and often recurrent or chronic. Considerable evidence now suggests that remission following an episode of major depression is associated with better function and better prognosis compared with response without remission. Among patients followed over 2 years after treatment for MDD, 68% of those achieving response without remission relapsed, compared with only 15% among those in remission at the end of treatment (Pintor, Gasto, Navarro, Torres, & Fananas, 2003); over 4 years, the rates were 92% and 50%, respectively (Pintor, Torres, Navarro, Matrai, & Gasto, 2004). In a cohort of patients with MDD who met criteria for response and were followed for at least 10 years, those with residual symptoms (absence of remission) suffered a recurrence 3 times more rapidly than those who were fully asymptomatic (Judd et al., 1998). In patients with MDD followed for over 18 months, the relative risk of making a suicide attempt was 2.5 times higher for those with residual symptoms compared with those in remission (Sokero et al., 2005). Monthly impairment ratings in patients with MDD followed for approximately 10 years revealed a graded relationship between remission status (grouped as asymptomatic, subthreshold, minor and major depression symptoms) and level of psychosocial impairment, with asymptomatic patients functioning best (Judd et al., 2000). Similar results were recently reported from the multisite Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study for patients followed for 1 year after one, two, and three different acute treatment steps (Rush, Trivedi, et al., 2006).
Thus, remission, as opposed to response, is now considered the preferred endpoint for treatment of major depression (Fava et al., 2003; Frank et al., 1991; Rush, Kraemer, et al., 2006; Rush & Ryan, 2002). Reflecting this preference is the increase in reporting of remission, variously defined, in efficacy studies conducted in the past decade (Beasley, Nilsson, Koke, & Gonzales, 2000; Goldstein et al., 2004; Koran et al., 2001; Montgomery, Huusom, & Bothmer, 2004; Perlis et al., 2004; Rush et al., 2004), including a few that targeted remission as the primary outcome (Entsuah, Huang, & Thase, 2001; Nelson, Mazure, Jatlow, Bowers, & Price, 2004; Thase, Entsuah, & Rudolph, 2001; Trivedi et al., 2006). Recommendations regarding optimal outcomes in depression research also stress the importance of remission as the primary outcome. These include, among others, the Agency for Health Care Policy and Research (Agency for Health Care Policy and Research, 1993), the British Association for Psychopharmacology (Anderson, Nutt, & Deakin, 2000), and, more recently, the American College of Neuropsychopharmacology (ACNP) Task Force on Response and Remission in Major Depressive Disorder (Rush, Kraemer, et al., 2006). The most recent recommendations propose that remission be defined by a minimal level of symptom severity for 3 consecutive weeks (Rush, Kraemer, et al., 2006). This definition is particularly relevant for patients with chronic depression for whom waxing and waning of symptoms are common.
The degree of suffering and disability associated with MDD makes it important to identify treatments that are associated with more rapid onset of remission. Therefore, the main aim of the present novel reanalysis of archival data is to compare the time to remission during 12 weeks of acute phase treatments of chronic depression following two monotherapies (antidepressant medication and psychotherapy) and their combination.
Because most depressed patients do not achieve remission, it is important to identify predictors (moderators) of remission. To date, very few studies have examined predictors of remission (Bosworth, McQuoid, George, & Steffens, 2002; Trivedi et al., 2006), though many have examined predictors of other outcomes, with mixed results. One factor that makes comparison of results across studies difficult is variability in the specific target outcome. Target outcome in past research included symptom reduction, response (traditionally defined as 50% reduction in symptom severity), remission (variously defined), relapse, recurrence, and improved quality of life. It is possible that predictors of these different outcomes are indeed different. For example, Moses, Leuchter, Cook, and Abrams (2006) examined a variety of clinical variables and presence of precipitating life events and concluded that predictors of symptomatic change should be distinguished from predictors of change in quality of life. Similarly, predictors of remission may be different than predictors of other outcomes that have been previously studied.
In sum, there is little research on predictors of remission in general and a virtual absence of studies on predictors of remission in chronic depression. As generalization from research on predictors of other depression outcomes and in samples of patients with nonchronic depression is questionable, we have taken a first step toward addressing this gap. Thus, our secondary aim was to explore potential predictors of remission from chronic depression during the acute phase of treatment. Taking advantage of this well-characterized sample, in the present predictor analysis we explore a number of potential moderators (predictors), including demographics, clinical features, early childhood adversity, psychological variables, and social functioning. We selected receiver operating characteristic curve (ROC) analysis for exploring predictors because it is a hypothesis-generating approach, which is particularly appropriate where insufficient data exist upon which to base a priori predictions (Kraemer, Frank, & Kupfer, 2006). Moreover, ROC is an empirically driven, nonparametric technique that offers more flexibility and potentially greater clinical relevance than regression analysis, as it identifies specific thresholds so that interaction between predictors can be more meaningfully interpreted.
Method
This report is a reanalysis of archival data from the acute treatment phase (12 weeks) of a large (n = 681) multicenter chronic depression study (Keller et al., 2000) in which participants were randomized to one of the following three treatments: (1) the antidepressant nefazodone (MED, n = 226), (2) cognitive-behavioral analysis system of psychotherapy (CBASP, n = 228), and (3) combined nefazodone and CBASP (COMB, n = 227). Figure 1 describes the flow of participants from screening to the analyzable sample. This report is based on data from 656 participants who had provided data for at least one postrandomization visit (218 in MED, 216 in CBASP, and 222 in MED + CBASP). The institutional review boards of each of the 12 participating academic institutions approved the study protocol. All participants gave written informed consent before study entry.
Figure 1.
Flow of participants. MED = antidepressant nefazodone; CBASP = cognitive-behavioral analysis system of psychotherapy.
Participants
Participants were adult outpatients (65.4% female) between the ages of 18 and 75 years (mean age = 43.5 years, SD = 10.7). Eligible participants had a score equal to or greater than 20 on the 24-item Hamilton Rating Scale for Depression (HRSD; Hamilton, 1967), met criteria for a major depressive episode (MDE) as determined by the Structured Clinical Interview for DSM–IV (SCID) Axis I Disorders (First, Spitzer, Gibbon, & Williams, 1994), and had one of the following three kinds of chronic mood disorder: (1) Criteria for MDE were met continuously for at least 2 years, with no antecedent dysthymia; (2) the MDE was superimposed on antecedent dysthymia; or (3) the MDE was recurrent, with incomplete interepisode recovery, and lasted at least 2 years. Participants were excluded if they had any other primary Axis I Diagnostic and Statistical Manual of Mental Disorders (4th ed.; American Psychiatric Association, 1994) diagnosis, organic mental disorder, or unstable medical condition. Additional details regarding the exclusion criteria and the larger sample can be found in Keller et al. (2000).
Treatments
Pharmacotherapy consisted of open-label nefazodone in two divided doses. The initial dose of 200 mg per day during the 1st week was titrated with incremental dose adjustments of 100 mg per day each week up to 600 mg per day until maximum efficacy and tolerability were achieved. To remain in the study, patients had to have reached a minimum dose of 300 mg per day by Week 3. Medication management followed a published manual (Fawcett, Epstein, Fiester, Elkin, & Autry, 1987) and was limited to 15–20 min. Visits were conducted weekly during the 1st month and biweekly thereafter.
Psychotherapy consisted of CBASP, which is based on an integrative model of psychotherapy that combines behavioral, cognitive, psychodynamic, and interpersonal procedures. CBASP is focused on improving patients’ interpersonal effectiveness by helping them understand the relationship between their own thoughts and behaviors and the outcomes they produce (McCullough, 2000). CBASP’s core procedure is situational analysis (SA). Each SA consists of a detailed analysis of a discrete interpersonal encounter (situation) that the patient identifies as stressful.
During the acute phase of treatment, participants received 16–20 sessions of CBASP, which were conducted twice a week during Weeks 1–4 and weekly through Week 12. Sessions could be held twice a week during Weeks 5–8 if the patient did not demonstrate mastery of the SA during the first session of that week (on the basis of the therapist’s rating of the patient’s mastery of SA on the Patient Performance Rating Form; Manber et al., 2003; McCullough, 2000).
Measures
The SCID Axis I Disorders (First et al., 1994) was used for determining psychiatric diagnosis at screening. The main outcome measure was the 24-item interview-administered HRSD (HRSD-24). Interviewers’ prompts were standardized within and across sites. All raters were certified in the administration of the HRSD and were blind to treatment condition. HRSD interviews were conducted at baseline and after 1, 2, 3, 4, 6, 8, 10, and 12 weeks of treatment.
The recent ACNP recommendations (Rush, Kraemer, et al., 2006, p. 1846) for defining remission states that “3 consecutive weeks must pass, during which each week is characterized by the virtual absence of depressive symptoms.” In the absence of SCID postrandomization data, we relied on the 24-item HRSD scores to define remission. Following past recommendations and conventions (Frank et al., 1991; Rush, Kraemer, et al., 2006), a participant was classified as remitted if her/his HRSD score was ≤7 during any 3 consecutive weeks. As the HRSD evaluates symptoms during the week preceding the interview, and because the HRSD was administered biweekly during the second half of the trial, the requirement of 3 consecutive weeks with HRSD scores below threshold used extrapolation. For example, if the patient’s HRSD score was ≤7 at both Week 6 and Week 8, we assumed that had an interview been conducted at Week 7, the HRSD score would have been ≤7. The time to remission was defined as the 1st week that remission was attained. Data were censored at the time of the last available information. Thus, we determined remission status for all participants in the sample using all available data. The ACNP recommendations further stated that “remission can end only (a) with a return of the index MDE (i.e., a relapse) or (b) with a new MDE (i.e., a recurrence)” (Rush, Kraemer, et al., 2006, p. 1846). We have tested whether those thus classified as remitters have subsequently lost their remission status within the acute phase of treatment, defining relapse conservatively as the presence of a 4-week period during which the HRSD score was 16 or above. Given that typical mean 17-item HRSD in ambulatory samples ranges from 18 to 22 (Thase, 2001), this cutoff is conservative. (As recurrence can be ascertained only after at least 4 months of remission, loss of remission due to loss of recovery was not relevant in the context of this study.) We found that none of the participants who achieved remission during the acute phase subsequently lost this status during the acute phase.
Predictor measures included the following 13 variables grouped by domain: (1) five demographic variables: age, gender, Caucasian race, marital status (in a cohabitating relationship or not), and employment status (employed or unemployed); (2) four clinical features: baseline levels of depression symptom severity as measured by the clinician rated HRSD-24, baseline levels of anxiety symptom severity as measured by the clinician-rated Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959), age of onset of the first depressive episode, and the duration of the current depressive episode; (3) presence or absence of early life adverse events in any of the following four domains: parental loss, physical abuse, sexual abuse, and neglect, as measured by the Childhood Trauma Scale adapted from Lizardi et al. (1995); (4) psychological features: the total score of the Attributional Style Questionnaire for Negative Events (Peterson et al., 1982), which is a measure of causal attributions for negative events; (5) social function, as measured by the Social Adjustment Scale (Paykel, Weissman, Prusoff, & Tonks, 1971; note that lower scores on the Social Adjustment Scale and its subscales represent lesser role dysfunction, i.e., better functioning); and (6) treatment group, coded as three dummy variables. To avoid multicollinearity, we did not include subscales in the model.
Analysis
Group differences in the time to remission were tested with Cox proportional hazard analysis with treatment group and site as covariates. The time to the event was defined as the time to remission for those who remitted and with censoring at the time of the last available observation for those who did not remit. An omnibus test with all three groups in the model was first assessed, followed by the three pairwise comparisons.
We used ROC analysis (Kraemer, 1992) to identify patient characteristics that predicted remission using the ROC4 program (found at http://mirecc.stanford.edu and described in Kraemer, 1992). ROC is a nonparametric technique that can evaluate multiple potential predictors and does not make assumptions about colinearity, additivity, or homoscedasticity of the predictors that are required of linear models. Another unique feature of the ROC program is that it allows the user to designate the criterion for identifying the best criterion variable by adjusting the weight in kappa to optimize sensitivity (i.e., emphasis placed on avoiding false negatives), specificity (i.e., emphasis placed on avoiding false positives), or efficiency (i.e., equal emphasis placed on both types of errors). The decision to adjust the weighted kappa is based on clinical importance of false negatives versus false positives. For each independent variable (IV), the program searches for a cut-point that optimizes the balance between sensitivity and specificity for predicting the outcome of interest (i.e., remission). Once the best predictor (and optimum cut-point) is identified, the group with the success criterion is tested against a stopping rule (cut-point significance set at p < .01 level). If it fails the stopping rule, no further action is taken. If the group passes the rule, the sample is divided into two subgroups on the basis of the predictor variable. The analyses are then restarted for each of the two subgroups in an iterative process until the stopping rule is encountered (either a subgroup has less than 10 participants or the optimal test is not statistically significant at the .01 level). In the present study, the outcome measure achieved remission status. The predictor variables are listed in the Measures section. Rather than identifying predictors separately for each treatment, we entered membership in each of the three treatment groups as independent predictors, allowing the model to determine the relevance of treatment group.
Results
Sample Characteristics
The demographic characteristics of the sample and baseline levels of all other predictor variables are depicted in Table 1, organized by treatment group. There were no statistically significant differences among the groups for any of these variables.
Table 1.
Baseline Characteristics of Predictors by Treatment Group
| Variable | MED (n = 218) | MED + CBASP (n = 222) | CBASP (n = 216) | Test type |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 42.5 ± 11.0 | 44.3 ± 10.3 | 43.6 ± 10.6 | F = 1.5; p = .22 |
| Female gender (%) | 65 | 69 | 62 | χ2 = 2.7; p = .26 |
| Caucasian race (%) | 88 | 92 | 91 | χ2 = 3.1; p = .21 |
| Married/cohabitating (%) | 44 | 43 | 43 | χ2 = 0.0; p = .98 |
| Employed (%) | 86 | 83 | 83 | χ2 = 1.1; p = .58 |
| Clinical features | ||||
| HRSD-24 | 27.9 ± 5.2 | 28.2 ± 5.1 | 27.7 ± 4.7 | F = 0.5; p = .58 |
| HAM-A | 17.4 ± 6.1 | 18.7 ± 6.2 | 18.2 ± 6.1 | F = 2.6; p = .07 |
| Age of onset of first MDD (years) | 25.8 ± 13.0 | 27.2 ± 13.1 | 27.5 ± 13.4 | F = 1.1; p = .34 |
| Current MDE duration (years) | 7.6 ± 9.1 | 8.0 ± 9.3 | 8.0 ± 10.3 | F = 0.2; p = .85 |
| Childhood trauma history (%) | 30 | 36 | 27 | χ2 = 3.8; p = .15 |
| Psychological features | ||||
| ASQ | 4.9 ± .8 | 5.0 ± .8 | 4.9 ± .8 | F = 1.1; p = .32 |
| Social functioning | ||||
| SAS | 2.5 ± .4 | 2.6 ± .4 | 2.5 ± .4 | F = 0.7; p = .49 |
Note. MED = antidepressant nefazodone; CBASP = cognitive-behavioral analysis system of psychotherapy; HRSD-24 = 24-item Hamilton Rating Scale for Depression; HAM-A = Hamilton Anxiety Rating Scale; MDD = major depressive disorder; MDE = major depressive episode; ASQ = Attribution Style Questionnaire for Negative Events; SAS = Social Adjustment Scale. There were no statistically significant group differences on any of these variables.
Primary Outcome
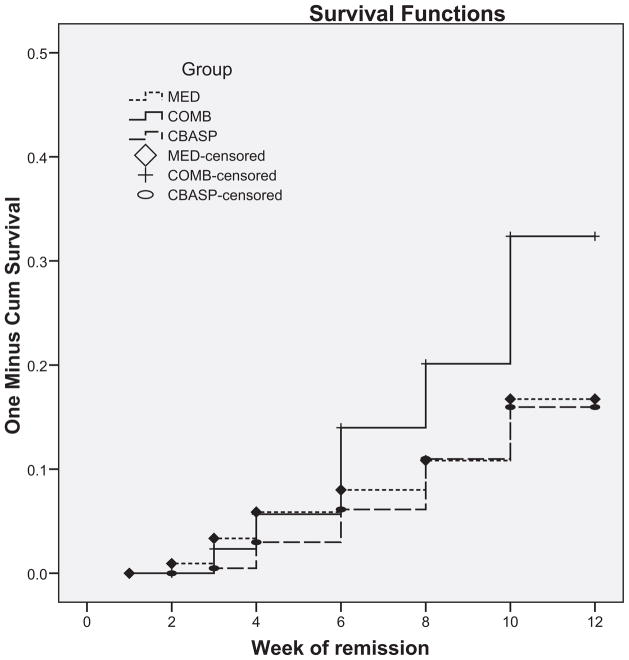
A total of 19.1% of the sample achieved remission. This includes 31 of the 218 participants in MED (14.2%), 30 of the 216 participants in CBASP (13.9%), and 64 of 222 participants in MED + CBASP (28.9%). The number to treat effect size (NNT) between MED and CBASP is 301.8, a very small effect size; the NNT for MED versus COMB is 6.8, and for CBASP versus COMB it is 6.7, both moderate effect sizes. The Cox proportional hazard survival analysis revealed significant group differences in the time to remission, χ2 = 38.2, p < .0001, with the COMB group demonstrating more rapid time to remission than both MED, Exp(B) = .50, 95% confidence interval (CI) = .33–.77, Wald = 9.8, p = .002, and CBASP, Exp(B) = .46, 95% CI = .30–.71, Wald = 12.4, p < .001. The effect for site (11 levels) approached significance, with Wald = 19.2, p = .058. Figure 2 depicts the time to remission in the three groups at each assessment week.
Figure 2.
The time to remission in the three groups at each assessment week (Kaplan–Meir survival plot). MED = antidepressant nefazodone; CBASP = cognitive-behavioral analysis system of psychotherapy; COMB = combined nefazodone and CBASP. There were significant group differences in the time to full remission, χ2 = 15.3, p < .0001, with the COMB group remitting sooner than the each therapy alone.
Secondary (Exploratory) Outcome
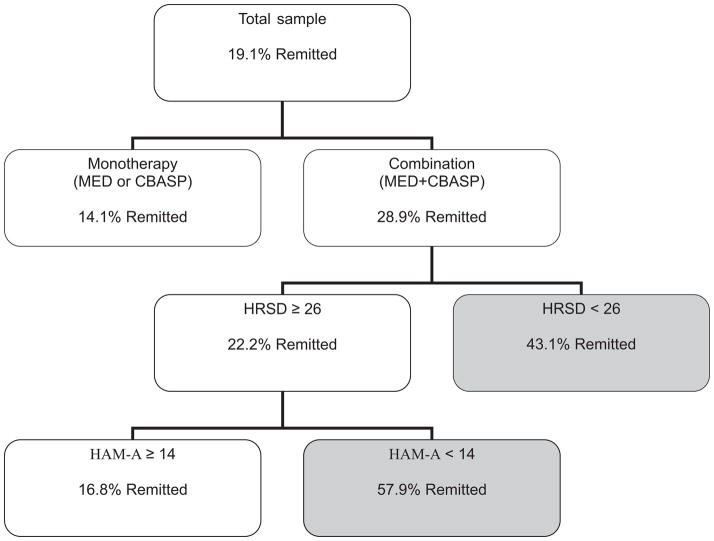
The first step of the ROC analysis identified receiving combination treatment as the best predictor, χ2 = 19.7, p < .001. For those receiving the combination treatment, the most likely to meet criteria for remission were those with low depression scores (HRSD < 26); 41.1% of those in this subgroup remitted. Furthermore, the ROC analysis revealed that among those with HRSD ≥ 26, the most likely to remit were those with low anxiety (HAM-A < 20), 57.9% of whom remitted. Those with high depression symptom severity (HRSD ≥ 26) and high anxiety (HAM-A ≥ 20) had only a 16.8% chance of remitting. For those receiving the mono-therapy, none of the predictors entered were significant. Table 2 depicts the proportion of participants in each treatment group by the three patterns above (low HRSD; high HRSD and low HAM-A; and high HRSD and high HAM-A). Figure 3 summarizes the results of the ROC analysis, depicted as a binary tree.
Table 2.
Proportion of Participants in Each Treatment Group by the Three Patterns of Low Depression, High Depression and Low Anxiety, and High Depression and High Anxiety
| Variable | MED | COMB | CBASP |
|---|---|---|---|
| Low depression (%) | 20.3 | 43.1 | 15.9 |
| High depression and low anxiety (%) | 24.1 | 57.9 | 11.5 |
| High depression and high anxiety (%) | 6.4 | 16.8 | 13.4 |
Note. MED = antidepressant nefazodone; CBASP = cognitive-behavioral analysis system of psychotherapy; COMB = combined nefazodone and CBASP; low depression = 24-item Hamilton Rating Scale for Depression (HRSD24) < 26; high depression and low anxiety = HRSD24 ≥ 26 and Hamilton Anxiety Rating Scale (HAM-A) < 14; high depression and high anxiety = HRSD24 ≥.26 and HAM-A ≥ 14.
Figure 3.
Results of the receiver operating characteristic curve (ROC) analysis depicted as a binary tree. The tree is truncated when the stopping rule for the ROC is reached. The shaded branches (paths) represent profiles that were associated with 20% or greater rate of remission. Each branching point represents a variable that emerged as the best predictor at a given branching point. Each box includes the rule and the percentage of participants who remitted. MED = antidepressant nefazodone; CBASP = cognitive-behavioral analysis system of psychotherapy; HRSD = Hamilton Rating Scale for Depression; HAM-A = Hamilton Anxiety Rating Scale.
Discussion
Remission is now widely endorsed as the goal of the initial or acute phase of depression treatment, although it has not been commonly used as a primary outcome measure in depression trials. Time to remission, a measure of the speed with which the treatment reduces suffering, is rarely reported as an outcome. The main results of this study indicate that the combination of medication and psychotherapy leads to faster remission from chronic depression than either of the single modality treatments, which did not differ from each other. Similar to the present study, the Treatment of Adolescents with Depression Study (TADS; Kratochvil et al., 2006) examined the time to response and found that, as in the present study, the combination of medication and psychotherapy accelerates response compared with psychotherapy alone. Although past research evaluating the relative efficacy of combined treatment relative to its single components has yielded mixed results, large scale studies and pooled analyses have found superior outcome with combined treatments, particularly for more severe or more difficult to treat forms of depression (de Maat, Dekker, Schoevers, & de Jonghe, 2007; Hollon et al., 2005; Keller et al., 2000; Kennard et al., 2006; Simon, Pilling, Burbeck, & Goldberg, 2006; Thase et al., 1997). In a previous analysis of the same data set, we reported that the combination treatment was associated with greater reduction of symptom severity and higher response and remission rates at study exit than monotherapy (Keller et al., 2000). In the current reanalysis, we focused on time to remission, using contemporary guidelines for defining remission and for the statistical analysis and for the reporting of remission (Rush, Kraemer, et al., 2006).
The secondary finding, which is based on the ROC analysis, confirmed that the major predictor of success was combination therapy. The ROC program went on to identify predictors for remission for those receiving combined treatment but did not identify significant predictors for those receiving monotherapy. Two profiles emerged as the best predictors of remission for those receiving combination treatment and were associated with 43% and 58% chance of attaining full remission. For those receiving combination treatment, the most likely to remit were those with low baseline depression (HRSD24 < 26) and those with high depression but low anxiety ((HRSD24 ≥ 26 and HAM-A < 14). High anxiety/high depression appeared to interact with group in the analysis, but its predictive power seems to go beyond just the COMB group, as indicated by the similar remission rate in the MED group and by the fact that the results replicated in the sample as a whole—that is, when we tested the model without group as a predictor.
Not all studies that examined anxiety (either the presence of a comorbid anxiety disorder or the severity of anxiety symptoms) found it to be a significant predictor of outcomes in MDD. In the context of chronic depression, which is most relevant to the present study, the presence of comorbid anxiety disorders was examined and found not to be a significant predictor of remission or response (Hirschfeld et al., 1998; Kocsis et al., 1989). A few studies of clinic-based samples, which are likely to have included a large number of patients with chronic depression, did find an association between higher baseline anxiety (variously defined) and poorer short- and long-term outcomes (Enns & Cox, 2005; Fava et al., in press; Parker, Wilhelm, Mitchell, & Gladstone, 2000; Trivedi et al., 2006). This suggests that the severity of anxiety symptoms might be more relevant to remission from depression than the presence of a comorbid anxiety disorder. The present study suggests further that high anxiety level might be particularly detrimental for those with high depression symptom severity. This finding, if replicated in different samples and different treatments, has clinical significance, as it identified a subgroup of patients with depression for whom current treatments are not optimal and new approaches might need to be developed.
Many factors have been previously examined as predictors of treatment outcome in depression. The advantage of the exploratory ROC approach of this study is that it allows the identification of multiple levels of interactions, seldom considered in hypothesis-testing analyses, and the identification of specific cutoff scores differentiating between those attaining the desired outcome criterion and those not attaining the outcome. This approach can help explain inconsistencies in the literature regarding predictors of outcome. For example, high level of anxiety has been identified as a predictor of poor outcome in some but not all studies of MDD. The current analysis suggests an interaction between anxiety and depression severity. Specifically, we found that the detrimental effect of anxiety on outcome was moderated by depression severity (HRSD24 scores greater than or equal to 26).
There are several limitations to the generalizability our main findings. It is not clear how well the main finding of faster remission with combined treatment compared with single modality treatment will generalize to other antidepressant medications, to other forms of psychotherapy, to nonchronic forms of depression, to more racially diverse samples, and to samples with greater psychiatric and medical comorbidities. In addition, given the archival nature of the data, our definition of remission is not fully consistent with the ACNP Task Force recommendations (Rush, Kraemer, et al., 2006), as it relies on the cutoff score of the HRSD-24 item rather than on the nine criterion symptom domains identified in the Diagnostic and Statistical Manual of Mental Disorders (text rev.; American Psychiatric Association, 2000) for the diagnosis of a MDE. However, both the use of 7 as a threshold criterion for the HRSD and the duration criteria we used are consistent with the recommendations of the ACNP Task Force.
The low rate of remission observed in this study could also hinder the interpretation of our results. Although 29% of the chronically depressed patients who received combination treatment in the present study remitted, a figure comparable with the recent results from the STAR*D trial (Trivedi et al., 2006) in which approximately three quarters of the participants experienced chronic depression, the overall remission rate when all conditions were combined was lower (19%). There is considerable variability in reported rates of MDD remission during acute phase treatment. Such variability is likely due to differences in the way remission was defined (most typically on the basis of the last observation, with different cutoff scores of different measures of symptom severity), the length of the acute phase, the sample studied (chronic depression is associated with lower rates of remission), and the treatments used. The low rates of remission might render the results of the predictor analysis, our secondary aim, unstable. Nevertheless significant predictors emerged only for the combination treatment, which was associated with a higher remission rate. Importantly, an ROC analysis is inherently an exploratory analysis. Therefore, the results cannot be considered definitive before they are subjected to hypothesis testing in a different sample.
Taken together, the results from the present study indicate that combined antidepressant medications and psychotherapy result in more rapid remission of MDD. The exploratory analysis suggests that the advantage of combined treatment over mono-therapy (in terms of remission rates) is limited among those patients with high levels of anxiety. This suggests that there is a need for a focused effort on developing treatments that can improve levels of remission in patients with high levels of depression and high anxiety.
Acknowledgments
This research was supported by Bristol-Myers Squibb (New York).
Contributor Information
Rachel Manber, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine.
Helena C. Kraemer, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine
Bruce A. Arnow, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine
Madhukar H. Trivedi, Department of Psychiatry, University of Texas Southwestern Medical Center
A. John Rush, Department of Psychiatry and Department of Clinical Sciences, University of Texas Southwestern Medical Center.
Michael E. Thase, Department of Psychiatry, University of Pennsylvania
Barbara O. Rothbaum, Department of Psychiatry, Emory University School of Medicine
Daniel N. Klein, Department of Psychology, State University of New York at Stony Brook
James H. Kocsis, Department of Psychiatry, Cornell University Medical College
Alan J. Gelenberg, Department of Psychiatry, University of Arizona College of Medicine
Martin E. Keller, Department of Psychiatry, Brown University
References
- Agency for Health Care Policy and Research. Clinical practice guideline Number 5: Depression in primary care, 2: Treatment of major depression. Rockville, MD: Author; 1993. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Anderson IM, Nutt DJ, Deakin JF. Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 1993 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology. 2000;14(1):3–20. doi: 10.1177/026988110001400101. [DOI] [PubMed] [Google Scholar]
- Beasley CM, Jr, Nilsson ME, Koke SC, Gonzales JS. Efficacy, adverse events, and treatment discontinuations in fluoxetine clinical studies of major depression: A meta-analysis of the 20-mg/day dose. Journal of Clinical Psychiatry. 2000;61(10):722–728. doi: 10.4088/jcp.v61n1003. [DOI] [PubMed] [Google Scholar]
- Bosworth HB, McQuoid DR, George LK, Steffens DC. Time-to-remission from geriatric depression: Psychosocial and clinical factors. American Journal of Geriatric Psychiatry. 2002;10(5):551–559. [PubMed] [Google Scholar]
- de Maat SM, Dekker J, Schoevers RA, de Jonghe F. Relative efficacy of psychotherapy and combined therapy in the treatment of depression: A meta-analysis. European Psychiatry. 2007;22(1):1–8. doi: 10.1016/j.eurpsy.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Enns MW, Cox BJ. Psychosocial and clinical predictors of symptom persistence vs. remission in major depressive disorder. Canadian Journal of Psychiatry. 2005;50(12):769–777. doi: 10.1177/070674370505001206. [DOI] [PubMed] [Google Scholar]
- Entsuah AR, Huang H, Thase ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. Journal of Clinical Psychiatry. 2001;62(11):869–877. doi: 10.4088/jcp.v62n1106. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert J, Balasubramani GK, Wisniewski SR, Carmin CN, et al. Do outpatients with anxious vs. non-anxious major depressive disorder have different treatment outcomes? American Journal of Psychiatry in press. [Google Scholar]
- Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatrics Clinics of North America. 2003;26(2):457–494. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management—Imipramine/placebo administration manual: NIMH Treatment of Depression Collaborative Research Program. Psychopharmacology Bulletin. 1987;23(2):309–324. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MG, Williams JBW. The Structured Clinical Interview for DSM–IV. New York: New York State Psychiatric Institute, Biometrics Research Department; 1994. [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer D, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: Remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Lu Y, Detke MJ, Wiltse C, Mallinckrodt C, Demitrack MA. Duloxetine in the treatment of depression: A double-blind placebo-controlled comparison with paroxetine. Journal of Clinical Psychopharmacology. 2004;24(4):389–399. doi: 10.1097/01.jcp.0000132448.65972.d9. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social Psychology. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Russell JM, Delgado PL, Fawcett J, Friedman RA, Harrison WM, et al. Predictors of response to acute treatment of chronic and double depression with sertraline or imipramine. Journal of Clinical Psychiatry. 1998;59(12):669–675. doi: 10.4088/jcp.v59n1205. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Jarrett RB, Nierenberg AA, Thase ME, Trivedi M, Rush AJ. Psychotherapy and medication in the treatment of adult and geriatric depression: Which monotherapy or combined treatment? Journal of Clinical Psychiatry. 2005;66(4):455–468. doi: 10.4088/jcp.v66n0408. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, et al. Major depressive disorder: A prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. Journal of Affective Disorders. 1998;50(2):97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Zeller PJ, Paulus M, Leon AC, Maser JD, et al. Psychosocial disability during the long-term course of unipolar major depressive disorder. Archives of General Psychiatry. 2000;57(4):375–380. doi: 10.1001/archpsyc.57.4.375. [DOI] [PubMed] [Google Scholar]
- Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, et al. A comparison of nefazodone, cognitive behavioral analysis system of psychotherapy, and their combination for the treatment of chronic depression. New England Journal of Medicine. 2000;342(20):1462–1470. doi: 10.1056/NEJM200005183422001. [DOI] [PubMed] [Google Scholar]
- Kennard B, Silva S, Vitiello B, Curry J, Kratochvil C, Simons A, et al. Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS) Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(12):1404–1411. doi: 10.1097/01.chi.0000242228.75516.21. [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Mason BJ, Frances AJ, Sweeney J, Mann JJ, Marin D. Prediction of response of chronic depression to imipramine. Journal of Affective Disorders. 1989;17(3):255–260. doi: 10.1016/0165-0327(89)90008-6. [DOI] [PubMed] [Google Scholar]
- Koran LM, Gelenberg AJ, Kornstein SG, Howland RH, Friedman RA, DeBattista C, et al. Sertraline versus imipramine to prevent relapse in chronic depression. Journal of Affective Disorders. 2001;65(1):27–36. doi: 10.1016/s0165-0327(00)00272-x. [DOI] [PubMed] [Google Scholar]
- Kraemer HC. Evaluating medical tests: Objective and quantitative guidelines. Newbury Park, CA: Sage; 1992. [Google Scholar]
- Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: Clinical, research, and policy importance. Journal of the American Medical Association. 2006;296(10):1286–1289. doi: 10.1001/jama.296.10.1286. [DOI] [PubMed] [Google Scholar]
- Kratochvil C, Emslie G, Silva S, McNulty S, Walkup J, Curry J, et al. Acute time to response in the Treatment for Adolescents with Depression Study (TADS) Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(12):1412–1418. doi: 10.1097/01.chi.0000237710.73755.14. [DOI] [PubMed] [Google Scholar]
- Lizardi H, Klein DN, Ouimette PC, Riso LP, Anderson RL, Donaldson SK. Reports of the childhood home environment in early-onset dysthymia and episodic major depression. Journal of Abnormal Psychology. 1995;104(1):132–139. doi: 10.1037//0021-843x.104.1.132. [DOI] [PubMed] [Google Scholar]
- Manber R, Arnow B, Blasey C, Vivian D, McCullogh JP, Blalock JA, et al. Patient’s therapeutic skill acquisition and response to psychotherapy, alone or in combination with medication. Psychological Medicine. 2003;33(4):693–702. doi: 10.1017/s0033291703007608. [DOI] [PubMed] [Google Scholar]
- McCullough JPJ. Treatment for chronic depression: Cognitive behavioral analysis system of psychotherapy. New York: Guilford Press; 2000. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Huusom AK, Bothmer J. A randomised study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorder. Neuropsychobiology. 2004;50(1):57–64. doi: 10.1159/000078225. [DOI] [PubMed] [Google Scholar]
- Moses T, Leuchter AF, Cook I, Abrams M. Does the clinical course of depression determine improvement in symptoms and quality of life? Journal of Nervous and Mental Disease. 2006;194(4):241–248. doi: 10.1097/01.nmd.0000207358.15230.80. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Mazure CM, Jatlow PI, Bowers MB, Jr, Price LH. Combining norepinephrine and serotonin reuptake inhibition mechanisms for treatment of depression: A double-blind, randomized study. Biological Psychiatry. 2004;55(3):296–300. doi: 10.1016/j.biopsych.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Parker G, Wilhelm K, Mitchell P, Gladstone G. Predictors of 1-year outcome in depression. Australian and New Zealand Journal of Psychiatry. 2000;34(1):56–64. doi: 10.1046/j.1440-1614.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Weissman M, Prusoff BA, Tonks CM. Dimensions of social adjustment in depressed women. Journal of Nervous and Mental Disease. 1971;152(3):158–172. doi: 10.1097/00005053-197103000-00002. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Iosifescu DV, Alpert J, Nierenberg AA, Rosenbaum JF, Fava M. Effect of medical comorbidity on response to fluoxetine augmentation or dose increase in outpatients with treatment-resistant depression. Psychosomatics. 2004;45(3):224–229. doi: 10.1176/appi.psy.45.3.224. [DOI] [PubMed] [Google Scholar]
- Peterson C, Semmel A, von Baeyer C, Abramson LY, Metalsky GI, Seligman MEP. The Attributional Style Questionnaire. Cognitive Therapy and Research. 1982;6:287–300. [Google Scholar]
- Pintor L, Gasto C, Navarro V, Torres X, Fananas L. Relapse of major depression after complete and partial remission during a 2-year follow-up. Journal of Affective Disorders. 2003;73(3):237–244. doi: 10.1016/s0165-0327(01)00480-3. [DOI] [PubMed] [Google Scholar]
- Pintor L, Torres X, Navarro V, Matrai S, Gasto C. Is the type of remission after a major depressive episode an important risk factor to relapses in a 4-year follow up? Journal of Affective Disorders. 2004;82(2):291–296. doi: 10.1016/j.jad.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, et al. Sequenced Treatment Alternatives to Relieve Depression (STAR*D): Rationale and design. Controlled Clinical Trials. 2004;25(1):119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Ryan ND. Current and emerging therapeutics for depression. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 1081–1095. [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. American Journal of Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Simon J, Pilling S, Burbeck R, Goldberg D. Treatment options in moderate and severe depression: Decision analysis supporting a clinical guideline. British Journal of Psychiatry. 2006;189(6):494–501. doi: 10.1192/bjp.bp.105.014571. [DOI] [PubMed] [Google Scholar]
- Sokero TP, Melartin TK, Rytsala HJ, Leskela US, Lestela-Mielonen PS, Isometsa ET. Prospective study of risk factors for attempted suicide among patients with DSM–IV major depressive disorder. British Journal of Psychiatry. 2005;186(4):314–318. doi: 10.1192/bjp.186.4.314. [DOI] [PubMed] [Google Scholar]
- Thase ME. The clinical, psychosocial, and pharmacoeconomic ramifications of remission. American Journal of Managed Care. 2001;7(Suppl 11):S377–S385. [PubMed] [Google Scholar]
- Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. British Journal of Psychiatry. 2001;178:234–241. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- Thase ME, Greenhouse JB, Frank E, Reynolds CF, III, Pilkonis PA, Hurley K, et al. Treatment of major depression with psychotherapy or psychotherapy–pharmacotherapy combinations. Archives of General Psychiatry. 1997;54(11):1009–1015. doi: 10.1001/archpsyc.1997.01830230043006. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. American Journal of Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]