Abstract
A wideband (WB) aural acoustical test battery of middle-ear status, including acoustic-reflex thresholds (ARTs) and acoustic-transfer functions (ATFs, i.e., absorbance and admittance) was hypothesized to be more accurate than 1-kHz tympanometry in classifying ears that pass or refer on a newborn hearing screening (NHS) protocol based on otoacoustic emissions. Assessment of middle-ear status may improve NHS programs by identifying conductive dysfunction and cases in which auditory neuropathy exists. Ipsilateral ARTs were assessed with a stimulus including four broadband-noise or tonal activator pulses alternating with five clicks presented before, between and after the pulses. The reflex shift was defined as the difference between final and initial click responses. ARTs were measured using maximum likelihood both at low frequencies (0.8–2.8 kHz) and high (2.8–8 kHz). The median low-frequency ART was elevated by 24 dB in NHS refers compared to passes. An optimal combination of ATF and ART tests performed better than either test alone in predicting NHS outcomes, and WB tests performed better than 1-kHz tympanometry. Medial olivocochlear efferent shifts in cochlear function may influence ARs, but their presence would also be consistent with normal conductive function. Baseline clinical and WB ARTs were also compared in ipsilateral and contralateral measurements in adults.
Keywords: acoustic reflex, middle-ear muscle, medial olivocochlear efferent, newborn hearing screening
Introduction
Present clinical methods to detect the acoustic reflex (AR), reviewed in Gelfand (2009), are limited by the relatively high activator levels employed to elicit a reflex shift in the level of a probe tone, the narrow frequency range over which a reflex is measured (typically at a single probe frequency of 0.226 kHz in adults or 1 kHz in infants), and the absence of an objective assessment of the acoustic reflex threshold (ART). The sound level of the activator used to elicit the reflex may be sufficiently high in some ears to cause permanent sensorineural hearing loss (Hunter et al., 1999), a concern that takes on greater significance when AR measurements are performed in infants. This report describes a new technique to measure AR shifts in a wideband (WB) probe signal, using an automated, objective procedure to detect threshold. As a first step in determining the feasibility of these new ART assessment tools, measurements of WB ARTs are described in newborns and adults, and adult WB ARTs are compared to clinically measured ARTs using a 0.226-kHz tone.
Sanford et al. (2009) reported that a WB aural acoustic transfer function (ATF) test, including energy absorbance and acoustic admittance, was more accurate than 1-kHz tympanometry in classifying ears that passed or referred on the basis of a distortion product otoacoustic emission (DPOAE) newborn hearing screening (NHS) exam. The present report builds on the data reported in Sanford et al. by assessing the accuracy with which a WB ART, as either a stand-alone test or as part of a battery composed of ART and ATF tests, classified ears that passed or referred on the NHS test. The development of an ART that can be used with infants may prove useful in programs for early detection of hearing impairment (EDHI). In contrast, current EDHI programs do not include AR testing in initial NHS exams, but AR testing is recommended in follow-up audiological evaluations of infants suspected of having a hearing loss at age > 6 months (JCIH, 2007).
A contralateral ART test based on WB reflectance and admittance was developed (Feeney and Keefe, 1999; Feeney and Keefe, 2001) that enabled the measurement of AR shifts from 0.25–8 kHz. The resulting WB ARTs were detected at activator levels approximately 13 dB lower than traditional ART measurements (Feeney et al., 2003). Unlike traditional measurements in which a clinician subjectively classifies a reflex as present or absent, the WB ART test classifies the response using an objective decision rule. The rule used a magnitude method to determine if the AR shift magnitude exceeded baseline variability and a synchrony method based on correlation to determine if the AR shift was repeatable. Contralateral AR shifts were measured in six-week-old infants and adults using a WB probe and broadband (BBN) activator (Feeney and Sanford, 2005). The AR shifts in ATFs were generally similar in infants and adults above 1 kHz, but infant measurements were noisy below 1 kHz, making them less reliable. Reflexes were more successfully detected in infants when the correlation was calculated from 1–8 kHz and in adults from 0.25–2 kHz.
AR responses have been measured using BBN and 1-kHz activators in 1- to 6-day-old infants; AR shifts were observed in 88% of infants for at least one condition, and ARTs were lower in ipsilateral than contralateral tests (Sprague et al., 1985). The fact that ARTs vary with probe frequency in newborns (McMillan et al., 1985) suggests that a WB test may assist in identifying the most sensitive frequencies at which to measure threshold. As with adults, ARTs for noise are lower than for pure tones (Bennett and Weatherby, 1982; Sprague et al., 1985; Margolis, 1993). The initial absence of an AR response in newborns may help identify the presence of a transient middle-ear condition that resolves within 12 hours of birth (Geddes, 1987). It might be expected that these transient conditions would be associated with a reduced OAE response.
An ipsilateral AR test is preferable to a contralateral test in evaluating newborn infants, particularly in a NHS protocol. A newborn usually sleeps, or lies flat, with the head resting on one side, i.e., with one ear easily accessible to insert a probe into the ear canal, and the other ear in contact with the bed. It would be difficult to perform a bilateral or contralateral test on a newborn with the probe in the ipsilateral ear and an earphone on the contralateral ear.
An ipsilateral WB ART test has been developed for adults using a 4-kHz tonal activator and probe signal presented simultaneously, but in which the probe bandwidth (0.2–2 kHz) was limited at higher frequencies to allow spectral separation between the activator frequency and probe bandwidth (Feeney et al., 2004). No significant differences between WB and clinical ARTs were reported, although the equipment limitation of a maximum activator SPL of 92 dB in the WB test limited the maximum activator level at which thresholds could be compared. Another ipsilateral AR test for adults used two identical tone bursts separated by 110 ms, such that the initial burst elicited an AR shift detected by calculating the difference waveform between the tone bursts (Neumann et al., 1996). A WB-elicitor form of this test was developed in which each of the two identical brief signals had a 0.1–8 kHz bandwidth, and the presence of a sufficiently large phase coherence at 1 kHz served as a synchrony test to detect any AR effect (Müller-Wehlau et al., 2005). An ipsilateral WB ART test has been evaluated in adults and children (mean age 57 months) in which a click train was presented before and after a tonal or BBN activator (Schairer et al., 2007). The mean click difference waveform in the two conditions assessed the AR shift, and the ART was measured using an AR magnitude test. ARTs were lower in adults for the WB probe signal than for clinical tests using a tonal probe signal, but no difference was found in children. The absence of a synchrony test in Schairer et al. may have limited the WB ART test performance.
Previous WB AR studies have been performed at ambient pressure in the ear canal. This is a limitation in testing older children and adults, inasmuch as clinical AR testing is usually performed by pressurizing the ear canal to an air pressure equal to the tympanometric peak pressure (TPP); this tends to maximize the magnitude of the AR shift, resulting in a lower ART than that measured at ambient pressure (Gelfand, 2009). This is not necessarily the case for AR testing in newborns, because the effect of pressurizing the ear canal changes the viscoelastic properties of the ear-canal wall (Holte, 1990; Keefe et al., 1993; Qi et al., 2006; Qi et al., 2008). For this reason, it is of interest to evaluate AR tests performed at ambient pressure in newborns.
In a NHS protocol in a well-baby nursery, the JCIH (2007) recommends the use of either or both OAE and automated auditory brainstem response (ABR) tests. In a neonatal intensive care unit (NICU), JCIH recommends an ABR test to identify neonates at risk for neural hearing loss, i.e., auditory neuropathy/auditory dyssynchrony (AN/AD). The ABR is abnormal in AN/AD patients whereas OAEs can be in the normal range (Starr et al., 1996), and it is thought that AN/AD is more common among graduates from NICUs. Neural-conduction disorders underlying patients with AN/AD also affect the AR, e.g., all 10 AN/AD patients in Starr et al. (1996) had an absence of AR shifts, and all 136 AN/AD patients in Berlin et al. (2005) had absent or elevated ARTs. In a feasibility study of highrisk infants screened in a NICU, an AR test with a 2 kHz activator tone and 0.8 kHz probe tone identified all 10 ears that failed an ABR screening, an AR response was present in 83 of 86 ears that passed the ABR screening, while AR data were not obtained due to patient movement in 22 ears and equipment failure in 9 ears (Hirsch, 1992). Thus, an initial NHS test battery including an OAE and an ART test may be advantageous in identifying increased risk for either cochlear or neural hearing loss. An absent or elevated ART might provide a rationale for a follow-up ABR test, even if the OAE response met screening criteria. The first step in considering such applications to NHS programs is to demonstrate that ART testing is feasible and accurate in infants. If feasible and accurate, an objective WB ART test might also be used in follow-up diagnostic exams in older infants in EDHI programs, and in screening and diagnostic testing of older children and adults.
The WB AR test used in the current study had the following properties to address limitations found in previous studies and issues specific to NHS: (1) ipsilateral and contralateral testing was used in adults while ipsilateral only testing was used in newborns, (2) ambient-pressure or TPP test types, (3) WB probe signal, (4) choice of BBN or tonal activators, (5) objective assessment to detect AR shift based on both magnitude and synchrony tests at multiple levels, (6) automatic calculation of ART by combining responses across levels, (7) and a sufficiently short test time (32 s) for use in NHS programs.
Materials and methods
The ear-canal probe and WB tympanometry hardware used for AR and ATF tests were provided by Interacoustics as described in Liu et al. (2008). A calibration provided by the manufacturer corrected for magnitude and phase variability in microphone sensitivity, and was used to convert the output voltage from the microphone into pressure. Data were recorded using custom software running on a personal computer with a CardDeluxe sound card (22.05 kHz sample rate, 24 bit converters).
Subjects and measurement procedures
Adults
Ipsilateral and contralateral WB AR data were collected in both ears (N=80) of 40 subjects with normal 0.226-kHz tympanometry and with normal hearing. Normal hearing was assessed based on air-conduction thresholds ≤ 15 dB HL for frequencies from 0.25 to 8 kHz, and air/bone gaps ≤ 10 dB between 0.25 and 4 kHz. Subjects were included only if the TPP was between −83 and 50 daPa; these limits are based on Ellison and Keefe (2005).
Clinical ARTs were measured in adults using a GSI Tympstar with a 226-Hz probe tone. Ipsilateral WB and clinical ARTs were measured using tonal (0.5, 1, 2 and 4 kHz) and BBN activators. Contralateral ARTs measured with an insert earphone were also measured at 0.5 and 1 kHz (denoted 0.5_C and 1_C, respectively). An ascending/descending activator level approach was used to find the ART. Activator presentations typically were initiated at 80 dB HL, then ascended in 5 dB steps until a visually detected change in admittance (≥ 0.03 mmho criterion) was observed (Stephenson et al., 1997). Activator level was then decreased by 10 dB, then increased in 5-dB steps until the criterion change in admittance was observed a second time. This process was continued until the lowest activator stimulus level which produced at least two changes ≥ 0.03 mmho in admittance was identified; this level was defined as the clinical ART.
Newborns
Ipsilateral WB AR data were collected from ears of one- and two-day-old infants (N=455 ears from 230 infants, 100 female and 130 male) who had received a DPOAE-based NHS screening test. Infants were recruited from a well-baby nursery, and also received WB ATF tests and 1-kHz tympanometry tests as described in Sanford et al. (2009).
Infants were classified as Pass or Refer by the initial NHS screening exam based on a DPOAE test, in what is termed the Day-1 test. DPOAE tests were performed using a Biologic AuDx Plus device and its associated ear tips. DPOAEs were measured for f2 frequencies of 2, 3, 4 and 6 kHz with f2/f1 = 1.22, f1 level = 65 dB SPL, and f2 level = 55 dB SPL. The DPOAE pass criteria were a signal-to-noise ratio (SNR) ≥ 6 dB and DPOAE level ≥ −6 dB SPL. The test outcome was classified as a Pass if DPOAE criteria were met for at least three f2 frequencies, and, otherwise, as a Refer. Some of the infants who referred on either ear on Day 1 were re-screened 24 hours later on Day 2. Infants passing the DPOAE test on either Day 1 (DP1-Pass) or Day 2 (DP2-Pass) were classified as passing the NHS. Infants who were referred based on the Day-2 DPOAE test (DP2-Refer), and those infants who were referred on the Day-1 DPOAE (DP1-Refer) test and left the hospital prior to further re-screening, were classified as refers. Infants in each of the Day-1 and Day-2 groups received AR, ATF and 1-kHz tympanometry tests immediately following the DPOAE test. The mean age (and standard deviation, SD) of the DP1-Pass group (N=375 ears) was 25.5 hours (h) (SD=8.0 h), and that of the DP1-Refer group (N=80 ears) was 22.2 h (SD=8.0 h). The mean age (SD) of the DP2-Pass group (N=53 ears) was 45.3 h (SD=7.1 h), and that of the DP2-Refer group (N=14 ears) was 45.7 h (SD=7.5 h).
The 1-kHz tympanograms were measured in newborns with a Madsen Otoflex 100 middle-ear analyzer with standard ear tips using a downwards sweep at a 500 daPa/s rate from +200 to −400 daPa. Based on analyses in Sanford et al. (2009), the 1-kHz tympanometry test outcome used in the present study was the difference in admittance magnitude (ΔYPos) between the peak and the most positive test pressure; this was the most accurate 1-kHz tympanometry test in predicting the functional status of the ear canal and middle ear on Day 1. Because the test accuracies of the ambient-pressure and tympanometric WB ATFs were similar in Sanford et al., only the ambient-pressure WB ART results were used in the present study.
Reflex test procedures
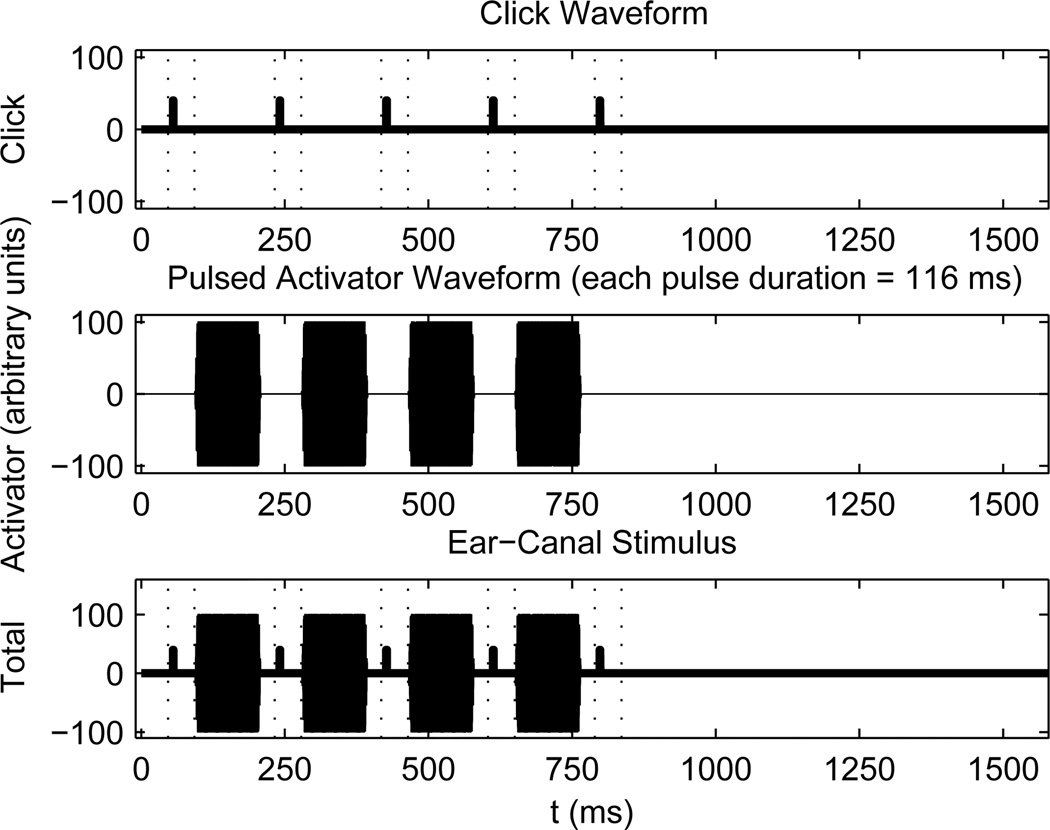
The AR test used a pulsed-activator stimulus set, in which a BBN or tonal activator eliciting the AR was pulsed on and off (see Fig. 1). The ipsilateral test stimulus was a sequence of four activator pulses alternating with five WB clicks (0.25–8 kHz bandwidth). The analysis buffer for each click was 1024 samples (46.4 ms), and was temporally separated from each pulse. The AR was detected by assessing the response difference between the initial, or baseline, click and any of the other clicks. At moderate-to-high activator levels, the AR was due to the action of the middle-ear muscle reflex (MEMR), which induces a contraction of the stapedius muscle to reduce energy transmission into the cochlea (Pang and Peake, 1985). The AR elicited at low activator levels may also have included medial olivocochlear efferent effects, which are discussed below. The time interval between successive clicks was 186 ms, and the duration of each activator pulse was 116 ms. Following the presentation of 5 clicks and 4 activator pulses, there was 790 ms of silence to allow the AR to return to baseline for an overall stimulus-set duration of 1580 ms. Data were acquired using two repetitions of the stimulus set at each of 10 increasing activator levels for a total test duration of 31.6 s. Activator levels varied by 4 dB for newborns and 5 dB for adults. In addition to ipsilateral AR testing in all subjects, contralateral AR tests were performed in adults only with pulsed activators delivered using an insert earphone.
Fig. 1.
[grey-scale, EPS]. The stimulus waveform of 5 clicks, which are output by a first probe receiver, is shown in the top panel. The stimulus waveform of 4 pulsed activators, which are output by a second probe receiver, is shown in the middle panel. The duration of each pulse is 116 ms. The stimulus waveform shown in the bottom panel is the linear combination of clicks and pulsed activators from each receiver. Each pair of dashed lines shows a 46.4 ms analysis buffer surrounding each click response. Each buffer is temporally separated from any pulse activator.
The peak-equivalent (pe) SPL of the click stimulus had a mean of 93.0 dB peSPL (with SD of 2.4 dB) in adults, and a mean of 99.1 dB peSPL (with SD of 2.4 dB) in the Day-1 pass group of infants. Because the electrical voltage applied to the probe receiver to generate the click stimulus was 1.8 dB lower in infant than adult ears, the peSPL would have been 7.9 dB higher in the infant than the adult ear if the input voltage level had been maintained constant. The temporal fine structure of the click-response waveforms in adult and infant ear canals differed, so that their peSPLs were not simply related.
Activator levels in both clinical and WB reflex testing were calibrated based on measurements in a 2 cm (HA-1) coupler. Although this coupler volume is much larger than the enclosed volume between the probe and tympanic membrane of a neonatal ear canal, this provided a uniform set of reference stimulus levels that are often used clinically. The maximum activator SPLs for clinical and WB testing, which are listed in Table 1 for measurements in the HA-1 coupler, were set 13–14 dB lower for safety reasons in testing newborns relative to adults. The activator level was also measured in each ear during the WB reflex test, so there was no essential dependence on the use of the HA-1 coupler. These in-the-ear levels are described below and reported in Table 2. For purposes of clarity, all ARTs are expressed as the SPL measured in the HA-1 coupler, except for levels measured in individual ears, for which the level is denoted as “in-the-ear” SPL.
Table 1.
Maximum SPL (referenced to a 2 cm3 HA-1 coupler) used for each activator type is listed for each subject group. The tonal activator type in each column is the activator frequency (kHz) for ipsilateral activators, and the activator frequency (kHz) to which is appended “_C” for contralateral activators; i.e., 0.5C and 1_C are the contralateral activators at 0.5 and 1 kHz, respectively. The top row includes maximum SPLs for the clinical ART test, the middle row includes those for the WB ART test in adults and children, and the bottom row includes those for the WB ART test in newborns.
| Maximum SPL (dB) for each activator type | |||||||
|---|---|---|---|---|---|---|---|
| Type | 0.5 | 0.5_C | 1 | 1_C | 2 | 4 | BBN |
| Clinical test (adults) | 105 | 105 | 105 | 105 | 105 | 100 | 95 |
| WB test (adults & children) | 103 | 98 | 103 | 109 | 103 | 100 | 89 |
| WB test (newborns) | 101 | -- | 89 | -- | 89 | -- | 76 |
Table 2.
The mean ± SD (with number of ears in parentheses) of the in-the-ear SPL (dB) measured in each test ear is listed at the maximum activator level specified in Table 1 (this maximum level was measured in terms of the SPL in a HA-1 coupler in Table 1). Results for WB tests are shown for each ipsilateral activator type in each subject group, adults and newborns, with separate statistics for Day-1 pass and refer groups of newborns. The tonal activator type in each column is the activator frequency (kHz), with a p6 added for activators in newborn ears in which the click level was elevated above the reference level by 6 dB (as described in the Methods and Materials section). For newborns, the 1 and 1p6 activator-type data provide a replication in different groups of subjects, because the activator level was fixed and only the click level was varied.
| Maximum in-the-ear SPL (dB) for each activator type | |||||||
|---|---|---|---|---|---|---|---|
| Type | 0.5 | 1 | 1p6 | 2 | 2p6 | 4 | BBN |
| Adults | 106.5±1.4 (80) | 103.6±1.4 (80) | -- | 103.8±2.4 (80) | -- | 101.7±4.4 (80) | 93.1±1.7 (80) |
| Newborns Day-1 pass | -- | 102.7±3.7 (204) | 103.1±3.0 (169) | -- | 100.6±2.9 (169) | -- | 94.0±1.8 (375) |
| Newborns Day-1 refer | -- | 106.2±2.6 (30) | 106.8±2.2 (23) | -- | 103.3±1.6 (23) | -- | 95.1±1.6 (53) |
| Newborns Day-2 pass | -- | 102.6±3.4 (42) | 103.1±3.2 (38) | -- | 100.9±2.6 (38) | -- | 93.9±1.7 (80) |
| Newborns Day-2 refer | -- | 105.9±3.0 (8) | 104.7±3.2 (6) | -- | 101.2±2.7 (6) | -- | 94.1±2.0 (14) |
In the ipsilateral reflex testing of newborn infants, the ARTs elicited by the 1-kHz activator were measured in some infants (N=204 ears in Day-1 pass group, and N=42 ears in Day-1 refer group) using the reference click level, and in other infants (N=169 in Day-1 pass group, and N=38 ears in Day-1 refer group) at a click level 6 dB higher than the reference in order to search for reference-level effects. The infants who received the ART test using the 1-kHz activator at a click level 6 dB higher than the reference also received an ART test using the 2-kHz activator at the same click level (6 dB higher). For example, the SNR of a low-level AR shift of a click response would be larger if the stimulus level of the click were increased by 6 dB, assuming that the noise background of the infant was stationary. The ART test using the BBN activator was performed at the reference click level for all infants (N =375ears in Day-1 pass group, and N=80 ears in Day-1 refer group).
Infants generally tolerated the experimental tests well. If an infant did become restless, attempts were made to soothe the infant or pause testing until the infant was quiet. While stimulus levels for the experimental tests were not expected to initiate a behavioral response, individuals collecting data monitored the infants’ state of arousal during stimulus presentations; no behavioral responses, coincident with the stimulus presentations, were noted during the experimental test sessions.
These studies were approved by the Institutional Review Boards of the study sites at Boys Town National Research Hospital and Bergan Mercy Medical Center (Omaha, NE).
Procedure to calculate WB ARTs
To calculate the ART, the first task was to determine whether an AR shift was present at each activator level, and the second was to estimate the threshold level of the activator producing an AR shift. For the first task, the following processing classified an AR shift as present or absent. A click-difference or AR-shift waveform was defined as the difference of the response to the final (5th) click relative to the baseline (1st) click. The click differences were calculated for the 2n through 5th clicks with respect to the baseline click. These click differences were used to assess the response similarity across the four click-difference waveforms (they also provided information about the temporal growth of response during the set of pulsed activators, but this growth rapidly saturated in most ears and no detailed results are presented). It was thereby possible to classify a shift as synchronous rather than due to some intermittent noise glitch. WB AR responses were acquired at ambient pressure in all subjects, but were also acquired in adults at the TPP. As described in Liu et al. (2008), this WB TPP was calculated from a WB energy absorbance tympanogram that was averaged over frequencies from 0.38–2 kHz.
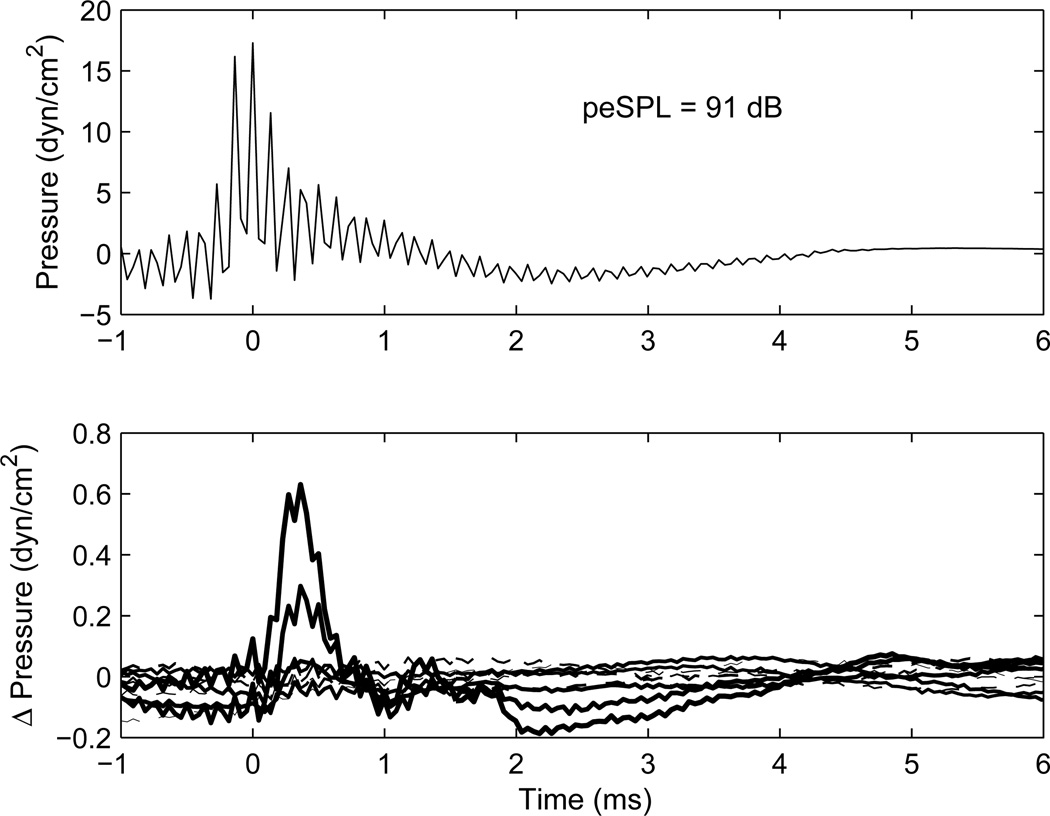
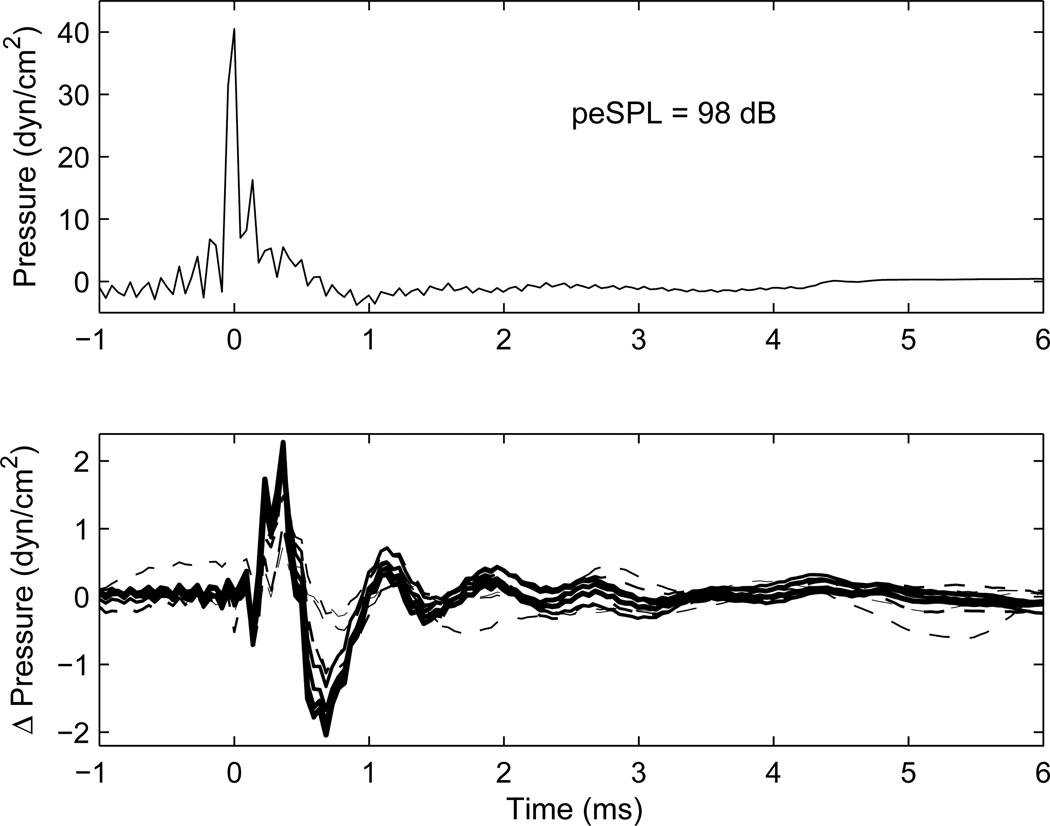
Results to illustrate the procedure with a BBN activator are shown in Fig. 2 for an adult and in Fig. 3 for a newborn with large reflex shifts at nearly all activator levels. Each shows the click baseline stimulus in the top panel and click difference response in the bottom panel. These transients show a unipolar type of click response, in contrast to the more bipolar responses observed in recordings using an electrical impulse delivered to typical receivers used in ear-canal probes. As summarized in Liu et al. (2008), the electrical stimulus to the sound source was constructed to produce a unipolar click response in a reflectionless tube, which was designed to approximate the impulse response of a digital filter with passband from 0.226–8 kHz. The ripples in the baseline stimulus waveform in the adult ear (Fig. 2) include effects of multiple internal reflections within the ear canal between the probe and the tympanic membrane. Ripples are much less evident in the waveform recorded in the ear of the newborn (Fig. 3) because the probe is much closer to the eardrum, and, hence, the internal reflections are more difficult to separate in time (in the absence of measurements at frequencies above 8 kHz). Another factor is that more energy is reflected from the adult eardrum at high frequencies, which contributes to the magnitude of any ripple.
Fig. 2.
[grey-scale, EPS]. The waveform response in an ear of an adult to the initial click is shown in the top panel, for which the peSPL in the ear was 91 dB. Time t=0 ms is located at the maximum amplitude of the click stimulus. The click difference waveform response (final click minus initial click) is shown in the bottom panel at each of the 10 BBN activator levels, with increasing line thickness corresponding to increasing activator level. The click differences for the 5 lower-level activators are plotted with dashed lines, and those for the 5 high-level activators are plotted with solid lines. Each click difference response is the average across the two repetitions at each activator level.
Fig. 3.
[grey-scale, EPS]. The waveform response in the ear of a newborn to the initial click is shown in the top panel, for which the peSPL in the ear was 98 dB. The format of the figure is otherwise similar to that in Fig. 2. A reflex response above baseline was observed in each trace.
The peak click stimulus amplitude occurred at a time labeled as 0 ms in these plots, and the MEMR shift in the click difference waveform increased with increasing activator level with peak response near 0.7–0.8 ms. This latency is much shorter than the onset latency of the acoustic reflex, which was elicited during the 743 ms between the initial and final clicks (see Fig. 1). This much shorter latency assesses how magnitude and phase effects in the action of the AR affected the transient response of the middle ear.
Reflex test: Detecting an AR shift as present
The AR shift in a given test was classified as present or absent based on the spectrum of the click-difference waveform. The spectrum was calculated using a 17.4-ms Hamming window duration (384 samples) sufficient to include the stimulus and any AR response (except for multiple internal reflections of any click-evoked OAE, which would be of low amplitude). The peak amplitude of the click was located 4 ms from the beginning of the window.
As expected based on past MEMR research, the largest AR shifts were observed at lower frequencies. The spectrum was divided into low- and high-frequency components to facilitate age-dependent measurements of the ART and supra-threshold growth at both low and high frequencies. A bandwidth to assess low-frequency AR shifts (AR-L) with ART of θL was defined as 0.38–2.8 kHz in adults, and 0.79–2.8 kHz in newborns. The sensitive reflex frequency range ≥ 0.8 kHz for newborns agrees with findings in Weatherby and Bennett (1980). A bandwidth to assess high-frequency AR shifts (AR-H) with ART of θH was defined as 2.8–8.0 kHz.
The present/absent AR decision was determined using 3 criteria:
The signal “energy” (i.e., energy in the signal-processing sense) of the AR-shift was calculated as the summed squared magnitude of the windowed signal spectrum over the analysis bandwidth. The noise energy was defined over the same bandwidth using a 17.4-ms (384-sample) Hamming window over times in which the click stimulus was absent, and any AR shifts were thus expected to be absent. The noise window began on the sample just after the end of the signal window. Each summed energy was converted to a level (in dB). An AR shift was judged present only if the AR shift SNR > 6 dB.
Correlations were calculated between the 6 pairs of the complex spectra of each of the 4 click difference responses in each stimulus set. An AR shift was present only if the maximum correlation significantly exceeded +0.7 (Fisher’s Z transformed test with α=0.05). The 116-ms duration of the first activator pulse (see Fig. 1) was often sufficiently long that any AR shift was saturated in later presentations of clicks and pulses. The AR similarity test searched for any pair of click-difference waveforms sufficiently similar to have a common origin. Criteria 1 and 2 mutually confirmed that a detected shift was due to an AR effect synchronous with each click presentation, rather than some noise glitch. Such a combination of magnitude and similarity tests to detect the AR has improved properties over either test alone (Feeney and Keefe, 2001).
Low-level noise responses were excluded. The relative response level of the AR shift was calculated as the SPL difference of the click-difference spectrum to the baseline click spectrum. An AR shift was present in the low- or high-frequency bandwidth if the relative AR level > −43 dB.
An AR shift was judged present if all 3 criteria were satisfied for either stimulus repetition, which handled the case of intermittent noise that occurred in one, but not both, of the repetitions.
It would be unlikely in the presence of excessive noise to satisfy criterion 2 for synchrony, but a real-time test of excessive noise was developed for use during data collection. The test classified the noise as excessive if the click-difference noise level calculated for criterion 1 was within −10 dB of the click-stimulus level. The squared spectral magnitudes of both responses were summed over the entire analysis bandwidth (0.8–8 kHz for newborns, 0.38–8 kHz otherwise) and converted to levels. Results were used during data collection in some subjects: the operator was notified when the noise was excessive, so that the reflex test could be repeated at whatever desired range of activator levels that was selected by the operator. However, the excessive-noise test was not yet implemented in software at the beginning of data collection, so that these results were not available for all subjects during data collection. Consequently, the final set of measurements used in group analyses included all data, i.e., tests with excessive noise were not discarded.
AR threshold and supra-threshold response
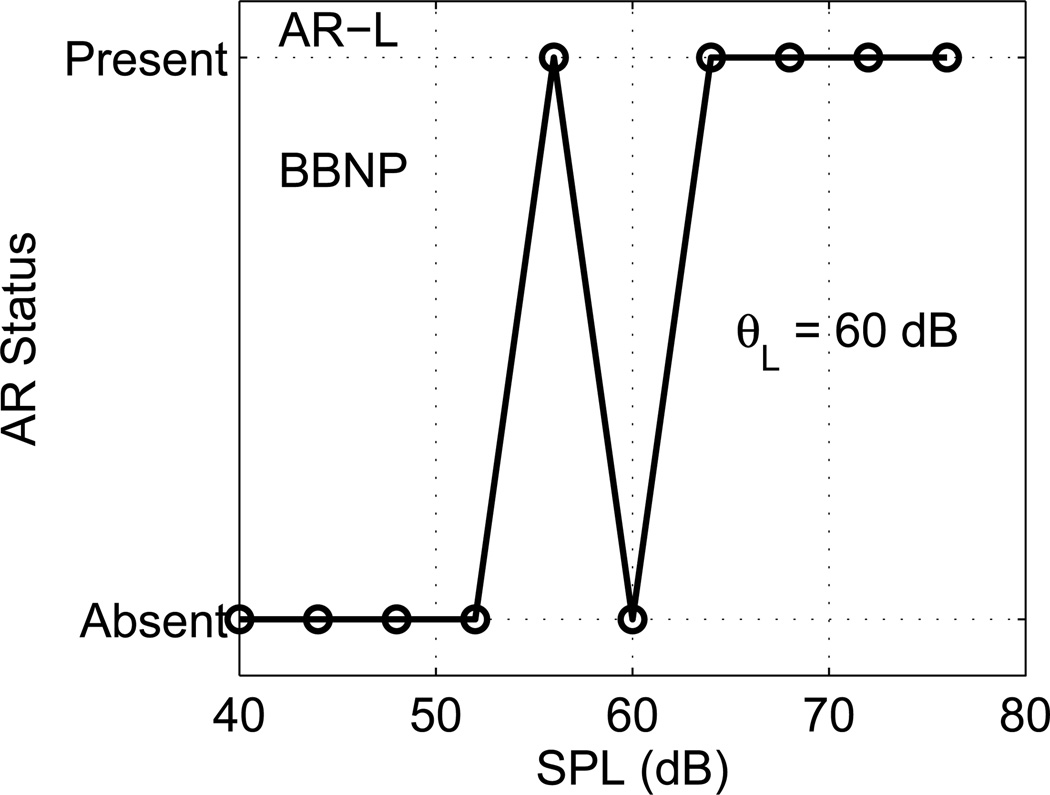
An example is shown in Fig. 4 for a newborn test ear for the low-frequency AR shift (AR-L) classified as present/absent at each BBN activator level. Similar AR status functions were calculated for the high-frequency AR shift (AR-H), although not shown in this figure. AR-L was absent at the lowest activator levels (40–52 dB SPL), and present at all higher activator levels except for 60 dB SPL. The anticipated shape of an “ideal” AR status function would be absent at low activator levels, and present at high activator levels at and above the AR threshold. In this example, the AR status function differed from the ideal case at 60 dB SPL. Such non-monotonic effects in the AR status functions of individual ears are relatively common. In clinical reflex testing, an audiologist would ordinarily repeat a threshold test as needed until sufficiently confident whether a reflex were present or absent. The challenge for an automated objective ART test, especially in the NHS setting, is to measure a threshold without evaluative input from an audiologist (or other skilled operator).
Fig. 4.
[grey-scale, EPS]. The AR status as determined for a BBN activator is plotted for a newborn test ear for AR-L. The ART for this ear was θL =60 dB SPL.
The procedure to measure the ART used a yes-no task and a maximum-likelihood approach generally similar to that used to measure behavioral thresholds (Green, 1993) and OAE thresholds (Keefe et al., 2009). The common property across these studies is the goal of measuring a threshold of response as a function of a monotonic stimulus variable, which in the case of the ART is the activator level. Green (1993) used an adaptive sequential approach with a set of model psychometric functions, whereas the present study used a fixed set of activator levels and a set of model probability functions. The psychometric function is the probability of behaviorally detecting a signal in noise as a function of the signal level. The probability varies between 0 and 1, and the stimulus level at which the probability is equal to 0.5 is termed the threshold. A model probability function is a smoothly varying monotonic function, in which a logistic function was used as in previous work. This logistic function is specified by its threshold and its slope in the vicinity of threshold. This slope was fixed in the present study at the same value used by Green (in addition, other slope values were used in preliminary studies, and the results obtained were nearly the same). The set of model probability functions is the set of logistic functions that vary only in their thresholds in 1-dB increments. The threshold value of the particular model that best fits the data, which are measurements of the response present or absent as a function of stimulus level, is the estimate of threshold. This threshold is calculated as the model with the maximum likelihood for explaining the measured data.
One reason for not using an adaptive control of the activator level, as would have been the direct analogy of the behavioral threshold procedure used by Green, was that the final form of the methods to calculate the AR shift as present or absent and the resulting threshold were not yet completed during the period in which data were acquired. This is in contrast to behavioral testing, in which the subject’s response in each stimulus trial is measured. Another reason was that a goal of the study was to measure AR input/output functions over a wide range of activator levels (36 dB range for newborns and 45 dB range for adults). If the maximum-likelihood threshold was calculated to lie between any two adjacent activator levels used in data collection, the ART was set to the maximum-likelihood threshold rounded up to the nearest higher activator level. This provided a slightly more conservative estimate of the ART. The maximum-likelihood procedure correctly calculated the ART for all AR status functions of the ideal form, and estimated the most likely ART for non-ideal functions. In the example shown in Fig. 4, the resulting low-frequency ART (θL) was 60 dB SPL, which is weighted between the lowest and next lowest levels (56 and 64 dB SPL, respectively) at which the AR shift was present.
The absolute value of the maximum likelihood was useful in preliminary studies to set the detection parameters: AR status functions of ideal form had the highest maximum likelihood, whereas non-monotonic AR status functions had the lowest. This use of maximum likelihood to establish the particular form of the detection test is outside the scope of psychophysical testing, in which a subject provides responses to stimuli presented during the test and the experimenter does not know how the subject makes each decision. For the ART test, the objective AR shift test is under the control of the investigator. The numerical values of the parameters in the three criteria used to classify an AR shift as present or absent were set based on the properties of the resulting maximum likelihood scores in the preliminary studies. A set of detection parameters resulting in a model with higher maximum-likelihood value was preferable to other sets of detection parameters resulting in models with lower maximum-likelihood values. This translates into more individual tests have a more monotonic shape to their AR status function (e.g., Fig. 4).
The detection parameters for AR status were the upper and lower 1/12th -octave frequencies of the analysis bandwidth, the SNR of the AR shift, the maximum correlation, and the relative AR level. These were varied based on preliminary analyses of data from normal-hearing adults (N=15 ears), and newborns in the Day-1 Pass (N=50 ears) and Day-1 Refer (N=42 ears) groups. Results were assessed in terms of the number of ears with low maximum-likelihood values, the number of normal-hearing ears with AR absent, and the average ART in the other ears. For normal-functioning ears, a test design was preferred that had relatively few ears with low maximum-likelihood levels, few ears with MEMR absent, and relatively low MEMR thresholds otherwise. For ears with an assumed impairment in sound conduction (i.e., any newborn in the Day-1 refer group), a test design was preferred that had comparatively more ears with MEMR absent and more elevated ARTs otherwise. All parameters were fixed in value before any analyses of the full data set, which are described below, were performed.
Results
Case study – AR response in newborn with low thresholds
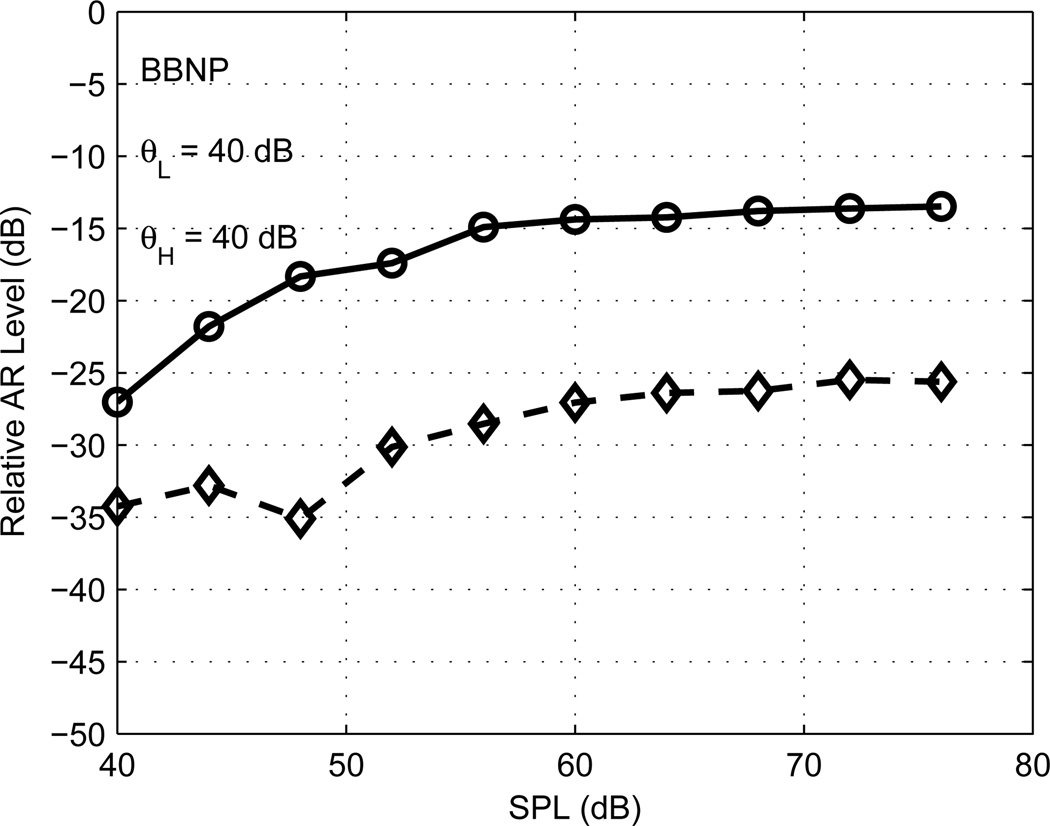
An example of the supra-threshold growth of ipsilateral AR-L and AR-H responses in a second newborn is shown in Fig. 5 (this is the same test ear for which results are plotted in Fig. 3). Both AR-L and AR-H shifts were present at every BBN activator level in this test. Supra-threshold growth is plotted as the relative AR level, which is the difference of the click-difference level and the stimulus level summed over low frequencies (solid line) and high frequencies (dashed line). The calculation of the click-difference level over the low- or high-frequency bandwidths was described in Methods (criterion 1 in the Reflex test subsection). The stimulus level was calculated in a similar manner: the click waveform was Fourier transformed, the magnitude squared of its Fourier spectral components were summed over low and high frequencies, and each sum was converted to a level (in dB). The supra-threshold growth saturated with increasing activator level for both AR-L and AR-H, while the relative AR level was larger for AR-L than for AR-H. These properties were commonly observed in ears of all ages. The relative AR level varied from −27 dB to −13 dB for AR-L and from −35 dB to −26 dB for AR-H, which were typical levels observed for other ears.
Fig. 5.
[grey-scale, EPS]. The relative AR levels are plotted for AR-L (solid line) and AR-H (dashed line) for a newborn ear as a function of the BBN activator SPL. The ARTs for this ear were the lowest activator test level, θL =θH =40 dB SPL.
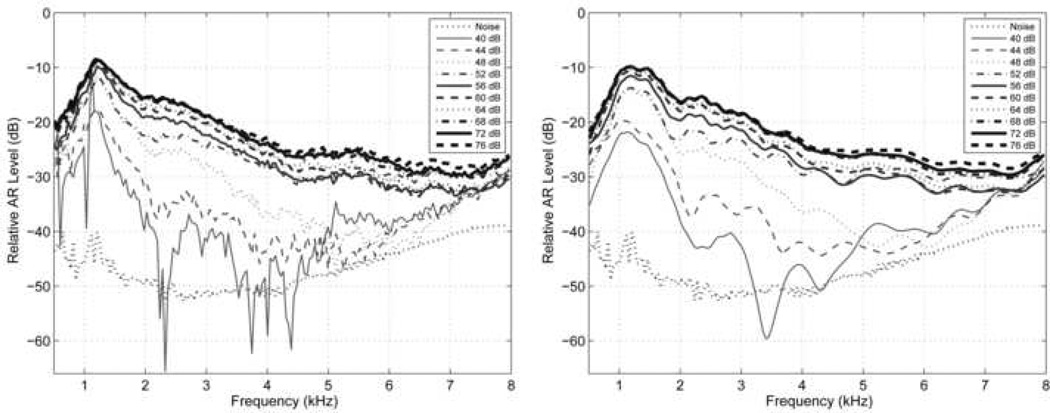
A detailed spectral level ΔLAR(f) representing the shift due to the AR was obtained by calculating the SPL of the click-difference response relative to the SPL of the baseline click response. For the same newborn whose data are shown in Figs. 3 and 5, ΔLAR(f) is plotted at each of the 10 BBN activator levels in Fig. 6A (left panel), in which 0 dB would represent an AR shift equal in level to the baseline click level. ΔLAR(f) increased with increasing activator level, and exceeded the noise level at even the lowest activator level. ΔLAR(f) at the maximum activator level exceeded −30 dB at all frequencies above 2 kHz. At the lowest activator level, it is unknown whether the initial click or the lowest level pulsed activator was the dominant signal eliciting the AR. Further research is needed to investigate this issue.
Fig. 6.
[grey-scale, TIF]. Left panel: The relative AR spectral levels (ΔLAR (f)) are plotted for a newborn test ear with separate line styles for each of the 10 BBN activator SPLs. These spectra were calculated from the windowed click-difference signals in Fig. 3. SPLs and line styles are listed in the legend. The nominal noise spectral level is plotted as a black dotted line. Right: The relative AR spectral levels are plotted with a similar format as in the left panel, except that these spectra were calculated from the windowed click-difference signals after removing the response energy that occurred 2 ms or later (relative to the time axis in Fig. 3).
The possibility exists that the shift in the spectrum of Fig. 6A may be due to the action of the medial olivocochlear reflex (MOCR) rather than the MEMR. An MOCR shift would be expected in a click-evoked otoacoustic emission (CEOAE) response at OAE latencies; these latencies are ~11 ms at 1 kHz. In typical clinical procedures, CEOAEs are measured by discarding the initial 2.5 ms of the response and MOCR shifts in CEOAEs are evaluated at times >2.5 ms after the click onset. A MOCR is expected to be absent in the baseline click and present in any subsequent click following the activator, so that the click-difference waveform would encode any MOCR effects. To eliminate the possible role of the MOCR in the present data at these longer latencies, as is typically measured using CEOAEs, each click difference waveform was windowed to discard the portion that was later than 2.0 ms after the peak of the click stimulus, eliminating all of what is typically considered to be the CEOAE. The window had a cosine-squared taper between 1.8–2.0 ms. This windowing restricted MOCR effects to those occurring only at latencies shorter than 2 ms. The resulting click-difference spectrum in Fig. 6B (left panel of Fig. 6) was nearly equal to that in Fig. 6A, except that the noise at times later than 2.0 ms was eliminated. This demonstrates that the click-difference response is dominated by a short-latency component at all frequencies, which is inconsistent with the action of the MOCR on CEOAEs as typically measured. The fine structure in ΔLAR(f) at the lowest activator level in Fig. 6A but not in Fig. 6B, e.g., close to 0.85 and 1 kHz, is consistent with the presence of click-synchronous spontaneous OAEs that were eliminated by discarding the response later than 2.0 ms. The fine structure in ΔLAR(f) at the lowest activator level in both Figs. 6A and Fig. 6B between 2–5 kHz may be related to MOCR suppression of an OAE response, but the mechanism remains unclear.
It remains possible that some combination of MEMR and MOCR processes may influence the relative AR shift in Fig. 6, through some mechanism acting on the click response in the initial 2 ms. ΔLAR(f) had a similar spectral shape and saturating behavior for activator levels between 52–76 dB SPL, consistent with a common mechanism. The maximum relative AR shift exceeded −20 dB only between 0.8–3.2 kHz, which is consistent with the generally accepted bandwidth of MEMR effects (Rabinowitz, 1977). Identifying the MEMR as the common mechanism at high levels, ΔLAR(f) exceeded −30 dB between 3.2–8 kHz, i.e., the action of the MEMR extended over a wide frequency range. At the three lowest activator levels (40–48 dB SPL), the maximum ΔLAR(f) remained close to 1 kHz, which appears consistent with a common mechanism, but its spectrum at higher frequencies was more different than the spectral shape at the higher activator levels. This leaves open the possibility of a contribution from the MOCR at lower levels. Because MOCR thresholds are thought to be lower than MEMR thresholds (Guinan, 2006), this might explain the presence of extremely low ARTs in this and some other ears (described below in group analyses). Alternatively, the MEMR threshold may be much lower than has been previously reported. The present results help frame the question of the extent to which MOCR and MEMR effects can be simply separated, but more research is needed to investigate these issues.
Group results
The activator SPL was measured in each test ear for each activator type. The mean and SD of the in-the-ear SPL measured in each group at the maximum activator level specified in Table 1, which were based on coupler measurements, are listed in Table 2. These in-the-ear SPL data are based on ipsilateral testing only inasmuch as the pressure was measured by the probe microphone placed in the ipsilateral test ear. The number of ears for each activator type and subject group is shown in parentheses in Table 2. For adults, the in-the-ear SPL was within 2 dB of the SPL measured in the HA-1 coupler for each activator type (compare Table 2 with the ipsilateral results in Table 1). The results for newborns are shown separately for the 1-kHz activator in which the click was presented at the reference level and elevated in level by 6 dB. Because the activator was presented at the same level in each case, the results assess the repeatability of distinct groups of infant ears. Results in these subgroups were highly repeatable.
Comparing adults with the Day-1 pass group of newborns, which was the largest infant test group, the maximum mean in-the-ear SPLs were within 1 dB at 1 kHz and for BBN, and 3.2 dB higher at 2 kHz. This near equality of in-the-ear SPLs between adults and newborns was a direct consequence of setting the maximum stimulus level applied to the receiver delivering the activator signal 14 dB lower in newborns at 1 and 2 kHz, and 13 dB lower for BBN (see coupler recording data in Table 1). Thus, reducing the input to the receiver resulted in a close match in the total SPL in the ear canal of adults and newborns.
The mean in-the-ear SPL was 3.6 dB higher at 1 kHz in the Day-1 refer ears than the Day-1 pass ears, 2.7 dB higher at 2 kHz, and 1.1 dB higher for BBN. These differences are highly significant given the SDs ranging from 1.6–3.7 dB and the large numbers of subjects in each subgroup. Day-1 ears that referred on a DPOAE test had higher in-the-ear activator levels for the same stimulus applied to the sound source, likely due to differences in sound-conduction status in pass and refer groups. The same outcome would be expected for the DPOAE stimuli, i.e., the same voltage applied to the sound source would generate a higher SPL in ears that were referred on DPOAEs than in ears that passed. This has significance for DPOAE test protocols in those NHS programs, which, as in the DPOAE protocol used in the present study, adjust the voltage applied to the sound source to generate a constant total SPL in the ear canal at each of the f1 and f2 stimulus frequencies. Such an in-the-ear calibration may be counter-productive, because an ear with sound-conduction dysfunction would have its voltage stimulus level reduced due to the conductive dysfunction relative to an ear with normal sound conduction. This would effectively reduce the DPOAE stimulus level by 3 dB at 1 and 2 kHz in ears with ear-canal and/or middle-ear dysfunction relative to a fixed voltage drive procedure. More research is needed to investigate this issue.
The mean in-the-ear SPLs of the pass groups on Day 1 and Day 2 differed by no more than 0.3 dB for any activator type. The mean in-the-ear SPLs of the refer group on Day 2 tended to be in between the SPLs of the Day-2 pass group and the Day-1 refer group. However, the relatively small numbers of test ears in the Day-2 refer group, which ranged across activator type from 6 to 14 ears, constrained the ability to evaluate the significance of these trends.
Because the ART was absent in some ears, the median ART was used rather than the mean ART as the measure of central tendency across ears. The median ART is well defined across all measurements, whereas the mean ART cannot be calculated for ears with absent AR responses.
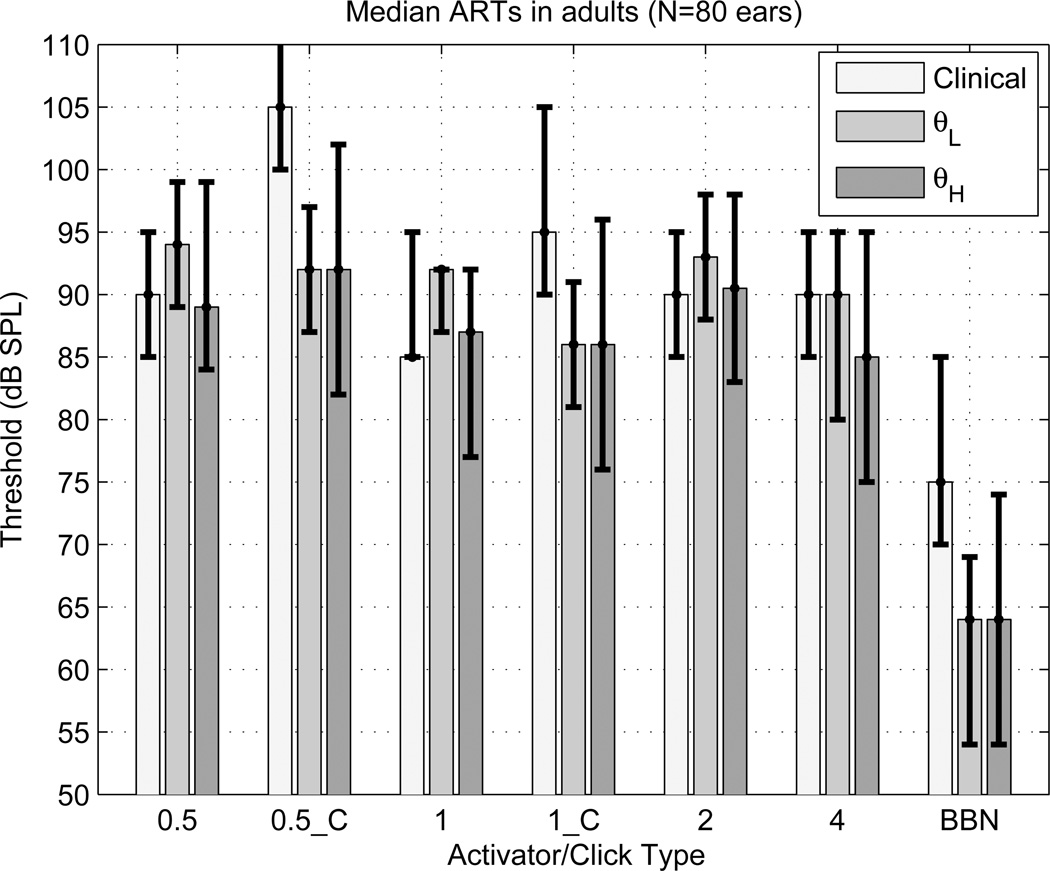
Clinical and WB measurements of ipsilateral and contralateral ARTs are compared in Fig. 7 on the same group of 80 adult ears in terms of the median ART and its interquartile range (IQR). The clinical and WB measurements of the median ipsilateral tonal ARTs (at 0.5, 1, 2 and 4 kHz) were similar with respect to their IQRs, and were also similar across activator frequency. The median clinical ARTs were more elevated than the median WB ARTs relative to their IQRs for contralateral activators (at 0.5 kHz denoted by 0.5_C, and 1 kHz denoted by 1_C) and for the ipsilateral BBN activator. The IQR of the clinical ART for BBN was completely separated and higher than the IQR of θL: the median clinical ART was 11 dB higher than the median θL.
Fig. 7.
[grey-scale, EPS]. The median ARTs (in dB SPL) measured in 80 normal-hearing adult ears are plotted for each activator type for clinical [left bar] and WB tests (θL and θH) [middle and right bars, respectively]. The associated IQRs are shown by the vertical error bars. The ipsilateral activator types include tonal activators (0.5, 1, 2 and 4 kHz) and BBN. The contralateral activators include tonal activators (0.5_C at 0.5 kHz, and 1_C at 1 kHz). The upper error bar extends above the plotting range for the clinical activator at 0.5_C because the 75th percentile was an absent AR at every activator level. The upper or lower bar value is plotted at the median value for those conditions in which the 75th or 25th percentile, respectively, of the ART was numerically equal to the median ART.
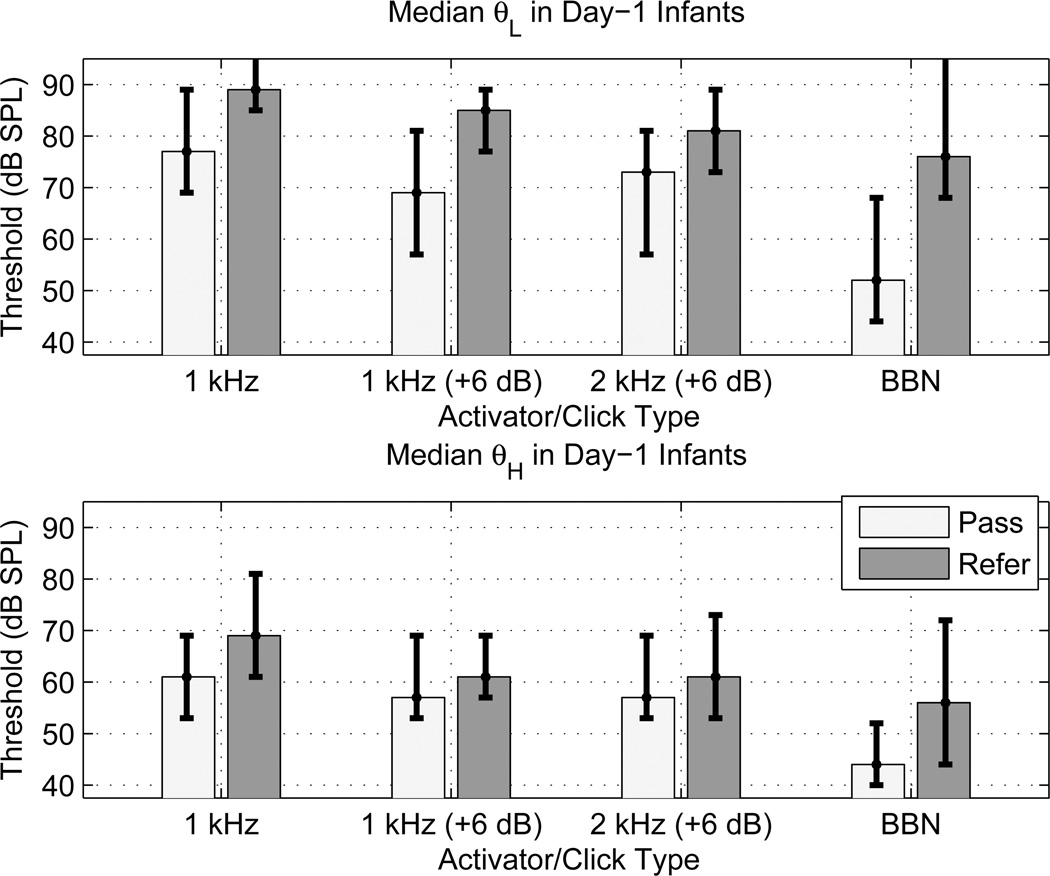
WB AR testing was completed and thresholds were measured in all 455 neonatal ears tested during the first day of life, and in all 67 ears tested during the second day. The median and IQR of the ART are plotted for the Day-1 Pass and Day-1 Refer groups of infants in Fig. 8 for θL (top panel) and θH (bottom panel). ART results using the BBN activator were measured in all infants in the Day-1 groups, whereas ART results for the tonal activators were measured in roughly half of each of the pass and refer groups (see Methods section for exact numbers). The tonal activator results were measured in distinct subsets of neonates so that the overall test time was not too long for a given infant; nonetheless, it was possible to assess effects of varying activator frequency by comparing results from different sub-groups. The overall effects were that the ARTs for θL were more elevated than θH, and all ARTs were elevated in the refer group compared to the corresponding pass group. The IQRs of pass and refer groups overlapped less for θL than for θH.
Fig. 8.
[grey-scale, EPS]. The median ARTs (in dB SPL) measured in the Day-1 newborn group of pass and refer ears are plotted as bars for each activator type for θL (top panel) and θH (bottom panel). The associated IQRs are shown by the error bars. The ipsilateral activator types include the 1 kHz tonal activator at the reference click level and at a level 6 dB higher than the reference (denoted 1 kHz and 1 kHz (+6 dB), respectively), the 2 kHz tonal activator at a level 6 dB higher than the reference (denoted 2 kHz (+6 dB)), and BBN. The upper error bar extends above the plotting range for θL in the refer group at 1 kHz and BBN because the 75th percentile was an absent AR at every activator level.
The effect of the reference level of the probe was assessed by measuring the ARTs elicited by the 1-kHz activator at two probe levels differing by 6 dB. The median ARTs tended towards slightly lower values at the higher click level (+6 dB) than at the reference level, but the degree of overlap between the IQRs may limit the practical significance of such a difference. The median ARTs at 1 and 2 kHz, which were measured at the same click level, were similar.
A nearly complete separation between the IQRs of the pass and refer groups occurred for θL using the BBN activator, for which the median θL was 24 dB higher in the refer group than in the pass group. Across all ears in which a threshold was detected at any activator level, the mean θL using BBN was 15 dB higher in the refer group than the pass group. Either θL or θH was measured in 97% of the infants in the Day-1 Pass group, and in 86% in the Day-1 Refer group. The clinical significance of such a reflex-threshold difference was further assessed in the analyses below, which also used the results obtained in the Day-2 groups with their smaller numbers of test ears.
ART test accuracy to predict sound-conduction dysfunction in newborns
An OAE test of cochlear function can be influenced by the abilities of the ear canal and middle ear to conduct sound in the forward direction from the probe to the cochlear, and in the reverse direction from the cochlear-source region of the OAE back to the ear-canal probe. The present study evaluated the accuracy of the WB ART test in predicting the status of the sound-conduction pathway for ears that passed or referred in a DPOAE-based NHS program; results are compared with those of Sanford et al. (2009), who evaluated the accuracy of WB acoustic transfer functions (ATFs) and 1-kHz tympanometry in predicting DPOAE status in the same group of subjects. As further described in Sanford et al., the DPOAE test outcome measure was treated as a “gold standard” for the functioning of the sound-conduction pathway, despite its limitations as a test of middle-ear status.
Test accuracy was assessed using the receiver operating characteristic (ROC) curve, which is a plot of the sensitivity versus one minus the specificity (i.e., the false-positive rate). The dimensionless summary measures of the ROC curve used to quantify test accuracy were the area under the ROC curve (AROC), and the symmetry (SYM) point on the ROC curve (the point at which the sensitivity and specificity were most nearly equal). More details on the properties of AROC and SYM are described in Sanford et al. (2009). The most important properties shared by AROC and SYM are that values of 0.50 represent chance performance, values of 1 represent perfect test performance, and a test with a larger value is more accurate than a test with a smaller value.
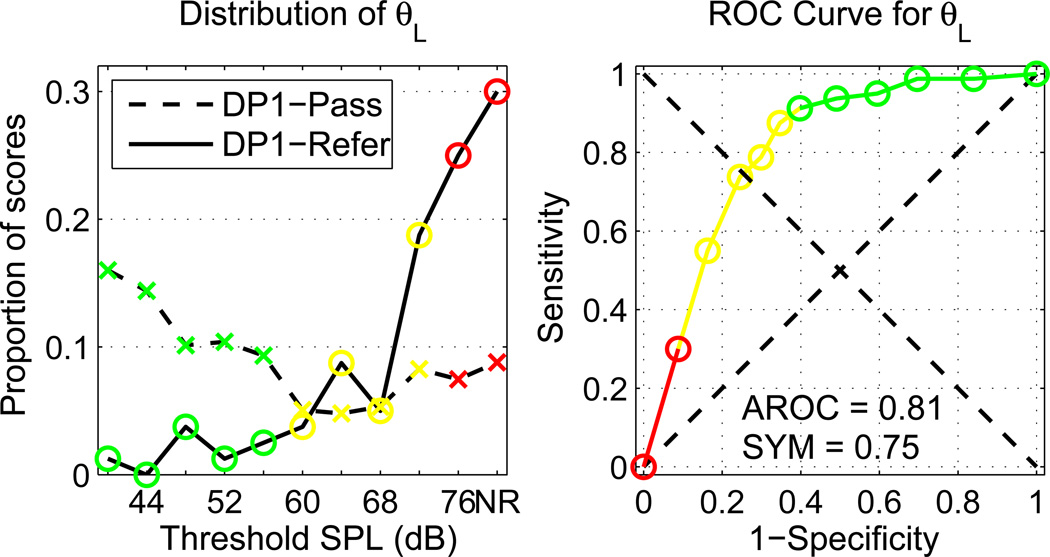
The proportions of infants in the DP1-Pass and DP1-Refer groups are plotted in the left panel of Fig. 9 as a function of each possible value of θL for the BBN activator, including the proportion with no response (NR) at any activator level. These results show the relationship between DPOAE pass and refers, and the reflex activator level. The DP1-Pass ears had a relatively flat distribution of thresholds, except for a slight elevation at the lowest activator levels. θL was measured in 91% of DP1-Pass ears (i.e., the right-most datum was 9%). The infant responses that were shown in Figs. 3, 5 and 6 were from an ear from DP1-Pass with θL =40 dB SPL; the proportion of responses at this lowest activator level was 0.16 (i.e., the left-most datum). Thus, a substantial proportion of ears in the DP1-Pass group had low reflex thresholds. The DP1-Refer ears had a larger proportion of θL at higher activator levels, with θL successfully measured in 70% of DP1-Refer ears (i.e., the right-most datum is 0.3).
Fig. 9.
[color, EPS]. Left panel: The proportions of infants in the DP1-Pass (dashed line) and DP1-Refer (solid line) group are plotted as a function of each possible value of θL, in which the right-most category NR represents no response, i.e., the AR was absent at every activator level. Right panel: the ROC curve is plotted for θL predicting sound-conduction status as DP1-Pass or DP1-Refer. In each panel, green symbols representing a test result of pass are defined by activator levels with a test sensitivity > 0.90; red symbols representing a test result of refer are defined by activator levels with a test specificity > 0.90; yellow symbols representing a test result of borderline are defined by any activator levels not in either of the other groups.
The ROC curve for the ability of the low-frequency reflex threshold to predict DPOAE status is shown in the right panel of Fig. 9; this curve was constructed based on the histogram data shown in the left panel of Fig. 9. The symbols in both panels of Fig. 9 are color-coded to denote one of the following illustrative screening test outcomes: pass (green), refer for a diagnostic evaluation (red), or borderline (yellow). These illustrative test outcomes are defined in terms of test specificity and sensitivity. In the example shown in Fig. 9, the pass outcome was assigned to thresholds with a sensitivity > 90%, the refer outcome was assigned to thresholds with a specificity > 90%, and the borderline outcome was assigned to all other possible thresholds. Depending on other clinical factors, the critical sensitivity and specificity might be independently varied to define ARTs according to a more or less conservative criterion.
Using WB reflex in tests to classify newborn hearing status
Measurements in the NHS population were also performed using 1-kHz tympanometry and aural WB ATF tests including absorbance and admittance. Results describing the ability of these WB tests to classify ears that passed or referred on the NHS DPOAE test have been reported (Sanford et al., 2009). The present study compares these results with the WB reflex test individually and in a test battery of these WB tests including the WB reflex test.
The ROC-curve summary measures for θL on infants tested on Day 1 were AROC=0.81 and SYM=0.75. The former metric described the sensitivity averaged across all specificities; the latter is equivalent to the statement that θL was able to classify sound-conduction status on the DPOAE exam with a specificity and sensitivity of 75%.
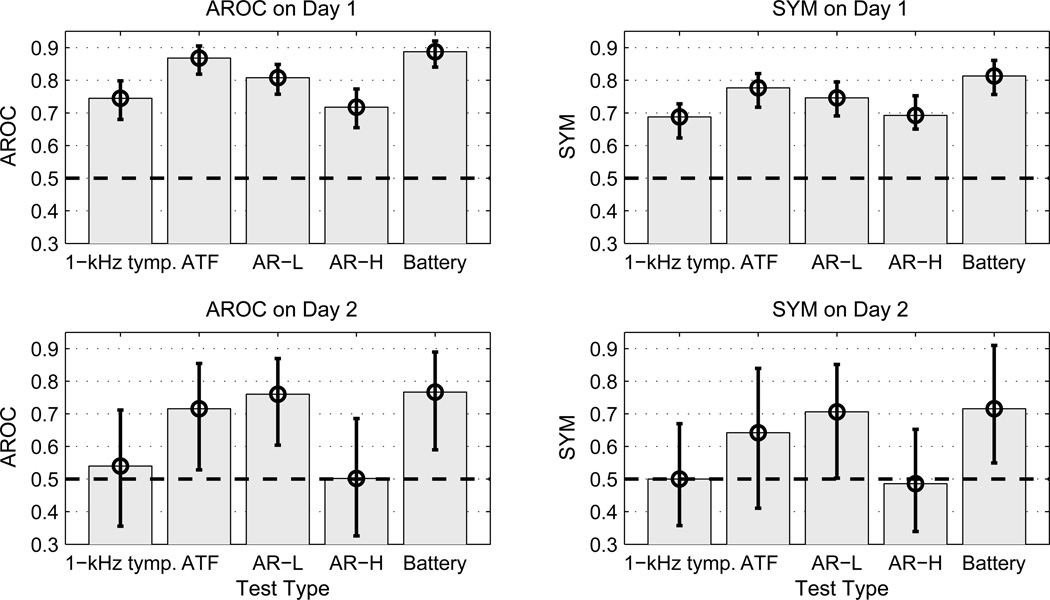
For predicting sound-conduction status in newborns on Days 1 and 2, the ROC summary measures are shown in Fig. 10 for 1-kHz tympanometry, WB ATF test, AR-L (i.e., θL), AR-H (i.e., θH), and a test battery combining the WB ATF and AR-L tests. The 95% confidence intervals of each ROC summary measure were calculated using a bootstrap procedure based on 20,000 re-samplings with replacement. This re-sampling was implemented in one procedure in which within-ear correlations between individual test results were retained, and in another simpler procedure in which the bootstrapped confidence intervals were calculated independently for each test. The confidence intervals obtained by the two procedures were essentially the same in all cases examined, so that results are reported using the simpler procedure. The best single WB ATF test at ambient pressure previously was found to be admittance phase (Sanford et al., 2009), and this predictor is used in Fig. 10 (denoted by ATF). The test battery predictor in Fig. 10 (denoted by Battery) was calculated by a weighted sum of the log likelihood-ratio (LLR) predictors for admittance phase, admittance magnitude, and AR-L. The use of the LLR to define univariate ATF predictors is described in Sanford et al. The weighting coefficients were determined using a nonlinear optimization method to maximize the AROC of the test-battery predictor for the Day-1 groups. The optimal combination of the admittance phase and magnitude LLRs was first determined, and then the optimal combination of the new admittance LLR and the AR-L predictor, which was transformed into a LLR according to methods described in Sanford et al., was determined. The resulting weighting coefficients were used to calculate the ROC summary measures for both Day-1 and Day-2 groups.
Fig. 10.
[grey-scale, EPS]. For predicting sound-conduction status in newborn infants, the ROC summary measures for 1-kHz tympanometry, WB ATF test, AR-L, AR-H and a test battery combining the WB ATF and AR-L tests are plotted, with AROC results in the left column, SYM results in the right column, Day-1 results in the top row, and Day-2 results in the bottom row. The black error bars show the 95% confidence intervals of each ROC summary measure. The dashed black line at 0.5 represents chance performance.
All predictors of sound-conduction status performed better than chance in the Day-1 group inasmuch as the lower bound of each of their confidence intervals exceeded 0.5. The higher values of AROC and SYM for AR-L compared to AR-H shows that AR-L was more accurate than AR-H at predicting sound-conduction status in infants on both days. The 1-kHz tympanometry and AR-H were the least accurate tests overall. The summary measures for AR-L were smaller than for ATF on Day 1, but the differences were not significant. The summary measures for the test-battery predictor were larger than for ATF, but these differences were also not significant.
The only predictors of sound-conduction status that performed better than chance in the Day-2 group were ATF, AR-L and Battery based on AROC, and AR-L and Battery based on SYM. The larger confidence intervals on Day 2 were due to the much smaller numbers of subjects in the pass and refer groups on that day. As on Day 1, AROC and SYM were largest for the test-battery predictor, but the differences were not significant. The Day-2 SYM results, in particular, provide evidence that an AR test helped detect sound-conduction dysfunction in infants receiving an initial NHS exam that no other single test was able to detect.
Discussion
Reflex effects were observed in the time domain in terms of the click-difference responses such as those shown in Fig. 2–Fig. 3 (bottom panels). To better understand the shape of these measured click-difference waveforms, a model of middle-ear mechanics was developed based on a simple oscillator model, in which the action of the MEMR was assumed to stiffen the tympanic membrane. The model formulation and predicted results are described in the Appendix. The main features of the measured click-difference responses were captured by this simple model. In particular, the time delay of its main positive-polarity lobe was explained as a temporal build-up of resonant response.
Analyses of group results
The finding in adults, that contralateral ARTs were lower in WB tests than clinical tests, confirmed past research. Feeney et al. (2003) reported mean contralateral ARTs to be 12–13.7 dB lower in WB tests using 1- and 2-kHz activators, whereas the contralateral median ARTs in the present study were 13 dB lower at 0.5 kHz and 9 dB lower at 1 kHz (see Fig. 7). The findings that ipsilateral thresholds for tonal activators were similar for WB and clinical tests supported past research in which the mean ART using a 4-kHz activator was similar in WB and clinical tests (Feeney et al., 2004). The median difference in the present study between the clinical and the WB ARTs was 0 dB using the 4-kHz activator. Given these results for ipsilateral tonal activators, it is notable in the present study that the median WB thresholds for the ipsilateral BBN test were 11 dB lower than the clinical ART (Fig. 7). In summary, ARTs can be measured at generally lower levels in adults using a contralateral or an ipsilateral WB probe with a BBN activator than with a standard clinical reflex test using a 0.226-kHz probe tone. Analyzing a reflex shift over a broad frequency range (as opposed to at a single probe frequency) is beneficial in that reliable measurements can be made with lower-level activating stimuli. The WB ARTs measured at ambient pressure and at TPP were similar, but this was expected given that the inclusion criteria for adults were an air-bone gap ≤ 10 dB and a TPP between −83 and 50 daPa. It would be interesting to compare clinical and WB ARTs measured at TPP in ears with pressures that deviated from ambient pressure, and/or TPPs varying over a wider range.
Both adult and infant AR responses extended to 8 kHz in some ears, even though the largest AR shifts occurred at lower frequencies. The AR shift was mainly localized to the initial 2 ms of the response following the time of the peak amplitude of the click stimulus (see Fig. 6), although a portion of the response extended to longer durations (bottom panels of Figs. 2 and 3). While MEMR effects would be expected to operate over these short durations, the present results did not exclude the possibility of an MOCR contribution, as reviewed in Guinan (2006). More research is needed to understand whether the 40–50 dB range of ARTs observed in both infant and adult ears is entirely due to MEMR effects, or to some combination of MOCR effects (possibly mixed with MEMR effects) at low activator levels and MEMR effects dominating at high activator levels.
The summary provided here demonstrates that WB ARTs can be reliably measured in infants as young as 1 day of age, that test time was sufficiently short to make the measurements clinically feasible, and that the measurements were useful in classifying middle-ear sound-conduction status in a NHS protocol. As expected, the median ARTs were lower using a BBN activator than a 1-kHz or 2-kHz activator (see Fig. 8). The BBN activator performed best in separating infant ears that passed or referred on a NHS exam, both in terms of the difference in the medians in the two groups on both Day 1 and Day 2 as well as in the degree of separation of the respective IQR (Fig. 8). The median θL was 24 dB higher and the median θH was 12 dB higher in the Day-1 refer group than the Day-1 pass group.
There were systematic differences between median θL and θH in the normal-hearing adult and the Day-1 infant pass groups (Figs. 7–8). Confining attention to the thresholds measured using an ipsilateral BBN activator, the median θL was 64 dB SPL in adults and 52 dB in infants, and the median θH was 64 dB SPL in adults and 44 dB in infants. Among the methodological factors that may have contributed to these differences are that the activator stimuli were calibrated in a 2 cm3 coupler and the maximum levels were separately adjusted for adults and newborns (see Table 1). The ear-canal volume between the probe and eardrum is much smaller in infants than in adults, but other factors influence the sound fields in infants and adults, such as maturational differences in the input admittance of the tympanic membrane, the input admittance associated with the ear-canal wall mobility, and the middle-ear transmittance (Abdala and Keefe, 2006; Keefe and Abdala, 2007). As described above, statistics on the activator SPLs measured in the ear for adults and infants are presented in Table 2. The corresponding maturational effects on ARTs, which also may involve post-natal development of neural function, remain a topic for future study.
In the Day-1 pass group, θL was measured in 91.2% of ears and θH was measured in 92.8% of ears, while at least one of θL and θH was measured in 97.1% of ears. Thus, only 3% of normal-functioning newborn ears had an absent AR. In the Day-2 pass group, θL was measured in 84.9% of ears and θH was measured in 94.3% of ears, while at least one of θL and θH was measured in 98.1% of ears. These results are similar to those reported by Mazlan et al. (2009), who found that 91.3% of ears from infants of age 24–192 hours had a measurable ipsilateral AR elicited by either BBN or a 2-kHz tone, in which the AR shift was detected using a 1-kHz probe tone. The inclusion criterion for these infants is that they passed a screening ABR test.
Most refer infants also had measurable ARTs. In the Day-1 refer group, θL was measured in 70.0% of ears and θH was measured in 86.3% of ears, while at least one of θL and θH was measured in 90.0% of ears. In the Day-2 refer group, θL was measured in 57.1% of ears and θH was measured in 92.9% of ears, while at least one of θL and θH was measured in 92.9% of ears. Thus, presence or absence of an ART did not suffice to predict whether infants would pass or refer on the DPOAE test. The fact that the median θL was 24 dB higher in the refer group than the normal group on Day 1 (Fig. 8) helps explain the DPOAE results as likely due to differences in sound conduction through the ear canal and middle ear in the two groups. θL was a more accurate predictor than θH of DPOAE outcome. The detailed results for θL showed that the DP1-Pass infants had lower thresholds than the DP1-Refer infants (see Fig. 9) The finding that some DP1-Pass0 thresholds extend to the highest activator levels used is consistent with a reduction in transmission through the middle ear in ears that nonetheless had DPOAEs in the normal range (i.e., in DP1-Pass ears). The finding is also consistent with a source of variability in ARTs that is unrelated to variability in DPOAEs.
A clinically important question is the extent to which ipsilateral ART measurements in infants prove useful in assessing their auditory status. The ART test was more accurate than 1-kHz tympanometry in classifying ears that passed or referred in a NHS exam based on DPOAE testing. In infants tested on Day 2, the θL test (labeled as AR-L in Fig.10) performed better than chance at classifying ears into pass and refer groups, while the 1-kHz tympanometry test was at chance performance, and the WB ATF test exceeded chance performance using AROC but not using S YM. The interpretation of the Day-2 results is limited by the small numbers of ears tested (53 ears for DP2-Pass, 14 ears for DP2-Refer).
A WB test battery composed of ATF and ART tests may have useful properties in a NHS exam, or in follow-up testing of infants who refer on the initial NHS exam. Such a test battery might be able to identify sound-conduction disorders and assist in the early identification of the type of hearing loss. A first possible outcome from such a test battery is that the ATF and ART tests may both indicate a possible sound-conduction problem consistent with middle-ear dysfunction. A test ear might refer on the NHS exam, so that the affected infant would need a follow-up hearing screening test to assess sensorineural status. Nevertheless, information provided by the ATF and ART tests might be helpful to medical staff and parents or guardians. Another possible outcome is that the ATF and ART tests may both indicate normal sound conduction consistent with normal middle-ear function. As explained by Sanford et al. (2009) for the case of an ATF test, a refer on the NHS exam in an ear with normal middle-ear function would signify an increased risk of a sensorineural hearing loss, and this would be clinically useful information. A third possible outcome is that the ART may be in the normal range while the ATF may refer on the basis of sound-conduction dysfunction. A NHS pass would be consistent with an ART in the normal range, so that an ATF indicating a possible conductive dysfunction would likely not affect the overall pass result from the NHS exam. A fourth possible outcome is a refer on the ART and a normal ATF test of sound conduction. As described in the Introduction, an infant might pass an OAE-based NHS test, but still be at risk for AN/AD. The fact that AN/AD patients also have absent ARTs suggests the possibility that a combination of ART and OAE testing might identify infants at an elevated risk for AN/AD. An ART refer would indicate added risk for AN/AD independent of the outcome of the OAE test (this situation also arises for the first possible outcome). An ABR test might be helpful in such a circumstance to evaluate the AN/AD risk, even in an ear with OAEs present. In a NHS protocol based on ABR testing, whether in the normal nursery or NICU, the ART test outcome would provide additional information to guide clinical management. These possibilities require further study to assess the potential value of using an expanded test battery in NHS programs.
Acknowledgements
Patrick Feeney provided helpful comments regarding the AR test design. John Ellison, Dawna Lewis, and Ryan McCreery assisted in data collection in this project; John Ellison also assisted in calibrations and data management. Research grant support was provided by the NIDCD grants DC003784, DC006607, DC00013 and DC004662.
Douglas Keefe has a commercial involvement related to the objectives of this research.
Abbreviations
- ABR
auditory brainstem response
- AN/AD
auditory neuropathy/auditory dyssynchrony
- AR
acoustic reflex
- AROC
area under the ROC curve
- ART
acoustic reflex threshold
- ATF
acoustic transfer function
- BBN
broadband noise
- CEOAE
click-evoked otoacoustic emission
- DPOAE
distortion product otoacoustic emission
- DP1
Infant group on Day 1 with DPOAE test result
- DP1-Pass
Infants passing DPOAE test on Day 1
- DP1-Refer
Infants referring on DPOAE test on Day 1
- DP2
Infant group on Day 2 with DPOAE test result
- DP2-Pass
Infants passing DPOAE test on Day 2
- DP2-Refer
Infants referring on DPOAE test on Day 2
- EDHI
early detection of hearing impairment
- IQR
inter-quartile range
- MEMR
middle-ear muscle reflex
- MOCR
medial olivocochlear reflex
- NHS
newborn hearing screening
- NICU
neonatal intensive care unit
- NR
no response
- OAE
otoacoustic emission
- peSPL
peak-equivalent sound pressure level
- ROC
receiver operating characteristic
- SD
standard deviation
- SPL
sound pressure level
- SYM
symmetry point on the ROC curve
- TM
tympanic membrane
- TPP
tympanometric peak pressure
- WB
wideband
- θL
Low-frequency ART
- θH
High-frequency ART
Appendix: Simple time-domain model of reflex effects
A time-domain model of reflex effects acting on the click-difference responses was developed and used to analyze individual-ear response such as those shown in Fig. 2–3 (bottom panels). The rationale was to provide qualitative insight into these waveform responses, even if the model was too simplified to provide a quantitatively accurate result.
The ear-canal pressure p(t) at the tympanic membrane (TM) of area A forces the TM to move in air with phase velocity c and equilibrium density ρ. The TM mechanics are modeled by a simple harmonic oscillator, with TM mass per unit area m, stiffness per unit area κ, and resistance per unit area βz. This resistance is expressed as the product of a dimensionless positive constant β and the specific impedance z = ρc of air. The oscillator equation for TM volume displacement V(t) is
| (1) |
Continuity between the acoustic signal and the TM volume displacement implies that the acoustic volume velocity u = dV / dt just in front of the TM. The acoustic pressure and volume velocity are expressed in terms of the incident and reflected acoustic pressure, pi and pr, respectively, by
| (2) |
The ear canal is modeled using cylindrical cross-section of area A, and effects of re-reflections from the ear-canal probe are not included. The reflected pressure is
| (3) |
so that the pressure is
| (4) |
The incident pressure is assumed to be a delta function source with impulse strength Ji, i.e.,
| (5) |
The oscillator equation takes the form,
| (6) |
The corresponding frequency-domain equation is discussed by Rabbitt (1990). The solution to this equation is expressed in terms of the radian resonance frequency ω0 and the damping coefficient b by (Morse and Ingard, 1968):
| (7) |
The step function is ε(t), which is zero for t < 0, and one otherwise.
The click-difference waveform Δp(t) is calculated as the difference in the pressure waveform p′(t) with the MEMR active to the pressure waveform p(t) with MEMR effects absent, i.e., Δp(t) = p′(t) − p(t). Because re-reflections from the probe have been neglected for simplicity, the incident pressure is the same in both conditions, so that
| (8) |
in which the reflected pressure with the MEMR active is The click-difference pressure is equal to the difference in reflected pressure waveforms. The resulting reflected pressure is,
| (9) |
A simple model of MEMR effects is adopted in which the TM stiffness is increased to κ′ > κ and the mass and damping are unchanged. The increase in stiffness increases the resonance frequency The predicted click-difference waveform is
| (10) |
A short-duration incident “click” with a non-zero pressure amplitude P0 over a time interval Δt has an impulse Ji = P0 Δt, and the delta-function solution is adequate as long as Δt is much less than the oscillator period T0 = 2π / ω0 (otherwise, an additional convolution is needed with an arbitrary incident-pressure waveform). Mean values calculated from equivalent rectangular amplitude fits were used for the impulse, i.e., Δt = 2.7 ms andP0 =5.0 dyne/cm2 in adults, and Δt = 3.0 ms andP0 =8.7 dyne/cm2 in infants. The mechanical properties of the TM were modeled in adults using κ= 3e5 dyne/cm, m = 4e −3 g/cm, and β= 0.4, and in infants using κ= 4e5 dyne/cm3m = 9e − 3 g/cm, and β= 0.1. The MEMR action was modeled by a relative increase in stiffness of 10, 20 and 30%. The calculations also included the round-trip travel time between microphone and probe based on a separation distance of 1 cm in adults and 0.3 cm in infants; results were relatively insensitive to these values.
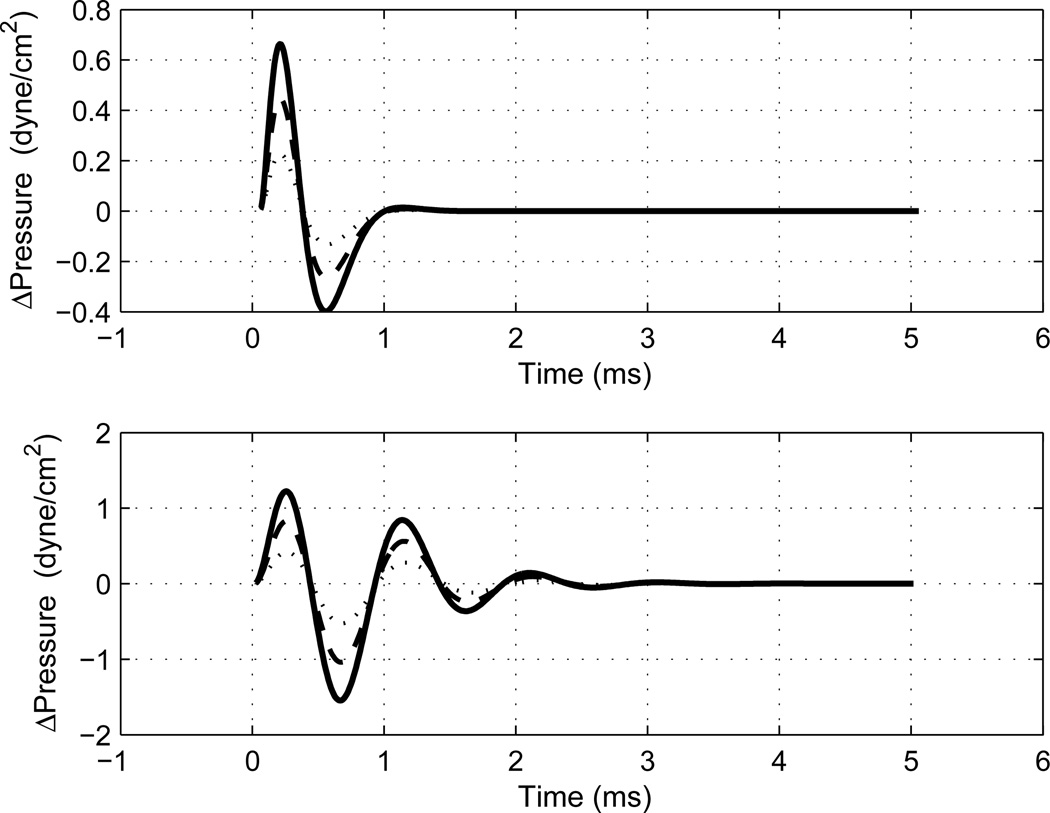
Model results (Fig. 11) show that the main features of the measured click-difference responses (bottom panels, Figs. 2–3) were adequately described. The modeled click-difference waveform has the correct order of magnitude, and the time delay of its main positive-polarity lobe can be interpreted as a temporal build-up of the resonant-mode response.
Fig. 11.
[grey-scale, EPS]. The click-difference waveforms are plotted that were generated by a time-domain simple-harmonic oscillator model of middle-ear response, including the round-trip travel time in an ear canal with cylindrical dimensions, based on an input signal with the same impulse strength as the measured click stimulus. Top: results modeling the measured adult-ear response in Fig. 2 (bottom). Bottom: results modeling the measured infant-ear response in Fig. 3 (bottom). Modeling results are shown for three stiffnesses, with the thickest solid line corresponding to the largest stiffness.
Footnotes
This report is based on a presentation at the 5th International Middle-Ear Mechanics in Research and Otology meeting, Stanford University, Palo Alto, CA (June 24–28, 2009).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Douglas H. Keefe, Email: keefe@boystown.org.
Denis Fitzpatrick, Email: fitzpatrickd@boystown.org.
Yi-Wen Liu, Email: liuy@boystown.org.
Chris A. Sanford, Email: sanfordc@boystown.org.
Michael P. Gorga, Email: gorga@boystown.org.
References
- Abdala C, Keefe DH. Effects of middle-ear immaturity on distortion-product otoacoustic emission suppression tuning in infant ears. J. Acoust. Soc. Am. 2006;120:3832–3842. doi: 10.1121/1.2359237. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Weatherby LA. Newborn acoustic reflexes to noise and pure-tone signals. J. Speech Hear. Res. 1982;25:383–387. doi: 10.1044/jshr.2503.383. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Morlet T, Wilensky D, St. John P, Montgomery E, Thibodaux M. Absent or elevated middle ear muscle reflexes in the presence of normal otoacoustic emissions: a universal finding in 136 cases of auditory neuropathy/dys- synchrony. J. Am. Acad. Audiol. 2005;16:546–53. doi: 10.3766/jaaa.16.8.3. [DOI] [PubMed] [Google Scholar]
- Ellison JC, Keefe DH. Audiometric predictions using stimulus-frequency otoacoustic emissions and middle ear measurements. Ear Hear. 2005;26:487–503. doi: 10.1097/01.aud.0000179692.81851.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney M, Keefe DH. Acoustic reflex detection using wide-band acoustic reflectance, admittance, and power. J. Speech Lang. Hear. Res. 1999;42:1029–1041. doi: 10.1044/jslhr.4205.1029. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH. Estimating the acoustic reflex threshold from wideband measures of reflectance, admittance, and power. Ear Hear. 2001;22:316–332. doi: 10.1097/00003446-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH, Marryott LP. Contralateral acoustic reflex thresholds for tonal activators using wideband energy reflectance and admittance. J. Speech Lang. Hear. Res. 2003;46:128–136. doi: 10.1044/1092-4388(2003/010). [DOI] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH, Sanford CA. Wideband reflectance measures of the ipsilateral acoustic stapedius reflex threshold. Ear Hear. 2004;25:421–430. doi: 10.1097/01.aud.0000145110.60657.73. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Sanford CA. Detection of the acoustic stapedius reflex in infants using wideband energy reflectance and admittance. J. Am. Acad. Audiol. 2005;16:278–290. doi: 10.3766/jaaa.16.5.3. [DOI] [PubMed] [Google Scholar]
- Geddes NK. Tympanometry and the stapedial reflex in the first five days of life. Int. J. Pediatr. Otorhinolaryngol. 1987;13:293–297. doi: 10.1016/0165-5876(87)90110-8. [DOI] [PubMed] [Google Scholar]
- Gelfand SA. The acoustic reflex. In: Katz J, Medwetsky L, Burkhard R, Hood LJ, editors. Handbook of Clinical Audiology. 6th edition. Baltimore: Lippincott Williams & Wilkins; 2009. pp. 189–221. Chapter 10. [Google Scholar]
- Green DM. A maximum-likelihood method for estimating thresholds in a yes-no task. J. Acoust. Soc. Am. 1993;93:2096–2105. doi: 10.1121/1.406696. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr. Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. Erratum, 2007, Ear Hear., 28, 129. [DOI] [PubMed] [Google Scholar]
- Hirsch JE, Margolis RH, Rykken JR. A comparison of acoustic reflex and auditory brain stem response screening of high-risk infants. Ear Hear. 1992;13:181–186. doi: 10.1097/00003446-199206000-00007. [DOI] [PubMed] [Google Scholar]
- Holte L, Cavanaugh RM, Jr., Margolis RH. Ear canal wall mobility and tympanometric shape in young infants. J. Pediatr. 1990;117:77–80. doi: 10.1016/s0022-3476(05)82448-5. [DOI] [PubMed] [Google Scholar]
- Hunter L, Ries DT, Schlauch RS, Levine SC, Ward WD. Safety and clinical performance of acoustic reflex tests. Ear Hear. 1999;20:506–514. doi: 10.1097/00003446-199912000-00006. [DOI] [PubMed] [Google Scholar]
- Joint Committee on Infant Hearing (JCIH) Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Abdala C. Theory of forward and reverse middle-ear transmission applied to otoacoustic emissions in infant and adult ears. J. Acoust. Soc. Am. 2007;121:978–993. doi: 10.1121/1.2427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Bulen JC, Hoberg K, Burns EM. Ear-canal impedance and reflection coefficient of human infants and adults. J. Acoust. Soc. Am. 1993;94:2617–2638. doi: 10.1121/1.407347. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Schairer KS, Ellison JC, Fitzpatrick DF, Jesteadt W. Use of stimulus-frequency otoacoustic emissions to investigate efferent and cochlear contributions to temporal overshoot. J. Acoust. Soc. Am. 2009;125:1595–1604. doi: 10.1121/1.3068443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YW, Sanford CA, Ellison JC, Fitzpatrick DF, Gorga MP, Keefe DH. Wideband absorbance tympanometry using pressure sweeps: System development and results on adults with normal hearing. J. Acoust. Soc. Am. 2008;124:3708–3719. doi: 10.1121/1.3001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RH. Detection of hearing impairment with the acoustic stapedius reflex. Ear Hear. 1993;14:3–10. doi: 10.1097/00003446-199302000-00002. [DOI] [PubMed] [Google Scholar]
- Mazlan R, Kei J, Hickson Test-retest reliability of the acoustic stapedial reflex test in healthy neonates. Ear Hear. 2009;30:295–301. doi: 10.1097/AUD.0b013e31819c3ea0. [DOI] [PubMed] [Google Scholar]
- McMillan PM, Bennett MJ, Marchant CD, Shurin PA. Ipsilateral and contralateral acoustic reflexes in neonates. Ear Hear. 1985;6:320–324. doi: 10.1097/00003446-198511000-00008. [DOI] [PubMed] [Google Scholar]
- Morse PM, Ingard KU. Theoretical Acoustics. New York: McGraw-Hill; 1968. [Google Scholar]
- Müller-Wehlau M, Mauermann M, Dau T, Kollmeier B. The effects of neural synchronization and peripheral compression on the acoustic-reflex threshold. J. Acoust. Soc. Am. 2005;117:3016–3027. doi: 10.1121/1.1867932. [DOI] [PubMed] [Google Scholar]
- Neumann J, Uppenkamp S, Kollmeier B. Detection of the acoustic reflex below 80 dB HL. Audiol. Neurootol. 1996;1:359–369. doi: 10.1159/000259219. [DOI] [PubMed] [Google Scholar]
- Pang XD, Peake WT. How do contractions of the stapedius muscle alter the acoustic properties of the ear? In: Allen J, Hall J, Hubbard A, Neely S, Tubis A, editors. Mechanics of Hearing Workshop. Peripheral Auditory Mechanisms. New York: Springer- Verlag; 1985. [Google Scholar]
- Qi L, Funnell WR, Daniel SJ. A nonlinear finite-element model of the newborn middle ear. J. Acoust. Soc. Am. 2008;124:337–347. doi: 10.1121/1.2920956. [DOI] [PubMed] [Google Scholar]
- Qi L, Liu H, Lutfy J, Funnell RJ, Daniel SJ. A nonlinear finite-element model of the newborn ear canal. J. Acoust. Soc. Am. 2006;120:3789–3798. doi: 10.1121/1.2363944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt RD. A hierarchy of examples illustrating the acoustic coupling of the eardrum. J. Acoust. Soc. Am. 1990;87:2566–2582. doi: 10.1121/1.399050. [DOI] [PubMed] [Google Scholar]
- Rabinowitz WM. Acoustic-reflex effects on the input admittance and transfer characteristics of the human middle ear. Cambridge, Massachusetts: Massachusetts Institute of Technology; 1977. Unpublished doctoral dissertation. [Google Scholar]
- Sanford CA, Keefe DH, Liu YW, Fitzpatrick DF, McCreery R, Lewis DE, Gorga MP. Sound-conduction effects on DPOAE screening outcomes in newborn infants: Test performance of wideband acoustic transfer functions and 1-kHz tympanometry. 2009 doi: 10.1097/AUD.0b013e3181b61cdc. In press, Ear Hear. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer KS, Ellison JC, Fitzpatrick, Keefe DH. Wideband ipsilateral measurements of middle-ear muscle reflex thresholds in children and adults. J. Acoust. Soc. Am. 2007;121:3607–3616. doi: 10.1121/1.2722213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague BH, Wiley TL, Goldstein R. Tympanometric and acoustic-reflex studies in neonates. J. Speech Hear. Res. 1985;28:265–272. doi: 10.1044/jshr.2802.265. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119:741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Stephenson H, Higson JM, Haggard MP, Dutson M, Rogers M, Schilder AG. The acoustic reflex in adults with histories of otitis media in childhood. Ear Hear. 1997;18:62–72. doi: 10.1097/00003446-199702000-00006. [DOI] [PubMed] [Google Scholar]
- Weatherby LA, Bennett MJ. The neonatal acoustic reflex. Scand. Audiol. 1980;9:103–110. doi: 10.3109/01050398009076343. [DOI] [PubMed] [Google Scholar]