Exercise intolerance is the primary symptom of chronic diastolic heart failure (DHF). It is part of the definition of heart failure and is intimately linked to its pathophysiology. Further, exercise intolerance affects the diagnosis and prognosis of heart failure. In addition, understanding the mechanisms of exercise intolerance can lead to developing and testing rational treatments for heart failure. This article focuses on the fundamental principles of exercise physiology and on the assessment, pathophysiology, and potential treatment of exercise intolerance in DHF.
IMPORTANCE OF EXERCISE INTOLERANCE
Heart failure is defined as a syndrome in which cardiac output is insufficient to meet metabolic demands. This definition implies that insufficient cardiac output will be expressed symptomatically. Heart failure often may manifest by occasional episodes of acute decompensation with overt systemic volume overload and pulmonary edema.1,2 Exertional fatigue and dyspnea, however, are the primary chronic symptoms in outpatients, even when well compensated and non-edematous, and whether associated with reduced or normal ejection fraction (EF).3 In addition, these symptoms and other consequences of exercise intolerance are potent determinants of health-related quality of life in patients who have heart failure. Several investigators have reported that objective measures and even subjective estimates of exercise tolerance are predictors of survival.4,5
Exercise intolerance can be quantified objectively using semiquantitative assessments, such as interview (New York Heart Association [NYHA] classification) and surveys (Minnesota Living with Heart Failure or Kansas City Cardiomyopathy questionnaires), and quantitative methods, including timed walking tests (6-minute walk distance) and graded exercise treadmill or bicycle exercise tests. Cardiopulmonary exercise testing on a tread-mill or a bicycle ergometer provides the most accurate, reliable, and reproducible assessments of exercise tolerance and yields multiple important outcomes, including metabolic equivalents, exercise time, exercise workload, blood pressure and heart rate responses, and rate–pressure product. Using commercially available instruments that perform automated concentration and volume analyses of expired gas, one can assess simultaneously measures of oxygen consumption (VO2), carbon dioxide generation, and ventilatory response both at rest and during exercise. Patient effort is an important modifier of data quality and can itself be assessed simultaneously, objectively by expired gas analysis (as the respiratory exchange ratio) and by the somewhat subjective but more easily obtained measures of perceived effort by the Borg scale and percent age-predicted maximal heart rate.
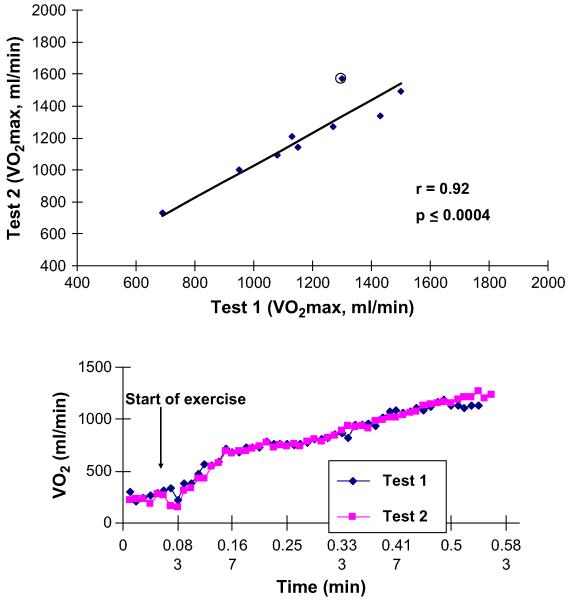
Submaximal exercise is in some ways a more important outcome variable than peak exercise capacity because it is more applicable to everyday life and is relatively independent of effort. Submaximal exercise capacity can be assessed as the ventilatory anaerobic threshold by expired gas analysis, using either the Wasserman-Whipp or the V-slope method. Cardiopulmonary exercise testing measurements and expired gas analysis with automated, commercially available instruments provides measures of both peak oxygen consumption and of ventilatory anaerobic threshold that are valid and highly reproducible in elderly patients who have DHF as well as in those who have systolic heart failure (SHF) (Fig. 1). Another variable provided by these methods, the ventilation/carbon dioxide production (VE/VCO2) slope, is a strong predictor of survival, independent of VO2.6 The VE/VCO2 slope is abnormal in patients who have DHF, although it is not as abnormal as it is in those who have SHF.7
Fig. 1.
Excellent reproducibility of peak exercise VO2 in older patients who have heart failure, including those who have LVEF. (Top panel) Group data. (Bottom panel) 15-Second averaged data from a representative patient. (From Marburger CT, Brubaker PH, Pollock WE, et al. Reproducibility of cardiopulmonary exercise testing in elderly heart failure patients. Am J Cardiol 1998;82:905-9; with permission.)
Submaximal exercise performance also can be assessed by timed and distance walk tests. These tests are simple to perform and are widely available. The authors have shown that the 6-minute walk distance is decreased considerably in elderly patients who have DHF. In group data, the reduction is in proportion to both peak exercise oxygen consumption and ventilatory anaerobic threshold. The authors’ published studies, however, suggest that 6-minute walk testing has only modest accuracy for predicting peak exercise capacity in individual patients compared with direct measurement with cardiopulmonary exercise testing with expired gas analyses and also is not as reproducible.8
PATHOPHYSIOLOGY OF EXERCISE INTOLERANCE
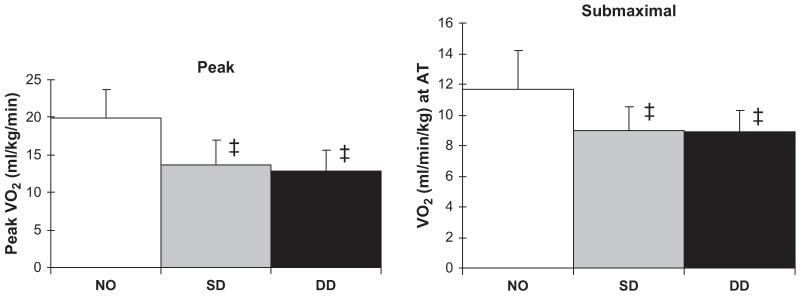
To understand the pathophysiology of exercise intolerance in DHF, the authors performed a comparative study of maximal exercise testing with expired gas in 119 older subjects in three distinct, well-defined groups: persons who had heart failure with severe left ventricular (LV) systolic dysfunction (mean EF, 30%); persons who had isolated DHF (EF ≥50% and no significant coronary, valvular, pericardial, or pulmonary disease and no anemia); and age-matched controls.3 In comparison with the controls, peak exercise VO2 was severely reduced in the patients who had DHF, to a degree similar to the reduction in those with SHF (Fig. 2).3 Submaximal exercise capacity, as measured by the ventilatory anaerobic threshold, was reduced in patients who had DHF versus those who had SHF, and this reduced exercise capacity was accompanied by reduced health-related quality of life.3
Fig. 2.
VO2 during peak exhaustive exercise (left panel) and during submaximal exercise at the ventilatory anaerobic threshold (right panel) in age-matched normal subjects (NO), elderly patients who have heart failure caused by systolic dysfunction (SD), and elderly patients who have heart failure with normal systolic function, that is, presumed diastolic dysfunction (DD). Exercise capacity is severely reduced in patients who have DHF compared with normal controls (P<.001), to a degree similar to the reduction in patients who have SHF. Overall, peak exercise VO2 was 33% lower in the women than in the men (not shown). (Data from Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002;288(17):2144-50.)
By the Fick equation, peak VO2 during exercise is the product of cardiac output and arteriovenous oxygen (A-VO2) difference, indicating that exercise intolerance is related to one or both of these factors and to the variables that influence them. Measurement of peak exercise VO2 and at least one of these other two factors (cardiac output or A-VO2 difference) allows one to calculate the remaining unknown factor and to begin to isolate specific factors that contribute to exercise intolerance in individual patients and within groups (Fig. 3).
Fig. 3.
Potential mechanisms of exercise intolerance from the factors of the Fick equation. EDV, enddiastolic volume; ESV, end-systolic volume.
Central Cardiac Response to Exercise
These principles were used to examine the determinants of exercise performance in normal persons and in patients who had heart failure. A series of cardiopulmonary exercise studies was performed using symptom-limited upright bicycle exercise with indwelling pulmonary artery and brachial artery catheters and simultaneous expired gas analysis and radionuclide ventriculography.9-17 Cardiac output was determined by the Fick principle for oxygen, and LV end-diastolic volume and end-systolic volume were calculated from the Fick stroke volume and the radionuclide EF (LVEF).
In healthy young and middle-aged male and female volunteers, VO2 increases 7.7-fold from rest to peak exercise during upright bicycle exercise,9,14 and this increase is achieved by a 3.2-fold increase in cardiac output and a 2.5-fold increase in A-VO2 difference. The increase in cardiac output results from a 2.5-fold increase in heart rate and a 1.4-fold increase in stroke volume. Stroke volume increases during low levels of exercise via the Frank-Starling mechanism; during higher levels of exercise, stroke volume increases predominantly because of increased contractility and even may decline slightly because of tachycardia and limited filling time.
Aging is accompanied by reduced peak exercise VO2 caused by an age-related decline in peak exercise cardiac output, heart rate, stroke volume, and LVEF.10,13 Thus, in normal subjects, stroke volume and end-diastolic volume response are important contributors to the increase in VO2 and cardiac output during upright exercise and are altered by normal aging but not by gender.
This information regarding the physiology of exercise in normal persons and changes with aging provides background for a series of studies the authors performed to understand the cardiovascular and peripheral mechanisms of the reduced exercise capacity in patients who have DHF. Invasive cardiopulmonary exercise testing was performed in seven patients who had severe but stable chronic heart failure, six of whom had had at least one episode of clinically and radiographically documented pulmonary edema.15 Patients had no significant coronary artery disease detected by angiography, normal LVEF (≥ 50%), no wall motion abnormalities, and no evidence of valvular or pericardial disease. Most, but not all, patients had a history of hypertension and increased LV mass. Ten age-matched and gender-matched healthy volunteers served as normal controls.
Patients who had DHF had marked exercise intolerance and a 48% reduction in peak oxygen consumption. In patients and normal subjects, exercise was limited primarily by leg fatigue, and dyspnea also was reported frequently.15 The peak respiratory exchange ratio was greater than 1.10 and was similar in patients who had DHF and normal subjects, suggesting good exercise effort in both groups. In both groups, arterial lactate concentration increased several fold from rest to peak exercise. During submaximal exercise at 50 watts, where oxygen consumption was similar in patients and controls, lactate concentration tended to be increased in the patients compared with the normal subjects (2.2 ± 1.1 vs 1.4 ± 0.7 mmol/L).
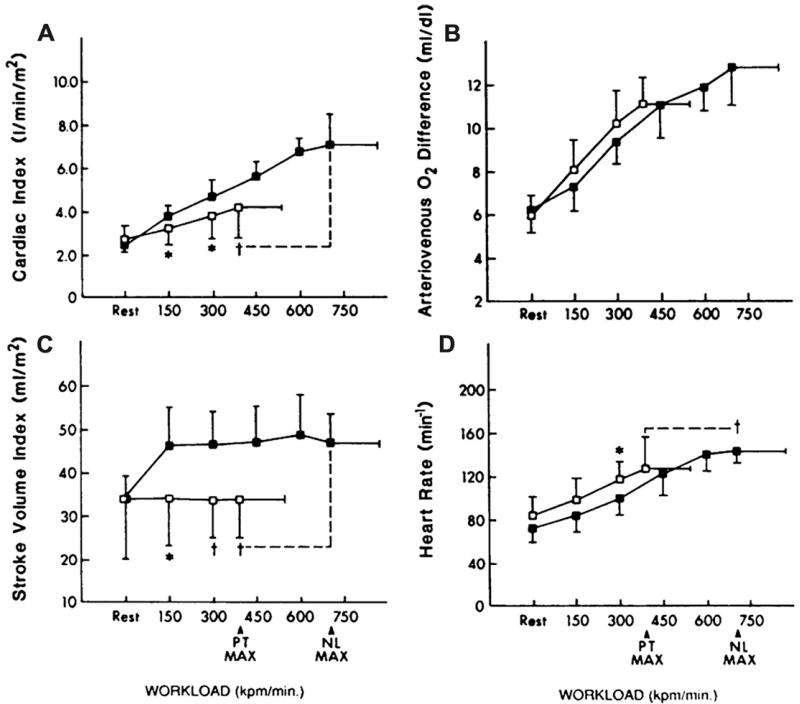
At rest, there were no intergroup differences between the two groups in cardiac output, central A-VO2 difference, stroke volume, or heart rate. Cardiac output was significantly reduced in the patients at submaximal workloads, however, and was severely reduced by 41% at peak exercise (Fig. 4A). Central A-VO2 difference was increased by approximately 10% in the patients during the submaximal exercise, partially compensating for the reduced cardiac index (Fig. 4B). At peak exercise, however, this mechanism was outstripped, and the A-VO2 difference was reduced by 13%. In the patients, the change in cardiac output from rest to peak exercise correlated closely with the increase in VO2 during exercise (r = 0.81; P<.03).
Fig. 4.
Cardiovascular function assessed by invasive cardiopulmonary exercise testing in patients who have heart failure and normal systolic function (open boxes) and age-matched normal controls (closed boxes). The primary components of the Fick equation for VO2, cardiac output, and A-VO2 difference are shown in panels A and B, respectively. The components of cardiac output, stroke volume, and heart rate are shown in panels C and D. The X-axis is exercise workload in kilopounds/min (kpm); 150 kpm is equivalent to 25 W. (From Kitzman DW, Higginbotham MB, Cobb FR, et al. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol 1991;17:1065-72; with permission. Copyright © 1991, American College of Cardiology.)
Stroke volume was reduced in the patients during submaximal exercise and was markedly reduced (−26%) at peak exercise (Fig. 4C).15 Like-wise, heart rate was reduced by 18% in patients compared with controls at peak exercise (Fig. 4D). The change in stroke volume correlated well with the increase in cardiac output during exercise, suggesting that reduced stroke volume was the primary factor for reduced cardiac output and for the 48% reduction in peak VO2 in the patients who had DHF.
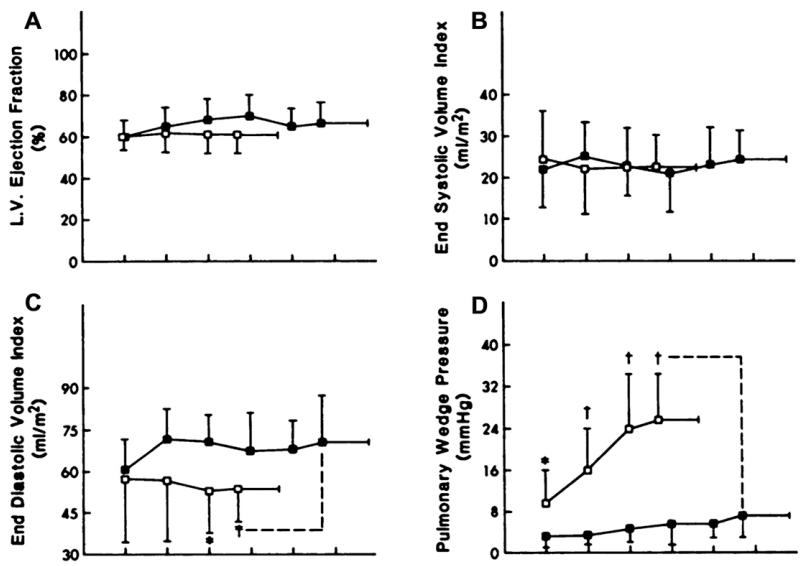
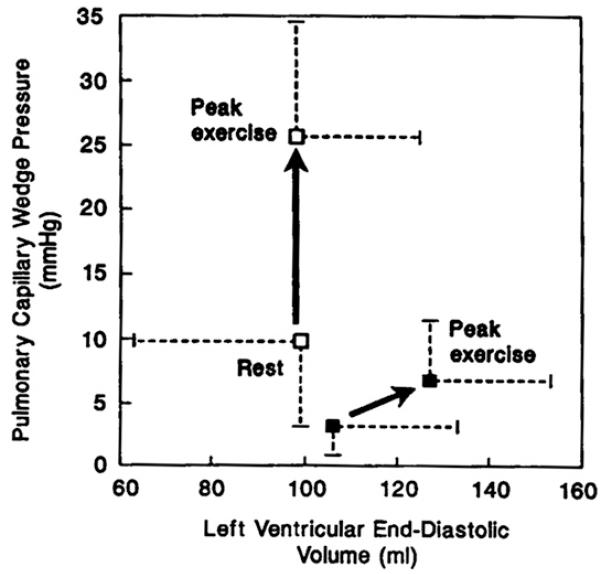
A number of factors might contribute to the abnormal stroke volume response in the patients (Fig. 5A-D). The LVEF and end-systolic volume index during rest and exercise were not different from those in normal subjects (see Fig. 5A, B), confirming that systolic function was within normal limits (see Fig. 5B). End-diastolic volume, in contrast, was reduced markedly during exercise, resulting in a flattened curve that was similar to the abnormal stroke volume response (see Fig. 5C). In the patients who had heart failure, the change in end-diastolic volume from rest to peak exercise correlated strongly with the change in stroke volume and in cardiac output.15
Fig. 5.
The components of the LV stroke volume response during exercise, LVEF, end-systolic volume, end-diastolic volume, and LV filling pressure are shown in panels A-D. Not shown are systolic and mean arterial pressures, which were not different between groups. The key is the same as in Fig. 4. (From Kitzman DW, Higginbotham MB, Cobb FR, et al. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol 1991;17:1065-72; with permission. Copyright © 1991, American College of Cardiology.)
Pulmonary wedge pressure as an estimate of LV filling pressure was mildly increased in the patients at rest and became severely increased during exercise (see Fig. 5D). Notably, however, the change in pulmonary wedge pressure from rest to peak exercise did not correlate significantly with the change in stroke volume or the increase in VO2 during exercise. The LV end-diastolic pressure-volume ratio tended to be elevated in the patients at rest and became markedly increased during exercise. The upward, left-shifted LV diastolic pressure-volume relationship in the patients who had DHF (Fig. 6)15 indicates that the patients did not use the Frank-Starling mechanism, probably primarily because of diastolic LV dysfunction. In contrast, patients who have heart failure and reduced systolic function have an operating pressure-volume relationship that is shifted upward and to the right during exercise.18
Fig. 6.
LV diastolic function assessed by invasive cardiopulmonary exercise testing. The key is the same as in Fig. 4. The pressure-volume relationship was shifted upward and leftward at rest. In the patients undergoing exercise testing, LV diastolic volume did not increase despite the marked increase in diastolic (pulmonary wedge) pressure. Because of diastolic dysfunction, failure of the Frank-Starling mechanism resulted in severe exercise intolerance. (From Kitzman DW, Higginbotham MB, Cobb FR, et al. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol 1991;17:1065-72; with permission. Copyright © 1991, American College of Cardiology.)
Although these invasively assessed LV filling pressures offers key insights into exercise intolerance, their invasive nature limits their overall utility. Noninvasive Doppler mitral filling indices, particularly the more recently developed tissue Doppler indices, can give insight into LV diastolic function. The time constant of isovolumic pressure decline (t) can be estimated noninvasively by measuring the early diastolic velocity of the mitral annulus (E′).19 Furthermore, the ratio of early LV diastolic filling velocity (E) to E′ correlates well with invasively measured LV end-diastolic pressures.20 Notably, an increased E/E′ ratio at rest has been correlated with maximal and submaximal exercise intolerance.21,22 In addition, an increase in E/E′ during exercise correlates with exercise intolerance.23
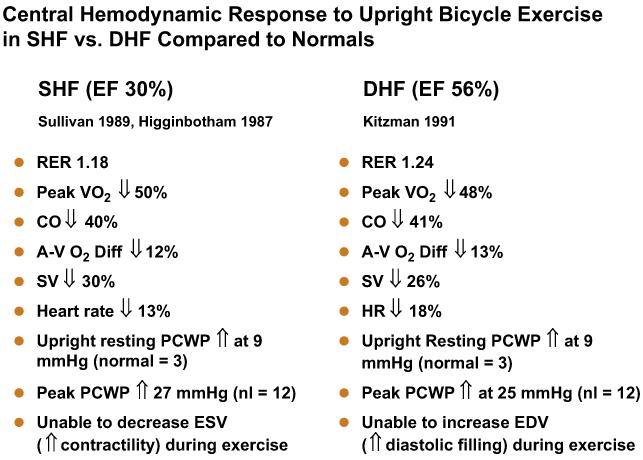
Comparison of the exercise cardiovascular responses in the two different groups of patients who had heart failure (those who had normal EFs15 and those who had reduced EFs11,13,15) can be instructive. Both had severe exertional symptoms and objective evidence of exercise intolerance, markedly reduced peak cardiac output and stroke volume, mildly reduced peak heart rate, and slightly reduced peak A-VO2 difference (Fig. 7). Both groups also had mildly increased resting and markedly elevated exercise mean pulmonary capillary wedge pressures. The means by which LV stroke volume was reduced differed, however. In one group patients had profound systolic contractile dysfunction and were able to use markedly increased LV filling pressure to produce greater-than-normal use of the Frank-Starling mechanism to compensate partially and maintain an increase in exercise stroke volume.11,13 In the other group, despite normal systolic contractile function and markedly increased LV filling pressure,15 patients were unable to use the Frank-Starling mechanism to increase stroke volume during exercise (see Fig. 7).24
Fig. 7.
Comparison of characteristic central and peripheral cardiovascular response to exercise in patients who have heart failure associated with severe LV systolic dysfunction (HFpEF) versus normal LVEF. See text for discussion. CO, carbon dioxide; EDV, end-diastolic volume; ESV, end-systolic volume; PCWP, pulmonary capillary wedge pressure; RER, respiratory exchange ratio; SV, stroke volume. (Data from Refs.11,12,15)
Heart Rate Response to Exercise
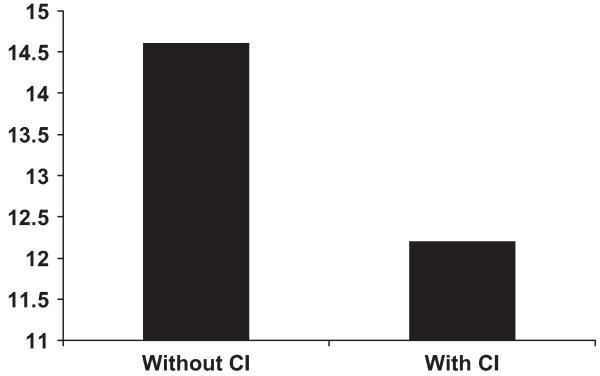
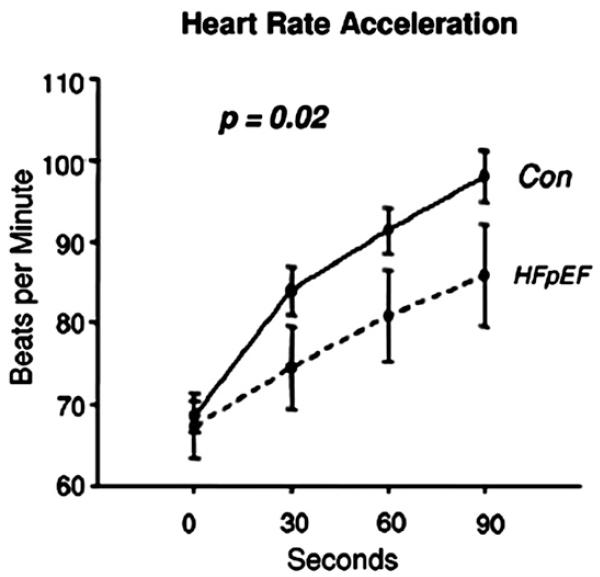
Decreased heart rate response also contributes to the reduced cardiac output at peak exercise and thus to reduced peak exercise VO2. Indeed, chronotropic incompetence has been a frequent finding during cardiopulmonary exercise studies in SHF, but data were lacking for older patients and particularly for those who had normal EFs. Therefore, the authors compared heart rate and expired gas analyses responses in elderly patients who had DHF with those in a group of age- and gender-matched patients who had SHF and in healthy normal controls. Using the most standard definition of chronotropic incompetence, the authors found that chronotropic incompetence was present in 20% to 25% of older patients who had heart failure, that the prevalence was similar in patients who had DHF and those who had SHF, that the presence of chronotropic incompetence contributed significantly to the degree of exercise intolerance, measured as maximal VO2, and that this contribution was independent of medications, including beta-adrenergic antagonists (Fig. 8).25 The important contribution of chronotropic incompetence to exercise intolerance in patients who had DHF was confirmed by Borlaug and colleagues,26 who studied a cohort of primarily elderly, African American women who had hypertension and heart failure with a preserved EF. They reported that significant reductions in the rate of heart rate increase during exercise were primary contributors to reduced peak cardiac index and maximal exercise VO2 (Fig. 9). The implication of this finding merits further investigation and has therapeutic implications.
Fig. 8.
Peak exercise VO2 (y-axis, in mL/kg/min) in patients who have heart failure with and without chronotropic incompetence (CI). Patients who have chronotropic incompetence have more severe exercise intolerance, suggesting a contributory role for CI. (Adapted from Brubaker PH, Joo KC, Steward KP, et al. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehab 2006;26:86-9; with permission.)
Fig. 9.
Heart rate acceleration during exercise in controls (Con) and patients who have heart failure and preserved EF (HFpEF). (From Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 2006;114:2144; with permission.)
Central and Peripheral Vascular Contributions to Exercise Intolerance
Abnormal afterload and abnormal ventricular-vascular coupling may contribute to the abnormal Frank-Starling response seen in the patients who have DHF. Nearly all such patients (88%) have a history of chronic systemic hypertension.27-29 In animal models, diastolic dysfunction develops early in systemic hypertension, and LV diastolic relaxation is sensitive to increased afterload,30-35 which can impair relaxation, leading to increased LV filling pressures and decreased stroke volume and could lead to symptoms of dyspnea and congestion.1,2,33
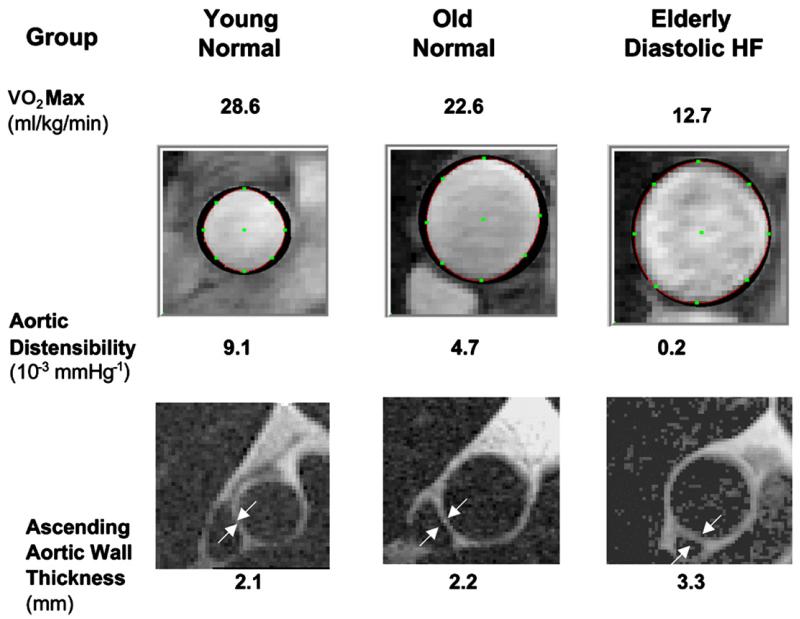
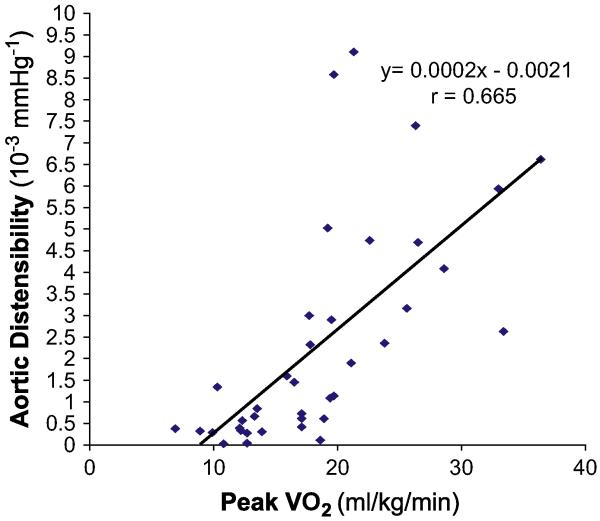
In animal models and in humans, chronic systolic hypertension accelerates and magnifies the age-related increase in fibrotic thickening of the aortic wall and resultant increase in aortic stiffness, which is a major determinant of LV after-load and ventricular-vascular coupling.36,37 To determine whether abnormally decreased aortic distensibility contributes to the severe exercise intolerance in heart failure with normal EF, the authors performed MRI and maximal exercise testing with expired gas analysis in a group of elderly patients who had isolated DHF, as defined earlier, with young healthy subjects and age-matched healthy subjects as normal controls. The patients who had DHF had severe exercise intolerance that was associated with increased pulse pressure and concentric hypertrophic LV remodeling. Thoracic aortic wall thickness was increased 50%, and there was markedly decreased aortic distensibility (Fig. 10). In univariate analysis, decreased aortic distensibility correlated closely with severely decreased peak exercise VO2 (Fig. 11).38 In multivariate analysis, decreased aortic distensibility was the strongest independent predictor of reduced exercise capacity. These data support a potentially important role of increased aortic stiffness, caused by underlying aging and amplified by chronic hypertension, in the pathophysiology of chronic heart failure symptoms.39
Fig. 10.
Data and images from representative subjects from healthy young persons, healthy elderly persons, and elderly patients who have diastolic heart failure (HF). Maximal exercise oxygen consumption (VO2max), aortic distensibility at rest, and the LV mass:volume ratio are shown. Patients who have DHF have severely reduced exercise tolerance (VO2max) and aortic distensibility and increased aortic wall thickness (arrows). (Adapted from Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle dependent changes in aortic area and aortic distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol 2001;38(3):796-802; with permission. Copyright © 2001, American College of Cardiology.)
Fig. 11.
There is a close relationship between peak exercise VO2 (horizontal axis) and proximal aortic distensibility (vertical axis) in a group of 30 subjects (10 healthy young persons, 10 healthy elderly persons, and 10 elderly patients who have DHF). Each symbol represents the data from one participant. (From Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle dependent changes in aortic area and aortic distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol 2001;38(3):796-802; with permission. Copyright © 2001, American College of Cardiology.)
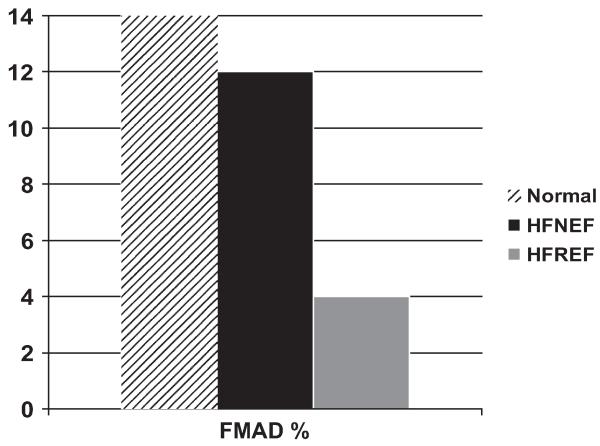
Peripheral arteries must dilate early during exercise to accommodate and facilitate the conveyance of increased nutritive blood flow to working skeletal muscle. Multiple lines of evidence suggest that in patients who have SHF this response is impaired and contributes to exercise intolerance and that this impairment is modifiable with exercise training and other interventions.40-43 The authors examined the flow-mediated arterial dilation (FMAD) response to ischemia-induced by 3- to 5-minute cuff inflation in the femoral artery in elderly patients who had DHF, patients who had SHF, and normal age-matched controls using phase-contrast MRI. They also performed cardiopulmonary exercise testing with expired gas analysis.44 Cardiopulmonary exercise testing again demonstrated severe exercise intolerance in the patients who had DHF that was similar in degree to that in patients who had SHF. The patients who had SHF had severely reduced femoral FMAD compared with normal subjects. In the patients who had DHF, however, the FMAD was relatively preserved and did not differ significantly from that in normal controls (Fig. 12). Thus, the authors concluded that abnormal FMAD was not present in DHF and was not a significant contributor to the severe exercise intolerance in these patients.
Fig. 12.
Flow-mediated arterial dilation (FMAD) of the femoral artery by phase-contrast MRI in normal subjects, elderly patients who have heart failure and normal EF (HFNEF), and patients who have heart failure and reduced EF (HFREF). FMAD is severely reduced in HFREF but is relatively preserved in HFNEF compared with age-matched healthy normal subjects. (From Hundley WG, Bayram E, Hamilton CA, et al. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular EF. Am J Physiol Heart Circ Physiol 2007;292(3):H1427-34; with permission.)
Primary Cause of Symptoms of Exercise Intolerance; Skeletal Muscle; Other Factors
Despite the many physiologic exercise studies that have been performed in patients who have heart failure, uncertainty remains regarding the final stimulus that causes patients to stop exercising at lower workloads than healthy subjects.45-48 It had been thought that increased exercise pulmonary wedge pressure and stimulation of pulmonary J-receptors cause reflex hyperventilation and hypoxia leading to the sensation of severe dyspnea, causing the patient to stop exercise prematurely. About 50% of heart patients who have either SHF or DHF, however, discontinue exercise primarily because of general fatigue or leg fatigue rather than because of dyspnea. In addition, investigators have demonstrated that arterial hypoxia does not occur during exercise in patients who have heart failure and that excess ventilation is related to pulmonary hypoperfusion and reduced cardiac output rather than elevated LV filling pressures.16,49 Furthermore, exercise intolerance, as measured objectively by peak VO2, is unrelated to invasively measured pulmonary capillary wedge pressures, including in patients who have DHF.15 The decreased exercise cardiac output probably causes skeletal muscle hypoperfusion, a potent stimulus for early anaerobic metabolism, and subsequent generation of muscle lactate and other metabolites that could produce the sensation of peripheral and central fatigue.50,51 Indeed, in the studies that reported lactate production during exercise, the production of lactate in persons who had heart failure was abnormal compared with that in normal persons.3,15
Based on the extensive experience in seeking to understand exercise intolerance in patients who have SHF, it is likely that several factors in addition to those discussed previously may contribute to exercise intolerance in patients who have DHF. Such potential contributors include anemia (which is highly prevalent in DHF, as it is in SHF),52 and skeletal muscle bulk, fiber type, and function.11,42,53-61 There have been particularly compelling findings regarding skeletal muscle remodeling in SHF11,56,62-73 but there are no data regarding skeletal muscle remodeling in DHF. Skeletal muscle could be an even more relevant factor in DHF than in SHF, given increasing data regarding the role of skeletal muscle atrophy and dysfunction in older patients who have a variety of disabling chronic syndromes. This area seems to be a particularly promising area for future investigation.74-78 In addition, the amount of adipose tissue between skeletal muscle bundles seems to be a potential modifier of skeletal muscle function and of exercise capacity as well. This area probably will also be a fruitful area for future research, particularly because skeletal muscle bulk and function seem to be potentially modifiable through nutrition and exercise interventions.79
INTERVENTIONS TO IMPROVE EXERCISE TOLERANCE
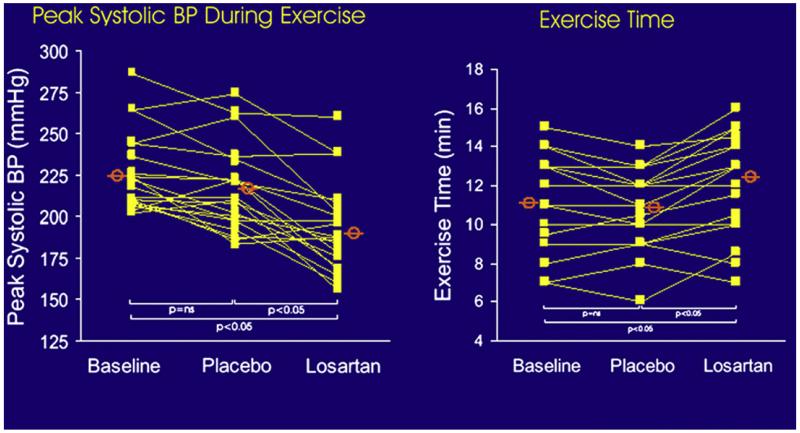
During exercise in normal subjects, systolic and pulse pressure increase substantially, and this response is magnified by increased arterial stiffness. Data from animal models suggest that the exercise-related increase in systolic blood pressure is mediated, in part, by exercise-induced increases in circulating angiotensin II. Indeed, in a randomized, double-blind, placebo-controlled cross-over trial, angiotensin receptor blockade reduced the exaggerated exercise increase in systolic and pulse pressures, resulting in significantly improved exercise treadmill time and quality of life (Fig. 13).80
Fig. 13.
Plots of peak systolic blood pressure (BP) and exercise duration during baseline, during placebo administration, and during losartan administration in a randomized, controlled, cross-over trial. Treatment with the angiotensin II antagonist losartan increased exercise time. (From Warner JG, Metzger C, Kitzman DW, et al. Losartan improves exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise. J Am Coll Cardiol 1999;33:1567-72; with permission. Copyright © 1999, American College of Cardiology.)
In a group of patients who had NYHA class III heart failure and presumed diastolic dysfunction (EF >50%), Aronow and Kronzon81 showed that the angiotensin-converting enzyme inhibitor enalapril significantly improved functional class, exercise duration, EF, diastolic filling, and LV mass.
In hypertrophic cardiomyopathy, a disorder in which diastolic dysfunction is common, verapamil seems to improve symptoms and objectively measured exercise capacity.82-85 This agent also improves ventricular vascular coupling and exercise performance in aged individuals who have hypertension.86 In laboratory animal models calcium antagonists, particularly dihydropyridines, prevent ischemia-induced increases in LV diastolicstiffness87 and improve diastolic performance in pacing-induced heart failure.88-90 Negative inotropic calcium antagonists significantly impair early relaxation,90-94 however, and in general have shown a tendency toward adverse outcome in patients who have SHF.90 Nonetheless, Setaro and colleagues95 examined 22 men (mean age, 65 years) who had clinical heart failure despite an EF greater than 45% in a randomized, double-blind, placebo-controlled cross-over trial of verapamil. There was a 33% improvement in exercise time, and there also were significant improvements in clinicoradiographic heart failure scoring and peak filling rate.
In a randomized, cross-over, blinded trial, Little and colleagues96 compared the calcium-channel antagonist verapamil with the angiotensin receptor antagonist candesartan using the outcomes of peak exercise blood pressure, exercise time, and quality of life. Although both agents blunted the peak systolic blood pressure response to exercise, only candesartan, but not verapamil, improved exercise time and quality of life.96
In a subsequent trial with a similar randomized, cross-over, blinded design, the diuretic hydrochlorothiazide was compared with the angiotensin receptor antagonist losartan using the outcomes of peak exercise blood pressure, exercise time, and quality of life.97 Although both agents blunted the peak systolic blood pressure response to exercise, only losartan, but not hydrochlorothiazide, improved exercise time and quality of life.97
The addition of low-dose spironolactone (12.5-50 mg daily) to standard therapy has been shown to improve exercise tolerance in patients who have severe SHF. Aldosterone antagonism has numerous potential benefits in patients who have DHF, including LV remodeling, reversal of myocardial fibrosis, and improved LV diastolic function and vascular function.98-100 Few data, however, are presently available regarding aldosterone antagonism in DHF. In one small study, low-dose spironolactone was well tolerated and seemed to improve exercise capacity and quality of life in older women who had isolated DHF.101 In another, spironolactone improved measures of myocardial function in hypertensive patients who had DHF.102
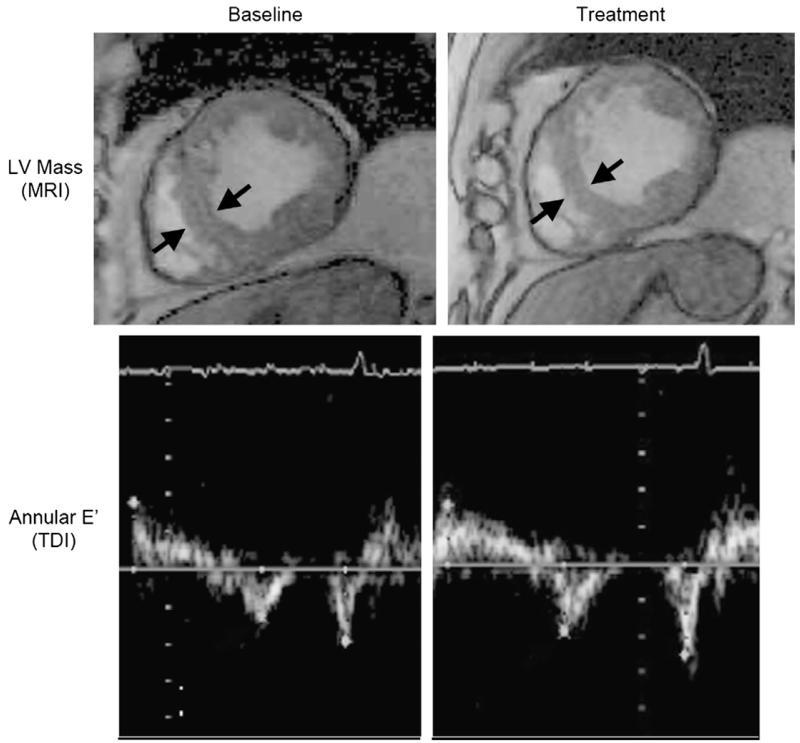
Glucose cross-links increase with aging and diabetes and cause increased vascular and myocardial stiffness. Alagebrium, a novel crosslink breaker, improved vascular and LV stiffness in dogs. In a small, open-label, 4-month trial of this agent in elderly patients, LV mass, quality of life, and tissue Doppler diastolic function indexes improved (Fig. 14), but there were no significant improvements in exercise capacity or aortic distensibility, the primary outcomes of the trial.103 A variety of other agents and strategies for this syndrome, including a selective endothelin antagonist, are being evaluated currently or are under consideration.
Fig. 14.
Effect of alagebrium on LV mass seen by MRI (top panel) and E′ seen by tissue Doppler imaging (TDI) in older patients who have DHF. (From Little WC, Zile MR, Kitzman DW, et al. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail 2005;11(3):191-5; with permission.)
The substantial chronotropic incompetence seen in patients who have DHF and its correlation with reduced exercise capacity described earlier provides a rationale for electronic pacing interventions to improve exercise capacity. Indeed, one modest-sized single-center study using such a strategy demonstrated substantially improved exercise performance in selected patients who had hypertensive LV hypertrophy with supranormal systolic ejection and distal cavity obliteration and who experienced debilitating exertional fatigue and dyspnea.104 These data merit confirmation in larger, multicenter, randomized, controlled trials.
Thus, a variety of pharmacologic and other interventions in small studies have shown improvements in exercise tolerance with verapamil,95 enalapril,81 angiotensin receptor antagonism,80,96 and aldosterone antagonism.101 It should be remembered that in patients who have SHF, some types of pharmacologic interventions that improve exercise tolerance have had paradoxical effects on long-term survival.105,106 Therefore the VE/VCO2 slope during exercise, which is a powerful predictor of survival independent of VO2, should be included in future intervention trials of exercise tolerance.6
Aerobic exercise training has the potential to improve a variety of key abnormalities in patients who have heart failure and normal EF, including LV diastolic compliance, aortic distensibility, blood pressure, and skeletal muscle function.72,107,108 Indeed, in SHF, aerobic exercise training has been shown to improve exercise tolerance, probably by favorable effects on multiple factors.17,109,110 A recent report indicates that LV diastolic compliance is preserved in older masters athletes compared with their age-matched and young counterparts, suggesting that exercise training may be beneficial in DHF as well.111 A preliminary report indicates that exercise training improves exercise tolerance and quality of life in older patients who have heart failure and normal EF.112 A recent report from a clinical exercise rehabilitation program suggests that exercise training also may benefit patients who have DHF.113 Although the role of exercise training in the clinical management of this syndrome remains to be defined, it would seem prudent to recommend regular, moderate physical activity as tolerated, as is the accepted practice in SHF. The effect of exercise training on survival in patients who have SHF is being examined in a large, multicenter, randomized, controlled trial sponsored by the National Institutes of Health (HF-ACTION). Presently, there is no trial examining mortality and exercise training in patients who have heart failure and normal EF.
SUMMARY
Even when stable and non-edematous, patients who have heart failure and normal EF have severe, chronic exercise intolerance. The pathophysiology of exercise intolerance in this syndrome is incompletely understood but probably is multifactorial. Presently available data suggest that important contributors include decreased LV diastolic compliance, decreased aortic distensibility, exaggerated exercise systolic blood pressure, relative chronotropic incompetence, and possibly anemia and skeletal muscle remodeling. Because it is a primary determinant of quality of life, can be quantified objectively, is reproducible, and is modifiable, exercise intolerance is an attractive therapeutic target. A number of pharmacologic and other interventions seem to improve exercise intolerance in DHF. Although it is unknown whether these interventions will be accompanied by improved survival, the parallel outcome of improved quality of life supports the clinical relevance of exercise performance outcomes.
Acknowledgments
This work was supported in part by National Institute on Aging Grants, R37-AG18915 (MERIT), Dennis Jahnigen Career Development and Paul Beeson Award (K08-AG026764) and Claude D. Pepper Older Americans Independence Center (P30 AG21332).
REFERENCES
- 1.Gandhi SK, Powers JE, Fowle KM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2000;344(1):17–22. doi: 10.1056/NEJM200101043440103. [DOI] [PubMed] [Google Scholar]
- 2.Powers JE, Gandhi SK, Kramer RK, et al. Predictors of poor outcome in patients with hypertensivepulmonary edema [abstract] J Am Coll Cardiol. 2004;43(5A):227A. [Google Scholar]
- 3.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288(17):2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 4.Bol E, de Vries WR, Mosterd WL, et al. Cardiopulmonary exercise parameters in relation to all-cause mortality in patients with chronic heart failure. Int J Cardiol. 2000;72:255–63. doi: 10.1016/s0167-5273(99)00195-3. [DOI] [PubMed] [Google Scholar]
- 5.Jones RC, Francis GS, Lauer MS. Predictors of mortality in patients with heart failure and preserved systolic function in the Digitalis Investigation Group trial. J Am Coll Cardiol. 2004;44(5):1025–9. doi: 10.1016/j.jacc.2004.05.077. [DOI] [PubMed] [Google Scholar]
- 6.Francis DP, Shamin W, Davies LC, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO2 slope and peak VO2. Eur Heart J. 2000;21:154–61. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 7.Moore B, Brubaker PH, Stewart KP, et al. VE/VCO2 slope in older heart failure patients with normal versus reduced ejection fraction compared with age-matched healthy controls. J Card Fail. 2007;13(4):259–62. doi: 10.1016/j.cardfail.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Maldonado-Martin S, Brubaker PH, Kaminsky LA, et al. The relationship of a 6-min walk to VO(2 peak) and VT in older heart failure patients. Med Sci Sports Exerc. 2006;38(6):1047–53. doi: 10.1249/01.mss.0000222830.41735.14. [DOI] [PubMed] [Google Scholar]
- 9.Higginbotham MB, Morris KG, Williams RS, et al. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–91. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 10.Higginbotham MB, Morris KG, Williams RS, et al. Physiologic basis for the age-related decline in aerobic work capacity. Am J Cardiol. 1986;57:1374–9. doi: 10.1016/0002-9149(86)90221-3. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan M, Knight JD, Higginbotham MB, et al. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure: muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–81. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 12.Higginbotham MB, Sullivan M, Coleman RE, et al. Regulation of stroke volume during exercise in patients with severe left ventricular dysfunction: importance of Starling mechanism. J Am Coll Cardiol. 1987;9:58A. [Google Scholar]
- 13.Kitzman DW, Sullivan M, Cobb FR, et al. Exercise cardiac output declines with advancing age in normal subjects. J Am Coll Cardiol. 1989;13(2):241A. [Google Scholar]
- 14.Sullivan M, Cobb FR, Knight JD, et al. Stroke volume increases by similar mechanisms in men and women. Am J Cardiol. 1991;67:1405–12. doi: 10.1016/0002-9149(91)90472-w. [DOI] [PubMed] [Google Scholar]
- 15.Kitzman DW, Higginbotham MB, Cobb FR, et al. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–72. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan M, Higginbotham MB, Cobb FR. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation. 1988;77:552–9. doi: 10.1161/01.cir.77.3.552. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan M, Higginbotham MB, Cobb FR. Exercise training in patients with chronic heart failure delays ventilatory anaerobic threshold and improves submaximal exercise performance. Circulation. 1989;79:324–9. doi: 10.1161/01.cir.79.2.324. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan M, Cobb FR. Central hemodynamic response to exercise in patients with chronic heart failure. Chest. 1992;101:340S–6S. doi: 10.1378/chest.101.5_supplement.340s. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30(6):1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 20.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 21.Hadano Y, Murata K, Yamamoto T, et al. Usefulness of mitral annular velocity in predicting exercise tolerance in patients with impaired left ventricular systolic function. Am J Cardiol. 2006;97(7):1025–8. doi: 10.1016/j.amjcard.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Skaluba SJ, Litwin SE. Mechanisms of exercise intolerance: insights from tissue Doppler imaging. Circulation. 2004;109(8):972–7. doi: 10.1161/01.CIR.0000117405.74491.D2. [DOI] [PubMed] [Google Scholar]
- 23.Ha JW, Oh JK, Pellikka PA, et al. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr. 2005;18(1):63–8. doi: 10.1016/j.echo.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 24.Kitzman DW, Sullivan M. Exercise intolerance in patients with heart failure: role of diastolic dysfunction. In: Grossman W, editor. Diastolic relaxation of the heart. Kluwer Academic Publishers; Boston: 1994. pp. 295–302. [Google Scholar]
- 25.Brubaker PH, Joo KC, Stewart KP, et al. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26(2):86–9. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–47. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 27.Kitzman DW, Gardin JM, Gottdiener JS, et al. Cardiovascular Health Study Research Group Importance of heart failure with preserved systolic function in patients > or 5 65 years of age. Am J Cardiol. 2001;87(4):413–9. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 28.Iriarte M, Murga N, Morillas M, et al. Congestive heart failure from left ventricular diastolic dysfunction in systemic hypertension. Am J Cardiol. 1993;71:308–12. doi: 10.1016/0002-9149(93)90796-f. [DOI] [PubMed] [Google Scholar]
- 29.Iriarte MM, Perez OJ, Sagastagoitia D, et al. Congestive heart failure due to hypertensive ventricular diastolic dysfunction. Am J Cardiol. 1995;76(13):43D–7D. doi: 10.1016/s0002-9149(99)80491-3. [DOI] [PubMed] [Google Scholar]
- 30.Little WC. Enhanced load dependence of relaxation in heart failure: clinical implications. Circulation. 1992;85(6):2326–8. doi: 10.1161/01.cir.85.6.2326. [DOI] [PubMed] [Google Scholar]
- 31.Gelpi RJ. Changes in diastolic cardiac function in developing and stable perinephritic hypertension in conscious dogs. Circ Res. 1991;68:555–67. doi: 10.1161/01.res.68.2.555. [DOI] [PubMed] [Google Scholar]
- 32.Shannon RP, Komamura K, Gelpi RJ, et al. Altered load: an important component of impaired diastolic function in hypertension and heart failure. In: Lorell BH, Grossman W, editors. Diastolic relaxation of the heart. Kluwer Academic Publishers; Norwell (MA): 1994. pp. 177–85. [Google Scholar]
- 33.Little WC, Braunwald E. Assessment of cardiac performance. In: Braunwald E, editor. Heart disease. W.B. Saunders Company; Philadelphia: 1996. pp. 421–44. [Google Scholar]
- 34.Hoit BD, Walsh RA. Diastolic dysfunction in hypertensive heart disease. Left ventricular diastolic dysfunction and heart failure. In: Gaasch WH, LeWinter MM, editors. Lea & Febiger; Philadelphia: 1994. pp. 354–72. [Google Scholar]
- 35.Little WC, Ohno M, Kitzman DW, et al. Determination of left ventricular chamber stiffness from the time for deceleration of early left ventricular filling. Circulation. 1995;92:1933–9. doi: 10.1161/01.cir.92.7.1933. [DOI] [PubMed] [Google Scholar]
- 36.Lakatta E. Cardiovascular aging research: the next horizons. J Am Geriatr Soc. 1999;47:613–25. doi: 10.1111/j.1532-5415.1999.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 37.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 38.Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle dependent changes in aortic area and aortic distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38(3):796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 39.Rerkpattanapipat P, Hundley WG, Link KM, et al. Relation of aortic distensibility determine by magnetic resonance imaging in patients 5 60 years of age to systolic heart failure and exercise capacity. Am J Cardiol. 2002;90(11):1221–5. doi: 10.1016/s0002-9149(02)02838-2. [DOI] [PubMed] [Google Scholar]
- 40.Drexler H, Hayoz D, Monzel T, et al. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69:1596–601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- 41.Hayoz D, Drexler H, Munzel T, et al. Flow-mediated arterial dilation is abnormal in congestive heart failure. Circulation. 1993;87 VII-92-6. [Google Scholar]
- 42.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93(2):210–4. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- 43.Hornig B, Arakawa N, Haussmann D, et al. Differential effects of quinaprilat and enalaprilat on endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;98(25):2842–8. doi: 10.1161/01.cir.98.25.2842. [DOI] [PubMed] [Google Scholar]
- 44.Hundley WG, Bayram E, Hamilton CA, et al. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292(3):H1427–34. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 45.Myers J, Froelicher V. Hemodynamic determinants of exercise capacity in chronic heart failure. Ann Intern Med. 1991;115:377–86. doi: 10.7326/0003-4819-115-5-377. [DOI] [PubMed] [Google Scholar]
- 46.Franciosa JA. Role of ventricular function in determining exercise capacity in patients with chronic left ventricular failure. Adv Cardiol. 1986;34:170–8. doi: 10.1159/000413049. [DOI] [PubMed] [Google Scholar]
- 47.Wilson JR, Rayos G, Yeoh TK, et al. Dissociation between exertional symptoms and circulatory function in patients with heart failure. Circulation. 1995;92(1):47–53. doi: 10.1161/01.cir.92.1.47. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 48.Clark AL, Sparrow JL, Coats AJ. Muscle fatigue and dyspnoea in chronic heart failure: two sides of the same coin? Eur Heart J. 1995;16(1):49–52. doi: 10.1093/eurheartj/16.1.49. [DOI] [PubMed] [Google Scholar]
- 49.Fink LI, Wilson JR, Ferraro N. Exercise ventilation and pulmonary artery wedge pressure in chronic stable congestive heart failure. Am J Cardiol. 1966;57:249–53. doi: 10.1016/0002-9149(86)90900-8. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan M, Cobb FR. The anaerobic threshold in chronic heart failure. Circulation. 1990;81 II-47-58. [PubMed] [Google Scholar]
- 51.Green HJ. Manifestations and sites of neuromus-cular fatigue. Biochem Exercise. 1990;VII:13–35. [Google Scholar]
- 52.Brucks S, Little WC, Chao T, et al. Relation of anemia to diastolic heart failure and the effect on outcome. Am J Cardiol. 2004;93(8):1055–7. doi: 10.1016/j.amjcard.2003.12.062. [DOI] [PubMed] [Google Scholar]
- 53.Deedwania PC, Gottlieb S, Ghali JK, et al. Efficacy, safety and tolerability of beta-adrenergic blockade with metoprolol CR/XL in elderly patients with heart failure. Eur Heart J. 2004;25(15):1300–9. doi: 10.1016/j.ehj.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 54.Sanders P, Kistler PM, Morton JB, et al. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110(8):897–903. doi: 10.1161/01.CIR.0000139336.69955.AB. [DOI] [PubMed] [Google Scholar]
- 55.Nagaya N, Moriya J, Yasumura Y, et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110(24):3674–9. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan JJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–27. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 57.Adamopoulos S, Coats A, Brunotte F, et al. Physical training improves skeletal muscle metabolism in patients with chronic heart failure. J Am Coll Cardiol. 1993;21:1101–6. doi: 10.1016/0735-1097(93)90231-o. [DOI] [PubMed] [Google Scholar]
- 58.Stratton J, Dunn JF, Adamopoulos S, et al. Training partially reverses skeletal muscle metabolic abnormalities during exercise in heart failure. J Appl Physiol. 1994;76:1575–82. doi: 10.1152/jappl.1994.76.4.1575. [DOI] [PubMed] [Google Scholar]
- 59.Kouba EJ, Hundley WG, Brubaker PH, et al. Skeletal muscle remodeling and exercise intolerance in elderly patients with diastolic heart failure [abstract] Am J Geriatr Cardiol. 2003;12(2):135. [Google Scholar]
- 60.Felker GM, Adams KF, Jr, Gattis WA, et al. Anemia as a risk factor and therapeutic target in heart failure. J Am Coll Cardiol. 2004;44(5):959–66. doi: 10.1016/j.jacc.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 61.Adams V, Jiang H, Yu J, et al. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol. 1999;33(4):959–65. doi: 10.1016/s0735-1097(98)00626-3. [DOI] [PubMed] [Google Scholar]
- 62.Wilson JR, Fink L, Maris J, et al. Evaluation of energy metabolism in skeletal muscle of patients with heart failure with gated phosphorus-31 nuclear magnetic resonance. Circulation. 1985;71(1):57–62. doi: 10.1161/01.cir.71.1.57. [DOI] [PubMed] [Google Scholar]
- 63.Minotti JR, Johnson EC, Hudson TL, et al. Skeletal muscle response to exercise training in congestive heart failure. J Clin Invest. 1990;86:751–8. doi: 10.1172/JCI114771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minotti JR, Christoph I, Oka R, et al. Impaired skeletal muscle function in patients with congestive heart failure. J Clin Invest. 1991;88:2077–82. doi: 10.1172/JCI115537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–73. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 66.Minotti JR, Christoph I, Massie BM. Skeletal muscle function, morphology, and metabolism in patients with congestive heart failure. Chest. 1992;101:333S–9S. doi: 10.1378/chest.101.5_supplement.333s. [DOI] [PubMed] [Google Scholar]
- 67.Wilson JR, Mancini DM. Skeletal muscle metabolic dysfunction. Implications for exercise intolerance in heart failure. Circulation. 1993;87 VII-104-9. [Google Scholar]
- 68.Minotti JR, Pillay P, Oka R, et al. Skeletal muscle size: relationship to muscle function in heart failure. J Appl Physiol. 1993;75(1):373–81. doi: 10.1152/jappl.1993.75.1.373. [DOI] [PubMed] [Google Scholar]
- 69.Kao W, Helpern JA, Goldstein S, et al. Abnormalities of skeletal muscle metabolism during nerve stimulation determined by 31P nuclear magnetic resonance spectroscopy in severe congestive heart failure. Am J Cardiol. 1995;76(8):606–9. doi: 10.1016/s0002-9149(99)80166-0. [DOI] [PubMed] [Google Scholar]
- 70.Lang CC, Chomsky DB, Rayos G, et al. Skeletal muscle mass and exercise performance in stable ambulatory patients with heart failure. J Appl Physiol. 1997;82:257–61. doi: 10.1152/jappl.1997.82.1.257. [DOI] [PubMed] [Google Scholar]
- 71.Harrington D, Anker SD, Chua TP, et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30(7):1758–64. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 72.Peters DG, Mitchell HL, McCune SA, et al. Skeletal muscle sarcoplasmic reticulum Ca(21)-ATPase gene expression in congestive heart failure. Circ Res. 1997;81(5):703–10. doi: 10.1161/01.res.81.5.703. [DOI] [PubMed] [Google Scholar]
- 73.Vescovo G, Volterrani M, Zennaro R, et al. Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes [see comments] Heart. 2000;84(4):431–7. doi: 10.1136/heart.84.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grimby G. Physical activity and effects of muscle training in the elderly. Ann Clin Res. 1988;20:62–6. [PubMed] [Google Scholar]
- 75.Fleg JL, Lakatta EG. Role of muscle loss in the ageassociated reduction in VO2 max. J Appl Physiol. 1988;65(3):1147–51. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- 76.Rice CL, Cunningham DA, Paterson DH, et al. Arm and leg composition determined by computed tomography in young and elderly men. Clin Physiol. 1989;9(3):207–20. doi: 10.1111/j.1475-097x.1989.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 77.Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82–8. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- 78.Buchner D, deLateur B. The importance of skeletal muscle strength to physical function in older adults. Ann Behav Med. 1991;13:4206–14. [Google Scholar]
- 79.Menshikova EV, Ritov VB, Toledo FGS, et al. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab. 2005;288(4):E818–25. doi: 10.1152/ajpendo.00322.2004. [DOI] [PubMed] [Google Scholar]
- 80.Warner JG, Metzger C, Kitzman DW, et al. Losartan improves exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise. J Am Coll Cardiol. 1999;33:1567–72. doi: 10.1016/s0735-1097(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 81.Aronow WS, Kronzon I. Effect of enalapril on congestive heart failure treated with diuretics in elderly patients with prior myocardial infarction and normal left ventricular ejection fraction. Am J Cardiol. 1993;71:602–4. doi: 10.1016/0002-9149(93)90520-m. [DOI] [PubMed] [Google Scholar]
- 82.Vandenberg VF, Rath LS, Stuhlmuller P, et al. Estimation of left ventricular cavity area with on-line, semiautomated echocardiographic edge detection system. Circulation. 1992;86:159–66. doi: 10.1161/01.cir.86.1.159. [DOI] [PubMed] [Google Scholar]
- 83.Bonow RO, Leon MB, Rosing DR, et al. Effects of verapamil and propranolol on left ventricular systolic function and diastolic filling in patients with coronary artery disease: radionuclide angiographic studies at rest and during exercise. Circulation. 1981;65:1337–50. doi: 10.1161/01.cir.65.7.1337. [DOI] [PubMed] [Google Scholar]
- 84.Bonow RO, Dilsizian V, Rosing DR, et al. Verapamil-induced improvement in left ventricular diastolic filling and increased exercise tolerance in patients with hypertrophic cardiomyopathy: short- and long-term effects. Circulation. 1985;72:853–64. doi: 10.1161/01.cir.72.4.853. [DOI] [PubMed] [Google Scholar]
- 85.Udelson J, Bonow RO. Left ventricular diastolic function and calcium channel blockers in hypertrophic cardiomyopathy. Left ventricular diastolic dysfunction and heart failure. In: Gaasch WH, editor. Lea & Febiger; Malvern, Pennsylvania: 1996. pp. 465–89. [Google Scholar]
- 86.Chen CH, Nakayama M, Talbot M, et al. Verapamil acutely reduces ventricular-vascular stiffening andimproves aerobic exercise performance in elderly individuals. J Am Coll Cardiol. 1999;33:1602–9. doi: 10.1016/s0735-1097(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 87.Serizawa T, Shin-Ichi M, Nagai Y, et al. Diastolic abnormalities in low-flow and pacing tachycardia-induced ischemia in isolated rat hearts-modification by calcium antagonists. Diastolic relaxation of the heart. In: Lorell BH, Grossman W, editors. Kluwer Academic Publishers; Norwell (MA): 1996. pp. 266–74. [Google Scholar]
- 88.Cheng CP, Pettersson K, Little WC. Effects of felo-dipine on left ventricular systolic and diastolic performance in congestive heart failure. J Pharma and Exper Thera. 1994;271:1409–17. [PubMed] [Google Scholar]
- 89.Cheng CP, Noda T, Ohno M, et al. Differential effects of enalaprilat and felodipine on diastolic function during exercise in dogs with congestive heart failure [abstract] Circulation. 1993;88(4):I–294. [Google Scholar]
- 90.Little WC, Cheng CP, Elvelin L, et al. Vascular selective calcium entry blockers in the treatment of cardiovascular disorders: focus on felodipine. Cardiovasc Drugs Ther. 1995;9(5):657–63. doi: 10.1007/BF00878548. [DOI] [PubMed] [Google Scholar]
- 91.Ten Cate FJ, Serruys PW, Mey S, et al. Effects of short-term administration of verapamil on left ventricular filling dynamics measured by a combined hemodynamic-ultrasonic technique in patients with hypertrophic cardiomyopathy. Circulation. 1983;68(6):1274–9. doi: 10.1161/01.cir.68.6.1274. [DOI] [PubMed] [Google Scholar]
- 92.Hess OM, Murakami T, Krayenbuehl HP. Does verapamil improve left ventricular relaxation in patients with myocardial hypertrophy? Circulation. 1996;74:530–43. doi: 10.1161/01.cir.74.3.530. [DOI] [PubMed] [Google Scholar]
- 93.Brutsaert DL, Rademakers F, Sys SU, et al. Analysis of relaxation in the evaluation of ventricular function of the heart. Prog Cardiovasc Dis. 1985;28:143–63. doi: 10.1016/0033-0620(85)90022-2. [DOI] [PubMed] [Google Scholar]
- 94.Brutsaert DL, Sys SU, Gillebert TC. Diastolic failure: pathophysiology and therapeutic implications. J Am Coll Cardiol. 1993;22:318–25. doi: 10.1016/0735-1097(93)90850-z. [DOI] [PubMed] [Google Scholar]
- 95.Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981–6. doi: 10.1016/0002-9149(90)90937-v. [DOI] [PubMed] [Google Scholar]
- 96.Little WC, Wesley-Farrington DJ, Hoyle J, et al. Effect of candesartan and verapamil on exercise tolerance in diastolic dysfunction. J Cardiovasc Pharmacol. 2004;43(2):288–93. doi: 10.1097/00005344-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 97.Little WC, Zile MR, Klein AL, et al. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and diastolic dysfunction. Am J Cardiol. 2006;98(3):383–5. doi: 10.1016/j.amjcard.2006.01.106. [DOI] [PubMed] [Google Scholar]
- 98.Pitt B, Reichek N, Willenbrock R, et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108(15):1831–8. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 99.Rajagopalan S, Pitt B. Aldosterone as a target in congestive heart failure. Med Clin North Am. 2003;87(2):441–57. doi: 10.1016/s0025-7125(02)00183-9. [DOI] [PubMed] [Google Scholar]
- 100.Zannad F, Alla F, Dousset B, et al. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized Aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102(22):2700–6. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 101.Daniel KR, Wells GL, Fray B, et al. The effect of spironolactone on exercise tolerance and quality of life in elderly women with diastolic heart failure [abstract] Am J Geriatr Cardiol. 2003;12(2):131. [Google Scholar]
- 102.Mottram PM, Haluska B, Leano R, et al. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110(5):558–65. doi: 10.1161/01.CIR.0000138680.89536.A9. [DOI] [PubMed] [Google Scholar]
- 103.Little WC, Zile MR, Kitzman DW, et al. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail. 2005;11(3):191–5. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 104.Kass DA, Chen CH, Talbot MW, et al. Ventricular pacing with premature excitation for treatment of hypertensive-cardiac hypertrophy with cavity-obliteration. Circulation. 1999;100(8):807–12. doi: 10.1161/01.cir.100.8.807. [DOI] [PubMed] [Google Scholar]
- 105.Packer M, Carver JR, Chesebro J, et al. Effect of oral milrinone on mortality in severe chronic heart failure: The Prospective Randomized Milrinone Survival Evaluation (PROMISE) N Engl J Med. 1991;325:1468–75. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 106.Creager MA, Massie BM, Faxon DP, et al. Acute and long-term effects of enalapril on the cardiovascular response to exercise and exercise tolerance in patients with congestive heart failure. J Am Coll Cardiol. 1985;6(1):163–73. doi: 10.1016/s0735-1097(85)80269-2. [DOI] [PubMed] [Google Scholar]
- 107.Sullivan M. Role of exercise conditioning in patients with severe systolic left ventricular dysfunction. In: Fletcher GF, editor. Cardiovascular response to exercise. Futura Publishing Company; Mount Kisco: 1994. pp. 359–72. [Google Scholar]
- 108.Vaitkevicius PV, Fleg J, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88(5):1456–62. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 109.Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107(8):1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 110.Coats A, Adamopoulos S, Radaelli A, et al. Controlled trial of physical training in chronic heart failure. Circulation. 1992;85:2119–31. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 111.Arbab-Zadeh A, Dijk E, Prasad A, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110(13):1799–805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 112.Kitzman DW, Brubaker PH, Abdelahmed A, et al. Effect of exercise training on exercise capacity, quality of life, and flow-mediated arterial dilation in elderly patients with diastolic heart failure [abstract] J Am Coll Cardiol. 2004;110(17):III–558. [Google Scholar]
- 113.Smart N, Haluska B, Jeffriess L, et al. Exercise training in systolic and diastolic dysfunction: effects on cardiac function, functional capacity, and quality of life. Am Heart J. 2007;153(4):530–6. doi: 10.1016/j.ahj.2007.01.004. [DOI] [PubMed] [Google Scholar]