Abstract
Urocortin 3 (Ucn 3) is a corticotrophin-releasing factor related neuropeptide with potent anorectic effects in the brain. Ucn 3 nerve fibers heavily innervate the hypothalamic ventromedial nucleus (VMH) and injection of Ucn 3 into the VMH suppresses feeding. Currently, the origin of the Ucn 3 afferent input into the VMH is unknown. In the present study, anatomical tracing shows the major Ucn 3 afferent input to the VMH resides in the anterior parvicellular part of the paraventricular nucleus of the hypothalamus (PVHap) and the adjacent posterior part of the bed nucleus of stria terminalis (pBNST). VMH also receives moderate Ucn 3 input from the medial amygdala. Interestingly, Ucn 3 neurons located immediately caudal to the PVHap/pBNST in the rostral perifornical hypothalamic area (rPFH) provide only minimal input. The paucity of rPFH-VMH Ucn 3 projection is consistent with the finding that only Ucn 3 neurons in the rPFH co-expressed Enkephalin (Enk) and Ucn 3/Enk double-labeled nerve fibers and terminals were observed predominately in the lateral septum (LS) while only a few double-labeled fibers were found in other brain areas including the VMH. Furthermore, retrograde tracing demonstrates that Ucn 3 neurons in the rPFH project to the LS. In conclusion, the present study determines the major Ucn 3 afferent into the VMH originates from the PVHap/pBNST. In addition, anatomical heterogeneity is observed in the major hypothalamic Ucn 3 neuron population as the rostral part (PVHap/pBNST) of the cell group projects to the VMH and the caudal part (rPFH) colocalizes with Enk and provides major afferent input to the LS.
Keywords: CRF, CRF receptors, feeding, enkephalin, stress
Introduction
Ucn 3 is a member of the corticotrophin-releasing factor (CRF) peptide family identified in humans and rodents (Hsu and Hsueh, 2001; Lewis et al., 2001). It displays high affinity for the type 2 CRF receptor (CRFR2) with minimal affinity for the type 1 CRF receptor (CRFR1) (Hsu and Hsueh, 2001; Lewis et al., 2001). In the central nervous system, Ucn 3-expressing neurons are primarily located in the hypothalamus and the medial amygdala (MeA) (Lewis et al., 2001; Li et al., 2002). In the hypothalamus, the major Ucn 3 cell population is found near the rostral perifornical hypothalamic area (rPFH) (Li et al., 2002). Specifically, Ucn 3-positive cells are observed around the fornix lateral to the paraventricular nucleus of the hypothalamus (PVH). This population continues rostrally and remains close to the fornix into the posterior part of the bed nucleus of the stria terminalis (pBNST) and medially into the anterior parvicellular part of the PVH (PVHap). A small group of Ucn 3-positive cells is found in the median preoptic nucleus (MePO) (Li et al., 2002). Ucn 3-immunoreactive nerve fibers and terminals are distributed mostly in the forebrain including the ventromedial nucleus of the hypothalamus (VMH), the lateral septum (LS), MeA and BNST (Li et al., 2002). Moreover, these areas also express high levels of CRFR2 (Chalmers et al., 1995; Van Pett et al., 2000). Taken together, the overlap between Ucn 3 and CRFR2 distribution and the high affinity of the peptide for the receptor suggest that Ucn 3 is an endogenous ligand for CRFR2.
Central administration of Ucn 3 induces a number of effects associated with energy adaption. These include suppression of food intake, stimulation of sympathetic nervous system (SNS) activity, and elevation of blood glucose levels (Fekete et al., 2007; Jamieson et al., 2006; Ohata and Shibasaki, 2004; Pelleymounter et al., 2004). A number of brain areas including the VMH, PVH, and amygdala which express CRFR2 and receive Ucn 3 innervation were investigated. It was found that injection of Ucn 3 into the VMH but not PVH or amygdala mimics the central effects of Ucn 3 in feeding (Chen et al., 2009; Fekete et al., 2007), SNS activity and blood glucose levels (Chen et al., 2009). These data thus argue the VMH is a major brain area mediating the central effects of Ucn 3 in energy homeostasis.
A key question remaining is the origin of Ucn 3 afferent into the VMH. Afferent inputs to the VMH have been previously examined and several brain areas including the BNST, amygdala, LS, preoptic nucleus, lateral hypothalamus, and perifornical hypothalamus have been shown to project to the VMH (Berk and Finkelstein, 1981; Fahrbach et al., 1989; Kita and Oomura, 1982; Luiten and Room, 1980; McBride and Sutin, 1977; Zaborszky, 1982). Ucn 3 neurons are found in a number of these areas and potentially provide the afferent input to the VMH. In the present study, retrograde tracing combined with in situ hybridization was employed to determine the origin of Ucn 3 neurons which project to the VMH.
The neuropeptide Enkephalin (Enk) is a member of the opioid peptide family with high affinity for the opioid delta receptor (Janecka et al., 2004). Similar to the CRF family peptides, Enk has been shown to modulate stress responses (Bilkei-Gorzo et al., 2008; Drolet et al., 2001). Enk expressing neurons are found throughout the brain. In particular, abundant Enk-positive neurons are found in the rPFH, PVH, and BNST (Merchenthaler et al., 1986) where the major hypothalamic Ucn 3 cell group is located. Anatomical studies have shown Enk neurons in the rPFH project to the lateral septum (LS) (Merchenthaler, 1991; Onteniente et al., 1989; Poulain et al., 1984; Sakanaka and Magari, 1989). Extensive Ucn 3 nerve fibers are also observed in the LS thus raising the possibility that Ucn 3 neurons in the rPFH are Enk-positive and project to the LS. Another brain area which receives intensive Enk innervation is the VMH (Merchenthaler et al., 1986). Although the origin of the Enk afferent inputs into the VMH has yet to be determined, it is conceivable that Ucn 3 neurons in the rPFH colocalize with Enk and project to brain areas such as the LS and VMH. To better understand the anatomical relationship between Ucn 3 and Enk and the projection of Ucn 3 neurons in the rPFH, a double-label immunofluorescent staining for Ucn 3 and Enk was first performed in the rat brain to determine whether Ucn 3-positive neurons, especially the rPFH cell group, are also Enk-positive and whether Ucn 3 nerve fibers and terminals in the LS and/or VMH are colocalized with Enk. Secondly, retrograde tracing combined with in situ hybridization was used to further determine if Ucn 3 cells in the rPFH project to the LS.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Charles River, Wilmington, MA) weighing 200–250 g were housed in a temperature-controlled room with light on between 0600–1800 hr. All experimental protocols and procedures were approved by The University of Virginia Animal Use and Care Committee.
Retrograde tracer injection
Rats anesthetized with a mixture of Ketamine (80 mg/kg) and xylazine (8 mg/kg) were placed in a stereotaxic apparatus and a glass micropipette filled with a retrograde tracer, FluoroGold (FG, 2% w/v in saline), was inserted into the VMH. The stereotaxic coordinates used for VMH were 3.3 mm caudal, 0.5 mm lateral to bregma, and 8.4 mm ventral to the dura. Coordinates for lateral septum were 0.12 mm caudal, 1.2 mm lateral to bregma and 5.4 mm ventral to the dura. FG was injected by iontophoresis with 5 μA positive current pulsed at 7-s intervals for 10 min. Ten days after FG injection, animals were perfused with 4% paraformaldehyde (pH 9.5) and the brains removed and sectioned coronally at 25 μm thickness and stored in cryoprotectant at −20°C until use.
Antibody characterization
See Table 1 for a list of all antibodies used in the study. The Ucn 3 antiserum recognized rodent Ucn 3 on immunohistochemical analyses (Li et al., 2002). Staining was eliminated by preincubation of diluted antiserum (1:200) with 3 μM of recombinant mouse Ucn 3. The enkephalin (Enk) monoclonal antibody recognized Leu-enkephalin and displayed about 40% cross-reactivity with Met-enkephalin with no cross-reactivity to β-endophorin or dynophorin by radioimmunoassay (Cuello et al., 1984). Immunohistochemical analysis showed the antibody labeled all sites that are known to express enkephalin and showed no cross-reactivity to β-endorphin or dynorphin (Cuello et al., 1984). The fluorogold (FG) antiserum recognized fluorogold (hydroxystilbamidine) and aminostilbamidine with immunohistochemical analyses (manufacturer’s datasheet) and has been extensively used to label the retrograde tracer (manufacturer’s datasheet). Li and colleagues have used this antibody to detect FG in rat brains with FG injected into the arcuate nucleus of the hypothalamus (Li et al., 1999).
Table 1.
Average number of FG-positive and Ucn3-positive and double-labeled cells identified in selective brain areas and percentage of double-labeled cells with respect to total FG-positive cells and to total Ucn 3-positive cells.
| Area | FG+ cells (cells/section) | Ucn 3+ cells (cells/section) | FG/Ucn 3 cells (cells/section) | % double labeled cells in FG+ cells | % double labeled cells in Ucn 3+ cells |
|---|---|---|---|---|---|
| MePO | 22.4 ± 4.03 | 10.6 ± 1.36 | 1.2 ± 0.37 | 6.6 ± 1.99 | 10.5 ± 3.3 |
| pBNST | 76.01 ± 12.4 | 16.8 ± 3 | 6.2 ± 0.86 | 8.8 ± 1.7 | 38 ± 2.67 |
| PVHap | 38.2 ± 4.3 | 16.4 ± 3.3 | 7.3 ± 1.8 | 20.3 ± 3.6 | 44.67 ± 3.28 |
| rPFH | 48 ± 7.9 | 28.4 ± 5.1 | 2.4 ± 0.75 | 4.9 ± 1.1 | 9.03 ± 2.3 |
| MeA | 125.2 ± 26.01 | 14.2 ± 1.4 | 3.6 ± 0.6 | 3.1 ± 0.44 | 25.9 ± 4.1 |
Data: Mean ± SEM.
Abbreviation: MeA: Medial amygdala, MePO: Median preoptic nucleus, pBNST: posterior part of the bed nucleus of stria terminalis, PVHap: anterior parvicellular part of the paraventricular nucleus of the hypothalamus, rPFH: rostral perifornical hypothalamic area.
Double- label immunohistochemistry and in situ hybridization
For FG immunohistochemistry, brain sections were washed with potassium phosphate buffered saline (KPBS) and were then incubated in rabbit anti-FG antibody (Millipore, 1:15,000) in KPBS with 0.4% Triton-X 100 for 48 hrs. After incubation, the sections were incubated in biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch, 1:600) for 1 hr at room temperature followed by a 1 hr incubation at room temperature in avidin-biotin complex solution (Vectastain ABC Elite Kit, Vector Laboratories). The FG antibody-peroxidase complex was visualized with 3,3 diaminobenzdine (DAB). Sections were then processed for in situ hybridization to detect Ucn 3 mRNA as previously described (Li et al., 2002). Briefly, a Ucn 3 complementary RNA (cRNA) probe was transcribed from a linearized vector containing full length rat Ucn 3 sequence and flanking 3 prime untranslated region (total 528bp) in the presence of the 33P-labeled UTP (Perkin Elmer) as previously described (Li et al., 2002). Brain sections were treated with a fresh solution containing 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0) followed by a rinse in 2X SSC. The sections were then exposed to the cRNA probe overnight at 55°C. After incubation, the brain sections were washed in SSC which increased in stringency, in RNase, and in 0.1XSSC at 60°C. The sections were then mounted onto glass slides, dipped in emulsion (Kodak), and incubated at 4°C for 7 days. The slides were then developed and coverslipped with DPX.
Double-label immunofluorescence staining
For Ucn 3 and Enk double-label immuofluorescence staining, rat brain sections were incubated in rabbit anti-human Ucn 3 serum (1:1000, generously provided by Dr. Wylie Vale, Salk Institute) and mouse anti-enkephalin antibody (1:1100; Millipore). The sections were then incubated in a mixture of biotinylated donkey anti-rabbit IgG (1:600, Jackson ImmunoResearch) and Cy3 conjugated donkey anti-mouse IgG (1:350, Jackson ImmunoResearch) followed by Cy2 conjugated streptavidin (1:1000). The sections were then mounted onto glass slides and coverslipped with buffered glycerol. Results were examined and captured by confocal microscopy (Nikon C1-plus).
Data analysis
Results from the FG/Ucn 3 double label study were examined and captured by a Nikon 80i microscope coupled with a QImaging CCD camera (BioVision, Exton, PA) controlled by iVision software (BioVision, Exton, PA). The images were cropped and adjusted to balance brightness and contrast in ImageJ (NIH) before import into Canvas (version 8.0) for assembly into plates. For data analysis, one-in-six series of the coronal brain sections that were processed for double label experiments were first anatomically matched across animals. Three sections that are evenly across the rostrocaudal levels of the MePO were used for cell count. For pBNST/PVHap, rPFH and MeA, three, five and seven sections were used, respectively, for cell count using similar criteria. FG-positive, Ucn 3 mRNA-positive and FG-Ucn 3 double-labeled cells in the area of interest were identified and counted using ImageJ. An FG-labeled neuron was considered to be double-labeled with Ucn 3 mRNA if the number of silver grains on top of the cell body was greater than three times background levels. Cell count results for each brain area are expressed as cells per section and are presented as mean ± SEM.
Results
Distribution of FluoroGold (FG)-labeled neurons following FG injection into the VMH
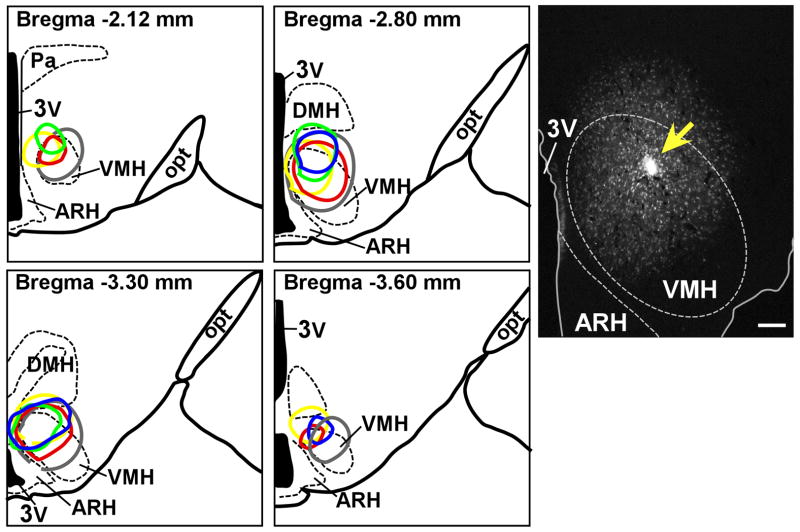
To determine Ucn 3 afferent inputs to the VMH, a retrograde tracer, FG, was injected unilaterally into the VMH of male rats. Five cases were identified with injection sites targeted to the dorsomedial part of the VMH (Fig. 1) where high levels of CRFR2 and a large concentration of Ucn 3-positive fibers are located (Li et al., 2002). As showin in Fig. 1, injection sites were centered primarily in the VMHdm with varying rostrocaudal extents and occasionally spread dorsally to include the ventral border of the DMH and ventrally to cover the adjacent ventral part of the VMH. Injections centered in neighboring structures, such as the dorsomedial nucleus (DMH) and the arcuate nucleus (ARH), served as control experiments.
Fig. 1.
Left: Schematic drawings of four rostral-to-caudal levels of the rat hypothalamus showing the distribution of the FG injection site of five successful cases. Distance from bregma is indicated at each level. Right: Representative fluorescence micrograph from case #35 (red circle in the drawings) showing fluorogold (FG) injection site (yellow arrow) in the dorsomedial part of the VMH. 3V: Third ventricle, ARH: Arcuate nucleus of hypothalamus, DMH: Dorsomedial nucleus of hypothalamus, opt: Optic tract, VMH: Ventromedial nucleus of hypothalamus. Scale bar = 100 μm.
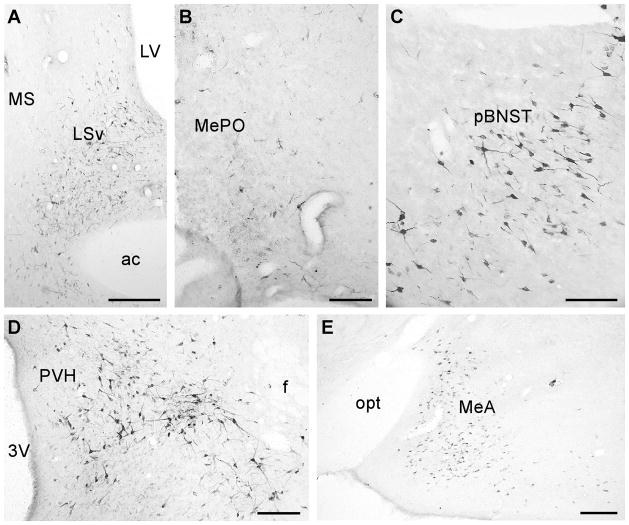
The pattern of FG-positive cells distribution was similar across the five cases. In the forebrain, FG-immunoreactive (ir) neurons were found mostly in the hypothalamus and the limbic system (Fig. 2 and 3) including the LS, the BNST, and the amygdala. In the LS, FG-positive cells were observed mostly in the ventral part of the nucleus. Very few cells were found in the medial septal nucleus. In the BNST, the majority of FG-positive cells were found scattered across all three subdivisions (medial, intermediate, and lateral) of the posterior part of the nucleus (Fig. 3). A few FG-positive cells were observed in the anterior part of the BNST. In the amygdala, the majority of retrograde labeled cells were found in the posteroventral (MePV) and posterodorsal parts (MePD) of the medial amygdala (MeA) (Fig. 2, 3) with moderate levels of FG-positive cells scattered in the anterodorsal part of the nucleus (MeAD) and in the amygdalohippocampal area (AHi). In the hypothalamus, abundant FG-ir neurons were observed in theanterior nucleus (AH), subparaventricular zone (SPa), rostral perifornical area (rPFH), periventricular nucleus (Pe), and PVHap (Fig. 2, 3). Moderate numbers of FG-ir cells were found in the medial (MPN) as well as median preoptic nucleus (MePO) and the DMH (Fig. 3). A few scattered FG-positive cells were found in the ARH and the lateral hypothalamic area (LH) (Fig. 3). In control experiments, abundant FG-positive cells in the hypothalamus and the limbic system were also observed in animals with injection sites outside the VMH; subtle differences were noted. For example, compared to VMH targeted cases, animals with a FG injection site centered in the DMH area showed more retrograde-labeled cells in the MPN, MePO and LH with less abundant FG-positive cells observed in the amygdaloid area. On the other hand, animals with FG deposits centered in the ARH area covering the ventral part of the VMH showed more FG-positive cells in the medial preoptic area (mPOA) and the paraventricular nucleus (PVH) in the hypothalamus compared to VMH injected animals (data not shown).
Fig. 2.
Representative photomicrographs showing FG-positive cells in the LSv (a), MePO (b), pBNST (c), perifornical hypothalamic area (d) and MeA (e). 3V: Third ventricle, ac: Anterior commissure, f: Fornix, LSv: Lateral septum, ventral part, LV: Lateral ventricle, MeA: Medial amygdala, MePO: Median preoptic nucleus, MS: Medial septum, pBNST: posterior part of the bed nucleus of stria terminalis, PVH: Paraventricular nucleus of hypothalamus, opt: Optical tract. Scale bar = 250 μm (a, e), 100 μm (b, c, d).
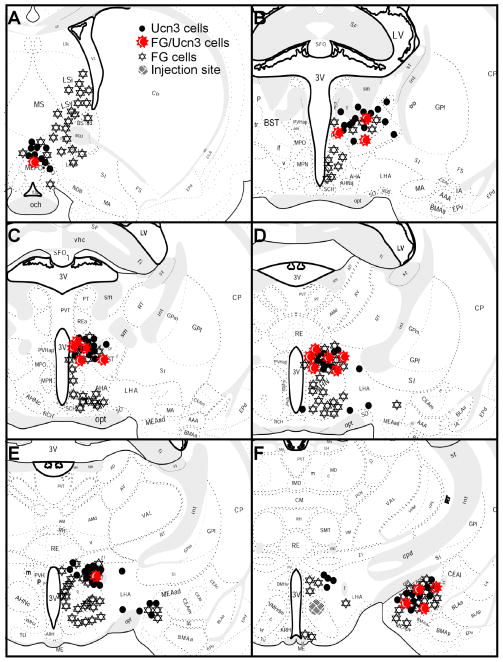
Fig. 3.
Schematic drawings of 25 μm coronal sections showing the distribution of FG-ir cells, Ucn 3 mRNA-positive cells and FG/Ucn 3 double-labeled cells in Ucn 3 expressing areas and surrounding regions. Each symbol for Ucn 3 (●) and for FG/Ucn 3 (
 ) represents approximately two labeled cells and each symbol for FG (
) represents approximately two labeled cells and each symbol for FG (
 ) represents approximately 8 cells.
) represents approximately 8 cells.
FG/Ucn 3 double-labeled neurons
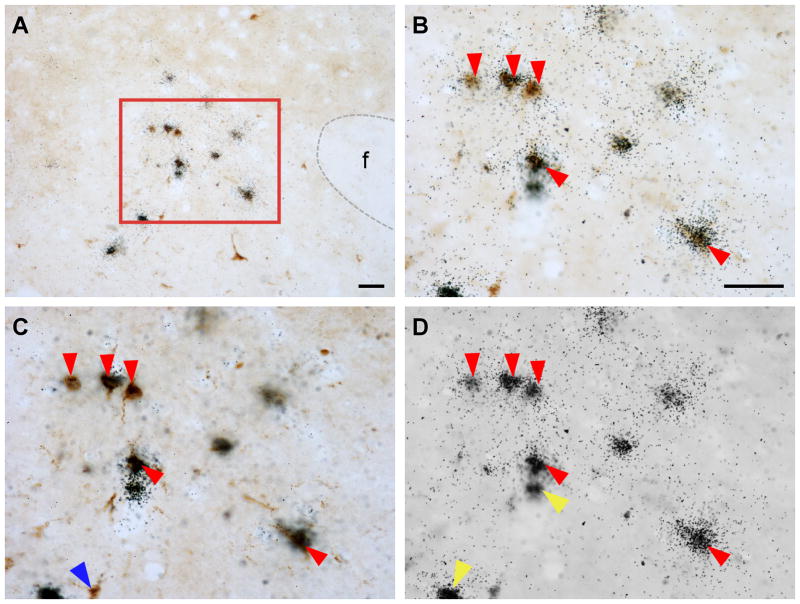
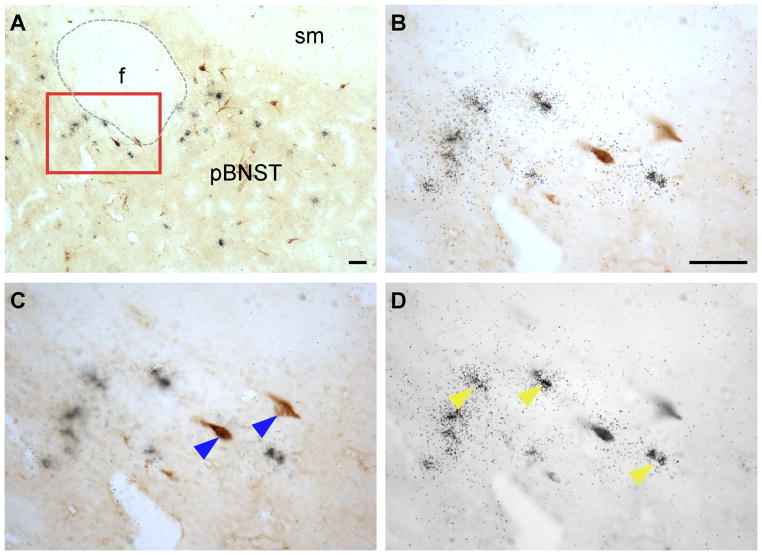
Double-label immunohistochemistry and in situ hybridization were performed to visualize FG and Ucn 3 mRNA simultaneously in brain sections from five cases in which the FG injection site was located in the proximity of the VMHdm. Consistent with earlier reports, Ucn 3 mRNA signals were observed in MePO, PVHap, rPFH, pBNST, and MeA (Fig. 3–6). When Ucn 3 mRNA signals were viewed simultaneously with FG-ir cells, FG and Ucn 3 double-labeled cells were observed in all Ucn 3 expressing areas (Fig. 3). However, there were differences in the abundance of FG/Ucn 3 double-labeled cells in different regions. FG/Ucn 3 double-labeled neurons were observed frequently in the PVHap (Fig. 3, 4) and the adjacent posterior part of BNST (pBNST) (Fig. 3, 5). Moderate numbers of double-labeled neurons were observed in the MeA (Fig. 3, 6). Only a few scattered double-labeled neurons were found in the rPFH (Fig. 3, 7) and MePO (Fig. 3).
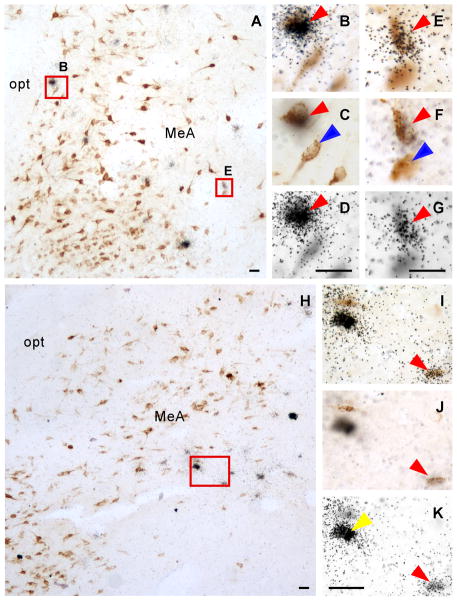
Fig. 6.
A,H: Photomicrographs showing FG-positive cells (dark brown color cells) and Ucn 3 mRNA signals (black dot clusters) in rostral (A) and caudal (H) MeA. B, E: High power magnification of boxed areas in A to show colocalization of FG immunoreactivity (brown color cells) and Ucn 3 mRNA signals (black dot clusters). C, D, F, G: Photomicrographs of B (C, D) and E (F, G) at different focal planes showing FG-positive cells (C, F) and Ucn 3 mRNA signals (silver grain clusters, D, G). I: High power magnification of boxed area in H showing colocalization of FG immunoreactivity and Ucn 3 mRNA signals. J,K: Photomicrographs of I at different focal planes showing FG-positive cells (J) and Ucn 3 mRNA signals (silver grain clusters, K). Representative double-labeled cells are indicated by red arrowhead. Single-labeled FG-positive cells are indicated by blue arrowheads and single-labeled Ucn 3 mRNA-positive cells were indicated by yellow arrowheads. MeA: Medial amygdala, opt: Optic tract. Scale bar: 25 μm.
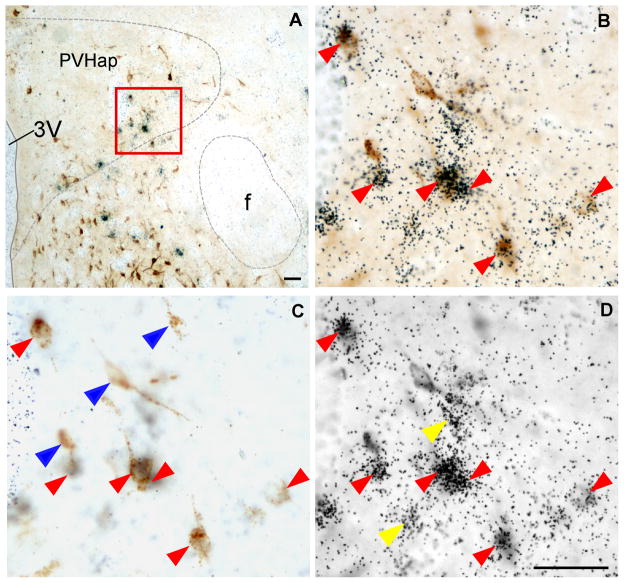
Fig. 4.
A: Representative photomicrograph showing FG-positive cells (dark brown color cells) and Ucn 3 mRNA-positive signals (black dot clusters) in the PVHap. B: High power magnification of boxed area in A showing colocalization of FG immunoreactivity and Ucn 3 mRNA signals. C,D: Photomicrographs of B at different focal planes showing FG-positive cells (C) and Ucn 3 mRNA signals (silver grain clusters, D). Representative double-labeled cells are indicated by red arrowheads. Representative single-labeled FG-positive cells in C are indicated by blue arrowheads and single-labeled Ucn 3 mRNA-positive cells in D are indicated by yellow arrowheads. 3V: Third ventricle, f: Fornix,, PVHap: anterior parvicellular part of the paraventricular nucleus of hypothalamus. Scale bar: 50 μm.
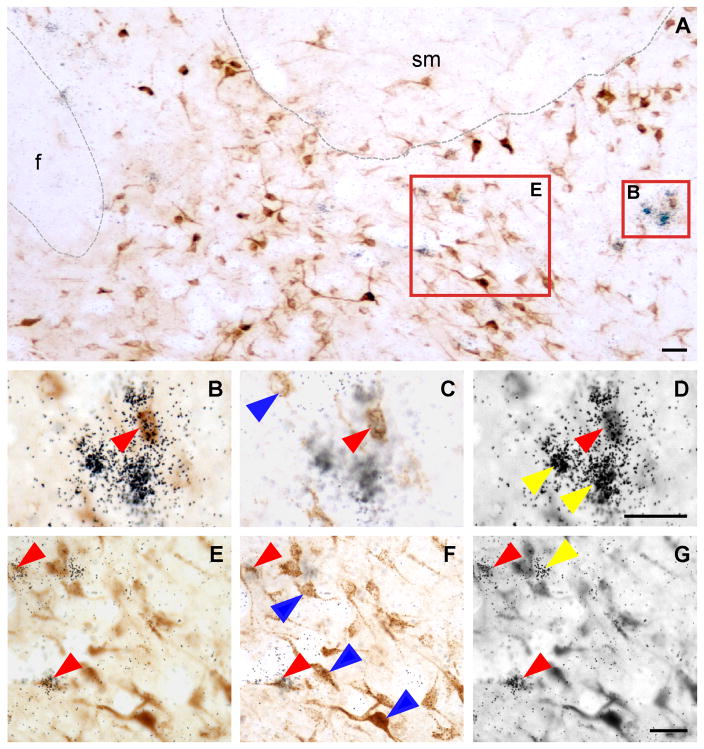
Fig. 5.
A. Bright-field photomicrograph showing FG-positive cells (dark brown color cells) and Ucn 3 mRNA-positive signals (black dot clusters) in the posterior part of the BNST. B,E: High power magnification of boxed areas in A showing colocalization of FG immunoreactivity and Ucn 3 mRNA signals. C, D, F, G: Photomicrographs of B (C, D) and E (F, G) at different focal planes showing FG-positive cells (C, F) and Ucn 3 mRNA signals (silver grain clusters, D, G). Representative double-labeled cells are indicated by red arrowheads. Representative single-labeled FG-positive cells in C and F are indicated by blue arrowheads and representative single-labeled Ucn 3 mRNA-positive cells in D and G are indicated by yellow arrowheads. f: Fornix, sm: Stria medullaris of thalamus. Scale bar: 25 μm.
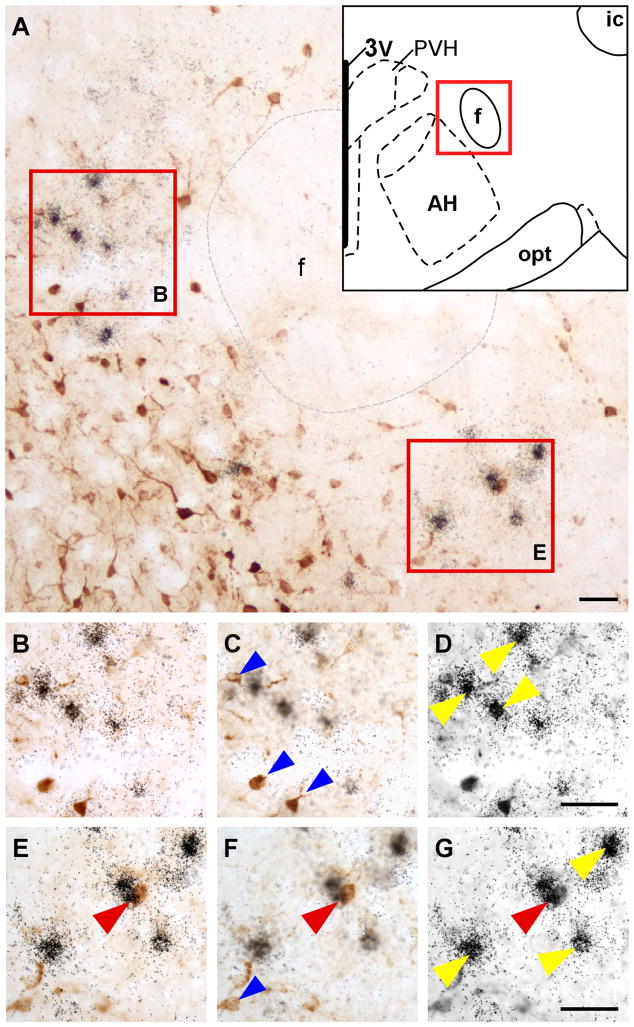
Fig. 7.
A: Representative low power photomicrograph showing FG-positive cells (dark brown color cells) and Ucn 3 mRNA signals (black dot clusters) in the rostral PFH indicated by the schematic drawing in the inset. B,E: High power magnification of boxed areas in A showing FG immunoreactivity (brown color cells) and Ucn 3 mRNA signals (silver grain clusters). Note that very few double-labeled cells were observed in this area. C, D, F, G: Photomicrographs of B (C, D) and E (F, G) at different focal planes showing FG-positive cells (C, F) and Ucn 3 mRNA signals (silver grain clusters, D, G). A double-labeled cell is indicated by red arrowhead. Single-labeled FG-positive cells are indicated by blue arrowheads and single-labeled Ucn 3 mRNA-positive cells were indicated by yellow arrowheads. 3V: Third ventricle, AH: anterior hypothalamus, ic: Internal capsule, f: Fornix, PVH: paraventricular nucleus of hypothalamus, opt: Optic tract. Scale bar: 100 μm (a), 50 μm (b).
Table 2 summarizes the numbers of FG-ir, Ucn 3-positive, and FG/Ucn 3 double-labeled cells identified in Ucn 3 expressing areas. In the PVHap, 20% of the retrograde labeled cells expressed Ucn 3 mRNA and the double-labeled cells accounted for nearly 45% of Ucn 3 neurons identified in this area (Fig. 3, Table 2). In the pBNST adjacent to the PVHap, we found about 8% of FG-positive cells expressed Ucn 3 mRNA which was approximately 38% of Ucn 3 cells found in this area (Fig. 3, Table 2). In the MeA, about 4% of FG-positive cells were also Ucn 3-positive and the double-labeled cells were 26% of Ucn 3-positive cells in this area (Fig. 3, Table 2). In the rPFH and MePO (Fig. 3, Table 2), 5% and 7% of retrograde labeled cells were also positive for Ucn 3 mRNA signal, respectively. However, the double-labeled cells accounted for approximately 10% of Ucn 3-positive cells identified in these areas (Table 2).
Table 2.
Primary Antibodies used in the study
| Antigen | Immunogen | Source of antibody | Dilution used |
|---|---|---|---|
| Ucn 3 | recombinant human Ucn 3 | Rabbit polyclonal, lot#6570, from Dr. Wylie Vale, The Salk Institute | 1:1,000 |
| Enkephalin | leu5-enkephalin conjugated to BSA | Millipore ( Billerica, MA), mouse monoclonal, #MAB350 | 1:1,100 |
| Fluoro-gold | fluorogold | Millipore ( Billerica, MA), rabbit polyclonal, #MAB153 | 1:15,000 |
Colocalization of Ucn 3 and Enk in rat brain
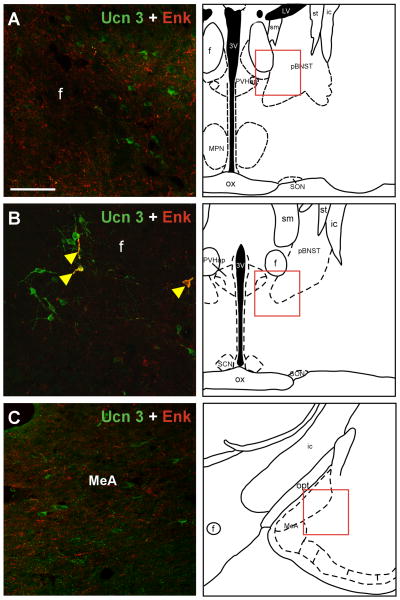
To determine the anatomical relationship between Ucn 3 and Enk, double-label immunofluorescence staining for Enk and Ucn 3 in the rat brain was performed. Enk-positive neurons were observed throughout the brain with abundant Enk neurons found in the rPFH, BNST, VMH, and POA. Confocal image analysis of double-label immunofluorescence for Enk and Ucn 3 revealed the majority of Ucn 3-positive cells in the perifornical area near the fornix (Fig. 8) were also positive for Enk. Both Ucn 3 and Enk cells were observed rostral to the rPFH in the PVHap/pBNST area (Fig. 9) but neurons positive for both Ucn 3 and Enk were rarely found. In the amygdala, the majority of the Ucn 3 cells were located in the MeA where only a few scattered single-labeled Enk-positive cells were observed (Fig. 9) and thus Ucn 3 cells in the MeA were mostly Enk-negative.
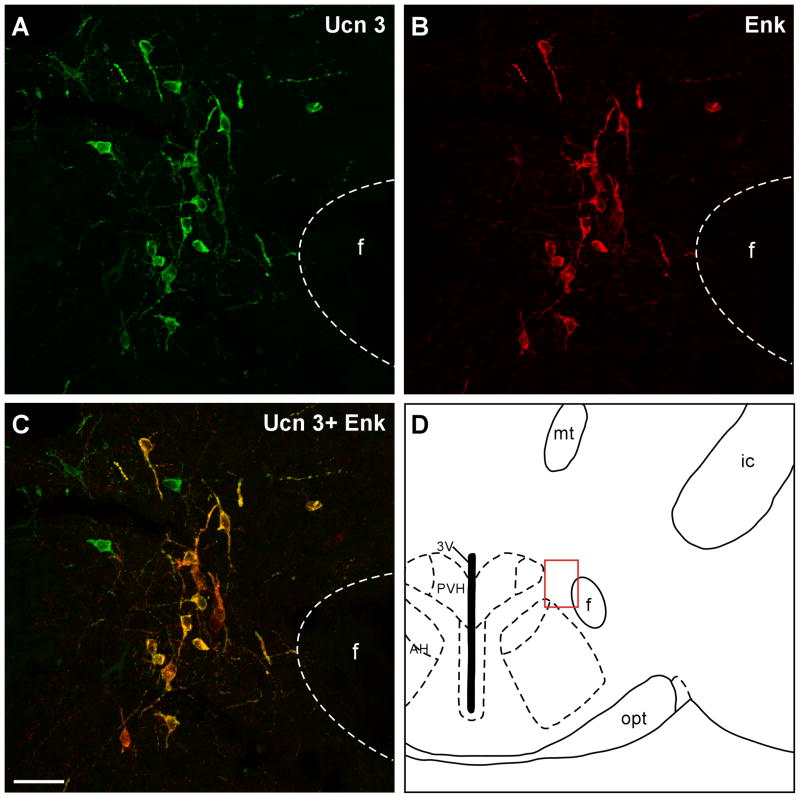
Fig. 8.
Representative stacked confocal images showing Ucn 3 (A) and Enk (B) immunostaining in the perifornical area indicated in D (red box). C: Merged images of Ucn 3 (A) and Enk (B) showing high degree of colocalization of the two materials in neurons and fibers in this brain area. 3V: Third ventricle, AH: Anterior hypothalamus, f: Fornix, ic: Internal capsule, mt: Mammillothalamic tract, PVH: Paraventricular nucleus of hypothalamus. opt: Optic tract. Scale bar: 25 μm.
Fig. 9.
a: Double-label immunofluorescent staining of Ucn 3 (green) and Enk (red) in the posterior part of the BNST (A), the PVHap (B) and in the medial amygdala (C). The location of each image is indicated by red box in the respective drawing. Note that both Ucn 3 and Enk-positive cells and fibers are evident in these areas, but double-label cells or fibers were rarely observed. A few double-labeled cells and fibers (indicated by yellow arrowheads) were observed in the PVHap (B). 3V: Third ventricle, f: Fornix, ic: Internal capsule, MPN: Medial preoptic nucleus, LV: Lateral ventricle, ox: Optic chiasm, MeA: Medial amygdala, pBNST: posterior part of the bed nucleus of stria terminalis, PVHap: Anterior parvicellular part of the paraventricular nucleus of hypothalamus, SCN: Suprachiasmatic nucleus of hypothalamus, SON: Supraoptic nucleus of hypothalamus, sm: Stria medullaris of thalamus, st: Stria terminalis. Scale bar: 50 μm.
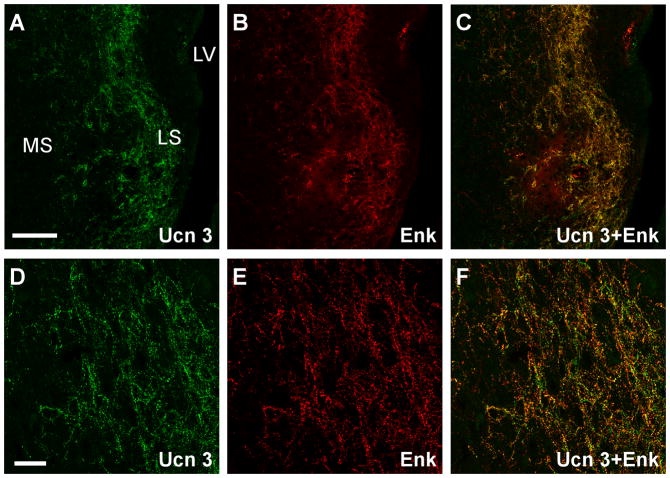
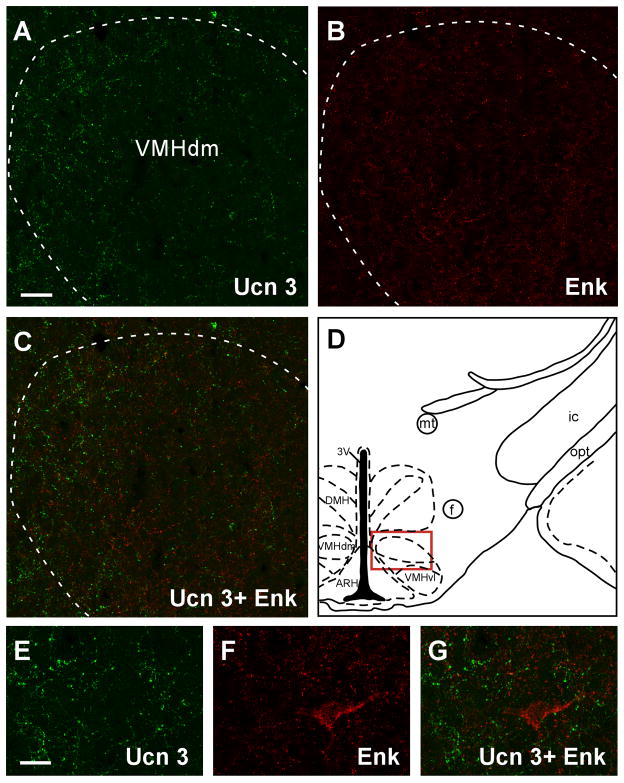
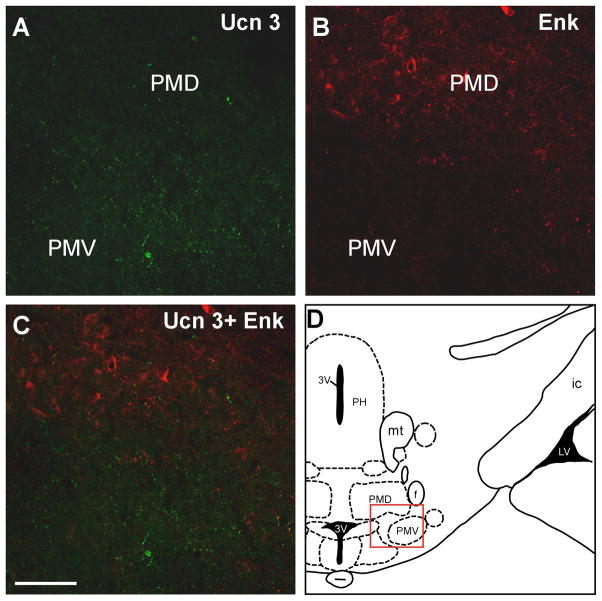
Among brain areas which receive Ucn 3 innervation, prominent Ucn 3/Enk double labeled fibers and terminals were only found in the intermediate part of the LS (Fig. 10). In the hypothalamus, both Ucn 3 and Enk-positive fibers were found in the VMH (Fig. 11) but Ucn 3/Enk double-labeled fibers were rarely found. In the ventral premammillary nucleus (Fig. 12) where Ucn 3 fibers were prominent, Enk-positive fibers were relatively less abundant and only scant double-labeled fibers were observed. Outside the hypothalamus, abundant single-labeled fibers for Ucn 3 and Enk were evident in the MeA but double-labeled fibers were rarely encountered (Fig. 9). No significant double-labeled fibers were observed in other brain areas which received moderate Ucn 3 innervation including the preoptic area and the ARH (data not shown).
Fig. 10.
Representative stacked confocal images showing double immunofluorescent labeling of Ucn 3 (A, D) and Enk (B, E) in the lateral septum. C, F: Merged images of A and B (C) and D and E (F) showing high degree of colocalization of the two neuropeptides in neuronal fibers and axonal terminals in this area. LS: Lateral septum, LV: Lateral ventricle, MS: Medial septum, Scale bar: 50 μm (A), 20 μm (D).
Fig. 11.
Fluorescent confocal images showing Ucn 3 (A) and Enk (B) immunostaining in the VMH. The location of the images was indicated by the red box in D. C: Merged image of A and B showing no significant colocalization of Ucn 3 and Enk in the VMH. E–G: High magnification of the dorsomedial part of the VMH showing the staining of Ucn 3 (E) and Enk (F). Merged image (G) of E and F further illustrates little colocalization of the two neurosubstrates in this brain area. 3V: Third ventricle, ARH: Arcuate nucleus of hypothalamus, DMH: Dorsomedial nucleus of hypothalamus, f: Fornix, ic: Internal capsule, mt: Mammillothalamic tract, opt: Optic tract, VMHdm and VMHvl: Dorsomedial part (VMHdm) and ventrolateral part (VMHvl) of the ventromedial nucleus of hypothalamus. Scale bar: 50 μm (A), 20 μm (E).
Fig. 12.
Fluoresecnt confocal images showing Ucn 3 (A) and Enk (B) in the premammillary area indicated by the red box in D. C: Merged image of A and B indicates that Ucn 3 immunoreacitivty was found mostly in the PMV where as prominent Enk staining was observed in the PMD and thus no significant colocalization of Ucn 3 and Enk was found in these brain regions. 3V: Third ventricle, f: Fornix, ic: Internal capsule, LV: Lateral ventricle, mt: Mammillothalamic tract, PH: Posterior hypothalamus, PMD: Dorsal premammillary nucleus, PMV: Ventral premammillary nucleus. Scale bar: 50 μm.
Afferent input to the LS from Ucn 3 neurons in the rPFH
A retrograde tracing experiment was conducted to further determine if Ucn 3 neurons in the rPFH project directly into the LS. Four animals with FG injection site targeted in the LS were used for double-label study to visualize FG and Ucn 3 mRNA simultaneously. The injection sites primarily centered in the intermediate part of the LS (LSi) (Fig. 13) with varying rostrocaudal extents and often covered the dorsal part of the LS (LSd) and occasionally spread ventrally into the ventral part of the lateral septum. In Ucn 3 expressing areas, FG-positive cells were observed in the rPFH (Fig. 14) and pBNST (Fig. 15). Only a few scattered FG-positive cells were found in the MePO and MeA (data not shown). The majority of FG/Ucn 3 mRNA double-labeled neurons were observed in the rostral perifornical area (Fig. 14). On the other hand, double-labeled neurons were rarely encountered in pBNST/PVHap area (Fig. 15).
Fig. 13.
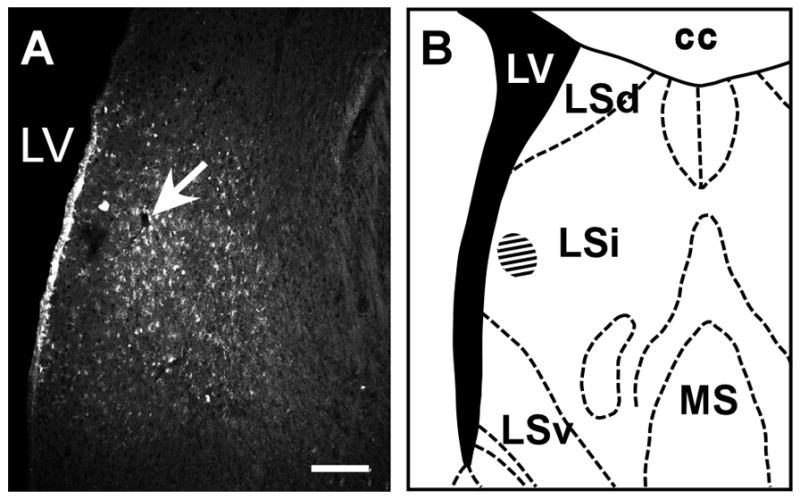
A: Fluorescent image showing FG tracer injection site (white arrow) in the septal region. The injection site centers near the intermediate part of the lateral septal nucleus (B, hatched area), which receives a prominent Ucn 3 fiber innervation. cc: Corpus callosum, LSd: Dorsal part of the lateral septal nucleus, LSi: Intermediate part of the lateral septal nucleus, LSv: Ventral part of the lateral septal nucleus, LV: Lateral ventricle, MS: Medial septal nucleus. Scale bar: 100 μm.
Fig. 14.
A: Bright-field photomicrograph showing double-labeling of FG-positive cells (dark brown cells) and Ucn 3 mRNA signals (black dot clusters) in the rostral perifornical hypothalamic area. B: High magnification photomicrograph of boxed area in A showing cells double-labeled with FG and Ucn 3 mRNA signals. C,D: Photomicrographs of B at different focal planes to show FG-positive cells (C) and Ucn 3 mRNA signals (silver grain clusters, D). Representative double-labeled cells are indicated by red arrowheads. FG single-labeled cells are indicated by blue arrowheads and Ucn 3 mRNA single-labeled cells are indicated by yellow arrowheads. f: Fornix. Scale bar: 50 μm.
Fig. 15.
A: Bright-field photomicrograph showing FG-positive cells (dark brown cells) and Ucn 3 mRNA-positive signals (black dot clusters) in the posterior part of the BNST. B: High magnification of boxed area in A. Note that no colocalization of FG and Ucn 3 mRNA signals in this area. C, D: Photomicrographs of B at different focal planes to show FG-positive cells (C) and Ucn 3 mRNA signals (silver grain clusters, D). Representative single-label FG cells were indicated by blue arrowheads (C) and single-label Ucn 3 mRNA signals were indicated by yellow arrowheads. f: Fornix, pBNST: Posterior part of the bed nucleus of stria terminalis, sm: Stria medullaris of thalamus. Scale bar: 50 μm.
Discussion
In the present study, FG retrograde tracing combined with in situ hybridization was used to determine the Ucn 3 afferent input to the VMH. The pattern of retrograde labeled neurons following FG injections into the VMH is similar to that described in earlier reports (Berk and Finkelstein, 1981; Fahrbach et al., 1989; Kita and Oomura, 1982; Luiten and Room, 1980; McBride and Sutin, 1977; Zaborszky, 1982). We found the majority of Ucn 3 afferent into the VMH was located in the PVHap and pBNST and Ucn 3 neurons in the MeA provide moderate projection to the VMH. Ucn 3 neurons in the rPFH located immediately caudal to the PVHap/pBNST Ucn 3 cell group and in the MePO provide only minor Ucn 3 input into the VMH. Consistent with the tracing result, double-label immunofluorescence for Ucn 3 and Enk showed most Ucn 3 neurons in the rPFH were also positive for Enk and Ucn 3/Enk double-labeled fibers and terminals were observed predominately in the LS. Ucn 3 cells and fibers found in other brain areas including the VMH showed little colocalization with Enk. Finally, a retrograde tracing study demonstrated Ucn 3 neurons in the rPFH project to the LS. Taken together, these results indicate the major Ucn 3 cell group in the hypothalamus is heterogeneous in terms of anatomical projection: the rostral part (PVHap/pBNST) projects to the VMH and the caudal part (rPFH) projects to the LS.
Heterogeneity of the major Ucn 3 neuronal population in the hypothalamus has previously been noted with a degree of co-expression of thyrotropin-releasing hormone (TRH): almost all Ucn 3 neurons in the rPFH also express TRH where only about half the Ucn 3 neurons in the PVHap/pBNST area are also TRH-positive (Wittmann et al., 2009). Furthermore, co-expression of Enk and TRH has been reported in PFH neurons and Enk/TRH neurons in the PFH project to the LS (Merchenthaler, 1991). Taken together with the results from the present study, we suggest Ucn 3 neurons in the PVHap/pBNST provide afferent input to the VMH and some of these neurons express TRH. On the other hand, Ucn 3 neurons in the PFH coexpress Enk and TRH and project to the LS.
The number of FG cells found in the PVHap after FG injection into the VMH was modest compared to areas such as the amygdala and pBNST where nearly 2–3 times more FG-positive cells were found. It is noteworthy that the relatively high percentage of the retrograde labeled cells (20%) was Ucn 3-positive in the PVHap whereas in the amygdala and pBNST, FG/Ucn 3 cells only accounted for 3% and 8% of total FG-positive cells identified in these respective areas. Importantly, FG/Ucn 3 cells found in the PVHap constitute approximately 45% of Ucn 3 cells identified in this area. This result indicates that Ucn 3 neurons are a major constituent of the afferent input to the VMH from the PVHap.
PVHap neurons have been associated with neuroendocrine regulation of pituitary hormone secretion. A number of hypophysiotropic peptides including CRF and TRH are expressed in this area (Merchenthaler et al., 1988; Merchenthaler et al., 1989; Sawchenko, 1987; Swanson et al., 1983). Importantly, PVHap has also been linked to the regulation of energy balance. TRH neurons in the PVHap have been suggested to play an important role in regulating appetite (Lechan and Fekete, 2006). Anatomical studies show that PVHap TRH neurons are innervated by α-melanocyte-stimulating hormone (α-MSH), agouti related protein (AGRP), neuropeptide Y (NPY), and galanin-like peptide (GALP) (Lechan and Fekete, 2006), suggesting PVHap neurons are involved in the arcuate nucleus energy regulatory system. Currently, precise downstream brain areas mediating the effect of PVHap neurons remain elusive. The present study demonstrates that Ucn 3 neurons in this area project to the VMH thus suggesting the VMH is a possible downstream target of PVHap Ucn 3 neurons in modulating appetite and energy balance. Although specific afferent projection to Ucn 3 neurons in the PVHap remains to be determined, it has been shown that this area receives afferent inputs from most of the hypothalamus (Larsen et al., 1994), suggesting the PVHap is in a unique position to control VMH function by integrating various hypothalamic inputs.
The posterior part of the BNST covers a large area and Ucn 3 neurons projecting to the VMH reside in the posteromedial and posterolateral part of the BNST near the fornix and lateral to the PVHap. This area receives heavy projections from the amygdala, especially the olfactory system including the piriform-amygdalar area, posterolateral part of the cortical nucleus of amygdala and the MeA (Dong et al., 2001). Afferent projection of gustatory relay neurons in the parabrachial nucleus into the pBNST has also been demonstrated (Li and Cho, 2006). Functionally, the BNST plays an important role in the integration of autonomic and behavioral responses to stress (Cecchi et al., 2002; Davis et al., 1997; Khoshbouei et al., 2002) and modulation of behaviors (Numan and Numan, 1996; Numan et al., 1998; Wood and Newman, 1995) and of sodium appetite (Reilly et al., 1994; Zardetto-Smith et al., 1994). Accumulating evidence has suggested this area may be involved in the regulation of appetite and energy balance. Lesions of the amygdala results in excessive weight gain and the obesity-inducing lesions also lead to severe degeneration of projections from the amygdala into several areas including the pBNST (King et al., 2003). Furthermore, a recent study has demonstrated the BNST is also important in mediating interaction between CRF and nociceptin/Orphanin FQ in regulating feeding (Ciccocioppo et al., 2003). Taken together, it is conceivable that the pBNST is involved in integrating sensory information (olfactory and gustatory) to modulate appetite. Our data provide anatomical evidence that the VMH is a downstream target of the pBNST and we identify a neurosubstrate (Ucn 3) which is potentially a molecular mediator acting in the VMH to regulate feeding.
The amygdala has received a great deal of attention for its role in relaying olfactory information as well as emotional control (Dong et al., 2001; Wood and Newman, 1995). This area has also been shown to play a role in the regulation of feeding (King et al., 1996; King et al., 2003). The present study provides anatomical evidence that the MeA may regulate feeding by modulating VMH activity and Ucn 3 is an underlying molecular mediator in this regulation. Our results show that FG/Ucn 3 double-labeled cells comprise 4% of total FG-positive cells found in this area but the double-labeled cells accounted for about 25% of total Ucn 3 cells observed in the MeA. This result indicates that Ucn 3 neurons constitute a small population of afferent input into the VMH from the MeA. It is noteworthy that restraint stress rapidly stimulates Ucn 3 mRNA expression in the MeA (Jamieson et al., 2006) raising the possibility that under certain conditions such as stress, MeA Ucn 3 afferent input to the VMH is elevated and subsequently may have a greater impact in these situations to modulate energy balance via the VMH. More studies are needed in order to resolve this issue.
In the present study, both FG-ir and Ucn 3 mRNA-positive cells were prominent in the rPFH but very few FG/Ucn 3 double-labeled neurons were found in this area. Consistent with this result, FG injection into the LS shows that rPFH Ucn 3 project to the LS while Ucn 3 neurons in the pBNST/PVHap provide only scant input to the LS. Furthermore, the majority of rPFH Ucn 3 cells were also positive for Enk while Ucn 3/Enk double-labeled fibers and nerve terminals were rarely observed in the VMH. Together, these data indicate that Ucn 3 cells in the rPFH provide only minor afferent input to the VMH and likely do not play an important role in regulating VMH activity.
Wittmann et al. shows the majority of Ucn 3 neurons in the rPFH also contain TRH (Wittmann et al., 2009). The present study further illustrates that Ucn 3 neurons in the same area also express Enk and this population of Ucn 3/Enk neurons projects predominately to the LS. It is likely all three neuropeptides are found in this unique population of neurons in the rPFH. It is not uncommon that two or more neuropeptides are co-expressed by the same cell group. It is conceivable these neuropeptides allow this cell group to exert distinct and subtle regulation on its target neurons in the LS.
In the LS, activation of CRFR2 by Urocortin peptides impairs fear conditioning associated learning (Radulovic et al., 1999; Todorovic et al., 2007). Kuperman et al. show that mice over-express Ucn 3 in the rPFH, which presumably leads to overstimulation of CRFR2 in the LS, display elevated anxiety-like behavior (Kuperman et al. 2010). Currently, the function of the other two neuropeptides in the LS is unclear. Interestingly, female preproenkephalin knockout mice display exaggerated responses to a fear-provoking environment (Ragnauth et al., 2001) and central injection of TRH suppresses conditioned fear (Thompson and Rosen, 2000). Anatomical studies have shown that Enk-positive fibers make close contacts on GABA neurons in the LS (Szeidemann et al., 1995). It is conceivable that Ucn 3/Enk/TRH neurons projecting to the LS regulate fear associated learning and possibly anxiety by modulating GABAergic activity.
In conclusion, the present study determines the major Ucn 3 afferent to the VMH resides in the PVHap/pBNST. Furthermore, we define heterogeneity of the major Ucn 3 cell group located in the hypothalamus: the rostral part, i.e. the cell group in the PVHap/pBNST, projects to the VMH while the caudal part of the cell group, which resides in the rPFH, coexpresses Enk and projects to the LS (Fig. 16).
Fig. 16.
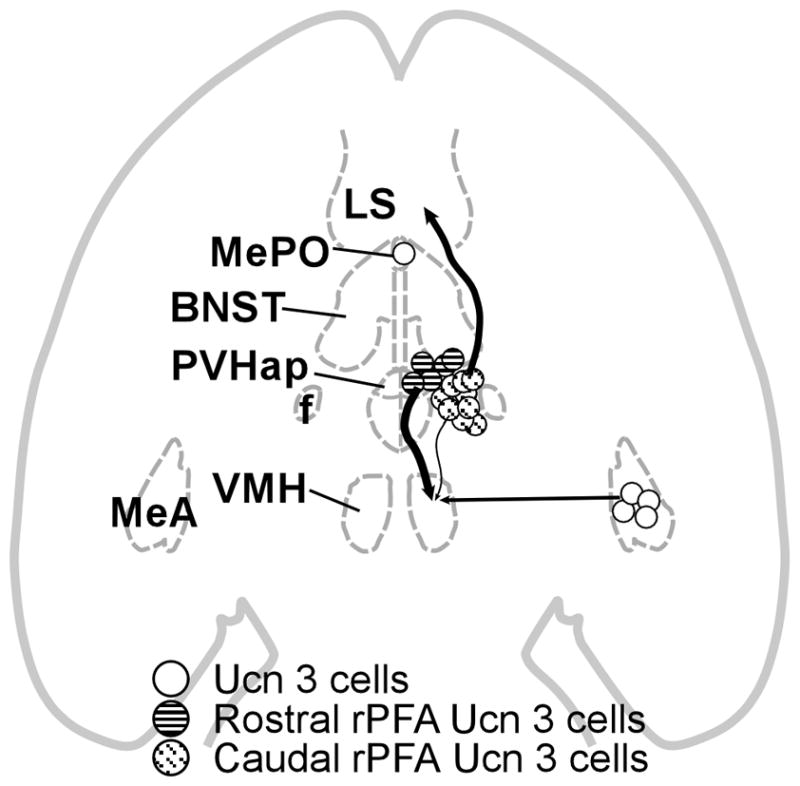
Organization of Ucn 3 projections to the VMH and LS. The magnitude of each pathway is roughly proportional to the thickness of the line representing it. Ucn 3 neurons in the PVHap/pBNST provide the major Ucn 3 afferent input to the VMH. Ucn 3 cells in the rPFH immediately caudal to the PVHap/pBNST Ucn 3 cell group project predominately to the LS. Ucn 3 cells in the MeA provide moderate afferent into the VMH. BNST: Bed nucleus of stria terminalis, f: Fornix, LS: Lateral septal nucleus, MeA: Medial amygdala, MePO: Median preoptic nucleus, PVHap: Anterior parvicellular part of the paraventricular nucleus of hypothalamus, VMH: Ventromedial nucleus of hypothalamus.
Acknowledgments
Studies included in this manuscript were supported by R01 DK-078049 and P01DK026741-25 from the National Institute of Diabetes and Digestive and Kidney Diseases
We thank Michael DiGruccio for technical assistance and Dr. Ruth Stornetta for comments on the manuscript.
References
- Berk ML, Finkelstein JA. Afferent projections to the preoptic area and hypothalamic regions in the rat brain. Neuroscience. 1981;6(8):1601–1624. doi: 10.1016/0306-4522(81)90227-x. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmuller D, Zimmer A. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology. 2008;33(4):425–436. doi: 10.1016/j.psyneuen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112(1):13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Chen P, Vaughan J, Donaldson C, Vale WW, Li C. Injection of Urocortin 3 into the ventromedial hypothalamus modulates feeding, blood glucose levels and hypothalamic POMC gene expression but not the HPA axis. American journal of physiology. 2009 doi: 10.1152/ajpendo.00402.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23(28):9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello AC, Milstein C, Couture R, Wright B, Priestley JV, Jarvis J. Characterization and immunocytochemical application of monoclonal antibodies against enkephalins. J Histochem Cytochem. 1984;32(9):947–957. doi: 10.1177/32.9.6086744. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Annals of the New York Academy of Sciences. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38(1–2):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Progress in neuro-psychopharmacology & biological psychiatry. 2001;25(4):729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Studies of ventromedial hypothalamic afferents in the rat using three methods of HRP application. ExpBrain Res. 1989;77(2):221–233. doi: 10.1007/BF00274980. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, Koob GF, Zorrilla EP. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32(5):1052–1068. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7(5):605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Li C, Kukura C, Vaughan J, Vale W. Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology. 2006;147(10):4578–4588. doi: 10.1210/en.2006-0545. [DOI] [PubMed] [Google Scholar]
- Janecka A, Fichna J, Janecki T. Opioid receptors and their ligands. Current topics in medicinal chemistry. 2004;4(1):1–17. doi: 10.2174/1568026043451618. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Morilak DA. Modulatory effects of galanin in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuropsychopharmacology. 2002;27(1):25–34. doi: 10.1016/S0893-133X(01)00424-9. [DOI] [PubMed] [Google Scholar]
- King BM, Cook JT, Dallman MF. Hyperinsulinemia in rats with obesity-inducing amygdaloid lesions. Am J Physiol. 1996;271(5 Pt 2):R1156–1159. doi: 10.1152/ajpregu.1996.271.5.R1156. [DOI] [PubMed] [Google Scholar]
- King BM, Cook JT, Rossiter KN, Rollins BL. Obesity-inducing amygdala lesions: examination of anterograde degeneration and retrograde transport. Am J Physiol Regul Integr Comp Physiol. 2003;284(4):R965–982. doi: 10.1152/ajpregu.00249.2002. [DOI] [PubMed] [Google Scholar]
- Kita H, Oomura Y. An HRP study of the afferent connections to rat medial hypothalamic region. Brain research bulletin. 1982;8(1):53–62. doi: 10.1016/0361-9230(82)90027-2. [DOI] [PubMed] [Google Scholar]
- Kuperman Y, Issler O, Regev L, Musseri I, Navon I, Neufeld-Cohen A, Gil S, Chen A. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(18):8393–8398. doi: 10.1073/pnas.1003969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Hay-Schmidt A, Mikkelsen JD. Efferent connections from the lateral hypothalamic region and the lateral preoptic area to the hypothalamic paraventricular nucleus of the rat. The Journal of comparative neurology. 1994;342(2):299–319. doi: 10.1002/cne.903420211. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Progress in brain research. 2006;153:209–235. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(13):7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Identification of neuronal input to the arcuate nucleus (ARH) activated during lactation: implications in the activation of neuropeptide Y neurons. Brain research. 1999;824(2):267–276. doi: 10.1016/s0006-8993(99)01217-2. [DOI] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22(3):991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Cho YK. Efferent projection from the bed nucleus of the stria terminalis suppresses activity of taste-responsive neurons in the hamster parabrachial nuclei. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R914–926. doi: 10.1152/ajpregu.00750.2005. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Room P. Interrelations between lateral, dorsomedial and ventromedial hypothalamic nuclei in the rat. An HRP study. Brain research. 1980;190(2):321–332. doi: 10.1016/0006-8993(80)90277-2. [DOI] [PubMed] [Google Scholar]
- McBride RL, Sutin J. Amygdaloid and pontine projections to the ventromedial nucleus of the hypothalamus. The Journal of comparative neurology. 1977;174(3):377–396. doi: 10.1002/cne.901740302. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I. Co-localization of enkephalin and TRH in perifornical neurons of the rat hypothalamus that project to the lateral septum. Brain research. 1991;544(1):177–180. doi: 10.1016/0006-8993(91)90903-9. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Csernus V, Csontos C, Petrusz P, Mess B. New data on the immunocytochemical localization of thyrotropin-releasing hormone in the rat central nervous system. The American journal of anatomy. 1988;181(4):359–376. doi: 10.1002/aja.1001810404. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Maderdrut JL, Altschuler RA, Petrusz P. Immunocytochemical localization of proenkephalin-derived peptides in the central nervous system of the rat. Neuroscience. 1986;17(2):325–348. doi: 10.1016/0306-4522(86)90250-2. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Meeker M, Petrusz P, Kizer JS. Identification and immunocytochemical localization of a new thyrotropin-releasing hormone precursor in rat brain. Endocrinology. 1989;124(4):1888–1897. doi: 10.1210/endo-124-4-1888. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Developmental psychobiology. 1996;29(1):23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Marzella SR, Palumbo A. Expression of c-fos, fos B, and egr-1 in the medial preoptic area and bed nucleus of the stria terminalis during maternal behavior in rats. Brain research. 1998;792(2):348–352. doi: 10.1016/s0006-8993(98)00257-1. [DOI] [PubMed] [Google Scholar]
- Ohata H, Shibasaki T. Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides. 2004;25(10):1703–1709. doi: 10.1016/j.peptides.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Onteniente B, Menetrey D, Arai R, Calas A. Origin of the met-enkephalinergic innervation of the lateral septum in the rat. Cell and tissue research. 1989;256(3):585–592. doi: 10.1007/BF00225608. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides. 2004;25(4):659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Poulain P, Martin-Bouyer L, Beauvillain JC, Tramu G. Study of the efferent connections of the enkephalinergic magnocellular dorsal nucleus in the guinea-pig hypothalamus using lesions, retrograde tracing and immunohistochemistry: evidence for a projection to the lateral septum. Neuroscience. 1984;11(2):331–343. doi: 10.1016/0306-4522(84)90027-7. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19(12):5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth A, Schuller A, Morgan M, Chan J, Ogawa S, Pintar J, Bodnar RJ, Pfaff DW. Female preproenkephalin-knockout mice display altered emotional responses. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(4):1958–1963. doi: 10.1073/pnas.041598498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JJ, Maki R, Nardozzi J, Schulkin J. The effects of lesions of the bed nucleus of the stria terminalis on sodium appetite. Acta neurobiologiae experimentalis. 1994;54(3):253–257. [PubMed] [Google Scholar]
- Sakanaka M, Magari S. Reassessment of enkephalin (ENK)-containing afferents to the rat lateral septum with reference to the fine structures of septal ENK fibers. Brain research. 1989;479(2):205–216. doi: 10.1016/0006-8993(89)91621-1. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE. Evidence for differential regulation of corticotropin-releasing factor and vasopressin immunoreactivities in parvocellular neurosecretory and autonomic-related projections of the paraventricular nucleus. Brain research. 1987;437(2):253–263. doi: 10.1016/0006-8993(87)91641-6. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Szeidemann Z, Shanabrough M, Leranth C. Hypothalamic Leu-enkephalin-immunoreactive fibers terminate on calbindin-containing somatospiny cells in the lateral septal area of the rat. The Journal of comparative neurology. 1995;358(4):573–583. doi: 10.1002/cne.903580410. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Rosen JB. Effects of TRH on acoustic startle, conditioned fear and active avoidance in rats. Neuropeptides. 2000;34(1):38–44. doi: 10.1054/npep.1999.0785. [DOI] [PubMed] [Google Scholar]
- Todorovic C, Radulovic J, Jahn O, Radulovic M, Sherrin T, Hippel C, Spiess J. Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety. The European journal of neuroscience. 2007;25(11):3385–3397. doi: 10.1111/j.1460-9568.2007.05592.x. [DOI] [PubMed] [Google Scholar]
- Wittmann G, Fuzesi T, Liposits Z, Lechan RM, Fekete C. Distribution and axonal projections of neurons coexpressing thyrotropin-releasing hormone and urocortin 3 in the rat brain. The Journal of comparative neurology. 2009;517(6):825–840. doi: 10.1002/cne.22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Integration of chemosensory and hormonal cues is essential for mating in the male Syrian hamster. J Neurosci. 1995;15(11):7261–7269. doi: 10.1523/JNEUROSCI.15-11-07261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L. Afferent connections of the medial basal hypothalamus. Advances in anatomy, embryology, and cell biology. 1982;69:1–107. doi: 10.1007/978-3-642-68289-6. [DOI] [PubMed] [Google Scholar]
- Zardetto-Smith AM, Beltz TG, Johnson AK. Role of the central nucleus of the amygdala and bed nucleus of the stria terminalis in experimentally-induced salt appetite. Brain research. 1994;645(1–2):123–134. doi: 10.1016/0006-8993(94)91645-4. [DOI] [PubMed] [Google Scholar]