Summary
Antiretroviral therapy (ART) is a life-saving intervention in human immunodeficiency virus (HIV) infection. Immune restoration after ART dramatically reduces the incidence and severity of opportunistic diseases and death. On some occasions, immune restoration may be erratic, leading to acute inflammatory responses (known as immune reconstitution inflammatory syndrome) shortly after ART initiation, or incomplete with residual inflammation despite chronic treatment, leading to non-infectious morbidity and mortality. We propose that ART may not always restore the perfect balance of innate and adaptive immunity in strategic milieus, predisposing HIV-infected persons to complications of acute or chronic inflammation. The best current strategy for fully successful immune restoration is early antiretroviral therapy, which can prevent acquired immunodeficiency syndrome (AIDS)-associated events, restrict cell subset imbalances and dysfunction, while preserving structural integrity of lymphoid tissues. Future HIV research should capitalize on innovative techniques and move beyond the static study of T-cell subsets in peripheral blood or isolated tissues. Improved targeted therapeutic strategies could stem from a better understanding of how HIV perturbs the environmental niches and the mobility and trafficking of cells that affect the dynamic cell to cell interactions and determine the outcome of innate and adaptive immune responses.
Keywords: IRIS, monocytes/macrophages, AIDS, T cells, cytokines
Introduction
The hallmark of infection with the human immunodeficiency virus (HIV) is a progressive depletion of CD4+ T cells and the risk of opportunistic infections and death in those who develop the acquired immunodeficiency syndrome (AIDS). Initiating combination antiretroviral therapy (ART) can achieve suppression of viral replication and an increase in CD4+ T-cell counts in the majority of patients (1), resulting in dramatic decreases in morbidity and AIDS-related mortality (2). The success of ART has been such that an HIV+ person who is diagnosed at age 30 and starts therapy when his CD4+ first falls below 500 cells/μL can now be expected to live to age 75 (3). Yet immune restoration may not always be a smooth transition to health or even fully successful. Starting ART may be initially accompanied by aberrant inflammatory responses termed immune reconstitution inflammatory syndrome (IRIS), wherein patients experience paradoxical deterioration in response to ART, despite efficient control of HIV viral replication and no apparent drug toxicity. Immune restoration on chronic ART may also be incomplete: there is considerable variability in both the extent of CD4+ T-cell recovery and the persistence of excess, for age, risk for morbidity and mortality. Relatively few individuals will achieve normal levels of CD4+ T cells in peripheral blood (PB), lymph nodes (LNs), or in effector sites, where the majority of CD4+ T cells reside. Up to 20% of patients may experience immunologic non-response despite HIV virologic suppression, with limited CD4+ T-cell increase or none at all (4–6). Strikingly, even those with recovery of near-normal CD4+ T-cell counts may maintain chronic immune activation that has been linked to an increased risk of non-AIDS related morbidity and mortality (7, 8), making it clear that the detrimental effects of HIV infection go beyond CD4+ T-cell depletion and immunodeficiency. In this review, we discuss to what extent ART may reverse the damage done by HIV to the function of the entire immune system, both innate and adaptive, and the clinical implications of the spectrum of immune restoration, including acute complications like IRIS after ART initiation, as well as chronic immune activation in those receiving long term therapy. We propose that both acute (IRIS) and persistent (chronic treated infection) inflammation in HIV may be the result of an imbalanced recovery of innate and adaptive immune responses, which may lead to adverse outcomes. Finally, we highlight areas of further research that may guide targeted therapeutic interventions to supplement the benefits of ART.
Early ART and IRIS
The effects of HIV infection on the human immune system lead to both immunodeficiency and immunosuppression eventually resulting in increased susceptibility to a wide range of opportunistic pathogens and diseases. Successful control of viral replication with ART greatly improves immune function and clinical outcomes in the majority of patients (1). Paradoxically, some patients beginning ART experience a clinical worsening, despite evidence of HIV virologic suppression and immunologic improvement, IRIS. IRIS is frequently characterized by both localized and systemic inflammatory symptoms of previously recognized and treated (paradoxical IRIS) or subclinical, untreated (unmasking IRIS) infections or HIV-associated malignancies (9). The incidence of IRIS can vary anywhere from 7–50% of HIV-infected patients beginning ART (10–12), with morbidity and mortality varying greatly based upon the pathogen, degree of immune compromise, site involved (i.e. central nervous system vs. lungs) and management (13). IRIS in HIV is strongly associated with severe immunodeficiency and preexisting clinical or subclinical opportunistic infections or diseases, mostly mycobacterial and fungal. The patients developing IRIS frequently require additional clinic visits or hospitalizations, complex diagnostic and therapeutic procedures, and supplementary medications. New guidelines recommending early initiation of ART in patients with tuberculosis (TB) and HIV co-infection are expected to further increase the incidence and burden of TB-IRIS (14). It is clear that approaches to prevent, diagnose early, and treat IRIS are urgently needed to allow the safe restoration of immunity in HIV-infected patients with severe comorbidities and opportunistic diseases. The mainstay of treatment is currently corticosteroids (15), although targeted immunomodulatory interventions guided by a better comprehension of pathogenesis would be preferable in an already immunocompromised host.
The mechanisms underlying IRIS are being elucidated, with the help of ongoing clinical and basic research and aided by a new animal model (16). The two strongest clinical predictors of IRIS are severe immunosuppression manifested by low CD4+ T-cell counts in HIV, and existence of a foreign antigen in the form of an opportunistic infection or tumor such as Kaposi’s sarcoma (17–19). Based on these observations, it was initially hypothesized that IRIS may represent a break of tolerance phenomenon similar to the autoimmune manifestations in lymphopenic mouse models (20) in which unrestrained lymphopenia induced proliferation leads to lack of self-recognition and autoimmunity. This was refuted by the fact that most IRIS patients have evidence of expanding effector memory T cells that express high levels of activation markers (PD-1 and Ki67) with a T-helper 1 (Th1) predilection (21), suggesting antigen-driven expansion and activation, rather than a predominance of homeostatic proliferation. In support of this, when antigen-specific responses were studied, CD4+ T cells from patients with IRIS events were more likely to have robust and polyfunctional [producing an array of cytokines, interleukin-2 (IL- 2), interferon γ (IFNγ), and tumor necrosis factor (TNF)] responses targeting specifically the underlying opportunistic pathogen (22, 23). Despite the augmented T-cell responses against the underlying pathogen linked to the IRIS event, responses to other co-pathogens including HIV itself were not impaired, implying that although IRIS is an exuberant and dysregulated response towards a specific pathogen, it does not cause a global immune dysfunction. The break of tolerance hypothesis could still be playing a role and ought to be revisited in patients with autoimmune manifestations of IRIS (sarcoidosis, thyroid disease, or arthritis), but studies in these patients are limited.
Serum cytokine measurements in IRIS patients have shown elevated Th1 and Th17 levels but have also consistently pointed to a strong innate signal with high levels of IL-1, IL-8, IL-6, and C-reactive protein (CRP) in many studies, regardless of the underlying pathogen (24–26). Interestingly, immunomodulatory cytokines, such as IL-10 and the related IL-22, have also been noted to be high in TB-IRIS, perhaps in a compensatory anti-inflammatory response (27). Although it is tempting to implicate a regulatory T-cell (Treg) dysfunction in the pathogenesis of IRIS, there are currently no clear data to support or dismiss their role. Characterization of Treg prior to ART initiation in some patients who developed IRIS showed a highly activated phenotype (21) that has been linked to loss of suppressive function and acquisition of either Th1 or Th17 effector function in other animal models and human studies (28–31). Clearly the role of Tregs requires further study, particularly in conjunction with T-cell interactions with antigen-presenting cells. In an attempt to reproduce the IRIS phenomenon in mice, T-cell receptor (TCR)αβ−/− mice with Mycobacterium avium complex (MAC) infection were used as a model of T-cell depletion and mycobacterial infection. Under normal conditions, these mice are heavily infected with MAC but do not develop disease. Transfer of naive CD4+ T cells leads to wasting and death of the lymphopenic but not T-cell-replete MAC-infected mice (16). In this model of mouse IRIS (mIRIS), we have shown that (i) the Th1 response is dominant as transfer of CD4+ T cells from IFNγ−/− mice can ameliorate the disease, (ii) antigen specificity is required, as transfer of transgenic ovalbumin specific T cells does not cause disease, (iii) immunosuppression (lack of antigen recognition) without CD4+ T-cell deficiency is sufficient to cause this phenomenon, as MAC-specific CD4+ T cells can cause mIRIS in MAC-infected mice that are replete with transgenic CD4+ T cells recognizing an irrelevant antigen, and finally (iv) myeloid cells play a role in the emergence of mIRIS. This last point appears to be in agreement with human data from several groups that are now unraveling the role of the innate system in the pathogenesis of IRIS with the most striking observations in TB and cryptococcal IRIS (32). A substudy from the CAMELIA trial has also shown that enhanced natural killer (NK) cell granulocytic activity even prior to ART is strongly associated with paradoxical TB-IRIS (33). Our group has recently found high levels of soluble CD163 and tissue factor in paradoxical TB-IRIS, supporting a possible role of monocyte or macrophage activation and dysfunction in its pathogenesis (authors’ unpublished observations). Recent data showing that apoptotic microparticles in HIV viremic patients may further stifle dendritic cell (DC) activity would further support this notion (34), suggesting that decreasing these microparticles with anti-HIV therapy could abruptly reverse the dysfunction of DCs.
Based on the existing data, our hypothesis is that IRIS results from the uncoupling of the innate and adaptive immune response during microbial infection: the cells of the innate immune system are primed and activated when antigen-specific CD4+ T cells are absent or inactive, and when these antigen-specific cells recover after ART initiation, discontinuation of immunosuppressive therapies such as corticosteroids or lymphocyte transfers in the animal models, they mount dysregulated immune responses that turn inflammatory and pathogenic (9). IRIS is not therefore limited to HIV, and in fact, HIV-related IRIS might just be one manifestation of a more general phenomenon of acute immunopathogenic response associated with rapid reversal of immunosuppression. The emergence of IRIS in patients with multiple sclerosis who had received and then discontinued natalizumab (blocking α4β7 integrins and thus trafficking of T cells in sites of inflammation) and developed progressive multifocal leukoencephalopathy clearly supports the concept that an uncoordinated T-cell response, not CD4 deficiency per se, is the necessary element in IRIS pathogenesis.
We thus propose that IRIS represents a dysregulated T-cell response that may stem from incongruous innate signals and can occur during reversal of immunosuppression. The diverse scenarios and presentations of IRIS are influenced by the specific pathogenic pathways involved for each underlying pathogen (bacterial or viral), the presence or absence of antimicrobial therapy, the anatomy of affected sites, and the underlying type and degree of immune suppression; CD4 deficiency alone is neither necessary nor sufficient for this phenomenon to occur. Based on the observed cytokines involved and the fact that the pathogens are ultimately cleared, we propose that T cells are central and proximal to the end effect and injury, a dysregulated inflammatory response leading to tissue destruction in organs of antigen abundance (Fig. 1A).
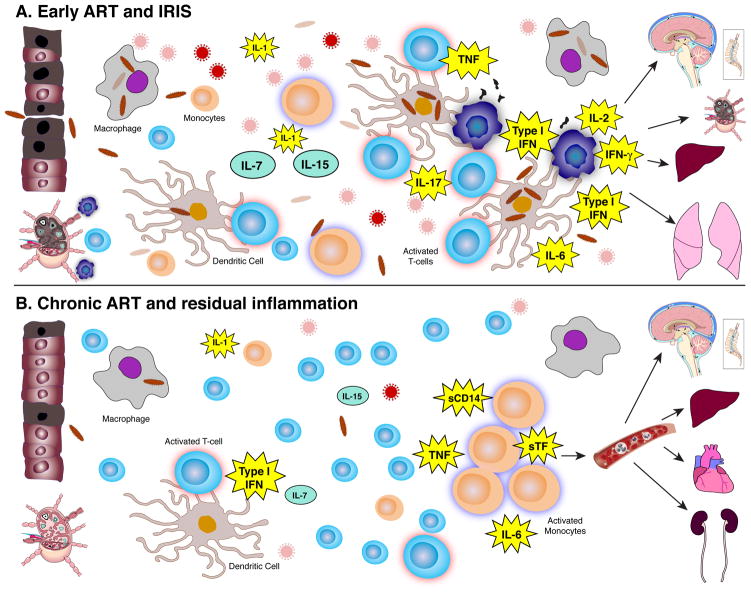
Fig. 1. Graphic depiction of the inflammatory environment of IRIS (top) during early ART and chronic residual inflammation (bottom) of long-term treated HIV.
(A) In IRIS, there is a contracted T-cell pool consisting mainly of highly activated effectors and a high antigen burden due to the uncontrolled underlying opportunistic disease. The injured mucosa further increases the antigen exposure and innate system activation, while the fibrosis in the lymph nodes restricts access to homeostatic cytokines that are underutilized leading to death of naive T cells. Apoptotic microparticles further blunt the function of antigen-presenting cells. As HIV viremia resolves, the activated effector T cells emerge and encounter primed antigen presenting cells in a disrupted milieu that fosters dysregulated inflammatory responses, and release cytokines (IFN-γ, TNF-α, IL-2, IL-17) that drive tissue damage in organs with abundance of foreign antigen such as lungs, liver, lymph nodes, or the central nervous system. (B) In chronic treated HIV, significantly lower foreign antigen burden (no opportunistic disease, partially restored leaky gut), continuing low levels of chronic viral stimulation, in the presence of a fuller T-cell pool with lower levels of T-cell activation and lower homeostatic forces, maintain innate system activation with activated monocytes and high levels of IL-6, soluble tissue factor, D-dimer, and soluble CD14 causing predominantly chronic inflammation and vascular endothelial damage that in turn results in end organ disease in the heart, brain, kidney, and liver.
Future research in IRIS would benefit from a more robust evaluation of cell to cell interactions, particularly of antigen-presenting cells with T cells, to elucidate the pathways that trigger the inflammatory responses. The role of Tregs should be further studied, and the trafficking of cells with accumulation in areas of antigen encounter should be better appreciated. A provocative recent study describing the destructive and frequently lethal fungal infection (white nose syndrome) caused by Geomyces destructans in little brown bats (35, 36) when they recover from hibernation also highlights the potential role of metabolism in immune function and of neutrophils in tissue destruction in IRIS, neither of which have been addressed in humans. Biomarkers have already been extensively studied and have identified potential candidates (TNF, INFγ, sand IL-6) for both risk stratification and as potential selection criteria or direct targets for interventional clinical trials. Pathogen-specific studies that further elucidate pathogenic pathways may help guide more targeted treatment interventions for IRIS to hopefully replace or, at a minimum, supplement corticosteroids.
Immune reconstitution during chronic ART: persistent immune activation and inflammation in treated HIV infection
Despite successful suppression of viral replication, some patients initiating ART fail to experience immune reconstitution. While the definition of incomplete CD4+ T-cell recovery or immunologic non-response may vary, it is estimated that half of ART treated patients may fail to reconstitute their CD4+ T-cell counts to levels above 500 cells/μL, and up to 16% may not achieve a CD4+ T-cell count greater than 200 cells/μL, even with long term therapy (6, 37). These individuals with lack of immunologic recovery despite suppression of viral replication are at higher risk for clinical events, including death, AIDS-defining events, malignancies, cardiovascular events, and severe infections compared to those with more substantial CD4+ T-cell increases (38, 39). Some of the risk factors for a limited CD4+ T-cell recovery include older age (40), lower CD4+ T-cell nadir prior to ART (41), and active co-infection, most notably with hepatitis C virus (HCV) (42, 43), but the strongest predictor remains a lower nadir CD4+ T-cell count before ART initiation.
The incomplete immune reconstitution observed in older patients or those who initiate ART at lower CD4+ T-cell nadirs likely reflects the impairment of T-cell homeostasis in severely lymphopenic states. Antigen-experienced CD4+ T cells, particularly those in the central memory compartment, appear to be preferentially lost in HIV infection, with newer evidence suggesting that antigen specificity may influence their susceptibility based on their degree of Th17 cytokine expression (44, 45). Replacement of mature memory CD4+ T cells, via antigen-specific clonal expansion of naive cells, and antigen-independent homeostatic turnover are also impaired or insufficient (46). HIV infection furthermore reduces the pool of naive CD4+ T cells through continuous depletion of naive T cells, both by maturing into memory T cells and because of decreased survival (47). With the initiation of ART, the accelerated differentiation of naive CD4+ T cells into memory cells is slowed (1, 48), leading to replenishment of the naive subset (44) and a restoration of the CD4+ TCR diversity (49). Data from our laboratory confirms an increase in CD4+ T-cell activation and cycling in HIV infection that depletes the overall CD4+ T-cell pool (50) and begins to normalize with ART when CD4+ T-cell counts recover (51). T cells of HIV-infected patients are also less responsive to IL-7, a necessary survival signal sustaining naive CD4+ T-cell populations, than are uninfected controls, and in ART-treated patients, IL-7 responsiveness correlates strongly with the extent of CD4+T-cell reconstitution (52, 53). In addition, reconstitution in the first two years of ART appears to be driven predominantly by T cells expressing the a chain of the IL-7 receptor (CD127) (54).
The inadequate IL-7 homeostatic signaling in immune restoration should also be studied within the tissue niche of naive lymphocytes, the lymph nodes. In 2002, Ashley Haase’s group (46) reported a strong negative correlation between both CD4+ T-cell counts and the degree of peripheral CD4+ T-cell reconstitution on ART with the extent of collagen deposition in the paracortical T-cell zone in LT of individuals with chronic HIV infection. As a result, they hypothesized that these LT were unable to support normal T-cell reconstitution. Further research into this area has revealed that the inflammation and activation of transforming growth factor β (TGFβ) pathway found in HIV-infected patients leads to fibrosis that disrupts the tissue architecture and impedes naive T-cell access to survival cytokines including IL-7, resulting in their apoptotic depletion (55). In addition, the death of naive T cells deprives the LT from production of lymphotoxin B, a critical survival factor for the reticular endothelial cells that in turn are the main producers of IL-7 in the lymph nodes. This leads to a vicious cycle of decreased production of IL-7 and underutilization caused by difficult access to it due to fibrosis. It is unclear whether this fibrosis is reversible with long term ART therapy. Interestingly, blocking the effects of TNFα in acute simian immunodeficiency virus (SIV) infection was shown to prevent fibrosis of LT, without any decrease in T-cell activation or change in CD4+ T-cell counts or SIV viremia (56). These data suggest the involvement of TNF pathway in tissue fibrosis but not necessarily in T-cell cycling and activation. Fibrosis in other sites, including mucosal effector sites, has also been shown in HIV infection, and this may correlate with the extent of immune recovery of these sites after ART (57, 58). Further investigations into specific anti-fibrotic therapies in HIV, both in acute and chronic infections, are ongoing. Intriguingly, another factor consistently associated with limited CD4+ T-cell reconstitution is chronic immune activation, which persists despite ART (59–61), although it is unclear whether this is a cause or result of impaired immune restoration. HIV-infected individuals, even those receiving long term therapy, are more likely to develop non-AIDS morbidity and die of non-AIDS events at an earlier age than the HIV-uninfected population (7, 8). Markers of chronic immune activation and inflammation, IL-6, D-dimer, C-reactive protein (CRP), and soluble CD14 (sCD14), are predictive of these events. These inflammatory biomarkers continue to be elevated in individuals receiving ART, and are predictors of both AIDS and non-AIDS associated morbidity and mortality after controlling for CD4+ T-cell counts and HIV viral load (62–65). It is not clear what drives chronic immune activation in treated HIV infection. Possible causes include persistent innate system activation from residual HIV replication, ongoing viral co-infections, or microbial translocation across a compromised gastrointestinal mucosa as well as persistent homeostatic signals.
Chronic viral stimulation
A regimen of combination antiretroviral therapy typically leads to plasma viremia suppression to below 50 copies/mL by conventional detection assays. Ultra-sensitive assays detecting levels of viremia between 1 and 49 copies/mL have repeatedly shown that lower levels of HIV can still be identified in patients on ART (66, 67). However, intensification of ART with the addition of alternative classes of medications has shown mostly negative results in further suppressing the viremia or T-cell activation (68, 69), with one study showing a transient decrease in markers of HIV integration with an associated decrease in T-cell activation markers in a subgroup of participants (70). Elite controllers spontaneously control viral replication, many of them without developing opportunistic infections or AIDS, in the absence of ART. While their viral loads are below the limit of detection in conventional assays (<50 copies/mL), they also frequently have measurable low level viremia, ongoing viral replication, and evolution of their viral reservoir (71). These persons also have elevated levels of inflammatory biomarkers, immune activation, and non-AIDS events, including atherosclerosis, in the absence of uncontrolled viremia or ART (72, 73), suggesting that the low (below detection by routine assays) HIV viremia may be playing a role in chronic inflammation in HIV.
Other chronic viral infections, such as herpes simplex virus (HSV) and viral hepatitis that are disproportionately common in HIV infected patients (74) as well as cytomegalovirus (CMV) with more than 90% of HIV+ patients seropositive for it (75), may contribute to immune activation. More specifically, CMV-specific T cells have been implicated in atherosclerosis, which has a well-studied component of innate immune activation (76, 77). Administration of anti-CMV therapy in HIV-infected patients, on ART for more than 6 months without significant immune reconstitution (CD4<350 cells/μL) and without CMV end organ disease, was found to reduce some markers of CD8+ T-cell activation but did not increase CD4+ T-cell counts (78). Randomized studies of anti-HSV therapy have shown reductions in HIV viral load by approximately half a log (79) and increases in CD4+ T-cell counts (80) in ART-naive patients, but it remains unclear if HSV remains a significant contributor to immune activation in treated HIV patients.
Immune restoration in mucosal sites
Another potential setback of successful immune restoration and driver of ongoing chronic immune activation on ART may be the HIV-induced injury in mucosal barriers. The gut-associated lymphoid tissue (GALT) is the most widely studied of these. GALT homes a large proportion of the body’s CD4+ T cells. During acute HIV (or SIV) infection, this tissue compartment is severely compromised, with massive depletion of T lymphocytes, altered gut microbiota, increased permeability, and extensive intestinal mucosal inflammation (81). It has been proposed that microbial translocation (MT) across this damaged mucosa and defective clearance leads to persistent innate system and T-cell activation (81, 82). Association of MT with T-cell activation (82) but lack of direct correlation with mortality suggests an important intermediate step that is most likely the activation of the innate system. Although treatment with ART can produce a remarkable restoration of peripheral CD4+ T cells, reduce CD4+ T-cell activation, and improve CD4+ T-cell populations at effector sites (51), some reports showed that restoration of CD4+ T cells in the lamina propria may be incomplete, even after up to five years on ART (83). Recent data suggest a possible defect in gut homing that may be contributing to this ineffective repopulation (84), highlighting how studies ought to extend beyond peripheral blood to assess tissue-homing receptors and cell trafficking in conditions of perturbed homeostasis (85). Elite controllers appear to have intact mucosal T cells (50), despite recent evidence of increased inflammatory biomarkers (sCD163, LPS) and possible cardiovascular disease, suggesting that the mucosal T cells are not the necessary injury that connects systemic inflammation with HIV, as was also highlighted in nonpathogenic models of SIV infection in sooty mangabeys (86).
Homeostatic drive and T-cell subsets
CD4+ T-cell lymphopenia is the characteristic defect of untreated HIV infection and is often attributed to direct mechanisms, including infection and viral cytopathic effect or killing by virus-specific cytotoxic CD8+ T cells, as well as indirect effects, including bystander apoptosis due to chronic immune activation (87) or pyroptosis, a pro-inflammatory induced cell death with release of caspases 1 and 3, as a consequence of abortive HIV infection (88) or deprivation of survival cytokines as described previously. The dynamics of CD4+ T-cell loss are increasingly understood to differently affect the PB, LT, and effector sites, where the majority (approximately 98%) of CD4+ T cells reside, as well as the individual T-cell subsets at these sites. New data from Thorston Mempel’s laboratory (89) has shown how infected T cells can migrate quickly in LN, forming complex tethering interactions with multiple uninfected cells, enabling viral dissemination while remaining sequestered in lymph nodes. It will be of great interest to study how ART affects these morphological and mobility alterations, both in the short and in the long-term, to get a better understanding of the dynamic tissue T-cell changes and trafficking after ART initiation.
Imbalances in T-cell subset restoration may also hinder a successful immune system recovery post-ART. Th17 cells, critical in mounting an inflammatory defense against bacterial and fungal microbes at mucosal surfaces, are preferentially lost from GALT in HIV infection but not from PB (90). Treg cells, the circulating subset of CD4+ T cells with suppressive activity implicated in immune tolerance, are relatively increased in frequency in PB, but overall their numbers are depleted, and it remains unclear whether this reflects redistribution between PB and LT (91, 92), preferential infection by HIV (93), or the inherent higher rates of turnover and apoptosis in this subset (94). One explanation is that plasmacytoid dendritic cells (pDCs) that have been exposed to and stimulated by HIV secrete high levels of IFNa that appear to promote the differentiation of naive T cells preferentially into Treg cells (95), suppressing T-cell effector functions and perhaps leading to the preferential loss of Th17 cells in HIV and pathogenic SIV infection (90, 96). T-follicular helper (Tfh) cells, which nearly exclusively reside in LT and specialize in providing B-cell help for antibody production, appear to be significantly expanded (approximately tenfold) in the LN of HIV-infected individuals, perhaps driving the hypergammaglobulinemia, increased IL-6, and generalized immune activation that has been identified in untreated HIV infection (97, 98). Finally, it is unclear how depletion and subsequent recovery with ART of T-memory stem cells, a recently described T-cell subset with robust proliferative and functional potential (99), may be affecting T-cell restoration in HIV.
The role of DCs and monocytes in immune reconstitution after ART
Studies of DC subsets in non-human primates and SIV models have supported the hypothesis that DC dysfunction, particularly increased IFNa production by pDCs, contributes to HIV pathogenesis and chronic immune activation (100). In nonpathogenic SIV infection models (sooty mangabeys), there is an initial type I IFN response spike during acute infection that is attenuated during chronic infection (101, 102). In contrast, in pathogenic models of SIV (rhesus macaques), there is an analogous IFN-induced response during acute infection that persists throughout the chronic phase of infection (103, 104). The effects of HIV infection on DC function appear to be only partially ameliorated with ART. After initiation of ART, pDC function remains impaired, with some evidence of enhanced type I IFN production in response to HIV and partial maturation to a phenotype of inflammatory cytokine production that can support effective T-cell responses (105). Chronic innate immune activation appears to be predictive of HIV-associated non-AIDS events, such as cardiovascular disease and stroke. The identification of elevated IL-6, secreted by activated monocytes and macrophages, and D-dimer, a marker of coagulation and fibrinolysis, as independent predictors of mortality in treated patients, has increased the research focus on the interaction between monocytes and the coagulopathy in chronic treated infection. Further studies have shown that cardiovascular disease risk is strongly associated with increased activation of monocytes (106, 107) and plasma levels of sCD14 (108, 109) as well as increased proportions of monocytes and platelets expressing cell-surface tissue factor (TF). Data from Suzanne Crowe’s laboratory (110) have shown that the phenotypic and functional profiles of monocytes of HIV-infected patients, both treated and untreated, more closely resemble those of elderly uninfected controls (approximately 20 years older) than age-matched uninfected controls, with impaired phagocytic function and shortened telomeres. In studies by our group, D-dimer and soluble TF (sTF) but not markers of T-cell activation were associated with development of cardiovascular disease and venous thromboembolic disease in long-term treated HIV infection (65, 111) and with biomarkers of coagulation in elite controllers (unpublished data). In agreement with these earlier observations, a higher proportion of patrolling, pro-inflammatory (CD16+) monocytes was found to be an independent predictor of coronary calcium score progression in a large study of predominantly treated HIV+ patients (106). Finally, higher levels of biomarkers linked to treated HIV morbidity and mortality (IL-6, D-dimer, sCD14, and CRP) were associated with monocyte activation but not with T-cell activation in a large study of mostly treated HIV+ patients (107). Thus, factors associated with innate immune activation and inflammation appear to trigger the coagulation cascade in chronic HIV infection and contribute to vascular complications that lead to chronic end organ damage.
Early ART is the most effective strategy for successful immune reconstitution
The mechanism underlying both early and chronic immune activation in HIV infection that we have proposed, that is the uncoupling of the innate and adaptive immune responses due to severe immunosuppression and their unbalanced recovery, has important implications for the management of HIV infection and areas of future investigation and interventions. Increasingly, there is recognition that earlier initiation of ART may offer the best opportunity for CD4+ T-cell recovery and immune system preservation. Early and effective ART clearly is associated with less overall CD4+ T-cell depletion, a lower rate of AIDS events or other infections, and a higher capacity for reconstitution of the immune system, with a reduced risk of developing the acute complication of IRIS, more common when initiating ART with low CD4+ T-cell counts and high antigen burdens.
The prospect for averting the long-term effects of chronic immune activation on ART, especially if started very early during infection, are increasingly appreciated. A trial of increased intensity ART (a regimen of tenofovir, emtricitabine, efavirenz, raltegravir, and maraviroc) started in early acute HIV infection (Fiebig stages I–III) found that this intensive early therapy was able to limit reservoir size at 24 weeks and partially restore gut mucosal T-cell counts (112). Another study showed that the initiation of ART in early HIV infection was associated with no measurable viral evolution in the GALT, suggesting that early therapy may effectively inhibit viral replication in this compartment (113). Data presented at the International AIDS Society conference (IAS 2012, Washington D.C) described 12 patients from the Virological and Immunological Studies in CONtrollers after Treatment Interruption (VISCONTI) cohort, who, after beginning therapy during acute HIV infection, went on to spontaneously control viral replication once treatment was interrupted (114). In-depth analysis of the viral reservoirs of these ‘induced controller’ patients revealed a durable viral reservoir with similar magnitude and distribution compared to Elite Controllers in short-lived memory CD4+ T cells. Finally, a recent cohort study found that within the first 4 months of HIV infection, there is a narrow ‘restorative time window’ in which the immune system equilibrates after initial CD4+ T-cell losses and thus is poised for recovery, and they observed that starting ART during this period augmented both the rate and extent of CD4+ T-cell reconstitution (115).
The emerging understanding of the interplay between homeostatic dysregulation and immune activation has led to studying interventions that attempt T-cell expansion and have shown mixed results. IL-2, an important homeostatic cytokine, led to substantial CD4+ T-cell expansions in peripheral blood (116, 117), but in randomized clinical trials, combining IL-2 therapy with ART showed no clinical benefit in terms of preventing AIDS- defining illnesses (118). An alternative candidate common chain cytokine, IL-7, which prevents apoptosis of T cells and is required for naive T-cell survival in vivo, has been shown to be well-tolerated (119) and to increase CD4+ T-cell counts in treated HIV patients (120). These T cells remain capable of responding to antigenic stimuli and producing cytokines after polyclonal and specific antigenic stimulation, and the repopulation appears to extend from thymus to PB, LT, and effector sites (121, 122). Further investigation of the effects of IL-7 is ongoing.
Treatment intensification with additional anti-retroviral drugs, to improve CD4+ T-cell counts or modulate immune responses, has proven disappointing. Maraviroc (MVC), a CCR5 blocker currently approved for treatment of HIV, also prevents the interaction of CCR5 with its primary ligand CCL5 (RANTES) and prevents receptor binding by CCL3 and CCL4 (known as monocyte inflammatory proteins 1 α and β, MIP-1 1 α and β), members of the β chemokine family that when bound to the CCR5 receptor can trigger T-cell activation and proliferation (123). MVC and another new agent under investigation in preliminary trials, Cenicriviroc (Tobira Therapeutics, San Francisco, CA), additionally antagonize CCL2, also known as monocyte chemotactic protein 1 (MCP-1), and monocytes treated in vitro with MVC showed a dose-dependent reduction in chemotaxis to MIP-1 β and MCP-1 (124). Initial studies have given inconclusive results: intensification with MVC did not increase CD4+ T-cell counts of HIV patients with poor immunologic response, but markers of T-cell activation, cell cycling, and apoptosis decreased during MVC therapy (125, 126). Studies into the short and long term anti-inflammatory effects of MVC, including in immunologic nonresponders, prevention of IRIS, and attenuation of liver fibrosis in HIV-hepatitis B or C co-infection, are ongoing. Intensification with the integrase inhibitor raltegravir has shown transient decreases in T-cell activation (70) and alterations in RANTES and MIP-1 β expression (127), but, again, without any associated improvements in CD4+ T-cell counts or other long term outcomes.
Other targeted interventions have been proposed and are in various stages of development. In an attempt to target the lymphoid fibrosis associated with chronic treated HIV infection, clinical trials are underway to evaluate medications that directly inhibit fibrosis, including angiotensin receptor blockers and angiotensin-converting enzyme inhibitors (128). Some experts have hypothesized that inhibitors of the TGFβ pathway (47) and medications that might help restore the fibroblastic reticular cell network of LT by activating the lymphotoxin B signaling receptor pathway (129) would be worthy of further investigation. Guided by the association of sTF and D-dimer with cardiovascular events and thromboembolic disease in HIV as well as the hypothesis that neurocognitive disease could be a microvascular complication of chronic HIV-associated immune activation, targeting the coagulation cascade with anti-coagulants has been considered. Interventions to counteract the products of MT, including sevelamer, as a binder of endotoxin, and rifaximin, to reduce the overall bacterial burden within the intestine, are also under investigation.
Other immunomodulating drugs, particularly those with anti-inflammatory activity including methotrexate, shown to reduce cardiovascular events in patients with rheumatoid arthritis (130), and statins (131) continue to be investigated, and as we have shown, studies should predominantly focus on targets related to innate immune activation, as these have been more directly linked to clinical events and mortality in ART-treated persons. One possible shortcoming of many immunomodulatory strategy trials has been a persistent use of the proportion of activated T cells as the primary end point. In our opinion, soluble biomarkers are more tightly linked to morbidity and mortality in treated HIV infection and should be further evaluated as surrogate markers. In addition, innate system activation, particularly monocyte activation, appears more proximal to the injury (specifically the non-infectious long term complications of chronic treated HIV) and thus may represent a better therapeutic target or measurable outcome (Fig. 1B).
Summary and concluding remarks
Although inflammation during IRIS and chronic treated HIV infection represent two disparate conditions, they highlight the spectrum of immune reconstitution in treated HIV infection and the complex interplay between innate and adaptive immune responses in the reconstituting immune system (Fig. 1). The best current approach to successfully restore the immune system is early ART, which can prevent AIDS associated events and limit the imbalances and dysfunction of leukocyte subsets, while preserving the structural integrity of lymphoid tissues. With increasingly widespread access to early ART and increased life expectancy in those treated, the population of HIV-infected patients receiving chronic therapy will expand. Future research should take advantage of innovative techniques and must move beyond the static study of T-cell subsets in peripheral blood or isolated tissues. A better understanding of how the entire immune system continues to recover and respond to ART will be necessary to address the challenges of an aging HIV+ treated population.
Acknowledgments
This work was supported by the Intramural Research Program of NIAID/NIH.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Autran B, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa F, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26:335–343. doi: 10.1097/QAD.0b013e32834dcec9. [DOI] [PubMed] [Google Scholar]
- 4.Robbins GK, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48:350–361. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valdez H, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–1866. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 6.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 7.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 8.Guaraldi G, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 9.Barber DL, Andrade BB, Sereti I, Sher A. Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nat Rev Microbiol. 2012;10:150–156. doi: 10.1038/nrmicro2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French MA. Immune reconstitution inflammatory syndrome: immune restoration disease 20 years on. Med J Australia. 2012;196:318–321. doi: 10.5694/mja12.10089. [DOI] [PubMed] [Google Scholar]
- 11.Shelburne SA, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 12.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418–427. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 13.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naidoo K, et al. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Ann Int Med. 2012;157:313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marais S, Wilkinson RJ, Pepper DJ, Meintjes G. Management of patients with the immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep. 2009;6:162–171. doi: 10.1007/s11904-009-0022-z. [DOI] [PubMed] [Google Scholar]
- 16.Barber DL, et al. Th1-driven immune reconstitution disease in Mycobacterium avium-infected mice. Blood. 2010;116:3485–3493. doi: 10.1182/blood-2010-05-286336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bower M, et al. Immune reconstitution inflammatory syndrome associated with Kaposi’s sarcoma. J Clin Oncol. 2005;23:5224–5228. doi: 10.1200/JCO.2005.14.597. [DOI] [PubMed] [Google Scholar]
- 18.Calabrese LH, Kirchner E, Shrestha R. Rheumatic complications of human immunodeficiency virus infection in the era of highly active antiretroviral therapy: emergence of a new syndrome of immune reconstitution and changing patterns of disease. Semin Arthritis Rheum. 2005;35:166–174. doi: 10.1016/j.semarthrit.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 20.Honig M, Schwarz K. Omenn syndrome: a lack of tolerance on the background of deficient lymphocyte development and maturation. Curr Opin Rheumatol. 2006;18:383–388. doi: 10.1097/01.bor.0000231907.50290.6f. [DOI] [PubMed] [Google Scholar]
- 21.Antonelli LR, et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood. 2010;116:3818–3827. doi: 10.1182/blood-2010-05-285080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahnke YD, et al. Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood. 2012;119:3105–3112. doi: 10.1182/blood-2011-09-380840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chahroudi A, Silvestri G. IRIS: the unfortunate rainbow of HIV. Blood. 2012;119:2971–2972. doi: 10.1182/blood-2012-01-403683. [DOI] [PubMed] [Google Scholar]
- 24.Sereti I, Rodger AJ, French MA. Biomarkers in immune reconstitution inflammatory syndrome: signals from pathogenesis. Current opinion in HIV and AIDS. 2010;5:504–510. doi: 10.1097/COH.0b013e32833ed774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonham S, Meya DB, Bohjanen PR, Boulware DR. Biomarkers of HIV Immune Reconstitution Inflammatory Syndrome. Biomark Med. 2008;2:349–361. doi: 10.2217/17520363.2.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant PM, et al. Elevated interleukin 8 and T-helper 1 and T-helper 17 cytokine levels prior to antiretroviral therapy in participants who developed immune reconstitution inflammatory syndrome during ACTG A5164. J Infect Dis. 2012;206:1715–1723. doi: 10.1093/infdis/jis604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tadokera R, et al. The role of interleukin-10 family of cytokines in HIV-tuberculosis associated immune reconstitution inflammatory syndrome. J Infect Dis. 2013 doi: 10.1093/infdis/jit002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyirenda MH, et al. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187:2278–2290. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- 30.Franceschini D, et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behrens F, et al. Imbalance in distribution of functional autologous regulatory T cells in rheumatoid arthritis. Ann Rheum Dis. 2007;66:1151–1156. doi: 10.1136/ard.2006.068320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boulware DR, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pean P, et al. Natural killer cell degranulation capacity predicts early onset of the immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients with tuberculosis. Blood. 2012;119:3315–3320. doi: 10.1182/blood-2011-09-377523. [DOI] [PubMed] [Google Scholar]
- 34.Frleta D, et al. HIV-1 infection-induced apoptotic microparticles inhibit human DCs via CD44. J Clin Invest. 2012;122:4685–4697. doi: 10.1172/JCI64439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorch JM, et al. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature. 2011;480:376–378. doi: 10.1038/nature10590. [DOI] [PubMed] [Google Scholar]
- 36.Meteyer CU, Barber D, Mandl JN. Pathology in euthermic bats with white nose syndrome suggests a natural manifestation of immune reconstitution inflammatory syndrome. Virulence. 2012;3:583–588. doi: 10.4161/viru.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufmann GR, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Int Med. 2003;163:2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 38.Lapadula G, et al. Risk of clinical progression among patients with immunological non response despite virological suppression after combination antiretroviral treatment. AIDS. 2013;27 doi: 10.1097/QAD.0b013e32835cb747. in press. [DOI] [PubMed] [Google Scholar]
- 39.Tan R, et al. Clinical outcome of HIV-infected antiretroviral-naive patients with discordant immunologic and virologic responses to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:553–558. doi: 10.1097/QAI.0b013e31816856c5. [DOI] [PubMed] [Google Scholar]
- 40.Kaufmann GR, Bloch M, Finlayson R, Zaunders J, Smith D, Cooper DA. The extent of HIV-1-related immunodeficiency and age predict the long-term CD4 T lymphocyte response to potent antiretroviral therapy. AIDS. 2002;16:359–367. doi: 10.1097/00002030-200202150-00007. [DOI] [PubMed] [Google Scholar]
- 41.Kaufmann GR, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41:361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 42.Greub G, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 43.Almeida M, Cordero M, Almeida J, Orfao A. Abnormal cytokine production by circulating monocytes and dendritic cells of myeloid origin in ART-treated HIV-1+ patients relates to CD4+ T-cell recovery and HCV co-infection. Curr HIV Res. 2007;5:325–336. doi: 10.2174/157016207780636524. [DOI] [PubMed] [Google Scholar]
- 44.Guihot A, Bourgarit A, Carcelain G, Autran B. Immune reconstitution after a decade of combined antiretroviral therapies for human immunodeficiency virus. Trends Immunol. 2011;32:131–137. doi: 10.1016/j.it.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Hu H, et al. Distinct gene expression profiles associated with the susceptibility of pathogen-specific CD4 T cells to HIV-1 infection. Blood. 2013;121:1136–1144. doi: 10.1182/blood-2012-07-446278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schacker TW, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–1139. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33:306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Pakker NG, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 49.Yin L, Kou ZC, Rodriguez C, Hou W, Goodenow MM, Sleasman JW. Antiretroviral therapy restores diversity in the T-cell receptor Vbeta repertoire of CD4 T-cell subpopulations among human immunodeficiency virus type 1-infected children and adolescents. Clin Vaccine Immunol. 2009;16:1293–1301. doi: 10.1128/CVI.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciccone EJ, et al. CD4+ T cells, including Th17 and cycling subsets, are intact in the gut mucosa of HIV-1-infected long-term nonprogressors. J Virol. 2011;85:5880–5888. doi: 10.1128/JVI.02643-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciccone EJ, et al. Cycling of gut mucosal CD4+ T cells decreases after prolonged anti-retroviral therapy and is associated with plasma LPS levels. Mucosal Immunol. 2010;3:172–181. doi: 10.1038/mi.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camargo JF, et al. Responsiveness of T cells to interleukin-7 is associated with higher CD4+ T cell counts in HIV-1-positive individuals with highly active antiretroviral therapy-induced viral load suppression. J Infect Dis. 2009;199:1872–1882. doi: 10.1086/598858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Catalfamo M, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci USA. 2008;105:19851–19856. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hodge JN, et al. Decreases in IL-7 levels during antiretroviral treatment of HIV infection suggest a primary mechanism of receptor-mediated clearance. Blood. 2011;118:3244–3253. doi: 10.1182/blood-2010-12-323600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng M, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabb B, et al. Reduced inflammation and lymphoid tissue immunopathology in rhesus macaques receiving anti-tumor necrosis factor treatment during primary simian immunodeficiency virus infection. J Infect Dis. 2013;207:880–892. doi: 10.1093/infdis/jis643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estes J, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198:456–464. doi: 10.1086/590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Read SW, Sereti I. HIV infection and the gut: scarred for life? J Infect Dis. 2008;198:453–455. doi: 10.1086/590113. [DOI] [PubMed] [Google Scholar]
- 59.Anthony KB, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J Acquir Immune Defic Syndr. 2003;33:125–133. doi: 10.1097/00126334-200306010-00002. [DOI] [PubMed] [Google Scholar]
- 60.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–242. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009;200:1212–1215. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- 62.Baker JV, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56:36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuller LH, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duprez DA, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Musselwhite LW, et al. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS. 2011;25:787–795. doi: 10.1097/QAD.0b013e3283453fcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palmer S, et al. New real-time reverse transcriptase-initiated PCR assay with single- copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piwowar-Manning EM, Henderson TA, Brisbin L, Jackson JB. A modified ultrasensitive assay to detect quantified HIV-1 RNA of fewer than 50 copies per milliliter. Am J Clin Pathol. 2003;120:268–270. doi: 10.1309/TRRQ-FWM9-LE9H-427M. [DOI] [PubMed] [Google Scholar]
- 68.Gandhi RT, et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59:229–235. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chege D, et al. Effect of raltegravir intensification on HIV proviral DNA in the blood and gut mucosa of men on long-term therapy: a randomized controlled trial. AIDS. 2012;26:167–174. doi: 10.1097/QAD.0b013e32834e8955. [DOI] [PubMed] [Google Scholar]
- 70.Llibre JM, et al. Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: a randomized 48-week study. Antivir Ther. 2012;17:355–364. doi: 10.3851/IMP1917. [DOI] [PubMed] [Google Scholar]
- 71.Mens H, et al. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol. 2010;84:12971–12981. doi: 10.1128/JVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pereyra F, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26:2409–2412. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hunt PW, et al. Relationship between T cell activation and CD4+ T cell count in HIV- seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taiwo B, Barcena L, Tressler R. Understanding and controlling chronic immune activation in the HIV-infected patients suppressed on combination antiretroviral therapy. Curr HIV/AIDS Rep. 2013;10:21–32. doi: 10.1007/s11904-012-0147-3. [DOI] [PubMed] [Google Scholar]
- 75.Quesnel A, et al. Antibodies to Epstein-Barr virus and cytomegalovirus in relation to CD4 cell number in human immunodeficiency virus 1 infection. J Med Virol. 1992;36:60–64. doi: 10.1002/jmv.1890360112. [DOI] [PubMed] [Google Scholar]
- 76.Sacre K, et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS. 2012;26:805–814. doi: 10.1097/QAD.0b013e328351f780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parrinello CM, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis. 2012;205:1788–1796. doi: 10.1093/infdis/jis276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hunt PW, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagot N, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 80.Roxby AC, et al. Effects of valacyclovir on markers of disease progression in postpartum women co-infected with HIV-1 and herpes simplex virus-2. PLoS One. 2012;7:e38622. doi: 10.1371/journal.pone.0038622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 83.Mehandru S, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mavigner M, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest. 2012;122:62–69. doi: 10.1172/JCI59011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hayes TL, et al. Impact of highly active antiretroviral therapy initiation on CD4+ T- cell repopulation in duodenal and rectal mucosa. AIDS. 2013 doi: 10.1097/QAD.0b013e32835d85b4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gordon SN, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 88.Doitsh G, et al. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murooka TT, et al. HIV-infected T cells are migratory vehicles for viral dissemination. Nature. 2012;490:283–287. doi: 10.1038/nature11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klatt NR, Brenchley JM. Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS. 2010;5:135–140. doi: 10.1097/COH.0b013e3283364846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andersson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 92.Mozos A, et al. Redistribution of FOXP3-positive regulatory T cells from lymphoid tissues to peripheral blood in HIV-infected patients. J Acquir Immune Defic Syndr. 2007;46:529–537. [PubMed] [Google Scholar]
- 93.Oswald-Richter K, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2:E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xing S, et al. Increased turnover of FoxP3high regulatory T cells is associated with hyperactivation and disease progression of chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2010;54:455–462. doi: 10.1097/QAI.0b013e3181e453b9. [DOI] [PubMed] [Google Scholar]
- 95.Favre D, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Favre D, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lindqvist M, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petrovas C, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bosinger SE, Sodora DL, Silvestri G. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Current opinion in HIV and AIDS. 2011;6:411–418. doi: 10.1097/COH.0b013e3283499cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mandl JN, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 102.Harris LD, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84:7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bosinger SE, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacquelin B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O’Brien M, et al. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. J Clin Invest. 2011;121:1088–1101. doi: 10.1172/JCI44960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baker J, et al. Monocyte Activation, but Not T-cell Activation, Predicts Progression of Coronary Artery Calcium in a Contemporary HIV Cohort. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA, USA. 2013. [Google Scholar]
- 107.Singh A, et al. Plasma Levels of IL-6, D-dimer, and CRP in HIV positive Adults are Associated with Monocyte Activation. Keystone Symposia: Immune Activation in HIV Infection; Breckenridge, CO, USA. 2013. [Google Scholar]
- 108.Funderburg NT, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayne E, et al. Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression. J Acquir Immune Defic Syndr. 2012;59:340–346. doi: 10.1097/QAI.0b013e3182439355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hearps AC, et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 2012;26:843–853. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- 111.Ford ES, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ananworanich J, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One. 2012;7:e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Evering TH, et al. Absence of HIV-1 evolution in the gut-associated lymphoid tissue from patients on combination antiviral therapy initiated during primary infection. PLoS Pathog. 2012;8:e1002506. doi: 10.1371/journal.ppat.1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bacchus C, et al. Distribution of the HIV reservoir in patients spontaneously controlling HIV infection after treatment interruption. 19th International AIDS Conference; Washington, D.C. 2012. [Google Scholar]
- 115.Le T, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kovacs JA, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996;335:1350–1356. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 117.Davey RT, Jr, et al. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: A randomized controlled trial. JAMA. 2000;284:183–189. doi: 10.1001/jama.284.2.183. [DOI] [PubMed] [Google Scholar]
- 118.Abrams D, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levy Y, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sereti I, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–6314. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cimbro R, et al. IL-7 induces expression and activation of integrin alpha4beta7 promoting naive T-cell homing to the intestinal mucosa. Blood. 2012;120:2610–2619. doi: 10.1182/blood-2012-06-434779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Levy Y, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012;55:291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Camargo JF, et al. CCR5 expression levels influence NFAT translocation, IL-2 production, and subsequent signaling events during T lymphocyte activation. J Immunol. 2009;182:171–182. doi: 10.4049/jimmunol.182.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rossi R, et al. In vitro effect of anti-human immunodeficiency virus CCR5 antagonist maraviroc on chemotactic activity of monocytes, macrophages and dendritic cells. Clin Exp Immunol. 2011;166:184–190. doi: 10.1111/j.1365-2249.2011.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilkin TJ, et al. A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4(+) T-cell recovery despite sustained virologic suppression: ACTG A5256. J Infect Dis. 2012;206:534–542. doi: 10.1093/infdis/jis376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gutierrez C, et al. Intensification of antiretroviral therapy with a CCR5 antagonist in patients with chronic HIV-1 infection: effect on T cells latently infected. PLoS One. 2011;6:e27864. doi: 10.1371/journal.pone.0027864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lichtenstein KA, et al. A pilot study to assess inflammatory biomarker changes when raltegravir is added to a virologically suppressive HAART regimen in HIV-1-infected patients with limited immunological responses. Antivir Ther. 2012;17:1301–1309. doi: 10.3851/IMP2350. [DOI] [PubMed] [Google Scholar]
- 128.Deeks SG. HIV infection, lymphoid fibrosis, and disease. Blood. 2012;120:1753–1754. doi: 10.1182/blood-2012-06-433987. [DOI] [PubMed] [Google Scholar]
- 129.Zeng M, et al. Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution. Blood. 2012;120:1856–1867. doi: 10.1182/blood-2012-03-418624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 131.Moore RD, Bartlett JG, Gallant JE. Association between use of HMG CoA reductase inhibitors and mortality in HIV-infected patients. PLoS One. 2011;6:e21843. doi: 10.1371/journal.pone.0021843. [DOI] [PMC free article] [PubMed] [Google Scholar]