Abstract
Zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) comprise a powerful class of tools that are redefining the boundaries of biological research. These chimeric nucleases are composed of programmable, sequence-specific DNA-binding modules linked to a non-specific DNA cleavage domain. ZFNs and TALENs enable a broad range of genetic modifications by inducing DNA double-strand breaks that stimulate error-prone non-homologous end joining or homology-directed repair at specific genomic locations. Here, we review achievements made possible by site-specific nuclease technologies and discuss applications of these reagents for genetic analysis and manipulation. In addition, we highlight the therapeutic potential of ZFNs and TALENs and discuss future prospects for the field, including the emergence of CRISPR/Cas-based RNA-guided DNA endonucleases.
Keywords: zinc-finger, TALE, CRISPR, nuclease, genome engineering
Classical and contemporary approaches for establishing gene function
With the development of new and affordable methods for whole-genome sequencing, and the design and implementation of large-scale genome annotation projects, scientists’ are poised to deliver upon the promises of the Genomic Revolution to transform basic science and personalized medicine. The resulting wealth of information presents researchers with a new primary challenge of converting this enormous amount of data into functionally and clinically relevant knowledge. Central to this problem is the need for efficient and reliable methods that enable investigators to determine how genotype influences phenotype. Targeted gene inactivation via homologous recombination is a powerful method capable of providing conclusive information for evaluating gene function [1]. However, the use of this technique has been hampered by several factors, including the low efficiency at which engineered constructs are correctly inserted into the chromosomal target site, the need for time-consuming and labor-insensitive selection/screening strategies, and the potential for adverse mutagenic effects. Targeted gene knockdown by RNA interference (RNAi) has provided researchers with a rapid, inexpensive and high-throughput alternative to homologous recombination [2]. However, knockdown by RNAi is incomplete, varies between experiments and laboratories, has unpredictable off-target effects, and provides only temporary inhibition of gene function. These restrictions impede researchers’ ability to directly link phenotype to genotype and limit the practical application of RNAi technology.
In the past decade, a new approach has emerged that enables investigators to directly manipulate virtually any gene in a diverse range of cell types and organisms. This core technology – commonly referred to as “genome editing” – is based on the use of engineered nucleases composed of sequence-specific DNA-binding domains fused to a non-specific DNA cleavage module [3, 4]. These chimeric nucleases enable efficient and precise genetic modifications by inducing targeted DNA double-strand breaks (DSBs) that stimulate the cellular DNA repair mechanisms, including error-prone non-homologous end joining (NHEJ) and homology-directed repair (HDR) [5]. The versatility of this approach is facilitated by the programmability of the DNA-binding domains that are derived from zinc-finger and transcription activator-like effector proteins. This combination of simplicity and flexibility has catapulted zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) to the forefront of genetic engineering. Here, we review recent advances in site-specific nuclease technologies and discuss applications of these reagents for targeted genome engineering and analysis in eukaryotic cells and model organisms. We also discuss the therapeutic potential of these technologies and examine future prospects, including the development and application of CRISPR/Cas-based RNA-guided DNA endonucleases.
Custom DNA-binding domains
The versatility of ZFNs and TALENs arises from the ability to customize the DNA-binding domain to recognize virtually any sequence. These DNA-binding modules can be combined with numerous effector domains to impact genomic structure and function (Box 1), including nucleases, transcriptional activators and repressors, recombinases, transposases, DNA histone methyltransferases and histone acetyltransferases. Thus, the ability to successfully execute genetic alterations depends largely on the DNA-binding specificity and affinity of designed zinc-finger and TALE proteins. Below, we highlight several of the most successful approaches for assembling these modular DNA-binding domains.
Box 1. Beyond nucleases: Recombinases, transposases, and transcription factors.
Site-specific nucleases are currently the most well-characterized, widely used and broadly applicable tool for inducing custom modifications in cells and model organisms. However, several limitations of targeted nucleases are driving the development of alternative types of programmable enzymes for genome engineering. For example, off-target effects created by site-specific nucleases can be toxic to cells, and difficult to comprehensively predict and monitor. Additionally, because targeted nucleases rely on non-homologous end joining (NHEJ) and homology-directed repair (HDR) to induce genetic alterations, this technology may be limited by the availability of the desired DNA repair mechanism in particular cell types. To address these concerns, zinc-finger proteins and TALEs have been fused to enzymatic domains, including site-specific recombinases [29, 110–112] and transposases [117], that catalyze DNA integration, excision, and inversion. Because these enzymes perform DNA cleavage and re-ligation autonomously, potentially toxic DNA double-strand breaks should not accumulate in the genome. Additionally, for applications that require targeted gene addition, recombinase and transposase activity is marked by the insertion of donor DNA into the genome, thereby enabling off-target effects to be monitored directly. Moreover, the mechanism of recombination and transposition is independent of cellular DNA repair pathways. As a result, these approaches should be functional in nearly any cell type and cell cycle stage. The efficiency of these processes can also be improved by directed evolution [118]. However, in order for recombinases and transposases to achieve the level of general utility afforded by site-specific nucleases, significant improvements in their performance and flexibility are needed. In particular, recombinase catalytic domains retain sequence specificity from the parental enzyme, and require significant re-engineering towards user-defined DNA targets [110, 112]. While transposase fusions demonstrate high-activity at their intended genomic targets, these chimeric proteins also suffer from significant off-target activity [119]. Finally, synthetic zinc-finger and TALE transcription factors offer an alternative approach for inducing targeted modifications by providing stringent control over gene expression [6, 8, 17, 27, 28, 115, 116]. Collectively, these proteins and enzymes represent an exciting suite of tools that can be customized for diverse genome engineering applications.
Cys2-His2 zinc-finger proteins
The Cys2-His2 zinc-finger domain is among the most common types of DNA-binding motifs found in eukaryotes and represents the second most frequently encoded protein domain in the human genome. An individual zinc-finger consists of approximately 30 amino acids in a conserved ββα configuration [6] (Figure 1a). Several amino acids on the surface of the α-helix typically contact three base pairs (bps) in the major groove of DNA, with varying levels of selectivity. The modular structure of zinc-finger proteins has made them an attractive framework for the design of custom DNA-binding proteins. Key to the application of zinc-finger proteins for specific DNA recognition was the development of unnatural arrays that contain more than three zinc-finger domains. This advance was facilitated by the structure-based discovery of a highly conserved linker sequence that enabled construction of synthetic zinc-finger proteins that recognized DNA sequences 9 to 18 bps in length [7]. Because 18 bps of DNA sequence can confer specificity within 68 billion bp of DNA, this method allowed for specific sequences to be targeted in the human genome for the first time[8, 9]. While initially controversial [10], this design has proven to be the optimal strategy for constructing zinc-finger proteins that recognize contiguous DNA sequences that are specific in complex genomes [6–9, 11–15].
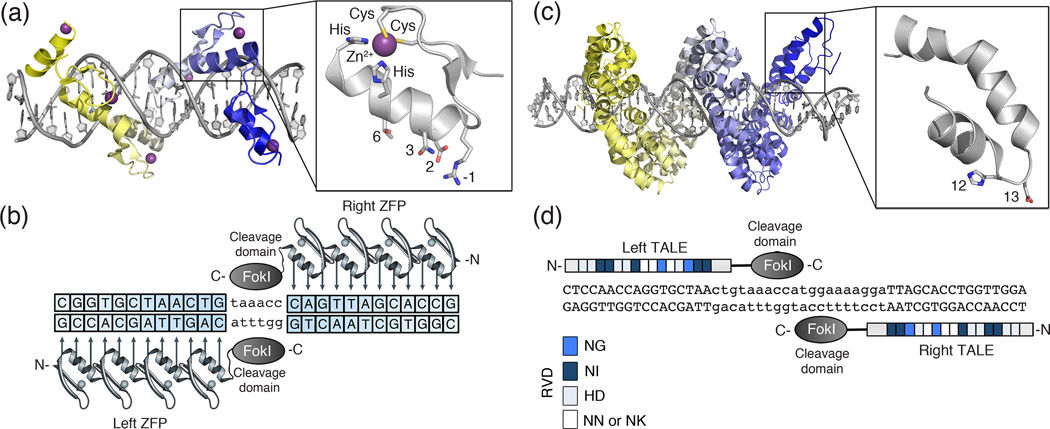
Figure 1. Structure of zinc-finger and transcription activator-like effectors.
(a) (Top) Designed zinc-finger protein in complex with target DNA (grey) (PDB ID: 2I13). Each zinc-finger consists of approximately 30 amino acids in an ββα arrangement (inset). Surface residues (−1, 2, 3 and 6) that contact DNA are shown as sticks. Each zinc-finger domain contacts 3–4 base pairs (bps) in the major groove of DNA. The side chains of the conserved Cys and His residues are depicted as sticks in complex with a Zn2+ ion (purple). (b) Cartoon of a zinc-finger nuclease (ZFN) dimer bound to DNA. ZFN target sites consist of two zinc-finger binding sites separated by a 5- to 7-bp spacer sequence recognized by the FokI cleavage domain. Zinc-finger proteins can be designed to recognize unique “left” and “right” half-sites. (c) (Top) TALE protein in complex with target DNA (grey) (PDB ID: 3UGM). Individual TALE repeats contain 33–35 amino acids that recognize a single bp via two hypervariable residues (repeat-variable diresidues; RVDs) (shown as sticks) (inset). (d) Cartoon of a TALE nuclease (TALEN) dimer bound to DNA. TALEN target sites consist of two TALE binding sites separated by a spacer sequence of varying length (12-to 20-bp). TALEs can be designed to recognize unique “left” and “right” half-sites. RVD compositions are indicated.
Following this proof-of-principle work, several methods for constructing zinc-finger proteins with unique DNA-binding specificity were developed. The “modular assembly” approach involves the use of a pre-selected library of zinc-finger modules generated by selection of large combinatorial libraries or by rational design [6, 16]. Because zinc-finger domains have been developed that recognize nearly all of the 64 possible nucleotide triplets, pre-selected zinc-finger modules can be linked together in tandem to target DNA sequences that contain a series of these DNA triplets [6, 8, 13–15, 17]. Alternatively, selection-based approaches, such as OPEN (Oligomerized Pool Engineering) can be used to select for new zinc-finger arrays from randomized libraries that take into consideration context-dependent interactions between neighboring fingers [18]. Approaches have also been developed that combine the methods described above, utilizing zinc-finger modules pre-selected for context-dependency to assemble longer arrays by modular assembly [19, 20]. For many years, zinc-finger protein technology was the only approach available to create custom site-specific DNA-binding proteins and enzymes. Engineered zinc-fingers are also available commercially; Sangamo Biosciences (Richmond, CA) has developed a propriety platform (CompoZr) for zinc-finger construction in partnership with Sigma-Aldrich (St. Louis, MO), allowing investigators to bypass zinc-finger construction and validation altogether and many thousands of proteins are already available. Broadly, zinc-finger protein technology enables targeting of virtually any sequence.
Transcription activator-like effectors
The recent discovery of a simple modular DNA recognition code by transcription activator-like effector (TALE) proteins [21, 22] has led to the explosive expansion of an alternative platform for engineering programmable DNA-binding proteins. TALEs are naturally occurring proteins from the plant pathogenic bacteria genus Xanthomonas, and contain DNA-binding domains composed of a series of 33–35 amino acid repeat domains that each recognizes a single bp (Figure 1b). TALE specificity is determined by two hypervariable amino acids that are known as the repeat-variable diresidues (RVDs) [23, 24]. Like zinc-fingers, modular TALE repeats are linked together to recognize contiguous DNA sequences. However in contrast to zinc finger proteins, there was no re-engineering of the linkage between repeats necessary to construct long arrays of TALEs with the ability to theoretically address single sites in the genome. Following nearly two decades of pioneering work based on zinc-finger proteins, numerous effector domains have been made available to fuse to TALE repeats for targeted genetic modifications, including nucleases [25–27], transcriptional activators [27, 28] and site-specific recombinases [29]. While the single base recognition of TALE-DNA binding repeats affords greater design flexibility than triplet-confined zinc-finger proteins, the cloning of repeat TALE arrays presents an elevated technical challenge due to extensive identical repeat sequences. To overcome this issue, several methods have been developed that enable rapid assembly of custom TALE arrays. These strategies include “Golden Gate” molecular cloning [30], high-throughput solid-phase assembly [31, 32] and ligation-independent cloning techniques [33]. Several large-scale, systematic studies utilizing various assembly methods have indicated that TALE repeats can be combined to recognize virtually any user-defined sequence [31, 33]. The only targeting limitation for TALE arrays for which there is consensus in the literature is that TALE binding site must (should) start with a T base. Indeed, the ease with which TALE repeats can be assembled is evident in a recent study reporting the construction of a library of TALENs targeting 18,740 human protein-coding genes [34], a technological feat that will not only facilitate numerous new studies, but will also encourage other, equally ambitious endeavors. Custom-designed TALE arrays are also commercially available through Cellectis Bioresearch (Paris, France), Transposagen Biopharmaceuticals (Lexington, KY) and Life Technologies (Grand Island, NY).
Genome editing with site-specific nucleases
Historically, targeted gene inactivation, replacement or addition has been achieved by homologous recombination; however, the low efficiency of homologous recombination in mammalian cells and model organisms dramatically limits the utility of this approach. Following the discovery that induction of a DSB increases the frequency of HDR by several orders of magnitude, targeted nucleases have emerged as the method of choice for improving the efficiency of HDR-mediated genetic alterations. By co-delivering a site-specific nuclease with a donor plasmid bearing locus-specific homology arms [35], single or multiple transgenes can be efficiently integrated into an endogenous locus (Figure 2a). Linear donor sequences with <50 base pairs of homology [36], as well as single-stranded DNA oligonucleotides [37], can also be used to induce mutations, deletions or insertions at the target site. Significantly, nuclease-mediated targeted integration normalizes for positional effects that typically confound many types of genetic analysis and enables study of structure-function relationships in the complex and native chromosomal environment. In addition to their role in facilitating HDR, site-specific nucleases also allow rapid generation of cell lines and organisms with null phenotypes; NHEJ-mediated repair of a nuclease-induced DSB leads to the introduction of small insertions or deletions at the targeted site, resulting in knockout of gene function via frame-shift mutations [38] (Figure 2b). Site-specific nucleases can also induce deletions of large chromosomal segments [39, 40]. This method has been shown to support large-scale chromosomal inversions [41] and translocations [42]. Finally, by synchronizing nuclease-mediated cleavage of donor DNA with the chromosomal target, large transgenes (up to 14 kb) have been introduced into various endogenous loci via NHEJ-mediated ligation [43, 44]. Together, these approaches support the study of gene function and the modeling of disease states by altering genes to mimic both known and as yet uncharacterized genotypes. Many of these approaches have been extended to progenitor cell types, including embryonic stem (ES) cells [45] and induced pluripotent stem (iPS) cells [46, 47], encouraging their further development for modeling a broad range of genetic conditions [48, 49] (Table 1). Extension of this technology to study the role of non-coding DNA in the regulation and expression of coding genes can also be envisioned [50, 51], including the use of multiplexed approaches as a means to identify unknown regulatory sites for genes of interest [52].
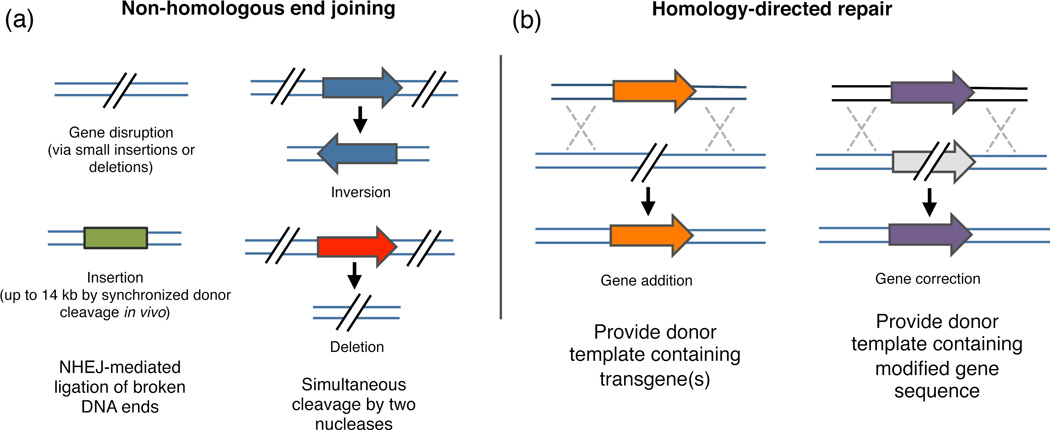
Figure 2. Overview of possible genome editing outcomes using site-specific nucleases.
Nuclease-induced DNA double-strand breaks (DSBs) can be repaired by homology-directed repair (HDR) or error-prone non-homologous end joining (NHEJ). (a) In the presence of donor plasmid with extended homology arms, HDR can lead to the introduction of single or multiple transgenes to correct or replace existing genes. (b) In the absence of donor plasmid, NHEJ-mediated repair yields small insertion or deletion mutations at the target that cause gene disruption. In the presence of double-stranded oligonucleotides or in vivo linearized donor plasmid, DNA fragments up to 14 kb have been inserted via NHEJ-mediated ligation. Simultaneous induction of two DSBs can lead to deletions, inversions and translocations of the intervening segment.
Table 1.
Abbreviated list of examples of ZFN, TALEN and CRISPR/Cas-mediated genome editing in human cells and model organisms.
| Type of modification |
Organism | Gene(s) | Nuclease(s) | Ref(s) |
|---|---|---|---|---|
| Gene disruption | Human | CCR5 | ZFN | [66, 92, 93] |
| TALEN | [26, 53] | |||
| CRISPR/Cas | [102] | |||
| Human | TCR (T-cell receptor) | ZFN | [95, 96] | |
| Zebrafish |
gol (Golden), ntl (No tail), kra |
ZFN | [67, 69] | |
| Pig |
GGTA1 (α1, 3- galactosyltransferase) |
ZFN | [78] | |
| LDLR (LDL receptor) | TALEN | [77] | ||
| Bovine | ACAN12, p65 | TALEN | [77] | |
| Human | EMX1, PVALB | CRISPR/Cas | [103] | |
| Rat | IgM, Rab38 | ZFN | [71] | |
| Arabidopsis | ADH1, TT4 | ZFN | [82] | |
| C. elegans | ben-1, rex-1, sdc-2 | ZFN/TALEN | [79] | |
| Hamster | DHFR | ZFN | [38] | |
| Drosophila | yellow | ZFN | [73] | |
| Rice | OsSWEET14 | TALEN | [85] | |
| Gene addition | Human | OCT4, PITX3 | ZFN/TALEN | [46, 47] |
| Human | CCR5 | ZFN | [98] | |
| Human |
F9 (Coagulation Factor IX) |
ZFN | [87] | |
| Mouse | Rosa26 | ZFN | [58] | |
| Human | AAVS1 | ZFN | [46, 97, 98] | |
| TALEN | [47] | |||
| CRISPR/Cas | [104] | |||
| Human | VEGF-A | ZFN | [18] | |
| Zebrafish |
th (tyrosine hydroxylase), fam46c smad5 |
TALEN | [81] | |
| Maize | IPK1 | ZFN | [83] | |
| Gene correction | Human | IL2RG | ZFN | [45, 86] |
| A1AT (α1-antitrypsin) | ZFN | [90] | ||
| HBB (β-globin) | ZFN | [88, 89] | ||
| SNCA (α-synuclein) | ZFN | [91] | ||
| Tobacco |
SuRA, SurRB (acetolactate synthase) |
ZFN | [84] | |
| Drosophila | yellow | ZFN | [72] |
Improving the performance of site-specific nucleases
In order for customizable nucleases to carry relevance for genetic analysis and clinical application, they must demonstrate strict specificity toward their intended DNA targets. Complex genomes, however, often contain multiple copies of sequences that are identical or highly homologous to the intended DNA target, leading to off-target activity and cellular toxicity. To address this problem, structure [53, 54] and selection-based [55, 56] approaches have been used to generate improved ZFN and TALEN heterodimers with optimized cleavage specificity and reduced toxicity. Our laboratory has utilized directed evolution to generate a hyperactivated variant of the FokI cleavage domain, Sharkey, that exhibits a >15-fold increase in cleavage activity in comparison to traditional ZFNs [56] and is directly compatible with various ZFN architectures [55]. Furthermore, there is mounting evidence to suggest that 4 to 6 zinc-finger domains for each ZFN half enzyme significantly enhances activity and specificity [13, 56–58]. Additional methods for improving ZFN activity include the use of transient hypothermic culture conditions to increase nuclease expression levels [59], co-delivery of site-specific nucleases with DNA end-processing enzymes [60], and the use of fluorescent surrogate reporter vectors that allow for the enrichment of ZFN and TALEN-modified cells [61]. The specificity of ZFN-mediated genome editing has been further refined by the development of zinc-finger nickases (ZFNickases) [62–64], which take advantage of the finding that induction of nicked DNA stimulates HDR [65] without activating the error-prone NHEJ repair pathway. Consequently, this approach leads to fewer off-target mutagenesis events than conventional DSB-induced methods for genome editing; however, the frequency of HDR by ZFNickases remains lower than those achieved with conventional ZFNs. Finally, conventional DNA and mRNA-based methods for delivering ZFNs into cells are restricted to certain cell types and are associated with undesirable side-effects, including insertional mutagenesis, toxicity and low efficiency (Box 2). To address these limitations, we recently developed a simple alternative based on the direct delivery of purified ZFN proteins into cells. This approach does not carry the risk of insertional mutagenesis and leads to comparatively fewer off-target effects than ZFN gene-delivery systems that rely on expression from nucleic acids [66]. This type of delivery platform thus may represent an optimal strategy for studies that require precision genome engineering in cells.
Box 2. Methods for delivering site-specific nucleases into cells.
Although site-specific nucleases provide a means for introducing diverse custom alterations at specific genomic locations, this technology is still limited by methods for delivering these enzymes into relevant cell types. Typically, nuclease-encoded genes are delivered into cells by plasmid DNA, viral vectors, or in vitro transcribed mRNA. The delivery method can be tailored to some degree toward the application or cell type of interest; however, the deficiencies of contemporary viral and non-viral gene delivery systems restrict the possible applications of site-specific nucleases. In particular, transfection of plasmid DNA or mRNA by electroporation or cationic lipid-based reagents can be toxic and restricted to certain cell types. Viral vectors also present limitations, as they are complex, difficult-to-produce, potentially immunogenic, and involve additional regulatory hurdles. Despite these difficulties, clinical trials based on adenoviral-mediated ZFN gene delivery into T lymphocytes are ongoing [92], however, future endeavors would benefit greatly from improved delivery methods.
Integrase-deficient lentiviral vectors (IDLVs) are an attractive alternative for delivering ZFNs into transfection-resistant cell types [45]; however, this method does not appear to be compatible with highly repetitive TALEN sequences [108]. Despite the apparent ease with which TALENs can be engineered, these enzymes may prove more difficult to deliver into cells than ZFNs. Adeno-associated virus (AAV) is a promising vector for ZFN delivery that has been used to enhance the efficiency of ZFN-mediated HDR [109, 120] and drive ZFN-mediated gene correction in vivo [87]. Efficient packaging of AAV occurs only for expression cassettes less than 4.2-kb in length. While this is sufficient to accommodate both ZFN monomers and an engineered donor construct, only a single TALEN monomer with a minimal promoter sequence can be inserted into this vector.
As an alternative to ZFN gene-delivery systems, our group recently reported that purified ZFN proteins are capable of crossing cell membranes and inducing endogenous gene disruption [66]. This approach has several advantages over gene-based delivery methods. First, this approach reduces off-target activity by limiting the time that cells are exposed to ZFNs and thus minimizing opportunities for off-target activity. Second, this method circumvents the cell-type dependency and toxicity of viral and non-viral gene delivery systems. Third, this approach overcomes several safety and regulatory hurdles for developing ZFN-based therapies by allowing the knockout of human genes without exposing cells to any genetic material. It remains unknown whether purified TALEN proteins can also introduced into cells in the same manner.
Site-specific nucleases in model organisms
Site-specific nucleases have enabled the introduction of targeted modifications in a number of model organisms common to biological research, including zebrafish [67–69], rats and mice [70, 71], Drosophila [72, 73], C. elegans [74], and many other species for various applications, including the monarch butterfly [75], frogs [76], and livestock [77, 78]. ZFNs and TALENs have also allowed investigators to compare gene function across related species, such as C. elegans and C. briggsae [79], shedding light on the similarities and differences between closely related organisms and making analyses between orthologous gene pairs possible. By micro-injecting single-cell embryos with TALEN mRNA and single-stranded DNA oligonucleotides [80] or donor plasmid with extended (>800 bp) homology-arms [81], TALENs have achieved targeted integration in zebrafish, enabling the generation of loxP engineered chromosomes and the possibility for conditional gene activation in this model organism. In addition to valuable animal models, both ZFNs and TALENs have been used to introduce targeted alterations in plants, including Arabidopsis [82] and several crop species [83, 84], allowing the incorporation of valuable traits, such as disease [85] and herbicide-resistance [83, 84]. The diversity of organisms modified by these site-specific nucleases will undoubtedly continue to grow, expanding the repertoire of model systems for basic research and knowledge of the intricacies and opportunities of genome biology.
Therapeutic applications of site-specific nucleases
The use of site-specific nucleases for therapeutic purposes represents a paradigm shift in gene therapy. Unlike conventional methods, which either temporarily address disease symptoms or randomly integrate therapeutic factors in the genome, ZFNs and TALENs are capable of correcting the underlying cause of the disease, therefore permanently eliminating the symptoms with precise genome modifications. To date, ZFN-induced HDR has been used to directly correct the disease-causing mutations associated with X-linked severe combined immune deficiency (SCID) [86], haemophilia B [87], sickle-cell disease [88, 89], and α1-antitrypsin deficiency [90]. Moreover, ZFNs have been used to genetically repair Parkinson's disease-associated mutations within the SNCA gene in patient-derived human iPS cells [91]. Targeted gene knockout via ZFN-induced NHEJ-mediated repair has also proven a potentially powerful strategy for combating HIV/AIDs. ZFNs have been used to confer HIV-1 resistance by disabling the HIV co-receptor C-C chemokine receptor type 5 (CCR5) in primary T cells [92] and hematopoietic stem/progenitor cells [93]. This approach is currently in clinical trials (NCT01252641, NCT00842634 and NCT01044654). More recently, ZFN-mediated targeted integration of anti- HIV restriction factors into the CCR5 locus has led to the establishment of T cells that show near-complete protection from both R5 and X4-tropic strains of HIV [94]. Additionally, ZFNs has been used to improve the performance of T cell-based immunotherapies by inactivating the expression of endogenous T cell receptor genes [95, 96], thereby enabling the generation of tumor-specific T cells with improved efficacy profiles. Finally, site-specific nucleases afford the unique possibility of safely inserting therapeutic transgenes into specific “safe harbor” locations in the human genome [97, 98]. While the overall utility of site-specific nucleases is currently limited in somatic cells, continued progress in stem cell research, including the production and manipulation of iPS cells, will ultimately open countless new directions for gene therapy, including treatments based on autologous stem cell transplantation.
Genome editing using programmable RNA-guided DNA endonucleases
Distinct from the site-specific nucleases described above, the CRISPR (Clustered Regulatory Interspaced Short Palindromic Repeats)/CRISPR-associated (Cas) system has recently emerged as a potentially facile and efficient alternative to ZFNs and TALENs for inducing targeted genetic alterations. In bacteria, the CRISPR system provides acquired immunity against invading foreign DNA via RNA-guided DNA cleavage [99]. In the Type II CRISPR/Cas system, short segments of foreign DNA, termed “spacers” are integrated within the CRISPR genomic loci and transcribed and processed into short CRISPR RNA (crRNAs). These crRNAs anneal to trans-activating crRNAs (tracrRNAs) and direct sequence-specific cleavage and silencing of pathogenic DNA by Cas proteins. Recent work has shown that target recognition by the Cas9 protein requires a “seed” sequence within the crRNA and a conserved dinucleotide-containing protospacer adjacent motif (PAM) sequence upstream of the crRNA- binding region [100]. The CRISPR/Cas system can thereby be re-targeted to cleave virtually any DNA sequence by re-designing the crRNA. Significantly, the CRISPR/Cas system has been shown to be directly portable to human cells by co-delivery of plasmids expressing the Cas9 endonuclease and the necessary crRNA components [101–104]. These programmable RNA-guided DNA endonucleases have demonstrated multiplexed gene disruption capabilities [103] and targeted integration in iPS cells [104]. Cas9 endonucleases have also been converted into nickases [103], enabling an additional level of control over the mechanism of DNA repair. In addition to human cells, CRISPR/Cas-mediated genome editing has been successfully demonstrated in zebrafish [105] and bacterial cells [106]; however, more exhaustive studies are required in order to thoroughly evaluate the utility of this system, including the potential for off-target effects. In particular, it remains unclear whether CRISPR/Cas system affords the requisite recognition selectivity necessary to ensure single-site specificity in complex genomes.
Future directions
ZFNs, TALENs and RNA-guided DNA endonucleases are transformative tools that have the potential to revolutionize biological research and impact personalized medicine. Indeed, these emerging technologies have dramatically expanded the ability to manipulate and study model organisms, and support the promise of correcting the genetic causes behind many diseases. However, in order to achieve the full potential of this technology, many important questions and challenges must be addressed (Box 3). Chief among these is the relative specificity of each nuclease platform. In the future, the use of high-throughput methods that enable comprehensive profiling of off-target cleavage sites [107] should provide insight into the stringency of target recognition inherent in each system. Questions also remain regarding the optimal methods for delivering these nucleases into cells and organisms. In particular, while adenoviral vectors can accommodate and deliver full-length TALEN genes into human cells, lentiviral plasmid vectors harboring TALEN sequences are prone to rearrangements after transduction [108]. Furthermore, the large size of TALENs may limit their delivery by size-restricted vectors such as recombinant adeno-associated virus (AAV), which have been shown to accommodate ZFN genes [109]. These findings suggest that the development of new TALEN delivery systems will be a critical area of future research. And while CRISPR/Cas systems show great promise and flexibility for genetic engineering, sequence requirements within the PAM sequence may constrain some applications. Directed evolution of the Cas9 protein should offer a path toward PAM-independence, and may also provide a means to generate an even more efficient Cas9 endonuclease. Additional studies will also be required to evaluate the specificity and toxicity of RNA-guided DNA endonucleases in vitro and in vivo. Finally, the continued development of conditional methods that rely on customizable recombinases [110–112] and transcription factors [6, 17, 113–116] for impacting genomic structure and function will complement existing and future nuclease technologies. Together, these technologies promise to expand our ability to explore and alter any genome and constitute a new and promising paradigm to understand and treat disease.
Box 3. Outstanding questions.
How effective are ZFNs and TALENs as therapeutic agents?
What are the best methods for delivering site-specific nucleases into cells, and how can TALENs be delivered into cells by lentivirus?
Can the Cas9 endonuclease be co-opted as a DNA-binding domain and be fused to enzymatic domains?
How specific and safe are CRISPR/Cas9 systems, and how does the efficiency of Cas9-mediated genome editing compare to ZFN and TALEN-based approaches?
What is the optimal RNA scaffold for application of CRISPR/Cas9 in mammalian cells?
HIGHLIGHTS.
-
➢
ZFNs, TALENs and CRISPR/Cas-based RNA-guided DNA endonucleases are programmable site-specific nucleases.
-
➢
Site-specific nucleases induce DNA double-strand breaks that stimulate non-homologous end joining and repair at targeted genomic loci.
-
➢
We discuss the therapeutic potential of site-specific nuclease technologies and discuss future prospects for the field.
ACKNOWLEDGENTS
The authors are supported by the National Institutes of Health (Pioneer Award DP1CA174426 (CB) and DP2OD008586 (CG) and National Science Foundation (CBET-1151035). T.G. was supported by National Institute of General Medicine Sciences fellowship (T32GM080209). We apologize to those investigators whose important contributions may have been omitted due to space constraints.
GLOSSARY
- ZFNs
Zinc-finger nucleases are fusions of the non-specific DNA cleavage domain from the FokI restriction endonuclease with zinc-finger proteins. ZFN dimers induce targeted DNA double-strand breaks (DSBs) that stimulate DNA damage response pathways. The binding specificity of the designed zinc-finger domain directs the ZFN to a specific genomic site.
- TALENs
Transcription activator-like effector (TALE) nucleases are fusions of the FokI cleavage domain and DNA-binding domains derived from TALE proteins. TALEs contain multiple 33–35 amino acid repeat domains that each recognizes a single base pair. Like ZFNs, TALENs induce targeted DSBs that activate DNA damage response pathways and enable custom alterations.
- CRISPR/Cas (CRISPR associated) systems
Clustered Regulatory Interspaced Short Palindromic Repeats or CRISPR are loci that contain multiple short direct repeats, and provide acquired immunity to bacteria and archaea. CRISPR systems rely on crRNA and tracrRNA for sequence-specific silencing of invading foreign DNA. Three types of CRISPR/Cas systems exist: In type II systems, Cas9 serves as an RNA-guided DNA endonuclease that cleaves DNA upon crRNA-tracrRNA target recognition.
- DSB
The product of ZFN, TALEN and CRISPR/Cas9 action, double-strand breaks are a form of DNA damage that occurs when both DNA strands are cleaved
- NHEJ
Non-homologous end joining is a DSB repair pathway that ligates or joins two broken ends together. NHEJ does not use a homologous template for repair and thus typically leads to the introduction of small insertions and deletions at the site of the break, often inducing frame-shifts that knockout gene function.
- HDR
Homology-directed repair is a template-dependent pathway for DSB repair. By supplying a homology-containing donor template along with a site-specific nuclease, HDR faithfully inserts the donor molecule at the targeted locus. This approach enables the insertion of single or multiple transgenes, as well as single nucleotide substitutions.
- RNAi
RNA interference is the process by which RNA molecules inhibit or knockdown gene expression. More broadly, RNAi is a natural mechanism that occurs in response to the introduction of many types of RNA molecules into cells.
- ZFNickases
Zinc-finger nickases are ZFNs that contain inactivating mutations in one of the two FokI cleavage domains. ZFNickases make only single-strand DNA breaks and induce HDR without activating the mutagenic NHEJ pathway.
- PAM
Proto-spacer adjacent motifs are short nucleotide motifs that occur on crRNA and are specifically recognized and required by Cas9 for DNA cleavage.
- crRNA
CRISPR RNA base pair with tracrRNA to form a two-RNA structure that guides the Cas9 endonuclease to complementary DNA sites for cleavage.
- tracrRNA
trans-activating chimeric RNA are non-coding RNA that promote crRNA processing and are required for activating RNA-guided cleavage by Cas9.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat. Rev. Genet. 2005;6:507–512. doi: 10.1038/nrg1619. [DOI] [PubMed] [Google Scholar]
- 2.McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 2002;3:737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- 3.Urnov FD, et al. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 4.Carroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011;188:773–782. doi: 10.1534/genetics.111.131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyman C, Kanaar R. DNA double-strand break repair: all's well that ends well. Annu. Rev. Genet. 2006;40:363–383. doi: 10.1146/annurev.genet.40.110405.090451. [DOI] [PubMed] [Google Scholar]
- 6.Beerli RR, Barbas CF., 3rd Engineering polydactyl zinc-finger transcription factors. Nat. Biotechnol. 2002;20:135–141. doi: 10.1038/nbt0202-135. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, et al. Design of polydactyl zinc-finger proteins for unique addressing within complex genomes. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5525–5530. doi: 10.1073/pnas.94.11.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beerli RR, et al. Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14628–14633. doi: 10.1073/pnas.95.25.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beerli RR, et al. Positive and negative regulation of endogenous genes by designed transcription factors. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1495–1500. doi: 10.1073/pnas.040552697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JS, Pabo CO. Getting a handhold on DNA: design of poly-zinc finger proteins with femtomolar dissociation constants. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2812–2817. doi: 10.1073/pnas.95.6.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segal DJ, et al. Structure of Aart, a designed six-finger zinc finger peptide, bound to DNA. J. Mol. Biol. 2006;363:405–421. doi: 10.1016/j.jmb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Neuteboom LW, et al. Effects of different zinc finger transcription factors on genomic targets. Biochem. Biophys. Res. Commun. 2006;339:263–270. doi: 10.1016/j.bbrc.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Bhakta MS, et al. Highly active zinc-finger nucleases by extended modular assembly. Genome Res. 2013;23:530–538. doi: 10.1101/gr.143693.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, et al. Preassembled zinc-finger arrays for rapid construction of ZFNs. Nat. Methods. 2011;8:7. doi: 10.1038/nmeth0111-7a. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez B, et al. Modular system for the construction of zinc-finger libraries and proteins. Nat. Protoc. 2010;5:791–810. doi: 10.1038/nprot.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segal DJ, et al. Toward controlling gene expression at will: selection and design of zinc finger domains recognizing each of the 5'-GNN-3' DNA target sequences. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2758–2763. doi: 10.1073/pnas.96.6.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beerli RR, et al. Positive and negative regulation of endogenous genes by designed transcription factors. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1495–1500. doi: 10.1073/pnas.040552697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeder ML, et al. Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol. Cell. 2008;31:294–301. doi: 10.1016/j.molcel.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sander JD, et al. Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA) Nat. Methods. 2011;8:67–69. doi: 10.1038/nmeth.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A, et al. An optimized two-finger archive for ZFN-mediated gene targeting. Nat. Methods. 2012;9:588–590. doi: 10.1038/nmeth.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boch J, et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 22.Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 23.Mak AN, et al. The crystal structure of TAL effector PthXo1 bound to its DNA target. Science. 2012;335:716–719. doi: 10.1126/science.1216211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng D, et al. Structural basis for sequence-specific recognition of DNA by TAL effectors. Science. 2012;335:720–723. doi: 10.1126/science.1215670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christian M, et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186:757–761. doi: 10.1534/genetics.110.120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mussolino C, et al. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 2011;39:9283–9293. doi: 10.1093/nar/gkr597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller JC, et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 2011;29:143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 28.Zhang F, et al. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat. Biotechnol. 2011;29:149–153. doi: 10.1038/nbt.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercer AC, et al. Chimeric TALE recombinases with programmable DNA sequence specificity. Nucleic Acids Res. 2012;40:11163–11172. doi: 10.1093/nar/gks875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cermak T, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyon D, et al. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 2012;30:460–465. doi: 10.1038/nbt.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briggs AW, et al. Iterative capped assembly: rapid and scalable synthesis of repeat-module DNA such as TAL effectors from individual monomers. Nucleic Acids Res. 2012;40:e117. doi: 10.1093/nar/gks624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid-Burgk JL, et al. A ligation-independent cloning technique for high-throughput assembly of transcription activator-like effector genes. Nat. Biotechnol. 2013;31:76–81. doi: 10.1038/nbt.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, et al. A library of TAL effector nucleases spanning the human genome. Nat Biotechnol. 2013;31:251–258. doi: 10.1038/nbt.2517. [DOI] [PubMed] [Google Scholar]
- 35.Moehle EA, et al. Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3055–3060. doi: 10.1073/pnas.0611478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlando SJ, et al. Zinc-finger nuclease-driven targeted integration into mammalian genomes using donors with limited chromosomal homology. Nucleic Acids Res. 2010;38:e152. doi: 10.1093/nar/gkq512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F, et al. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat. Methods. 2011;8:753–755. doi: 10.1038/nmeth.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santiago Y, et al. Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5809–5814. doi: 10.1073/pnas.0800940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HJ, et al. Targeted chromosomal deletions in human cells using zinc finger nucleases. Genome Res. 2010;20:81–89. doi: 10.1101/gr.099747.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sollu C, et al. Autonomous zinc-finger nuclease pairs for targeted chromosomal deletion. Nucleic Acids Res. 2010;38:8269–8276. doi: 10.1093/nar/gkq720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HJ, et al. Targeted chromosomal duplications and inversions in the human genome using zinc finger nucleases. Genome Res. 2012;22:539–548. doi: 10.1101/gr.129635.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunet E, et al. Chromosomal translocations induced at specified loci in human stem cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10620–10625. doi: 10.1073/pnas.0902076106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cristea S, et al. In vivo cleavage of transgene donors promotes nuclease-mediated targeted integration. Biotechnol. Bioeng. 2013;110:871–880. doi: 10.1002/bit.24733. [DOI] [PubMed] [Google Scholar]
- 44.Maresca M, et al. Obligate Ligation-Gated Recombination (ObLiGaRe): Custom-designed nuclease-mediated targeted integration through nonhomologous end joining. Genome Res. 2013;23:539–546. doi: 10.1101/gr.145441.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lombardo A, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 2007;25:1298–1306. doi: 10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- 46.Hockemeyer D, et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hockemeyer D, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat. Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Q, et al. A TALEN Genome-Editing System for Generating Human Stem Cell-Based Disease Models. Cell Stem Cell. 2013;12:238–251. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou J, et al. Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells. Cell Stem Cell. 2009;5:97–110. doi: 10.1016/j.stem.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanyal A, et al. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–113. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutschner T, et al. Noncoding RNA gene silencing through genomic integration of RNA destabilizing elements using zinc finger nucleases. Genome Res. 2011;21:1944–1954. doi: 10.1101/gr.122358.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunham I, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller JC, et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat. Biotechnol. 2007;25:778–785. doi: 10.1038/nbt1319. [DOI] [PubMed] [Google Scholar]
- 54.Szczepek M, et al. Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nat. Biotechnol. 2007;25:786–793. doi: 10.1038/nbt1317. [DOI] [PubMed] [Google Scholar]
- 55.Doyon Y, et al. Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures. Nat. Methods. 2011;8:74–79. doi: 10.1038/nmeth.1539. [DOI] [PubMed] [Google Scholar]
- 56.Guo J, et al. Directed evolution of an enhanced and highly efficient FokI cleavage domain for zinc finger nucleases. J. Mol. Biol. 2010;400:96–107. doi: 10.1016/j.jmb.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sood R, et al. Efficient Methods for Targeted Mutagenesis in Zebrafish Using Zinc-Finger Nucleases: Data from Targeting of Nine Genes Using CompoZr or CoDA ZFNs. PLoS ONE. 2013;8:e57239. doi: 10.1371/journal.pone.0057239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez-Pinera P, et al. Gene targeting to the ROSA26 locus directed by engineered zinc finger nucleases. Nucleic Acids Res. 2012;40:3741–3752. doi: 10.1093/nar/gkr1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doyon Y, et al. Transient cold shock enhances zinc-finger nuclease-mediated gene disruption. Nat. Methods. 2010;7:459–460. doi: 10.1038/nmeth.1456. [DOI] [PubMed] [Google Scholar]
- 60.Certo MT, et al. Coupling endonucleases with DNA end-processing enzymes to drive gene disruption. Nat. Methods. 2012;9:973–975. doi: 10.1038/nmeth.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim H, et al. Surrogate reporters for enrichment of cells with nuclease-induced mutations. Nat. Methods. 2011;8:941–943. doi: 10.1038/nmeth.1733. [DOI] [PubMed] [Google Scholar]
- 62.Kim E, et al. Precision genome engineering with programmable DNA-nicking enzymes. Genome Res. 2012;22:1327–1333. doi: 10.1101/gr.138792.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, et al. Targeted gene addition to a predetermined site in the human genome using a ZFN-based nicking enzyme. Genome Res. 2012;22:1316–1326. doi: 10.1101/gr.122879.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramirez CL, et al. Engineered zinc finger nickases induce homology-directed repair with reduced mutagenic effects. Nucleic Acids Res. 2012;40:5560–5568. doi: 10.1093/nar/gks179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McConnell Smith A, et al. Generation of a nicking enzyme that stimulates site-specific gene conversion from the I-AniI LAGLIDADG homing endonuclease. Proc. Natl. Acad. Sci. U S A. 2009;106:5099–5104. doi: 10.1073/pnas.0810588106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaj T, et al. Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nat. Methods. 2012;9:805–807. doi: 10.1038/nmeth.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doyon Y, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sander JD, et al. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat. Biotechnol. 2011;29:697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meng X, et al. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tesson L, et al. Knockout rats generated by embryo microinjection of TALENs. Nat. Biotechnol. 2011;29:695–696. doi: 10.1038/nbt.1940. [DOI] [PubMed] [Google Scholar]
- 71.Geurts AM, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bibikova M, et al. Enhancing gene targeting with designed zinc finger nucleases. Science. 2003;300:764. doi: 10.1126/science.1079512. [DOI] [PubMed] [Google Scholar]
- 73.Bibikova M, et al. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morton J, et al. Induction and repair of zinc-finger nuclease-targeted double-strand breaks in Caenorhabditis elegans somatic cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16370–16375. doi: 10.1073/pnas.0605633103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merlin C, et al. Efficient targeted mutagenesis in the monarch butterfly using zinc-finger nucleases. Genome Res. 2013;23:159–168. doi: 10.1101/gr.145599.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young JJ, et al. Efficient targeted gene disruption in the soma and germ line of the frog Xenopus tropicalis using engineered zinc-finger nucleases. Proc. Natl. Acad. Sci. U. S. A. 2011;108:7052–7057. doi: 10.1073/pnas.1102030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carlson DF, et al. Efficient TALEN-mediated gene knockout in livestock. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17382–17387. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hauschild J, et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12013–12017. doi: 10.1073/pnas.1106422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wood AJ, et al. Targeted genome editing across species using ZFNs and TALENs. Science. 2011;333:307. doi: 10.1126/science.1207773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bedell VM, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zu Y, et al. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat Methods. 2013;10:329–331. doi: 10.1038/nmeth.2374. [DOI] [PubMed] [Google Scholar]
- 82.Zhang F, et al. High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12028–12033. doi: 10.1073/pnas.0914991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shukla VK, et al. Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature. 2009;459:437–441. doi: 10.1038/nature07992. [DOI] [PubMed] [Google Scholar]
- 84.Townsend JA, et al. High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature. 2009;459:442–445. doi: 10.1038/nature07845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li T, et al. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012;30:390–392. doi: 10.1038/nbt.2199. [DOI] [PubMed] [Google Scholar]
- 86.Urnov FD, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 87.Li H, et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 2011;475:217–221. doi: 10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zou J, et al. Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease. Blood. 2011;118:4599–4608. doi: 10.1182/blood-2011-02-335554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sebastiano V, et al. In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells. 2011;29:1717–1726. doi: 10.1002/stem.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yusa K, et al. Targeted gene correction of alpha1-antitrypsin deficiency in induced pluripotent stem cells. Nature. 2011;478:391–394. doi: 10.1038/nature10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soldner F, et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perez EE, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 2008;26:808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holt N, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol. 2010;28:839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Voit RA, et al. Generation of an HIV Resistant T-cell Line by Targeted “Stacking” of Restriction Factors. Mol Ther. 2013;21:786–795. doi: 10.1038/mt.2012.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Torikai H, et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119:5697–5705. doi: 10.1182/blood-2012-01-405365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Provasi E, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 2012;18:807–815. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.DeKelver RC, et al. Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome Res. 2010;20:1133–1142. doi: 10.1101/gr.106773.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lombardo A, et al. Site-specific integration and tailoring of cassette design for sustainable gene transfer. Nat. Methods. 2011;8:861–869. doi: 10.1038/nmeth.1674. [DOI] [PubMed] [Google Scholar]
- 99.Wiedenheft B, et al. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- 100.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jinek M, et al. RNA-programmed genome editing in human cells. elife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cho SW, et al. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- 103.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mali P, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hwang WY, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jiang W, et al. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gabriel R, et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat. Biotechnol. 2011;29:816–823. doi: 10.1038/nbt.1948. [DOI] [PubMed] [Google Scholar]
- 108.Holkers M, et al. Differential integrity of TALE nuclease genes following adenoviral and lentiviral vector gene transfer into human cells. Nucleic Acids Res. 2013;41:e63. doi: 10.1093/nar/gks1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ellis BL, et al. Zinc-finger nuclease-mediated gene correction using single AAV vector transduction and enhancement by Food and Drug Administration-approved drugs. Gene Ther. 2013;20:35–42. doi: 10.1038/gt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gaj T, et al. A comprehensive approach to zinc-finger recombinase customization enables genomic targeting in human cells. Nucleic Acids Res. 2013;41:3937–3946. doi: 10.1093/nar/gkt071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gersbach CA, et al. Targeted plasmid integration into the human genome by an engineered zinc-finger recombinase. Nucleic Acids Res. 2011;39:7868–7878. doi: 10.1093/nar/gkr421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gaj T, et al. Structure-guided reprogramming of serine recombinase DNA sequence specificity. Proc. Natl. Acad. Sci. U. S. A. 2011;108:498–503. doi: 10.1073/pnas.1014214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Beerli RR, et al. Chemically regulated zinc finger transcription factors. J. Biol. Chem. 2000;275:32617–32627. doi: 10.1074/jbc.M005108200. [DOI] [PubMed] [Google Scholar]
- 114.Polstein LR, Gersbach CA. Light-inducible spatiotemporal control of gene activation by customizable zinc finger transcription factors. J. Am. Chem. Soc. 2012;134:16480–16483. doi: 10.1021/ja3065667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Perez-Pinera P, et al. Synergistic and tunable human gene activation by combinations of synthetic transcription factors. Nat Methods. 2013;10:239–242. doi: 10.1038/nmeth.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maeder ML, et al. Robust, synergistic regulation of human gene expression using TALE activators. Nat Methods. 2013;10:243–245. doi: 10.1038/nmeth.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yant SR, et al. Site-directed transposon integration in human cells. Nucleic Acids Res. 2007;35:e50. doi: 10.1093/nar/gkm089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gersbach CA, et al. Directed evolution of recombinase specificity by split gene reassembly. Nucleic Acids Res. 2010;38:4198–4206. doi: 10.1093/nar/gkq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Owens JB, et al. Chimeric piggyBac transposases for genomic targeting in human cells. Nucleic Acids Res. 2012;40:6978–6991. doi: 10.1093/nar/gks309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Handel EM, et al. Versatile and efficient genome editing in human cells by combining zinc-finger nucleases with adeno-associated viral vectors. Hum. Gene Ther. 2012;23:321–329. doi: 10.1089/hum.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]