Abstract
Purpose
Hypoxia and acidosis develop in in situ tumors as cellular expansion increases the diffusion distance of substrates and metabolites from blood vessels deep to the basement membrane. Prior studies of breast and cervical cancer revealed that cellular adaptation to microenvironmental hypoxia and acidosis is associated with the transition from in situ to invasive cancer. We hypothesized that decreased acidosis in intraductal tumors would alter environmental selection pressures for acid adapted phenotypes and delay or prevent evolution to invasive cancer.
Materials and Methods
A total of 37 C57BL/6 TRAMP mice were randomized to a control group or to 1 of 4 treatment groups. In the latter groups 200 mM sodium bicarbonate were added to drinking water starting between ages 4 and 10 weeks.
Results
In all 18 controls prostate cancer developed that was visible on 3-dimensional ultrasound at a mean age of 13 weeks. They died within 52 weeks (median 37). When sodium bicarbonate therapy commenced before age 6 weeks in 10 mice, all reached senescence (age 76 weeks) without radiographic evidence of prostate cancer. Histological sections of the prostates in this cohort showed hyperplasia but no cancer in 70% of mice and minimal well differentiated cancer in the remainder. When therapy commenced after age 6 weeks in 9 mice, prostate cancer development was no different from that in controls.
Conclusions
Immunohistochemical staining for carbonic anhydrase 9 in regions of ductal hyperplasia showed increased expression in controls vs the early treatment group. Regional pH perturbation in in situ tumors may be a simple, inexpensive and effective cancer prevention strategy.
Keywords: prostate, prostatic neoplasms, sodium bicarbonate, hydrogenion concentration, clonal evolution
Invasive cancer develops during a prolonged period through multiple pre-malignant states,1–4 a process often characterized as somatic evolution. Most conceptual models view evolution during carcinogenesis as a sequence of heritable changes in oncogenic pathways.4 We have proposed a complementary microenvironmental model of carcinogenesis5,6 in which nascent cancer cells overcome barriers to cellular proliferation at different stages of cancer evolution due to selection forces that result from the distinctive anatomy and physiology of epithelial surfaces.
A novel prediction using this model is that hypoxia and acidosis are strong selection forces during the later stages of in situ carcinogenesis due to increasing separation of evolving tumor cells from underlying stroma and blood vessels. Hypoxia requires a cellular switch to anaerobic glucose metabolism. The subsequent increased production of acid causes extracellular acidosis, which requires adaptation to acid mediated toxicity. Regional acidosis promotes tumor growth and invasion through toxicity of nonadapted cells, increased extracellular matrix degradation and inhibited T-cell function, decreasing the immune response.5 Computational models predicted that this evolutionary sequence would promote transition from in situ to invasive tumor growth.7–9
The presence of hypoxia in intraductal cancer is supported by frequent pathological observations of central necrotic areas, for example in breast ductal carcinoma in situ. Increased expression of the hypoxia induced glucose transporter glucose transporter 1 and CA isoform IX was observed adjacent to necrotic zones in ductal carcinoma in situ as well as up-regulation of glucose transporter 1 and sodium hydrogen exchanger, a marker of extracellular acidosis, in microinvasion regions.8 Notably adaptation to hypoxia and acidosis is critical for the transition from in situ to invasive human cervical cancer.10
We hypothesized that decreasing regional acidosis in in situ cancer would perturb the evolutionary sequence and delay or prevent cancer emergence at a critical preinvasive stage. Computer simulations supported the hypothesis, showing that changes in microenvironmental pH slowed the rate of in situ cancer evolution.11
We chose PC for the initial in vivo test of this hypothesis. PC typically evolves from intraductal premalignant PIN, in which cellular proliferation away from the basement membrane is sufficient to produce regional hypoxia and acidosis. Since PC is generally a disease of older men, a prevention strategy that achieved even a modest delay in carcinogenesis could substantially decrease the health effects of the disease.
Sodium bicarbonate added to drinking water was chosen as the systemic buffer. We previously noted that, as a physiological buffer, it does not alter the pH of blood or other normal tissue. Furthermore, using fluorescence microscopy in dorsal window chambers we previously reported that PC PC3 xenografts export acid into surrounding normal tissue12 and adding bicarbonate to the drinking water of tumor bearing mice decreased tumor associated acidity and the development of metastasis.13,14
MATERIALS AND METHODS
Animal Models
Prostate tumors uniformly develop in TRAMP mice due to expression of the oncoprotein SV40 T antigen, which is under transcriptional control of the probasin promoter. The TRAMP system is a well recognized animal model in which to examine spontaneous tumors and test tumor prevention strategies.15–18 For these experiments mice were generated using breeding pairs, that is by crossing female C57BL/6 TRAMP mice with male C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine). Breeding was done at our center according to institutional animal care and use committee regulations. In the C57BL/6 inbred strain prostate tumors are typically multinodular, develop in the lateral lobes and invade surrounding tissue. Metastasis is observed in about half of the mice before death.
Study Design and Bicarbonate Supplementation
Mice confirmed to carry the transgene by polymerase chain reaction were randomly divided into a control group and groups that received 200 mM sodium bicarbonate in drinking water. This dose was based on prior computer simulations and in vivo observations that it increases intratumor pH between 0.1 and 0.15 pH U without changing systemic pH.13,14
After weaning at age 3 weeks treated animals were randomly divided into 4 treatment groups, commencing at ages 4, 5, 7 and 10 weeks, respectively. Timing was clustered at these points since preliminary observations and the extant literature indicate that the transition from in situ to microinvasive tumor occurs at ages 5 to 8 weeks. Each animal was evaluated weekly by palpation and 3D ultrasound. Mice and water bottles were weighed weekly. MRI was done at weeks 28 and 35, and immediately before sacrifice.
After confirmation of a prostate tumor by US the mouse was observed daily for clinical evidence of tumor progression. Sacrifice was done if the mouse appeared moribund, or showed a palpable prostate tumor or tumor at another site greater than 1 cm in diameter, hunched posture, isolation from cage mates, altered gait, lethargy or inability to move, unresponsiveness to stimuli, shallow, rapid or labored breathing, inability to groom, soiled fur or anogenital area, or unusual vocalization. Prostate pH was measured in vivo in anesthetized mice immediately before sacrifice.
Histology
Necropsy examination included visual inspection for metastatic disease in the liver, lung and abdominopelvic lymph nodes. At necropsy the primary tumor (prostate) and the seminal vesicles were collected, photographed and weighed for each animal. Lungs and any tissue that appeared abnormal visually or on prior MRI were also collected.
Tissues were stored in buffered formalin until processing within 2 weeks. Tissues were processed and embedded in paraffin, and 4 to 5 μm tissue slices were obtained. Slides were stained with hematoxylin and eosin, and graded by a pathologist (MB) for the presence and percent of tumor tissue. Paraffin embedded primary tumors (prostate) were also cut in 4 to 5 μm slices. Immunohistochemistry was done using goat polyclonal primary antibodies against mouse CAIX (R & D Systems®). The primary antibody was incubated at 1.7 μg/ml for 12 hours at 4C. Tissue was then stained with the Anti-Goat HRP-DAB Cell and Tissue Staining Kit (R & D Systems).
Image Analysis
Histological slides of TRAMP mouse prostates stained with hematoxylin and eosin were scanned using the Scan-Scope® XT with a 20×/0.8 numerical aperture objective lens at 2 minutes per slide using a Tri-linear array (Basler, Exton, Pennsylvania). 1) Images were analyzed by a Genie™, version 1 customized algorithm to first segment regions of various morphologies, including benign lesions, hyperplasia, and well and poorly differentiated tumors in conjunction with the pathologist review for a quantified investigation of percent malignancy. 2) Nuclei in each malignant region were identified and counted using an optimized Nuclear v9 algorithm from IHC Toolbox (Aperio®), including averaging radius = 1, segmentation type = 2 cytoplasmic rejection, threshold type = 1-edge threshold with weighted trimming, minimum/maximum nuclear size = 20/1,000,000 μm2 and red/green/blue stain = 0.69686/0.64307/0.31756. The sum of the number of malignant cells per sample was considered to be the tumor burden in that individual histology sample. Each algorithm was applied to the entire slide digital image and quality controlled by a pathologist. Nonpaired data were analyzed for mean differences by the 2-tailed Student t test.
RESULTS
To determine the effects of increased system buffers on PC progression in TRAMP mice, groups of mice were fed tap water or water containing 200 mM sodium bicarbonate beginning at age 4, 5, 7 or 10 weeks. Water consumption was identical in the groups. Treated cohorts maintained stable weight and showed normal behavioral characteristics.
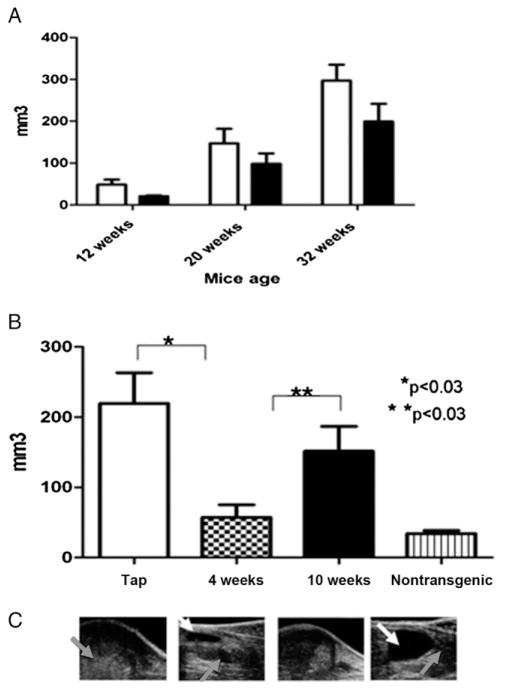
In all 18 controls prostate tumors developed that were visible on 3D US at an average age of 13 weeks. In TRAMP mice PC typically invades all prostate regions, resulting in progressive organ enlargement.15 US revealed progressive prostate enlargement after the emergence of the prostate tumor. This served as a measure of tumor progression and invasion (fig. 1, C).
Figure 1.
Effect of adding 200 mM sodium bicarbonate to drinking water on PC progression in TRAMP mice. A, mean ± SEM prostate tumor volume on 3D US at ages 12, 20 and 32 weeks. Volume in cohort treated at age 1, 4, 5 or 7 weeks was less than in controls but difference was not statistically significant (p <0.3). Open bars indicate tap water. Black bars indicate bicarbonate. B, time of therapy onset dramatically affected results, as shown by mean ± SEM prostate volume at 32 weeks in control, 4 and 10-week groups, and nontransgenic group with no cancer (p <0.05). C, representative US images show TRAMP mouse pelvic region in same groups. Gray arrow indicates prostate. White arrow indicates bladder.
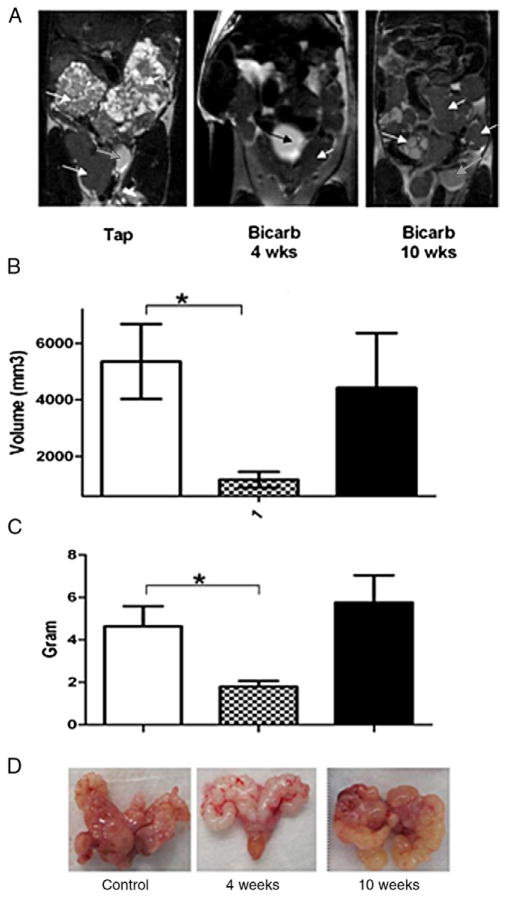
Figure 1, A shows US measured prostate volume in control and all bicarbonate treated mice regardless of when therapy was initiated. When bicarbonate was begun at age 4 weeks, US measured prostate volume was significantly less than in mice started on therapy at age 10 weeks (fig. 1, B). Although mean volume was lower in 10-week-old mice than in controls, the difference was not significant. At ages 28 and 35 weeks prostate volume was measured by MRI (fig. 2, A). As with US results, compared to controls MRI at each time point showed significantly lower prostate volume in mice that began therapy at age 4 weeks and no difference in those that started therapy at age 10 weeks. These data were confirmed by postmortem weighing of excised prostate and seminal vesicle organs (figs. 2, C and D). These data show that, when bicarbonate therapy was initiated at age 4 weeks, primary PC progression was significantly retarded, as measured by size.
Figure 2.
Further difference between early and late treatment. A, T2-weighted coronal MRI at age 35 weeks reveals control nontreated mice (Tap) and mice treated with 200 mM sodium bicarbonate (Bicarb) starting at age 4 or 10 weeks. Note bladder (white and black arrows) for reference. Control and 10-week treated groups show enlarged prostate and seminal vesicles (white arrows). Heterogeneous image shows homogenous contrast throughout seminal vesicles and prostate. B, as calculated from MRI, prostate and seminal vesicle volume at 35-week end point had much smaller volume in 4-week than in 10-week treated mice and controls. Asterisk indicates p <0.009. C, ex vivo weight demonstrated statistically significant reduction of prostate and seminal vesicle mass in 4-week treated group vs controls but 10-week treated group showed no effect. Asterisk indicates p <0.005. D, ex vivo images reveal differences in prostate and seminal vesicle color and morphology in representative control, and 4 and 10-week treated mice. Progressive prostate tumors were often associated with seminal vesicle enlargement. Dilated seminal vesicle infection was common cause of morbidity and mortality in treated and untreated mice.
All controls died by age 52 weeks (median 37). Histological sections of the prostate from these mice revealed diffuse tumor infiltration with a mixture of well and poorly differentiated cancer (figs. 3 and 4). The clinical course of treated mice depended highly on the age when therapy commenced. When the buffer was added at ages greater than 6 weeks, as in 9 mice, primary tumor growth characteristics and histology were not statistically different from those of controls. However, when sodium bicarbonate was added at ages less than 6 weeks, as in 10 mice, all animals attained senescence and were sacrificed at age 76 weeks without US evidence of prostate tumor. 3D US revealed that prostate volume in the group in which therapy began before age 6 weeks was minimally larger than but not statistically different from that in nonTRAMP C57BL/6 mice (fig. 1).
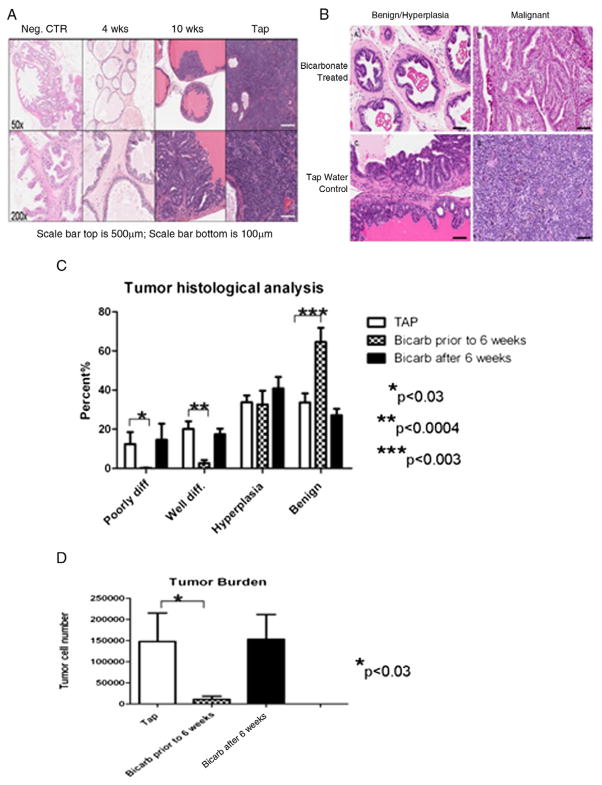
Figure 3.
Histological analysis of treated TRAMP mice. A, tap water control (CTR), early and late bicarbonate treated mice, and nontransgenic mice. Neg., negative. H&E. B, tissue from bicarbonate and tap water treated mice reveals morphology changes in same tumor classification of benign lesion/hyperplasia and malignancy. Bicarbonate did not stop malignant cell growth but cells seemed to maintain semblance of normal tissue structure compared to those of mice with tap water. Benign cells in treated mice appeared to maintain anatomical tissue structure even during hyperplasia while tap water mice showed hyperplasia, which appeared much less structured. H&E. C, tumor histological analysis reveals that mice started on bicarbonate (Bicarb) before age 6 weeks had significantly fewer poorly and well differentiated (diff) tumor cells than controls (TAP) or animals that started treatment later. D, mice that started treatment before age 6 weeks also showed fewer tumor cells than controls and mice treated after age 6.
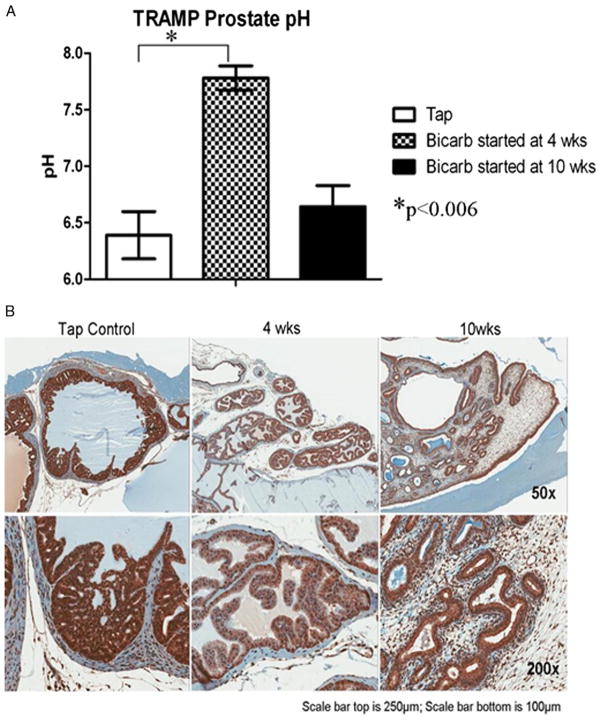
Figure 4.
Immunohistochemical and pH differences between treated and untreated animals. A, immediately before sacrifice prostate tumor showed significant increase in prostate pH in mice that started bicarbonate (Bicarb) at age 4 vs 10 weeks and vs mice on tap water during experiment. B, immunohistochemistry staining for CAIX expression in normal prostate tissue of control and treated mice reveal stronger staining in untreated mice.
After sacrifice histological sections were quantified as described to categorize individual regions as poorly or well differentiated, hyperplastic or benign (fig. 3, A to C). Categorical proportions were similar in controls and mice begun on therapy after age 6 weeks. In contrast, when therapy was begun before age 6 weeks, only a minimal amount of poorly or well differentiated cancer was observed and most areas were benign.
Quantitative histology was also used to measure the tumor burden in sections. Similar to macroscopic observations, the microscopic tumor burden, as determined by the number of pixels in the region of interest, was minimal in mice in which therapy began before age 6 weeks. This was determined by the number of malignant cells counted per sample (fig. 3, D).
Immediately before sacrifice prostate extracellular pH was measured by microelectrode. It was substantially lower in controls than in the group that began treatment at age 4 weeks (fig. 4, A). Prostate pH in the group that began treatment at age 10 weeks was not significantly different from that of controls. Stronger CAIX staining was observed in the hyperplastic regions of untreated and late treated mice compared to those in which treatment began at age 4 weeks (fig. 4, B). This suggests greater adaptation to acidosis.
DISCUSSION
We propose that while early somatic evolution of cancer is dominated by mutations in critical signaling pathways, the later stages of intraductal carcinogenesis are governed by adaptations to changes in the physical microenvironment. This is the result of tumor growth into the ductal lumen, which increases the distance from the blood vessels. Thus, they remain on the other side of the intact basement membrane. The resulting diffusion-reaction kinetics cause regional hypoxia, which promotes an evolutionary sequence, including cellular adaptation with up-regulation of glycolysis, consequent regional acidosis and then cellular adaptation to acid mediated toxicity. The resulting phenotype has a substantial proliferative advantage since it can generate an acidic environment that is toxic to competing epithelial cells. It also promotes the release of proteolytic enzymes, which facilitates invasion by degrading the extracellular matrix. This advantage promotes constitutive up-regulation of glycolysis, ie aerobic glycolysis or the Warburg effect, and facilitates breach of the basement membrane. The latter is a critical step in the transition from in situ to invasive cancer. The hypothesis is supported by prior computer simulations, in vitro experiments and clinical observations in patients with breast and cervical cancer.5–10
As an extension of this carcinogenesis model, we examined the possibility that increased systemic buffering of pH would delay or prevent the transition from in situ to invasive cancer. Results revealed that when oral sodium bicarbonate was administered to TRAMP mice at age less than 6 weeks, they survived until senescence without PC that was detectable by physical examination, US or MRI. At necropsy this cohort universally had evidence of prostatic hyperplasia and 30% had small cancer foci on histological section. In contrast, cancer developed in all controls with tumor detected by US at a mean age of 13 weeks and all died of the disease before age 52 weeks (mean 37). Mice treated with oral sodium bicarbonate at ages greater than 6 weeks had a clinical course and prostate histological prostate identical to those of controls.
The hypothesis that sodium bicarbonate acts by increasing pH in intraductal tumors is supported by greater CAIX expression in controls than in the treatment cohort. Pre-sacrifice needle measurement of the prostate showed that pH in the control and late treated groups was much lower (1 to 1.5 pH U) than in the early treated group. This may simply reflect acidosis related to extensive tumor infiltration in the former groups and the absence of large tumors in the latter.
Data clearly demonstrate that the time of therapy initiation is critical. When sodium bicarbonate therapy began after age 6 weeks, it had no effect on primary PC development. PIN develops in TRAMP mice before age 5 weeks and invasive cancer is typically observed around ages 5 to 8 weeks.15 While it was not possible to observe that actual transition in our cohort, it is reasonable to assume that this was the typical transition age in our cohort since tumors large enough (3 to 4 mm in diameter) to be visualized by US were apparent at a mean age of 13 weeks. Thus, it appears that sodium bicarbonate has a limited effect on local tumor progression after the transition from in situ to invasive cancer has occurred.
Our study has a number of limitations. Although statistical significance was achieved, the number of animals per treatment group was relatively small. Only 1 concentration (200 mM) of sodium bicarbonate was administered. Lower doses administered earlier or a higher dose administered later might have generated similar results. While many features of prostate tumors in TRAMP mice are identical to those of the human disease, some TRAMP tumors have neuroendocrine features that may limit the clinical applicability of these results.19 Furthermore, few if any clinical studies of human PC have specifically sought evidence for hypoxia and acidosis in clinical PIN lesions. Finally, the equivalent human daily sodium bicarbonate dose is about 0.4 gm/kg daily. While this dose has been administered for sickle cell disease for 1 year in a clinical trial,20 it is unlikely that it would be practicable in a cancer prevention study. However, notably our results likely represent a generalized effect of pH buffering and not a specific effect of bicarbonate. In other studies we noted that nonvolatile buffers given orally, such as imidazole or lysine, can also exert a potent antimetastatic effect.21 Thus, it is likely that a buffer more tolerable than sodium bicarbonate would be needed for long-term clinical prevention trials. Also, groups have noted antitumor effects by altering tumor pH with drugs that affect proton pumps,22,23 CAIX24,25 and Na/H exchangers.26,27
CONCLUSIONS
Results suggest that pH perturbation in in situ tumors can prevent or substantially delay the evolution of invasive PC. This principle could result in new approaches to clinical prevention studies of PC. Also, our general hypothesis that hypoxia and acidosis promote evolution of the invasive phenotype of in situ cancer should in principle apply to any epithelial malignancy. Thus, further study of perturbations of the physical microenvironment to prevent other cancer types appears warranted.
Acknowledgments
Supported by Grant 1U54 CA 143970-01.
Abbreviations and Acronyms
- 3D
3-dimensional
- CAIX
carbonic anhydrase 9
- MRI
magnetic resonance imaging
- PC
prostate cancer
- PIN
prostate intraepithelial neoplasia
- TRAMP
transgenic adenocarcinoma of mouse prostate
- US
ultrasound
References
- 1.Heng HH, Stevens JB, Bremer SW, et al. The evolutionary mechanism of cancer. J Cell Biochem. 2010;109:1072. doi: 10.1002/jcb.22497. [DOI] [PubMed] [Google Scholar]
- 2.Beerenwinkel N, Antal NT, Dingli D, et al. Genetic progression and the waiting time to cancer. PLoS Comput Biol. 2007;3:225. doi: 10.1371/journal.pcbi.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozic I, Antal T, Ohtsuki TH, et al. Accumulation of driver and passenger mutations during tumor progression. Proc Natl Acad Sci U S A. 2010;107:18545. doi: 10.1073/pnas.1010978107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 5.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature Review Cancer. 2004;4:891. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 6.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8:56. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 7.Silva AS, Gatenby RA, Gillies RJ, et al. A quantitative theoretical model for the development of malignancy in ductal carcinoma in situ. J Theor Biol. 2010;262:601. doi: 10.1016/j.jtbi.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Gatenby RA, Smallbone K, Maini PK, et al. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer. 2007;97:646. doi: 10.1038/sj.bjc.6603922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smallbone K, Gatenby RA, Gillies RJ, et al. Metabolic changes during carcinogenesis: potential impact on invasiveness. J Theor Biol. 2007;244:703. doi: 10.1016/j.jtbi.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Lee WY, Huang SC, Hsu KF, et al. Roles for hypoxia-regulated genes during cervical carcinogenesis: somatic evolution during the hypoxia-glycolysis-acidosis sequence. Gynecol Oncol. 2008;108:377. doi: 10.1016/j.ygyno.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Smallbone K, Maini P, Gatenby R. Episodic, transient systemic acidosis delays evolution of the malignant phenotype: Possible mechanism for cancer prevention by increased physical activity. Biology Direct. 2010;5:2. doi: 10.1186/1745-6150-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatenby RA, Gawlinski ET, Gmitro AF, et al. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66:5216. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 13.Silva AS, Yunes JA, Gillies RJ, et al. The potential roles of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Res. 2009;69:2677. doi: 10.1158/0008-5472.CAN-08-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robey IF, Baggett BK, Kirkpatrick ND, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S, Hastak K, Ahmad N, et al. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wechter WJ, Leipold DD, Murray ED, et al. E-7869 (R-flurbiprofen) inhibits progression of prostate cancer in the TRAMP mouse. Cancer Res. 2000;60:2203. [PubMed] [Google Scholar]
- 18.Wang J, Eltoum IE, Lamartiniere CA. Genistein chemoprevention of prostate cancer in TRAMP mice. J Carcinog. 2007;6:3. doi: 10.1186/1477-3163-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiaverotti T, Couto SS, Donjacour A, et al. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008;172:236. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann JR, Stuart TH. Sodium bicarbonate prophylaxis of sickle cell crisis. Pediatrics. 1974;53:414. [PubMed] [Google Scholar]
- 21.Ibrahim-Hashim A, Cornnell HH, Coelho-Ribeiro MD, et al. Reduction of metastasis using a non-volatile buffer. Clin Exp Metastasis. 2011;28:841. doi: 10.1007/s10585-011-9415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luciani F, Spada M, De Milito A, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- 23.Fais S, De Milito A, You H, et al. Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res. 2007;67:10627. doi: 10.1158/0008-5472.CAN-07-1805. [DOI] [PubMed] [Google Scholar]
- 24.Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71:3364. doi: 10.1158/0008-5472.CAN-10-4261. [DOI] [PubMed] [Google Scholar]
- 25.Morris JC, Chiche J, Grellier C, et al. Targeting hypoxic tumor cell viability with carbohydrate-based carbonic anhydrase IX and XII inhibitors. J Med Chem. 2011;54:6905. doi: 10.1021/jm200892s. [DOI] [PubMed] [Google Scholar]
- 26.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373. [PubMed] [Google Scholar]
- 27.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5:786. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]