Abstract
Many important questions in developmental biology increasingly interface with related questions in other biological disciplines such as evolutionary biology and ecology. In this article, we review and summarize recent progress in the development of horned beetles and beetle horns as study systems amenable to the integration of a wide range of approaches, from gene function analysis in the laboratory to population ecological and behavioral studies in the field. Specifically, we focus on three key questions at the current interface of developmental biology, evolutionary biology and ecology: (1) the developmental mechanisms underlying the origin and diversification of novel, complex traits, (2) the relationship between phenotypic diversification and the diversification of genes and transcriptomes, and (3) the role of behavior as a leader or follower in developmental evolution. For each question we discuss how work on horned beetles is contributing to our current understanding of key issues, as well as highlight challenges and opportunities for future studies.
INTRODUCTION
Many important questions in developmental biology increasingly interface with related questions in other biological disciplines such as evolutionary biology and ecology. The resulting interdisciplinary research is advanced through the use of model systems conducive to a wide range of approaches, from gene function analysis in the laboratory to population ecological studies in the field. Here, we discuss recent progress in the development of horned beetles and beetle horns as a study system able to be utilized across, as well as integrate between, diverse levels of biological organization and analysis. Specifically, we focus on three key questions at the current interface of developmental biology, evolutionary biology and ecology. For each question we discuss how work on horned beetles is contributing to our current understanding of key issues, as well as highlight challenges and opportunities for future studies. We will begin, however, with a brief overview to the biology and natural history of horned beetles and beetle horns.
A Short Biology of Horned Beetles and Beetle Horns
Species in at least seven beetle families have evolved horns or horn-like structures, with the majority of species and morphological diversity being concentrated in two subfamilies within the Scarabaeidae, the Dynastinae (rhinoceros beetles) and Scarabaeinae (true dung beetles).1 In both subfamilies, thousands of species develop horns, including many cases of extreme elaboration (Figure 1). In almost all species, horns extend predominantly from either the dorsal head or the pronotum, the dorsal portion of the first thoracic segment. As such they develop in body regions that normally do not produce appendages or any other type of outgrowth. Consequently, beetle horns lack obvious homology to other structures in insects or non-insect arthropods.2
FIGURE 1.
Examples of the exuberance and diversity of horn phenotypes across genera. From top to bottom: Phanaeus imperator, Onthophagus watanabei, Eupatorus gracilicornis, Trypoxylus (Allomyrina) dichotoma, Golofa claviger. (Reprinted after Ref 3. Copyright 2008 Armin Moczek)
Horn development varies on a variety of levels. For instance, among species horns differ dramatically in location, size, number, and shape. Much diversity also exists within species, predominantly on two levels: first, sexes are commonly highly dimorphic, with horn development being restricted to—or greatly elaborated in—males (Figure 2). Second, males are frequently dimorphic themselves, such that only large males express fully developed horns whereas smaller males develop no or only rudimentary horns (Figure 2).4 Male dimorphisms can be so extreme, and discrete, that they have resulted in male morphs being described as belonging to separate species.5 Importantly, while in many species both females and small males are essentially hornless, the genetic mechanisms underlying these differences are at least in part rather different: female ‘hornlessness’ is the result of sex-specific development following XY sex-determination (males are the heterogametic sex). All females, regardless of their body size, are hornless. The ‘hornlessness’ of small males, however, is entirely mediated by larval feeding conditions: male larvae with access to good feeding conditions exceed a certain threshold size prior to pupation and initiate horn development, whereas male larvae with access to poor conditions develop into smaller pupae and adults without initiating horn growth.
FIGURE 2.
(a) Examples of alternative male morphs in Onthophagus taurus (top) and O. nigriventris (bottom). Large males are shown on the left and small males on the right. Note that females (not shown) are entirely hornless in both species. (b) Rare reversed sexual dimorphism in O. sagittarius. Males (shown on left) also lost ancestral male dimorphism. (Reprinted with permission from Ref 17. Copyright 2012 Hindawi Publishers)
Despite the tremendous diversity in horn placement, shape, number, and size, all horns—when present—appear to be used for the same purpose: as a weapon to monopolize females against rival males. In each species studied thus far males were found to use their horns to push, block, prod, stab, lift, dislodge or otherwise impede rival males from accessing females.1 Fights are often intense, appear energetically expensive, and take time, yet are rarely injurious. Horns are effective, and males with larger horns typically enjoy an advantage in fights.6 However, not all males rely on horns and aggressive behaviors to access females. In species with pronounced male dimorphisms, smaller males lack horns, invest heavily into testes development and sperm competition and employ non-aggressive sneaking behaviors to circumvent physically superior rival males. ‘Hornlessness’ improves agility of sneaker males and is thought to represent an alternative morphological adaptation to the particular behavior niche inhabited by small males otherwise unable to successfully compete in direct male combat (reviewed in1).
In summary, the horns of beetles are extraordinarily diverse, evolutionarily novel, and ecologically important as weapons of sexual selection. As such they offer tremendous opportunity to investigate the causes, mechanisms, and consequences of phenotypic innovation and diversification. For the remainder of this manuscript we will address three major questions in evolutionary developmental biology—using horned beetles as a focal taxon—that investigate the nature of innovation and diversification from a diversity of perspectives. Specifically, we will explore (1) the developmental mechanisms underlying the origin and diversification of novel, complex traits, (2) the relationship between phenotypic diversification and the diversification of genes and transcriptomes, and lastly (3) the role of behavior as a leader or follower in developmental evolution. For each question we will discuss how work on horned beetles has extended previous understanding derived from studies in other taxa, as well as highlight challenges and opportunities for future studies on these remarkable organisms.
THEORIGINANDDIVERSIFICATIONOF COMPLEX TRAITS IN DEVELOPMENT AND EVOLUTION—INSIGHTS FROM BEETLE HORNS
Beetle horns develop in body regions in which insects normally do not produce any outgrowths, and thus cannot be easily homologized with other traits. As such beetle horns clearly embody something novel—major projections existing alongside traditional appendages, providing their bearers with a novel weapon in male combat. At the same time, on a histological level the development of horns shares many similarities with that of legs, mouthparts or antennae: all originate from epidermal tissue which detaches from the larval cuticle late in larval development, undergoes rapid cell proliferation and extensive folding prior to the larval-to-pupal molt.7 This prepupal growth phase is then followed by a pupal remodeling phase, during which horns, again like legs, mouthparts and antennae, become externally visible as the animal molts into a pupa, and subsequently remodeled and sculpted into the final adult morphology prior to the last, pupal-to-adult molt.8 This pupal remodeling phase can be extreme for some types of horns, notably those produced by the thorax, causing horned pupae of many species to molt into completely hornless adults, an observation we will return to in greater detail later in this section. A growing number of studies now suggest that the many general similarities in the development of horns and traditional appendages also extend to the developmental genetic processes known to instruct the formation of traditional appendages in insects. We therefore, begin this section with a summary of what has been learned about the regulation of horn development from candidate gene-and pathway studies informed by our understanding of appendage formation in other insects (Table 1). We end this section by summarizing our understanding of the conditions that may have led to the evolution of the very first adult horns. Throughout, we highlight the most significant open questions for future studies on beetle horn development.
TABLE 1.
Developmental Pathways Currently Implicated in the Development and Diversification of Beetle Horns. Listed are Pathways and Focal Genes Studied thus Far, Observed or Inferred Functions during Horn Development, Whether Functions Appeared to Exhibit Distinct Differences Depending on Horn Type (Head- vs. Thoracic Horn, or Resorbed vs. Maintained), Sex, or Species, and Corresponding References
| Pathway | Genes | Functions During Horn Development | Specificity
|
References | ||
|---|---|---|---|---|---|---|
| Horn type | Sex | Species | ||||
| Leg patterning | Dll, dac, hth, exd, dac, al | p/d axis patterning | ✓ | ✓ | ✓ | 7,9,10 |
| Hox | Scr | Growth, resorption | ✓ | ✓ | ✓ | 11 |
| TGF-β | dpp | Growth | ✓ | ✓ | ✓ | 12 |
| Wnt | pan | Growth | ✓ | ✓ | ✓ | 13 |
| Programmed cell death | caspase, br, E93 | Resorption, sculpting | ✓ | ✓ | ✓ | 8 |
| Insulin-signaling | foxo | Growth | ? | ? | ? | 14 |
| Juvenile hormone-signaling | N/A | Growth, sculpting | ✓ | ✓ | ✓ | 15 |
Developmental Genetics of Horn Development I—Axis Patterning
On one side horns can be thought of as highly simplified appendages: hollow cuticular projections lacking joints, nerves, or muscles. On the other side, horns and traditional appendages share important developmental properties such as a proximo-distal, medio-lateral, and dorso-ventral polarities. To date, no study has investigated how the latter two polarities might be established during horn formation, however, gene expression and functional analyses suggest that proximo-distal (p/d) axis formation may be achieved largely similar in horns compared to traditional appendages. Specifically, expression studies early on implicated the leg gap genes Distal-less (Dll), dachshund (dac), extradenticle (exd) and homothorax (hth) in the establishment of the p/d axis: Dll expression marked the development of future distal horn regions, whereas both exd and hth were confined in their expression to proximal horn regions, very similar to what is typically observed during insect appendage development (Figure 3).7,9 However, dac expression deviated from this pattern and instead of being confined to a predicted medial domain was expressed throughout developing horn primordia.
FIGURE 3.
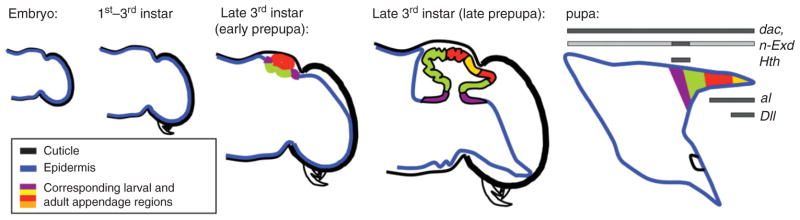
Schematic of thoracic horn development and summary of expression data for proximo-distal patterning genes during horn formation from embryo to pupa. Colors indicate tissue types and regional relationships between immature and mature appendage. (Reprinted with permission from Ref 2. Copyright 2009 Elsevier/Academic Press)
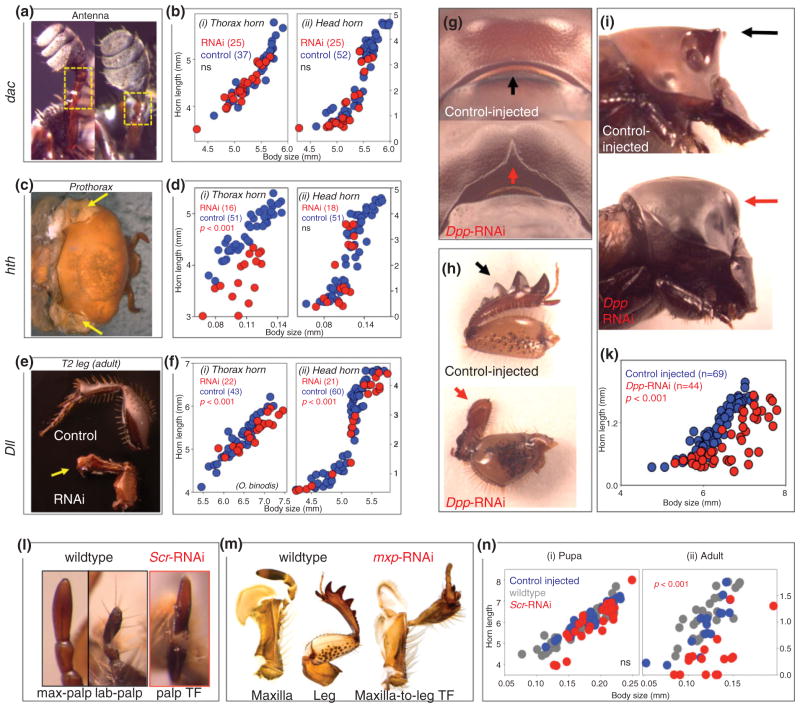
A role of leg gap genes in horn development was further confirmed through larval RNA interference-mediated transcript depletion (larval RNAi). Larval RNAi is highly effective in Onthophagus beetles, and examples of results obtained through larval RNAi approaches across a diversity of studies are given in Figure 4(a)–(n). In the context of p/d axis formation, larval RNAi for Dll, hth, and dac resulted in profound developmental defects in the formation of legs, mouthparts, and antennae matching phenotypes described in previous studies on Drosophila, Tribolium, and Oncopeltus (Figure 4(a)–(f)).10 However, only Dll-RNAi and hth-RNAi, but not dac-RNAi reliably affected horn formation. Intriguingly, degree and nature of RNAi-mediated effect on horn development differed depending on horn location (head vs. thorax), sex, and species, a finding shared by many subsequent studies.
FIGURE 4.
Examples of RNAi-mediated gene function analyses in horned beetles. (a)–(f) Proximo-distal (P/D) axis patterning genes, (g)–(k) TGFβ-signaling; (l)–(n) Hox genes. (a)–(f) Analysis of P/D axis patterning genes in O. taurus for dachshund (dac) (a, b), homothorax (hth) (c, d), and Distal-less (Dll) (e, f). (a, c, e) RNAi results in characteristic phenotypes in traditional appendages also observed in other insect models (a = deletion of medial antenna; c = induction of ectopic T1 wings; e = loss of distal leg regions). (b, d, f) Effects on scaling relationship between body size (x-axis) and horn length (y-axis; Reprinted with permission from Ref 10. Copyright 2009 Proceedings of the National Academy of Sciences). (g)–(k) Down-regulation of the TGFβ-signaling pathway member Decapentaplegic (dpp) reduces horn growth in O. binodis. (g, h) Typical Onthophagus Dpp knock-down phenotypes similar to those seen in other insects (g, notal cleft; h, leg deformation). (i, k) typical horn phenotype and allometry in control-injected and RNAi individuals (Reprinted with permission from Ref 12. Copyright 2011 Springer Publishers). (l)–(n) Examples of Hox-RNAi phenotypes in O. binodis. (l) labial-to-maxillary palp transformation (TF) following-down regulation of Sex combs reduced (Scr). (m) Maxilla-to-leg transformation following maxillopedia-RNAi. Phenotypes match those reported for other insects. (n) Scr-RNAi does not affect (i) prepupal growth but alters (ii) pupal remodeling of thoracic horns in O. nigriventris (Reprinted with permission from Ref 11. Copyright 2011 Wiley Publishers and Moczek and Simonnet, unpublished).
Developmental Genetics of Horn Development II—Size, Shape, and Position
Horn development in beetles varies primarily along three major axes: position, relative size, and shape. Studies on horn positioning have thus far focused on the role of Hox genes in regulating the segment-or body region-specific elaboration of horns. Here, the Hox gene Sex-combs reduced (Scr) was found to play a key role in the development of thoracic-, but not head horns, consistent with its conserved domain of expression in the prothoracic segment and gnathal mouthparts of insects.11 Specifically, Scr-RNAi moderately reduced prepupal growth of thoracic horns, but massively elevated the resorption of pupal horn primordia prior to the adult molt (Figure 4(l) and (n)). Furthermore, the two Onthophagus species studied in this context differed markedly in the sex most affected by Scr-RNAi.11 Follow-up studies have since implicated programmed cell death (PCD) in the sex-and species-specific resorption of horns during the pupal stage, suggesting that Scr may regulate PCD during horn formation, and that diversification of this regulatory interaction may have contributed to the diversification of thoracic horns.8 No such insights presently exist for horns that develop from the heads of horned beetles. Unpublished data for the anterior Hox genes maxillopedia, labial, and deformed suggest that none of them play a role in horn formation, and that the dorsal head surface may be patterned without regulatory input from Hox genes. Thus, the developmental genetic mechanisms underlying the positioning of head horns remain to be characterized.
The second major axis of horn variation constitutes relative size. Relative horn size varies remarkably among species, between sexes, as well as within sexes as a function of larval nutrition. A series of studies, all using comparative gene expression and larval RNAi approaches, have now begun to implicate a diversity of developmental pathways in the regulation, and in part integration, of horn size during beetle development (Table 1). Pathways implicated thus far include TGFβ-signaling,12 the wingless pathway,13 PCD,8 and most recently the insulin-signaling pathway.14 The latter study is particularly noteworthy because it provides the first insights to date into how the relative sizes of multiple growing organs may be regulated and integrated during horned beetle development. Recall that in many species large males invest heavily into horn growth rather than genitalia, whereas small males halt any resource allocation to horn growth and invest instead into enlarged testes and the production of large ejaculate volumes.16 Females do not show any corresponding response to nutritional variation. RNAi-mediated down-regulation of foxo, a growth inhibitor central to the insulin-signaling pathway, significantly affected body size and development time in males, but not females, consistent with a conservation of foxo function in the regulation of nutrition-dependent development.14 Most importantly, foxo-RNAi affected the relationship between body size and the relative sizes of horns and genitalia compared to untreated or control-injected animals: specifically, foxo-RNAi resulted in moderately enlarged horns, while genitalia size drastically declined with increasing body size. This raises the possibility that tissue-specific foxo expression underlies the integration of horn and genitalia development from small to large body sizes in male horned beetles.
Lastly, very recent work has begun to explore the role of methylation as an epigenetic mechanism in the regulation of nutrition-responsive development. At the present, we know that Onthophagus beetles possess the full complement of demethyltransferases also found in other insects (but not Drosophila or Tribolium; reviewed in17). Furthermore, a pilot study now suggests that differential methylation—measured using a methylation-sensitive AFLP assay—is associated with nutritional conditions in at least one species, O. gazella, and correlated with performance - measured as body size attained on a given diet—across nutritional environments.18 Combined, these data are at least consistent with the hypothesis that facultative methylation underlies adaptive, plastic responses to variation in nutritional environment. However, the functional significance, if any, of nutrition-associated methylation remains entirely unclear.
Much less is known about the regulation of the third major axis of horn variation—shape. Horn shape ranges from straight to curved, single to paired or forked, with or without raised shields, serrations, etc.19 Yet the developmental mechanisms underlying this diversity are presently almost entirely unclear. The only exception are a limited amount of expression data and histological observations that are consistent with a role of PCD in the shaping of adult horns during the pupal remodeling stage.8
Developmental Origin of First Horns
The studies summarized above clearly demonstrate that beetle horn evolution was mediated at least in part by the recruitment of a wide range of preexisting developmental processes. The conditions and mechanisms that enabled this recruitment from ancestral variation are of obvious interest to evolutionary developmental biologists, yet thus far remain overall poorly understood. The first origin of head horns in particular, is thus far completely unclear; no obvious putative ancestral or intermediary structures exist from which head horns might have derived. This is not true for thoracic horns, where several studies on diverse Onthophagus species have begun to piece together how adult thoracic horns might have originated, with some surprising insights into the unusual routes developmental evolution may take in the genesis of novel traits.
All Onthophagus species studied to date express thoracic horns as pupae and regardless of sex, but only a relatively small subset of species retains them into the adult stage.20,21 In those species in which thoracic horns are retained, such retention is almost exclusively restricted to males. In all other instances pupal thoracic horns are resorbed via PCD prior to the pupal-to-adult molt.8,20 This raises the question as to why so many species engage in transient thoracic horn development and invest into growing large projections seemingly just to remove them prior to adulthood. A combination of phylogenetic, histological, and experimental studies now suggests that thoracic horns originated from pupal projections that originally carried out a very different function: facilitating the shedding of the thick, tough, and highly sclerotized head capsule during the larval-to-pupal molt.21 Extant thoracic horns still carry out this function, which if compromised leaves larval heads unable to eclose. In addition, however, in a subset of lineages pupal thoracic horns now also function to form the precursors of adult structures used later in life as weapons in male combat. Phylogenetic studies suggest that the molting function of pupal horns predates their function as insipient adult weapons.21 This dual function of thoracic horns would explain why horn development is maintained in the larval stages of so many species despite the absence of horns in the corresponding adult. More generally, these data raise the possibility that adult thoracic horns may have originated secondarily through a possibly accidental carry-over into the adult stage, perhaps as the byproduct of incomplete resorption through PCD.8 We will discuss in the final section of this review how expression in the adult stage then exposed thoracic horns to a selective environment in which they may have proven immediately adaptive and able to launch one of the most dramatic radiations of secondary sexual traits in the animal kingdom.
In summary, the origin and diversification of beetle horns provides a rich and rewarding microcosm for exploring the mechanisms that enable innovation and diversification in nature. Studies thus far have in large part been driven, and thus biased, by candidate gene and pathway approaches informed by our understanding of patterning and appendage development in Drosophila and Tribolium. Clearly, future advances will depend on the development and application of unbiased transcriptomic and genomic approaches. The next section summarizes the current status of such efforts.
INTEGRATING GENOMICS, TRANSCRIPTOMICS AND DEVELOPMENTAL GENETICS TO ELUCIDATE THE ORIGINS OF HORNED BEETLE DIVERSITY
Horned beetles, most notably in the genus Onthophagus, represent an excellent model organism for identifying the genetic and developmental underpinnings of morphological diversity and innovation. In this section, we will review (1) what has been learned from transcriptome-wide studies in Onthophagus beetles to date, (2) the current genomic resources available for horned dung beetles, (3) the resources currently being developed, and (4) what we hope to learn in the next few years by integrating genomics and developmental genetics.
Trancriptome-wide Studies in Horned Beetles: What have We Learned thus Far?
There have been three transcriptome-wide studies in Onthophagus beetles conducted to date resulting in a number of interesting and complementary findings, as well as the development of several genomic resources.22–24 In the first, Kijimoto et al.22 Sanger-sequenced several thousand ESTs and developed a spotted microarray for transcriptome-wide gene expression measurements. Comparing the transcriptional profiles of developing head horns, thoracic horns, and legs, the authors made two significant discoveries (Figure 5). First, horns and legs share widespread similarities, yet also notable differences in transcriptional profiles. This result suggests that the origin of horns, a novel trait, was enabled in large part by the cooption of existing genes and pathways involved in traditional appendage development, but also the evolution or integration of genes and pathways previously thought to be unrelated to the formation of appendages. Second, the authors found that head-and thoracic horns exhibit different transcriptional profiles consistent with their independent evolutionary origin rather than strict serial homology across body segments.22
FIGURE 5.
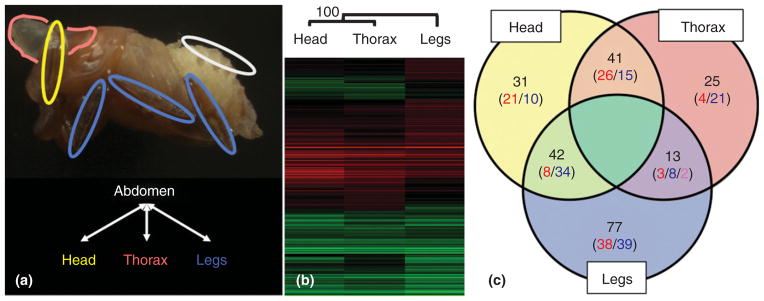
Example of a microarray study in horned beetles designed to identify similarities and differences in gene expression profiles between developing head horns, thoracic horns, and legs of the horned beetle O. taurus. (Reprinted after Ref 22. Copyright 2010 Armin Moczek). (a) Microarray experimental design. The pupal tissues used in this study are indicated in the upper panel, whereas the general array hybridization scheme is illustrated in the lower panel. Head horn (head), thoracic horn (thorax), and legs are labeled yellow, pink, and blue, respectively. RNA from each tissue was competitively hybridized with RNA from abdominal epithelium (abdomen, white). (b) Hierarchical clustering of differentially expressed genes indicates an overall high level of similarity in gene expression patterns between developing head and thoracic horns and, to a slightly lesser degree, legs. 1367 spots showed significant and differential expression, and are shown clustered based on their M-values when compared to abdominal epithelium. Each row represents a single spot and each column represents the sample. Relative magnitude of gene expression level is indicated by color brightness; red indicates enriched compared to abdominal epithelium whereas green indicates depleted relative to abdominal epithelium. Bootstrap value was obtained after 5000 trials. Branch lengths represent relative distances between the samples. (c) Categorization of genes that exhibited significantly differential expression, illustrating the unique components of head- and thoracic horn transcriptomes. Significant differential expression was defined by a P-value < 0.05 and > twofold difference in expression levels. Labels on each category represent tissue types (head = head horns, thorax = prothoracic horns, and legs = legs). Numbers indicated in the Venn diagram represent counts of non-redundant sequences in each category. Numbers in parentheses indicate counts of sequences that showed enriched or depleted expression relative to abdominal epithelium, where: red = enriched, blue = depleted, and pink = mixed (i.e., enriched in thoracic horns and depleted in legs).
A second study utilized the same cDNA microarray platform to compare gene expression patterns between alternative male morphs and both sexes to determine whether differences in transcription profiles among morphs were similar in nature and magnitude as those observed between sexes,23 a concept referred to as developmental decoupling.25 Specifically, Snell-Rood et al.23 compared gene expression profiles in pupal head horns, thoracic horns, legs, as well as brains and prothoracic ganglia, and found that patterns of gene expression were at times as divergent among morphs as they were among sexes. However, overall similarities in gene expression patterns far outweighed differences between morphs and sexes. When this effort was replicated for a second species, this study observed that the patterns themselves were evolutionary labile; instead similarities and differences in transcription profiles among morphs and sexes partly mirrored species-specific differences observed on a histological level. For instance, only large male O. nigriventris maintain their pupal horn into adulthood, whereas small males and all females resorb their horn during the pupal stage. These similarities were mirrored in their respective transcription profiles, which were most similar between females and small males. This constellation was different in O. taurus. In this species, all males and females resorb their thoracic horns, but males grow much larger thoracic horns prior to resorption, than do females. Correspondingly, transcription profiles were most similar among male morphs than when either morph was compared to females. Combined these results suggest that morph development can indeed be similarly decoupled, or divergent, at least on a transcriptional level, as sex-specific development. However, as gene expression patterns were overall largely similar across morphs and sexes, these data also raise the possibility that the major phenotypic differences that exist among morphs, sexes and species may be enabled by overall relatively modest changes in the timing, tissue-specificity or magnitude of gene expression.23
In a separate sequence-based analysis the same study also identified a possibly important evolutionary consequence of morph biased expression: genes with pronounced expression differences among morphs were more evolutionarily divergent at the amino acid level from the flour beetle Tribolium than genes showing no such expression differences. These results support the idea that genes that have more restricted function or expression (e.g., expression in only a single morph) show greater evolutionary lability, possibly due to reduced pleiotropic constraints or mutation accumulation via relaxed selection (reviewed in26).
Finally, Choi et al.24 generated a comprehensive transcriptome sequence for O. taurus utilizing 454 pyrosequencing. The authors sequenced cDNA from whole bodies (excluding the larval gut) from both sexes across 11 stages of development to generate over 50,000 non-redundant transcript sequences representing ~10,000 genes of the estimated 20,000 genes across the genome. Comparing the protein functions of identified gene transcripts to those of Tribolium castaneum, the closest species with a sequenced genome, revealed that the O. taurus transcriptome represents with a similar diversity of biological functions. Choi et al.24 also validated findings of relaxed pleiotropic constraint in genes with more restricted function presented in the Snell-Rood et al.23 study, supporting the idea that genetic diversity within a species is negatively related to the magnitude of gene expression and the number of tissues in which a gene is expressed. The primary contribution of this study, however, is the generation of many high quality annotated transcript sequences and a polymorphism database that is now available for the unbiased study of candidate genes involved in diverse contexts, from horn development to behavioral ecology.
Genomic Resources Currently Available for Horned Beetles
Transcriptome-wide studies on Onthophagus beetles thus far have resulted in a number of publically available genomic resources. Specifically, these resources include: (1) 2781 non-redundant transcript sequences from O. taurus larvae and pupae,22 (2) a cDNA spotted microarray from 3756 cDNA clones from the above O. taurus sequences,22 (3) gene expression data utilizing this microarray platform for early pupal head-and thoracic horns, legs, and abdominal tissues of O. taurus,22 (4) gene expression data utilizing this microarray platform for early pupal head-and thoracic horns, legs, brain, and abdomen tissues of O. taurus, and thoracic horn and abdominal epidermis of O. nigriventris,23 and (5) 454-sequence data for 50,080 non-redundant sequences representing about 10,000 genes of the estimated 20,000 genes across the O. taurus genome.24 Sequences were generated from normalized cDNA libraries from 32 O. taurus individuals of both sexes assayed across 11 stages of development from late 3rd larval instar to day 4-adults,24 further permitting the development of (6) a database of ~92,000 single nucleotide poymorphisms (SNPs) across 10,000 genes.24 Furthermore, these efforts resulted in the development of (7) a high-density custom oligonucleotide array from ~42,000 contigs from the 454 sequence data (Onthophagus array group, unpublished), and (8) extensive gene expression data utilizing this high-density microarray platform to assay gene expression during the development of head-and thoracic horns, legs and abdominal tissues from O. taurus males and females from high and low nutritional states (Onthophagus array group, unpublished).
Horned Beetle Genomics: Current Efforts and Future Opportunities
Several promising avenues of investigation are currently being pursued to build on these existing resources and to apply them toward a better understanding the genetic underpinnings of phenotypic diversity within and among Onthophagus species. For instance, in addition to the already published 454-transcriptome for O. taurus, comprehensive transcriptome sequences will soon be available for two more Onthophagus species. Specifically, a transcriptome sequence generated with 454-pyrosequencing is forthcoming for O. nigriventris (Warren, Corley and Emlen, in prep), a species with pronounced thoracic-rather than head horns (Figure 2(b)). Though horn position, number and shape differ dramatically from O. taurus, both species exhibit extreme sexual-and male dimorphism (Figure 2(b)). In addition, using Illumina paired-end, 100 base pair read sequencing, a transcriptome sequence is currently being developed for O. sagittarius, a species closely related to O. taurus yet highly divergent in patterns of phenotype expression: male dimorphism has been lost in this species, and sexual dimorphism has been reversed, such that females possess far more prominent horns than males.20 The diversity in sex-and morph-specific phenotypes encompassed across these three species will provide extensive substrate for fruitful comparisons toward the identification of mechanisms underlying the genesis of novelty and diversity within and between species.
Similar efforts are being pursued on the level of diverging populations. As detailed in the next section, approximately 50 years ago, populations of O. taurus were introduced to Eastern and Western Australia and the Eastern United States from their native Mediterranean habitat. Populations have since diverged dramatically in the allometric scaling relationship between body size and horn length, with important correlated divergences in endocrine control mechanisms and the timing of life history events (reviewed in20). Importantly, divergences among these populations parallel differences normally only observed between species (see Figure 4 in27). Identifying the early evolutionary mechanisms underlying such between-population divergences can lead to great insights in the mechanisms of diversification among species before evolutionary time and random genetic drift have had the opportunity to obscure relevant signatures in the genome. Both this population genomic comparison and the comparative transcriptomic studies described above will be integrated with studies on the timing-and tissue-specificity of gene expression as well as gene function studies to identify and characterize candidate genes and pathways underlying the phenotypic diversity of horned beetle morphology, physiology, and behavior.
THE ROLE OF BEHAVIOR AS A LEADER OR FOLLOWER IN DEVELOPMENTAL EVOLUTION
A longstanding question in evolutionary biology concerns the interplay between behavior and morphology in the evolution of novel traits. Does behavior drive the evolution of novel morphologies, or do novel morphologies enable the diversification of behavior? Horned beetles provide an interesting substrate for addressing this question, with some surprising outcomes. Specifically, in this section we highlight two case studies on horned beetle diversification, one contemporary and one ancient, that illuminate the interplay between behavioral and morphological evolution in these organisms.
The Role of Behavior in Facilitating the Evolution of Adult Weapons from Pupal Molting Devices
Recall that phylogenetic, histological, and functional studies support the hypothesis that adult thoracic horns are derived from pupal projections that originally functioned solely as molting devices designed to remove the larval head capsule during the larval-to-pupal molt.21 These results raised the possibility that the very first adult thoracic horns may have appeared simply due to the failure to remove pupal horns, for instance through a failure to activate PCD during the pupal stage, a phenomenon observed to occur at very low frequency in natural populations.8,21 If correct, this scenario begs the question as to the nature of the selective environment that may have enabled the occasional expression of adult thoracic horns to spread and eventually become constitutive in a population. As detailed next, a series of studies suggest that the behavioral ecology of scarabaeine beetles may have preadapted these organisms to utilize adult projections as weapons as soon as they became available, which in turn enabled the subsequent diversification of horn types and alternative morphologies.
Several studies illustrate that horn possession is not a prerequisite for fighting. Instead, aggressive pushing and prodding between rival males can be observed in numerous taxa lacking adult horns of any kind (reviewed in1). Similarly, in species with alternative horned and hornless morphologies, fighting can be observed among small, hornless ‘sneaker’ males as long as they are competing against similar-sized small, hornless males.6 Thus, aggressive fighting very likely characterized the nature of interactions between rival males of present-day horned taxa prior to the origin of the first adult horns. This suggests that the first adult horns that may have been expressed (possibly due to a failure to resorb pupal projections8) could have been immediately useable, and adaptive, in a preexisting behavioral context. Such horns would initially likely have been small, but at least two studies on Onthophagus beetles now illustrate that even the possession of very small horns, or subtly enlarged horns, can bring about measureable increases in fighting success.6,28 This scenario suggests that the behavioral repertoire of male scarabaeine beetles preadapted these organisms toward the exaptation (sensu29) of pupal thoracic horns, from their original function as a molting device to their derived function as an adult weapon.
A second behavioral–ecological context likely further facilitated and shaped the origin and diversification of horns as weapons in scarabaeine beetles. Almost all Scarbaeinae utilize dung as a resource during larval and adult development, but do so broadly in three different ecological guilds (reviewed in30): dwellers oviposit eggs below dung pads and provide minimal parental care; rollers carve out dug segments, shape them into balls and roll them away from competitors and to a suitable burying site; and finally, tunnelers, dig deep tunnels underneath dung pads and build brood balls underground. Competition for resources and mates is generally severe in all three groups, but only in tunnelers do males possess the ability to monopolize females by guarding and by defending access to tunnels against rivals.31 Furthermore, tunnels restrict rival males to face each other in serial one-on-one encounters in contrast to open space encounters above ground.31 Thus, tunneling likely set the stage for the evolution of one-on-one fighting in tunneling taxa, such as Onthophagus, and the evolution of weaponry, such as horns, that is useful in one-on-one combat and in the blocking of narrow tunnels to prevent other males from accessing the female.6,26 Consistent with this scenario, horn possession is widespread and elaborate in tunneling scarabs, where it appears to have evolved independently several times, yet is virtually absent in any other group of non-tunneling scarabaeine beetles.31
Lastly, the interplay between behavior and morphology in phenotypic diversification likely continued beyond the appearance and elaboration of the first adult horns. Once adult horns appeared, the use of horns as weapons likely intensified sexual selection within populations, discriminating especially against smaller males, whose body size already placed them at a competitive disadvantage in one-on-one fights. The resulting extreme reproductive skew subsequently led to the evolution of a flexible reproductive strategy in many species: expression of alternative morphs depending on the nutritional environment experienced during larval development. Accordingly, only male larvae that experience ample food resources develop into large, fully horned males that fight for access to females, whereas limited larval food availability leads to the expression of smaller, and nearly completely hornless males that engage in non-aggressive sneaking behavior that relies on a high degree of agility (Figure 6(a) and (b)).32 Horn possession reduces agility,6 and existing data suggest that ‘hornlessness’ is adaptive given the behavioral tactic employed by small sneaker males.6,33 It is worth noting that while the morphological differences between alternative morphs rival those observed between species, they do not reflect a genetic polymorphism—instead they reflect a single individual’s or genotype’s ability to express two discretely different phenotypes in response to variation in larval feeding conditions,32 akin to the development of castes in social insects,34–36 or seasonal morphs in butterflies.37,38 Here, the interplay between behavioral and morphological evolution has resulted in the diversification not just of form (as in the evolution of hornless morphs) or behavior (such as the evolution of sneaking) but also of plasticity itself, as species readily diverge in the degree and nature of responses to nutritional variation. An especially illuminating example of plasticity evolution is discussed in the next section.
FIGURE 6.
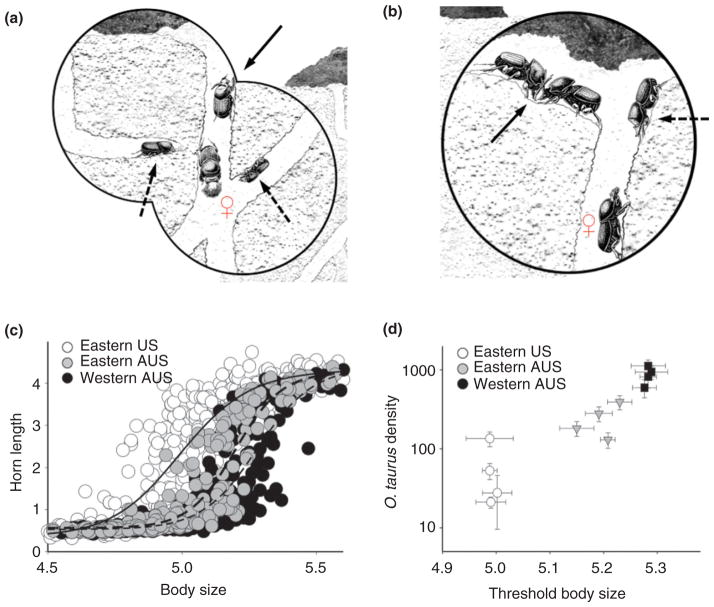
Divergences in the social context of male horn dimorphism appear to drive morphological divergences in exotic populations of Onthophagus taurus. (a and b) Alternative reproductive tactics of horned (solid arrow) and hornless (dashed arrow) males. Horned males guard (a) and fight over (b) access to tunnel entrances containing breeding females. Hornless males utilize non-aggressive sneaking behaviors such as digging of interception tunnels (a, left dashed arrow), using naturally existing tunnel connections (a, right dashed arrow), or waiting as satellite males until guarding males are distracted in order to gain access to females (b, dashed arrow). Figures (a) and (b); reprinted with permission from Ref 39. Copyright 2009 Science Publishers. (c) Scaling relationship between body size (x-axis) and horn length (y-axis) in three exotic, geographically isolated populations. (d) Correlation between body size thresholds (x-axis) and densities (y-axis) in local populations across the same three exotic ranges. Theoretical models (see text) predict that body size thresholds (c) can diverge as a result of differences in local densities of competing males (d), i.e., high density populations exhibit higher size thresholds than low density populations. Data in (c) and (d); reprinted with permission from Ref 40. Copyright 2003 Oxford University Press.
Rapid Contemporary Evolution of Horn Allometries in Exotic Beetle Populations
Recall from the above sections that male horn dimorphism functions in the context of two alternative reproductive tactics to secure mating opportunities: fighting (using horns as weapons) and sneaking (using ‘hornlessness’ to enhance agility). Recent work on one species suggests that changes in this context can drive rapid morphological and developmental diversification.
Onthophagus taurus is a species with a substantial male dimorphism with respect to both morphology and reproductive behavior (Figure 2(a)). It was introduced in the early 1970s from its native Mediterranean range41 to the Eastern U.S. as well as Eastern and Western Australia.42,43 Across these three exotic ranges males encounter very different intensities of male–male competition40: Eastern U.S. males face moderate competition such that most males have to contend with very few other males. In contrast, Western Australian males face high local densities, often 2–3 orders of magnitude above those seen in the U.S. As a consequence, individual males have to compete with a large number of males for a limited number of females. Lastly, Eastern Australian populations exhibit intermediate intensities of male competition.
Examination of archival historical collections suggest that the average scaling relationship between body size and horn length (i.e., horn allometry) has been largely invariant within the native range of this species in the Mediterranean, as well as the source populations from this range that were used for the introduction of this species into Australia.27 In contrast, the U.S. introduction was accidental and the geographic origin of the source population(s) is unknown. In other words, across this species’ native range all males smaller than approximately 5 mm in body size are hornless, while those larger than this size threshold tend to be fully horned.27
Since introduction to its exotic ranges, however, the divergent social conditions in which horned (fighting) and hornless (sneaking) males function appear to have driven a rapid divergence in the size threshold of horn development. Selection models predict that individual genotypes should switch from expressing the hornless (sneaking) to the horned (fighting) phenotype at the body size at which the fitness gained from sneaking and lacking horns is beginning to be outweighed by the fitness gained from fighting and the possession of horns. These same models predict, however, that the optimal switch point should depend on local ecological conditions that may influence the respective fitness gains of each tactic in a given population, such as the intensity of local male–male competition. For instance, high local densities should elevate the intensity of male–male competition for breeding females and allow only males with the largest body sizes to defend and monopolize females by means of fighting. Consistent with these predictions, males from the low-competition Eastern U.S. populations develop the fully horned morph already at surprisingly small body sizes, whereas males from the extremely high-competition Western Australian populations develop horns only at significantly larger body sizes (Figure 6(c) and (d)).40 Eastern Australian males, which are subject to intermediate levels of male–male competition, start to express horns at intermediate body sizes in relation to the other two populations (Fig. 6(c) and (d)).40 These divergences are maintained under common garden conditions,44 are mediated by heritable divergences in the endocrine regulation of morph determination,45 and are of a nature and magnitude similar to what is normally observed among more distantly related taxa.27 Furthermore, population-specific divergences in horn development appear to have resulted in diverse correlated divergences, from the duration of larval development45 to divergences in relative resource allocation to other body parts, such as genitalia.46–48 Most generally, these divergences highlight how the social context of behavior can drive morphological and developmental diversification among isolated populations over an extraordinarily short time period.
In summary, the case studies highlighted in this section illustrate how the behavior of individuals and the social contexts produced by groups of interacting individuals can generate selective environments that favor morphological and developmental diversification and innovation. At the same time, these examples illustrate that behavior may only facilitate the evolution of those traits that developmental genetic and physiological processes can generate in the first place. Whether the evolution of behavior therefore necessarily leads developmental evolution, or is instead enabled by it remains an open question, and perhaps one whose answer is largely in the eye of the beholder.
CONCLUSIONS
Horned beetles are rich in natural history, feature incredible phenotypic diversity, and are increasingly experimentally accessible. As such they represent a rewarding microcosm within which to explore the mechanisms underlying innovation and diversification in the natural world. At the same time, many horned beetle species are easily and inexpensively maintained in captivity, and some of the most interesting species are broadly distributed geographically, i.e., researchers in many countries are likely to find at least some species occurring naturally in their backyard. Research conducted thus far has only begun to scratch the surface of what these remarkable organisms can teach us about the interplay between development, ecology, behavior and evolution, and we hope that the present manuscript will encourage future research efforts into the biology of horned beetles and beetle horns.
Acknowledgments
We thank the editors for the opportunity to contribute this review article. Research presented here was made possible in large part through funding from the National Science Foundation.
References
- 1.Snell-Rood EC, Moczek AP. Horns and the role of development in the evolution of beetle contests. In: Hardy ICW, Briffa M, editors. Animal Contests. UK: Cambridge University Press; 2012a. [Google Scholar]
- 2.Moczek AP. The origin and diversification of complex traits through micro-and macro-evolution of development: Insights from horned beetles. In: Jeffrey W, editor. Current Topics in Developmental Biology (CTDB) Vol. 6. New York: Elsevier/Academic Press; 2009. pp. 135–162. [DOI] [PubMed] [Google Scholar]
- 3.Moczek AP. Phenotypic plasticity and diversity in insects. In: Minelli A, Fusco G, editors. From polyphenism to complex metazoan life cycles Philosophical Transactions of the Royal Society of London. Vol. 365. 2010. pp. 593–603. Series B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emlen DJ, Hunt J, Simmons LW. Evolution of sexual dimorphism and male dimorphism in the expression of beetle horns: phylogenetic evidence for modularity, evolutionary lability, and constraint. Am Nat. 2005;166:S42–S68. doi: 10.1086/444599. [DOI] [PubMed] [Google Scholar]
- 5.Paulian R. Le polymorphisme des males de coléopteres. In: Tessier G, editor. Exposés de biométrie et statistique biologique IV, Actualités scientifiques et industrielles. Vol. 255. Paris, France: Hermann and Cie; 1935. pp. 1–33. [Google Scholar]
- 6.Moczek AP, Emlen DJ. Male horn dimorphism in the scarab beetle Onthophagus taurus: do alternative reproductive tactics favor alternative phenotypes? Anim Behav. 2000;59:459–466. doi: 10.1006/anbe.1999.1342. [DOI] [PubMed] [Google Scholar]
- 7.Moczek AP, Nagy LM. Diverse developmental mechanisms contribute to different levels of diversity in horned beetles. Evol Dev. 2005;7:175–185. doi: 10.1111/j.1525-142X.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 8.Kijimoto T, Andrews J, Moczek AP. Programmed cell death shapes the expression of horns within and between species of horned beetles. Evol Dev. 2010;12:449–458. doi: 10.1111/j.1525-142X.2010.00431.x. [DOI] [PubMed] [Google Scholar]
- 9.Moczek AP, Rose D, Sewell W, Kesselring BR. Conservation, innovation, and the evolution of horned beetle diversity. Dev Genes Evol. 2006a;216:655–665. doi: 10.1007/s00427-006-0087-2. [DOI] [PubMed] [Google Scholar]
- 10.Moczek A, Rose D. Differential recruitment of limb patterning genes during development and diversification of beetle horns. Proc Natl Acad Sci USA. 2009;106:8992. doi: 10.1073/pnas.0809668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasik BR, Rose DJ, Moczek AP. Beetle horns are regulated by the Hox gene, Sex combs reduced, in a species-and sex-specific manner. Evol Dev. 2010;12:353–362. doi: 10.1111/j.1525-142X.2010.00422.x. [DOI] [PubMed] [Google Scholar]
- 12.Wasik B, Moczek AP. decapentaplegic (dpp) regulates the growth of a morphological novelty, beetle horns. Dev Genes Evol. 2011;221:17–27. doi: 10.1007/s00427-011-0355-7. [DOI] [PubMed] [Google Scholar]
- 13.Wasik B, Moczek AP. pangolin expression influences the development of a morphological novelty: beetle horns. Genesis. 2012;50:404–414. doi: 10.1002/dvg.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snell-Rood EC, Moczek AP. Insulin signaling as a mechanism underlying developmental plasticity: the role of FOXO in a nutritional polyphenism. PLoS ONE. 2012b;7:e34857. doi: 10.1371/journal.pone.0034857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelby JA, Madewell R, Moczek AP. Juvenile hormone mediates sexual dimorphism in horned beetles. J Exp Zool B. 2007;308B:417–427. doi: 10.1002/jez.b.21165. [DOI] [PubMed] [Google Scholar]
- 16.Simmons LW, Emlen DJ, Tomkins J. Sperm competition games between sneaks and guards: a comparative analysis using dimorphic male beetles. Evolution. 2007;61:2684–2692. doi: 10.1111/j.1558-5646.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 17.Valena S, Moczek AP. Epigenetic mechanisms underlying developmental plasticity in horned beetles. The Epigenetics of Emerging and Non-model Organisms. Genet Res Int. 2012 doi: 10.1155/2012/576303. Article ID 576303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snell-Rood EC, Troth A, Moczek AP. DNA methylation as a mechanism of nutritional plasticity: insights from horned beetles. J Exp Zool. doi: 10.1002/jez.b.22479. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emlen DJ, Marangelo J, Ball B, Cunningham CW. Diversity in the weapons of sexual selection: Horn evolution in the beetle genus Onthophagus (Coleoptera: Scarabaeidae) Evolution. 2005b;59:1060–1084. [PubMed] [Google Scholar]
- 20.Moczek AP. Pupal remodeling and the development and evolution of sexual dimorphism in horned beetles. Am Nat. 2006;168:711–729. doi: 10.1086/509051. [DOI] [PubMed] [Google Scholar]
- 21.Moczek AP, Cruickshank TE, Shelby JA. When ontogeny reveals what phylogeny hides: gain and loss of horns during development and evolution of horned beetles. Evolution. 2006b;60:2329–2341. [PubMed] [Google Scholar]
- 22.Kijimoto T, Costello J, Tang Z, Moczek A, Andrews J. EST and microarray analysis of horn development in Onthophagus beetles. BMC Genomics. 2009;10:504. doi: 10.1186/1471-2164-10-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snell-Rood E, Cash A, Han M, Kijimoto T, Andrews J, Moczek A. Developmental decoupling of alternative phenotypes: Insights from the transcriptomes of horn polyphenic beetles. Evolution. 2010;65:231–245. doi: 10.1111/j.1558-5646.2010.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JH, Kijimoto T, Snell-Rood E, Tae H, Yang Y, Moczek A, Andrews J. Gene discovery in the horned beetle Onthophagus taurus. BMC Genomics. 2010;11:703. doi: 10.1186/1471-2164-11-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West-Eberhard MJ. Phenotypic plasticity and the origins of diversity. Annu Rev Ecol Syst. 1989;20:249–278. [Google Scholar]
- 26.Pál C, Papp B, Lercher MJ. An integrated view of protein evolution. Nat Rev Gen. 2006;7:337–348. doi: 10.1038/nrg1838. [DOI] [PubMed] [Google Scholar]
- 27.Moczek AP, Nijhout HF. Rapid evolution of a polyphenic threshold. Evol Dev. 2003;5:259–268. doi: 10.1046/j.1525-142x.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- 28.Emlen DJ. Alternative reproductive tactics and male dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae) Behav Ecol Sociobiol. 1997;41:335–341. [Google Scholar]
- 29.Gould SJ, Vrba ES. Exaptation-a missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- 30.Simmons LW, Ridsdill-Smith TJ. Reproductive competition and its impact on the evolution and ecology of dung beetles. In: Simmons LW, Ridsdill-Smith JT, editors. Dung Beetle Ecology and Evolution. Chapter 1 Oxford: Wiley-Blackwell; 2011. [Google Scholar]
- 31.Emlen DJ, Philips TK. Phylogenetic evidence for an association between tunneling behavior and the evolution of horns in dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) Coleopterists Soc Monogr. 2006;5:47–56. [Google Scholar]
- 32.Moczek AP, Emlen DJ. Proximate determination of male horn dimorphism in the beetle Onthophagus taurus (Coleoptera: Scarabaeidae) J Evol Biol. 1999;12:27–37. [Google Scholar]
- 33.Madewell R, Moczek AP. Horn possession reduces maneuverability in a horn-polyphenic beetle. J Insect Sci. 2006;6:21. doi: 10.1673/2006_06_21.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheeler DE, Nijhout HF. Soldier determination in Pheidole bicarinata Effect of methophrene on caste and size within castes. J Insect Physiol. 1983;29:847–854. [Google Scholar]
- 35.Wheeler DE, Nijhout HF. Soldier determination in the ant Pheidole bicarinata Inhibition by adult soldiers. J Insect Physiol. 1984;30:127–135. [Google Scholar]
- 36.Hölldobler B, Wilson EO. The Ants. MA: Harvard University Press; 1990. [Google Scholar]
- 37.Shapiro AM. Seasonal polyphenism. Evol Biol. 1976;9:259–333. [Google Scholar]
- 38.McLeod L. Seasonal polyphenism in African Precis butterflies. In: Vane-Wright RI, Ackery PR, editors. The biology of butterflies. London: Academic Press; 1984. [Google Scholar]
- 39.Moczek AP. Developmental plasticity and the origins of diversity: a case study on horned beetles. In: Ananthakrishnan TN, Whitman D, editors. Phenotypic Plasticity in Insects: Mechanisms and Consequences. Vol. 3. Plymouth: Science Publishers, Inc; 2011. pp. 81–134. [Google Scholar]
- 40.Moczek AP. The behavioral ecology of threshold evolution in a polyphenic beetle. Behav Ecol. 2003;14:831–854. [Google Scholar]
- 41.Balthasar V. Coprinae. Prag: Verlag der tschechoslowakischen Akademie der Wissenschaften; 1963. Monographie der Scaraba eidae und Aphodiidae der palaearktischen und orientalischen Region (Coleoptera: Lamellicornia) (Band 2) [Google Scholar]
- 42.Fincher GT, Woodruff RE. A European dung beetle, Onthophagus taurus Schreber, new to the U.S. (Coleoptera: Scarabaeidae) Coleopts Bull. 1975;29:349–350. [Google Scholar]
- 43.Tyndale-Biscoe M. Technical Report No 62. Canberra, ACT: CSIRO, Division of Entomology; 1990. Australia’s introduced dung beetles: Original releases and redistributions. [Google Scholar]
- 44.Moczek AP, Hunt J, Emlen DJ, Simmons LW. Threshold evolution in exotic populations of a polyphenic beetle. Evol Ecol Res. 2002;4:587–601. [Google Scholar]
- 45.Moczek AP, Nijhout HF. Developmental mechanisms of threshold evolution in a polyphenic beetle. Evol Dev. 2002;4:252–264. doi: 10.1046/j.1525-142x.2002.02014.x. [DOI] [PubMed] [Google Scholar]
- 46.Parzer HF, Moczek AP. Rapid antagonistic coevolution between primary and secondary sexual characters in horned beetles. Evolution. 2008;62:2423–2428. doi: 10.1111/j.1558-5646.2008.00448.x. [DOI] [PubMed] [Google Scholar]
- 47.Pizzo A, Roggero A, Palestrini C, Moczek AP, Rolando A. Rapid shape divergences between natural and introduced populations of a horned beetle partly mirror divergences between species. Evol Dev. 2008;10:166–175. doi: 10.1111/j.1525-142X.2008.00224.x. [DOI] [PubMed] [Google Scholar]
- 48.Macagno ALM, Pizzo A, Parzer HF, Palestrini C, Rolando A, Moczek AP. Shape—but not size—codivergence between male and female copulatory structures in Onthophagus beetles. PLoS ONE. 2011;6:e28893. doi: 10.1371/journal.pone.0028893. [DOI] [PMC free article] [PubMed] [Google Scholar]