Summary
Despite complete or near-complete suppression of human immunodeficiency virus (HIV) replication with combination antiretroviral therapy, both HIV and chronic inflammation/immune dysfunction persist indefinitely. Untangling the association between the virus and the host immune environment during therapy might lead to novel interventions aimed at either curing the infection or preventing the development of inflammation-associated end-organ disease. Chronic inflammation and immune dysfunction might lead to HIV persistence by causing virus production, generating new target cells, enabling infecting of activated and resting target cells, altering the migration patterns of susceptible target cells, increasing the proliferation of infected cells, and preventing normal HIV-specific clearance mechanisms from function. Chronic HIV production or replication might contribute to persistent inflammation and immune dysfunction. The rapidly evolving data on these issues strongly suggest that a vicious cycle might exist in which HIV persistence causes inflammation that in turn contributes to HIV persistence.
Keywords: AIDS, immunodeficiency diseases, dell activation, cell differentiation, cell proliferation, inflammation
Introduction
With the recent optimization of antiretroviral drugs, most motivated human immunodeficiency virus (HIV)-infected patients with access to therapy can achieve durable and perhaps life-long viral suppression. Although these drugs improve quality of life, prevent acquired immunodeficiency syndrome (AIDS), and reduce overall mortality, they do not fully restore health. Treatment-mediated immune reconstitution is often incomplete, even after many years of viral suppression (1–3). Inflammation and T-cell activation remain elevated, and CD4+ T-cell counts often fail to achieve normal levels (4–8). Limited immune reconstitution is particularly notable in mucosal lymphoid tissues (9–14) and may result in a diminished capacity of the adaptive immune system to function effectively (15, 16). As compared to age-matched uninfected adults, treated HIV-infected adults have higher risk of developing a number of non-AIDS-related non-immunological diseases, including cardiovascular disease, cancer, kidney disease, liver disease, neurologic disease, and bone diseases (17). Some (but not all) studies have argued that the life-span of the typical long-term treated adult is not normalized by effective combination antiretroviral therapy. Chronic immune dysfunction, immune activation, and inflammation predict and likely contribute to this excess risk of morbidity and mortality (18–23). Defining the mechanisms for persistent inflammation during combination antiretroviral therapy is key question for the field.
Many factors contribute to persistent inflammation and immune dysfunction during therapy in HIV-infected individuals. Deposition of collagen in lymphoid organs during untreated disease causes irreversible tissue fibrosis, which likely contributes to failed T-cell homeostasis (12, 24), persistent immunodeficiency, excess levels of various pathogens such as cytomegalovirus (CMV) (25), destruction of mucosal surfaces (26), and persistent inflammation all likely contribute to ongoing dysfunction. HIV-associated mucosal immune dysfunction and loss of immunoregulatory mucosal cells such as interleukin-17 (IL-17)-producing cells also likely persists during therapy, leading to failed control of microbial translocation and consequent persistent inflammation (27–30). Although less well-studied, traditional risk factors such as central obesity, treatment-mediated effects on metabolism leading to the metabolic syndrome, and substance abuse likely also contribute to inflammation during combination antiretroviral therapy.
One of the more controversial areas in HIV medicine pertains to the association between persistent HIV infection and chronic inflammation during long-term effective antiretroviral therapy (where effective is defined as having maintained undetectable plasma HIV RNA levels using conventional assays for several years)(31). As outlined in detail below, a positive correlation exists between measures of immune activation (particularly those based on CD4+ T-cell phenotype) and HIV persistence (as measured in cells and tissues) among long-term treated adults. Whether immune activation is a cause, a consequence, or both a cause and a consequence of HIV persistence is unknown. Understanding the mechanisms for this association could lead to the optimization of strategies aimed at curing HIV infection and/or at reducing inflammation-associated disease. In this review, we summarize what is known about immune activation and HIV persistence during antiretroviral therapy and describe ongoing studies in humans and non-human primates that examine how the virus and immune system interact once treatment-mediated control of HIV replication is achieved.
Pathogenesis of HIV-associated immune activation
The central role of immune activation in HIV disease progression was noted in the earliest observations of the clinical disease (32) (Fig. 1). In the 1990s, Janis Giorgi and her colleagues (33) performed seminal work which argued that HIV-associated alterations in T-cell phenotype (as defined by expression of ‘activation’ markers such as CD38 and HLA-DR) predicted disease progression independently of other factors. After the resolution of primary infection, an apparently steady-state level or ‘set-point’ of T-cell activation is achieved; this level predicts the rate of CD4+ T-cell decline (34). Importantly, as the level of T-cell activation is strongly and consistently correlated with level of HIV replication during untreated disease, defining with precision the independent effects of T-cell activation on outcome has been challenging (35). Perhaps the strongest evidence that the inflammatory response to the virus is a critical determinant of pathogenesis comes from study of natural host for the simian version of the virus (SIV). Although SIV replication is high in these animals, immune activation/inflammation and disease progression are both limited. Preservation of central memory CD4+ T cells due to low CCR5 expression, rapid downregulation of type interferon I response post-infection, preservation of lymphoid tissues, maintenance of mucosal barrier integrity, and lower tissue burden of virus are consistent correlates of protection in these models (36–38). Indeed, all of these factors can be a cause or effect (or both) of chronic immune activation and disease progression in untreated HIV infection and thus likely underlie lack of disease progression in natural hosts.
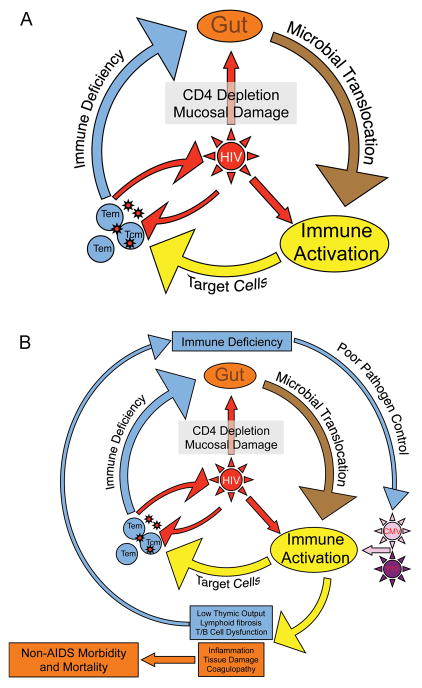
Fig. 1. HIV initiates and sustains a ‘vicious cycle’.
(A). Acute HIV infection causes damage to the mucosal integrity of the gastrointestinal tract, resulting in continuous local and systemic exposure to gut microbial products. HIV and these microbial products cause activation of T cells and expansion of CD4+ T cells, resulting in more target cells and higher levels of HIV replication. Direct and indirect mechanisms lead to CD4+ T-cell loss, broad immunodeficiency, and higher levels of both HIV and microbial translocation. (B). Chronic activation of the immune system results in direct damage to lymphoid tissues, which in turn contributes to failure to regenerate T cells and overall decrease in function of adaptive and innate immune systems. The resulting immunodeficiency results in excess pathogens (e.g. HIV, gut microbes, herpes viruses) and, as a result, yet more immune activation. This chronic inflammatory state predicts and presumably causes development of AIDS and non-AIDS conditions such as early cardiovascular disease.
In untreated HIV disease, there is a striking and consistent association between activation of T cells and plasma HIV RNA levels. In some studies, the association between viremia and T-cell activation is more consistent in CD8+ T cells than in CD4+ T cells, while the peripheral CD4+ T-cell count is a more consistent predictor of CD4+ T-cell activation (39–42). These latter observations have been used to argue that viremia drives CD8+ T-cell activation directly, while homeostatic signals associated with low CD4+ T-cell counts upregulate certain markers on circulating CD4+ T cells.
The expression of many of the negative regulators of T-cell activation such as programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4) is also strongly correlated with viremia (43–50), while in other studies the frequency of forkhead box protein 3 (FoxP3)+ and T-regulatory (Treg) cells are correlated with viremia (28, 29, 50, 51). As is common in these types of studies, it is impossible to define whether HIV replication drives an immunoregulatory response or whether the immune-suppressive effects of these responses causes increased viremia, perhaps by preventing an effective HIV-specific immune response. Untreated HIV infection is also associated with activation and dysregulation of B cells (52–58). B-cell dysfunction prevents optimal antibody production in both primary and secondary immune responses, leading to compromised responses to infection and vaccination (52, 54, 57, 59). Furthermore, innate cells such as natural killer (NK) cells, dendritic cells, macrophages, and other key regulators of immune function are also affected by HIV infection and may underlie the high plasma levels of proinflammatory cytokines and biomarkers observed during infection (60). Indeed, monocytes and macrophages have been shown to be hyper-activated and have reduced propensity for phagocytosing pathogens such as bacteria, further promoting microbial translocation and activation (58, 61, 62). Altered dendritic cell subsets have also been observed during HIV infection, including abnormal plasmacytoid and myeloid dendritic cells, and (in some studies) loss of mucosal CD103+ dendritic cells (27, 63, 64). NK cells are also dysfunctional with both altered cytokine production and homing to tissues observed in HIV-infected individuals (64–66).
A major driver of immune activation and dysfunction during HIV infection is damage to the mucosal barriers and lymphoid structures (Fig. 1). Densely populated CCR5-expressing CD4+ T cells in the gastrointestinal tract are likely the preferred infection targets of HIV/SIV during early stages of the infection (9, 11, 13, 67–71). HIV/SIV-associated breaches in the tight epithelial barrier of the gastrointestinal tract allows microbial products to translocate across the barrier, resulting in local and systemic activation (27, 61, 72–75). This local activation in the GI tract contributes to increased numbers of inflammatory cells such as plasmacytoid dendritic cells (pDCs), neutrophils, and monocytes and decreased numbers of cells that are essential for mucosal regulation, including IL-17 and IL-22-producing lymphocytes and CD103+ dendritic cells (27, 63, 64, 76–78). The loss of IL-17 and IL-22-producing CD4+ T cells may be particularly problematic, as these cells regulate epithelial homeostasis and thus loss of these subsets likely directly contributes to breakdown of the mucosal barrier (27, 30, 79–85).
In addition to direct infection, a major mechanism underlying lack of reconstitution of CD4+ T cells activation-induced collagen deposition in lymphoid tissues (12, 24) (Fig. 1). Thus, the vicious cycle of HIV replication and immune activation induces and maintains mucosal immune dysfunction during HIV infection, which further drives systemic activation.
Inflammation and immune activation during combination antiretroviral therapy
The level of T-cell activation declines rapidly and durably during combination antiretroviral therapy but rarely achieves a normal steady state (5, 86). Although the frequency of HLA-DR and CD38-expressing T cells is a strong predictor of disease progression in untreated disease, the clinical implications of these cells during treated disease remains largely undefined, although some studies have suggested they retain some prognostic significance (20, 87). The immunologic profile of circulating T cells during effective antiretroviral therapy is also often characterized by lower than normal expression levels of CD28, elevated levels of CD57 and elevated levels of PD-1, a profile that is consistent with T-cell ‘senescence’ and dysfunction (87–91).
Data on B-cell and innate cell activation during combination antiretroviral therapy is not as complete as in untreated infection. While partial functional restoration may occur during treatment, these immune cells are still clearly dysfunctional compared to uninfected individuals. Indeed, NK cells remain activated despite virus suppression by combination antiretroviral therapy, and defective antibody-dependent cell-mediated cytotoxicity (ADCC) signaling by NK cells persists during treatment (92). Monocyte dysfunctions also persist despite treatment, whereby monocytes maintain decreased phagocytosis and resemble those isolated from elderly individuals, even in very young patients (93). Furthermore, sCD163, a marker of monocyte activation, remains elevated despite combination antiretroviral therapy and is associated with increased risk for atherosclerosis (94, 95). During treatment, the numbers and function of pDCs and mDCs remain altered as well, and plasma factors have been demonstrated to contribute to dsyfunctionality despite long-term combination antiretroviral therapy (96, 97). Finally, it has been demonstrated that even during long-term combination antiretroviral therapy treatment, innate responses to other pathogens, such as malaria, remain dysfunctional (98).
There has been a recent shift in clinical research from studies focused on T-cell phenotype to studies focused on plasma biomarkers of inflammation. This shift occurred in part because the number of clinical events in HIV-infected patients has declined, making it difficult for those cohorts which store peripheral blood mononuclear cells (PBMCs) to define with adequate power the role of T-cell activation and/or dysfunction during therapy in predicting subsequent morbidity and mortality. Also, the biology of chronic inflammation during treated disease is almost certainly unique from that in untreated disease. Many of the biomarkers that seem to be most strongly associated with disease progression in the modern era reflect innate immune activation [e.g. IL-1, IL-6, tumor necrosis factor (TNF), C-reactive protein](18, 19, 99). Markers specific to monocyte activation and/or microbial translocation (e.g. soluble CD14, CD163) and the coagulation cascade (D-dimers, fibrinogen) are also consistent correlates or predictors of disease (18, 19, 94, 99–108). Long-term effective combination antiretroviral therapy reduces many of these markers, but the treatment effect is less consistent than that observed with T-cell activation outcomes, suggesting factors other than HIV replication contribute to those pathways associated with these biomarkers (109–111).
Mechanisms for HIV persistence during antiretroviral therapy
Several non-mutually exclusive mechanisms underlie HIV persistence in adults who have received suppressive combination antiretroviral therapy for extended periods of time. The best characterized and potentially the most paramount mechanism for persistence is the generation and maintenance of a ‘silent’ provirus in resting memory CD4+ T cells (112–114). The memory CD4+ T-cell compartment where HIV largely resides is heterogeneous. Using markers such as CD45RA (a tyrosine phosphatase), CCR7 (a lymph node homing receptor), and CD27 (a member of the TNF superfamily critical for the long term maintenance of immunological memory)(115, 116), it has been possible to demonstrate that HIV primarily persists in three memory T-cell subsets endowed with distinct functional and survival capacities, namely central memory (TCM)(CD45RA−CCR7+CD27+), transitional memory (TTM)(CD45RA−CCR7−CD27+) and effector memory (TEM)(CD45RA−CCR7−CD27−) CD4+ T cells (117).
The distinct T-cell subsets which harbor HIV have unique functional and phenotypic properties, suggesting that cellular reservoirs might support viral persistence through different mechanisms. For instance, the drastic differences in the activation status of TCM and TEM cells (115, 117) suggest that the former may represent an ideal reservoir for latent HIV, whereas the latter may be more prone to support residual levels of viral replication in the face of combination antiretroviral therapy. In one study, approximately 85% of the circulating cells harboring integrated HIV DNA displayed a TCM or a TTM phenotype, with TEM representing only 15% of the pool of latently infected cells. The distribution of virus in mucosal T-cell subsets is less well-characterized. The rate at which these unique cell populations decay during therapy is unknown, but the total resting memory cell reservoir decays slowly, with an estimated half-life in chronically infected adults of 40 to 44 months, indicating that more than 70 years of intensive therapy would be required for its eradication (118).
Other reservoirs for HIV persistence have been described, but their contribution after many years of effective antiretroviral therapy is less certain. Naive CD4+ T cells, macrophage/monocytes, astrocytes, and microglial cells are possible reservoirs (31). Most of these cellular and tissues reservoirs which persist during therapy are assumed to have been generated prior to treatment. As outlined in detail below, there are some emerging data that suggest that the suppression of viral replication by combination antiretroviral therapy may be incomplete in some if not most individuals. Theoretically, low-level replication allows the continuous replenishment of a small pool of infected cells (119).
Association between immune activation and HIV persistence during therapy
The association between HIV burden and immune activation during effective therapy remains controversial. Progress in untangling this association has been limited by the lack of well-validated measures of viral load during effective therapy. HIV RNA can often be detected in the plasma of individuals receiving antiretroviral therapy, but the levels are very low (i.e. 0.1 to 5 copies RNA/mL) and near the limit of quantification for even the most sensitive assays. HIV can often be more easily detected in cells residing in lymphoid and mucosal tissues, but such tissues are hard to access, and it is unclear as to whether one should measure the frequency of virus in all cells, in all CD4+ T cells, or in all resting memory CD4+ T cells.
Despite these limitations, a number of consistent trends have emerged. There appears to be no consistent association between T-cell activation (as defined by CD38 and HLA-DR expression) and the level of HIV RNA in plasma during effective therapy. Although some small studies have suggested a positive correlation (120), the vast majority of studies have found no association (89, 121–125). These observations suggest that T-cell activation is unlikely to be major determinant of plasma HIV RNA levels and that whatever process causes release of HIV RNA into plasma is not related to a quantifiable level of activated T cells circulating in blood; also, these observations suggest that HIV production is probably only a minor determinant of the level of residual T-cell activation that is observed during treatment.
In contrast to the largely negative associations between T-cell activation and viremia in treated disease, there is a consistent association between T-cell activation and level of cell-associated HIV DNA or HIV RNA (89). This effect may be even more apparent in tissues, where the majority of the virus resides (126–129). Other markers of T-cell activation and dysfunction that remain elevated despite long-term effective therapy include PD-1 and CTLA-4 (89, 130). A positive correlation between PD-1-expressing CD4+ T cells and frequency of infected cells has also been noted in a few studies (89, 117).
Immune activation as a cause of HIV persistence during therapy
The mechanism for the consistent association between T-cell activation and cell-associated HIV burden during therapy is not known. Indeed, it is not clear if higher levels of T-cell activation cause higher levels of HIV burden or whether higher viral burdens cause higher levels of immune activation. As outlined in this and the following sections, it is likely that the both pathways are active during treated disease, and that a ‘vicious cycle’ might exist during treatment that results in maintenance of both immune activation and HIV persistence (Fig. 2).
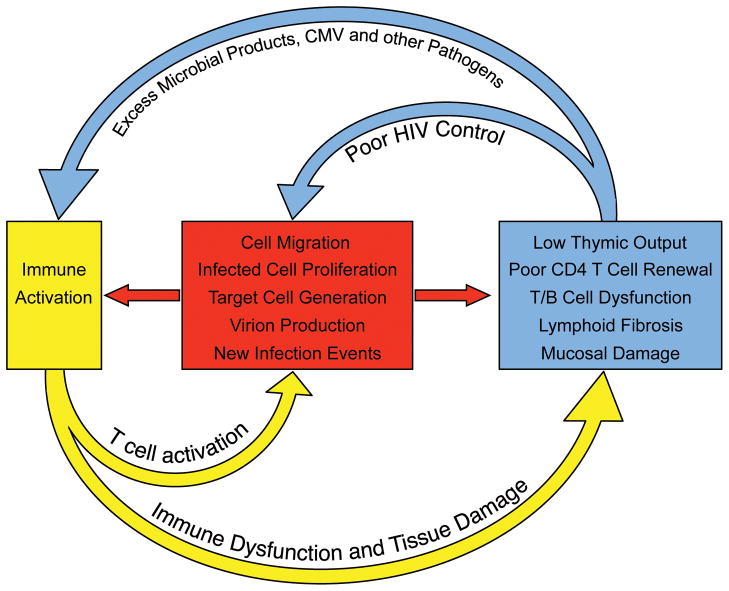
Fig. 2. Immune activation sustains HIV persistence during antiretroviral therapy.
HIV-associated damage to the lymphoid system is only partially reversible. During long-term antiretroviral therapy, the residual immune dysfunction (which is due in part to loss of thymic tissue, fibrosis in germinal centers of secondary lymphoid structure, hematopoietic stem cell loss, and mucosal barrier breakdown) results in immunodeficiency, excess amounts of pathogens and chronic immune activation. The immune activation in turn leads to migration of CD4+ T cells to foci of HIV replication, generation of activated and susceptible target cells, and production of virus from latently infected cells (all of which enable more efficient cell-to-cell spread of HIV and replenishment of infected cells). The inflammatory environment also leads to proliferation and maintenance of latently infected cells. The residual HIV replication/production in turn contributes to sustained tissue damage and immune activation.
Inflammation and target cell generation
Theoretically, persistently high levels of CD4+ T-cell activation during combination antiretroviral therapy may contribute to HIV persistence by continuously providing a pool of cellular targets for the virus to infect (131–133)(Figs 2 and 3). The recent observation that CD8+ T-cell activation during effective therapy predicts subsequent episodes of low-level detectable viremia is generally consistent with this possibility (125). In one early study of long-term treated adults, higher levels of HIV DNA were found in activated compared to resting CD4+ T cells; phylogenetic analyses suggested ongoing rounds of de novo infection events between these distinct populations (134). Similarly, it has been argued that the continuous production of HIV antigens from any cell source may lead to the generation of activated HIV-specific CD4+ T cells, which are being continually primed to migrate to foci of virus production, thereby providing the virus with a potential source of target cells. Although experimental data from such a model is lacking during treated disease, there are data from untreated individuals, which support this possibility (135, 136).
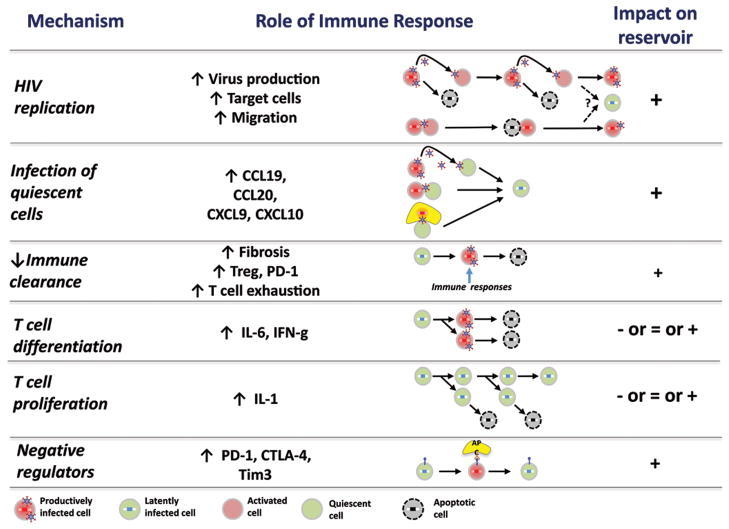
Fig. 3. Mechanisms by which immune activation causes HIV persistence.
The chronic immune dysfunction of antiretroviral-treated HIV infection contributes to HIV persistence by (1) enabling HIV replication via generation of activated CD4+ T cells, (2) enabling infection of resting cells, (3) reducing the capacity of the adaptive immune system to clear infected cells, (4) causing differentiation and proliferation of infected cells, and (5) increasing expression of cell-surface negative regulators, which in turn contributes to persistence of latently infected cells. Detailed knowledge regarding the mechanisms which contributes to each of these steps might lead to the development of immune-based therapeutics which could contribute to an HIV cure.
While resting CD4+ T cells are resistant to in vitro infection by HIV compared to activated CD4+ T cells, resting memory CD4+ T cells with integrated HIV DNA can be stimulated ex vivo and presumably in vivo to produce infectious virions (137–140). Multiple inflammatory stimuli can cause production of virus from resting cells, including many known to be elevated during treated HIV disease such as IL-2, TNF, IL-6, IL-12 and IL-18 (141–144). Furthermore, exposure to a combination of certain chemokines (i.e. CCL19 and CCL21) renders resting CD4+ T cells susceptible to infection and the establishment of latency ex vivo (144, 145). Many of these pro-inflammatory stimuli are known to remain elevated during treated HIV disease. While the role of these cytokines and chemokines in promoting infection and generation of latency in vivo is unknown, the increased permissibility to HIV infection that occurs on exposure to these cytokines/chemokines indicates that an inflammatory environment in the host might make CD4+ T cells more susceptible to infection(146).
Many of the activated T cells during untreated and perhaps treated HIV infection target herpes viruses. CMV-specific CD4+ and CD8+ T-cell responses, for example, are much higher in HIV-infected adults than age-matched uninfected adults (147). If these cells are preferentially activated, then they may be more likely to become infected and hence enriched for HIV during untreated and eventually treated disease. In one recent survey of untreated men presenting with early HIV infection, the presence of detectable CMV in semen or PBMCs was associated with higher HIV DNA content in PBMCs (148).
Although many have argued that activation-induced production of virus from latently infected cells might lead to their destruction and ultimately a cure (149–152), this hypothesis is dependent on HIV-producing cells dying through some clearance mechanisms and on all susceptible target cells being protected by antiretroviral therapy. Both of these assumptions are now being challenged (153).
Inflammation and migration of target cells to sites of HIV spread
HIV/SIV spread to new target cells is likely localized, with virions only able to infect cells which are nearby (154) (Figs 2 and 3). This is likely to be particularly true when other factors such as strong immunity (as seen in elite controllers) or antiretroviral therapy place additional constraints on HIV replication. Indeed, it has been argued that any residual replication of HIV during potent antiretroviral therapy will be via direct cell-to-cell contact, which allows such high concentrations of spreading virions that standard concentrations of antiretroviral drugs in cells fail to inhibit replication (155). The finding that raltegravir intensification reduced HIV levels and inflammation in lymphoid tissue-rich ileum but not in blood is consistent with this emerging model of HIV persistence (127).
HIV-associated damage to the mucosal barrier causes localized inflammation in gastrointestinal tract tissues (27, 61, 74). This inflammation drives migration of T cells to mucosal tissues, where the higher concentration of activated target cells should make HIV replication more efficient (49, 156, 157). The concentration of highly-susceptible gut-homing activatedα4β7high CD4+ T cells in mucosal surfaces likely contribute to development of an optimal environment for cell-to-cell spread (158, 159). Reduced penetration of certain antiretroviral drugs into these tissues might also allow localized rounds of HIV replication (160, 161). In addition, although the translocation of microbial products from a ‘leaky gut’ is reduced during antiretroviral therapy (26), abnormally high levels of bacterial products often persist and may contribute to HIV persistence by inducing production of pro-inflammatory cytokines known to enhance cell cycling and/or HIV replication (such as IL-1 and IFN)(129, 162). This may result in increased levels of residual viral replication and in the cycling of infected cells, thereby promoting viral spread and possibly persistence. Tissue-based studies of lymphoid tissue-rich areas of the gut will be needed to define the precise role of localized inflammatory environment in lymphoid structures of the gut as a cause of persistence.
Reeves and colleagues (163) have recently found that interferon-α-producing plasmacytoid dendritic cells (pDCs) accumulate in the gut mucosa during untreated SIV disease and that the level of viremia correlated with frequency of these cells (as defined by expression of retention integrinα4β7). They argued that pDC activation in mucosa might cause trafficking and retention of CCR5-expressing CD4+ T cells to foci of localized inflammation (perhaps by secretion of MIP-1β and other chemokines), thereby enabling more efficient transmission of virus. Similarly, Favre and colleagues (28, 29) have found that activation of mDCs results in upregulation of indoleamine 2,3-dioxygenase (IDO) and a shift in local T-cell populations, with loss of Th17 cells (which protect against microbial translocation and regulate epithelial cell regeneration) and increased Treg cells (which have complex effects, including potentially blunting local clearance mechanisms for HIV and other pathogens). Activation of this complex pathway appears to contribute to ongoing microbial translocation, which in turn activates dendritic cells, resulting in ongoing cycles of localized inflammation. Consistent with these various observations, Chege and colleagues (68) observed persistent loss of duodenal Th17 cells during effective antiretroviral therapy. The loss of these cells was associated with higher levels of microbial translocation (as expected), while higher levels of microbial translocation (as defined by plasma lipopolysaccharide levels) was associated with higher levels of HIV DNA in gut. The observed correlation between markers of microbial translocation, T-cell activation, and HIV DNA levels in the gut of treated individuals is largely consistent with this model (126, 129). The critical role of location in driving the impact of inflammation on HIV persistence is well illustrated by recent studies of T-follicular helper cells (Tfh cells). These cells largely reside in the germinal centers of lymph nodes, are defined phenotypically by the expression of CXCR5, PD-1, and Bcl-6, produce high levels of IL-21, and regulate antibody development by nearby B cells. The chronic inflammatory response to SIV infection appears to drive the expansion and activation of these cells in lymph nodes (164). In untreated SIV and HIV disease, the frequency of activated Tfh cells appears to be expanded (165), and these cells appear to be enriched for HIV DNA (as compared to other CD4+ T-cell populations)(57, 166, 167). The degree to which this process persists during long-term effective antiretroviral therapy is not known.
The theoretical model that inflammation-associated release of chemokines could lead to migration of CD4+ T cells to these foci of inflammation was the rationale for at least one of the maraviroc intensification studies (168). Other interventions aimed specifically at addressing these mucosa-based pathways are in development, including the potential use of IDO inhibitors and inhibitors of the interferon-αpathway(169).
Inflammation and the immunoregulatory response
Successful integration of HIV DNA into genomes of resting memory T cells occurs very early during acute infection (170–172). Acute HIV infection is associated with a potent inflammatory response marked by the production of excess amounts of a number of pro-inflammatory cytokines, including interferon-α, interferon-γ, TNF, IL-6, IL-8, IL-15, and CXCL10 (173, 174). This inflammatory response causes a potent and sustained immunoregulatory response, with the production of cytokines such as IL-10 observed very early during acute infection (173). Treg cell numbers and responses also occur, which along with other cells release strong anti-inflammatory cytokines such as transforming growth factor-β (TGF-β). These immunoregulatory responses to chronic inflammation persist during untreated chronic infection and even during long-term effective antiretroviral therapy (175–177).
These anti-inflammatory responses may contribute to establishment and maintenance of latent infection. IL-10 might inhibit T-cell activation, allowing resting cells containing integrated DNA to persist indefinitely (178). Upregulation of negative regulators of T-cell activation, which are aimed at containing the inflammatory response and preventing tissue damage, may act to prevent recently infected cells from dying, leading to persistence. The observation that PD-1-expressing CD4+ cells are enriched for HIV and associated with reservoir size is consistent with this hypothesis (89, 117). Inflammation-associated upregulation of Treg cells and local release of TGF-β may initiate a cascade of events in lymphoid tissues resulting in collagen deposition, lymphoid fibrosis, and irreversible immune dysfunction (179, 180), leading to a chronic inflammatory state and persistent HIV through multiple mechanisms outlined in this review.
Inflammation and dysfunction of adaptive and innate immune responses
Not all HIV-mediated immune responses are harmful. CD8+ T-cell responses are the quintessential immune cell that protects from virus replication. Although HIV-specific cytotoxic activities are decreased or dysfunctional during chronic untreated disease, a small subset of individuals appear to effectively control their virus in the absence of therapy due to the generation and preservation of effective immunity (44, 181–184). Polyfunctional CD8+ T-cell responses and proliferation capacity are consistent correlates of virus control (185, 186). Certain ‘protective’ class I HLA alleles such as B5701 are highly enriched in controllers, providing strong evidence for positive role of active CTL activation in virus control (186–189), although HLA may be mediating its effect via other mechanisms, including directing the activity of NK cells and other immune responses (190, 191).
CD4+ T-helper cell responses are also vital to adaptive immunity against infections. Although less well characterized than CD8+ T-cell responses, HIV-specific CD4+ T-cell function and proliferation seems to predict virus control in the absence of therapy (133, 192). This beneficial effect may be mediated through direct killing/control of the virus, or indirectly through enhanced CD8+ T-cell and B-cell activities (53, 54, 57, 193). More recently, chronic activation and HIV/SIV infection of CD4+ Tfh in lymphoid tissues has been recently demonstrated to result in dysfunctional B-cell responses (57, 194, 195).
Although the influence of the adaptive immune response on HIV replication in the absence of combination antiretroviral therapy has been extensively studied, surprising few studies have focused on the impact of these responses during therapy. In the early combination antiretroviral therapy era, a substantial proportion of treated patients failed to achieve complete viral suppression. Many of these ‘virologic failures’ were able to maintain partial suppression of viral replication for months to years, even as high-level drug resistance emerged. Strong HIV-specific CD8+ and CD4+ T cells were often present in such individuals, with levels that were in comparable to those observed in elite controllers, suggesting that the adaptive immune response could contribute to virus control virus when used with combination antiretroviral therapy (196–198).
There has as of yet been no comprehensive assessment of the role of HIV-specific immunity in determining the size of the reservoir during combination antiretroviral therapy. In one cross-sectional analysis of individuals of long-term treated adults with undetectable viremia who were enrolled in a treatment intensification study, strong HIV-specific CD8+ and CD4+ T cells (as defined by the co-expression of IL-2 and interferon-γ) in the rectal mucosa was associated with lower frequency of infected cells (122). T-cell vaccine-mediated reduction in frequency of infected cells in one study also argues that effective adaptive immunity during therapy may affect the size of the reservoir (199), perhaps because a greater than expected proportion of ‘resting’ cells make low levels of HIV proteins (200).
Another consequence of chronic inflammation that is likely to contribute to HIV persistence is the deleterious impact that immune activation has on HIV-specific T-cell responses (Figs 2 and 3). Indeed, it is well described that chronic exposure to antigens leads to T-cell exhaustion (43, 45, 47). Although HIV-specific CD8+ T cells that persist after prolonged combination antiretroviral therapy may regain some function (201, 202), it is clear that their frequency is extremely low and that they may not migrate or persist in the compartments in which HIV replication still occurs (203). In a recent study, Shan and colleagues (153) demonstrated that HIV-specific cytotoxic T lymphocytes (CTLs) from the blood of virally suppressed subjects are inefficient at eliminating CD4+ T cells in which HIV replication occurs. Of note, the killing capacity of these cells may be restored after in vitro stimulation with HIV peptides (204). This observation suggests that the extremely low frequency of HIV-specific CD8+ T cells after prolonged therapy is unable to control residual levels of HIV replication and that, assuming the problem of virus epitope escape can be surmounted, strategies aiming at increasing these frequencies (through vaccination for example) may be needed to eliminate or at least control the small pool of productively infected CD4+ T cells that persists during combination antiretroviral therapy.
The inflammatory environment of treated HIV infection stimulates a compensatory response aimed at blunting any inflammation-associated harm. For example, HIV-associated inflammation causes increased numbers of Treg cells(205). During HIV infection, Tregs are dysfunctional and accumulate in high numbers, particularly compared to T-cell subsets such as Th17 cells (28, 29, 206). The increased levels of Tregs may, in turn, suppress the capacity of the adaptive immune system to clear virus.
Another potential driver of HIV latency may be inappropriate antigen presentation and innate immune cell function due to immune activation. As discussed above, several antigen-presenting cells, including monocytes/macrophages, B cells, and dendritic cells, are dysregulated, hyper-activated, and exhausted during HIV infection (55, 56, 62, 63, 108, 207, 208). Given that antigen-presenting cells are responsible for inducing antigen-specific responses in T cells via major histocompatibility complex (MHC):T-cell receptor (TCR) interactions, dysfunctionality of these cells may lead to inappropriate activation and exhaustion of T cells.
Another innate immune system factor that may affect HIV persistence is the relationship between restriction factors and innate immune activation. Recent studies have demonstrated that restriction factors (e.g. TRIM5α and APOBEC3G) play a role in the innate immune response to HIV and may alter the viral replication life cycle in this process (209–212). Thus, restriction factor inhibition of complete HIV replication may result in non-productively or latently infected cells, which may later be inducible to re-establish productive HIV replication. While preliminary evidence exists for the mechanisms described here, these are hypothetical ideas for how immune activation may induce and maintain HIV persistence and latency, and further studies are required.
Common γ chain cytokines and HIV persistence
IL-7-induced cycling of CD4+ T cells (which is distinct from the T-cell proliferation induced by inflammatory cytokines discussed above) has also been associated with reservoir size during long-term effective therapy. In one study of long-term treated adults, the frequency of CD4+ T cells expressing Ki67 (a cell cycle marker) but not the frequency of cells expressing classical activation markers such as CD25, HLA-DR, and CD71 was significantly associated with frequency of CD4+ T cells harboring HIV DNA (117). Subjects with low CD4+ T-cell counts and higher levels of IL-7-mediated proliferation had higher levels of Ki67 expressing cells. They also had levels of HIV DNA in those cell populations which emerge from such proliferation events (i.e. transitional memory CD4+ T cells)(117). In a related analysis from a separate cohort, a lower pre-treatment CD4+ T-cell count nadir was associated with higher frequency of infected cells, a finding that is consistent with a model in which homeostatic proliferation of CD4+ T cells contributes to HIV persistence(213).
The stability of the TCM reservoir is ensured by the intrinsic capacity of these cells to survive for decades and to self-renew upon antigenic stimulation (Figs 2 and 3) (116). The survival of TCM cells depends, at least in part, on the activation and phosphorylation of signal transducer and activator of transcription 5a (STAT5a) and FOXO3a. Signaling via both the TCR and γ-chain (γc) cytokine receptors (such as CD127, the receptor for IL-7) leads to FOXO3a phosphorylation and drives the survival of TCM cells. The TTM reservoir also appears to be maintained in part by the effect of IL-7 on homeostatic proliferation, a natural mechanism ensuring the long-term persistence of immunological memory. The importance of this mechanism for the persistence of latently infected CD4+ T cells was originally predicted by a mathematical model (214) and is now supported by several studies that indicate a role for this cytokine in the maintenance of a stable pool of latently infected cells.
The impact which IL-7 might have on the latent reservoir during effective therapy is the focus of intense interest. IL-7 is produced in lymphoid organs by stromal cells, which have yet to be fully characterized. In a recent study, fibroblastic reticular cells and lymphatic endothelial cells were identified as the major producers of IL-7 during lymph node remodeling after viral infection in mice and humans (220). Unlike many other cytokines that act on lymphocytes, IL-7 production by stromal cells is not substantially affected by extrinsic stimuli. The amount of available IL-7 protein is thought to be regulated by the rate that it is scavenged by T cells (221). In states of chronin lymphopenia (such as HIV disease), less consumption of IL-7 leads to higher levels of this cytokine. As a consequence, the remaining T cells encounter abundant IL-7, which induces expansion in the depleted niche. In HIV disease, this model of ‘regulation through consumption’ is supported by the strong negative correlation between CD4+ T-cell counts and plasma IL-7 levels (217) and by the high frequencies of CD4+ T cells undergoing cell proliferation in subjects displaying abnormally low CD4+ T-cell counts, independently of their plasma viremia (42, 222).
Although the impact of such a mechanism on the pool of reservoir cells is still unclear, it is likely that IL-7 not only expands uninfected T cells but also expands T cells harboring integrated HIV DNA. In vitro, physiological concentrations of IL-7 induce homeostatic proliferation of latently infected cells without viral production (223), suggesting that IL-7 does not disrupt viral latency as originally proposed (224, 225) but rather induces proliferation of latently infected cells. In addition, a recent study examining the sequences of viruses recovered during viral blip episodes upon IL-7 administration showed that these viral particles reflect predominantly transient induction of virus from a pre-existing pool of productively infected cells rather than activation of silent quasispecies from stable reservoirs (226). In line with this model, incomplete T-cell recovery and elevated IL-7 levels would be predicted to cause increased levels of T-cell proliferation and with stability of the HIV reservoir in its size and genetic diversity over time (117). These findings collectively argue that T-cell division of latently infected cells in the absence of viral production is likely to be a major mechanism contributing to the restoration of the CD4+ T-cell compartment and to the persistence of a pool of reservoir cells during suppressive combination antiretroviral therapy.
Other cytokines involved in the maintenance of memory CD4+ T cells may contribute to HIV persistence by promoting survival and/or proliferation of latently infected cells during treatment: Several γc cytokines (IL-2, IL-7, IL-15 and IL-21) have been shown to induce the expression of PD-1 at the surface of T cells (227), a marker associated with CD8+ T-cell dysfunction that identifies HIV infected cells in the CD4+ T-cell compartment in treated and untreated lentiviral infections (117, 228, 229). In addition to their potential role in the establishment and maintenance viral latency, these cytokines are important immunomodulators and may contribute to the control of residual levels of viral replication during antiretroviral therapy by enhancing antiviral T-cell responses, particularly in tissue reservoirs. For instance, administration of IL-15 to SIV-infected macaques has originally been shown to induce CD4+ effector memory T-cell production and tissue emigration (230). However, a recent study in which the cytokine was administered concomitantly with combination antiretroviral therapy indicates that although IL-15 is able to transiently promote the proliferation of antigen-specific CD8+ T cells in the peripheral blood, it failed to boost antiretroviral treatment-induced CD4+ T-cell recovery both in the blood and in peripheral tissues and delayed viral suppression (231). Taken together, these studies suggest that IL-15 may have a positive impact on antiviral T-cell responses when viremia is fully suppressed by combination antiretroviral therapy, whereas the administration of the cytokine may have no impact or even a deleterious effect when residual levels of viral replication persist. These IL-15 studies emphasize the difficulty to predict the beneficial of deleterious effect of a γc cytokine-based therapy during combination antiretroviral therapy and suggest that the degree of viral suppression may be a critical parameter to monitor before initiating such therapies.
Impact of immune-based therapeutics on HIV persistence
The consistent observation that immune activation and measures of HIV persistence are positively correlated is intriguing and consistent with a number of theoretical models. However, establishing causal biological pathways to explain these associations in humans will require controlled clinical trials in which the pathways are interrupted or enhanced in a precise manner, and the size of the reservoir measured. The recent development of effective antiretroviral treatment regimens for non-human primates allows this model to be used to explore the role of agents aimed at interrupting the inflammatory response on viral persistence. For example, probiotic/prebiotic supplementation of combination antiretroviral therapy decreased the frequency of cycling CD4+ T cells in the colon of SIV-infected macaques; although not tested, this effect would be expected to result in reduce levels of SIV persistence (232). Many such studies in nonhuman primates are now being developed.
Defining the role of host environment in HIV persistence will ultimately require performance of controlled clinical trials in which long-term treated individuals receive an immune-based therapeutic and the impact of this intervention on HIV persistence quantified. Several questions remain as to the optimal design of such studies. How long should antiretroviral treatment be administered before enrolling subjects? Will response to such regimens differ in those individuals treated during acute versus chronic infection? How should HIV persistence be quantified? Which tissues (if any) need to be sampled? When should primary outcomes be measured? Will promising agents that might work in only in combinations with other modalities be allowed to move forward in combination regimens even if monotherapy studies fail to detect an effect? Finally, given that HIV is generally no longer a fatal disease in the setting of antiretroviral therapy, ethical questions will likely arise as to the benefit conferred by using potentially toxic interventions to manipulate the immune response in an attempt to cure an infected individual.
HIV persistence as cause of immune activation
As emphasized throughout this review, the directionality of any observed associations between HIV persistence and immune activation is difficult to define in vivo. Although the focus of this review is how immune activation might contribute to HIV persistence, HIV production/replication can cause immune activation through several mechanisms (Figs 1–3). Most of the activated CD4+ T cells during untreated disease are not HIV-specific. More specifically, a higher than expected frequency of activated cells are specific for persistent herpes virus antigens (147, 233). Mechanistically, it has been proposed that HIV activates dendritic cells and that these cells are more likely to activate CD4+ T cells in the presence of commonly prevalent antigens, such as those associated with Herpes simplex virus, Epstein-Barr virus, and CMV (234). The remarkably high levels of CMV-specific T cells in untreated and treated HIV infection is consistent with this model (147, 235, 236). These observations might argue that much of the latent reservoir during combination antiretroviral therapy is in herpes-specific T cells, a hypothesis which should be testable.
The strongest evidence that HIV causes immune activation during therapy comes from recent therapy intensification studies. Although some studies have failed to find an impact of intensification on T-cell activation in blood or rectal mucosa (122, 237), one study found that the addition of raltegravir reduced the frequencies of infected cells and of activated cells in ileum (which is densely populated with T cells)(127, 128). In one randomized study of raltegravir intensification, a decline in T-cell activation was more readily detectable in those individuals who exhibited a virologic response to the intervention (238, 239). Other studies have found an impact of raltegravir intensification on immune activation (240, 241). These data provide compelling evidence that HIV persistence during antiretroviral therapy causes immune activation, although a larger more definitive study will be needed to address remaining uncertainty on this issue.
The lack of clear temporal evolution in viral sequences during suppressive therapy reported in some studies (242, 243) as well as the failure of most clinical trials to demonstrate an appreciable effect of combination antiretroviral therapy intensification on HIV persistence (122, 244–246) suggest that persistent low levels of HIV RNA in plasma and tissues primarily reflect continuous production from stable reservoir. However, increased levels of 2-LTR circles (238) as well as a significant reduction in the amount of cell associated RNA in the gut of combination antiretroviral therapy subjects upon intensification with raltegravir (127) suggest that complete cycles of viral replication may still occur during combination antiretroviral therapy, at least in a subset of individuals. Novel therapeutic strategies aimed at abrogating these low levels of viral replication/production are clearly needed. For instance, selecting a combination of antiretroviral drugs endowed with the ability to penetrate in anatomical reservoirs such as the CNS and the gut may prove to be beneficial in reducing residual replication and inflammation in virally suppressed subjects.
Conclusion
Despite the effectiveness of combination antiretroviral therapy in suppressing virus replication, immunological abnormalities persist, even after years of suppressive therapy (5, 7, 247–249). Combination antiretroviral therapy does not always restore normal CD4+ T-cell counts, and in a substantial fraction of virally suppressed subjects, levels of immune activation remain elevated compared to uninfected individuals (216, 250). The association observed between residual levels of immune activation and viral persistence suggests that these two phenomena may be reciprocally connected (216, 251). However, whether immune activation is a cause or a consequence of HIV persistence is still unclear. It is likely to be both.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (R01 AI087145, K24 AI069994), the DARE: Delaney AIDS Research Enterprise (DARE; U19AI096109), the American Foundation for AIDS Research (amfAR), and the Intramural Program of the National Institute of Allergy and Infectious Diseases.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Valdez H, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–1866. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 2.Aiuti F, Mezzaroma I. Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under HAART. AIDS Rev. 2006;8:88–97. [PubMed] [Google Scholar]
- 3.Kelley CF, et al. Incomplete Peripheral CD4(+) Cell Count Restoration in HIV-Infected Patients Receiving Long-Term Antiretroviral Treatment. Clin Infect Dis. 2009;48:787–794. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009;200:1212–1215. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- 5.Hunt PW, et al. T Cell Activation Is Associated with Lower CD4+ T Cell Gains in Human Immunodeficiency Virus-Infected Patients with Sustained Viral Suppression during Antiretroviral Therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 6.Neuhaus J, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchetti G, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 8.Lederman MM, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes TL, et al. Impact of highly active antiretroviral therapy initiation on CD4+ T-cell repopulation in duodenal and rectal mucosa. AIDS. 2013 doi: 10.1097/QAD.0b013e32835d85b4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tincati C, et al. Early initiation of highly active antiretroviral therapy fails to reverse immunovirological abnormalities in gut-associated lymphoid tissue induced by acute HIV infection. Antivir Ther. 2009;14:321–330. [PubMed] [Google Scholar]
- 11.Gordon SN, et al. Disruption of intestinal CD4+ T cell homeostasis is a key marker of systemic CD4+ T cell activation in HIV-infected individuals. J Immunol. 2010;185:5169–5179. doi: 10.4049/jimmunol.1001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estes J, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198:456–464. doi: 10.1086/590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guadalupe M, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lederman MM, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange CG, Valdez H, Medvik K, Asaad R, Lederman MM. CD4+ T-lymphocyte nadir and the effect of highly active antiretroviral therapy on phenotypic and functional immune restoration in HIV-1 infection. Clin Immunol. 2002;102:154–161. doi: 10.1006/clim.2001.5164. [DOI] [PubMed] [Google Scholar]
- 17.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22:2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodger AJ, et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009;200:973–983. doi: 10.1086/605447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuller LH, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt PW, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25:2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandler NG, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulware DR, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203:1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledwaba L, et al. Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS One. 2012;7:e24243. doi: 10.1371/journal.pone.0024243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol. 2008;20:181–186. doi: 10.1016/j.smim.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt PW, et al. Valganciclovir Reduces T Cell Activation in HIV-infected Individuals With Incomplete CD4+ T Cell Recovery on Antiretroviral Therapy. J Infect Dis. 2011;203:1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 27.Klatt NR, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012;5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favre D, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Favre D, et al. Critical Loss of the Balance between Th17 and T Regulatory Cell Populations in Pathogenic SIV Infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klatt NR, Brenchley JM. Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS. 2010;5:135–140. doi: 10.1097/COH.0b013e3283364846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deeks SG, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12:607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottlieb MS, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305:1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Cumberland WG, Hultin LE, Kaplan AH, Detels R, Giorgi JV. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:332–340. doi: 10.1097/00042560-199808010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Deeks SG, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 35.Mellors JW, et al. Prognostic value of HIV-1 RNA, CD4 cell count, and CD4 Cell count slope for progression to AIDS and death in untreated HIV-1 infection. JAMA. 2007;297:2349–2350. doi: 10.1001/jama.297.21.2349. [DOI] [PubMed] [Google Scholar]
- 36.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. Natural SIV hosts: showing AIDS the door. Science. 2012;335:1188–1193. doi: 10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenchley JM, et al. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 2012;120:4172–4181. doi: 10.1182/blood-2012-06-437608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenchley JM, Silvestri G, Douek DC. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity. 2010;32:737–742. doi: 10.1016/j.immuni.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan RC, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasula S, et al. Differential effects of HIV viral load and CD4 count on proliferation of naive and memory CD4 and CD8 T lymphocytes. Blood. 2011;118:262–270. doi: 10.1182/blood-2011-02-335174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catalfamo M, et al. CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. J Immunol. 2011;186:2106–2116. doi: 10.4049/jimmunol.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Catalfamo M, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci USA. 2008;105:19851–19856. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 44.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Souza M, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 46.Estes JD, et al. Early Resolution of Acute Immune Activation and Induction of PD-1 in SIV-Infected Sooty Mangabeys Distinguishes Nonpathogenic from Pathogenic Infection in Rhesus Macaques. J Immunol. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dyavar Shetty RD, et al. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest. 2012;122:1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garber DA, et al. Blockade of T cell costimulation reveals interrelated actions of CD4+ and CD8+ T cells in control of SIV replication. J Clin Invest. 2004;113:836–845. doi: 10.1172/JCI19442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji J, Cloyd MW. HIV-1 binding to CD4 on CD4+CD25+ regulatory T cells enhances their suppressive function and induces them to home to, and accumulate in, peripheral and mucosal lymphoid tissues: an additional mechanism of immunosuppression. Int Immunol. 2009;21:283–294. doi: 10.1093/intimm/dxn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nigam P, et al. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J Immunol. 2010;184:1690–1701. doi: 10.4049/jimmunol.0902955. [DOI] [PubMed] [Google Scholar]
- 51.Dunham RM, et al. CD127 and CD25 Expression Defines CD4+ T Cell Subsets That Are Differentially Depleted during HIV Infection. J Immunol. 2008;180:5582–5592. doi: 10.4049/jimmunol.180.8.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moir S, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci USA. 2001;98:10362–10367. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moir S, et al. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc Natl Acad Sci USA. 2003;100:6057–6062. doi: 10.1073/pnas.0730819100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malaspina A, et al. Compromised B cell responses to influenza vaccination in HIV- infected individuals. J Infect Dis. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 55.Moir S, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klatt NR, et al. SIV infection of rhesus macaques results in dysfunctional T- and B-cell responses to neo and recall Leishmania major vaccination. Blood. 2011;118:5803–5812. doi: 10.1182/blood-2011-07-365874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Grevenynghe J, et al. Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL-mediated apoptosis. J Clin Invest. 2011;121:3877–3888. doi: 10.1172/JCI59211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dawood MR, et al. Association of phenotypic changes in B cell lymphocytes and plasma viral load in human immunodeficiency virus-infected patients. J Clin Immunol. 1998;18:235–240. doi: 10.1023/a:1020539207593. [DOI] [PubMed] [Google Scholar]
- 60.Connolly NC, Riddler SA, Rinaldo CR. Proinflammatory cytokines in HIV disease-a review and rationale for new therapeutic approaches. AIDS Rev. 2005;7:168–180. [PubMed] [Google Scholar]
- 61.Estes JD, et al. Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections. PLoS Pathog. 2010;6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wallet MA, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS. 2010;24:1281–1290. doi: 10.1097/QAD.0b013e328339e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reeves RK, et al. SIV infection induces accumulation of plasmacytoid dendritic cells in the gut mucosa. J Infect Dis. 2012;206:1462–1468. doi: 10.1093/infdis/jis408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwa S, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2011;118:2763–2773. doi: 10.1182/blood-2011-02-339515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alter G, et al. Increased natural killer cell activity in viremic HIV-1 infection. J Immunol. 2004;173:5305–5311. doi: 10.4049/jimmunol.173.8.5305. [DOI] [PubMed] [Google Scholar]
- 66.Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. CD16- natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehandru S, et al. Lack of Mucosal Immune Reconstitution during Prolonged Treatment of Acute and Early HIV-1 Infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chege D, et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS. 2011;25:741–749. doi: 10.1097/QAD.0b013e328344cefb. [DOI] [PubMed] [Google Scholar]
- 69.Mavigner M, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest. 2012;122:62–69. doi: 10.1172/JCI59011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 71.Veazey RS, et al. Gastrointestinal Tract as a Major Site of CD4+ T Cell Depletion and Viral Replication in SIV Infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 72.Klatt NR, et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2010;3:387–398. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Canary LA, et al. Rate of AIDS Progression is Associated with Gastrointestinal Dysfunction in SIV-infected Pigtail Macaques. J Immunol. 2013 doi: 10.4049/jimmunol.1202319. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nigam P, Kwa S, Velu V, Amara RR. Loss of IL-17-Producing CD8 T Cells during Late Chronic Stage of Pathogenic SIV Infection. J Immunol. 2011;186:745–753. doi: 10.4049/jimmunol.1002807. [DOI] [PubMed] [Google Scholar]
- 77.Reeves RK, et al. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood. 2011;118:3321–3330. doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol. 2012;5:658–669. doi: 10.1038/mi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugimoto K, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanos SL, Vonarbourg C, Mortha A, Diefenbach A. Control of epithelial cell function by interleukin-22-producing RORgammat+ innate lymphoid cells. Immunology. 2011;132:453–465. doi: 10.1111/j.1365-2567.2011.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guglani L, Khader SA. Th17 cytokines in mucosal immunity and inflammation. Curr Opin HIV AIDS. 2010;5:120–127. doi: 10.1097/COH.0b013e328335c2f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 84.Brenchley JM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paiardini M. Th17 cells in natural SIV hosts. Curr Opin HIV AIDS. 2010;5:166–172. doi: 10.1097/COH.0b013e328335c161. [DOI] [PubMed] [Google Scholar]
- 86.Robbins GK, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48:350–361. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaplan RC, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tassiopoulos K, et al. CD28-negative CD4+ and CD8+ T cells in antiretroviral therapy-naive HIV-infected adults enrolled in adult clinical trials group studies. J Infect Dis. 2012;205:1730–1738. doi: 10.1093/infdis/jis260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hatano H, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2012 doi: 10.1093/infdis/jis630. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sauce D, et al. HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood. 2011;117:5142–5151. doi: 10.1182/blood-2011-01-331306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 92.Lichtfuss GF, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol. 2012;189:1491–1499. doi: 10.4049/jimmunol.1200458. [DOI] [PubMed] [Google Scholar]
- 93.Hearps AC, et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 2012;26:843–853. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- 94.Burdo TH, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burdo TH, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller EA, et al. Plasma factors during chronic HIV-1 infection impair IL-12 secretion by myeloid dendritic cells via a virus-independent pathway. J Acquir Immune Defic Syndr. 2012;61:535–544. doi: 10.1097/QAI.0b013e31826afbce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chehimi J, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 98.Finney CA, et al. HIV infection deregulates innate immunity to malaria despite combination antiretroviral therapy. AIDS. 2013;27:325–335. doi: 10.1097/QAD.0b013e32835b3dfa. [DOI] [PubMed] [Google Scholar]
- 99.Lichtfuss GF, Hoy J, Rajasuriar R, Kramski M, Crowe SM, Lewin SR. Biomarkers of immune dysfunction following combination antiretroviral therapy for HIV infection. Biomark Med. 2011;5:171–186. doi: 10.2217/bmm.11.15. [DOI] [PubMed] [Google Scholar]
- 100.Torre D, Pugliese A. Interleukin 18 and cardiovascular disease in HIV-1 infection: a partner in crime? AIDS Rev. 2010;12:31–39. [PubMed] [Google Scholar]
- 101.Iannello A, et al. Potential role of IL-18 in the immunopathogenesis of AIDS, HIV-associated lipodystrophy and related clinical conditions. Curr HIV Res. 2010;8:147–164. doi: 10.2174/157016210790442713. [DOI] [PubMed] [Google Scholar]
- 102.Monsuez JJ, Escaut L, Teicher E, Charniot JC, Vittecoq D. Cytokines in HIV-associated cardiomyopathy. Int J Cardiol. 2007;120:150–157. doi: 10.1016/j.ijcard.2006.11.143. [DOI] [PubMed] [Google Scholar]
- 103.Pandrea I, et al. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood. 2012;120:1357–1366. doi: 10.1182/blood-2012-03-414706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Funderburg NT, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mayne E, et al. Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression. J Acquir Immune Defic Syndr. 2012;59:340–346. doi: 10.1097/QAI.0b013e3182439355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sandler NG, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marchetti G, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 2011;25:1385–1394. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 108.Funderburg NT, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baker JV, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56:36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McComsey GA, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. Aids. 2012;26:1371–1385. doi: 10.1097/QAD.0b013e328354f4fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eastburn A, et al. Association of low level viremia with inflammation and mortality in HIV-infected adults. PLoS One. 2011;6:e26320. doi: 10.1371/journal.pone.0026320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 113.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 114.Chun TW, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 116.Riou C, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Siliciano JD, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 119.Chun TW, et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J Clin Invest. 2005;115:3250–3255. doi: 10.1172/JCI26197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mavigner M, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One. 2009;4:e7658. doi: 10.1371/journal.pone.0007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steel A, et al. HIV-1 viral replication below 50 copies/ml in patients on antiretroviral therapy is not associated with CD8+ T-cell activation. Antivir Ther. 2007;12:971–975. [PubMed] [Google Scholar]
- 122.Hatano H, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011;203:960–968. doi: 10.1093/infdis/jiq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chun TW, et al. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2011;204:135–138. doi: 10.1093/infdis/jir208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gandhi RT, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010:7. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taiwo B, et al. CD8+ T-cell activation in HIV-1-infected patients experiencing transient low-level viremia during antiretroviral therapy. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0b013e3182895af4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sheth PM, et al. Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal Immunol. 2008;1:382–388. doi: 10.1038/mi.2008.23. [DOI] [PubMed] [Google Scholar]
- 127.Yukl SA, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24:2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yukl SA, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202:1553–1561. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.d’Ettorre G, et al. HIV persistence in the gut mucosa of HIV-infected subjects undergoing antiretroviral therapy correlates with immune activation and increased levels of LPS. Curr HIV Res. 2011;9:148–153. doi: 10.2174/157016211795945296. [DOI] [PubMed] [Google Scholar]
- 130.Stone SF, Price P, French MA. Dysregulation of CD28 and CTLA-4 expression by CD4 T cells from previously immunodeficient HIV-infected patients with sustained virological responses to highly active antiretroviral therapy. HIV Med. 2005;6:278–283. doi: 10.1111/j.1468-1293.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 131.Douek DC, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 132.Ribeiro RM, Mohri H, Ho DD, Perelson AS. In vivo dynamics of T cell activation, proliferation, and death in HIV-1 infection: why are CD4+ but not CD8+ T cells depleted? Proc Natl Acad Sci USA. 2002;99:15572–15577. doi: 10.1073/pnas.242358099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klatt NR, Silvestri G. CD4+ T Cells and HIV: A Paradoxical Pas de Deux. Sci Trans Med. 2012;4:123ps124. doi: 10.1126/scitranslmed.3003862. [DOI] [PubMed] [Google Scholar]
- 134.Chun TW, et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J Clin Invest. 2005;115:3250–3255. doi: 10.1172/JCI26197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Douek DC, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 136.Hunt PW, et al. HIV-specific CD4+ T cells may contribute to viral persistence in HIV controllers. Clin Infect Dis. 2011;52:681–687. doi: 10.1093/cid/ciq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 138.Unutmaz D, KewalRamani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vandergeeten C, Fromentin R, Chomont N. The role of cytokines in the establishment, persistence and eradication of the HIV reservoir. Cytokine Growth Factor Rev. 2012;23:143–149. doi: 10.1016/j.cytogfr.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Finzi D, Siliciano RF. Viral dynamics in HIV-1 infection. Cell. 1998;93:665–671. doi: 10.1016/s0092-8674(00)81427-0. [DOI] [PubMed] [Google Scholar]
- 141.Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saleh S, et al. Expression and reactivation of HIV in a chemokine induced model of HIV latency in primary resting CD4+ T cells. Retrovirology. 2011;8:80. doi: 10.1186/1742-4690-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iannello A, et al. HIV-1 causes an imbalance in the production of interleukin-18 and its natural antagonist in HIV-infected individuals: implications for enhanced viral replication. J Infect Dis. 2010;201:608–617. doi: 10.1086/650314. [DOI] [PubMed] [Google Scholar]
- 144.Cameron PU, et al. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc Natl Acad Sci USA. 2010;107:16934–16939. doi: 10.1073/pnas.1002894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood. 2007;110:4161–4164. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- 146.van Grevenynghe J, et al. Foxo3a: an integrator of immune dysfunction during HIV infection. Cytokine Growth Factor Rev. 2012;23:215–221. doi: 10.1016/j.cytogfr.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Naeger DM, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5:e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gianella S, et al. CMV DNA in semen and blood is associated with higher levels of proviral HIV DNA. J Infect Dis. 2013;207:898–902. doi: 10.1093/infdis/jis777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chun TW, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti- retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 150.Kulkosky J, et al. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J Infect Dis. 2002;186:1403–1411. doi: 10.1086/344357. [DOI] [PubMed] [Google Scholar]
- 151.Prins JM, et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1- infected patients on potent antiretroviral therapy. AIDS. 1999;13:2405–2410. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 152.Lafeuillade A, et al. Pilot study of a combination of highly active antiretroviral therapy and cytokines to induce HIV-1 remission. J Acquir Immune Defic Syndr. 2001;26:44–55. doi: 10.1097/00126334-200101010-00006. [DOI] [PubMed] [Google Scholar]
- 153.Shan L, et al. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grossman Z, Feinberg MB, Paul WE. Multiple modes of cellular activation and virus transmission in HIV infection: a role for chronically and latently infected cells in sustaining viral replication. Proc Natl Acad Sci USA. 1998;95:6314–6319. doi: 10.1073/pnas.95.11.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sigal A, et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–98. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 156.Wacleche VS, et al. The colocalization potential of HIV-specific CD8+ and CD4+ T-cells is mediated by integrin beta7 but not CCR6 and regulated by retinoic acid. PLoS One. 2012;7:e32964. doi: 10.1371/journal.pone.0032964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang X, et al. Monitoring alpha4beta7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal CD4+ T-cell loss in SIV infection. Mucosal Immunol. 2009;2:518–526. doi: 10.1038/mi.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arthos J, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 159.Cicala C, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci USA. 2009;106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Patterson KB, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re114. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bourry O, et al. Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology. 2010;7:78. doi: 10.1186/1742-4690-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ciccone EJ, et al. Cycling of gut mucosal CD4+ T cells decreases after prolonged anti-retroviral therapy and is associated with plasma LPS levels. Mucosal Immunol. 2010;3:172–181. doi: 10.1038/mi.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reeves RK, et al. SIV infection induces accumulation of plasmacytoid dendritic cells in the gut mucosa. J Infect Dis. 2012;206:1462–1468. doi: 10.1093/infdis/jis408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Petrovas C, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lindqvist M, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fukazawa Y, et al. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med. 2012;18:1673–1681. doi: 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Perreau M, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hunt PW, et al., editors. The Immunomodulatory Effects of Maraviroc Intensification in HIV-infected Individuals with Incomplete CD4+ T Cell Recovery during Suppressive ART. Program and Abstracts of the 18th Conference on Retroviruses and Opportunistic Infections; February 27–March 2, 2011; Boston, MA, USA. p. Abstract 153LB. [Google Scholar]
- 169.Dunham RM, et al. Preclinical evaluation of HIV eradication strategies in the simian immunodeficiency virus-infected rhesus macaque: a pilot study testing inhibition of indoleamine 2,3-dioxygenase. AIDS Res Hum Retroviruses. 2013;29:207–214. doi: 10.1089/aid.2012.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Archin NM, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci USA. 2012;109:9523–9528. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Strain MC, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 173.Stacey AR, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nilsson J, Kinloch-de-Loes S, Granath A, Sonnerborg A, Goh LE, Andersson J. Early immune activation in gut-associated and peripheral lymphoid tissue during acute HIV infection. AIDS. 2007;21:565–574. doi: 10.1097/QAD.0b013e3280117204. [DOI] [PubMed] [Google Scholar]
- 175.Mendez-Lagares G, Pozo-Balado MM, Genebat M, Garcia Perganeda A, Leal M, Pacheco YM. Severe immune dysregulation affects CD4(+)CD25(hi)FoxP3(+) regulatory T cells in HIV-infected patients with low-level CD4 T-cell repopulation despite suppressive highly active antiretroviral therapy. J Infect Dis. 2012;205:1501–1509. doi: 10.1093/infdis/jis230. [DOI] [PubMed] [Google Scholar]
- 176.Simonetta F, et al. Early and long-lasting alteration of effector CD45RA(-)Foxp3(high) regulatory T-cell homeostasis during HIV infection. J Infect Dis. 2012;205:1510–1519. doi: 10.1093/infdis/jis235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood. 2013;121:29–37. doi: 10.1182/blood-2012-07-409755. [DOI] [PubMed] [Google Scholar]
- 178.Vandergeeten C, Fromentin R, Chomont N. The role of cytokines in the establishment, persistence and eradication of the HIV reservoir. Cytokine Growth Factor Rev. 2012;23:143–149. doi: 10.1016/j.cytogfr.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Estes JD, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency vrus infection. J Infect Dis. 2006;193:703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 180.Zeng M, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Klatt NR, et al. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 2010;6:e1000747. doi: 10.1371/journal.ppat.1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kuroda MJ, et al. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and cell-associated viral RNA levels in distinct lymphoid compartments of SIVmac-infected rhesus monkeys. Blood. 2000;96:1474–1479. [PubMed] [Google Scholar]
- 183.Benito JM, Lopez M, Soriano V. The role of CD8+ T-cell response in HIV infection. AIDS Rev. 2004;6:79–88. [PubMed] [Google Scholar]
- 184.Wong JK, et al. In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 2010;6:e1000748. doi: 10.1371/journal.ppat.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Betts MR, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Casado C, et al. Host and viral genetic correlates of clinical definitions of HIV-1 disease progression. PLoS One. 2010;5:e11079. doi: 10.1371/journal.pone.0011079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kaslow RA, et al. Polymorphisms in HLA class I genes associated with both favorable prognosis of human immunodeficiency virus (HIV) type 1 infection and positive cytotoxic T-lymphocyte responses to ALVAC-HIV recombinant canarypox vaccines. J Virol. 2001;75:8681–8689. doi: 10.1128/JVI.75.18.8681-8689.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pereyra F, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fadda L, Alter G. KIR/HLA: genetic clues for a role of NK cells in the control of HIV. Adv Exp Med Biol. 2011;780:27–36. doi: 10.1007/978-1-4419-5632-3_3. [DOI] [PubMed] [Google Scholar]
- 191.Tomescu C, et al. Impact of protective killer inhibitory receptor/human leukocyte antigen genotypes on natural killer cell and T-cell function in HIV-1-infected controllers. AIDS. 2012;26:1869–1878. doi: 10.1097/QAD.0b013e32835861b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Soghoian DZ, et al. HIV-Specific Cytolytic CD4 T Cell Responses During Acute HIV Infection Predict Disease Outcome. Sci Trans Med. 2012;4:123ra125. doi: 10.1126/scitranslmed.3003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Darrah PA, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 194.Lindqvist M, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Perreau M, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 197.Lederman MM. Immune restoration and CD4+ T-cell function with antiretroviral therapies. AIDS. 2001;15 (Suppl):S11–15. doi: 10.1097/00002030-200102002-00003. [DOI] [PubMed] [Google Scholar]
- 198.Stratov I, Dale CJ, Chea S, McCluskey J, Kent SJ. Induction of T-cell immunity to antiretroviral drug-resistant human immunodeficiency virus type 1. J Virol. 2005;79:7728–7737. doi: 10.1128/JVI.79.12.7728-7737.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Persaud D, et al. Effect of therapeutic HIV recombinant poxvirus vaccines on the size of the resting CD4+ T-cell latent HIV reservoir. AIDS. 2011;25:2227–2234. doi: 10.1097/QAD.0b013e32834cdaba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pace MJ, et al. Directly infected resting CD4+T cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Pathog. 2012;8:e1002818. doi: 10.1371/journal.ppat.1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Marchetti G, et al. Skewed T-cell maturation and function in HIV-infected patients failing CD4+ recovery upon long-term virologically suppressive HAART. AIDS. 2010;24:1455–1460. doi: 10.1097/QAD.0b013e328339cf40. [DOI] [PubMed] [Google Scholar]
- 202.Janbazian L, et al. Clonotype and repertoire changes drive the functional improvement of HIV-specific CD8 T cell populations under conditions of limited antigenic stimulation. J Immunol. 2012;188:1156–1167. doi: 10.4049/jimmunol.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wacleche VS, et al. The colocalization potential of HIV-specific CD8+ and CD4+ T-cells is mediated by integrin β7 but not CCR6 and regulated by retinoic acid. PLoS One. 2012;7:e32964. doi: 10.1371/journal.pone.0032964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sewell AK, Harcourt GC, Goulder PJ, Price DA, Phillips RE. Antagonism of cytotoxic T lymphocyte-mediated lysis by natural HIV-1 altered peptide ligands requires simultaneous presentation of agonist and antagonist peptides. Eur J Immunol. 1997;27:2323–2329. doi: 10.1002/eji.1830270929. [DOI] [PubMed] [Google Scholar]
- 205.Sempere JM, Soriano V, Benito JM. T regulatory cells and HIV infection. AIDS Rev. 2007;9:54–60. [PubMed] [Google Scholar]
- 206.Zhang Z, et al. Relationship of frequency of CD4+CD25+Foxp3+ regulatory T cells with disease progression in antiretroviral-naive HIV-1 infected Chinese. Jpn J Infect Dis. 2008;61:391–392. [PubMed] [Google Scholar]
- 207.Bosinger SE, Sodora DL, Silvestri G. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Curr Opin HIV AIDS. 2011;6:411–418. doi: 10.1097/COH.0b013e3283499cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bosinger SE, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Laguette N, Benkirane M. How SAMHD1 changes our view of viral restriction. Trends Immunol. 2012;33:26–33. doi: 10.1016/j.it.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Goila-Gaur R, Strebel K. HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology. 2008;5:51. doi: 10.1186/1742-4690-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Williams KC, Burdo TH. HIV and SIV infection: the role of cellular restriction and immune responses in viral replication and pathogenesis. APMIS. 2009;117:400–412. doi: 10.1111/j.1600-0463.2009.02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Grutter MG, Luban J. TRIM5 structure, HIV-1 capsid recognition, and innate immune signaling. Curr Opin Virol. 2012;2:142–150. doi: 10.1016/j.coviro.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Boulassel MR, Chomont N, Pai NP, Gilmore N, Sekaly RP, Routy JP. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J Clin Virol. 2012;53:29–32. doi: 10.1016/j.jcv.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 214.Kim H, Perelson AS. Viral and latent reservoir persistence in HIV-1-infected patients on therapy. PLoS Comput Biol. 2006;2:e135. doi: 10.1371/journal.pcbi.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Anthony KB, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J Acquir Immune Defic Syndr. 2003;33:125–133. doi: 10.1097/00126334-200306010-00002. [DOI] [PubMed] [Google Scholar]
- 216.Marchetti G, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS. 2006;20:1727–1736. doi: 10.1097/01.aids.0000242819.72839.db. [DOI] [PubMed] [Google Scholar]
- 217.Napolitano LA, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 218.Camargo JF, et al. Responsiveness of T cells to interleukin-7 is associated with higher CD4+ T cell counts in HIV-1-positive individuals with highly active antiretroviral therapy-induced viral load suppression. J Infect Dis. 2009;199:1872–1882. doi: 10.1086/598858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Benito JM, Lopez M, Lozano S, Gonzalez-Lahoz J, Soriano V. Down-regulation of interleukin-7 receptor (CD127) in HIV infection is associated with T cell activation and is a main factor influencing restoration of CD4(+) cells after antiretroviral therapy. J Infect Dis. 2008;198:1466–1473. doi: 10.1086/592716. [DOI] [PubMed] [Google Scholar]
- 220.Onder L, et al. IL-7-producing stromal cells are critical for lymph node remodeling. Blood. 2012;120:4675–4683. doi: 10.1182/blood-2012-03-416859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 222.Catalfamo M, et al. CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. J Immunol. 2011;186:2106–2116. doi: 10.4049/jimmunol.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bosque A, Famiglietti M, Weyrich AS, Goulston C, Planelles V. Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathog. 2011;7:e1002288. doi: 10.1371/journal.ppat.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Scripture-Adams DD, Brooks DG, Korin YD, Zack JA. Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. J Virol. 2002;76:13077–13082. doi: 10.1128/JVI.76.24.13077-13082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang FX, et al. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J Clin Invest. 2005;115:128–137. doi: 10.1172/JCI22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Imamichi H, et al. HIV-1 viruses detected during episodic blips following interleukin-7 administration are similar to the viruses present before and after interleukin-7 therapy. AIDS. 2011;25:159–164. doi: 10.1097/QAD.0b013e328340a270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kinter AL, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 228.Perreau M, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV–1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fukazawa Y, et al. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med. 2012;18:1673–1681. doi: 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Picker LJ, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lugli E, Mueller YM, Lewis MG, Villinger F, Katsikis PD, Roederer M. IL-15 delays suppression and fails to promote immune reconstitution in virally suppressed chronically SIV-infected macaques. Blood. 2011;118:2520–2529. doi: 10.1182/blood-2011-05-351155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Klatt NR, et al. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J Clin Invest. 2013 doi: 10.1172/JCI66227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Papagno L, et al. Immune Activation and CD8(+) T-Cell Differentiation towards Senescence in HIV-1 Infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Haas A, et al. HIV-1 replication activates CD4+ T cells with specificities for persistent herpes viruses. EMBO Mol Med. 2010;2:231–244. doi: 10.1002/emmm.201000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Harari A, Vallelian F, Pantaleo G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur J Immunol. 2004;34:3525–3533. doi: 10.1002/eji.200425324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Doisne JM, et al. CD8+ T Cells Specific for EBV, Cytomegalovirus, and Influenza Virus Are Activated during Primary HIV Infection. J Immunol. 2004;173:2410–2418. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- 237.Hatano H, et al. A randomized, controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infection. J Acquir Immune Defic Syndr. 2012;61:317–325. doi: 10.1097/QAI.0b013e31826e7d0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Buzon MJ, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 239.Llibre JM, et al. Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: a randomized 48-week study. Antivir Ther. 2012;17:355–364. doi: 10.3851/IMP1917. [DOI] [PubMed] [Google Scholar]
- 240.Massanella M, et al. Raltegravir intensification shows differing effects on CD8 and CD4 T cells in HIV infected HAART-supressed individuals with poor CD4 T-cell recovery. AIDS. 2012;26:2285–2293. doi: 10.1097/QAD.0b013e328359f20f. [DOI] [PubMed] [Google Scholar]
- 241.Vallejo A, et al. The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients. AIDS. 2012;26:1885–1894. doi: 10.1097/QAD.0b013e3283584521. [DOI] [PubMed] [Google Scholar]
- 242.Sedaghat AR, Siliciano JD, Brennan TP, Wilke CO, Siliciano RF. Limits on replenishment of the resting CD4+ T cell reservoir for HIV in patients on HAART. PLoS Pathog. 2007;3:e122. doi: 10.1371/journal.ppat.0030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Joos B, et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci USA. 2008;105:16725–16730. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dinoso JB, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.McMahon D, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 2010;50:912–919. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yilmaz A, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55:590–596. doi: 10.1097/QAI.0b013e3181f5b3d1. [DOI] [PubMed] [Google Scholar]
- 247.Jiang W, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rajasuriar R, et al. Biological determinants of immune reconstitution in HIV-infected patients receiving antiretroviral therapy: the role of interleukin 7 and interleukin 7 receptor alpha and microbial translocation. J Infect Dis. 2010;202:1254–1264. doi: 10.1086/656369. [DOI] [PubMed] [Google Scholar]
- 249.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011;117:5582–5590. doi: 10.1182/blood-2010-12-322453. [DOI] [PubMed] [Google Scholar]
- 250.Marziali M, et al. T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS. 2006;20:2033–2041. doi: 10.1097/01.aids.0000247588.69438.fd. [DOI] [PubMed] [Google Scholar]
- 251.Chun TW, et al. Relationship between the size of the human immunodeficiency virus type 1 (HIV-1) reservoir in peripheral blood CD4+ T cells and CD4+:CD8+ T cell ratios in aviremic HIV-1-infected individuals receiving long-term highly active antiretroviral therapy. J Infect Dis. 2002;185:1672–1676. doi: 10.1086/340521. [DOI] [PubMed] [Google Scholar]