Abstract
GB virus B (GBV-B), a flavivirus closely related to HCV, has previously been shown to infect and replicate to high titers in tamarins (Saguinus sp.). This study describes the use of GBV-B infection and replication in the common marmoset (Callithrix jacchus) for the successful development and validation of a surrogate animal model for hepatitis C virus (HCV). Infection of marmosets with GBV-B produced a viremia that peaked at 108 to 109 genome copies/ml for a period of 40 to 60 days followed by viral clearance at 60 to 80 days postinfection. Passage of the initial tamarin-derived GBV-B in marmosets produced an infectious stock that gave a more reproducible and consistent infection in the marmoset. Titration of the virus stocks in vivo indicated that they contained 1 infectious unit for every 1,000 genome copies. Cultures of primary marmoset hepatocytes were also successfully infected with GBV-B, with high levels of virus detected in supernatants and cells for up to 14 days postinfection. Treatment of GBV-B-infected hepatocyte cultures with a novel class of HCV protease inhibitor (pyrrolidine 5,5 trans-lactams) reduced viral levels by more than 2 logs. Treatment of GBV-B-infected marmosets with one such inhibitor resulted in a 3-log drop in serum viral titer over 4 days of therapy. These studies provide the first demonstration of the in vivo efficacy of a small-molecule inhibitor for HCV in an animal model and illustrate the utility of GBV-B as a surrogate animal model system for HCV.
For many years the discovery and development of novel therapies for hepatitis C virus (HCV) has been hampered by a lack of both a small animal model and a robust in vitro viral replication system. Although the recently developed HCV replicon system (3, 20) has proven extremely valuable for preclinical testing of antiviral compounds, there is still a need for virus-based culture systems and animal models. The restricted host range of HCV means that apart from humans, it will only infect chimpanzees. However, ethical considerations and cost mitigate the use of chimpanzees in drug discovery programs. HCV murine models in which transgenic or mutant mice implanted with human hepatocytes were found to be able to support the replication of HCV have been described previously (16, 21). However, these systems are laborious, require specialist expertise, and have yet to be reproduced. In the absence of an HCV animal model, researchers have looked to surrogate viruses (in particular, GB virus B [GBV-B]) to provide an alternative model system.
GBV-B was first described over 30 years ago, following the inoculation of tamarins with sera from a patient suffering from acute hepatitis. Serial passage of sera from these animals into further tamarins resulted in hepatitis. Although initially thought to be a human pathogen, this “GB agent” is now known to be of primate origin (9). When the molecular sequence of the GB agent was first characterized, two separate but closely related viruses, GBV-A and GBV-B, were identified (22, 32). GBV-A appears to replicate only in lymphocytes and is not associated with any clinical symptoms of disease. GBV-B, however, is a hepatotropic virus that causes an acute resolving hepatitis in infected tamarins (7, 8, 31).
Both GBV-A and GBV-B are members of the Flaviviridae family and are closely related to HCV (19, 22, 23). There is a high degree of both structural and biochemical homology between the GBV-B and HCV replicative processes (10, 14, 23, 26, 28, 29, 33, 34). We have recently described a series of compounds with activity against both the HCV and GBV-B NS3 proteases (V. Chung et al., unpublished data). In addition, it has also been shown that the core protein of GBV-B shares common features with HCV (15), indicating that similarities to HCV might extend to structural components of the virus. Finally, with the development of GBV-B infectious clones (8, 27) and subgenomic replicons (12) there are now several molecular tools with which the biology of GBV-B can be investigated and related to HCV.
Several groups have reported transmission and replication of GBV-B in a variety of tamarin species (5, 8, 31). However, captive-bred tamarins are difficult to source, breed, and handle. Literature reports in the 1970s suggested that GBV-B had a very restricted host range and could only replicate in tamarins (24, 25). More recently, however, GBV-B has been shown to replicate in owl monkeys, which are members of the Cebidae family of New World monkeys (9). This observation prompted us to investigate whether a GBV-B animal model could be developed using the common marmoset, a New World primate closely related to tamarins. Marmosets are easier to breed in captivity than tamarins, are smaller, and are already regularly used for drug metabolism, pharmacokinetic, and toxicology studies for drug development, making them an ideal species for antiviral efficacy studies.
In this report we show that the common marmoset is susceptible to GBV-B infection (an observation recently confirmed by Lanford and colleagues) (17) and compare virus replication and disease characteristics in both marmosets and tamarins. We also show that marmoset hepatocytes support the replication of GBV-B in cultures and that these cultures can be used to test the efficacy of HCV protease inhibitors. Finally, we use the GBV-B marmoset model to provide the first demonstration of the in vivo potency of a small-molecule inhibitor of HCV.
MATERIALS AND METHODS
Virus stocks.
An aliquot of Deinhardt's original passage 11 GB agent sera (11) was inoculated into four red-bellied tamarins. One of these tamarins (G17) was culled at day 32 postinfection (p.i.) when serum liver enzyme levels began to rise. This serum was aliquoted and stored at −80°C and used as the initial tamarin-derived infectious inoculum.
Animals.
Marmosets (Callithrix jacchus) were bred and housed at GlaxoSmithKline Medicines Research Centre. Red-bellied tamarins (Saguinus labiatus) were bred and housed at National Institute for Biological Standards and Control, South Mimms, Potters Bar, United Kingdom. Animal use was in accordance with United Kingdom Animals (Scientific Procedures) Act 1986 and was approved by internal ethical review. Animals were housed in groups and given appropriate environmental enrichment. Juvenile male and female tamarins and marmosets were used in the studies. The average weight of tamarins was 650 g and of marmosets was 350 g. Animals were infected intravenously into the right femoral vein with 0.5 to 1 ml of GBV-B infectious serum diluted in sterile saline. Blood samples (0.6 to 1 ml) were taken from either the left or right femoral vein at 15 min and 1 day and then at weekly time points thereafter. The sera were then analyzed for the presence of GBV-B RNA by quantitative PCR (Taqman) and for biochemical evidence of hepatitis by measuring alanine transferase (ALT) and glutamate dehydrogenase (GLDH) liver enzyme levels.
Biochemical analysis.
ALT and GLDH levels were measured using standard methods and a clinical autoanalyzer.
Quantitative analysis of GBV-B.
QIAamp viral RNA Minispin columns (Qiagen) were used to extract GBV-B RNA from serum (50 μl), cells (1 million), or culture supernatant (140 μl). RNA was eluted into a final volume of 25 μl and stored at −80°C. GBV-B was quantified using real-time PCR (Taqman) and a primer-probe combination that recognized the GBV-B core gene. The primers (forward, 5′-AAC GAG CAA AGC GCA AAG TC-3′, and reverse, 5′-CAT CAT GGA TAC CAG CAA TTT TGT-3′; Invitrogen) were used at concentrations of 900 nm (forward primer) and 300 nm (reverse primer) per 50 μl of reaction mixture. The probe (5′ 6FAM-AGCGCGATGCTCGGCCTCGTAAT-TAMRA 3′; Applied Biosystems) concentration was 200 nm per 50 μl of reaction mixture. Reactions were performed in triplicate using an EZ-RT PCR kit (Applied Biosystems) and consisted of a 45 min, 60°C reverse transcription step followed by 5 min at 95°C and 50 cycles of amplification (20 s of a 94°C denaturation step followed by 1 min of a 62°C annealing-extension step). Quantification of GBV-B RNA genomes was achieved by comparison of data to a standard curve generated from serial 10-fold dilutions (from 108 to 102 copies/μl) of in vitro-transcribed RNA corresponding to a 407-nucleotide region of GBV-B core protein. Results were expressed as genome equivalents (ge) per milliliter.
Isolation, culture, and infection of primary hepatocytes.
A standard two-step collagenase procedure was used to isolate primary hepatocytes from euthanized tamarins or marmosets. Briefly, isolated lobes of liver were flushed with perfusion buffer (Hanks balanced salt solution [HBSS], 10 mM HEPES, 50 μg of gentamicin/ml) for 5 min followed by a 10-min perfusion with chelating buffer (HBSS, 0.5 mM EGTA, 10 mM HEPES, 50 μg of gentamicin/ml). The liver was then digested for 30 min with collagenase solution (1 mg of type 1 collagenase [Worthington]/ml and 2 mM CaCl2 in HBSS). Forceps were used to tease hepatocytes out of the digested lobe and into attachment medium (Williams A medium containing 5% fetal calf serum and 2 mM penicillin, streptomycin, and glutamine). Cells were strained through sterile nylon bolting cloth (64 μm) and then washed three times in attachment medium by centrifugation at 50 × g for 3 min at 4°C. The viability and yield of hepatocytes were assessed by trypan blue dye exclusion. Cells were then plated at 1 million cells per well into type 1 rat tail collagen-coated 6-well plates (Biocoat; Becton Dickinson) and incubated at 37°C. After allowing 4 h for cells to attach, the medium was replaced with 2 ml of serum-free medium (SFM)/well (18). The medium was replaced every 2 to 3 days throughout the course of the experiments. Between 1 and 5 days postplating, hepatocyte cultures were infected with GBV-B infectious serum (0.5 ml of inoculum per well). In some experiments, the virus inoculum was UV inactivated (120 mJ/cm2 in a Stratagene UV cross-linker) before being added to cultures. Virus was allowed to adsorb for 2 h; then, the supernatant was removed and cultures were washed three times with phosphate-buffered saline (PBS) before SFM was added. Cultures were incubated for up to 2 weeks p.i., during which time supernatants and cells were regularly sampled and stored at −20°C.
In vitro inhibition studies.
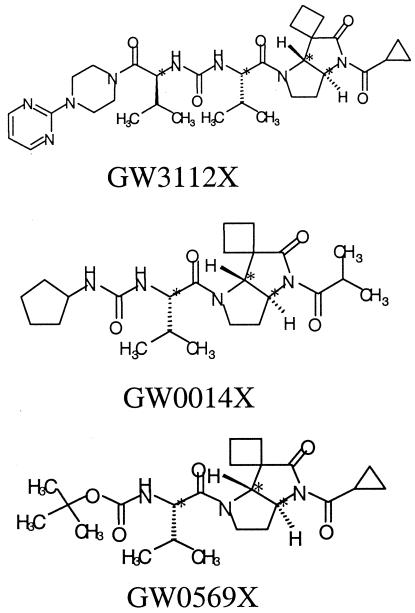
Following virus adsorption, the supernatant was removed and cells were washed with PBS and then grown in SFM containing pyrrolidine 5,5 trans-lactam, a novel class of HCV NS3 protease inhibitor (1, 2), at various concentrations. Three trans-lactams (GW3112X, GW0014X, and GW0569X), the structures for which are shown in Table 1, were used in these experiments. Compounds were initially dissolved in dimethyl sulfoxide to give a stock concentration of 40 mM. Further dilutions of this stock were carried out in SFM. Culture supernatants and cells were sampled as described above.
TABLE 1.
Activity of trans-lactams in various biological assays
| trans-lactam | Activity
|
|||
|---|---|---|---|---|
| HCV NS3 Kobs/I (M−1 s−1)a,b | GBV-B NS3 (% inhibition)c | HCV replicon IC50 (μM)b | GBV-B virus in culture (% inhibition)c | |
 |
7,761 | 85 | 0.45 | 96 |
| NDd | ND | 0.3 | 92 | |
| 301 | 24 | 9 | 23 | |
The Kobs/I values were calculated by dividing the Kobs value by the molar concentration used. In this case, the larger the value the greater the inhibitory activity of the compound under test.
data from Andrews et al. (1) and V. Chung et al. (unpublished data).
Values were determined at a drug concentration of 10 μM.
ND, not determined.
In vivo efficacy studies.
Nine male marmosets (all approximately 2 years old) were used in a chemotherapy study composed of two phases. Phase 1 of the study involved the dosing of animals during the very earliest stages of GBV-B infection. Four randomly selected animals were dosed subcutaneously twice a day with GW0014X at 30 mg/kg of body weight, while the remaining five marmosets received the vehicle (corn oil) only. The first dose was given 4 h prior to infection with GBV-B (5 × 106 ge per animal), and then dosing continued for 7 days. Serum samples were obtained from the animals on days 4, 7, and 15 p.i., and GBV-B RNA levels were quantified using Taqman assays.
Phase 2 of the chemotherapy study involved the treatment of marmosets during the peak period of GBV-B replication. The five marmosets from the vehicle control-treated group from the phase 1 studies were rested for 3 weeks, and then two serum samples were obtained on days 29 and 34 p.i. GBV-B RNA levels were measured, and three animals that consistently had viral RNA levels over 108 ge/ml were entered into the second phase of the experiment. One animal received GW0014X (30 mg/kg in corn oil), while two control animals were given vehicle only. The dosing regimen was exactly as described for phase 1 of the experiment but was shortened to only 4 days of dosing. Serum samples were obtained from the animals on the day before treatment began, in the middle of therapy, and at 1 day and 1 week posttherapy (days 39, 42, 44, and 49 p.i., respectively). GBV-B RNA levels in these serum samples were measured using Taqman assays as described previously.
Pharmacokinetic analysis of GW0014X.
Blood samples (approximately 300 μl) were collected from the vehicle control and GW0014X-treated marmosets on day 4 of the 7-day, 30-mg/kg bid dosing regimen at 2 and 8 h after the administration of the first dose. The blood was mixed in heparinized containers and centrifuged to yield plasma, which was stored frozen at −80°C. Plasma samples were extracted by protein precipitation with acetonitrile, and concentrations of GW0014X were determined by liquid chromatography and mass spectrography. The limit of quantitation was 0.5 ng/ml.
RESULTS
GBV-B replication in tamarins.
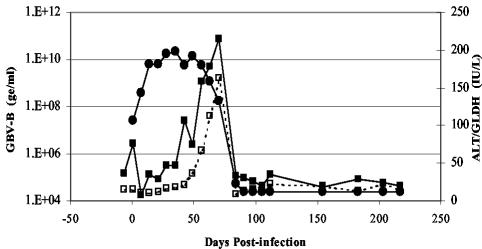
We used a historic serum sample of GBV-B (passage 11) (originally obtained from the American Type Culture Collection) to generate a stock of GBV-B infectious serum (termed tam-G17) by passage in a red-bellied tamarin. This stock virus was used to characterize GBV-B replication and disease in further tamarins. Tamarins were infected with 2.5 × 109 ge (as measured using Taqman assays). Weekly blood samples were obtained from the animals for up to 200 days, and the serum was assayed for viral load and liver enzyme levels. The results for a representative animal (G15) are shown in Fig. 1. GBV-B levels reached a peak in the serum very quickly. By 14 days p.i., virus levels had reached 109 to 1010 ge/ml; this viremia level was maintained for 50 to 80 days p.i. before declining rapidly. This decline correlated exactly with elevated levels of GLDH and ALT. Generally, by day 100 p.i. serum viral levels had decreased to below detectable levels and serum transaminases were restored to preinfection baseline levels. Histological examination of livers of infected tamarins during acute infection and following viral clearance showed evidence of periportal infiltration of lymphocytes and mononuclear cells (data not shown).
FIG. 1.
Infection of tamarins with GBV-B infectious serum (tam-G17). GBV-B serum levels (measured using Taqman assays and indicated in ge per milliliter) (•) and liver damage results (measured according to serum levels of ALT [▪] and GLDH [□]) and indicated in international units per liter [IU/L]) are shown.
GBV-B replication in common marmosets.
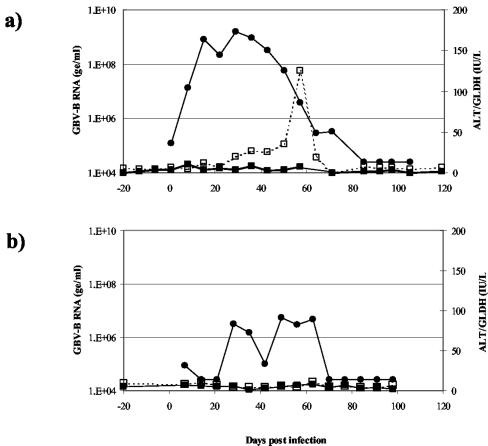
The same GBV-B infectious stock (tam-G17) used in the tamarin experiments as described above was then used to infect common marmosets. Again, 2.5 × 109 ge of virus were inoculated intravenously. Marmosets were bled once a week for 100 days p.i., and serum samples were assayed for levels of virus and liver enzymes. GBV-B replication in some marmosets followed a similar pattern to that seen in infected tamarins. Figure 2a shows the replication profile of a GBV-B-infected marmoset (Y307). Serum viral RNA levels reached 109 ge/ml by 21 days p.i. and were maintained for approximately 3 weeks before declining rapidly. In contrast to the results seen for tamarins, this viral clearance coincided with a small transient rise in GLDH levels and ALT levels remained normal. By 100 days p.i., viral RNA was below detectable levels in the serum and serum transaminases were at preinfection baseline levels.
FIG. 2.
Comparison of replication profiles of two marmosets infected with GBV-B (tam-G17 infectious serum). (a) Marmoset Y037 results, a typical example of robust GBV-B replication. (b) Marmoset Y191 results, an example of poor GBV-B replication with delayed and low-level viremia. GBV-B serum levels (measured using Taqman assays and indicated in ge per milliliter) (•) and liver damage results (measured according to serum levels of ALT [▪] and GLDH [□]) and indicated in international units per liter [IU/L]) are shown.
However, this pattern of replication was not consistent in all infected marmosets. Some GBV-B-infected marmosets attained serum virus titers 1 or 2 logs lower than expected and exhibited episodes of partial clearance (for an example, see the results for Y191 shown in Fig. 2b). Also, there were some animals in which virus remained below detectable levels in the serum at every time point measured during the experiment (data not shown).
To increase the infection rate and achieve consistent replication, we passaged our tamarin-derived GBV-B G17 infectious serum through marmosets. The resulting virus stock (termed marm-Y243) was derived from the second passage of our original tam-G17 virus. When tamarin-derived GBV-B virus stock (G17) was used to infect tamarins and marmosets at an inoculum level of 2.5 × 109 ge/ml, 17 of 17 tamarins and 13 of 20 marmosets showed consistent infections. When the same inoculum level of marmoset-derived GBV-B virus stock (Y243) was used to infect marmosets, 15 of 15 showed consistent infections. Thus, when our tamarin-derived GBV-B was used to infect tamarins we achieved consistent, reproducible infections in all 17 animals tested so far. However, when this same virus stock was used to infect marmosets, approximately one-third of the animals (7/20) displayed either no or suboptimal replication. In contrast, the new marmoset-derived Y243 virus stock gave consistent infection rates and replication profiles in all marmosets.
Titration of GBV-B in marmosets.
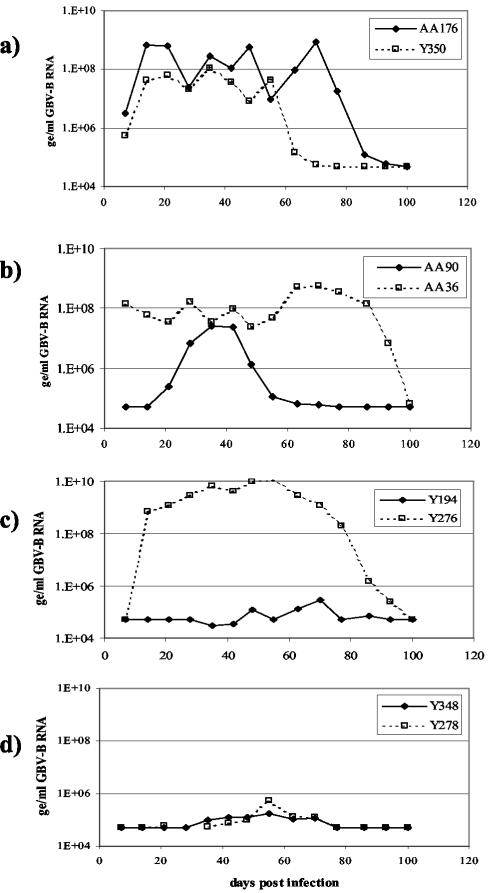
GBV-B (marm-Y243; Taqman titer, 109ge/ml) infectious serum was serially diluted 10-fold in saline and inoculated into four groups of marmosets. Virus replicated to anticipated levels in all animals infected with 5,000 ge or more of GBV-B (≤10−5 dilution of infectious serum), as shown in Fig. 3a and b. The 50% infectious dose (ID50) was seen in animals infected with 500 ge of GBV-B (10−6 dilution of infectious serum), with one out of two marmosets infected (Fig. 3c).
FIG. 3.
Titration of GBV-B infectious sera (marm-Y243) from marmosets to determine infectious dose. GBV-B serum levels are shown for animals infected with the following amounts of virus: 500,000 ge (AA176) or 50,000 ge (Y350) (a), 5,000 ge (b), 500 ge (c), and 50 ge (d).
The results from this experiment were used to calculate the infectious dose (ID50) for the virus stock, using the Spearman-Karber method. Marm-Y243 GBV-B virus stock had an infectious titer of 106 infectious units per ml. In a similar manner, the tamarin-derived tam-G17 GBV-B virus (stock titer, 1010ge/ml) was titrated in tamarins. Three out of four tamarins that received 10,000 ge of GBV-B (10−6 dilution of infectious serum) became infected, while none of the animals that received 1,000 ge (10−7 dilution of GBV-B serum) became infected (data not shown). This gives the tam-G17 virus stock a calculated ID50 value of 2 × 106 infectious units per ml. The infectivity of both GBV-B virus stocks can thus be estimated at approximately 1 infectious unit per 1,000 ge in their respective hosts.
Rechallenge of marmosets with homologous virus.
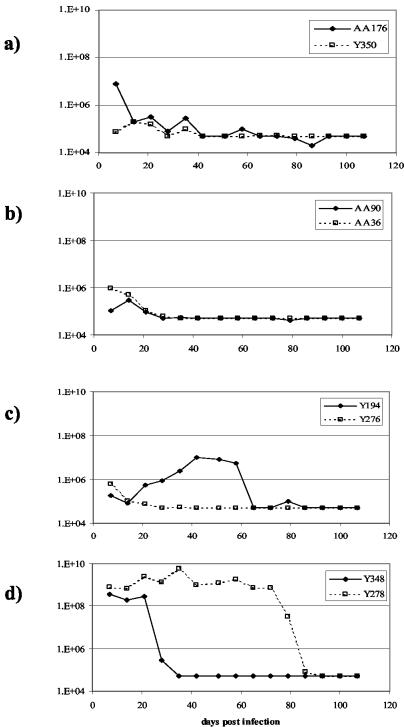
Marmosets that had been previously infected with various dilutions of marm-Y243 sera in the titration experiment described above were allowed to clear virus from the sera and rested for 3 months. All marmosets were then rechallenged with 5 × 106 ge per animal (10−2 dilution of the original sera) and monitored for GBV-B replication and serum liver transaminase responses (data not shown). As depicted in Fig. 4, the marmosets that had shown no evidence of replication after primary inoculation with GBV-B all had high levels of GBV-B viremia following the secondary challenge. In contrast, all the marmosets that were infected following primary inoculation with GBV-B showed a pattern of restricted viral replication upon rechallenge. Marmosets were not totally protected from reinfection, and a low level of GBV-B replication (<106 ge/ml) was observed which resolved quickly over a 2- to 3-week period.
FIG. 4.
Rechallenge of marmosets previously infected with GBV-B. GBV-B serum levels were measured for animals rechallenged with 500,000 ge; the animals had initially received the following amounts of homologous virus stock as the primary infection: 500,000 ge (AA176) or 50,000 ge (Y350) (a), 5,000 ge (b), 500 ge (c), and 50 ge (d).
Replication of GBV-B in cultured tamarin and marmoset hepatocytes.
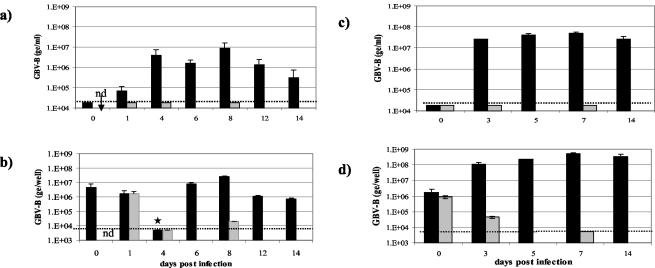
Tamarin and marmoset hepatocyte cultures were infected with GBV-B (108 ge of tam-G17 virus per well). To act as a negative control, hepatocyte cultures were also inoculated with UV-irradiated GBV-B. After 2 h was allowed for virus adsorption, the supernatant was removed and cultures were washed three times with PBS; then, SFM was applied. As depicted in Fig. 5, viral RNA was detected in the supernatants and cells of GBV-B-infected cultures from day 1 p.i. and reached a peak around 7 days p.i. In marmoset cultures (Fig. 5a and b), GBV-B levels began to decline after 12 days p.i.; tamarin cultures (Fig. 5c and d) continued to support high levels of GBV-B replication up to 14 days p.i. (when the study ended). Throughout the experiment, GBV-B RNA levels in the tamarin cultures were approximately 10-fold higher than the levels observed in the marmoset cultures. No viral RNA was detected in supernatants from cultures infected with UV-irradiated GBV-B. In the cell pellets (Fig. 5b and d), high levels of GBV-B RNA in hepatocytes inoculated with either the infectious or UV-irradiated virus were detected on days 0 and 1 p.i. In GBV-B-infected cells, viral RNA was maintained at high levels for up to 14 days p.i. and little or no viral RNA was detected in the cells treated with UV-irradiated virus. A low level of GBV-B RNA (2 × 104 ge per million cells) was detected in the marmoset cultures inoculated with UV-irradiated virus on day 8 p.i., which probably reflected the incomplete inactivation of all the virus particles by irradiation, leaving a small number of particles able to replicate.
FIG. 5.
Replication of GBV-B in primary hepatocytes. Hepatocytes from a marmoset (a and b) or tamarin (c and d) were inoculated with either infectious (black bars) or UV-irradiated (grey-shaded bars) GBV-B (tam-G17 sera). Hepatocytes were cultured in SFM, and supernatants and cell pellets were harvested periodically for the next 14 days. (a and c) GBV-B RNA levels (quantified using Taqman assays) measured in culture supernatants and expressed as ge per milliliter of supernatant. Cultures had a total volume of 2 ml per well. (b and d) GBV-B RNA levels measured in cell pellets and expressed as ge per well (approximately 1 million cells per well). Error bars represent the standard deviations between duplicate cultures. The limit of detection in this experiment is represented by the dotted line. In a subsequent experiment, the GBV-B RNA level in cells on day 4 was measured as 1.7 × 106 ge/well (★), which is more consistent with other data. nd, not done due to limitations of hepatocyte numbers.
In vitro titration of GBV-B.
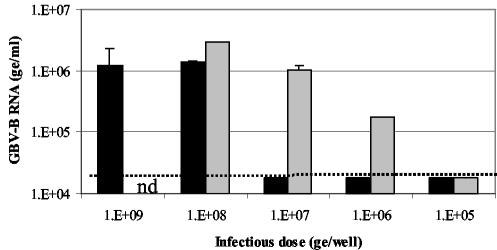
In vitro titration experiments were performed to determine the infectivity of our tamarin-derived and marmoset-derived GBV-B stocks in marmoset hepatocyte cultures. As Fig. 6 depicts, tam-G17 only gave a productive infection when either 109 or 108 ge/ml were used as the inoculum. However, for marm-Y243, replication was seen in cultures infected with a virus inoculum as low as 106 ge/ml. This suggests that the marm-Y243 virus stock is 1,000-fold more infectious in the marmoset hepatocytes than the tam-G17 virus.
FIG. 6.
Titration of tamarin- and marmoset-derived GBV-B stocks in marmoset hepatocyte cultures. Marmoset hepatocyte cultures were infected with either tam-G17 (black bars) or marm-Y243 (gray-shaded bars) in 10-fold dilutions from 109 to 105 ge per well. At 8 days p.i., GBV-B RNA levels in supernatants were measured using Taqman assays and are expressed as ge per milliliter. Error bars represent the standard deviations between duplicate cultures. nd, not done. The limit of detection in this experiment is represented by the dotted line.
In vitro inhibition studies.
GBV-B-infected tamarin and marmoset hepatocyte cultures were maintained in the presence of a small-molecule HCV NS3 protease inhibitor (GW3112X) at various concentrations or of medium alone. GBV-B RNA levels were measured in the supernatants after several days of culture growth. Table 2 shows that in both culture systems, GW3112X (at a concentration of 10 μM) reduced GBV-B viral titers by 2 logs or more.
TABLE 2.
Effect of the presence of an HCV NS3 protease inhibitor on GBV-B-infected tamarin and marmoset hepatocytes
| GW3112X concn (μM) | GBV-B RNA level (ge [±SD]/ml) in hepatocyte culture supernatants
|
|
|---|---|---|
| Tamarina | Marmosetb | |
| 0 | 2.00 (± 2.19) × 108 | 5.25 (± 1.06) × 106 |
| 10 | 7.81 (± 3.17) × 105 | 4.00 (± 3.96) × 104 |
Tamarin culture values for supernatants sampled 5 days p.i.
Marmoset culture values for supernatants sampled 8 days p.i.
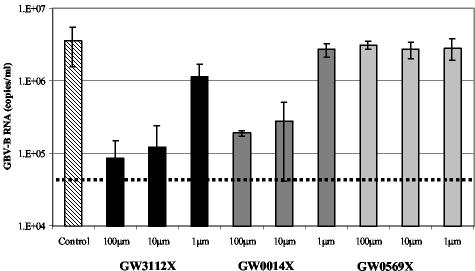
Following the successful inhibition of GBV-B replication by GW3112X, two further compounds in the chemical series were assessed for activity against GBV-B. All three compounds had previously been shown to have activity against both HCV and GBV-B proteases in biochemical assays and against the HCV subgenomic replicon, as exemplified in Table 1. GW3112X and GW0014X inhibited GBV-B replication in a dose-dependent manner (Fig. 7). Cultures treated with the highest doses of compounds had viral RNA levels 1.5 to 2 logs lower than the untreated control cultures. GW0569X had no effect on GBV-B RNA replication in this system. This assay ranked the activity of the three trans-lactams in the same order of potency as the biochemical enzyme assay and the HCV subgenomic replicon assay, as shown in Table 1.
FIG. 7.
Inhibition of GBV-B replication in primary marmoset hepatocytes following treatment with 5,5 pyrrolidine trans-lactams. Cultures of primary marmoset hepatocytes were inoculated with GBV-B (marm-Y243). After removal of the inoculum, cells were cultured in either SFM alone or SFM containing various concentrations of one of three trans-lactams. At 3 days later, supernatants were harvested; GBV-B RNA levels were measured using Taqman assays and are expressed as ge per milliter. Error bars represent the standard deviations between duplicate cultures.
In vivo efficacy studies.
Following the successful demonstration of GBV-B inhibition by the pyrrolidine 5,5 trans-lactams in the in vitro hepatocyte culture system, studies were performed to investigate the efficacy of a protease inhibitor in vivo. Unfortunately, GW3112X, the most active trans-lactam, was in limited supply and so the animal studies were undertaken with GW0014X. Nine marmosets were enrolled onto a study composed of two phases. Phase 1 was to determine the efficacy of the trans-lactam when given during the very early stage of GBV-B infection. The marmosets were allocated into two groups. One group of four animals received GW0014X at 30 mg/kg of body weight by subcutaneous injection twice a day, while the remaining five animals were given vehicle using the same regimen. Following one prophylactic dose of compound or vehicle, all marmosets were then infected with GBV-B and dosing continued for 7 days. Serum samples were obtained at days 4 and 7 p.i., and viral RNA levels were measured using Taqman assays. The results for this study are shown in Fig. 8. At 4 days p.i., GBV-B RNA could not be detected in two of the four treated animals. The remaining two marmosets in the group had very low levels of GBV-B RNA in the serum (1.8 × 105 and 2.2 × 105 ge/ml). In the untreated control group, GBV-B RNA levels ranged from 1.09 × 106 to 4.07 × 108 ge/ml (group mean, 2.25 × 107 ge/ml). After 7 days of therapy, GBV-B RNA levels in all four treated marmosets were below the limit of detection of 5 × 104 ge/ml. In contrast, the viral load range in the control group was between 4.38 × 106 and 3.46 × 109 ge/ml (group mean, 1.51 × 108 ge/ml). Upon completion of treatment, the animals were rested for 7 days and then a further blood sample was taken 15 days p.i. Treated animals were euthanized at this point. At the 15 days p.i. time point GBV-B RNA was undetectable in the sera of two out of the four animals treated with GW0014X. The control untreated animals had a mean serum viral load approaching 109ge/ml. While one of the treated animals had a very low level of viremia (just above the limit of detection of 5 × 104 ge/ml), the other animal (which had shown no detectable GBV-B RNA in its sera at days 4 and 7 p.i.) had a viral load of 3.14 × 106 ge/ml at day 15 p.i.
FIG. 8.
Efficacy of trans-lactam GW0014X in the GBV-B marmoset model following treatment during the early phase of infection. Marmosets received one prophylactic subcutaneous dose of either GW0014X (30 mg/kg of body weight; n = 4) or vehicle (corn oil; n = 5) 4 h prior to infection with GBV-B marm-Y243 stock. Dosing then continued at twice a day for 7 days, as indicated by the black line at the top of the chart. Serum GBV-B RNA levels (measured using Taqman assays) were determined at days 4, 7, and 15 p.i. Marmosets that received GW0014X are represented individually by open symbols, while the average viral loads for the control marmosets (dosed with vehicle only) are represented by black circles. The error bars represent the standard deviations from the means for this group. The limit of detection for the Taqman assay is 5 × 104 ge/ml and is represented by the dotted line.
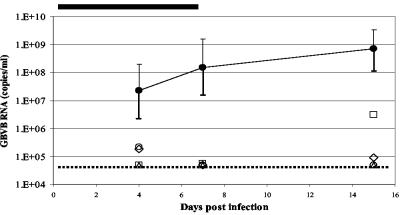
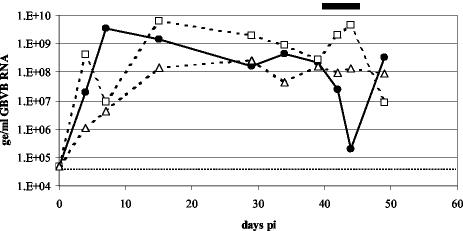
Phase 2 of the study was the evaluation of GW0014X given therapeutically during the period of peak viral replication. Following a 3-week rest period, two serum samples were taken from the marmosets in the control, untreated group over a 7-day period and GBV-B RNA levels were measured. Three animals that consistently had viral RNA levels higher than 108 ge/ml were entered into the second phase of the experiment. One animal received GW0014X, while two control animals were given vehicle only. The dosing regimen was exactly as described for the earlier experiment but was shortened to only 4 days of dosing. Serum samples obtained from the animals on the day before treatment began, in the middle of therapy, and at 1 day and 1 week posttherapy were analyzed for GBV-B RNA levels. The two control marmosets maintained high levels of viremia throughout the dosing period, while the marmoset that received GW0014X had viral RNA levels that were 3 logs lower by the end of therapy compared to the pretreatment value (Fig. 9). Upon cessation of treatment, however, GBV-B levels in the serum had returned to pretherapy levels by 1 week posttreatment.
FIG. 9.
Efficacy of trans-lactam GW0014X in the GBV-B marmoset model following treatment during the peak of virus replication. One marmoset (•) received 30 mg of GW0014X/kg of body weight, while two control marmosets (□ and Δ) received vehicle (corn oil). Compounds were given subcutaneously twice a day for 4 days between days 39 and 43 p.i., as indicated by the black line at the top of the chart. Serum GBV-B RNA levels (measured using Taqman assays) were determined on the day prior to therapy, in the middle of therapy, and then 1 day and 1 week after the completion of therapy. The limit of detection for the Taqman assay is 5 × 104 ge/ml and is represented by the dotted line.
Pharmacokinetic analysis of GW0014X.
Plasma drug levels of GW0014X were determined during phase 1 of the efficacy study. Blood samples were taken at 2 and 8 h postdose on day 4 of the 7 day, 30 mg/kg subcutaneous, bid dosing regimen. As shown in Table 3, GW0014X was observed in the plasma of all marmosets dosed with the compound. The average plasma concentration at 2 h after the administration of the first dose on day 4 was 902 ng/ml, and the average plasma concentration at 8 h after the first dose on day 4 was 596 ng/ml. Subcutaneous exposure levels (as expressed using a value for the area under the curve from 0 to 8 h of 5,400 h · ng/ml and a value for the average steady-state plasma concentration of 675 ng/ml) were approximated from the limited sampling times. The average steady-state plasma concentration of 675 ng/ml was threefold lower than the 50% inhibitory concentration [IC50] of GW0014X for GBV-B in culture (IC50, ∼5 μM [equivalent to a steady-state plasma concentration of 2,230 ng/ml]).
TABLE 3.
Pharmacokinetic analysis of GW0014X in treated marmosets
| Subcutaneous pharmacokinetic parameters of GW0014X | Concn (ng/ml) ± SDa |
|---|---|
| Avg plasma concn at 2 h postdose | 902 ± 246 |
| Avg plasma concn at 8 h postdose | 596 ± 134 |
| Cmaxb | 902 |
| Avg steady-state plasma concn | 675c |
Limit of detection = 0.5 ng/ml.
Cmax, maximum concentration of drug in serum.
Approximated value based on limited sampling times.
DISCUSSION
It is well established that GBV-B can infect and replicate in tamarins (Saguinus species) (5, 6). The findings of Bright et al. confirm that tamarins experimentally infected with GBV-B develop an acute resolving hepatitis characterized by sustained viremia followed by clearance after 10 to 12 weeks (H. Bright, D. Mesogiti, M. Cleveland, N. Parry, and A. R. Carroll, European Virology 2000, abstr. O-013, J. Clin. Virol., vol. 18, no. 1-3, 2000). Unfortunately, the use of tamarins as an animal model for drug discovery poses a number of disadvantages; for example, they are difficult to source and breed and they are relatively large animals. In this report, we present an alternative surrogate animal model for HCV based on the infection and replication of GBV-B in common marmosets (Callithrix sp.), which are in the same family (Callitrichidae) as tamarins. We have shown that it is possible to generate a robust GBV-B infection in this species and that this model can be used to test the in vivo and in vitro efficacy of anti-HCV NS3 protease compounds.
In investigations of GBV-B-infected marmosets, high levels of virus were seen in the serum for a number of weeks before clearance; resolution of disease was often accompanied by a short episode of raised serum GLDH levels. These results are contrary to those in early reports in the literature (24, 25) that suggested that GB virus infections were confined to tamarins. However, the advent of sensitive, specific real-time PCR, which can quantitate serum virus RNA, has conclusively demonstrated that GBV-B replicates in the common marmoset, as previously reported by Bright et al. and Carroll et al. (H. Bright, D. Mesogiti, P. A. Watts, A. R. Carroll, and R. J. Fenton, 15th Int. Conf. Antivir. Res., Antivir. Res. 53[3]:A39, abstract 013, 2002, and A. R. Carroll, H. Bright, D. Mesogiti, P. A. Watts, N. Gray, and R. J. Fenton, 15th Int. Conf. Antivir. Res., Antivir. Res. 53[3]:A39, abstract 012, 2002) and more recently by others (17).
There are a number of differences between the observations presented here and those reported in the preliminary studies on two marmosets presented by Lanford and colleagues (17). They surmise that the lack of increases in ALT levels in marmosets and the lack of correlation between increases in ALT levels and viral clearance in tamarins suggests that the destruction of infected hepatocytes is not the primary mechanism of viral clearance. However, the present report shows that ALT is a poor marker for viral-induced hepatitis in tamarins and marmosets. We show that when another serum liver enzyme, GLDH, is monitored, evidence of hepatic damage is observed in all tamarins and in some, although not all, marmosets. Furthermore, this GLDH flare is consistently associated with clearance of GBV-B from the serum of infected animals; this finding represents evidence that destruction of infected hepatocytes does play a role in viral clearance. The preliminary study carried out by Lanford et al. (17) also showed that GBV-B infections in marmosets and tamarins are similar. Having compared GBV-B replication in over (in total) 50 tamarins and marmosets, however, we have observed that when the tamarin-derived GBV-B infectious serum is used, virus titers in marmosets are around 10-fold lower than those seen in tamarins. Indeed, some marmosets display atypical infections, with low serum viral titers and episodes of partial viral clearance and virus rebound. We achieved consistent viremia profiles for marmosets only with marmoset-passaged GBV-B serum. The results of the in vitro experiments conducted in the present study confirm that the tamarin-derived GBV-B (tam-G17) replicated to levels in marmoset hepatocytes approximately 10-fold lower than those seen in tamarin cultures. We also observed differences between tamarin- and marmoset-derived GBV-B virus stocks. When we used a high-titer inoculum to infect marmoset hepatocytes, both tam-G17 and marm-Y243 virus stocks gave similar peak viral titers in culture supernatants (an observation also made by Lanford and colleagues). However, when the viral inoculum was titrated down, it became apparent that the marmoset-derived GBV-B was much more efficient at infecting marmoset hepatocytes than the tamarin-derived GBV-B stock. The tamarin-derived virus (tam-G17) could establish an infection in the marmoset hepatocytes only when an inoculum of 108 or 107 ge per well was used, while the marm-Y243 virus was still able to replicate in the hepatocytes when an inoculum of 106 ge per well was used.
A body of evidence is now accumulating which suggests that GBV-B is able to infect a wide range of New World monkeys. In addition to infecting tamarins and marmosets (both members of the Callitrichidae family), GBV-B is able to replicate in owl monkeys, which are members of Cebidae, a separate family of New World monkeys (9). GBV-B appears to replicate most efficiently in tamarins, generating peak serum RNA titers that are approximately 1 log higher than the levels seen in infected marmosets and 3 logs higher than those seen in owl monkeys. GBV-B cannot be transmitted to chimpanzees, which are Old World primates (9). The present study also demonstrated that a more consistent infection could be achieved in marmosets by using a marmoset-passaged GBV-B stock. In addition, the results of the in vitro titration experiments undertaken in this study suggest that in marmoset hepatocytes the marmoset-derived GBV-B is 100-fold more infectious than the GBV-B derived from tamarins (even though they exhibit the same infectivity in vivo characteristics in their respective hosts). It is interesting that the tamarin-derived G17 virus does not titrate as one might expect in the marmoset hepatocytes, the pattern being suggestive of an all-or-nothing effect. One possibility is that the tam-G17 virus level needs to be above a threshold level for it to infect the marmoset hepatocytes.
Taken together, these data support the theory that GBV-B is a primate hepatitis virus able to infect and replicate in a number of species of New World monkeys (although GBV-B has yet to be isolated from a wild monkey). There are many questions about the natural history of GBV-B which still remain unanswered. It is not clear whether the virus is able to induce chronic disease. We have performed more than 50 experimental GBV-B infections in tamarins and marmosets, and in the majority of cases we have observed viral clearance before the end of the study. However, we have on occasion had animals with measurable GBV-B RNA levels in their serum samples at the end of the study at day 100 (unpublished results). It is possible that these animals could have maintained a persistent infection, but it is also possible that they would have resolved their infection normally over an extended time frame. Like others (5), we have shown that primary infection does not induce complete protection to subsequent virus challenge; this absence of complete protection is also the case with HCV infections in the chimpanzee model (4). The data shown in Fig. 4d representing the results of rechallenge of two animals are interesting and difficult to explain. Figure 3d shows that neither animal appeared to replicate GBV-B to any extent upon primary challenge with virus but that one animal went on to develop typical high-titer viremia following rechallenge and the other animal had high-titer viremia for only a very short period of time. This difference in patterns may be due to animal variability, since the marmosets are an outbred laboratory species. Thus, reinfection with GBV-B induces a low-level, transient replication that may be sufficient to ensure survival of the virus in the wild.
Infectivity studies in which both tamarin-derived and marmoset-derived GBV-B virus stocks were titrated in their respective host species have revealed that both virus stocks have an ID50 value of approximately 106 units/ml. Since the Taqman analysis gives a titer of 109 ge/ml, this results in an infectivity ratio of 1 infectious particle for every 1,000 ge copies. Infectivity values quoted for HCV from chimpanzee titration experiments range from 1:1 to 1:104, depending on the particular isolate used (30). For example, the infectivity ratio for HCV isolate H77 ratio has been estimated to be 1 infectious particle to 100 genome copies. Therefore, the value obtained for GBV-B in the present study falls within this range.
Having been used to characterize GBV-B replication in the marmosets and in cultures of primary hepatocytes, these systems were used to extend our knowledge of a novel series of HCV NS3 protease inhibitors. We have shown that compounds that are active against the HCV NS3 protease (as measured by biochemical enzyme assays and HCV subgenomic replicon assays) also have activity against GBV-B. In fact, the trans-lactams were found to have the same activity ranking when tested against the GBV-B NS3 protease and in the in vitro replication assay, which underlines the value of GBV-B as a surrogate virus during the optimizing of anti-HCV compounds.
When the in vivo efficacy of one of the trans-lactams, GW0014X, was assessed in the GBV-B marmoset model, it was found that when given at the start of the infection, the compound completely prevented the virus from replicating in all four animals after 7 days of dosing. Furthermore, after removal of treatment, virus rebounded in only one of the four treated animals. When therapy was delayed until viremia was well established, serum viral levels were reduced by more than 3 logs after only 4 days of treatment. This is the first demonstration of in vivo efficacy of an HCV antiviral in an animal model. In this model the trans-lactam was surprisingly effective, considering that its IC50 in vitro for primary marmoset hepatocytes was estimated to be around 5 to 10 μM. Indeed, pharmacokinetic analysis of GW0014X exposure in the treated marmosets estimates a steady-state plasma concentration of 675 ng/ml, which is threefold lower than the IC50 of the compound against the virus in culture.
These data are interesting in the light of the recent observation that the NS3 protease of HCV blocks the phosphorylation and effector action of interferon regulatory factor-3, which is a key cellular antiviral signaling molecule (13). This raises the possibility that by not only directly inhibiting viral replication but also by restoring the host's own antiviral response, inhibition of the NS3 protease can have a dual effect in vivo.
The validation of the GBV-B marmoset model with GW0014X formally links the inhibitor activity through increasingly complex biological assay systems: biochemical, subgenomic replicon, in vitro virus replication, and, finally, in vivo efficacy. GBV-B therefore now offers a clear route for the compound progression and optimization of HCV NS3 protease inhibitors. The in vitro and in vivo systems described in this paper also offer the opportunity to study the biology of GBV-B, particularly with regard to virus and host cell interactions, an area that is very difficult to explore with HCV.
Acknowledgments
We thank Bob Woollen and Robin Hull, National Institute of Biological Standards and Control, United Kingdom, for their kind assistance and technical expertise with studies undertaken with red-bellied tamarins. We also thank Malcolm Ward and the Clinical Pathology Unit at GSK, Ware, United Kingdom, for undertaking the liver enzyme analysis of all tamarin and marmoset serum samples. We acknowledge Tony Savage for histological analysis of tamarin and marmoset liver samples and Anne Cheasty for undertaking the pharmacokinetic analysis. Finally we thank Martin Slater, David Andrews, and J. Leahy for providing the pyrrolidine 5,5 trans-lactams.
Footnotes
Dedicated to the memory of Paul Watts, who died suddenly.
REFERENCES
- 1.Andrews, D. M., H. M. Chaignot, B. A. Coomber, M. D. Dowle, S. L. Hind, M. R. Johnson, P. S. Jones, G. Mills, A. Patikis, T. J. Pateman, et al. 2003. The design of potent, non-peptidic inhibitors of hepatitis C protease. Eur. J. Med. Chem. 38:339-343. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, D. M., S. Carey, H. Chaignot, B. A. Coomber, N. M. Gray, S. L. Hind, P. S. Jones, G. Mills, J. E. Robinson, and M. J. Slater. 2002. Pyrrolidine-5,5-trans-lactams. 1. Synthesis and incorporation into inhibitors of hepatitis C virus NS3/4A protease. Org. Lett. 4:4475-4478. [DOI] [PubMed] [Google Scholar]
- 3.Bartenschlager, R. 2002. Hepatitis C virus replicons: potential role for drug development. Nat. Rev. Drug Discov. 1:911-916. [DOI] [PubMed] [Google Scholar]
- 4.Bassett, S. E., B. Guerra, K. Brasky, E. Miskovsky, M. Houghton, G. R. Klimpel, and R. E Lanford. 2001. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33:1479-1487. [DOI] [PubMed] [Google Scholar]
- 5.Beames, B., D. Chavez, and R. E. Lanford. 2001. GB virus B as a model for hepatitis C virus. Ilar J. 42:152-160. [DOI] [PubMed] [Google Scholar]
- 6.Beames, B., D. Chavez, B. Guerra, L. Notvall, K. M. Brasky, and R. E. Lanford. 2000. Development of a primary tamarin hepatocyte culture system for GB virus-B: a surrogate model for hepatitis C virus. J. Virol. 74:11764-11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukh, J., and C. L. Apgar. 1997. Five new or recently discovered (GBV-A) virus species are indigenous to New World monkeys and may constitute a separate genus of the Flaviviridae. Virology 229:429-436. [DOI] [PubMed] [Google Scholar]
- 8.Bukh, J., C. L. Apgar, and M. Yanagi. 1999. Toward a surrogate model for hepatitis C virus: an infectious molecular clone of the GB virus-B hepatitis agent. Virology 262:470-478. [DOI] [PubMed] [Google Scholar]
- 9.Bukh, J., C. L. Apgar, S. Govindarajan, and R. H. Purcell. 2001. Host range studies of GB virus-B hepatitis agent, the closest relative of hepatitis C virus, in New World monkeys and chimpanzees. J. Med. Virol. 65:694-697. [DOI] [PubMed] [Google Scholar]
- 10.Butkiewicz, N., N. Yao, W. Zhong, J. Wright-Minogue, P. Ingravallo, R. Zhang, J. Durkin, D. N. Standring, B. M. Baroudy, D. V. Sangar, S. M. Lemon, J. Y. Lau, and Z. Hong. 2000. Virus-specific cofactor requirement and chimeric hepatitis C virus/GB virus B nonstructural protein 3. J. Virol. 74:4291-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deinhardt, F., A. W. Holmes, R. B. Capps, and H. Popper. 1967. Studies on the transmission of human viral hepatitis to marmoset monkeys. J. Exp. Med. 125:673-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Tomassi, A., M. Pizzuti, R. Graziani, A. Sbardellati, S. Altamura, G. Paonessa, and C. Traboni. 2002. Cell clones selected from the Huh7 human hepatoma cell line support efficient replication of a subgenomic GB virus B replicon. J. Virol. 76:7736-7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foy, E., K. Li, C. Wang, R. Sumpter, Jr., M. Ikeda, S. M. Lemon, and M. Gale, Jr. Regulation of interferon regulatory factor-3 by the hepatitis c virus serine protease. Science 300:1145-1148. [Online.] [DOI] [PubMed]
- 14.Grace, K., M. Gartland, P. Karayiannis, M. J. McGarvey, and B. Clarke. 1999. The 5′ untranslated region of GB virus B shows functional similarity to the internal ribosome entry site of hepatitis C virus. J. Gen. Virol. 80:2337-2341. [DOI] [PubMed] [Google Scholar]
- 15.Hope, R. G., D. J. Murphy, and J. McLauchlan. 2002. The domains required to direct core proteins of hepatitis C virus and GB virus-B to lipid droplets share common features with plant oleosin proteins. J. Biol. Chem. 277:4261-4270. [DOI] [PubMed] [Google Scholar]
- 16.Ilan, E., J. Arazi, O. Nussbaum, A. Zauberman, R. Eren, I. Lubin, L. Neville, O. Ben-Moshe, A. Kischitzky, A. Litchi, I. Margalit, J. Gopher, S. Mounir, W. Cai, N. Daudi, A. Eid, O. Jurim, A. Czerniak, E. Galun, and S. Dagan. 2002. The hepatitis C virus (HCV)-Trimera mouse: a model for evaluation of agents against HCV. J. Infect. Dis. 185:153-161. [DOI] [PubMed] [Google Scholar]
- 17.Lanford, R. E., D. Chavez, L. Notvall, and K. M. Brasky. 2003. Comparison of tamarins and marmosets as hosts for GBV-B infections and the effect of immune suppression on the duration of viremia. Virology 311:72-80. [DOI] [PubMed] [Google Scholar]
- 18.Lanford, R. E., and L. Estlack. 1998. A cultivation method for highly differentiated primary chimpanzee hepatocytes permissive for hepatitis c virus replication. Methods Mol. Med. 19:501-515. [DOI] [PubMed] [Google Scholar]
- 19.Leary, T. P., S. M. Desai, J. C. Erker, and I. K. Mushahwar. 1997. The sequence and genomic organization of a GB virus A variant isolated from captive tamarins. J. Gen. Virol. 78:2307-2313. [DOI] [PubMed] [Google Scholar]
- 20.Lohman, V., F. Korner, J. O. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 21.Mercer, F. M., D. F. Schiller, J. F. Elliott, D. N. Douglas, C. Hao, A. Rinfret, W. R. Addison, K. P. Fischer, T. A. Churchill, J. R. T. Lakey, D. L. J. Tyrrell, and N. M. Kneteman. 2001. Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 7:927-933. [DOI] [PubMed] [Google Scholar]
- 22.Muerhoff, A. S., T. P. Leary, J. N. Simons, T. J. Pilot-Matias, G. J. Dawson, J. C. Erker, M. L. Chalmers, G. G. Schlauder, S. M. Desai, and I. K. Mushahwar. 1995. Genomic organization of GB viruses A and B: two new members of the Flaviviridae associated with GB agent hepatitis. J. Virol. 69:5621-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohba, K., M. Mizokami, J. Y. Lau, E. Orito, K. Ikeo, and T. Gojobori. 1996. Evolutionary relationship of hepatitis C, pesti-, flavi-, plantviruses, and newly discovered GB hepatitis agents. FEBS Lett. 378:232-234. [DOI] [PubMed] [Google Scholar]
- 24.Parks, W. P., and J. L. Melnick. 1969. Attempted isolation of hepatitis viruses in marmosets. J. Infect. Dis. 120:539-547. [DOI] [PubMed] [Google Scholar]
- 25.Parks, W. P., J. L. Melnick, W. R. Voss, D. B. Singer, H. S. Rosenberg, J. Alcott, A. M. Casazza. 1969. Characterization of marmoset hepatitis virus. J. Infect. Dis. 120:548-559. [DOI] [PubMed] [Google Scholar]
- 26.Rijnbrand, R., G. Abell, and S. M. Lemon. 2000. Mutational analysis of the GB virus B internal ribosome entry site. J. Virol. 74:773-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sbardellati, A., E. Scarselli, E. Verschoor, A. De Tomassi, D. Lazzaro, and C. Traboni. 2001. Generation of infectious and transmissible virions from a GB virus B full-length consensus clone in tamarins. J. Gen. Virol. 82:2437-2448. [DOI] [PubMed] [Google Scholar]
- 28.Sbardellati, A., E. Scarselli, V. Amati, S. Falcinelli, A. S. Kekule, and C. Traboni. 2000. Processing of GB virus B non-structural proteins in cultured cells requires both NS3 protease and NS4A cofactor. J. Gen. Virol. 81:2183-2188. [DOI] [PubMed] [Google Scholar]
- 29.Scarselli, E., A. Urbani, A. Sbardellati, L. Tomei, R. De Francesco, and C. Traboni. 1997. GB virus B and hepatitis C virus NS3 serine proteases share substrate specificity. J. Virol. 71:4985-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu, Y. K., R. H. Purcell, and H. Yoshikura. 1993. Correlation between the infectivity of hepatitis c virus in vivo and its infectivity in vitro. Proc. Natl. Acad. Sci. USA 90:6037-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simons, J. N., T. J. Pilot-Matias, T. P. Leary, G. J. Dawson, S. M. Desai, G. G. Schlauder, A. S. Muerhoff, J. C. Erker, S. H. Buijk, M. L. Chalmers, C. L. Van Sant, and I. K. Mushahwar. 1995. Identification of two flavivirus-like genomes in the GB hepatitis agent. Proc. Natl. Acad. Sci. USA 92:3401-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simons, J. N., T. P. Leary, G. J. Dawson, T. J. Pilot-Matias, A. S. Muerhoff, G. G. Schlauder, S. M. Desai, and I. K. Mushahwar. 1995. Isolation of novel virus-like sequences associated with human hepatitis. Nat. Med. 1:564-569. [DOI] [PubMed] [Google Scholar]
- 33.Zhong, W., P. Ingravallo, J. Wright-Minogue, A. S. Uss, A. Skelton, E. Ferrari J. Y. Lau, and Z. Hong. 2000. RNA-dependent RNA polymerase activity encoded by GB virus-B non-structural protein 5B. J. Viral Hep. 7:335-342. [DOI] [PubMed] [Google Scholar]
- 34.Zhong, W., P. Ingravallo, J. Wright-Minogue, A. Skelton, A. S. Uss, R. Chase, N. Yao, J. Y. Lau, and Z. Hong. 1999. Nucleoside triphosphatase and RNA helicase activities associated with GB virus B nonstructural protein 3. Virology 261:216-226. [DOI] [PubMed] [Google Scholar]