Abstract
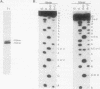
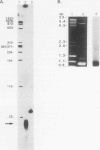
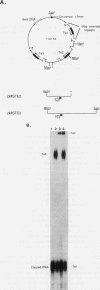
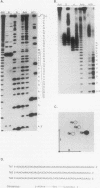
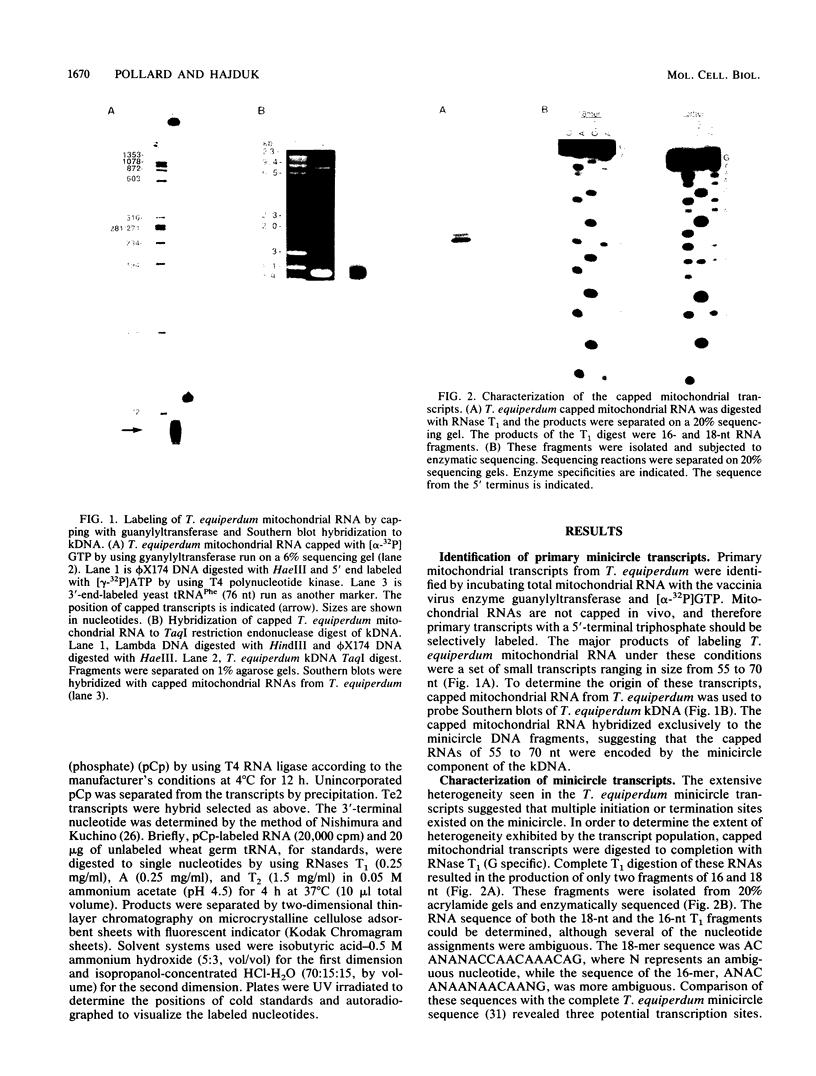
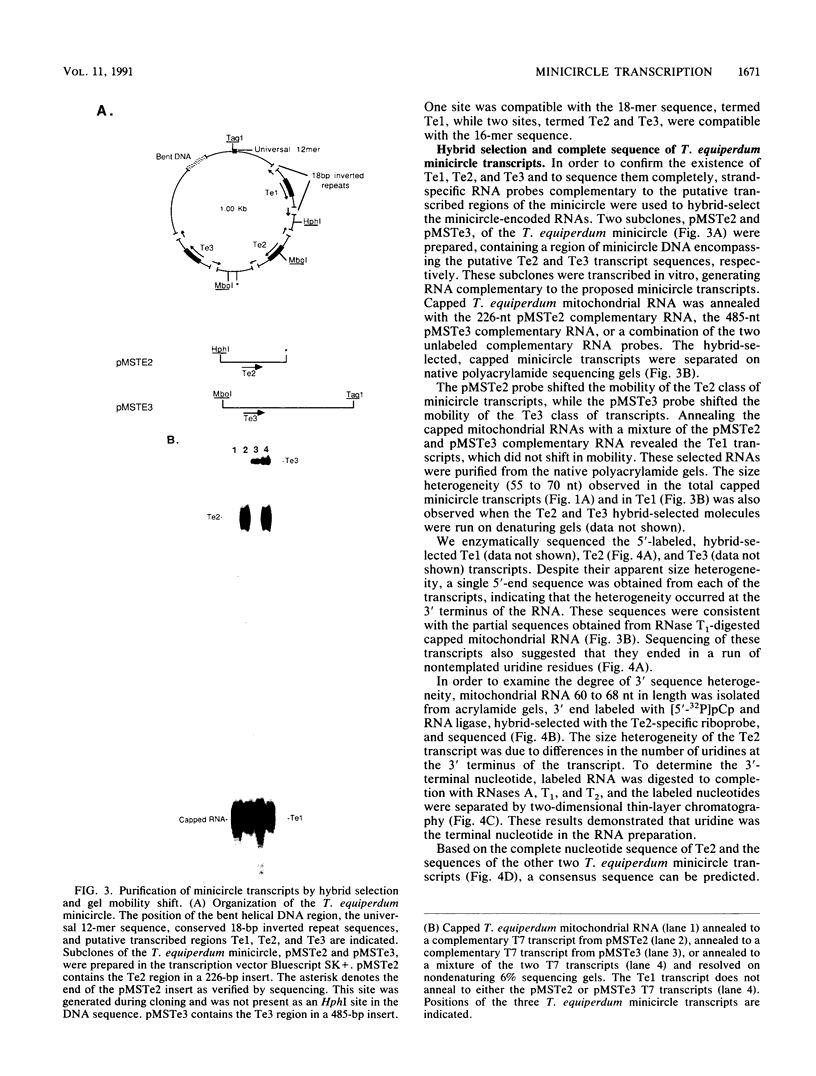
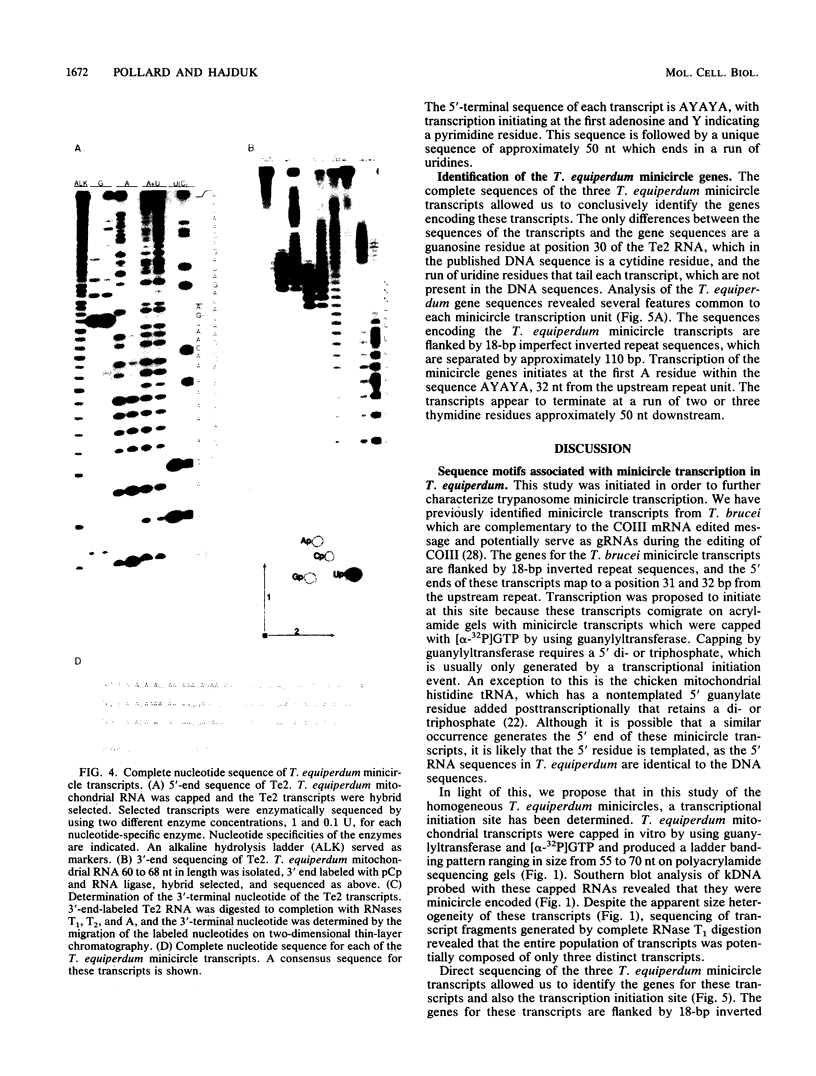
The mitochondrial DNA of trypanosomes is composed of maxicircle and minicircle DNAs catenated into a network, called the kinetoplast. Maxicircles encode proteins and RNAs necessary for mitochondrial assembly. Minicircles encode small transcripts which are believed to serve as guide RNAs in the process of RNA editing of maxicircle transcripts. Trypanosoma equiperdum minicircles contain three transcription units which produce three distinct transcripts. The genes for these transcripts are flanked by imperfect 18-bp repeats separated by approximately 110 bp. The transcripts have a 5' triphosphate, indicating that they are primary transcripts. Minicircle transcription initiates at a purine within a conserved sequence, 5'-AYAYA-3', where Y is a pyrimidine, 32 bp from the upstream inverted repeat, suggesting that the repeats may function in transcript initiation. Transcripts from a single minicircle transcription unit range in size from 55 to 70 nucleotides. This size heterogeneity within a single sequence class is due to the variable length of nontemplated uridine residues composing a 3' tail. The size range and heterogeneous polyuridylate 3' end of the minicircle transcripts appear to be conserved features and may be related to transcript function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Barrois M., Riou G., Galibert F. Complete nucleotide sequence of minicircle kinetoplast DNA from Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3323–3327. doi: 10.1073/pnas.78.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R. RNA-editing in trypanosome mitochondria. Biochim Biophys Acta. 1989 Mar 1;1007(2):131–139. doi: 10.1016/0167-4781(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Benne R., Van den Burg J., Brakenhoff J. P., Sloof P., Van Boom J. H., Tromp M. C. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986 Sep 12;46(6):819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- Bhat G. J., Koslowsky D. J., Feagin J. E., Smiley B. L., Stuart K. An extensively edited mitochondrial transcript in kinetoplastids encodes a protein homologous to ATPase subunit 6. Cell. 1990 Jun 1;61(5):885–894. doi: 10.1016/0092-8674(90)90199-o. [DOI] [PubMed] [Google Scholar]
- Blum B., Bakalara N., Simpson L. A model for RNA editing in kinetoplastid mitochondria: "guide" RNA molecules transcribed from maxicircle DNA provide the edited information. Cell. 1990 Jan 26;60(2):189–198. doi: 10.1016/0092-8674(90)90735-w. [DOI] [PubMed] [Google Scholar]
- Blum B., Simpson L. Guide RNAs in kinetoplastid mitochondria have a nonencoded 3' oligo(U) tail involved in recognition of the preedited region. Cell. 1990 Jul 27;62(2):391–397. doi: 10.1016/0092-8674(90)90375-o. [DOI] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Braly P., Simpson L., Kretzer F. Isolation of kinetoplast-mitochondrial complexes from Leishmania tarentolae. J Protozool. 1974 Nov;21(5):782–790. doi: 10.1111/j.1550-7408.1974.tb03752.x. [DOI] [PubMed] [Google Scholar]
- Chen K. K., Donelson J. E. Sequences of two kinetoplast DNA minicircles of Tryptanosoma brucei. Proc Natl Acad Sci U S A. 1980 May;77(5):2445–2449. doi: 10.1073/pnas.77.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker C. J., Sollner-Webb B. RNA editing involves indiscriminate U changes throughout precisely defined editing domains. Cell. 1990 Jun 15;61(6):1001–1011. doi: 10.1016/0092-8674(90)90065-m. [DOI] [PubMed] [Google Scholar]
- Donelson J. E., Majiwa P. A., Williams R. O. Kinetoplast DNA minicircles of Trypanosoma brucei share regions of sequence homology. Plasmid. 1979 Oct;2(4):572–588. doi: 10.1016/0147-619x(79)90055-6. [DOI] [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Weislogel P. O., Hoeijmakers J. H., Borst P. Isolation and characterization of kinetoplast DNA from bloodstream form of Trypanosoma brucei. J Cell Biol. 1978 Feb;76(2):293–309. doi: 10.1083/jcb.76.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Abraham J. M., Stuart K. Extensive editing of the cytochrome c oxidase III transcript in Trypanosoma brucei. Cell. 1988 May 6;53(3):413–422. doi: 10.1016/0092-8674(88)90161-4. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Jasmer D. P., Stuart K. Developmentally regulated addition of nucleotides within apocytochrome b transcripts in Trypanosoma brucei. Cell. 1987 May 8;49(3):337–345. doi: 10.1016/0092-8674(87)90286-8. [DOI] [PubMed] [Google Scholar]
- Frasch A. C., Hajduk S. L., Hoeijmakers J. H., Borst P., Brunel E., Davison J. The kinetoplast DNA of Trypanosoma equiperdum. Biochim Biophys Acta. 1980 May 30;607(3):397–410. doi: 10.1016/0005-2787(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Harris M. E., Moore D. R., Hajduk S. L. Addition of uridines to edited RNAs in trypanosome mitochondria occurs independently of transcription. J Biol Chem. 1990 Jul 5;265(19):11368–11376. [PubMed] [Google Scholar]
- Jasmer D. P., Stuart K. Sequence organization in African trypanosome minicircles is defined by 18 base pair inverted repeats. Mol Biochem Parasitol. 1986 Mar;18(3):321–331. doi: 10.1016/0166-6851(86)90089-7. [DOI] [PubMed] [Google Scholar]
- Kidane G. Z., Hughes D., Simpson L. Sequence heterogeneity and anomalous electrophoretic mobility of kinetoplast minicircle DNA from Leishmania tarentolae. Gene. 1984 Mar;27(3):265–277. doi: 10.1016/0378-1119(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Koslowsky D. J., Bhat G. J., Perrollaz A. L., Feagin J. E., Stuart K. The MURF3 gene of T. brucei contains multiple domains of extensive editing and is homologous to a subunit of NADH dehydrogenase. Cell. 1990 Sep 7;62(5):901–911. doi: 10.1016/0092-8674(90)90265-g. [DOI] [PubMed] [Google Scholar]
- L'Abbé D., Lang B. F., Desjardins P., Morais R. Histidine tRNA from chicken mitochondria has an uncoded 5'-terminal guanylate residue. J Biol Chem. 1990 Feb 15;265(5):2988–2992. [PubMed] [Google Scholar]
- Lanham S. M. Separation of trypanosomes from the blood of infected rats and mice by anion-exchangers. Nature. 1968 Jun 29;218(5148):1273–1274. doi: 10.1038/2181273a0. [DOI] [PubMed] [Google Scholar]
- Marini J. C., Levene S. D., Crothers D. M., Englund P. T. Bent helical structure in kinetoplast DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7664–7668. doi: 10.1073/pnas.79.24.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntambi J. M., Marini J. C., Bangs J. D., Hajduk S. L., Jimenez H. E., Kitchin P. A., Klein V. A., Ryan K. A., Englund P. T. Presence of a bent helix in fragments of kinetoplast DNA minicircles from several trypanosomatid species. Mol Biochem Parasitol. 1984 Jul;12(3):273–286. doi: 10.1016/0166-6851(84)90084-7. [DOI] [PubMed] [Google Scholar]
- Pollard V. W., Rohrer S. P., Michelotti E. F., Hancock K., Hajduk S. L. Organization of minicircle genes for guide RNAs in Trypanosoma brucei. Cell. 1990 Nov 16;63(4):783–790. doi: 10.1016/0092-8674(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Rohrer S. P., Michelotti E. F., Torri A. F., Hajduk S. L. Transcription of kinetoplast DNA minicircles. Cell. 1987 Jun 5;49(5):625–632. doi: 10.1016/0092-8674(87)90538-1. [DOI] [PubMed] [Google Scholar]
- Shaw J. M., Feagin J. E., Stuart K., Simpson L. Editing of kinetoplastid mitochondrial mRNAs by uridine addition and deletion generates conserved amino acid sequences and AUG initiation codons. Cell. 1988 May 6;53(3):401–411. doi: 10.1016/0092-8674(88)90160-2. [DOI] [PubMed] [Google Scholar]
- Silver L. E., Torri A. F., Hajduk S. L. Organized packaging of kinetoplast DNA networks. Cell. 1986 Nov 21;47(4):537–543. doi: 10.1016/0092-8674(86)90618-5. [DOI] [PubMed] [Google Scholar]
- Simpson L. Kinetoplast DNA in trypanosomid flagellates. Int Rev Cytol. 1986;99:119–179. doi: 10.1016/s0074-7696(08)61426-6. [DOI] [PubMed] [Google Scholar]
- Simpson L., Shaw J. RNA editing and the mitochondrial cryptogenes of kinetoplastid protozoa. Cell. 1989 May 5;57(3):355–366. doi: 10.1016/0092-8674(89)90911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert M., Van Assel S. Sequence heterogeneity in kinetoplast DNA: reassociation kinetics. Plasmid. 1980 Jan;3(1):7–17. doi: 10.1016/s0147-619x(80)90030-x. [DOI] [PubMed] [Google Scholar]
- Stuart K. Kinetoplast DNA, mitochondrial DNA with a difference. Mol Biochem Parasitol. 1983 Oct;9(2):93–104. doi: 10.1016/0166-6851(83)90103-2. [DOI] [PubMed] [Google Scholar]
- Stuart K. RNA editing: new insights into the storage and expression of genetic information. Parasitol Today. 1989 Jan;5(1):5–8. doi: 10.1016/0169-4758(89)90211-1. [DOI] [PubMed] [Google Scholar]
- Sturm N. R., Simpson L. Kinetoplast DNA minicircles encode guide RNAs for editing of cytochrome oxidase subunit III mRNA. Cell. 1990 Jun 1;61(5):879–884. doi: 10.1016/0092-8674(90)90198-n. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985 Apr;41(2):105–114. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]