This work demonstrates that D6PK AGC kinases and PIN auxin transporters are required for phototropic hypocotyl bending. The findings suggest that D6PK-mediated phosphorylation of PIN transporters promotes auxin transport in the hypocotyl to ensure proper phototropic hypocotyl bending.
Abstract
Phototropic hypocotyl bending in response to blue light excitation is an important adaptive process that helps plants to optimize their exposure to light. In Arabidopsis thaliana, phototropic hypocotyl bending is initiated by the blue light receptors and protein kinases phototropin1 (phot1) and phot2. Phototropic responses also require auxin transport and were shown to be partially compromised in mutants of the PIN-FORMED (PIN) auxin efflux facilitators. We previously described the D6 PROTEIN KINASE (D6PK) subfamily of AGCVIII kinases, which we proposed to directly regulate PIN-mediated auxin transport. Here, we show that phototropic hypocotyl bending is strongly dependent on the activity of D6PKs and the PIN proteins PIN3, PIN4, and PIN7. While early blue light and phot-dependent signaling events are not affected by the loss of D6PKs, we detect a gradual loss of PIN3 phosphorylation in d6pk mutants of increasing complexity that is most severe in the d6pk d6pkl1 d6pkl2 d6pkl3 quadruple mutant. This is accompanied by a reduction of basipetal auxin transport in the hypocotyls of d6pk as well as in pin mutants. Based on our data, we propose that D6PK-dependent PIN regulation promotes auxin transport and that auxin transport in the hypocotyl is a prerequisite for phot1-dependent hypocotyl bending.
INTRODUCTION
Throughout their growth and development, plants are constantly exposed to changing light environments. To optimize photosynthetic light capture, plants have evolved different light receptor and signaling systems that allow them to optimally adjust their growth to the specific light qualities and quantities of their environment. In this context, the phototropin (phot) blue light receptors serve to optimize photosynthesis in low-light conditions and to avoid photodamage in high-light conditions (Christie, 2007). In Arabidopsis thaliana, two phototropins, phot1 and phot2, have been identified that differentially contribute to the known blue light–regulated responses (Christie et al., 1998; Kagawa et al., 2001): phot1, and in high fluence light also phot2, influence chloroplast accumulation, leaf positioning, and phototropic responses, such as phototropic hypocotyl bending (Christie et al., 1998; Folta and Spalding, 2001; Briggs and Christie, 2002). Whereas in low-light conditions phot1 mainly regulates chloroplast accumulation at the upper cell surface to optimize photosynthesis, phot2 regulates the chloroplast high-light avoidance movement that serves to prevent photodamage to the photosynthetic apparatus (Kagawa et al., 2001; Sakai et al., 2001). phot1 and phot2 also redundantly promote blue light–induced stomatal opening to regulate CO2 uptake for photosynthesis and water loss by transpiration (Kinoshita et al., 2001). Phototropins also regulate aspects of vegetative growth in that they mediate leaf curling and thereby affect the exposure to light in leaves of adult plants (Takemiya et al., 2005). Thus, phot1 and phot2 differentially regulate various processes that help plants to adjust their growth in unfavorable environments, such as weak or strong sunlight as well as during drought.
phot blue light receptors are composed of an AGC (cAMP-dependent kinase, cGMP-dependent kinase, phospholipid-dependent protein kinase C) kinase domain as well as two photosensory LOV (light-oxygen-voltage) domains (Christie et al., 1998; Galván-Ampudia and Offringa, 2007). Photoexcitation of the LOV domain by blue light leads to phot1 autophosphorylation, which results in the reduced electrophoretic mobility of the excited photoreceptor (Christie et al., 1998; Sakai et al., 2001). In addition to phot1, a number of other proteins have been found to be essential for blue light–induced hypocotyl bending: NONPHOTOTROPIC HYPOCOTYL3 (NPH3) is a phot1-interacting protein required for phototropism that becomes dephosphorylated after blue light photoexcitation in a manner that negatively correlates with the phosphorylation of phot1 (Motchoulski and Liscum, 1999; Pedmale and Liscum, 2007). NPH3 was recently characterized as a subunit of an E3 ubiquitin ligase complex that regulates phot1 protein turnover (Roberts et al., 2011). Interestingly, the NPH3-related protein ROOT PHOTOTROPISM2 also interacts with phot1 and participates in blue light–dependent hypocotyl bending as well as in stomatal opening (Inada et al., 2004). Furthermore, phototropic hypocotyl bending requires the PHYTOCHROME KINASE SUBSTRATE (PKS) proteins PKS1, PKS2, and PKS4, which are proteins of unknown biochemical function that interact with phot1 as well as with NPH3 but also with the phytochrome red light photoreceptors (Fankhauser et al., 1999; Lariguet et al., 2006; Schepens et al., 2008; de Carbonnel et al., 2010). At least one member of the PKS protein family, PKS4, is transiently phosphorylated in a blue light– and phot1-dependent manner with kinetics that are highly similar to those seen for phot1 autophosphorylation and NPH3 dephosphorylation (Demarsy et al., 2012). Since PKS4 can also be phosphorylated by phot1 in vitro, PKS4 has been proposed to be a component of the early blue light phototropic response pathway (Demarsy et al., 2012).
Phototropic hypocotyl bending but not other blue light–dependent responses also depend on the transcription factor NPH4/AUXIN RESPONSE FACTOR7 (ARF7) and the auxin-induced degradation of its auxin/indole-3-acetic acid (IAA) inhibitor MASSUGU2/INDOLE-3-ACETIC ACID19 (Harper et al., 2000; Tatematsu et al., 2004). nph4/arf7 mutants are defective in phototropic bending, positive root gravitropism, and negative gravitropism of the hypocotyl (Stowe-Evans et al., 1998; Harper et al., 2000). Interestingly, the hypocotyl gravitropism defects of nph4/arf7 mutants but not their root gravitropism defects can be partially suppressed by ethylene treatments, suggesting that ethylene signaling acts downstream of NPH4/ARF7 in the hypocotyl or that ethylene promotes another limiting step during phototropic hypocotyl bending (Harper et al., 2000).
Following blue light perception, phototropic hypocotyl bending is mediated by an increase in the growth of the hypocotyl on the shaded side of the hypocotyl stem (Friml et al., 2002; Ding et al., 2011). It is expected that this process is controlled by the lateral distribution of auxin as a response to the phototropic stimulus. Indeed, several auxin transport mutants have been described to have phototropic response phenotypes: Mutants with a defect in the protein ATP BINDING CASSETTE B19 (ABCB19/MDR1/PGP19), which maintains long-distance auxin transport through vascular tissues, exhibit enhanced phototropic curvature that may be explained by an accumulation of auxin in the hypocotyl elongation zone or the apical hook region (Noh et al., 2003; Nagashima et al., 2008; Christie et al., 2011). Intriguingly, phot1 can phosphorylate ABCB19 and transiently inactivates ABCB19 auxin transport activity. Thus, there appears to be a direct signaling mechanism between blue light perception by phot1 and phototropic hypocotyl bending (Christie et al., 2011). In addition, three auxin efflux facilitators have been implicated in phototropic bending, namely, PIN1, PIN3, and PIN7 (Friml et al., 2002; Blakeslee et al., 2004; Nagashima et al., 2008; Christie et al., 2011; Ding et al., 2011; Haga and Sakai, 2012a, 2012b). Interestingly, the contributions of the individual PIN genes to phototropic signaling are rather subtle (Friml et al., 2002; Blakeslee et al., 2004). A recent detailed analysis of various PIN gene mutants found that the contributions of PIN1, PIN3, and PIN7 to phototropic hypocotyl bending become relatively obvious when dark-grown seedlings are exposed to a short blue light pulse (pulse-induced first positive phototropism). Strikingly, these phototropism defects become much weaker when seedlings are exposed to long-term blue light treatments (second positive phototropism), such as those employed in most phototropism studies (Haga and Sakai, 2012a, 2012b). At least in the case of PIN3, it was proposed that a phot1-dependent PIN3 polarity change is a prerequisite for phototropic bending in Arabidopsis hypocotyl cells (Ding et al., 2011). This polarity change is dependent on the three closely related AGCVIII kinases PINOID, WAG1, and WAG2 that were shown to regulate PIN protein polarity through phosphorylation at conserved Ser residues of the PIN proteins (Dhonukshe et al., 2010; Huang et al., 2010; Ding et al., 2011). Thus, differential distribution of PIN3 and possibly other PINs may represent a major regulatory step for the redistribution of auxin and phototropic hypocotyl bending. Although this may imply that phot1 is the protein kinase that drives PIN3 lateralization, the signal transduction from phot1 to PIN3 is seemingly indirect because PID but not phot1 can phosphorylate PIN3 in vitro (Ding et al., 2011). The accumulation of auxin at the shaded side of the hypocotyl of dark-grown seedlings following blue light photoexcitation was reported to correlate with the formation of a local maximum of the phytohormone auxin (Friml et al., 2002). Since this lateralization is impaired in pin3 mutants, auxin distribution correlates with PIN3 lateralization, and this may thus indeed be essential for phototropic hypocotyl bending (Friml et al., 2002; Ding et al., 2011).
We previously described a family of four Arabidopsis AGCVIII kinases that we named D6 PROTEIN KINASEs (D6PKs). D6PK, the founding member of the family, colocalizes with the PIN auxin transport facilitators, and D6PK phosphorylates PIN proteins in vitro and PIN1 in vivo (Zourelidou et al., 2009). d6pk mutants have various phenotypes that are indicative of a role for D6PK in the control of auxin transport, and we therefore proposed that D6PKs regulate the auxin transport activity of PINs by direct phosphorylation (Zourelidou et al., 2009). Here, we show that D6PKs are important regulators of phototropic hypocotyl bending and leaf flattening but not of other phot-mediated processes. Interestingly, basipetal auxin transport is reduced in d6pk mutants, and this decrease correlates with a decrease in PIN3 phosphorylation. We therefore propose that D6PK-mediated PIN phosphorylation promotes auxin transport in the hypocotyl and that this is a prerequisite for proper phototropic hypocotyl bending.
RESULTS
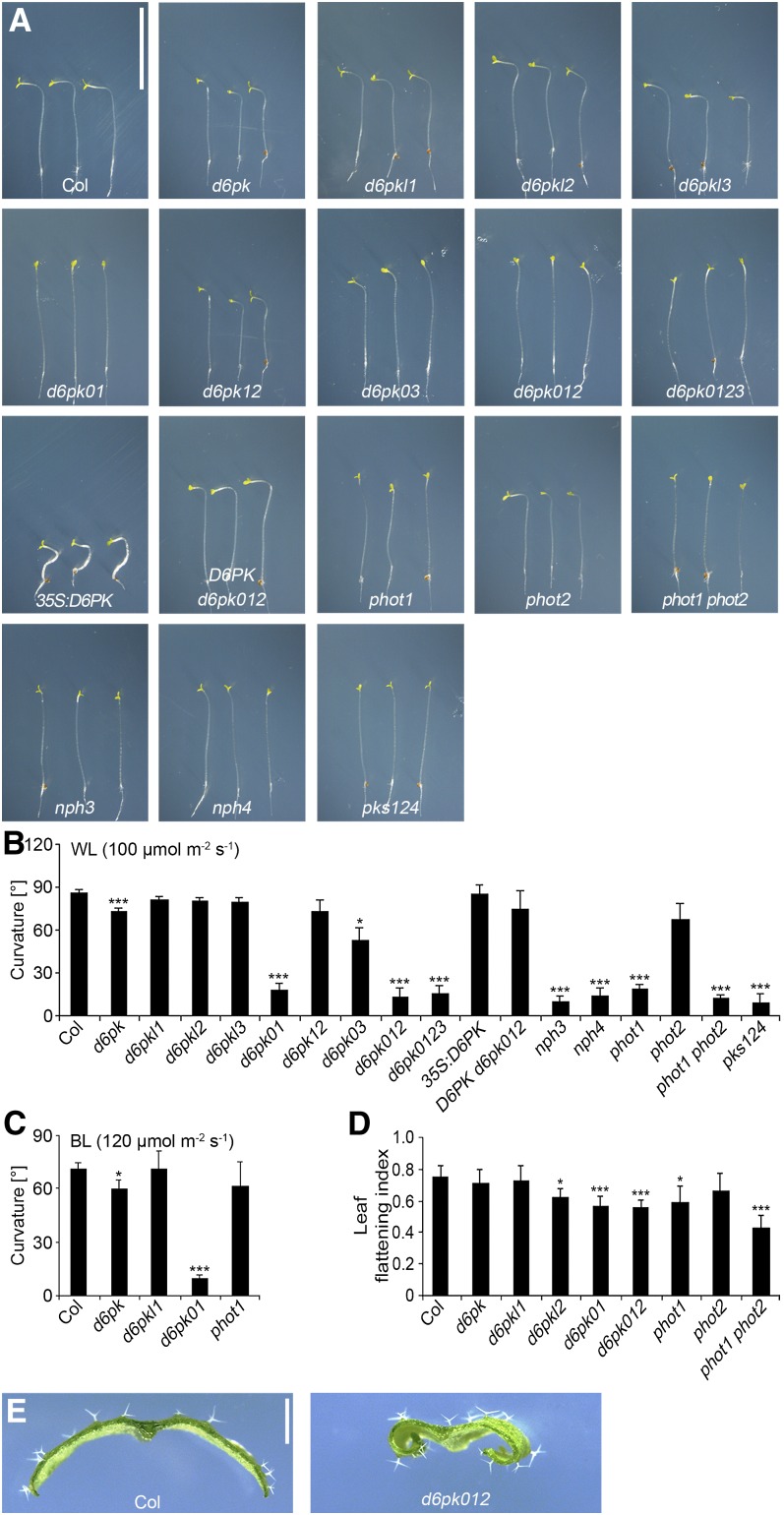
D6PK Is Required for Blue Light–Dependent Hypocotyl Bending
We previously characterized mutants deficient in the function of D6PK and its paralogs D6PKL1, D6PKL2, and D6PKL3 (Zourelidou et al., 2009). During our further phenotypic analyses of the available set of d6pk loss-of-function mutant combinations, it became apparent that d6pk d6pkl1 (d6pk01) double, d6pk d6pkl1 d6pkl2 (d6pk012) triple, and d6pk d6pkl1 d6pkl2 d6pkl3 (d6pk0123) quadruple mutants were strongly impaired in phototropic hypocotyl bending when exposed to lateral white light (100 µmol m−2 s−1) (Figures 1A and 1B). More detailed analyses then revealed that weak but significant phototropism defects could already be observed in the d6pk single mutant and that this phenotype was enhanced not only in d6pk d6pkl1 (d6pk01) but also in the d6pk d6pkl3 (d6pk03) double mutant (Figures 1A and 1B). In agreement with an important role for the founding member of the D6PK gene family, D6PK, in mediating phototropic hypocotyl bending, we further found that the phototropism defect of d6pk012 mutant was largely suppressed when we expressed D6PK as an N-terminally yellow fluorescent protein (YFP)–tagged D6PK cDNA under control of a 2-kb D6PK promoter fragment in this mutant (D6PK d6pk012; Figures 1A and 1B). Conversely, we found that phototropic hypocotyl bending was still functional when we overexpressed an N-terminally YFP-tagged D6PK cDNA under the control of the cauliflower mosaic virus 35S promoter in the wild-type background (35S:D6PK; Figures 1A and 1B). When we compared this phototropism defect with that of other previously identified phototropism mutants, we found that the mutant phenotype of the d6pk01 mutants was as severe as those observed in phot1/nph1, nph3, and nph4 single mutants or in pks1 pks2 pks4 (pks124) triple mutants (Figures 1A and 1B).
Figure 1.
D6PK Is Required for Phototropic Growth Responses.
(A) Representative photographs of 5-d-old wild-type and mutant seedlings after exposure for 20 h to unilateral white light (100 µmol m−2 s−1). Please note that variations in the position of the bending zone on the hypocotyl do not reflect variations in the phenotype but are the result of the respective position of the seedling on the plate and its distance to the light source. Col, Columbia. Bar = 10 mm.
(B) and (C) Quantification of hypocotyl bending in 5-d-old etiolated seedlings exposed for 20 h to unilateral white light (WL; 100 µmol m−2 s−1) (B) or unilateral blue light (BL; 120 µmol m−2 s−1) (C). The graphs show the average and sd of three representative experiments with at least 12 seedlings. Agravitropically growing seedlings were reoriented toward the gravity vector in safe green light before phototropism response experiments were initiated.
(D) Average and sd of the leaf flattening index determined from leaf number 5 from 25-d-old plants. The leaf flattening index is the ratio of the maximum width before and after manual flattening of the leaf.
(E) Representative photographs of cross-sectioned leaves of the wild type and d6pk012 mutants. Student’s t test, compared with Col: *0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001. Bar = 1 mm.
We then confirmed, using low-intensity blue light (5 µmol m−2 s−1), that the observed defects in phototropic bending were true defects in blue light signaling (see Supplemental Figure 1 online). Furthermore, because PHOT2 can confer phototropic hypocotyl bending in high-light conditions, also in the absence of PHOT1, we examined the phototropic responses of d6pk mutants in high-intensity blue light (120 µmol m−2 s−1 blue light) (Sakai et al., 2001). Interestingly, we found that the d6pk01 double mutant remained nonphototropic even in these experimental conditions that were sufficient to induce phototropic hypocotyl bending in phot1 mutants (Figure 1C).
Leaf flattening is another phot-dependent phenotype and phot1 as well as phot1 phot2 double mutants have characteristically inward-curled leaves when compared with the wild type (Figure 1D) (Takemiya et al., 2005). Interestingly, d6pkl2 mutants and the strongly nonphototropic d6pk01 and d6pk012 mutants had increased leaf curling, suggesting that D6PK may act in the same signaling pathway with phot in this growth response as well (Figure 1D).
To test whether the D6PK genes are expressed in the hypocotyl of dark-grown seedlings, we made use of lines expressing the β-glucuronidase (GUS) reporter under control of 2-kb D6PK, D6PKL1, D6PKL2, and D6PKL3 promoter fragments. In line with a functional role for D6PKs in the hypocotyl, we found that all four D6PK genes are expressed in the hypocotyl (see Supplemental Figure 2A online). We also examined whether blue light irradiation has an influence on D6PK transcript abundance and examined the expression regulation of D6PKs by histological GUS reporter staining and quantitative RT-PCR after exposure for 2 and 4 h. We observed only minor changes in D6PK gene expression and none that could reasonably be correlated with the strong defects in phototropic hypocotyl bending observed in the mutants (see Supplemental Figure 2 online). We thus consider it unlikely that transcriptional regulation of D6PKs is part of/involved in phototropism signaling.
We also examined whether light irradiation changes the stability or abundance of D6PK using the 35S:D6PK line, which expresses a functional YFP-tagged D6PK protein. However, we did not observe any light-dependent changes in YFP:D6PK abundance in the dark or in the light in the presence of the protein biosynthesis inhibitor cycloheximide (see Supplemental Figure 3 online). We thus concluded that light does not regulate D6PK protein levels.
In summary, our findings suggest that members of the D6PK family act redundantly to regulate the phot-mediated growth responses of hypocotyl bending and leaf flattening. Furthermore, our results indicate that neither D6PK transcriptional regulation nor regulation of D6PK protein abundance can explain the phototropic bending defects of the d6pk mutants. Since leaf curling was determined from plants that, for technical reasons, were grown in white light, we cannot conclude with certainty that the leaf flattening phenotype of these mutants is the result of impaired blue light and phot signaling.
D6PKs Are Not Essential for the Regulation of Stomatal Aperture and Chloroplast Relocation
phots also mediate blue light–induced stomata opening and chloroplast relocation (Kagawa et al., 2001; Kinoshita et al., 2001; Sakai et al., 2001). Since the phototropism assay had suggested a role of D6PKs in phot-mediated processes, we also examined the potential role of D6PKs in blue light–induced stomata opening. To this end, we performed gas exchange experiments using d6pk, d6pkl1, and d6pkl2 single, d6pk01 and d6pk12 double, and d6pk012 triple mutants as well as the 35S:D6PK overexpressor line (see Supplemental Figure 4 online). However, in comparison to the strong defects in phototropic hypocotyl bending that we had observed in the d6pk01 double and d6pk012 triple mutants, we detected no defects in stomatal opening either in these d6pk mutants or in the D6PK overexpressor (see Supplemental Figure 4 online). The only noteworthy observation from this analysis was an increase in the speed of stomatal opening in d6pk012 triple mutants when compared with the wild type. This phenotype was unexpected since it was, on the one hand, opposite to the decreased blue light response of the phot1 phot2 double mutant and, on the other hand, not observed in less complex d6pk mutant combinations (see Supplemental Figure 4 online). Regardless of the potential physiological explanation of this d6pk mutant response, we concluded that D6PKs are not essential for blue light–induced stomatal opening, but may affect, directly or indirectly, this process.
Blue light–induced chloroplast avoidance movement depends on phot2 (Kagawa et al., 2001) (see Supplemental Figure 5 online). When we tested whether this chloroplast movement response also requires D6PKs, we observed no major difference after exposure to low or high-intensity blue light when using light transmittance measurements as a readout for chloroplast relocation in d6pk, d6pkl1, and d6pkl2 single, d6pk01 and d6pk12 double, or d6pk012 triple mutants (see Supplemental Figure 5 online). We noted only small increases in the magnitude of the chloroplast movement response induced by low blue light (from 1 to 10 µmol m−2 s−1) in the d6pk mutants as well as small increases in the light intensity required to induce the chloroplast movement response in high-intensity light (>100 µmol m−2 s−1) (see Supplemental Figure 5 online). In the range from 1 to 10 µmol m−2 s−1, this difference was greatest between the wild type and the d6pk01 double and the d6pk012 triple mutant and least pronounced between the wild type and the d6pkl2 mutant (see Supplemental Figure 5 online). There was thus a correlation between the severity of this phenotype and d6pk mutant complexity that we had also observed for other d6pk mutant phenotypes (Figures 1A and 1B; Zourelidou et al., 2009). Although these chloroplast movement phenotypes are different from the phenotypes of the phot2 mutants, based on this correlation, we do not want to rule out that these subtle phenotypes are physiologically relevant. However, since the phenotype of none of the tested d6pk mutants was as severe as that recorded for the phot2 mutant, we concluded that D6PKs are not required for proper chloroplast movement in response to blue light.
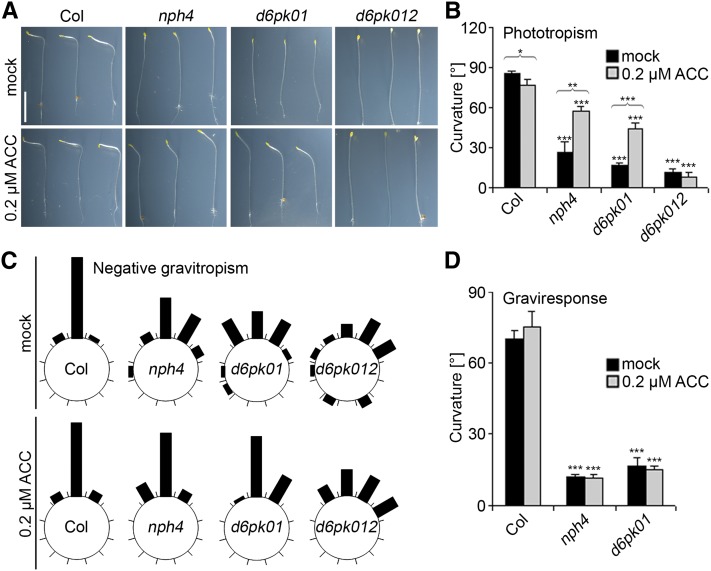
Ethylene Promotes Phototropic Bending in d6pk Mutants
Ethylene treatment had previously been reported to partially suppress the nonphototropic phenotype of nph4/arf7 mutants (Harper et al., 2000). To test whether ethylene treatment can also compensate for the loss of D6PK gene function, we performed phototropism experiments with d6pk mutant seedlings grown on media containing the ethylene precursor 1-aminocyclopropane-1-carboxylic-acid (ACC), which is converted into the active gaseous ethylene when taken up by the plant. Interestingly, ACC treatment was sufficient to promote phototropic bending in the d6pk01 double mutant but failed to do so in the d6pk012 triple mutant (Figures 2A and 2B). Similarly to nph4/arf7 mutants, d6pk01 double and d6pk012 triple mutants were defective in maintaining the negative gravitropic growth of their hypocotyls when grown in the dark (Harper et al., 2000) (Figure 2C). Interestingly, this phenotype, which can be suppressed by ACC treatment in nph4/arf7 mutants, was also partially suppressed in ACC-grown d6pk01 and to a minor extent also in d6pk012 mutant seedlings (Figure 2C). We went on to test the negative gravitropism response of d6pk01 mutants by measuring hypocotyl curvature following a change of the gravitropic vector by 90°. Intriguingly, nph4/arf7 and d6pk01 mutants were deficient in this negative gravitropism response, and the phenotype could not be suppressed by ACC treatment over the time course of this experiment (Figure 2D). We thus concluded that d6pk01 mutants share defects in phototropism and negative gravitropism with nph4/arf7 mutants, including in the effects of ethylene on these responses, and that, at least with regard to phototropism, the d6pk012 triple mutants are profoundly less sensitive to ethylene than are the d6pk01 mutants or nph4/arf7.
Figure 2.
ACC Treatment Can Partially Suppress Tropism Defects of d6pk Mutants.
(A) Representative photographs of three 5-d-old wild-type and mutant seedlings grown in the absence and presence of 0.2 µM ACC and exposed to unilateral white light (100 µmol m−2 s−1) for 20 h. Bar = 5 mm.
(B) Quantification of hypocotyl bending in 5-d-old etiolated seedlings as shown in (A).
(C) Quantification of negative gravitropism of the hypocotyls of 5-d-old seedlings. Each segment corresponds to a 30° angle window.
(D) Quantification of graviresponse of 5-d-old wild-type and mutant seedlings grown in the dark in the absence or presence of 0.2 µM ACC. Hypocotyl gravitropism was determined 24 h after changing the gravity vector by 90°.
In (A), (B), and (D), the agravitropically growing seedlings were reoriented toward the gravity vector in safe green light before phototropism response ([A] and [B]) and graviresponse (D) experiments were initiated. Average and sd were determined from at least 12 seedlings. Student’s t test, compared with Columbia (Col) unless otherwise indicated by brackets: *0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001.
[See online article for color version of this figure.]
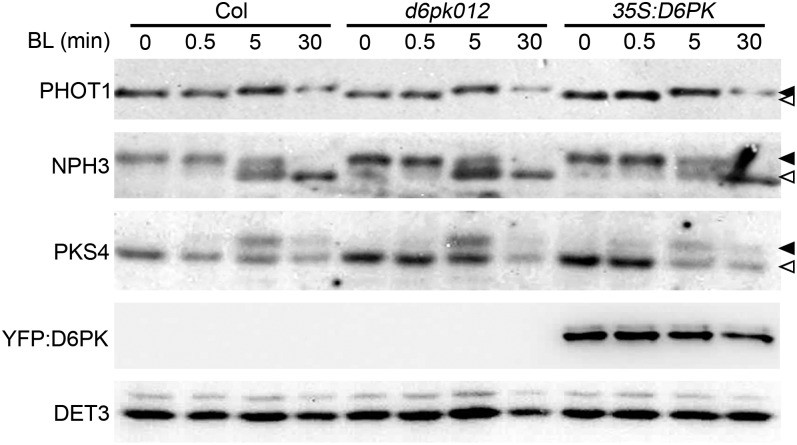
Blue Light–Induced Phosphorylation and Dephosphorylation Events Are Independent of D6PKs
At the molecular level, early blue light–induced phototropic bending is characterized by a rapid and transient phosphorylation of phot1 and PKS4 as well as a concomitant dephosphorylation of NPH3 (Liscum and Briggs, 1995; Christie et al., 1998; Pedmale and Liscum, 2007; Demarsy et al., 2012). Both phot proteins and D6PKs belong to the family of AGCVIII kinases, and we hypothesized that D6PK may potentially contribute to these phot1-dependent early signaling events. To test this hypothesis, we examined phot1 and PKS4 phosphorylation as well as NPH3 dephosphorylation in the d6pk012 triple mutants and the 35S:D6PK overexpression line. However, we failed to detect reproducible differences in the phosphorylation of phot1 or PKS4 or the dephosphorylation of NPH3 that were sufficiently pronounced to explain the strong defect in blue light–induced hypocotyl bending of the d6pk012 mutants (Figure 3). We thus concluded that the D6PK AGC kinases do not participate in the early blue light–induced phosphorylation and dephosphorylation events and that they act independently from the AGC kinases and blue light receptors phot1 and phot2.
Figure 3.
Early Phosphorylation Events of Phototropism Signaling Are Unaffected in d6pk012 Mutants and D6PK Overexpressors.
Immunoblot analyses of phot1, NPH3, PKS4, YFP:D6PK (anti-GFP), and DET3 (loading control) of protein extracts prepared from dark-grown seedlings after 0, 0.5, 5, and 30 min of blue light illumination (1.5 µmol m−2 s−1). Closed arrowheads, top band, phosphorylated form(s); open arrowhead, bottom band.
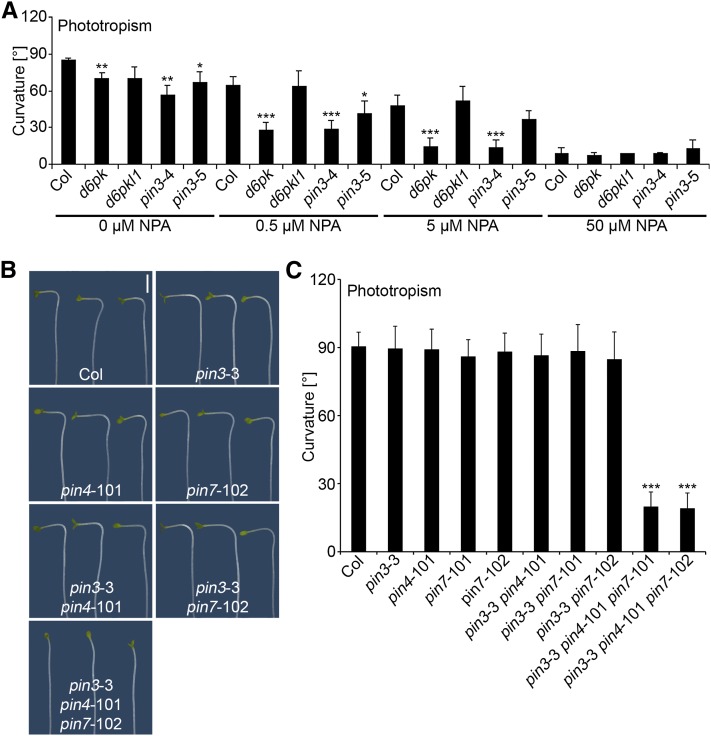
Phototropic Hypocotyl Bending Is Dependent on the Auxin Efflux Carriers PIN3, PIN4, and PIN7
Several studies have demonstrated a role for polar auxin transport in phototropic hypocotyl bending, and it has recently been shown that PIN proteins, including PIN3, are required for proper phototropic hypocotyl bending (Friml et al., 2002; Ding et al., 2011; Haga and Sakai, 2012b). Since we had previously proposed that D6PKs regulate auxin transport and that PIN auxin efflux facilitators may be direct targets of D6PKs, we reasoned that phototropic hypocotyl bending in d6pk and pin3 mutants may be hypersensitive to treatments with the auxin transport inhibitor naphthylphthalamic acid (NPA) if these mutants had a critical defect in auxin transport. Indeed, we measured a strong impairment of phototropism in pin3 and d6pk mutants at concentrations of NPA (0.5 and 5 µM) that had only a minor effect on phototropic responses in the wild type (Figure 4A). Based on these results, we hypothesized that D6PKs may function together with PIN3 and possibly other PINs in the control of auxin transport during hypocotyl bending. Since the phototropism defects of d6pk mutants are much stronger than those observed in pin3 single mutants or more complex pin mutant combinations (Haga and Sakai, 2012b), we further hypothesized that several members of the PIN gene family may act in a redundant manner and that the loss of multiple PIN genes is required to fully impair phototropic hypocotyl bending. In line with this hypothesis, work published while our study was in progress described phototropism response defects in a range of pin mutants that were strongest in pin3 pin7 double and pin1 pin3 pin7 triple mutant seedlings (Haga and Sakai, 2012b). Intriguingly, these defects were most pronounced after a short-term blue light pulse treatment (first degree phototropism) but only weak when seedlings were illuminated with continuous blue light (second degree phototropism). For our study, we generated and examined a set of pin3, pin4, and pin7 mutant allele combinations including two pin3 pin4 pin7 triple mutants. In Arabidopsis, PIN3, PIN4, and PIN7 form a specific subclade within the PIN family of proteins (De Smet et al., 2011). Interestingly, we discovered that these pin3 pin4 pin7 triple mutants were strongly impaired in phototropism after long-term unilateral light treatment (Figures 4B and 4C). We thus concluded that PIN3, PIN4, and PIN7 act redundantly in mediating phototropic hypocotyl bending.
Figure 4.
Phototropism Is Dependent on Auxin Transport and PIN Auxin Efflux Facilitators.
(A) Quantification of hypocotyl bending in 5-d-old etiolated seedlings grown in the absence and presence of the auxin transport inhibitor NPA. The graphs show averages and sd of three representative experiments with at least 12 seedlings. Col, Columbia.
(B) Representative photographs of 5-d-old wild-type and pin mutant seedlings. Bar = 2 mm.
(C) Quantification of hypocotyl bending in 5-d-old etiolated pin mutant seedlings. Average and sd were determined from at least 12 seedlings. Student’s t test, compared with Columbia: *0.01 < P < 0.05; **0.001 < P<0.01; ***P < 0.001.
Since our analysis of d6pk mutants had shown that ethylene treatment could partially suppress d6pk01 mutant phenotypes, we were also interested in examining the effect of ethylene on pin3 pin4 pin7 triple mutants. Similar to the ethylene insensitivity of the d6pk012 triple mutants with regard to phototropic bending, we found that the phototropism defect of pin3 pin4 pin7 triple mutants also was insensitive to ethylene treatment (see Supplemental Figure 6 online).
PIN3 Phosphorylation Is Reduced in d6pk Mutants
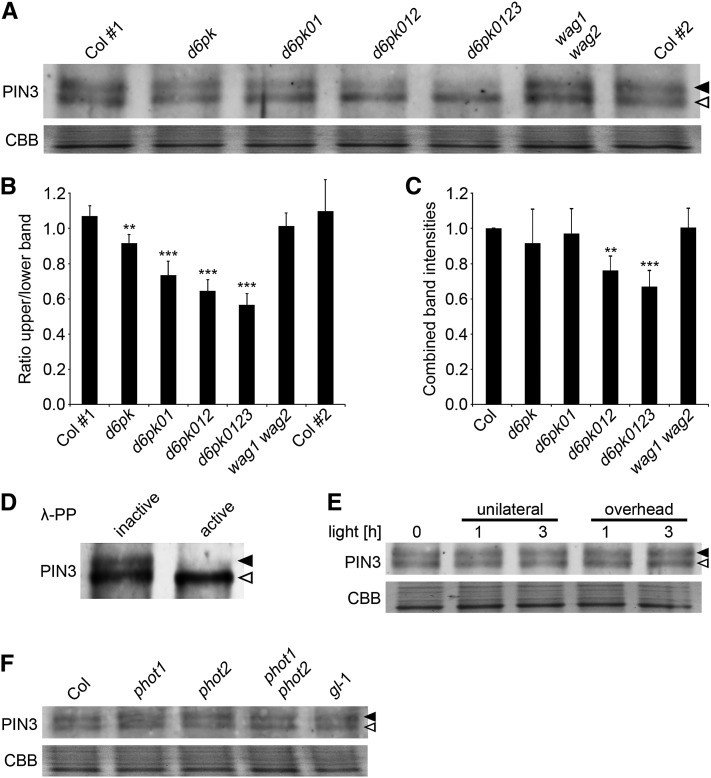
Our previous analysis of D6PK indicated that PIN3 may be a direct target for D6PK phosphorylation (Zourelidou et al., 2009). In support of D6PKs and PIN3 acting together in the context of phototropic hypocotyl bending, we found here that both D6PK and PIN3 localized to the basal end of the same cells of the hypocotyl stem (see Supplemental Figure 7A online). Furthermore, D6PK and D6PKL1 directly phosphorylated the intracellular hydrophilic loop of the PIN proteins PIN1 and PIN3 in in vitro phosphorylation experiments with purified recombinant proteins (see Supplemental Figure 7B online). We then made use of an antibody against PIN3 (Willige et al., 2012) to examine the behavior of PIN3 in dark-grown wild-type and d6pk mutant seedlings. Interestingly, we were able to resolve at least two forms of PIN3 in membrane extracts prepared from the wild type (Figure 5A). When we examined the abundance of these PIN3 protein forms in different d6pk mutant combinations, we observed a stepwise decrease in the abundance of the more slowly migrating upper form with increasing d6pk mutant complexity (Figures 5A and 5B). Based on the quantification of four independent immunoblots, we determined the ratio between the upper and the lower band for different d6pk mutant genotypes. This quantitative analysis revealed that their ratio was close to 1 in the Columbia wild type but decreased to ∼0.6 in the d6pk0123 quadruple mutant (Figure 5B). At the same time, there were no changes in the relative abundance of the two PIN3 forms in a double mutant of the AGC kinase genes WAG1 and WAG2 that are related to D6PKs and that had previously also been shown to phosphorylate PIN proteins (Figures 5A and 5B) (Dhonukshe et al., 2010; Willige et al., 2012). Since we hypothesized that the change in abundance of the more slowly migrating PIN3 form is the result of decreased PIN3 phosphorylation in the d6pk mutants, we expected the decrease in the abundance of the upper band to possibly result in an increase of the lower band. Indeed, the combined intensities measured for both PIN3 forms were very similar in d6pk and d6pk01 mutants to those observed in the wild type (Figure 5C). In the d6pk012 and d6pk0123 mutants, the combined band intensities were additionally reduced by ∼25%, suggesting that the abundance of PIN3 also was reduced in these complex d6pk mutants (Figure 5C). Incubation of protein extracts with the dephosphorylating enzyme λ protein phosphatase confirmed that the upper PIN3 band corresponded to one or several phosphorylated forms of PIN3 and that the lower band corresponded to dephosphorylated, possibly unphosphorylated, form(s) of PIN3 (Figure 5D). Since the phototropism defect was apparent in d6pk01 mutants, where PIN3 abundance was not significantly altered when compared with the wild type, we considered that the reduction of PIN3 phosphorylation may be causal for the phototropism defect of the d6pk mutants.
Figure 5.
Differential Phosphorylation of PIN3 in d6pk Mutants.
(A) Immunoblot with an anti-PIN3 antibody of membrane protein extracts prepared from 4-d-old dark-grown seedlings. Col #1 and Col #2 are two independent Columbia wild-type controls that serve as controls for uniform blotting. CBB, Coomassie blue.
(B) and (C) Ratio between the intensities of the top and bottom band (B) and combined band intensities of the top and bottom band (C) as determined by densitometric analysis from four independent replicate experiments. The graphs show averages and sd. Coomassie blue staining of a control gel was used to normalize for equal loading. The values in (C) were normalized to the Columbia wild-type control.
(D) Immunoblot with anti-PIN3 of membrane protein extracts that had been treated with boiled (inactive) or native (active) λ-phosphatase (λ-PP).
(E) and (F) Anti-PIN3 immunoblots with samples from 4-d-old dark-grown seedlings that had been exposed with unilateral or overhead white light (100 µmol m−2 s−1) for the times indicated. Closed arrowhead, top band, phosphorylated form(s) of PIN3; open arrowhead, bottom band. CBB, Coomassie blue, loading control. Student’s t test compared with Col#1: **0.001 < P < 0.01; ***P < 0.001.
To test whether differential PIN3 phosphorylation is part of a signaling cascade that is triggered during activation of phot signaling, we analyzed PIN3 phosphorylation in dark-grown seedlings after exposure to unilateral and overhead light for 1 and 3 h. However, neither treatment resulted in apparent differential phosphorylation of PIN3, suggesting that PIN3 phosphorylation is not directly activated during phot signaling (Figure 5E). Along the same lines, we did not observe any differences in the abundance of the two PIN3 bands when we compared phot single and double mutants with the wild type (Figure 5F). This further substantiated our conclusion that D6PK-dependent PIN3 phosphorylation, as visualized in our immunoblots, is independent from phot-dependent signaling.
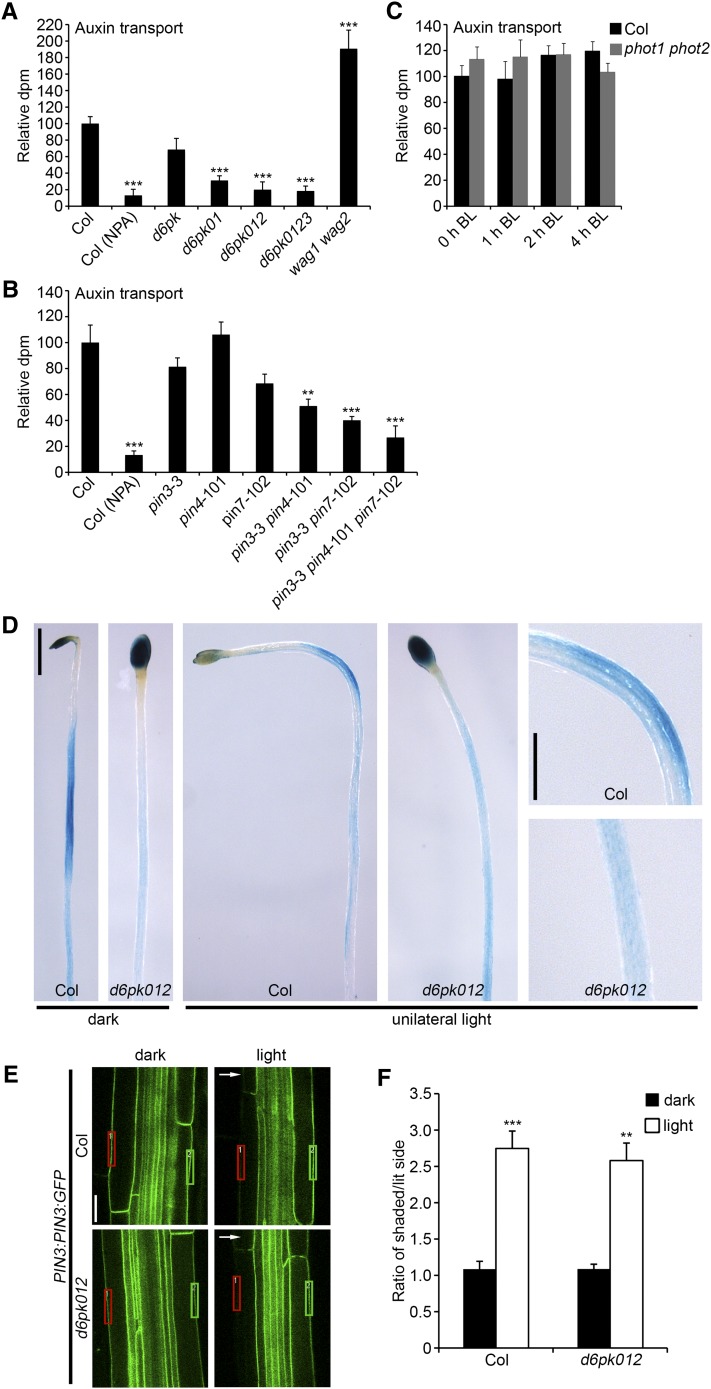
Basipetal Auxin Transport Is Altered in the d6pk and pin Mutants
As part of our previous study on d6pk, we had shown that basipetal auxin transport is impaired in stems of d6pk012 mutants (Zourelidou et al., 2009). Since changes in auxin transport may also be causative for the phototropism defects of the d6pk and pin mutants, we measured basipetal auxin transport in the hypocotyls of dark-grown seedlings in a range of d6pk and pin mutants. To this end, we applied radiolabeled IAA to the cotyledons of 4-d-old dark-grown seedlings and quantified the abundance of this radiolabeled auxin in the basal segment of the hypocotyl after 6 h. To ensure that the auxin transport measured was the result of active auxin transport, we performed control treatments with wild-type seedlings that were treated with the auxin transport inhibitor NPA. In our analysis, we observed a stepwise decrease in auxin transport in the various d6pk mutants that was most severe in d6pk012 triple and d6pk0123 quadruple mutants (Figure 6A). When we measured auxin transport in pin mutants, we also observed a decrease in auxin transport that was most severe in the pin3 pin4 pin7 mutants (Figure 6B). Thus, both loss of D6PK and PIN gene functions result in reduced auxin transport. Interestingly, loss of the two AGC kinases WAG1 and WAG2, which previously had been implicated in determining PIN polarity (Dhonukshe et al., 2010), resulted in increased basipetal auxin transport in wag1 wag2 mutant hypocotyls (Figure 6A). This indicates that WAG kinases are not required for the actication of basipetal auxin transport. The increased basipetal transport, conversely, may potentially reflect the fact that more PIN protein is basally localized in the wag1 wag2 mutant and, thus, is available for basipetal auxin transport.
Figure 6.
d6pk Mutants Are Defective in Auxin Transport.
(A) to (C) Basipetal auxin transport measurements of 4-d-old dark-grown seedlings in d6pk mutants (A), pin mutants (B), and phot mutants (C). In (C), seedlings were irradiated with blue light (BL; 5 µmol m−2 s−1) for 1, 2, and 4 h prior to the auxin transport experiment. Average and se were determined from at least eight biological replicate samples for each genotype. Student’s t test compared with Columbia (Col): ** 0.001 < P < 0.01; ***P < 0.001.
(D) Representative photographs of 5-d-old dark-grown and light-irradiated (20 h unilateral white light; 100 µmol m−2 s−1) GUS-stained Columbia wild type and d6pk012 triple mutants carrying the auxin response reporter DR5:GUS. Bar = 1 mm in left panels and 0.5 mm in right panels.
(E) Representative confocal images of 4-d-old dark-grown and light-irradiated (8 h unilateral white light; 6 µmol m−2 s−1) Columbia wild type and d6pk012 triple mutants carrying the reporter construct PIN3:PIN3:GFP. A white arrowhead indicates the direction of light treatment. Bar = 20 µm.
(F) Ratio of the signal intensities at the shaded (green rectangle) and lit (red rectangle) side of the outer hypocotyl endodermal membranes. Mean and se of at least 30 images taken from 18 seedlings for each genotype and treatment are shown. Student’s t test compared with dark: **0.001 < P <0.01; ***P < 0.001. Bar = 20 µm.
Since this finding also suggested that the activation of phot signaling could induce auxin transport, we measured auxin transport in dark-grown wild-type and phot mutant seedlings following irradiation with blue light for 1, 2, and 4 h. In line with our observation that PIN3 phosphorylation was unaltered following light irradiation (Figure 5E), we also did not detect any changes in basipetal auxin transport in light-irradiated wild-type seedlings or in a comparison between wild-type seedlings and phot1 phot2 double mutants, suggesting that unilateral light does not influence basipetal auxin transport (Figure 6C).
Earlier studies on the role of PIN3 in phototropic responses suggested that the proposed auxin transport defect of the pin3 mutants results in a failure to establish a lateral auxin maximum at the shaded side of the hypocotyl after exposure to unilateral light (Friml et al., 2002; Ding et al., 2011). To examine the contribution of D6PK to the establishment of this lateral auxin maximum, we introduced the reporter DR5:GUS into d6pk012 mutants, which allowed the visualization of local auxin maxima by measuring the activity of the auxin response machinery (Sabatini et al., 1999). Using this reporter, we detected increased DR5:GUS expression at the shaded side of hypocotyls from wild-type seedlings that had been exposed to unilateral light (Figure 6D). At the same time, we did not detect this lateral DR5:GUS signal in d6pk012 mutants, suggesting that D6PK participates in the auxin transport process that results in the lateral accumulation of auxin (Figure 6D). We also noted that the strong DR5:GUS staining observed in cotyledons of the wild type and of d6pk012 mutants decreased in the wild type following treatment with unilateral light but remained stable in d6pk012. This may indicate that an auxin pool from the cotyledons cannot be properly transported in the d6pk012 mutants due to this mutant’s defect in auxin transport. Interestingly, we observed similar DR5:GUS activity in seedlings that had been grown in the presence of the auxin transport inhibitor NPA, thus providing further support for the notion that defects in auxin transport may be the basis of the phototropism defects in d6pk mutants (see Supplemental Figure 8 online).
We next examined the distribution of PIN3 in d6pk01 mutants using a transgenic line that expresses PIN3:GFP (for green fluorescent protein) under the control of a PIN3 promoter fragment. While we found PIN3 to be equally distributed at both lateral membranes of endodermal cells in elongating hypocotyl cells of dark-grown wild-type seedlings, we observed, as previously reported (Ding et al., 2011), a shift in the distribution of PIN3 to the shaded side of the hypocotyl following exposure to unilateral light (Figures 6E and 6F). Importantly, this light-induced lateralization was not impaired in d6pk012 mutants (Figures 6E and 6F). We thus excluded the possibility that lack of PIN3 lateralization is the cause for the failure to establish the lateral auxin maximum in d6pk mutants.
Ethylene Does Not Affect PIN3 Phosphorylation or Basipetal Auxin Transport
Finally, we examined whether ACC treatments, which we had found to partially suppress the phototropism and gravitropism defects of d6pk01 mutants (Figure 2C) and alter the phosphorylation of PIN3 and possibly auxin transport. We reasoned that an increase in the abundance of the upper PIN3 phosphorylated form, possibly due to activation of the D6PK protein kinase, may explain the stimulating effect of ethylene in the phototropism response of these mutants. However, ethylene treatments affected neither the absolute abundance of PIN3 in immunoblots nor the relative abundance of the two PIN3 forms, and we therefore concluded that ethylene does not act by activating PIN3 phosphorylating kinases (see Supplemental Figures 9A and 9B online). Along the same lines, we did not find a stimulating effect of ACC on basipetal auxin transport in d6pk01 or d6pk012 mutants but rather an ACC-induced decrease in basipetal auxin transport (see Supplemental Figure 9C online). In summary, we concluded that the suppressive effect of ACC on d6pk mutant growth cannot be explained by an ACC-induced change in PIN3 phosphorylation or an activation of auxin transport.
DISCUSSION
In this study, we identified the AGCVIII protein kinases D6PK, D6PKL1, D6PKL2, and D6PKL3 as essential regulators of phototropic hypocotyl bending in Arabidopsis (Figure 1). D6PKs and phot blue light receptors belong to the same plant-specific family of AGCVIII protein kinases, which also includes the PIN regulatory kinases of the PINOID (PID) clade, including PID, PID2, WAG1, and WAG2 (Galván-Ampudia and Offringa, 2007). The evolutionary relationship of these kinases invites the hypothesis that they may have partially conserved biochemical functions (e.g., share target proteins and target protein regulation and share biological functions in mediating the same processes). Based on their evolutionary relationship and the shared phototropism defect of d6pk and phot mutants, we initially tested the hypothesis that D6PK may function by regulating the same target proteins as phot blue light receptors, possibly by acting together with phot kinases in the phosphorylation of the phot target proteins, thus, phot1 itself and PKS4. However, our analysis of early blue light signaling events in d6pk mutants and overexpressors indicated that D6PKs are required neither for phot1 and PKS4 phosphorylation nor for NPH3 dephosphorylation (Figure 3). Based on these observations, we thus rule out that D6PKs act at the level of early blue light signaling.
Our previous work suggested that D6PK-type AGCVIII kinases regulate the activity of PIN proteins (Zourelidou et al., 2009). This hypothesis was based on the observations that d6pk mutants, particularly d6pk012 triple and d6pk0123 quadruple mutants, have a number of developmental defects that are strongly reminiscent of phenotypes observed in mutants with reduced auxin transport, such as fused cotyledons, agravitropic root growth, and a strong impairment in lateral root formation. Second, D6PK colocalizes with PINs at the basal end of the plasma membrane and recombinant D6PK can phosphorylate PIN proteins in vitro and, when overexpressed in protoplast cells, also detectably in vivo. Finally, d6pk012 mutants have reduced auxin transport in the stems of adult plants (Zourelidou et al., 2009). In the context of this study, the strong phototropism defects of d6pk mutants in comparison to the comparatively weak phototropism defects reported previously by others for pin mutants after long-term exposure to blue light would suggest that D6PKs may have other targets that participate in phototropism signaling rather than solely the PINs (Friml et al., 2002; Blakeslee et al., 2004; Nagashima et al., 2008; Christie et al., 2011; Ding et al., 2011; Haga and Sakai, 2012a, 2012b). However, through the analysis of a triple mutant deficient in the function of the closely related PIN proteins PIN3, PIN4, and PIN7, which form a subclade within the Arabidopsis PIN protein family (De Smet et al., 2011), we were able to identify a pin triple mutant with a severe defect in phototropism (Figure 4). We are thus now able, at least at the genetic level, to potentially explain the strong phototropism defect of higher order d6pk mutants by a loss of PIN function. This thus suggests that phototropic hypocotyl bending depends on D6PK as well as PIN protein function. The availability of an antibody recognizing the endogenous PIN3 protein allowed us to visualize PIN3 in two forms in Arabidopsis membrane protein extracts, and we were further able to establish a correlation between absence of D6PK gene function and decrease in PIN3 phosphorylation, thus providing a further link between these two protein classes (Figures 5A to 5D). Since PIN3 is phosphorylated in phot1 phot2 mutants and since light irradiation does not alter the ratio of the two PIN3 forms that are distinguishable by immunoblot, we would exclude that PIN3 phosphorylation is part of phototropic signaling (Figures 5E and 5F).
Our analysis of auxin transport further substantiated our hypothesis that decreased auxin transport may be the basis of the reduced phototropism of d6pk and pin mutants (Figure 6). First, we found that basipetal auxin transport is reduced in the hypocotyls of dark-grown seedlings; second, we found that the lateral auxin distribution that is required for phototropic bending and that can be monitored using the auxin response reporter DR5:GUS does not take place in d6pk mutants. The lateral distribution of auxin during phototropic bending was previously described to correlate with the light-dependent relocalization of PIN3 to the shaded side of the plasma membrane of hypocotyl cells (Friml et al., 2002; Ding et al., 2011). Interestingly, lateral PIN3 protein localization is promoted by members of the PID subclade of AGCVIII kinases (Friml et al., 2004; Michniewicz et al., 2007; Ding et al., 2011). Our own previous analysis of D6PK as well as another systematic study of AGCVIII kinases further suggested that the ability to promote PIN repolarization is specific to AGCVIII kinases of the PID subclade (Zourelidou et al., 2009; Dhonukshe et al., 2010). In line with these observations, we found here that d6pk012 mutants, which are severely impaired in phototropic responses, are still able to direct PIN3 to the lateral plasma membrane following phototropic signaling but are unable to distribute auxin to the shaded side of the hypocotyl (Figures 6E and 6F). Since the inability to relocalize PIN3 is apparently not the cause for the absence of phototropic bending of d6pk mutants, it seems likely that D6PKs promote PIN-mediated auxin transport. Consistent with the defect in basipetal auxin transport, we noted that the DR5:GUS signal remained strong in the cotyledons of d6pk012 mutants before and after light irradiation, while the signal intensity decreased in the cotyledons of the wild type where basipetal auxin transport was active. A similar DR5:GUS signal distribution was observed when auxin transport was inhibited with the auxin transport inhibitor NPA.
While the accumulation of the DR5:GUS signal at the shaded side of the phototropic hypocotyl has been used as an indication of the redistribution of auxin during phototropism, we noticed that the appearance of the DR5:GUS signal at the lateral side was strongly delayed compared with the visible occurrence of hypocotyl bending (Figure 6D). Similarly, DR5:GFP lateralization was found by others to be delayed when compared with the kinetics of phototropism. Haga and Sakai (2012b) report rapid phototropic bending in their experimental conditions that is accompanied by a comparative delay in the accumulation of DR5:GFP at the lateral side of the hypocotyl. While this comparative delay may, on the one hand, be explained by a delay in the accumulation of DR5 reporter signals due to the de novo transcription, translation, and accumulation of the reporters in the respective cells, we also consider the possibility that these DR5 signals do not represent an auxin pool that promotes hypocotyl elongation. Rather, DR5 reporter accumulation in the cells of the shaded side of the hypocotyl may reflect a strong accumulation of auxin that could serve to restrict elongation and thereby prevent overbending (overshooting) of the phototropic hypocotyl.
During our analysis, we also examined the role of ethylene in promoting hypocotyl bending. This experiment was triggered by the phenotypic similarity between d6pk mutants and nph4/arf7 mutants, which are defective in the function of ARF7, with regard to nonphototropic and agravitropic hypocotyl growth (Figure 2). In nph4/arf7 mutants, these hypocotyl growth defects can be partially suppressed by ethylene (Harper et al., 2000). Interestingly, the hypocotyl growth defects of d6pk mutants also can be partially suppressed by ethylene (Figure 2). Since we had at the same time found that d6pk mutants are impaired in PIN3 phosphorylation and auxin transport, we reasoned that ethylene may act by promoting PIN3 phosphorylation and auxin transport. However, since neither process appeared to be stimulated by ethylene, we concluded that ethylene acts independently from PIN3 phosphorylation and the promotion of basipetal auxin transport. It has previously been shown that the transcription of the ARF7 paralog ARF19 is induced by ethylene (Li et al., 2006). Based on this, it had been proposed that the ethylene effect on the nph4/arf7 mutants is mediated by ARF19 (Stone et al., 2008). In line with this hypothesis, it was found that the ethylene suppression of nph4/arf7 is impaired in nph4/arf7 arf19 mutants. Since we did not see a stimulation of PIN phosphorylation or auxin transport by ethylene, our experimental data do not allow us to suggest an alternative model to explain the stimulatory effects of ethylene on the tropic responses that are defective in d6pk mutants and nph4/arf7.
Our study emphasizes the role of auxin transport mediated by D6PKs and their proposed PIN targets in phototropic hypocotyl growth. Since the early signaling events of phototropism are not disturbed in the d6pk mutants, we argue that a functional D6PK- and PIN-dependent auxin transport system is a prerequisite for phototropism. Since PIN3 lateralization is not impaired in d6pk mutants, we also argue that the activation of PIN auxin efflux function due to a lack of PIN phosphorylation, as demonstrated here with PIN3, is the primary cause for these growth defects. Importantly, a member of another class of auxin efflux mediators, namely, ABCB19/MDR1/PGP19, also participates in phototropic responses. ABC transporters are thought to maintain long-distance auxin transport and mutants with a defect in ABCB19 are hyperphototropic (Noh et al., 2003). Since the auxin efflux function of ABCB19 is negatively regulated by phot1-dependent phosphorylation, it has been proposed that the hyperphototropic phenotype of the abcb19 mutant is a consequence of increased auxin abundance in the hypocotyl as a consequence of reduced basipetal auxin transport. Interestingly, compared with the strong effects of pin3 pin4 pin7 mutations on phototropism reported here (Figure 4), the defects of abcb19 mutants are comparatively subtle and may be explained by the accumulation rather than the depletion from auxin in the bending tissue (Christie et al., 2011). Taken together, these findings thus invite the hypothesis that PIN-mediated auxin transport is a prerequisite for phototropic hypocotyl bending, while the direct control of auxin efflux through the phot-controlled ABC transporters may affect auxin distribution locally and allow fine-tuning of auxin distribution and hypocotyl bending during phototropic responses. Additionally, the availability of PIN activity and PIN polarity-regulating kinases, such as D6PKs and members of the PID subclade of AGCVIII protein kinases, respectively, may be prerequisites for a functional PIN auxin transport network. Both processes may potentially be linked since ABC transporters were found to interact with PINs (Blakeslee et al., 2007) and since PIN plasma membrane localization was found to be impaired in single and double mutants of the ABC transporter mutants mdr1 and pgp1 (Noh et al., 2003; Titapiwatanakun et al., 2009).
In summary, we report here on the essential role of D6PKs and their assumed phosphorylation targets PIN3, PIN4, and PIN7 as essential regulators of auxin transport in phototropic growth responses. Mutants with a defect in D6PKs and PIN function share a number of phenotypes, including, and most relevant here, defects in phototropic and gravitropic hypocotyl growth. This distinguishes d6pk and pin mutants from phot mutants, which are impaired in a number of other phot-dependent defects (chloroplast movement and stomatal opening) but are not impaired in gravitropic responses. In that respect, d6pk and pin mutants are more similar to the previously described phototropism mutant nph4/arf7. Thus, similarly to NPH4/ARF7, D6PKs and PINs are more likely involved in the execution of tropic responses initiated by phot signaling rather than being an integral part of the blue light and phot-triggered signaling cascade per se.
METHODS
Biological Material
The following Arabidopsis thaliana genotypes were used for the analyses presented in this article: single, double, triple, and quadruple mutants of d6pk-1 (d6pk0; SALK_061847), d6pkl1-1 (d6pk1; SALK_056618), d6pkl2-2 (d6pk2; SALK_086127), d6pkl3-2 (d6pk3; SALK_047347), and D6PK promoter GUS reporter lines as previously described (Zourelidou et al., 2009); single and double mutants of phot1-5 and phot2-1 (Kinoshita et al., 2001; Aihara et al., 2008); nph3-1 (Motchoulski and Liscum, 1999); nph4-1 (Harper et al., 2000); single, double, and triple mutants of pin3-3 (Friml et al., 2002), pin3-4 (SALK_038609), pin3-5 (SALK_005544), pin4-101 (GABI_593F01), pin7-101 (SALK_048791), and pin7-102 (SALK_062056); and pks1-1 pks2-1 pks4-1 (pks124) triple mutants (Schepens et al., 2008). The single and double mutants of phot1 and phot2 also carry the glabra-1 (gl-1) mutation as a phenotypic marker (Kinoshita et al., 2001; Aihara et al., 2008). Since no phototropism phenotypes have been described for gl-1, the wild type was used as the respective control to reduce the complexity of the physiological experiments. The DR5:GUS line was obtained from Tom Guilfoyle (University of Columbia, MO) (Sabatini et al., 1999), and PIN3:PIN3:GFP is a gift from Masao Tasaka (Nara Institute of Science and Technology, Nara, Japan) (Ding et al., 2011).
35S:YFP:D6PK, referred to in some figures as 35S:D6PK, was previously described and expresses the D6PK cDNA under control of the cauliflower mosaic virus 35S promoter in the vector pEXTAG-YFP-GW (Zourelidou et al., 2009). D6PK:YFP:D6PK, referred to as D6PK, expresses the D6PK cDNA under control of a 2-kb D6PK promoter fragment. To obtain D6PK:YFP:D6PK, a 2-kb D6PK promoter fragment was obtained by PCR amplification from genomic DNA of Arabidopsis ecotype Columbia and subcloned into pCR2.1 (Invitrogen). The promoter fragment was then transferred as a KpnI-XhoI fragment to pGREEN0229(BAR) carrying an XhoI-NotI YFP:D6PK insert from the 35S:YFP:D6PK construct described above.
Cloning of GST:D6PK, GST:PIN1, and GST:PIN3 was previously described (Zourelidou et al., 2009; Willige et al., 2012). GST:D6PKL1 was cloned in an analogous manner using the primers D6PKL1-FW and D6PKL1-RV. A list of primers and primer sequences for genotyping of novel alleles and cloning procedures is provided in Supplemental Table 1 online.
Physiological Experiments
For phototropism experiments, seedlings were grown in the dark at 22°C on vertically oriented half-strength Murashige and Skoog (MS) plates for 3 to 4 d. Etiolated seedlings were then transferred to a FloraLED chamber or GroBanks (CLF Plant Climatics) and illuminated with unilateral or overhead blue light (FloraLED) and white light (GroBanks) as specified in the figure legends. Agravitropically growing seedlings were reoriented toward the gravity vector in safe green light 2 to 4 h before phototropism response experiments were initiated. Plates were subsequently scanned and the inner hypocotyl angle was measured for each seedling using the NIH ImageJ software. The curvature angle was calculated as the difference between 180° and the measured value. Negative gravitropism angles of etiolated hypocotyls were determined from 4-d-old dark-grown seedlings, and seedlings were grouped in 30° angle categories. In experiments with ACC, seedlings were grown on 0.2 µM ACC containing half-strength MS media. NPA was included in half-strength MS media at concentrations indicated in the figure legends.
Gas Exchange Measurements
Plants were grown in a climate room for 6 to 8 weeks illuminated with white light fluorescent tubes (Philips F32T8/TL741) at a photon flux density of 100 µmol m−2 s−1 with relative humidity at 60% with a 12-h-day (22°C)/12-h-night (16°C) cycle. Twelve hours before the experiment was started, plants were transferred to the laboratory and kept in the dark. For the experiment, whole plants were placed in temperature-controlled measuring chambers (24°C), and their rosettes were exposed to an air flow of 1 liter min−1 with a CO2 concentration of 350 ppm and a relative humidity of 40%. Evaporation of water from the soil into the air stream was prevented by covering pots with polyethylene foil and imposing a laminar flow of air (150 mL min−1) toward the bottom of the chamber. The experiments with the d6pk mutants were performed with light provided by two halogen bulbs (HLX 150W; Osram) passed through infrared filters in combination with color glass filters: blue short-pass λ1/2 468 nm and red long-pass λ1/2 623 nm (Schott). The experiments with the phot mutants were performed with red (650 nm; Winger WEPDR3-S1) and blue light (455 nm; Philips, Luxeon) light-emitting diodes. All light sources were projected on the top of the measuring chambers with light guides, with intensities of 100 µmol m−2 s−1 red and 5 µmol m−2 s−1 blue light, respectively. The transpiration was measured with infrared gas analyzers incorporated into a customized gas exchange system (HCM-1000; Walz).
Chloroplast Movement Assay
Chloroplast movement was assessed photometrically by measuring changes in red light transmittance of leaves through time (Walczak et al., 1990; Jarillo et al., 2001; DeBlasio et al., 2003, 2005) using a microprocessor-controlled system based on the design of Berg et al. (2006). Plants were grown at 24°C under a 10 -h light photoperiod, and 100 to 120 µmol m−2 s−1 white light was provided by a cool-white fluorescent tubes. When plants were ∼35 d old, one adult leaf per plant was detached, its petiole was placed between two wet Whatman strips, and a region of the lamina between the midvein and the margin was positioned over a light sensor. Epinastic leaf laminas were gently uncurled by making a small section in the margin. Leaves were covered by a black plastic cover containing built-in red/blue light-emitting diodes and were dark-adapted overnight. Red light transmittance (measured every 5 min with a 100-ms pulse) was monitored for 1 h in the absence of blue light before chloroplast relocalization was triggered by 10 increments of blue light (0.1 to 120 µmol m−2 s−1). Blue light–induced chloroplast movement was determined by calculating the percentage change in red light transmittance relative to the dark position. Percentage change in red light transmittance (%Δt) was determined as %Δt = (Tt − TD)/I × 100, where Tt was the transmitted red light at time t, TD was the mean transmitted red light in dark-acclimated leaves (mean value over the first hour of measurement), and I was the incident red light. To account for differences in leaf transmittance, all data were scaled to have an initial transmittance of 10%.
Immunoblot Analyses
For the immunoblot analyses of phot1, NPH3, PKS4, and DEETIOLATED3 (DET3; loading control) after blue light irradiation (1.5 µmol m−2 s−1), 10 µg of total protein extracted from 20 mg (fresh weight) seedling material was ground in 100 μL 2× Laemmli buffer, separated on 8% SDS-PAGE gels, and transferred onto nitrocellulose with 100 mM CAPS, pH 11, 10% (v/v) ethanol. The blots were probed with anti-phot1, anti-NPH3, anti-PKS4, monoclonal anti-GFP, and anti-DET3 antibodies as previously described (Lariguet et al., 2006).
Membrane protein extracts for PIN3 immunoblots were prepared as previously reported (Willige et al., 2011) using the following extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM MG132, 0.1 mM phenylmethylsulfonyl fluoride, complete EDTA-free protease inhibitor cocktail [Roche], and PhosStop phosphatase inhibitor cocktail [Roche]) and probed with anti-PIN3 antibody (1:3000; Nottingham Arabidopsis Stock Centre). For dephosphorylation of PIN3 with λ-phosphatase, the phosphatase (350 units) was incubated with 30 µg membrane protein extract for 30 min at room temperature. For the respective negative control experiment, λ-phosphatase was heat inactivated by boiling the enzyme for 10 min. The dephosphorylation reaction was stopped by addition of 5× Laemmli buffer. RGA and YFP:D6PK immunoblots were performed as previously described using anti-RGA or anti-GFP antibodies (Willige et al., 2007). Coomassie Brilliant Blue–stained gels were used to control for equal loading.
Phosphorylation Experiments
In vitro phosphorylation experiments were performed using purified recombinant GST:D6PK and GST:D6PKL1 in combination with purified recombinant GST:PIN proteins. The recombinant proteins were incubated for 30 min in phosphorylation buffer (20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 10 mM MgCl2, 0.01% Triton X-100, 1 mM DTT, and 50 μM ATP) supplemented with 10 μCi [γ-32P]ATP. The reaction was stopped by adding 5× Laemmli buffer, and the reaction mix was separated on a 10% SDS-PAGE. GST:D6PK autophosphorylation and GST:PIN transphosphorylation were detected by autoradiography, and protein loading was controlled by Coomassie Brilliant Blue staining.
Auxin Transport Experiments
For auxin transport experiments, tritiated IAA ([3H]IAA; specific activity 20 Ci/mmol; 1 mCi/mL) (American Radiolabeled Chemicals) was dissolved to a final concentration of 417 nM [3H]IAA, 11 Bq in 5 mM MES, and 1% glycerol. Four-day-old seedlings grown in the dark on square plates containing half-strength MS without Suc were transferred to new plates containing a Parafilm stripe and positioned such that 5 mm of the apical parts of the seedlings were positioned on the Parafilm. Subsequently, a 0.5-μL drop of [3H]IAA solution was applied to the cotyledons and meristem of each seedling. NPA-treated (100 µM) wild-type seedlings were used as a negative control in this experiment. After 6 h (4 h for the experiment depicted in Figure 6C), the part of each seedling below the apical 5-mm segment was dissected, and five dissected seedlings were pooled together and measured in 3 mL of QuicksafeA scintillation liquid in a liquid scintillation analyzer (Tri Carb 2100TR; Perkin-Elmer). The results presented are the average and sd of at least four biological replicate measurements following background subtraction and normalization to the wild-type control.
Gene Expression Analyses
For GUS staining, etiolated seedlings were incubated in GUS staining solution [100 mM Na-phosphate buffer, pH 7.0, 2 mM K4Fe(CN)6, 2 mM K3Fe(CN)6, 0.1% Triton X-100, and 1 mg/mL X-Gluc]. DR5:GUS staining was performed as previously reported using vacuum infiltration (Willige et al., 2011). GUS-stained seedlings were photographed using a Leica MZ16 stereomicroscope with a PLAN-APOX1 objective (Leica).
Quantitative real-time PCR detection of D6PK gene transcripts was performed as previously described using UBC21 for normalization (Willige et al., 2012). Shown is the average and se of two pooled biological and four technical replicates.
Cell Biology
Microscopy of PIN3:GFP was performed to determine the PIN3:GFP relocalization index on 4-d-old etiolated seedlings grown at 19°C on half-strength MS media in a vertical orientation as previously described (Ding et al., 2011). Before photoexcitation, the agravitropically growing d6pk012 seedlings were reoriented toward the gravity vector in safe green light. Seedlings were then illuminated with unilateral white light (6 µmol m−2 s−1) for 8 h. Subsequently, seedlings were analyzed in batches of four seedlings using an Olympus FV1000/IX81 laser scanning confocal microscope. Three confocal images were acquired from endodermal and vascular cells from the hypocotyl region below the apical hook and throughout the bending zone. Average pixel intensities of the outer membranes of endodermal cells were determined using the Olympus FV1000 software by drawing a rectangle along the outer membranes of the endodermis as outlined in Figure 6E. For each experiment, the PIN3:GFP relocalization indices were determined from at least six hypocotyls and at least two images per hypocotyl by calculating the ratio of the lit (red rectangle) and the shaded side (green rectangle). Shown is the average and se of at least 30 images taken from 18 hypocotyls from three independent experiments.
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: D6PK (AT5G55910), D6PKL1 (At4G26610), D6PKL2 (At5G47750), D6PKL3 (At3G27580), DET3 (AT1G12840), NPH3 (AT5G64330), NPH4/ARF7 (AT5G20730), PIN3 (AT1G70940), PIN4 (AT2G01420), PIN7 (AT1G23080), PHOT1 (AT3G45780), PHOT2 (AT5G58140), PKS1 (AT2G02950), PKS2 (AT1G14280), PKS4 (AT5G04190), RGA (AT2G01570), WAG1 (AT1G53700), and WAG2 (AT3G14370).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Phototropism Defects of d6pk Mutants in Weak Blue Light.
Supplemental Figure 2. Analysis of D6PK Gene Expression in Response to Blue Light Irradiation.
Supplemental Figure 3. Regulation of D6PK Stability and Abundance in the Dark and in the Light.
Supplemental Figure 4. D6PKs Are Not Essential for Stomatal Closure.
Supplemental Figure 5. Chloroplast Relocation Does Not Require D6PKs.
Supplemental Figure 6. Ethylene Does Not Promote Hypocotyl Bending in pin3 pin4 pin7 Mutants.
Supplemental Figure 7. D6PK and PIN3 Colocalize and PINs Are Phosphorylation Targets of D6PK and D6PKL1 in Vitro.
Supplemental Figure 8. NPA Treatment of DR5:GUS Seedlings Exposed to a Phototropic Stimulus Mimics the d6pk Mutant Phenotype.
Supplemental Figure 9. ACC Treatment Does Not Have Obvious Effects on PIN3 Phosphorylation or Basipetal Auxin Transport.
Supplemental Table 1. List of Primers Used in This Study.
Supplementary Material
Acknowledgments
We thank Benjamin Weller for critical reading of the article, various colleagues for sharing published material, and Jutta Elgner and Lisa Gruber for excellent technical support. This work was supported by a grant from the Deutsche Forschungsgemeinschaft to C.S. (SCHW751/8-1).
AUTHOR CONTRIBUTIONS
B.C.W., P.A.D., R.H., M.R.G.R., C.F., and C.S. designed the research. S.A., B.C.W., I.C.R.B., M.Z., E.D., M.T., and P.A.D. performed the research. S.A., B.C.W., I.C.R.B., M.Z., P.A.D., M.R.G.R., and C.S. analyzed the data. B.C.W. and C.S. wrote the article.
Glossary
- IAA
indole-3-acetic acid
- YFP
yellow fluorescent protein
- GUS
β-glucuronidase
- ACC
1-aminocyclopropane-1-carboxylic-acid
- NPA
naphthylphthalamic acid
- MS
Murashige and Skoog
- [3H]IAA
tritiated indole-3-acetic acid
References
- Aihara Y., Tabata R., Suzuki T., Shimazaki K., Nagatani A. (2008). Molecular basis of the functional specificities of phototropin 1 and 2. Plant J. 56: 364–375 [DOI] [PubMed] [Google Scholar]
- Berg R., Königer M., Schjeide B.M., Dikmak G., Kohler S., Harris G.C. (2006). A simple low-cost microcontroller-based photometric instrument for monitoring chloroplast movement. Photosynth. Res. 87: 303–311 [DOI] [PubMed] [Google Scholar]
- Blakeslee J.J., et al. (2007). Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19: 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee J.J., Bandyopadhyay A., Peer W.A., Makam S.N., Murphy A.S. (2004). Relocalization of the PIN1 auxin efflux facilitator plays a role in phototropic responses. Plant Physiol. 134: 28–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W.R., Christie J.M. (2002). Phototropins 1 and 2: Versatile plant blue-light receptors. Trends Plant Sci. 7: 204–210 [DOI] [PubMed] [Google Scholar]
- Christie J.M. (2007). Phototropin blue-light receptors. Annu. Rev. Plant Biol. 58: 21–45 [DOI] [PubMed] [Google Scholar]
- Christie J.M., Reymond P., Powell G.K., Bernasconi P., Raibekas A.A., Liscum E., Briggs W.R. (1998). Arabidopsis NPH1: A flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701 [DOI] [PubMed] [Google Scholar]
- Christie J.M., et al. (2011). phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol. 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carbonnel M., Davis P., Roelfsema M.R., Inoue S., Schepens I., Lariguet P., Geisler M., Shimazaki K., Hangarter R., Fankhauser C. (2010). The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol. 152: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBlasio S.L., Luesse D.L., Hangarter R.P. (2005). A plant-specific protein essential for blue-light-induced chloroplast movements. Plant Physiol. 139: 101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBlasio S.L., Mullen J.L., Luesse D.R., Hangarter R.P. (2003). Phytochrome modulation of blue light-induced chloroplast movements in Arabidopsis. Plant Physiol. 133: 1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarsy E., Schepens I., Okajima K., Hersch M., Bergmann S., Christie J., Shimazaki K., Tokutomi S., Fankhauser C. (2012). Phytochrome Kinase Substrate 4 is phosphorylated by the phototropin 1 photoreceptor. EMBO J. 31: 3457–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I., et al. (2011). Unraveling the evolution of auxin signaling. Plant Physiol. 155: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P., Huang F., Galvan-Ampudia C.S., Mähönen A.P., Kleine-Vehn J., Xu J., Quint A., Prasad K., Friml J., Scheres B., Offringa R. (2010). Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development 137: 3245–3255 [DOI] [PubMed] [Google Scholar]
- Ding Z., Galván-Ampudia C.S., Demarsy E., Łangowski L., Kleine-Vehn J., Fan Y., Morita M.T., Tasaka M., Fankhauser C., Offringa R., Friml J. (2011). Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat. Cell Biol. 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Yeh K.C., Lagarias J.C., Zhang H., Elich T.D., Chory J. (1999). PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284: 1539–1541 [DOI] [PubMed] [Google Scholar]
- Folta K.M., Spalding E.P. (2001). Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Friml J., et al. (2004). A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865 [DOI] [PubMed] [Google Scholar]
- Galván-Ampudia C.S., Offringa R. (2007). Plant evolution: AGC kinases tell the auxin tale. Trends Plant Sci. 12: 541–547 [DOI] [PubMed] [Google Scholar]
- Haga K., Sakai T. (2012a). Differential roles of auxin efflux carrier PIN proteins in hypocotyl phototropism of etiolated Arabidopsis seedlings depend on the direction of light stimulus. Plant Signal. Behav. 8: e22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Sakai T. (2012b). PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. Plant Physiol. 160: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper R.M., Stowe-Evans E.L., Luesse D.R., Muto H., Tatematsu K., Watahiki M.K., Yamamoto K., Liscum E. (2000). The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Zago M.K., Abas L., van Marion A., Galván-Ampudia C.S., Offringa R. (2010). Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell 22: 1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada S., Ohgishi M., Mayama T., Okada K., Sakai T. (2004). RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 16: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo J.A., Gabrys H., Capel J., Alonso J.M., Ecker J.R., Cashmore A.R. (2001). Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410: 952–954 [DOI] [PubMed] [Google Scholar]
- Kagawa T., Sakai T., Suetsugu N., Oikawa K., Ishiguro S., Kato T., Tabata S., Okada K., Wada M. (2001). Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Doi M., Suetsugu N., Kagawa T., Wada M., Shimazaki K. (2001). Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Lariguet P., Schepens I., Hodgson D., Pedmale U.V., Trevisan M., Kami C., de Carbonnel M., Alonso J.M., Ecker J.R., Liscum E., Fankhauser C. (2006). PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc. Natl. Acad. Sci. USA 103: 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Dai X., Zhao Y. (2006). A role for auxin response factor 19 in auxin and ethylene signaling in Arabidopsis. Plant Physiol. 140: 899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E., Briggs W.R. (1995). Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M., et al. (2007). Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130: 1044–1056 [DOI] [PubMed] [Google Scholar]
- Motchoulski A., Liscum E. (1999). Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Nagashima A., Uehara Y., Sakai T. (2008). The ABC subfamily B auxin transporter AtABCB19 is involved in the inhibitory effects of N-1-naphthyphthalamic acid on the phototropic and gravitropic responses of Arabidopsis hypocotyls. Plant Cell Physiol. 49: 1250–1255 [DOI] [PubMed] [Google Scholar]
- Noh B., Bandyopadhyay A., Peer W.A., Spalding E.P., Murphy A.S. (2003). Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423: 999–1002 [DOI] [PubMed] [Google Scholar]
- Pedmale U.V., Liscum E. (2007). Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1-interacting protein NPH3. J. Biol. Chem. 282: 19992–20001 [DOI] [PubMed] [Google Scholar]
- Roberts D., Pedmale U.V., Morrow J., Sachdev S., Lechner E., Tang X., Zheng N., Hannink M., Genschik P., Liscum E. (2011). Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3(NPH3). Plant Cell 23: 3627–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S., Beis D., Wolkenfelt H., Murfett J., Guilfoyle T., Malamy J., Benfey P., Leyser O., Bechtold N., Weisbeek P., Scheres B. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Sakai T., Kagawa T., Kasahara M., Swartz T.E., Christie J.M., Briggs W.R., Wada M., Okada K. (2001). Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens I., Boccalandro H.E., Kami C., Casal J.J., Fankhauser C. (2008). PHYTOCHROME KINASE SUBSTRATE4 modulates phytochrome-mediated control of hypocotyl growth orientation. Plant Physiol. 147: 661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone B.B., Stowe-Evans E.L., Harper R.M., Celaya R.B., Ljung K., Sandberg G., Liscum E. (2008). Disruptions in AUX1-dependent auxin influx alter hypocotyl phototropism in Arabidopsis. Mol. Plant 1: 129–144 [DOI] [PubMed] [Google Scholar]
- Stowe-Evans E.L., Harper R.M., Motchoulski A.V., Liscum E. (1998). NPH4, a conditional modulator of auxin-dependent differential growth responses in Arabidopsis. Plant Physiol. 118: 1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya A., Inoue S., Doi M., Kinoshita T., Shimazaki K. (2005). Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 17: 1120–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu K., Kumagai S., Muto H., Sato A., Watahiki M.K., Harper R.M., Liscum E., Yamamoto K.T. (2004). MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16: 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titapiwatanakun B., et al. (2009). ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J. 57: 27–44 [DOI] [PubMed] [Google Scholar]
- Walczak T., Gabrys H., Appenroth K.J. (1990). Is there a third photoreceptor involved in the control of chloroplast movements in mougeotia? Plant Physiol. 94: 221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige B.C., Ghosh S., Nill C., Zourelidou M., Dohmann E.M., Maier A., Schwechheimer C. (2007). The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 19: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige B.C., Isono E., Richter R., Zourelidou M., Schwechheimer C. (2011). Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. Plant Cell 23: 2184–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige B.C., Ogiso-Tanaka E., Zourelidou M., Schwechheimer C. (2012). WAG2 represses apical hook opening downstream from gibberellin and PHYTOCHROME INTERACTING FACTOR 5. Development 139: 4020–4028 [DOI] [PubMed] [Google Scholar]
- Zourelidou M., Müller I., Willige B.C., Nill C., Jikumaru Y., Li H., Schwechheimer C. (2009). The polarly localized D6 PROTEIN KINASE is required for efficient auxin transport in Arabidopsis thaliana. Development 136: 627–636 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.