This study determines the quantitative dynamic parameters associated with actin filament growth and disappearance at pollen tube tips and identifies villin as a key regulator of actin dynamics in this region. Real-time visualization of single filaments showing that villin regulates actin dynamics via its severing activity supports a direct link between villin and filament severing in plants.
Abstract
Apical actin filaments are crucial for pollen tube tip growth. However, the specific dynamic changes and regulatory mechanisms associated with actin filaments in the apical region remain largely unknown. Here, we have investigated the quantitative dynamic parameters that underlie actin filament growth and disappearance in the apical regions of pollen tubes and identified villin as the major player that drives rapid turnover of actin filaments in this region. Downregulation of Arabidopsis thaliana VILLIN2 (VLN2) and VLN5 led to accumulation of actin filaments at the pollen tube apex. Careful analysis of single filament dynamics showed that the severing frequency significantly decreased, and the lifetime significantly increased in vln2 vln5 pollen tubes. These results indicate that villin-mediated severing is critical for turnover and departure of actin filaments originating in the apical region. Consequently, the construction of actin collars was affected in vln2 vln5 pollen tubes. In addition to the decrease in severing frequency, actin filaments also became wavy and buckled in the apical cytoplasm of vln2 vln5 pollen tubes. These results suggest that villin confers rigidity upon actin filaments. Furthermore, an observed decrease in skewness of actin filaments in the subapical region of vln2 vln5 pollen tubes suggests that villin-mediated bundling activity may also play a role in the construction of actin collars. Thus, our data suggest that villins promote actin turnover at pollen tube tips and facilitate the construction of actin collars.
INTRODUCTION
Tip growth is an extreme form of polarized cell growth that occurs exclusively from a single site. Importantly, this type of growth is crucial for development and morphogenesis of eukaryotic organisms. Well-documented examples of tip growth systems include animal neuronal axons and fungi hyphae, as well as moss protonemata, root hairs, and pollen tubes in plants (Lowery and Van Vactor, 2009; Berepiki et al., 2011; Rounds and Bezanilla, 2013). Pollen tubes provide a passage for the delivery of two nonmotile sperm cells to the ovule in order to facilitate double fertilization in higher plants, and this system is ideal for study of the molecular mechanisms underlying polarized cell growth. Pollen tube growth is extremely rapid, with a growth rate of up to 1 cm/h (Bedinger et al., 1994). To support such rapid single-celled tip growth, efficient delivery of new materials to the expanding point of the pollen tube tip is essential. It is well established that small GTPase molecular switches, actin dynamics, and a tip-focused calcium gradient all play important roles in regulating the secretory activity in the apical cytoplasm that supports growth (Yang, 1998; Hepler et al., 2001; Cole and Fowler, 2006; Cheung and Wu, 2008; Yang, 2008; Qin and Yang, 2011). These components are interconnected in the apical cytoplasm and mark the tip-growing domain, which regulates the velocity and direction of pollen tube growth. However, how the activities of these components are coordinated to properly regulate pollen tube growth remains largely unknown. Studies from Yang’s group have established the importance of two interlinked mechanisms, dynamic Rho signaling and the tip-focused calcium gradient. Both mechanisms target the actin cytoskeleton and are in turn regulated by actin in a feedback loop (Gu et al., 2005). Together, these proteins constitute an intricate system known as the LENS (for localization-enhancing network, self-sustaining) signaling network (Cole and Fowler, 2006). This network may represent a unifying mechanism that facilitates polarized growth in tip-growing cells. Therefore, understanding the state of actin filaments and the regulation of actin dynamics in the apical cytoplasm will provide insight into the integrated role of the actin cytoskeleton in the LENS signaling network.
The actin cytoskeleton has been definitively shown to play an essential role in tip growth (Gibbon et al., 1999; Vidali and Hepler, 2001; Vidali et al., 2001; Ye et al., 2009; Staiger et al., 2010). Actin filaments have also been shown to be present in distinct types of arrays in pollen tubes (Cheung and Wu, 2008; Chen et al., 2009; Staiger et al., 2010). Though the presence of filamentous actin at growing pollen tube tips has been a matter of controversial debate for decades, its existence within the tip domain has been established unambiguously using several independent approaches (Kost et al., 1998; Gibbon et al., 1999; Fu et al., 2001; Lovy-Wheeler et al., 2005). Treatment with a low dose of latrunculin B arrests pollen tube growth but does not affect cytoplasmic streaming, consistent with a critical role for apical and subapical actin filaments in pollen tube growth (Gibbon et al., 1999; Vidali et al., 2001; Cárdenas et al., 2008). Further support for this role comes from the observation that loss of FH5 specifically affects actin filaments in the apical and subapical regions and alters the direction of pollen tube growth (Cheung et al., 2010). To some extent, apical actin filaments have been shown to participate in localized regulation of vesicle docking and fusion, as well as to facilitate endocytosis (Hwang et al., 2008; Lee et al., 2008; Kroeger et al., 2009; Bou Daher and Geitmann, 2011). Experiments using low doses of latrunculin B (Gibbon et al., 1999; Vidali et al., 2001; Cárdenas et al., 2008) have also provided indirect evidence that apical and subapical actin filaments turn over rapidly. However, the details associated with actin dynamics and regulation in this region remains poorly understood. In particular, a detailed description of the parameters associated with filament growth and disappearance is lacking.
The dynamic status of actin filaments has been shown to be intimately associated with tip-focused calcium gradients in the pollen tube. The Yang group has identified two counteracting actin regulatory pathways mediated by the small GTPase Rho of plants 1, in which Rop-interactive CRIB (for Cdc42/Rac-interactive binding) motif-containing protein 4 (RIC4) promotes actin assembly, and RIC3 induces calcium-mediated actin disassembly (Gu et al., 2005). Several calcium-sensitive, actin binding proteins (ABPs), such as profilin and gelsolin, were assumed to function downstream of RIC3 (Gu et al., 2005).
Villin is another potential candidate that may regulate actin dynamics in concert with calcium signaling, since it has been shown to sever actin filaments under physiological calcium concentrations (Khurana et al., 2010; Zhang et al., 2010a; Zhang et al., 2011; Bao et al., 2012). Villin homologs were originally isolated from lily (Lilium longiflorum) pollen by biochemical approaches in plants (Nakayasu et al., 1998; Yokota et al., 1998, 2003). In addition, a role for villin together with calcium in regulating actin dynamics in the apical cytoplasm of pollen tubes has previously been proposed (Hepler et al., 2001; Vidali and Hepler, 2001; Holdaway-Clarke et al., 2003; Yokota et al., 2005). However, to date, no direct genetic and cytological evidence has been identified to support this hypothesis. Our previous study showed that loss of VLN5 affected pollen tube growth and caused actin filament instability (Zhang et al., 2010a). However, how VLN5, or other villin isovariants, regulates actin dynamics at the single filament level and how this protein acts in concert with the calcium gradient in the pollen tube remains to be determined.
Here, we employed high spatiotemporal live-cell imaging technology to visualize the dynamics of actin filaments within the apical cytoplasm of pollen tubes. Our study is the first to provide a detailed analysis of parameters associated with actin filament dynamics in the apical cytoplasm of pollen tubes. Further characterization identified villin as the major player that drives rapid turnover of actin filaments at pollen tube tips. Thus, we propose that villin acts in concert with the tip-focused calcium gradient to regulate actin dynamics and plays a general role in driving the rapid turnover of actin filaments in tip-growing cells.
RESULTS
VLN2 and VLN5 Decorate Filamentous Structures at Pollen Tube Tips
Based on the biochemical nature of VLN5, we proposed that it may act in concert with the tip-focused calcium gradient to regulate actin dynamics in pollen tubes (Zhang et al., 2010a). However, loss of VLN5 does not affect the overall actin organization in pollen tubes (Zhang et al., 2010a). Therefore, we hypothesized that VLN2, which is expressed in pollen (https://www.genevestigator.com/gv/index.jsp; Honys and Twell, 2003; Pina et al., 2005), acts in a redundant manner with VLN5 to regulate actin dynamics in the pollen tube. To test this hypothesis, we generated vln2 vln5 double mutants by crossing vln5-2 (Zhang et al., 2010a) with either vln2-1 or vln2-2 (Bao et al., 2012). Results from this analysis revealed that loss of VLN2 alone had no overt effects on pollen tube growth (see Supplemental Figures 1D and 1K online) but that loss of VLN2 enhanced the effects of loss of VLN5 (see Supplemental Figures 1F and 1K online). These results indicate that VLN2 acts redundantly with VLN5 to regulate pollen tube elongation. Compared with the wild type, vln2 or vln5 pollen tubes (see Supplemental Figures 1G to 1I online), vln2 vln5 pollen tubes became curled and wider at some regions along the tube (see Supplemental Figure 1J online). The width of pollen tubes increased significantly in vln2 vln5 pollen tubes (see Supplemental Figure 1L online). Thus, these data suggest that VLN2 acts synergistically with VLN5 to regulate polarized pollen tube growth.
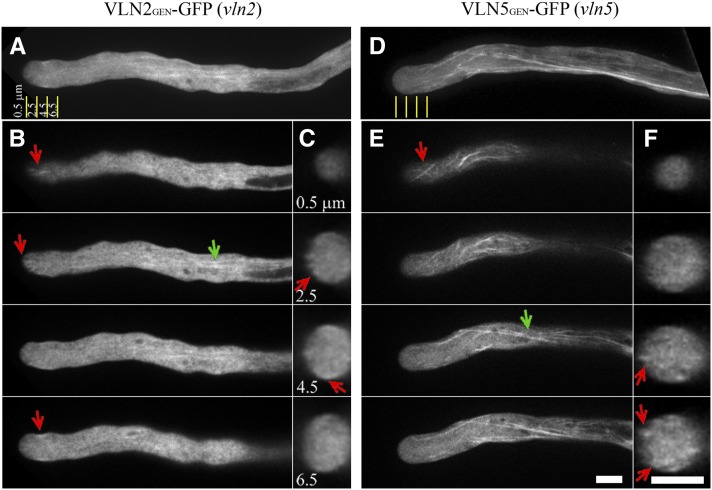
To determine the role of villin in the regulation of actin dynamics in the apical region, it is important to first determine where it is localized in the pollen tube. Constructs containing the green fluorescent protein (GFP) coding sequence fused with genomic VLN2 (VLN2GEN-GFP) or VLN5 (VLN5GEN-GFP) rescue the growth phenotype of vln2 vln5 pollen tubes (see Supplemental Figure 2 online), indicating that these GFP fusion proteins are functional and suggesting that they faithfully represent the subcellular localization of VLN2 and VLN5, respectively. As shown in Figure 1, both VLN2 and VLN5 decorated filamentous structures in the shanks of pollen tubes, but the signal associated with VLN5-GFP was more prominent, consistent with the fact that VLN5 is expressed at much higher levels than VLN2 in pollen (Zhang et al., 2010a). However, both VLN2-GFP and VLN5-GFP were present in the apical and subapical regions of pollen tubes, where they formed filamentous structures (Figure 1). Thus, these data indicate that VLN2 and VLN5 decorate filamentous structures found throughout the pollen tube, including the apical and subapical regions.
Figure 1.
VLN2 and VLN5 Decorate Filamentous Structures in Pollen Tubes.
VLN2GEN-GFP or VLN5GEN-GFP was transformed into vln2 or vln5 lines, respectively, to assess the localization of VLN2 or VLN5 in pollen tubes.
(A) to (C) VLN2 localization in the pollen tube.
(D) to (F) VLN5 localization in the pollen tube.
(A) The projection showed that VLN2 decorates filamentous structures in the shank as well as the subapex and apex. Yellow lines indicate distances distal from the apex, and the transverse sections were captured at the positions indicated in (C).
(B) Selected single optical sections. Red arrows indicate VLN2-decorated filamentous structures at the apex and subapex. Green arrow indicates VLN2-decorated filamentous structures in the shank.
(C) Transverse sections of the pollen tube taken at the positions indicated by the yellow lines at specific distances distal from the apex. Red arrows indicate VLN2-GFP fluorescence in the cortical region.
(D) Projections revealed that VLN5 decorates filamentous structures throughout the pollen tube. Yellow lines indicate distances distal from the apex identical to those shown in (A).
(E) Selected single optical sections. Red and green arrows indicate VLN5-decorated filamentous structures at the tip and shank, respectively. Bar = 5 μm in (E) for (A), (B), (D), and (E).
(F) Transverse sections of the pollen tube taken at the positions indicated by the yellow lines at specific distances distal from the apex. Red arrows indicate VLN5-GFP fluorescence in the cortical region. Bar = 5 μm in (F) for (C) and (F).
Highly Dynamic Actin Filaments Are Constantly Generated from the Apical Membrane within the Apical Dome
To visualize the dynamics of actin filaments within the apical dome, it is important to have a live cell probe capable of labeling actin filaments in this region. Lifeact-EGFP represents an ideal actin probe that labels actin filaments in tip-growing cells (Vidali et al., 2009). Lifeact-EGFP labeled actin filaments in Arabidopsis thaliana pollen tubes, revealing a typical distribution pattern (see Supplemental Figure 3 online). Therefore, using this methodology, it is possible to visualize the dynamics of actin filaments and determine the parameters associated with filament growth and disappearance within the apical dome in Arabidopsis pollen tubes. Visualization of z-series optical sections revealed that actin filaments are present within the apical dome (see Supplemental Figure 4A online). To determine the site of origination of these filaments, we visualized time-lapse, z-series projection images of actin filaments, which revealed that most of the actin filaments grew out from the apical membrane (see Supplemental Figures 4B and 4C and Supplemental Movies 1 and 2 online). These filaments are most likely nucleated by membrane-anchored formins (Cheung and Wu, 2004; Cheung et al., 2010). To further characterize the details associated with actin filament dynamics, we observed actin filaments at the cortex and the central portion of the pollen tube within the apical dome. Real-time visualization of actin filaments in the medial optical section clearly demonstrated that actin filaments originate and grow out from the membrane at the extreme apex, some of which shift from the apex to the apical flank (see Supplemental Figure 4D and Supplemental Movie 3 online). The newly generated actin filaments are highly dynamic, exhibiting rapid growth and shrinkage and frequent severing (see Supplemental Figures 4D and 4E and Supplemental Movies 3 and 4 online). The dynamic traits associated with actin filaments may explain why they are less abundant at the apex. Supplemental Figure 4F online (with the associated Supplemental References 1 online) shows a schematic diagram of actin filament growth and disappearance within the apical dome, proposing a model in which actin filaments are generated constantly from the membrane and turned over rapidly. The extreme tip, which contains fewer actin filaments, always faces toward the direction of growth (see Supplemental Figure 5 online), suggesting that the state of actin filaments at the extreme tip may be involved in the control of growth direction.
Loss of VLN2 and VLN5 Results in Accumulation of Actin Filaments at the Apex
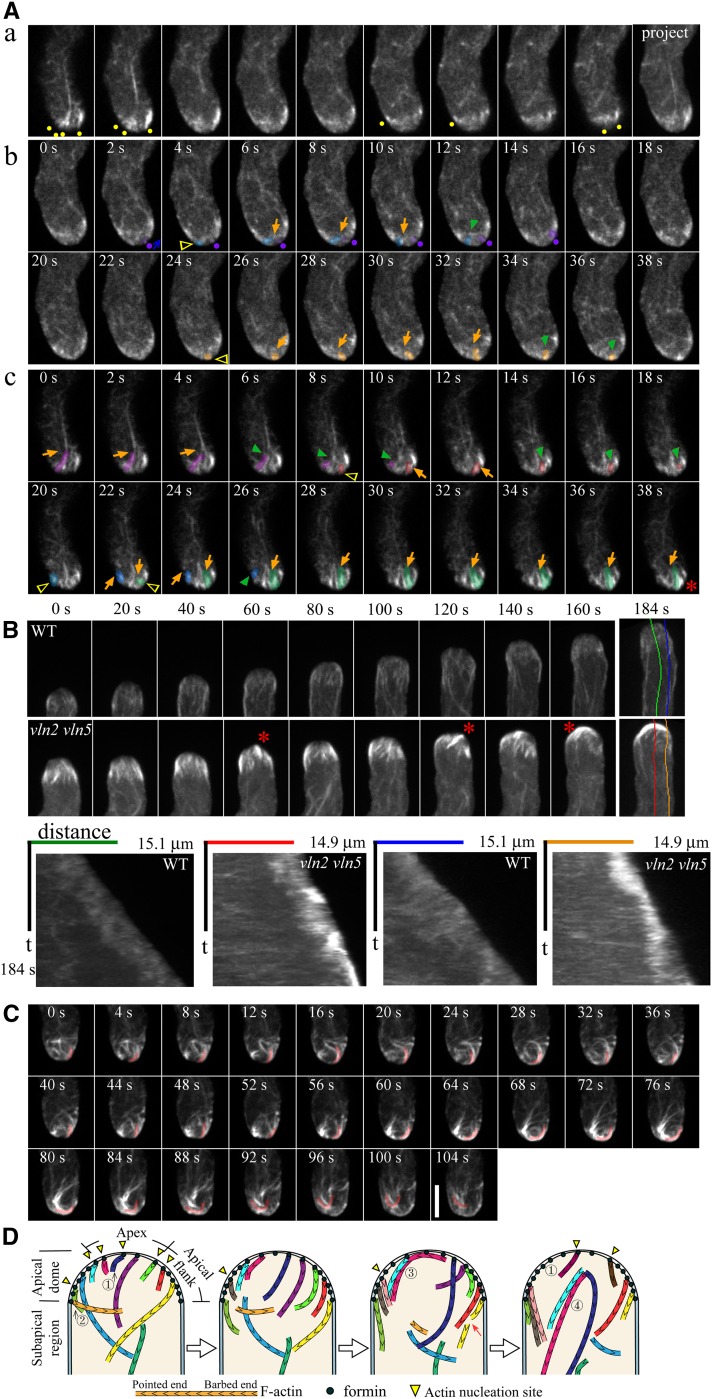
To explore whether VLN2 and VLN5 cooperate with the tip-focused calcium gradient (Holdaway-Clarke et al., 1997; Messerli et al., 2000) to regulate actin dynamics, we analyzed actin filament dynamics within the apical dome of vln2 vln5 pollen tubes. Similar to wild-type pollen tubes, actin filaments were detected in the apical region of the vln2 vln5 pollen tube (Figure 2A, a). Direct visualization of actin filament dynamics in both the middle and cortical regions of vln2 vln5 pollen tubes revealed that actin filaments originate from the apical membrane (Figure 2A, b and c; see Supplemental Movies 5 and 6 online). However, unlike wild-type pollen tubes (Figure 2B; see Supplemental Movie 7 online), actin filaments accumulated in the tips of vln2 vln5 pollen tubes (Figure 2B; see Supplemental Movie 8 online). Kymographs of actin filaments in pollen tube tips showed that actin filaments accumulated at both the apex and the apical flank (Figure 2B). These results suggest that actin nucleation at the membrane of the apex is reasonably active. However, in contrast with wild-type pollen tubes, the loss of the membrane-anchored actin filaments via severing was rarely observed. In fact, after substantial accumulation of actin filaments at the apex of the vln2 vln5 pollen tube, the intertwined filaments were instead observed to depart from the membrane as a unit (Figure 2C; see Supplemental Movie 9 online). A schematic diagram describing actin filament growth and dynamics within the apical dome of the vln2 vln5 pollen tube is shown in Figure 2D. The diagram shows that long, intertwined membrane-originated and -anchored actin filaments remain intact within the apical dome of the vln2 vln5 pollen tube and that they depart from the membrane via an unknown mechanism.
Figure 2.
Downregulation of VLN2 and VLN5 Causes Filamentous Actin to Accumulate at Pollen Tube Tips.
(A) Visualization of actin filaments in the apical region of vln2 vln5 pollen tubes. (a) z-series optical sections and projections revealed that actin filaments are present at the apex and apical flank of the vln2 vln5 pollen tube. Yellow dots indicate the positions of actin filaments close to the membrane. (b) Time-lapse images of actin filaments in the medial section of the apical dome of a vln2 vln5 pollen tube. Three actin filaments with different behaviors were pseudocolored purple, blue, and yellow. Yellow triangles indicate the origination sites of actin filaments, and purple dots indicate the position of anchorage of an actin filament that moves from the apex to the apical flank. Blue arrow indicates the moving direction of actin filaments. Orange and green arrows indicate growing and shrinking actin filaments, respectively. See Supplemental Movie 5 online for the entire series. (c) Time-lapse images of actin filaments in the cortical region of the apical dome of a vln2 vln5 pollen tube. Four actin filaments with distinct behaviors were pseudocolored purple, orange, blue, and green. Yellow triangles indicate the association sites of actin filaments with the membrane. Orange and green arrows indicate growing and shrinking actin filaments, respectively. The red asterisk indicates an accumulation of actin filaments. See Supplemental Movie 6 online for the entire series.
(B) Actin filaments accumulate at the apex and the apical flank of vln2 vln5 pollen tubes. The top panel shows time-lapse images of actin filaments in a wild-type (WT) pollen tube (see Supplemental Movie 7 online for the entire series) and a vln2 vln5 pollen tube (see Supplemental Movie 8 online for the entire series). Red asterisks indicate the position of actin filament accumulation. Kymographs were plotted based on lines parallel to the growth axes of pollen tubes, demonstrating that actin filaments accumulate in vln2 vln5 pollen tube tips.
(C) Time-lapse images of actin filaments from another vln2 vln5 pollen tube showed that actin filaments generated at the apical membrane were not severed and turned over. Consequently, they accumulated and intertwined near the membrane. They then departed from the membrane as a unit (see the represented pink pseudocolored filament), reducing the amount of actin filaments at pollen tube tips. Bar = 5 μm for images in (A) to (C).
(D) Schematic diagram of actin filament dynamics within the apical and subapical regions of vln2 vln5 pollen tubes. Similar to wild-type pollen tubes, nucleation of actin filaments is probably mediated by membrane-anchored formin(s). However, unlike wild-type pollen tubes, actin filaments accumulated and intertwined near the membrane within the apical dome of vln2 vln5 pollen tubes (see 3 marked actin filaments). Actin filaments are able to be nucleated on membrane at both the apex (see 1 marked actin filaments) and the apical flanks (see 2 marked actin filaments). Some intertwined actin structures leave the membrane as a unit via an unknown mechanism (see 4 marked actin filaments). Red arrow indicates the severing event.
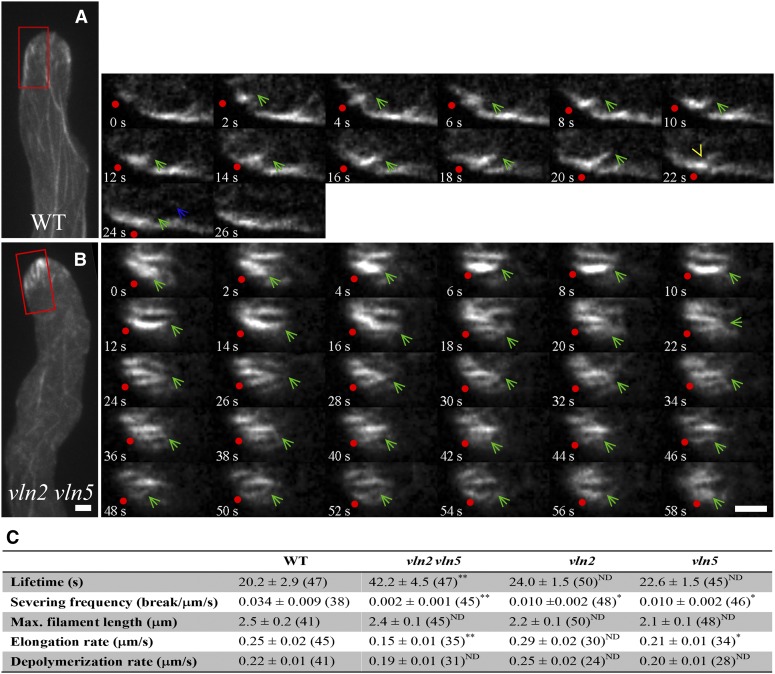
To characterize the defect in actin dynamics within the apical dome of vln2 vln5 pollen tubes on a single filament level, we quantified the dynamic parameters associated with single actin filaments within the apical domes of vln2 vln5 pollen tubes and compared these parameters with those of filaments in wild-type pollen tubes. As shown in Figure 3, actin filament growth can easily be tracked, and severing events can be detected in wild-type pollen tubes (Figure 3A; see Supplemental Movie 10 online). By contrast, actin filament severing events were rarely detected in vln2 vln5 pollen tubes (Figure 3B; see Supplemental Movie 11 online). In comparison to filaments in wild-type pollen tubes, the lifetimes of actin filaments significantly increased, and the filament severing frequency significantly decreased in vln2 vln5 pollen tubes (Figure 3C), suggesting that loss of villin-mediated severing activity at least partially accounts for the accumulation of actin filaments in vln2 vln5 pollen tubes. Additionally, the average severing frequency decreased significantly in both vln2 and vln5 single mutants (Figure 3C), suggesting that both VLN2 and VLN5 are required for severing actin filaments in the pollen tube. In wild-type pollen tubes, the elongation rate of actin filaments can reach 0.25 μm/s, consistent with the presence of a high concentration of actin/profilin complexes in pollen (Vidali and Hepler, 1997; Gibbon et al., 1999; Snowman et al., 2002). By contrast, the actin elongation rate was decreased significantly in vln5 and vln2 vln5 pollen tubes (Figure 3C), likely due to the efficient conversion of monomeric actin into filamentous actin to consequently decrease the concentration of the actin/profilin pool locally and/or to upregulate the activity of another capping factor, such as At-CP (Huang et al., 2003). Thus, these data suggest that the severing activity of villin promotes the rapid turnover of actin filaments in the apical regions of pollen tubes.
Figure 3.
Actin Filament Severing Frequency Is Decreased in vln2 vln5 Pollen Tubes.
(A) Dynamics of single actin filaments in the apical cytoplasm of a wild-type (WT) pollen tube. The right panels show an enlarged view of the region in the red box rotated 90° counterclockwise. Red dots indicate actin filament ends near the membrane, green arrows indicate actin filament ends in the cytoplasm, and yellow arrow indicates severing event. Blue arrow indicates the released filament from severing. See Supplemental Movie 10 online for the entire series.
(B) Dynamics of single actin filaments in the apical cytoplasm of a vln2 vln5 pollen tube. The right panels show an enlarged view of the region in the red box, rotated 90° counterclockwise. Red dots indicate actin filament ends near the membrane, and green arrows indicate actin filament ends in the cytoplasm. Severing events are hardly detected within the time window of image acquisition for this representative pollen tube. Bar = 2 μm for images in (A) and (B). See Supplemental Movie 11 online for the entire series.
(C) Quantification of parameters associated with single actin filament dynamics in wild-type, vln2, vln5, and vln2 vln5 pollen tubes. *P < 0.05 and **P < 0.01; ND, no significant difference by a Student’s t test.
Loss of VLN2 and VLN5 Affects the Formation of Actin Collars
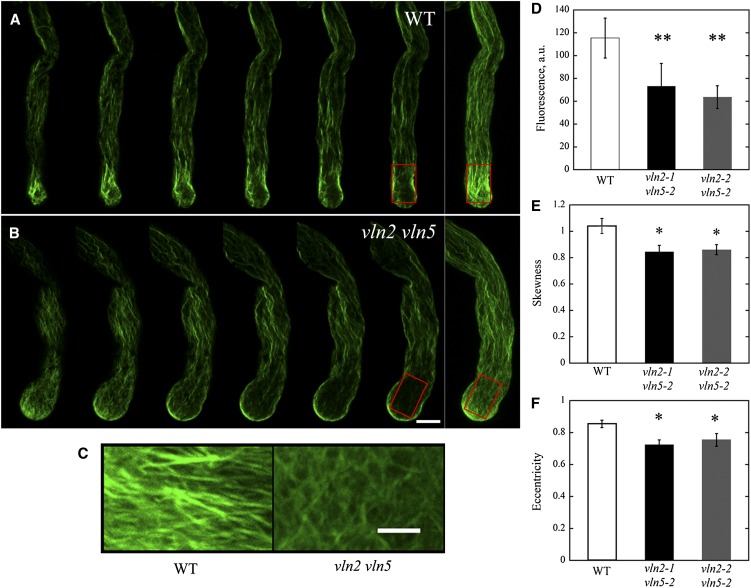
Apical membrane-nucleated actin filaments have previously been demonstrated to be required for the construction of subapical actin collars (Cheung et al., 2010). Thus, we hypothesized that the accumulation of actin filaments within the apical region of vln2 vln5 pollen tubes might affect the formation of actin collars at the subapex. Actin filaments typically formed bright, highly bundled structures in the subapical regions of wild-type pollen tubes (Figure 4A, boxed region). The organization of actin filaments in these structures was quite regular (Figure 4C), consistent with previous findings (Kost et al., 1998; Fu et al., 2001; Lovy-Wheeler et al., 2005). By contrast, the fluorescence associated with actin filament staining in the corresponding region of vln2 vln5 pollen tubes was quite dim (Figure 4B, boxed region), and the distribution of actin filaments was relatively irregular (Figure 4C). The significant decrease in fluorescence pixel intensity associated with F-actin staining in the boxed regions of vln2 vln5 pollen tubes (Figure 4D) suggests that VLN2 and VLN5 are required for stabilizing actin filaments at the subapex. Additionally, the decrease in skewness at the subapex in vln2 vln5 pollen tubes (Figure 4E) suggests that the bundling activity of VLN2 and VLN5 may be involved in the construction of actin collars. We also found that, compared with that in the wild type, the width of actin cables decreased and the angles formed between actin filaments and the pollen tube growth axis increased in the shank of vln2 vln5 pollen tubes (see Supplemental Figure 6 online), suggesting that VLN2 and VLN5 are also required for bundling actin filaments as well as maintaining the longitudinal arrangement of actin cables in the shank region. The distribution of actin filaments in the subapex region of pollen tubes was assessed by measuring the value of “eccentricity.” This measure has previously been successfully used to assess the uniformity of cellulose microfibers (Marga et al., 2005) and the organization of actin filaments (Vidali et al., 2007). By analyzing the elliptical shape of the fast Fourier transform (FFT) of actin filament images, we calculated eccentricity values. A larger eccentricity value (approaching 1) indicates that the orientation of the actin filaments is more ordered. The eccentricity value was significantly lower in vln2 vln5 pollen tubes compared with wild-type pollen tubes (Figure 4F). This observation indicates that the distribution of actin filaments is more random in vln2 vln5 pollen tubes, suggesting that VLN2 and VLN5 are required for the construction of actin collars.
Figure 4.
Actin Collars Do Not Form Properly in vln2 vln5 Pollen Tubes.
(A) Actin filaments in a wild-type (WT) pollen tube. Selected optical sections and a projection (far right panel) are shown. The red box marks the region containing actin structures corresponding to actin collars.
(B) Actin filaments in a vln2-2 vln5-2 pollen tube. Selected optical sections and a projection (far right panel) are shown. Bar = 5 μm for images in (A) and (B). The red box marks the region containing actin structures corresponding to actin collars.
(C) Enlarged images of actin filaments from the boxed regions of (A) and (B). Bar = 2 μm.
(D) The average fluorescence pixel intensity in arbitrary units (a.u.) associated with actin staining decreased significantly in vln2 vln5 pollen tubes. **P < 0.01 by a Student’s t test.
(E) The skewness decreased significantly in vln2 vln5 pollen tubes. *P < 0.05 by a Student’s t test.
(F) The eccentricity of actin filaments in the subapical region of vln2 vln5 pollen tubes decreased significantly, suggesting that the orientation of actin filaments was more irregular in the subapical region of vln2 vln5 pollen tubes. *P < 0.05 by a Student’s t test.
Decreases in the Severing Frequency and Rigidity of Actin Filaments May Underlie the Defect in the Construction of Actin Collars
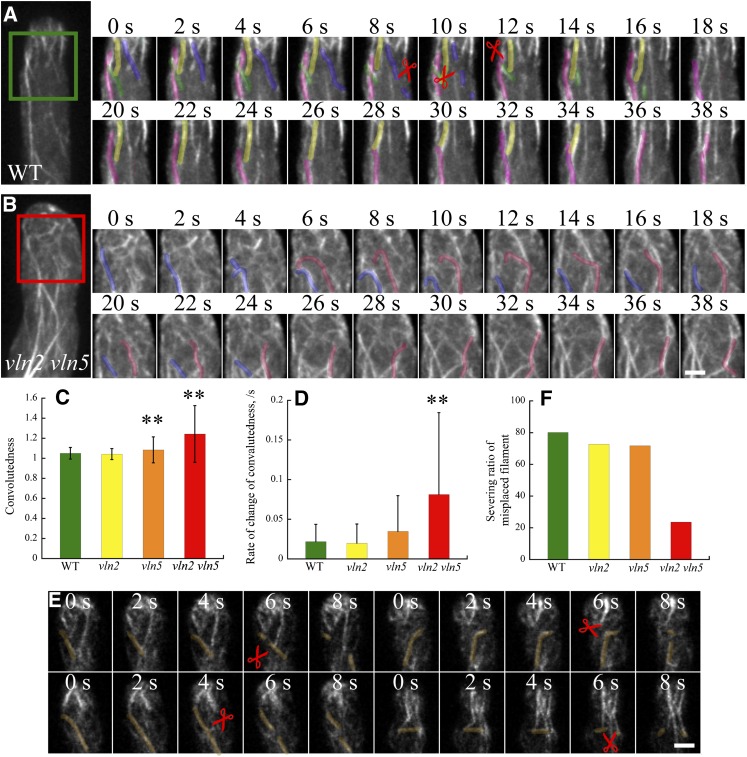
We next performed live imaging of actin filaments to visualize the dynamic parameters underlying the defect in the construction of actin collars. Consistent with previous findings (Cheung et al., 2010), most actin filaments present at the subapex traveled from the apical membrane. In wild-type pollen tubes, linear actin filaments dissociated from the apical membrane via severing (Figure 5A; see Supplemental Movie 12 online). By contrast, actin filaments became wavy and buckled in vln2 vln5 pollen tubes (Figure 5B; see Supplemental Movie 13 online). To quantify the wavy structure of these actin filaments, we assessed the “convolutedness,” defined by Staiger et al. (2009) as the ratio of traced actin filament length divided by the longest length of a bounding rectangle. We found that the convolutedness and the rate of change of convolutedness of actin filaments was significantly increased in vln2 vln5 pollen tubes compared with wild-type tubes (Figures 5C and 5D). These observations suggest that the rigidity of actin filaments was decreased, resulting in buckling. We also noticed that at the subapex of wild-type pollen tubes, actin filaments forming wide angles with the growth axis of the pollen tube were frequently severed (Figure 5E). This finding suggests that severing activity may facilitate the construction of actin collars by eliminating improperly placed actin filaments. By contrast, in vln2 vln5 pollen tubes, the percentage of actin filaments forming wide angles (>60°) with the growth axis that underwent severing was substantially decreased (Figure 5F). Thus, these data suggest that VLN2 and VLN5 regulate the construction of actin collars by increasing rigidity of actin filaments, bundling actin filaments, and severing actin filaments.
Figure 5.
Actin Filaments Are More Wavy in the Subapical Region of vln2 vln5 Pollen Tubes.
(A) Actin dynamics in the subapical region of a wild-type (WT) pollen tube. The right panels show enlarged time-lapse images of actin filaments from the green-boxed region shown in the left panel. Pink, yellow, purple, and green pseudocolored actin filaments denote filaments that grew in a linear manner out of the apical region. Scissors indicate actin filament severing events. See Supplemental Movie 12 for the entire series.
(B) Actin dynamics in the subapical region of a vln2 vln5 pollen tube. The right panels show enlarged time-lapse images of actin filaments from the red boxed region. Pink and blue pseudocolored actin filaments denote filaments in the subapical region that exhibit a wavy morphology and are not subject to severing. Bar = 2 μm for (A) and (B). See Supplemental Movie 13 for the entire series.
(C) The convolutedness of actin filaments increased substantially in vln2 vln5 pollen tubes. Data represent mean ± sd. **P < 0.01 by a Student’s t test.
(D) The average rate of change in the convolutedness of actin filaments increased substantially in vln2 vln5 pollen tubes. Data represent mean ± sd. **P < 0.01 by a Student’s t test.
(E) Time-lapse images of actin filament dynamics in the subapical region of two different wild-type pollen tubes. Pseudocolored actin filaments grew out of the apical region but did not align longitudinally. These filaments were normally subjected to severing, and scissors indicate the severing events. Bar = 2 μm.
(F) The percentage of actin filaments forming angles greater than 60° with the growth axis that were severed was decreased substantially in vln2 vln5 pollen tubes. Only actin filaments with alignments that formed angles greater than 60° were selected for quantification in wild-type, vln2, vln5, and vln2 vln5 pollen tubes.
The Distribution of Transport Vesicles Was Altered in vln2 vln5 Pollen Tubes
Subapical actin structures have been suggested to play an important role in organizing the vesicles in an inverted cone-shaped area found in the apical region of the pollen tube. We therefore wondered whether the distribution of vesicles at the subapex was altered in vln2 vln5 pollen tubes. RabA4b is a Rab GTPase that specifically decorates vesicles found in this inverted area of the apical region of pollen tubes (Lee et al., 2008; Zhang et al., 2010b) and Arabidopsis root hairs (Preuss et al., 2004). Consistent with previous findings (Lee et al., 2008; Zhang et al., 2010b), YFP-RabA4b accumulated at the apical region of wild-type pollen tubes, forming a concentrated inverted “V” shape (see Supplemental Figure 7A online). However, RabA4b-labeled vesicles at the subapex in vln2 vln5 pollen tubes were more uniformly distributed and dimmer (see Supplemental Figure 7A online). Real-time visualization of RabA4b-labeled vesicles showed that despite the fact that they are transported rapidly, the concentrated inverted “V” shape of the vesicle pool is maintained in the wild-type pollen tube subapex (see Supplemental Figure 7B and Supplemental Movie 14 online). By contrast, the distribution of RabA4b-positive vesicles was less focused in the subapex of vln2 vln5 pollen tubes (see Supplemental Figure 7C and Supplemental Movie 15 online). To quantify the distribution of RabA4b-labeled vesicles in the pollen tube, we calculated the ratio of the fluorescence of RabA4b-labeled vesicles within the apical and subapical regions to the fluorescence of RabA4b-labeled vesicles at the shanks of pollen tubes. This ratio was significantly decreased in vln2 vln5 pollen tubes (see Supplemental Figure 7D online). These data indicate that the vesicle distribution was altered in vln2 vln5 pollen tubes compared with wild-type pollen tubes, which may partially explain the decreased rate of pollen tube growth in vln2 vln5 plants.
DISCUSSION
The lack of information about the dynamic properties of actin filaments at the apical region has been a barrier to understanding the role and underlying mechanism of actin in regulating pollen tube tip growth. To overcome this limitation, we performed high-resolution spatiotemporal live-cell imaging of actin filament dynamics within the apical dome of pollen tubes, providing a detailed characterization of the parameters associated with actin filament growth and disappearance at pollen tube tips. We identified villin as a key regulator of actin filament dynamics in the apical dome of pollen tubes that acts via its severing activity in concert with the tip-focused calcium gradient. Our study reports a direct link between villin and actin filament severing events in vivo. Furthermore, our results provide insight into the role of actin filaments in regulating tip growth.
Actin Filaments Originate from the Apical Membrane and Are Highly Dynamic within the Apical Dome
To date, the precise organization and dynamics of actin structures at the pollen tube apex remain largely unknown. In particular, a detailed description of the parameters associated with actin filament growth and disappearance is lacking. This deficiency, to some extent, is due to the absence of a functional fluorescent fusion protein that decorates actin filaments within this region to facilitate monitoring actin dynamics in real time. Thus Lifeact-EGFP served as the reporter of choice in this study, since it decorates actin filaments in the apical region of tip-growing cells (Vidali et al., 2009). We first verified that Lifeact-EGFP labeled actin filaments, showing arrays typical of Arabidopsis pollen tubes (see Supplemental Figure 3 online), as previously revealed by staining with fluorescent phalloidin (Ye et al., 2009; Wu et al., 2010; Zhang et al., 2010a; Su et al., 2012). High-resolution spatiotemporal imaging of actin filament dynamics using spinning disk confocal microscopy showed that actin filaments were constantly generated from the apical membrane within the apical dome (see Supplemental Figure 4 online). Actin filaments grew out of the membrane quite linearly (Figure 4). We did not observe the growth of actin filaments from preexisting filaments, a type of growth characteristic of actin networks regulated by the Arp2/3 complex (Pollard, 2007). This observation suggests that nucleation of actin filaments within the apical dome is not likely mediated by Arp2/3, although this mechanism was previously proposed by Mathur and Hülskamp (2001). They proposed that the apical region of tip-growing cells contains a branching network of actin filaments (Mathur and Hülskamp, 2001). By contrast and consistent with our observations, Lovy-Wheeler et al. (2005) claimed that the organization of actin filaments within the tip domain of pollen tubes differs from that of the leading edge of motile mammalian cells. The nucleation of actin filaments within the apical dome is most likely mediated by membrane-anchored formins (Cheung et al., 2010). Overexpression of At-FH1 resulted in excessive formation of actin filaments originating from the membrane and was associated with curvature of the apical membrane at pollen tube tips (Cheung and Wu, 2004), further supporting this assertion. Our study provides direct evidence that actin filaments can originate from the extreme apex of pollen tubes. The outgrowing actin filaments are highly dynamic and elongate rapidly, with elongation rates reaching 0.25 μm/s, consistent with the presence of a high concentration of actin/profilin complexes in pollen (Chen et al., 2009; Staiger et al., 2010). These findings are similar to those observed in hypocotyl cells (Staiger et al., 2009; Henty et al., 2011; Li et al., 2012). Actin filaments originating from the apical membrane were severed frequently (Figure 3; see Supplemental Figure 4 online). These severing events may allow for the departure of actin filaments from their membrane anchors and their subsequent use in the construction of actin arrays, as proposed by Cheung et al. (2010). Additionally, we noticed that membrane-originated and -anchored actin filaments found at the extreme apex can move to the apical flanks, most likely due to the fusion of membrane-containing exocytic vesicles at the extreme apex of pollen tubes (Lee et al., 2008). Consequently, apical membrane-originated actin filaments may guide vesicle fusion and docking events to regulate pollen tube growth. In summary, these dynamic behaviors of actin filaments result in a decreased abundance of actin filaments at the apex, and this organization of actin filaments appears to be involved in regulating the direction of pollen tube growth (see Supplemental Figure 5 online).
Villin May Act in Concert with the Calcium Gradient to Confer the Dynamic Properties Associated with Actin Filaments in the Apical Dome
Villin/gelsolin family members have been proposed to act coordinately with calcium signaling to regulate actin dynamics at pollen tube tips (Gu et al., 2005; Cárdenas et al., 2008; Zhang et al., 2010a). For instance, several potential splice variants of lily villin, such as ABP29 and ABP41, have been implicated in actin cytoskeleton remodeling in the pollen tube (Xiang et al., 2007; Wang et al., 2008). However, to date, no direct genetic evidence has been discovered that firmly supports this speculation. Our observations showed that loss of VLN2 and VLN5 led to the accumulation of actin filaments in the apical region (Figure 2), suggesting that villin is the major player that drives the rapid turnover of actin filaments at pollen tube tips. Considering that both VLN2 and VLN5 can sever actin filaments in the presence of physiological calcium concentrations (Zhang et al., 2010a; Bao et al., 2012), the accumulation of actin filaments is likely due to a decrease in VLN2- and VLN5-mediated severing events. Indeed, we showed that severing events were frequently detected within the apical dome of wild-type pollen tubes, but rarely detected within the apical dome of vln2 vln5 pollen tubes (Figures 2 and 3, see Supplemental Figure 4 online). Furthermore, Cheung et al. (2010) demonstrated that actin filaments nucleated by the apical membrane-anchored protein FH5 exit the apical region and serve as a basis for the construction of subsequent actin arrays. Therefore, VLN2 and VLN5 may be important for severing actin filaments to facilitate their departure from the apical region so that they may be used for the construction of actin collars (described below). Considering that VLN2 and VLN5 bind to the barbed end of actin filaments after severing (Zhang et al., 2010a; Bao et al., 2012), it is easy to imagine that these proteins may act in concert with the monomer sequestering activity of profilin to drive rapid actin turnover, especially since the activity of profilin is also calcium dependent (Kovar et al., 2000). These observations make profilin a likely candidate in driving rapid actin turnover in the apical cytoplasm of pollen tubes, as previously proposed (Gu et al., 2005). This potential role for profilin is supported by evidence from in vitro reconstitution experiments demonstrating that the gelsolin-like protein Papaver rhoeas ABP80 (Pr-ABP80) works with profilin to induce massive net actin depolymerization in the presence of micromolar levels of calcium (Huang et al., 2004). Certainly, other ABPs, such as actin-depolymerizing factor (ADF) (Allwood et al., 2002; Chen et al., 2002; Augustine et al., 2008), actin-interacting protein 1 (AIP1) (Ketelaar et al., 2004; Augustine et al., 2011; Shi et al., 2013), and cyclase-associated protein (Chaudhry et al., 2007; Deeks et al., 2007) may also participate in driving the rapid turnover of actin filaments within the apical dome, though ADF is assumed to be inactive within this region (Allwood et al., 2002). Additionally, it was shown that class XI myosin localized at the apical region of root hairs and moss protonemal cells (Peremyslov et al., 2010; Vidali et al., 2010; Peremyslov et al., 2012; Furt et al., 2013), and loss of class XI myosin caused the accumulation of actin filaments at the apical region of these cells (Peremyslov et al., 2010; Vidali et al., 2010). Therefore, it is possible that class XI myosin functions in promoting the transport of actin filaments that are fragmented by villin away from pollen tube tips. It was shown that several class XI myosins are pollen specific (https://www.genevestigator.com/gv/index.jsp; Honys and Twell, 2003; Pina et al., 2005), and future reverse genetic analysis of these myosins is going to test this hypothesis. A recent study showed that shank plasma membrane–localized calcium responsive MAP18 severs actin filaments and regulates actin dynamics in the apical region of pollen tubes (Zhu et al., 2013). Therefore, it could be possible that VLN2 and VLN5, to some extent, may act coordinately with MAP18 to regulate apical actin filaments in response to calcium signaling. Considering that both VLN2 and VLN5 are versatile actin regulatory proteins (Zhang et al., 2010a; Bao et al., 2012), future studies are needed to dissect the precise contributions of their individual activities to actin filament dynamics at pollen tube tips.
Villin Is Involved in the Construction of Actin Collars
At the subapex of the pollen tube, cortical actin filaments form a dense collar, located in a region beginning 1 to 5 μm distal from the tip and extending basally 5 to 10 μm dependent upon the organism (Cheung and Wu, 2008; Chen et al., 2009; Staiger et al., 2010). The collar is filled with short actin bundles that are packed even more densely than those in the shank (Lenartowska and Michalska, 2008). Several actin binding proteins have been implicated in the formation of this actin-based structure, including ADF/AIP1 (Lovy-Wheeler et al., 2006) and fimbrin (Wu et al., 2010; Su et al., 2012). However, the molecular mechanisms underlying the generation and maintenance of actin collars remain poorly understood. Identification of additional ABPs involved in this process will provide clues regarding these underlying mechanisms. Given that the collar is filled with tightly packed actin bundles, bundling factors may be involved in this process. Therefore, the activity of villin may be relevant, though it has been assumed that villin would periodically degrade this actin structure due to the presence of micromolar levels of free cytosolic calcium (Su et al., 2012). Indeed, we found that villin is an important player in the construction of actin collars (Figure 4). In addition to the easily imagined contribution of the bundling activity of villin to collar formation (Figures 4C and 4E), we believe that the severing activity of villin also contributes to the formation of actin collars at different locations within pollen tubes. In the apical region, villin severs actin filaments to allow the release of apical membrane-anchored actin filaments, as described above. In the subapical region, villin-mediated severing may facilitate the construction of actin collars by eliminating improperly placed actin filaments (Figures 5E and 5F). Direct visualization of actin filaments at the apex and subapex revealed that actin filaments become very wavy and buckled in vln2 vln5 pollen tubes (Figures 5A to 5D), implying that VLN2 and VLN5 confer rigidity upon actin filaments, allowing them to grow in a linear manner and to be packed into actin collars at the subapex. Again, future studies are needed to dissect the precise contributions of the individual activities of VLN2 and VLN5 to the construction of actin collars. Our study also revealed that the distribution of transport vesicles was altered in vln2 vln5 pollen tubes (see Supplemental Figure 7 online), supporting the hypothesis that actin collars function in organizing transport vesicles.
A Simple Model Describing Villin-Mediated Regulation of Actin Dynamics within the Apical Dome
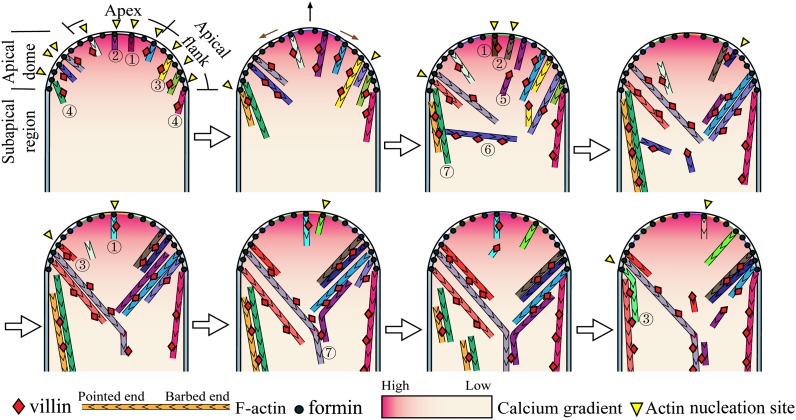
Based on our findings, we propose a simplified model explaining actin filament growth and disappearance within the apical dome and describing the potential roles of villin in this process (Figure 6). Actin filaments are generated from the apical membrane within the apical dome, likely via nucleation by the apical membrane-anchored FH5 (Cheung et al., 2010) or other membrane-anchored formins. These apical membrane-originated actin filaments are instantly bundled by bundling factors, such as villin, which provide the filaments with rigidity and allow them to grow out from the membrane in a straight manner. Due to the presence of the tip-focused calcium gradient, the free calcium concentration reaches micromolar levels in the apical region (Pierson et al., 1994; Holdaway-Clarke et al., 1997; Messerli et al., 2000). These calcium levels activate the severing activity of villin, allowing actin filaments to be turned over locally or released from their membrane anchors to be used for the construction of actin collars. Some apex-originated actin filaments move to the apical flank, presumably due to the fusion of exocytic vesicles at the extreme apex, resulting in membrane flow. The dynamic traits associated with these actin filaments result in a decrease in abundance of actin filaments at the extreme apex. In addition to the role of pushing actin filaments away from the apical membrane, villin-mediated bundling activity may be directly involved in organizing actin filaments into highly structured actin collars. Furthermore, villin-mediated severing activity may facilitate the construction of actin collars by eliminating actin filaments that do not align longitudinally at the subapex.
Figure 6.
Simplified Model Describing VILLIN-Mediated Regulation of Actin Dynamics at Pollen Tube Tips.
Actin filaments are nucleated by membrane-anchored formins within the apical dome of the pollen tube. Because formins bind the barbed end of actin filaments, we propose that the actin filaments are oriented with their pointed ends facing the cytoplasm. After nucleation, the actin filaments are instantly decorated with actin filament binding proteins, such as villin, which confer rigidity and allow the filaments to grow in a linear manner toward the cytoplasm. At the apex, membrane-anchored actin filaments can be turned over rapidly via severing (see 1 marked actin filaments), which is presumably mediated by villin acting in concert with the tip-focused calcium gradient. Some apex-originated actin filaments move to the apical flank (see 2 marked actin filaments), likely due to the fusion of exocytic vesicles at the apex of the pollen tube. The combination of these events leads to the outcome that actin filaments are less abundant at the pollen tube apex. In addition to actin filaments that move from the apex to the apical flank, actin filaments can also be nucleated at the membranes of the apical flank (see 3 marked actin filaments). Apical flank membrane-anchored actin filaments can be used for the construction of actin collars (see 4 marked actin filaments). In addition to conferring rigidity upon actin filaments and promoting linear growth toward the subapical cytoplasm, calcium-triggered, villin-mediated severing also promotes relocation of apical flank membrane-anchored actin filaments to actin collars (see 5 marked actin filament). Additionally, at the subapex, villin-mediated severing may facilitate the construction of actin collars by eliminating actin filaments that are not oriented longitudinally (see 6 marked actin filaments). The bundling activity of villin may also be directly involved in the construction of actin collars (see 7 marked actin filaments). Black arrow indicates pollen tube growth direction, and brown arrows indicate the direction of membrane flow.
In summary, our study showed that VLN2 and VLN5 regulate the organization and dynamics of actin filaments within the apical dome, presumably acting in concert with the tip-focused calcium gradient via severing actin filaments in pollen tubes. As tip-focused calcium gradients are present in many tip-growing cells, similar mechanisms may operate in related systems. Therefore, we propose that regulation of the organization and dynamics of actin filaments through coordination of calcium-responsive ABPs (villin in this case) with the calcium gradient may represent a unifying mechanism that underpins the regulation of tip growth in diverse tip-growing cells.
METHODS
Plasmid Construction and Arabidopsis thaliana Transformation
To examine the subcellular localization of VLN2 and VLN5 in pollen tubes, VLN2 and VLN5 genomic DNAs were amplified using primer pairs V2GENF/V2GENR and V5GENF/V5GENR (see Supplemental Table 1 online), respectively. The amplified fragments were subsequently cloned into the pDONR221 entry vector (Life Technologies) through a Gateway BP reaction according to the manufacturer’s instructions. After verification of the sequence of the amplified fragments by DNA sequencing, the sequences were transferred into the pK7FWG2 destination vector, in which the 35S promoter had been deleted, via the Gateway LR reaction according to the manufacturer’s instructions. This procedure resulted in the generation of the VLN2GEN-GFP and VLN5GEN-GFP constructs, in which GFP was fused to the C terminus of VLN2 or VLN5, respectively. These plasmids were then transformed into Agrobacterium tumefaciens strain GV3101 and introduced into vln2-2, vln5-1, or vln2-2 vln5-2 plants by the floral dip method (Clough and Bent, 1998).
RT-PCR Analysis
Freshly opened flowers derived from the wild type, vln2, vln5, and vln2 vln5 were collected to isolate total RNA using TRIzol reagent (Invitrogen), as described in the manufacturer’s instructions. To synthesize cDNA, the isolated total RNA was used for reverse transcription with MMLV reverse transcriptase (Promega), according to the manufacturer’s recommendations. Full-length VLN2 and VLN5 were amplified with primer pairs V2F1/V2R1 and V5F1/V5R1 (see Supplemental Table 1 online), respectively. eIF4A was used as an internal control amplified with primer pair eIF4AF/eIF4AR (see Supplemental Table 1 online). The PCR products were separated by agarose gel electrophoresis.
Pollen Germination and Growth Measurement
In vitro pollen germination was performed as previously described (Ye et al., 2009; Wu et al., 2010; Zhang et al., 2010a). To quantify pollen tube growth rate and pollen tube width, pollen grains derived from wild-type Columbia-0, single mutant (vln2 or vln5), or vln2 vln5 double mutant plants were germinated for 2 h and 3 to 4 h, respectively, to allow the average lengths of the pollen tubes to reach ∼200 μm. More than 50 individual pollen tubes were selected to determine the growth rate, and more than 200 individual pollen tubes were selected to determine the width at the widest region of the pollen tubes. A minimum of three independent experiments was conducted for each assay.
Actin Staining and Quantification
Staining of actin in pollen tubes with Alexa-488 phalloidin was performed according to the methods described by Zhang et al. (2010a). The actin cytoskeleton in the pollen tube was observed under a Leica TCS SP5 confocal laser scanning microscope equipped with a ×100 (1.46 NA HC PLAN) objective. Alexa-488 phalloidin was excited with the 488-nm line of an argon laser with the emission set to 550 to 600 nm. The step size was set to 0.5 μm to collect optical sections. To quantitatively compare the amount of F-actin between Columbia-0 and mutant pollen tubes, all optical sections were acquired under identical conditions. Quantification of actin structures was performed using Image J (http://rsbweb.nih.gov/ij/, version 1.46g), and part of the resulting data were processed using R programming language (http://www.R-project.org, version 2.13.1). To quantify actin structures in the subapical region, a 5 × 8-μm2 region located ∼1 μm from the pollen tube tip was selected. To quantify F-actin levels, the average gray value of the fluorescent phalloidin staining in the selected region was analyzed for more than 30 pollen tubes. Characterization of the orientation of actin filaments in the selected region was performed using ImageJ (version 1.38X) with the “fit ellipse 3c” plugin (Christopher Coulon; www.theGAIAgroup.org) as previously described (Marga et al., 2005; Vidali et al., 2007). Maximally projected images were initially transformed into FFT images. The FFT images were then thresholded at different values and followed by shape analysis by fitting an ellipse to the Fourier spectra in “fit ellipse 3c” in order to calculate the eccentricity. The threshold value was initially set to 100, and the value was increased by one at each step, until 800 black pixels remained. A series of values was generated for each image, and the last 10 to 14 values were selected and averaged to yield the eccentricity value. More than nine pollen tubes were analyzed for each line, and three independent experiments were performed. The extent of actin filament bundling in the selected region was determined by measuring the value of skewness according to published methods (Higaki et al., 2010; van der Honing et al., 2012). The z-stack optical sections from the 5 × 8-μm2 area of interest in the subapical region were processed by background subtraction with the rolling ball radius set to 15 pixels and the Gaussian blur radius set to 1.0. A minimum of 24 pollen tubes from each genotype was selected to determine the average skewness of the actin filaments. Quantification of the angles formed between actin cables and the pollen tube growth axis as well as the width of actin cables in the shank of pollen tubes was according to the method described by Wu et al. (2010).
Visualization of YFP-RabA4b
The distribution of transport vesicles in pollen tubes was analyzed in plants expressing Lat52:YFP-RabA4b (Zhang et al., 2010b). After the average length reached ∼200 μm, the pollen tubes were observed under an Olympus FV1000MPE multiphoton laser scanning confocal microscope equipped with a ×100 objective (numerical aperture of 1.4). Samples were excited under a 488-nm argon laser, and the emission wavelength range was set to 505 to 545 nm. To trace the dynamics of YFP-RabA4b–labeled vesicles, time-lapse, wide-field fluorescence microscopy was performed using an Olympus IX81 inverted microscope equipped with a ×100 UAPON objective (numerical aperture of 1.49). Time-lapse series images were captured every 2 s with a Photometrics Cascade II: 512 EMCCD camera (Major Instruments) driven by Micro-Manager software (www.micro-manager.org). To quantify the distribution pattern of YFP-RabA4b–labeled vesicles in pollen tubes, the average fluorescence intensity of YFP-RabA4b at the apical and subapical regions was divided by that of the shank region to yield a ratio. More than 50 pollen tubes were analyzed.
Visualization and Quantification of Actin Filament Dynamics in Pollen Tubes
Pollen tubes expressing Lat52:Lifeact-EGFP were subjected to observation with an Olympus BX51 microscope equipped with an Andor Revolution XDh spinning disk confocal system. Only the growing pollen tubes were selected for imaging. Images were acquired using Andor's IQ2 software. GFP was excited using a 488-nm argon laser, and the emission wavelengths were captured from 505 to 545 nm. Time-lapse, z-stack images (step size, 0.5 μm) were taken every 2 s. Rotation of pollen tube images was performed using the IQ2 software package. Other image manipulations, such as generation of montages and projections of z-stack images, were performed using Image J (version 1.46g). Kymographs were generated with the MultipleKymograph plugin in Image J (version 1.46g). To quantify single actin filament dynamics within the apical domes of pollen tubes, parameters such as filament lifetime, maximum filament length, elongation rate, depolymerization rate, and convolutedness were calculated as previously described (Staiger et al., 2009; Henty et al., 2011). Only filaments that could be tracked over four successive frames were considered. To quantify the frequency at which actin filaments were severed at the subapexes of pollen tubes, only actin filaments forming greater than a 60° angle with the growth axis were selected. More than 100 such actin filaments from more than 18 pollen tubes were counted to determine the percentage of actin filaments subjected to severing.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative under accession numbers At2g41740 (VLN2) and At5g57320 (VLN5).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. VLN2 and VLN5 Coordinately Regulate Polarized Pollen Tube Growth.
Supplemental Figure 2. VLN2GEN-GFP and VLN5GEN-GFP Are Functional.
Supplemental Figure 3. Lifeact-EGFP Decorates Distinct Actin Arrays in Arabidopsis Pollen Tubes.
Supplemental Figure 4. Actin Filaments Are Less Abundant at the Apex and Are Highly Dynamic within the Apical Dome of Wild-Type Pollen Tubes.
Supplemental Figure 5. The Extreme Apex Has Fewer Actin Filaments and Faces the Direction of Growth.
Supplemental Figure 6. The Width of Actin Cables Was Decreased and Angles Formed between Actin Cables and The Growth Axis of Pollen Tubes Increased in vln2 vln5 Pollen Tubes.
Supplemental Figure 7. The Distribution of Rab4Ab-Labeled Vesicles Is Altered in vln2 vln5 Pollen Tubes.
Supplemental Table 1. Primer Sequences Used in This Study.
Supplemental Movie 1. Actin Filament Dynamics in the Apical Region of a Wild-Type Pollen Tube.
Supplemental Movie 2. Actin Filament Dynamics in the Apical Region of a Wild-Type Pollen Tube.
Supplemental Movie 3. Actin Filament Dynamics at the Medial Section of a Wild-Type Pollen Tube.
Supplemental Movie 4. Actin Filament Dynamics at the Cortical Region of a Wild-Type Pollen Tube.
Supplemental Movie 5. Actin Filament Dynamics at the Medial Section of a vln2 vln5 Pollen Tube.
Supplemental Movie 6. Actin Filament Dynamics at the Cortical Region of a vln2 vln5 Pollen Tube.
Supplemental Movie 7. Actin Dynamic at the Apical Region of a Wild-Type Pollen Tube.
Supplemental Movie 8. Actin Dynamic at the Apical Region of a vln2 vln5 Pollen Tube.
Supplemental Movie 9. Actin Filament Dynamics at the Apical Region of a vln2 vln5 Pollen Tube.
Supplemental Movie 10. Dynamics of Single Actin Filaments in the Apical Region of a Wild-Type Pollen Tube.
Supplemental Movie 11. Dynamic of Single Actin Filaments in the Apical Region of a vln2 vln5 Pollen Tube.
Supplemental Movie 12. Actin Filament Dynamics at the Subapical Region of a Wild-Type Pollen Tube.
Supplemental Movie 13. Actin Filament Dynamics at the Subapical Region of a vln2 vln5 Pollen Tube.
Supplemental Movie 14. Dynamic Distribution of YFP-RabA4b-labeled Vesicles in a Wild-Type Pollen Tube.
Supplemental Movie 15. Dynamic Distribution of YFP-RabA4b–Labeled Vesicles in vln2 vln5 Pollen Tube.
Supplemental References 1. References for the Supplemental Data.
Supplementary Material
Acknowledgments
We thank the ABRC and the Nottingham Arabidopsis Stock Centre for providing T-DNA insertion lines and members of the Huang lab for helpful discussion. We also thank Yan Zhang (Shandong Agricultural University) for the marker line expressing YFP-RabA4b in pollen. X.Q. thanks Hui Zhang (Institute of Botany, Chinese Academy of Sciences) for the advice on image analysis. This work was supported by grants from the Ministry of Science and Technology of China (2013CB945100 and 2011CB944600) and the National Natural Science Foundation of China (31125004, 31071179, and 31121065).
AUTHOR CONTRIBUTIONS
S.H. and X.Q. conceived and designed the research. X.Q., H.Z., Y.X., J.W., and N.C. performed the experiments. X.Q. and S.H. analyzed the data. S.H. wrote the article.
Glossary
- GFP
green fluorescent protein
- FFT
fast Fourier transform
References
- Allwood E.G., Anthony R.G., Smertenko A.P., Reichelt S., Drobak B.K., Doonan J.H., Weeds A.G., Hussey P.J. (2002). Regulation of the pollen-specific actin-depolymerizing factor LlADF1. Plant Cell 14: 2915–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine R.C., Pattavina K.A., Tüzel E., Vidali L., Bezanilla M. (2011). Actin interacting protein1 and actin depolymerizing factor drive rapid actin dynamics in Physcomitrella patens. Plant Cell 23: 3696–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine R.C., Vidali L., Kleinman K.P., Bezanilla M. (2008). Actin depolymerizing factor is essential for viability in plants, and its phosphoregulation is important for tip growth. Plant J. 54: 863–875 [DOI] [PubMed] [Google Scholar]
- Bao C., Wang J., Zhang R., Zhang B., Zhang H., Zhou Y., Huang S. (2012). Arabidopsis VILLIN2 and VILLIN3 act redundantly in sclerenchyma development via bundling of actin filaments. Plant J. 71: 962–975 [DOI] [PubMed] [Google Scholar]
- Bedinger P.A., Hardeman K.J., Loukides C.A. (1994). Travelling in style: The cell biology of pollen. Trends Cell Biol. 4: 132–138 [DOI] [PubMed] [Google Scholar]
- Berepiki A., Lichius A., Read N.D. (2011). Actin organization and dynamics in filamentous fungi. Nat. Rev. Microbiol. 9: 876–887 [DOI] [PubMed] [Google Scholar]
- Cárdenas L., Lovy-Wheeler A., Kunkel J.G., Hepler P.K. (2008). Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol. 146: 1611–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F., Guérin C., von Witsch M., Blanchoin L., Staiger C.J. (2007). Identification of Arabidopsis cyclase-associated protein 1 as the first nucleotide exchange factor for plant actin. Mol. Biol. Cell 18: 3002–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y., Wong E.I., Vidali L., Estavillo A., Hepler P.K., Wu H.M., Cheung A.Y. (2002). The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell 14: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Qu X., Wu Y., Huang S. (2009). Regulation of actin dynamics in pollen tubes: Control of actin polymer level. J. Integr. Plant Biol. 51: 740–750 [DOI] [PubMed] [Google Scholar]
- Cheung A.Y., Niroomand S., Zou Y., Wu H.M. (2010). A transmembrane formin nucleates subapical actin assembly and controls tip-focused growth in pollen tubes. Proc. Natl. Acad. Sci. USA 107: 16390–16395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A.Y., Wu H.M. (2004). Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell 16: 257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A.Y., Wu H.M. (2008). Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu. Rev. Plant Biol. 59: 547–572 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cole R.A., Fowler J.E. (2006). Polarized growth: Maintaining focus on the tip. Curr. Opin. Plant Biol. 9: 579–588 [DOI] [PubMed] [Google Scholar]
- Bou Daher F., Geitmann A. (2011). Actin is involved in pollen tube tropism through redefining the spatial targeting of secretory vesicles. Traffic 12: 1537–1551 [DOI] [PubMed] [Google Scholar]
- Deeks M.J., Rodrigues C., Dimmock S., Ketelaar T., Maciver S.K., Malhó R., Hussey P.J. (2007). Arabidopsis CAP1 - A key regulator of actin organisation and development. J. Cell Sci. 120: 2609–2618 [DOI] [PubMed] [Google Scholar]
- Fu Y., Wu G., Yang Z. (2001). Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J. Cell Biol. 152: 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furt F., Liu Y.C., Bibeau J.P., Tüzel E., Vidali L. (2013). Apical myosin XI anticipates F-actin during polarized growth of Physcomitrella patens cells. Plant J. 73: 417–428 [DOI] [PubMed] [Google Scholar]
- Gibbon B.C., Kovar D.R., Staiger C.J. (1999). Latrunculin B has different effects on pollen germination and tube growth. Plant Cell 11: 2349–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Fu Y., Dowd P., Li S., Vernoud V., Gilroy S., Yang Z. (2005). A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 169: 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henty J.L., Bledsoe S.W., Khurana P., Meagher R.B., Day B., Blanchoin L., Staiger C.J. (2011). Arabidopsis actin depolymerizing factor4 modulates the stochastic dynamic behavior of actin filaments in the cortical array of epidermal cells. Plant Cell 23: 3711–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P.K., Vidali L., Cheung A.Y. (2001). Polarized cell growth in higher plants. Annu. Rev. Cell Dev. Biol. 17: 159–187 [DOI] [PubMed] [Google Scholar]
- Higaki T., Kutsuna N., Sano T., Kondo N., Hasezawa S. (2010). Quantification and cluster analysis of actin cytoskeletal structures in plant cells: Role of actin bundling in stomatal movement during diurnal cycles in Arabidopsis guard cells. Plant J. 61: 156–165 [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke T.L., Feijo J.A., Hackett G.R., Kunkel J.G., Hepler P.K. (1997). Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9: 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke T.L., Weddle N.M., Kim S., Robi A., Parris C., Kunkel J.G., Hepler P.K. (2003). Effect of extracellular calcium, pH and borate on growth oscillations in Lilium formosanum pollen tubes. J. Exp. Bot. 54: 65–72 [DOI] [PubMed] [Google Scholar]
- Honys D., Twell D. (2003). Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol. 132: 640–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Blanchoin L., Chaudhry F., Franklin-Tong V.E., Staiger C.J. (2004). A gelsolin-like protein from Papaver rhoeas pollen (PrABP80) stimulates calcium-regulated severing and depolymerization of actin filaments. J. Biol. Chem. 279: 23364–23375 [DOI] [PubMed] [Google Scholar]
- Huang S., Blanchoin L., Kovar D.R., Staiger C.J. (2003). Arabidopsis capping protein (AtCP) is a heterodimer that regulates assembly at the barbed ends of actin filaments. J. Biol. Chem. 278: 44832–44842 [DOI] [PubMed] [Google Scholar]
- Hwang J.U., Vernoud V., Szumlanski A., Nielsen E., Yang Z. (2008). A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr. Biol. 18: 1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelaar T., Allwood E.G., Anthony R., Voigt B., Menzel D., Hussey P.J. (2004). The actin-interacting protein AIP1 is essential for actin organization and plant development. Curr. Biol. 14: 145–149 [DOI] [PubMed] [Google Scholar]
- Khurana P., Henty J.L., Huang S., Staiger A.M., Blanchoin L., Staiger C.J. (2010). Arabidopsis VILLIN1 and VILLIN3 have overlapping and distinct activities in actin bundle formation and turnover. Plant Cell 22: 2727–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B., Spielhofer P., Chua N.H. (1998). A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 16: 393–401 [DOI] [PubMed] [Google Scholar]
- Kovar D.R., Drøbak B.K., Staiger C.J. (2000). Maize profilin isoforms are functionally distinct. Plant Cell 12: 583–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger J.H., Daher F.B., Grant M., Geitmann A. (2009). Microfilament orientation constrains vesicle flow and spatial distribution in growing pollen tubes. Biophys. J. 97: 1822–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Szumlanski A., Nielsen E., Yang Z. (2008). Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol. 181: 1155–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowska M., Michalska A. (2008). Actin filament organization and polarity in pollen tubes revealed by myosin II subfragment 1 decoration. Planta 228: 891–896 [DOI] [PubMed] [Google Scholar]
- Li J., Henty-Ridilla J.L., Huang S., Wang X., Blanchoin L., Staiger C.J. (2012). Capping protein modulates the dynamic behavior of actin filaments in response to phosphatidic acid in Arabidopsis. Plant Cell 24: 3742–3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler A., Kunkel J.G., Allwood E.G., Hussey P.J., Hepler P.K. (2006). Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of lily. Plant Cell 18: 2182–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler A., Wilsen K.L., Baskin T.I., Hepler P.K. (2005). Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta 221: 95–104 [DOI] [PubMed] [Google Scholar]
- Lowery L.A., Van Vactor D. (2009). The trip of the tip: Understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 10: 332–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marga F., Grandbois M., Cosgrove D.J., Baskin T.I. (2005). Cell wall extension results in the coordinate separation of parallel microfibrils: Evidence from scanning electron microscopy and atomic force microscopy. Plant J. 43: 181–190 [DOI] [PubMed] [Google Scholar]
- Mathur J., Hülskamp M. (2001). Cell growth: How to grow and where to grow. Curr. Biol. 11: R402–R404 [DOI] [PubMed] [Google Scholar]
- Messerli M.A., Créton R., Jaffe L.F., Robinson K.R. (2000). Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev. Biol. 222: 84–98 [DOI] [PubMed] [Google Scholar]
- Nakayasu T., Yokota E., Shimmen T. (1998). Purification of an actin-binding protein composed of 115-kDa polypeptide from pollen tubes of lily. Biochem. Biophys. Res. Commun. 249: 61–65 [DOI] [PubMed] [Google Scholar]
- Peremyslov V.V., Klocko A.L., Fowler J.E., Dolja V.V. (2012). Arabidopsis myosin XI-K localizes to the motile endomembrane vesicles associated with F-actin. Front. Plant Sci. 3: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov V.V., Prokhnevsky A.I., Dolja V.V. (2010). Class XI myosins are required for development, cell expansion, and F-Actin organization in Arabidopsis. Plant Cell 22: 1883–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson E.S., Miller D.D., Callaham D.A., Shipley A.M., Rivers B.A., Cresti M., Hepler P.K. (1994). Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell 6: 1815–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina C., Pinto F., Feijó J.A., Becker J.D. (2005). Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiol. 138: 744–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T.D. (2007). Regulation of actin filament assembly by Arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 36: 451–477 [DOI] [PubMed] [Google Scholar]
- Preuss M.L., Serna J., Falbel T.G., Bednarek S.Y., Nielsen E. (2004). The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16: 1589–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Yang Z. (2011). Rapid tip growth: Insights from pollen tubes. Semin. Cell Dev. Biol. 22: 816–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds C.M., Bezanilla M. (2013). Growth mechanisms in tip-growing plant cells. Annu. Rev. Plant Biol. 64: 243–265 [DOI] [PubMed] [Google Scholar]
- Shi M., Xie Y., Zheng Y., Wang J., Su Y., Yang Q., Huang S. (2013). Oryza sativa actin-interacting protein 1 is required for rice growth by promoting actin turnover. Plant J. 73: 747–760 [DOI] [PubMed] [Google Scholar]
- Snowman B.N., Kovar D.R., Shevchenko G., Franklin-Tong V.E., Staiger C.J. (2002). Signal-mediated depolymerization of actin in pollen during the self-incompatibility response. Plant Cell 14: 2613–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger C.J., Poulter N.S., Henty J.L., Franklin-Tong V.E., Blanchoin L. (2010). Regulation of actin dynamics by actin-binding proteins in pollen. J. Exp. Bot. 61: 1969–1986 [DOI] [PubMed] [Google Scholar]
- Staiger C.J., Sheahan M.B., Khurana P., Wang X., McCurdy D.W., Blanchoin L. (2009). Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array. J. Cell Biol. 184: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Zhu J., Cai C., Pei W., Wang J., Dong H., Ren H. (2012). FIMBRIN1 is involved in lily pollen tube growth by stabilizing the actin fringe. Plant Cell 24: 4539–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Honing H.S., Kieft H., Emons A.M., Ketelaar T. (2012). Arabidopsis VILLIN2 and VILLIN3 are required for the generation of thick actin filament bundles and for directional organ growth. Plant Physiol. 158: 1426–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., Augustine R.C., Kleinman K.P., Bezanilla M. (2007). Profilin is essential for tip growth in the moss Physcomitrella patens. Plant Cell 19: 3705–3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., Burkart G.M., Augustine R.C., Kerdavid E., Tüzel E., Bezanilla M. (2010). Myosin XI is essential for tip growth in Physcomitrella patens. Plant Cell 22: 1868–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., Hepler P.K. (1997). Characterization and localization of profilin in pollen grains and tubes of Lilium longiflorum. Cell Motil. Cytoskeleton 36: 323–338 [DOI] [PubMed] [Google Scholar]
- Vidali L., Hepler P.K. (2001). Actin and pollen tube growth. Protoplasma 215: 64–76 [DOI] [PubMed] [Google Scholar]
- Vidali L., McKenna S.T., Hepler P.K. (2001). Actin polymerization is essential for pollen tube growth. Mol. Biol. Cell 12: 2534–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., Rounds C.M., Hepler P.K., Bezanilla M. (2009). Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. PLoS ONE 4: e5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Xiang Y., Hou J., Ren H. (2008). ABP41 is involved in the pollen tube development via fragmenting actin filaments. Mol. Plant 1: 1048–1055 [DOI] [PubMed] [Google Scholar]
- Wu Y., Yan J., Zhang R., Qu X., Ren S., Chen N., Huang S. (2010). Arabidopsis FIMBRIN5, an actin bundling factor, is required for pollen germination and pollen tube growth. Plant Cell 22: 3745–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Huang X., Wang T., Zhang Y., Liu Q., Hussey P.J., Ren H. (2007). ACTIN BINDING PROTEIN 29 from Lilium pollen plays an important role in dynamic actin remodeling. Plant Cell 19: 1930–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. (1998). Signaling tip growth in plants. Curr. Opin. Plant Biol. 1: 525–530 [DOI] [PubMed] [Google Scholar]
- Yang Z. (2008). Cell polarity signaling in Arabidopsis. Annu. Rev. Cell Dev. Biol. 24: 551–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Zheng Y., Yan A., Chen N., Wang Z., Huang S., Yang Z. (2009). Arabidopsis formin3 directs the formation of actin cables and polarized growth in pollen tubes. Plant Cell 21: 3868–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E., Takahara K.-i., Shimmen T. (1998). Actin-bundling protein isolated from pollen tubes of lily. Biochemical and immunocytochemical characterization. Plant Physiol. 116: 1421–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E., Tominaga M., Mabuchi I., Tsuji Y., Staiger C.J., Oiwa K., Shimmen T. (2005). Plant villin, lily P-135-ABP, possesses G-actin binding activity and accelerates the polymerization and depolymerization of actin in a Ca2+-sensitive manner. Plant Cell Physiol. 46: 1690–1703 [DOI] [PubMed] [Google Scholar]
- Yokota E., Vidali L., Tominaga M., Tahara H., Orii H., Morizane Y., Hepler P.K., Shimmen T. (2003). Plant 115-kDa actin-filament bundling protein, P-115-ABP, is a homologue of plant villin and is widely distributed in cells. Plant Cell Physiol. 44: 1088–1099 [DOI] [PubMed] [Google Scholar]
- Zhang H., Qu X., Bao C., Khurana P., Wang Q., Xie Y., Zheng Y., Chen N., Blanchoin L., Staiger C.J., Huang S. (2010a). Arabidopsis VILLIN5, an actin filament bundling and severing protein, is necessary for normal pollen tube growth. Plant Cell 22: 2749–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., He J., Lee D., McCormick S. (2010b). Interdependence of endomembrane trafficking and actin dynamics during polarized growth of Arabidopsis pollen tubes. Plant Physiol. 152: 2200–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xiao Y., Du F., Cao L., Dong H., Ren H. (2011). Arabidopsis VILLIN4 is involved in root hair growth through regulating actin organization in a Ca2+-dependent manner. New Phytol. 190: 667–682 [DOI] [PubMed] [Google Scholar]
- Zhu L., Zhang Y., Kang E., Xu Q., Wang M., Rui Y., Liu B., Yuan M., Fu Y. (2013). MAP18 regulates the direction of pollen tube growth in Arabidopsis by modulating F-actin organization. Plant Cell 25: 851–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.