Abstract
Objective
Long-term survival for patients with advanced endometrial carcinoma is poor, and limited options exist for the management of recurrent disease. Our goal was to investigate the activity of doxorubicin in the second-line setting in patients who progressed after paclitaxel/carboplatin adjuvant treatment.
Methods
We conducted a retrospective analysis of patients with recurrent endometrial carcinoma who were treated at Memorial Sloan-Kettering Cancer Center from 1995-2009, and who received paclitaxel/carboplatin adjuvant chemotherapy followed by second-line doxorubicin therapy at time of recurrence. The median PFS and OS times following paclitaxel/carboplatin and following second-line doxorubicin therapy were estimated using the Kaplan-Meier method. Toxicity was assessed by the treating physician at each visit and graded using version 4.0 of Common Terminology Criteria for Adverse Events (CTCAE). Patient presentation, treatment, patterns of recurrence, and patient outcomes were summarized.
Results
Seventeen patients were included in study analyses. The median PFS from completion of paclitaxel/carboplatin was 8.0 months (95% CI: 4.5-13.6 months). At the time of recurrence, all 17 patients were treated with doxorubicin as second-line therapy. No patient achieved objective response of stable disease. The median PFS of this cohort following doxorubicin treatment was 2.1 months (95% CI: 0.95-2.7) months. Median OS was 5.8 months (95% CI: 1.0-15.0 months). There is only one patient still alive; her median follow-up time is 49.4 months. Predominant doxorubicin-related grade 2 toxicities included nausea/vomiting (18.8%), fatigue (18.8%), and neutropenia (12.5%). No grade 3 or 4 toxicities occurred.
Conclusions
Among patients with advanced endometrial carcinoma who had received adjuvant paclitaxel/carboplatin, treatment with doxorubicin at time of disease recurrence failed to achieve any objective responses and was associated with a very short (2 months) time to progression. Doxorubicin may be considered inactive as second-line therapy in this endometrial carcinoma population.
Keywords: Doxorubicin, Paclitaxel/Carboplatin, Recurrent Endometrial Carcinoma
Introduction
Endometrial carcinoma is the most common gynecologic malignancy in the United States, accounting for an estimated 47,130 new cases and 8,010 deaths in 2012 (1). While the majority of patients with early-stage disease are cured with surgery and/or radiation therapy, resulting in a 5-year survival rate of 83%, advanced disease continues to portend a poor prognosis, with only a 17% 5-year survival rate. Outside of local disease failures, limited options are available for the majority of patients with distant disease. Investigations focusing on novel approaches to improve outcomes in this patient population are warranted. Several randomized studies by the Gynecologic Oncology Group (GOG) have been performed to address optimal therapy for patients with advanced, completely resected endometrial carcinoma. These trials limited eligibility to patients with stage III or IV disease who had undergone surgical resection and had less than or equal to 2 cm residual disease and no distant metastases. In GOG-122, patients were randomly assigned to whole abdominal radiation therapy or doxorubicin (60mg/m2) plus cisplatin (50mg/m2) (AP). Patients assigned to AP had significantly improved progression-free survival (PFS) and overall survival (OS) (2). The disease-free survival (DFS) rate for the AP arm at 5 years was 51%. In GOG-184, patients were treated with tumor-directed radiation therapy (pelvic +/− para-aortic +/− intravaginal brachytherapy) followed by either AP or doxorubicin (45mg/m2) and cisplatin (50mg/m2) on day 1, followed by paclitaxel (160mg/m2) on day 2 (with GCSF support) (TAP). Relapse-free survival appeared similar between the two arms (62% vs 64%) (3).
The MSKCC experience with paclitaxel/carboplatin (TC) in the adjuvant treatment of patients with high-risk stage III and IV endometrial carcinoma was recently reported in a retrospective study (4). In that study, patients with stage III/IV endometrial carcinoma with less than 2 cm of disease following surgery who received TC in the adjuvant setting were evaluated. Median time to progression was 13 months (95% CI: 10-18), with a 3-year OS rate of 56%. In a prospective study, GOG-0209, TC was compared to TAP (paclitaxel, doxorubicin, cisplatin) in a non-inferiority designed trial to address optimal therapy for patients with advanced endometrial carcinoma. The results showed that TC and TAP are equivalent in terms of PFS and OS (5). The results of GOG 184 and GOG-0209 are likely to lead to the wide adoption of TC as first-line therapy for stage III and IV endometrial carcinoma. With increasing numbers of endometrial carcinoma patients receiving TC as first-line therapy for advanced or high-risk completely resected disease, and since at least half of such patients will suffer recurrence/disease progression, the question of best second-line therapy becomes critically important.
Trials evaluating cytotoxic agents in the second-line setting have shown that cisplatin (4%), docetaxel (7.7%), gemcitabine (4%), ifosfamide (15%), ixabepilone (12%), oxaliplatin (13.5%), and pegylated liposomal doxorubicin (9.5%) have only modest activity (6-12). While doxorubicin has had a role in combination therapy as first-line treatment in endometrial carcinoma, we do not know whether doxorubicin in the second-line setting for the management of endometrial carcinoma that has progressed after TC therapy results in meaningful clinical improvement. Since doxorubicin chemotherapy may be associated with significant morbidity, it is important to discern whether doxorubicin after TC therapy achieves objective responses or is associated with a prolonged PFS; if not, other potentially active agents could be utilized, or patients may be enrolled in clinical trials to identify active second-line agents. This retrospective study evaluates the efficacy of second-line doxorubicin in the treatment of advanced/recurrent endometrial carcinoma that has progressed after adjuvant TC therapy among patients treated at MSKCC between 1995 and 2009.
Patients and methods
Patient eligibility
Following Institutional Review Board approval, patients with stage I-IV endometrial carcinoma who had received adjuvant TC at MSKCC between 1995 and 2009 were identified. MSKCC electronic medical records were reviewed for patient age, diagnosis date, type of primary surgery, residual disease at completion of primary surgery, stage, treatment (chemotherapy and radiation therapy), dates of progression and death, site(s) of first recurrence, and toxic side effects. Patients had to have undergone total abdominal hysterectomy, bilateral salpingo-oophorectomy, and maximal resection of all gross intra-abdominal/pelvic disease, including macroscopically involved para-aortic and pelvic lymph nodes. Peritoneal cytology and lymph node dissection were optional if there were no intraoperative clinical manifestations of residual intra-abdominal disease. All patients had to have histologic confirmation of endometrial carcinoma at MSKCC. Patients were allowed to have received intravaginal radiation therapy (IVRT)/pelvic RT as part of their adjuvant treatment. Patients with carcinosarcoma were not included. From this dataset, we then identified the patients who had progression of disease after treatment with TC and who received doxorubicin as second-line therapy. All patients in this group were required to have measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST 1.1) (13) at the time of treatment with doxorubicin. Toxicity was determined by review of clinical documentation. Toxicity was assessed by the treating physician at each visit and graded using version 4.0 of Common Terminology Criteria for Adverse Events (CTCAE).
Statistical considerations
As no early-stage patients were identified, patients were analyzed as one group: advanced endometrial carcinoma treated with TC adjuvant chemotherapy, followed by second-line doxorubicin chemotherapy for recurrent, measurable disease. Sites of first recurrence after TC therapy and sites of progression after doxorubicin therapy were classified as vaginal, pelvic, liver abdomen, lung, peritoneal carcinomatosis, inguinal lymph nodes, para-aortic lymph nodes, and retroperitoneal lymph nodes. Patients with multiple sites of first recurrence were counted separately for each site.
PFS following TC/doxorubicin therapy was defined as the start date of TC/doxorubicin treatment to the corresponding date of progression. OS following TC/doxorubicin chemotherapy was defined as the time elapsed from the start date of TC/doxorubicin treatment to the date of death or the date of last follow-up. The median OS and PFS time as well as the corresponding 95% CIs were estimated using the Kaplan-Meier method. The analyses were performed using SAS 9.1.
Treatment
All patients received paclitaxel (175mg/m2) and carboplatin (AUC 6) intravenously (IV) once every 3 weeks as adjuvant treatment following surgical management of endometrial carcinoma. One patient with stage IIIB, high-grade serous carcinoma received IVRT for 2100 cGy in 3 fractions in conjunction with adjuvant TC.
At the time of measurable progression of disease all 17 patients included in this study received doxorubicin (60mg/m2) IV once approximately every 3 weeks.
Results
Patient characteristics
Patient characteristics are detailed in Table 1. Seventeen patients were included in the study analyses (25 potentially eligible patients were identified. Eight patients were excluded for the following reasons: 1 patient was excluded for receiving only one cycle of TC with progression of disease that was treated with second-line doxorubicin/cisplatin; 1 for having received multiple lines of hormonal therapy after recurrence on 1 cycle of TC prior to treatment with 1 cycle of doxorubicin; 4 for not having undergone resection of endometrial carcinoma at diagnosis; 1 for receiving neoadjuvant TC followed by resection; and 1 for diagnosis of carcinosarcoma). Stage distribution following surgical resection at the time of diagnosis of endometrial carcinoma was: 17.6% (n=3) stage III and 82.4% (n=14) stage IVB. The median age was 56 years (range: 36-78). Histologic subtypes were 29.4% (n=5) high grade serous, 29.4% (n=5) endometrioid (1 grade 1; 4 grade 3), 23.5% (n=4) mixed cell type, 11.8% (n=2) undifferentiated, and 5.9% (n=1) clear cell. Patient race distribution was 94.1% (n=16) white and 5.9% (n=1) black. Eastern Cooperative Oncology Group (ECOG) performance status was 47.0% (n=8) ECOG 0, 41.2% (n=7) ECOG 1, and 11.8% (n=2) ECOG 2. CA-125 level at diagnosis was <35 U/ml in 76.5% (n=13) patients and >35 U/ml in 23.5% (n=4) patients.
Table 1.
Patient Characteristics
| Patient Demographics | N (= 17) | % |
|---|---|---|
| Vital Status | ||
| Alive | 1 | 5.9 |
| Dead | 16 | 94.1 |
| Progressed | 17 | 100 |
| Age at Diagnosis, years | ||
| Median | 57.0 | |
| Range | 36-78 | |
| FIGO Stage | ||
| IIIA | 1 (serous histology) | 5.9 |
| IIIB | 1 (serous histology) | 5.9 |
| IIIC | 1 (serous histology) | 5.9 |
| IVB | 14 | 82.4 |
| Histologic sub-type | ||
| Endometrioid | 5 | 29.4 |
| Grade 1 | 1 | 5.9 |
| Grade 2 | 0 | |
| Grade 3 | 4 | 23.5 |
| Serous | 5 | 29.4 |
| Mixed Cell Type | 4 | 23.5 |
| Undifferentiated | 2 | 11.8 |
| Clear Cell | 1 | 5.9 |
| Race | ||
| White | 16 | 94.1 |
| Black | 1 | 5.9 |
| ECOG Performance Status | ||
| 0 | 8 | 47.0 |
| 1 | 7 | 41.2 |
| 2 | 2 | 11.8 |
| CA-125 | ||
| < 35 U/ml | 13 | 76.5 |
| > 35 U/ml | 4 | 23.5 |
FIGO, International Federation of Gynecology and Obstetrics; ECOG, Eastern Cooperative Oncology Group
Sixteen patients completed a median of 6 (range: 5-7) cycles of TC chemotherapy. One patient developed a hypersensitivity reaction (facial flushing, shortness of breath, rectal pain) to paclitaxel with the third cycle, and therefore only received 2 cycles of TC. The median number of doxorubicin cycles completed was 2.5 (range: 2-6). The lung and abdomen were the most common sites of recurrence following adjuvant TC treatment, and were also the most common sites of disease progression on second-line doxorubicin therapy, as shown in Tables 2 and 3.
Table 2.
Patterns of Recurrence Following TC Chemotherapy
| Site(s) of recurrence | N (=36) | % |
|---|---|---|
| Lung | 7 | 41.2 |
| Peritoneal Carcinomatosis | 7 | 41.2 |
| Pelvis | 6 | 35.3 |
| Abdomen | 7 | 41.2 |
| Liver | 1 | 5.9 |
| Vagina | 1 | 5.9 |
| Inguinal LN | 1 | 5.9 |
| Para-aortic LN | 4 | 23.5 |
| Retroperitoneal LN | 2 | 11.8 |
LN, lymph node
Table 3.
Patterns of Progression during Doxorubicin Chemotherapy
| Site(s) of progression | N (=23) | % |
|---|---|---|
| Lung | 7 | 41.2 |
| Abdomen | 8 | 47.0 |
| Pelvis | 5 | 29.4 |
| Retroperitoneal LN | 3 | 17.6 |
LN, lymph node
Of the patients who received third-line chemotherapy, 6/17 (35.3%) received paclitaxel at 60-80mg/m2 weekly 3 weeks on 1 week off; 4/17 (23.5%) patients received gemcitabine 800mg/m2 weekly 2 weeks on 1 off; 2/17 (11.8%) received docetaxel 75mg/m2 once every 3 weeks; 1/17 (5.9%) received topotecan 2.5mg/m2 weekly 3 weeks on 1 week off.
The frequency and severity of treatment-related adverse events are summarized in Table 4. Predominant grade 2 toxicities during doxorubicin treatment included nausea/vomiting (18.8%), fatigue (18.8%), and neutropenia (12.5%). No grade 3 or 4 toxicities occurred in these patients, as shown in Table 4.
Table 4.
Toxicities from Doxorubicin Treatment
| Toxicity | Grade | % | |
|---|---|---|---|
| 1 | 2 | ||
| Anorexia | 2 | 0 | 6.3 |
| Constipation | 2 | 2 | 23.5 |
| Diarrhea | 2 | 0 | 11.7 |
| Fatigue | 5 | 3 | 47.1 |
| HSN | 0 | 0 | 0 |
| Musculoskeletal | 1 | 1 | 11.8 |
| Nausea/Vomiting | 3 | 4 | 41.2 |
| Neutropenia | 0 | 2 | 11.7 |
| Peripheral Neuropathy | 1 | 1 | 11.7 |
| Pulmonary | 0 | 0 | 0 |
| Renal | 0 | 0 | 0 |
HSN, hypersensitivity reaction
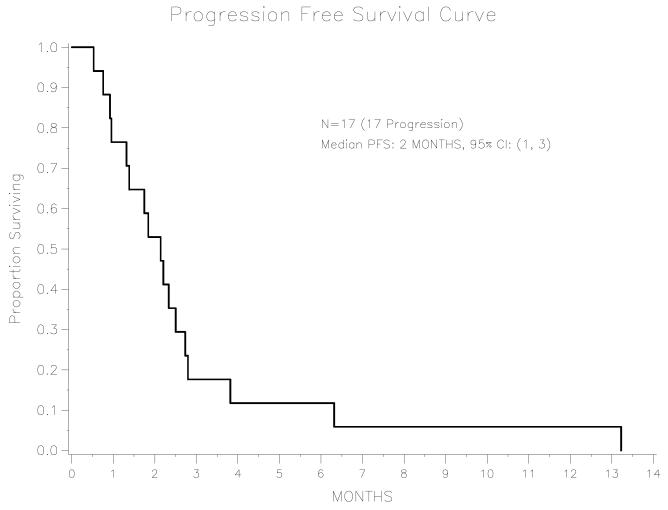
Among the 17 patients, median PFS following adjuvant TC treatment was 8.0 months (95% CI: 4.5-13.6 months). Median PFS of this cohort following doxorubicin treatment for recurrent disease was 2.1 months (95% CI: 0.9-2.7 months).
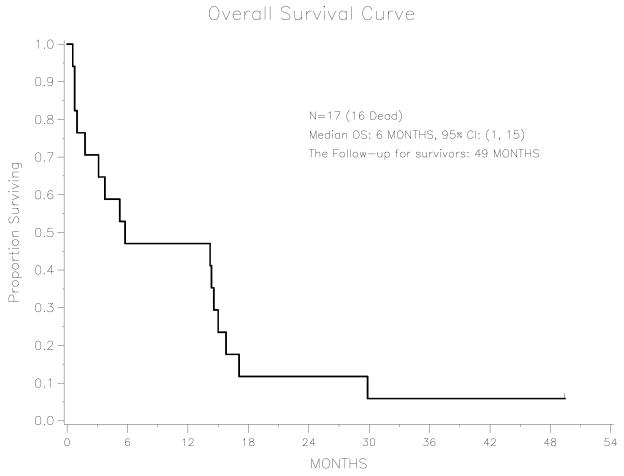
The median OS following adjuvant TC treatment was 12.8 months (95% CI: 8.0-30.2 months). There is only one patient alive, with a follow-up time of 115.0 months. Median OS following doxorubicin treatment for recurrent disease was 5.8 months (95% CI: 1.0-15.0 months). There is only one patient alive; her follow-up time after receiving the doxorubicin treatment is 49.4 months. For the entire cohort, the PFS and OS following doxorubicin chemotherapy are shown in Figures 1 and 2. Objective responses or disease stabilization to doxorubicin treatment were not seen in any of the patients in this cohort. Only one patient received 6 cycles of doxorubicin treatment, with no mid-treatment imaging studies to evaluate for response therapy. The first post-treatment CT scan revealed progression of disease.
Figure 1.
Progression-free survival among advanced endometrial cancer patients treated with second-line doxorubicin, n=17
Figure 2.
Overall survival among advanced endometrial cancer patients treated with second-line doxorubicin, n=17
Discussion
Anthracyclines have been one of the most widely utilized class of anticancer agents for both solid and hematologic malignancies since the 1960s (14). Doxorubicin has been utilized in the management of advanced gynecologic malignancies as first-line and salvage therapy. Doxorubicin is associated with toxicities, including myelosuppression, nausea, vomiting, stomatitis, alopecia, radiation recall, extravasation risk, and cumulative cardiac toxicity (15). In the management of advanced or recurrent endometrial carcinoma in chemotherapy-naïve patients, doxorubicin has been associated with objective and complete responses varying from 19 to 37% and 5 to 25%, respectively (16,17). Additional agents with activity in this setting include pegylated liposomal doxorubicin (response rates [RR] 12-36%) and ifosfamide (RR 26%) (18-20). However, based on the recent results of GOG-0209 (5), TC will likely become the standard regimen for first-line management of advanced/recurrent endometrial carcinoma.
In the second line setting, only docetaxel (RR 31%) administered once every 3 weeks and weekly paclitaxel (RR 27%) have shown to be efficacious (21, 22). While a myriad of other cytotoxic agents have been investigated in the second line setting including cisplatin (RR 4%), weekly docetaxel RR 7.7%) , ixabepilone (RR 12%), pegylated liposomal doxorubicin (RR 19.5%), non-pegylated doxorubicin (RR 0%), pemetrexed (RR 4%), dactinomycin (RR12%), gemcitabine (RR 4%), topotecan (RR 9%), ifosfamide (RR 15%), oxaliplatin (RR 13.5%), none have revealed positive results. (6-12, 23-26)
It is not known whether doxorubicin has efficacy in the second-line setting for the management of endometrial carcinoma that has progressed following adjuvant TC chemotherapy. In this study, we intended to determine the activity of doxorubicin in the second-line treatment setting of patients with endometrial carcinoma who had recurred after first-line, adjuvant treatment with TC. Seventeen cases were identified. No patient achieved objective response to doxorubicin therapy, PFS was short, and the frequency of grade 2 toxicities was high.
Our findings suggest that doxorubicin’s utility in the second-line setting for advanced/recurrent endometrial carcinoma is limited, but prospective testing of this agent is warranted. In the near future, an open-label randomized phase III trial of second line AEZS-108 (doxorubicin linked to LHRH analog) versus doxorubicin in patients progressing after platinum/taxane-based chemotherapy will be open to accrual, and will define the activity of second-line doxorubicin in malignancy. Although our retrospective analysis is limited by evaluation of a small number of patients, we feel that patients with recurrent endometrial carcinoma should be encouraged to consider clinical trials of novel therapies in recurrent endometrial carcinoma since there is no standard second-line therapy with established efficacy.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Randall M, Filiaci V, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24(1):36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 3.Homesley H, Filiaci V, Gibbons SK, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin or with or without paclitaxel: A GOG study. Gynecol Oncol. 2009;112:543–52. doi: 10.1016/j.ygyno.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiess AP, Damast S, Makker v, et al. Five-year outcomes of adjuvant carboplatin/paclitaxel chemotherapy and intravaginal radiation for stage I-II papillary serous endometrial cancer. Gynecol Oncol. 2012 Jul 28; doi: 10.1016/j.ygyno.2012.07.112. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Miller D, Filiaci V, Fleming G, et al. Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771–3. (Abstract) [Google Scholar]
- 6.Thigpen JT, Blessing JA, Lagasse LD, et al. Phase II trial of cisplatin as second-line chemotherapy in patients with advanced or recurrent endometrial carcinoma. A Gynecologic Oncology Group study. Am J Clin Oncol. 1984;7:253–256. doi: 10.1097/00000421-198406000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Garcia AA, Blessing JA, Nolte S, et al. Gynecologic Oncology Group A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: a study by the Gynecologic Oncology Group. Gynecol Oncol. 2008;111:22–26. doi: 10.1016/j.ygyno.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Tait DL, Blessing JA, Hoffman JS, et al. A phase II study of gemcitabine (Gemzar, LY188011) in the treatment of recurrent or persistent endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol. 2011;121:118–121. doi: 10.1016/j.ygyno.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Sutton GP, Blessing JA, Homesley HD, et al. Phase II study of ifosfamide and mesna in refractory adenocarcinoma of the endometrium. A Gynecologic Oncology Group study. Cancer. 1994;73:1453–1455. doi: 10.1002/1097-0142(19940301)73:5<1453::aid-cncr2820730521>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Dizon DS, Blessing JA, McMeekin DS, et al. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: gynecologic oncology group trial 129-P. J Clin Oncol. 2009;27:3104–3108. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fracasso PM, Blessing JA, Molpus KL, et al. Phase II study of oxaliplatin as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;103:523–526. doi: 10.1016/j.ygyno.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Lincoln S, Blessing JA, Lee RB, et al. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:277–281. doi: 10.1016/s0090-8258(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 13.Rustin GJ, Vergote I, Eisenhauer E, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 14.Stewart CF, Ratain MJ. Topoisomerase interactive agents. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer. Lippincott-Raven, Inc.; Philadelphia: 1997. pp. 452–67. [Google Scholar]
- 15.Maluf FC, Spriggs D. Anthracyclines in the treatment of gynecologic malignancies. Gynecol Oncol. 2002;85:18–31. doi: 10.1006/gyno.2001.6355. [DOI] [PubMed] [Google Scholar]
- 16.Horton J, Begg CB, Arseneault J, et al. Comparison of Adriamycin with cyclophosphamide in patients with advanced endometrial cancer. Cancer Treat Rep. 1978;62:159–61. [PubMed] [Google Scholar]
- 17.Thigpen JT, Blessing JA, DiSaia PJ, et al. A randomized comparison of doxorubicin alone versus doxorubicin plus cyclophosphamide in the management of advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 1994;12:1408–14. doi: 10.1200/JCO.1994.12.7.1408. [DOI] [PubMed] [Google Scholar]
- 18.Homesley HD, Blessing JA, Sorosky J, et al. Phase II trial of liposomal doxorubicin at 40 mg/m(2) every 4 weeks in endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2005;98(2):294–298. doi: 10.1016/j.ygyno.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Balbi G, Visconti S, Monteverde A, et al. Liposomal doxorubicin: a phase II trial. Acta Biomed. 2007;78(3):210–3. [PubMed] [Google Scholar]
- 20.Sutton GP, Blessing JA, DeMars LR, et al. A phase II Gynecologic Oncology Group trial of ifosfamide and mesna in advanced or recurrent adenocarcinoma of the endometrium. Gynecol Oncol. 1996;63(1):25–7. doi: 10.1006/gyno.1996.0272. [DOI] [PubMed] [Google Scholar]
- 21.Katsumata N, Noda K, Nozawa S, et al. Phase II trial of docetaxel in advanced or metastatic endometrial cancer: a Japanese Cooperative Study. Br J Cancer. 2005;93(9):999–1004. doi: 10.1038/sj.bjc.6602817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Homesley HD, Meltzer NP, Nieves L, et al. A phase II trial of weekly 1-hour paclitaxel as second-line therapy for endometrial and cervical cancer. Int J Clin Oncol. 2008;13(1):62–5. doi: 10.1007/s10147-007-0731-5. [DOI] [PubMed] [Google Scholar]
- 23.DiLegge A, Trivellizzi IN, Moruzzi MC, et al. Phase 2 trial of nonpegylated doxorubicin (Myocet) as second-line treatment in advanced or recurrent endometrial cancer. Int J Gynecol Cancer. 2011;21(8):1446–51. doi: 10.1097/IGC.0b013e31822d754e. [DOI] [PubMed] [Google Scholar]
- 24.Miller DS, Blessing JA, Drake RD, et al. A phase II evaluation of pemetrexed (Alimta, LY231514, IND #40061) in the treatment of recurrent or persistent endometrial carcinoma: a phase II study of the Gynecologic Oncology. Gynecol Oncol. 2009;115(3):443–6. doi: 10.1016/j.ygyno.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Moore DH, Blessing JA, Dunton C, et al. Dactinomycin in the treatment of recurrent or persistent endometrial carcinoma: a Phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 1999;75:473–475. doi: 10.1006/gyno.1999.5652. [DOI] [PubMed] [Google Scholar]
- 26.Miller DS, Blessing JA, Lentz SS, et al. A phase II trial of topotecan in patients with advanced, persistent, or recurrent endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol. 2002;87:247–251. doi: 10.1006/gyno.2002.6804. [DOI] [PubMed] [Google Scholar]