Abstract
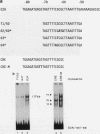
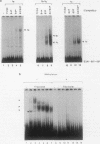
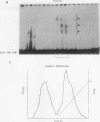
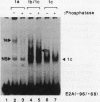
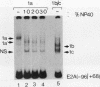
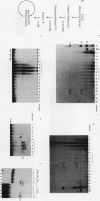
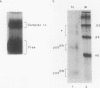
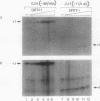
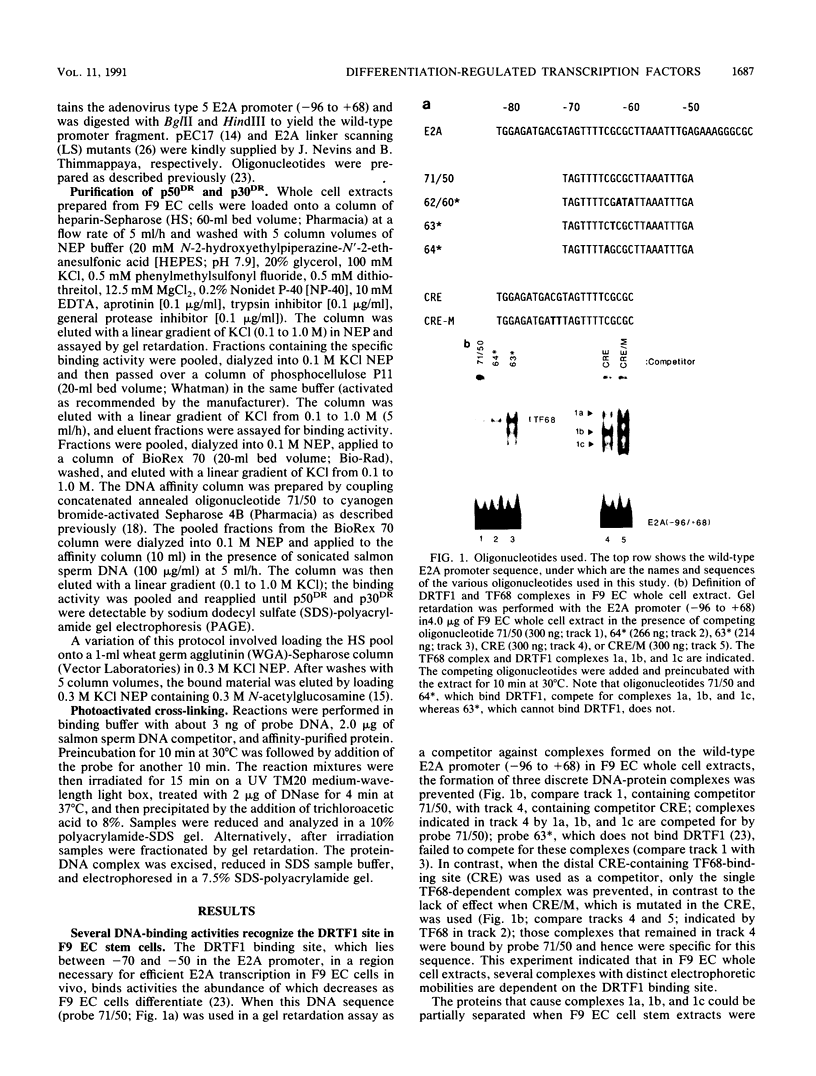
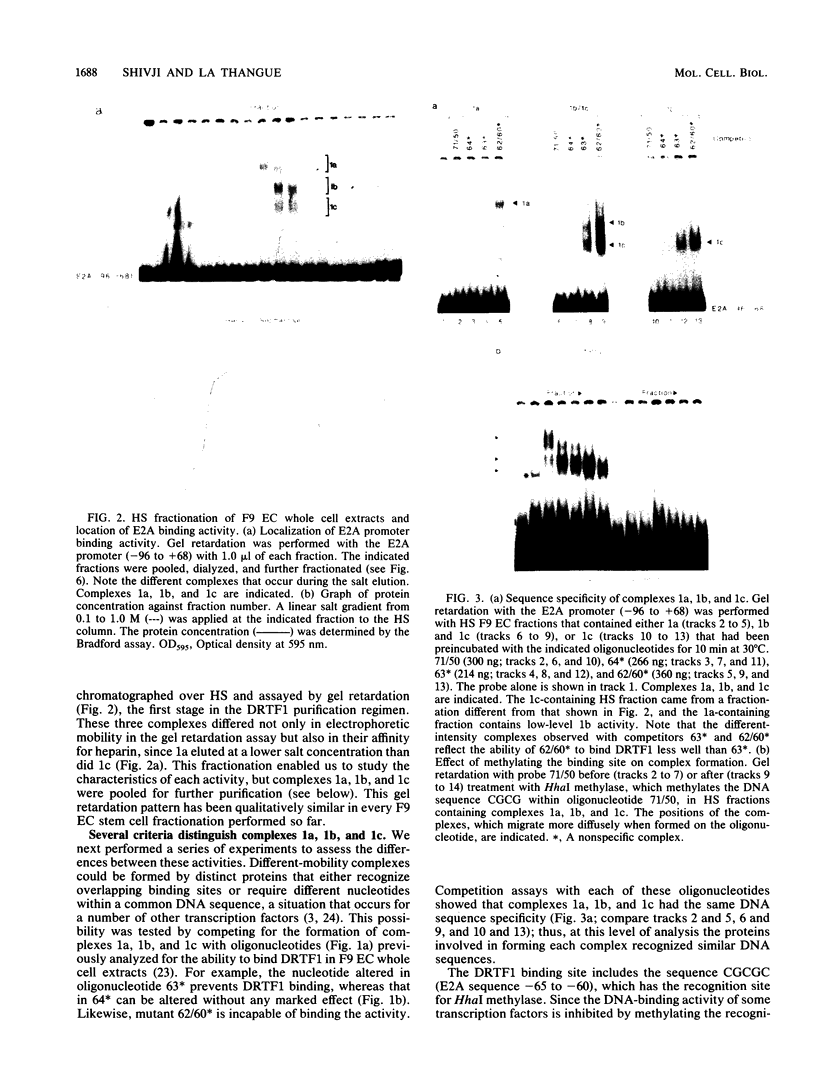
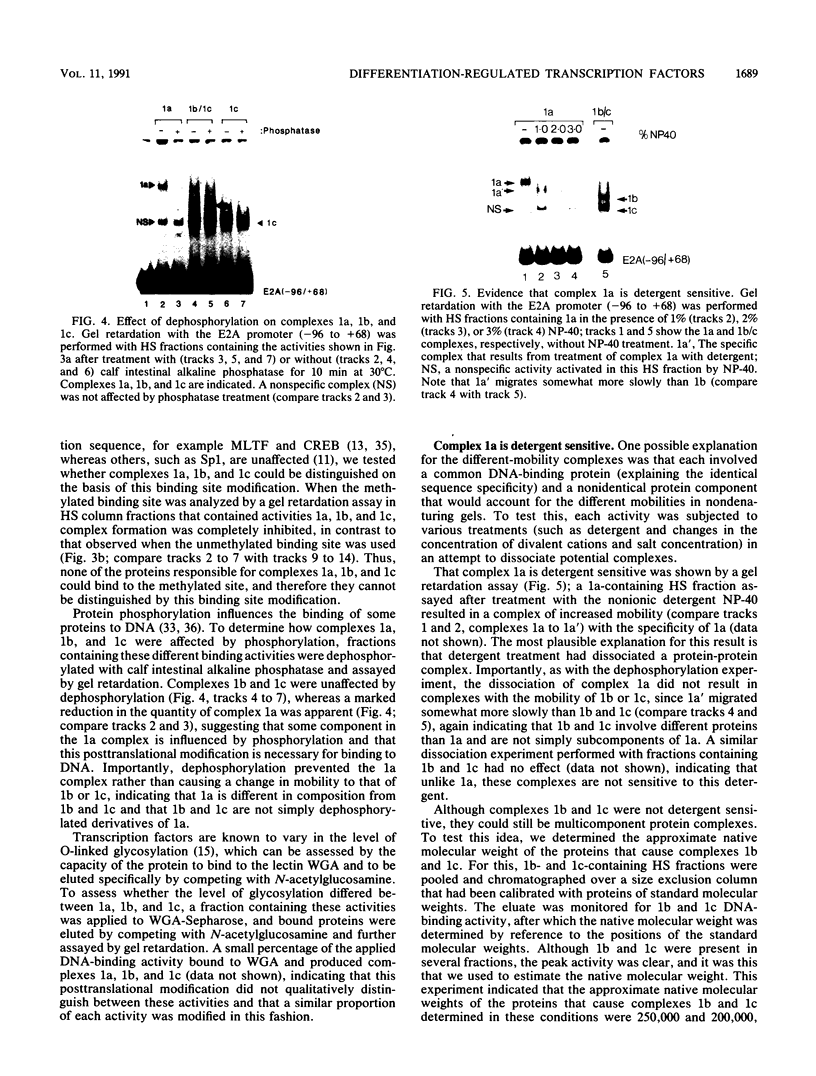
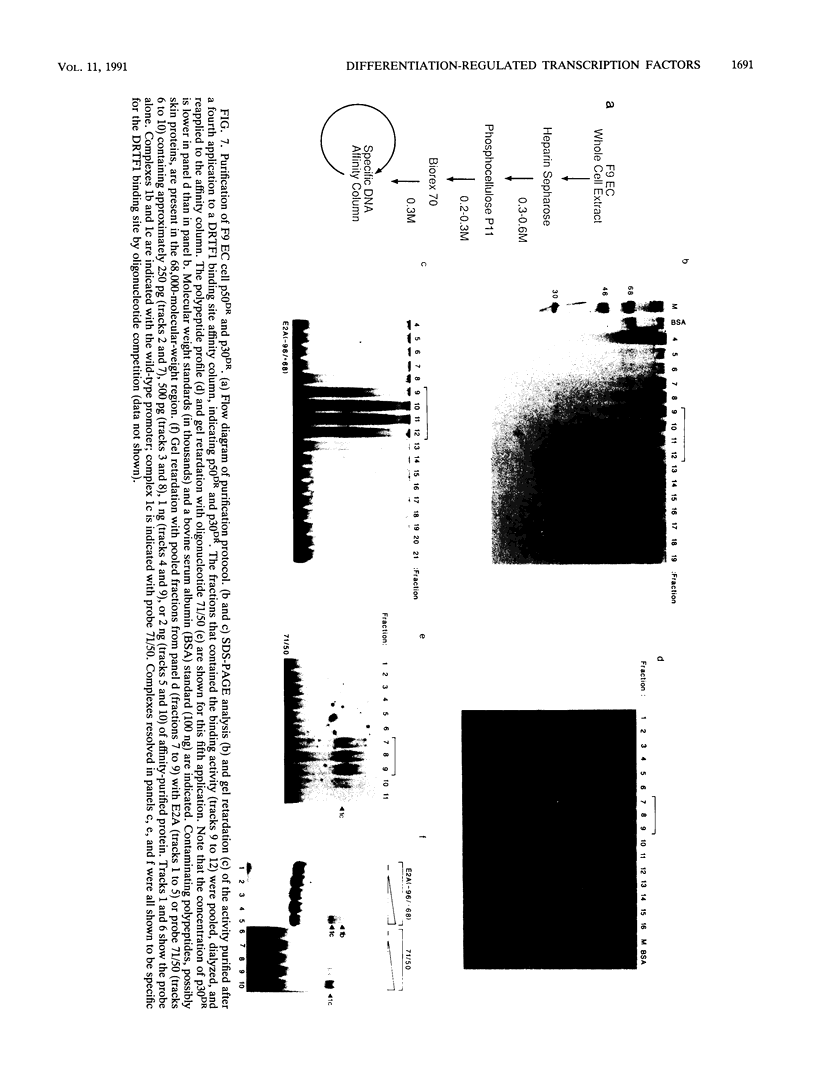
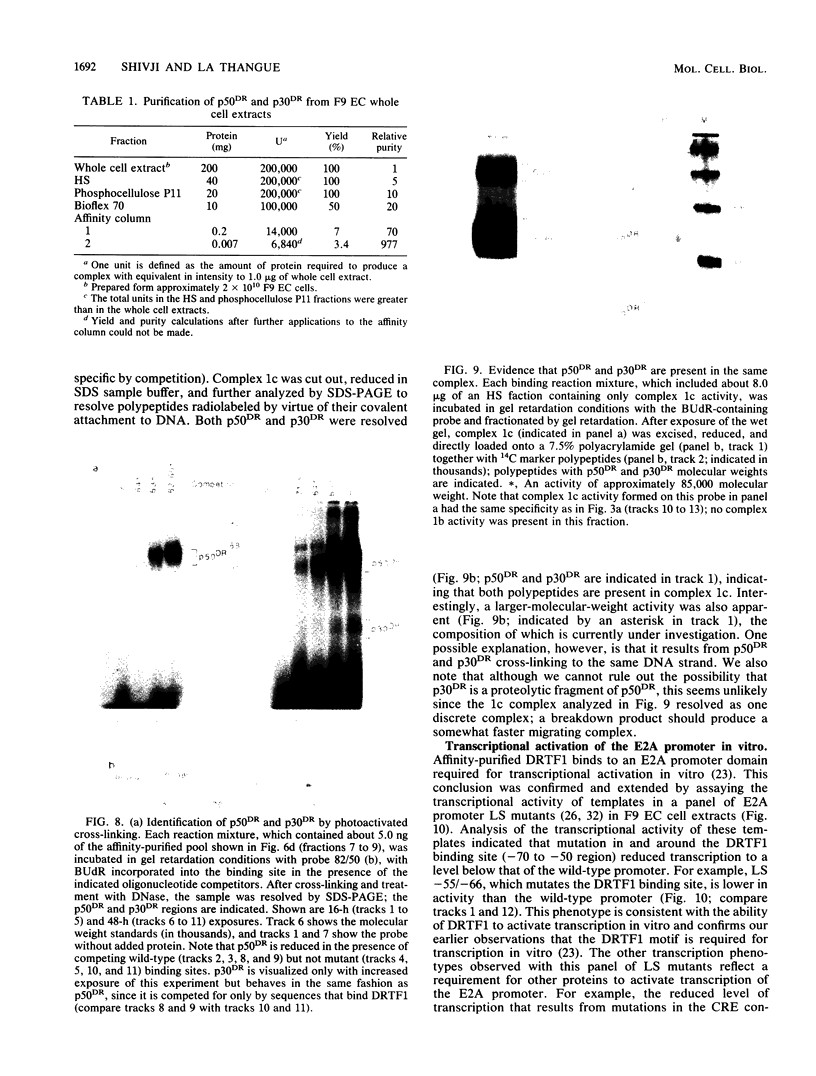
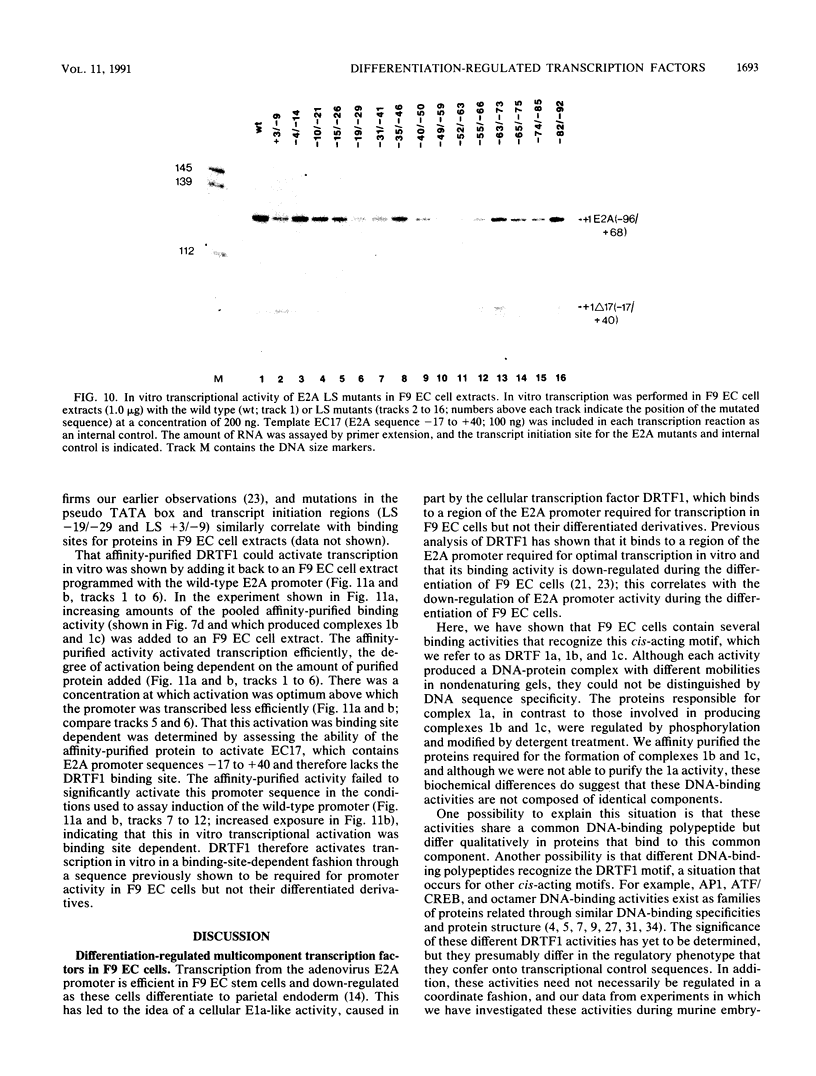
Murine F9 embryonal carcinoma (F9 EC) stem cells have an E1a-like transcription activity that is down-regulated as these cells differentiate to parietal endoderm. For the adenovirus E2A promoter, this activity requires at least two sequence-specific transcription factors, one that binds the cyclic AMP-responsive element (CRE) and the other, DRTF1, the DNA-binding activity of which is down-regulated as F9 EC cells differentiate. Here we report the characterization of several binding activities in F9 EC cell extracts, referred to as DRTF 1a, 1b and 1c, that recognize the DRTF1 cis-regulatory sequence (-70 to -50 region). These activities can be chromatographically separated but are not distinguishable by DNA sequence specificity. Activity 1a is a detergent-sensitive complex in which DNA binding is regulated by phosphorylation. In contrast, activities 1b and 1c are unaffected by these treatments but exist as multicomponent protein complexes even before DNA binding. Two sets of DNA-binding polypeptides, p50DR and p30DR, affinity purified from F9 EC cell extracts produce complexes 1b and 1c. Both polypeptides appear to be present in the same DNA-bound protein complex and both directly contact DNA. These affinity-purified polypeptides activate transcription in vitro in a binding-site-dependent manner. These data indicate the in F9 EC stem cells, multicomponent differentiation-regulated transcription factors contribute to the cellular E1a-like activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi S., Raychaudhuri P., Nevins J. R. Phosphorylation-dependent activation of the adenovirus-inducible E2F transcription factor in a cell-free system. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4352–4356. doi: 10.1073/pnas.86.12.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. Transcription factors and the control of Drosophila development. Trends Genet. 1989 Nov;5(11):377–383. doi: 10.1016/0168-9525(89)90173-x. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Cortes P., Buckbinder L., Leza M. A., Rak N., Hearing P., Merino A., Reinberg D. EivF, a factor required for transcription of the adenovirus EIV promoter, binds to an element involved in EIa-dependent activation and cAMP induction. Genes Dev. 1988 Aug;2(8):975–990. doi: 10.1101/gad.2.8.975. [DOI] [PubMed] [Google Scholar]
- Fletcher C., Heintz N., Roeder R. G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987 Dec 4;51(5):773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Allegretto E. A., Karin M., Green M. R. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev. 1988 Oct;2(10):1216–1226. doi: 10.1101/gad.2.10.1216. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Hardy S., Shenk T. E2F from adenovirus-infected cells binds cooperatively to DNA containing two properly oriented and spaced recognition sites. Mol Cell Biol. 1989 Oct;9(10):4495–4506. doi: 10.1128/mcb.9.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Huang M. M., Hearing P. The adenovirus early region 4 open reading frame 6/7 protein regulates the DNA binding activity of the cellular transcription factor, E2F, through a direct complex. Genes Dev. 1989 Nov;3(11):1699–1710. doi: 10.1101/gad.3.11.1699. [DOI] [PubMed] [Google Scholar]
- Höller M., Westin G., Jiricny J., Schaffner W. Sp1 transcription factor binds DNA and activates transcription even when the binding site is CpG methylated. Genes Dev. 1988 Sep;2(9):1127–1135. doi: 10.1101/gad.2.9.1127. [DOI] [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Schaffner W. CpG methylation of the cAMP-responsive enhancer/promoter sequence TGACGTCA abolishes specific factor binding as well as transcriptional activation. Genes Dev. 1989 May;3(5):612–619. doi: 10.1101/gad.3.5.612. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jansen-Durr P., Boeuf H., Kédinger C. Cooperative binding of two E2F molecules to an Ela-responsive promoter is triggered by the adenovirus Ela, but not by a cellular Ela-like activity. EMBO J. 1989 Nov;8(11):3365–3370. doi: 10.1002/j.1460-2075.1989.tb08499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Role of an adenovirus E2 promoter binding factor in E1A-mediated coordinate gene control. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2180–2184. doi: 10.1073/pnas.84.8.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Thangue N. B., Rigby P. W. An adenovirus E1A-like transcription factor is regulated during the differentiation of murine embryonal carcinoma stem cells. Cell. 1987 May 22;49(4):507–513. doi: 10.1016/0092-8674(87)90453-3. [DOI] [PubMed] [Google Scholar]
- La Thangue N. B., Rigby P. W. The regulation of SV40 early gene expression in embryonal carcinoma stem cells--faithful transcriptional regulation in vitro. Nucleic Acids Res. 1988 Dec 23;16(24):11417–11430. doi: 10.1093/nar/16.24.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Thangue N. B., Thimmappaya B., Rigby P. W. The embryonal carcinoma stem cell Ela-like activity involves a differentiation-regulated transcription factor. Nucleic Acids Res. 1990 May 25;18(10):2929–2938. doi: 10.1093/nar/18.10.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- Mauxion F., Sen R. Alteration of a single nucleotide allows efficient binding of H2TF1/KBF1 to the immunoglobulin kappa enhancer B motif. Mol Cell Biol. 1989 Aug;9(8):3548–3552. doi: 10.1128/mcb.9.8.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S. C., Bhat G. P., Thimmappaya B. Adenovirus EIIA early promoter: transcriptional control elements and induction by the viral pre-early EIA gene, which appears to be sequence independent. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2230–2234. doi: 10.1073/pnas.82.8.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Ogata R., Gilbert W. Contacts between the lac repressor and the thymines in the lac operator. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4973–4976. doi: 10.1073/pnas.74.11.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel R., Kovesdi I., Nevins J. R. Developmental control of a promoter-specific factor that is also regulated by the E1A gene product. Cell. 1987 Feb 13;48(3):501–506. doi: 10.1016/0092-8674(87)90200-5. [DOI] [PubMed] [Google Scholar]
- Reichel R., Neill S. D., Kovesdi I., Simon M. C., Raychaudhuri P., Nevins J. R. The adenovirus E4 gene, in addition to the E1A gene, is important for trans-activation of E2 transcription and for E2F activation. J Virol. 1989 Sep;63(9):3643–3650. doi: 10.1128/jvi.63.9.3643-3650.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Hatzopoulos A. K., Balling R., Suzuki N., Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989 Sep;8(9):2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SivaRaman L., Thimmappaya B. Two promoter-specific host factors interact with adjacent sequences in an EIA-inducible adenovirus promoter. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6112–6116. doi: 10.1073/pnas.84.17.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Lewis M. J., Pelham H. R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Watt F., Molloy P. L. Cytosine methylation prevents binding to DNA of a HeLa cell transcription factor required for optimal expression of the adenovirus major late promoter. Genes Dev. 1988 Sep;2(9):1136–1143. doi: 10.1101/gad.2.9.1136. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]