Abstract
Herpes simplex virus type 1 (HSV-1) has an intricate association with cellular nuclear structures known as ND10 or promyelocytic leukemia protein (PML) nuclear bodies. Parental viral genomes initially become juxtaposed to ND10, and then viral replication compartments develop from the ND10-associated genomes. Viral immediate-early (IE) regulatory protein ICP0 colocalizes with ND10 and then induces the degradation of critical ND10 component protein PML and therefore the release and dispersal of other ND10 proteins. The IE transcriptional regulatory protein ICP4 also forms foci at early times of infection, many of which are juxtaposed to ND10 and later develop into replication compartments, indicating that at least some of the initial ICP4 foci contain parental viral genomes. Here we report that the ICP4 foci also contain ICP27 and that their formation occurs extremely rapidly at locations just inside the nuclear envelope. By examining developing plaques or thinly seeded cells infected at high multiplicity, we found evidence to suggest that at least some of the ND10-viral nucleoprotein complex association could be attributed to de novo formation of ND10-like structures in response to incoming viral genomes. The ICP4 complexes associated efficiently with ND10 in cells infected with an ICP0-null mutant virus at high but not at low multiplicity, and the degree of association was reduced by the proteasome inhibitor MG132. Therefore, the interaction between viral nucleoprotein complexes and ND10 is in part due to a dynamic response by the cell. This response is modulated by functional ICP0, and cells that are productively or nonproductively infected in the absence of functional ICP0 can be distinguished by the relative locations of ICP4 foci and ND10 proteins.
The herpes simplex virus type 1 (HSV-1) immediate-early (IE) regulatory proteins ICP4, ICP0, and ICP27 play crucial roles in the regulation of productive infection (for reviews, see reference 23). ICP4 is essential for the activation of transcription of viral early and late genes and functions by binding to viral DNA and interacting with components of the host transcriptional apparatus. ICP27 is also essential for HSV-1 infection and has transcriptional and posttranscriptional roles, functioning in the processing and efficient export of viral mRNAs, especially at the later stages of infection. Although ICP0 is not essential for lytic infection, in its absence the virus is much more likely to establish quiescent infections (reviewed in references 13, 14, and 39). The activities of these proteins depend not only on their various cellular interacting partners but also on their location with respect to the viral genomes and transcripts with which they interact. The HSV-1 genomes themselves, like those of several other DNA viruses, can associate with cellular nuclear structures known as promyelocytic leukemia protein (PML) nuclear bodies or ND10 (reviewed in references 12 and 29). In the case of human cytomegalovirus, viral transcripts emanating from the ND10-associated genomes have also been detected, suggesting that the ND10-associated genomes are biologically active (22). Recent work has shown that viral amplicon genomes that are associated with ND10 have an increased probability of being replicated (46).
The linkage between protein function and cellular location has been greatly illuminated by the use of autofluorescent labels based on enhanced green fluorescent protein and its spectrally shifted derivatives enhanced yellow fluorescent protein (EYFP) and enhanced cyan fluorescent protein (ECFP). Using viruses expressing ICP4 fused to either EYFP or ECFP, ICP4 was shown during the early stages of infection to form punctate nuclear foci that were associated with ND10 in a manner reminiscent of the behavior of viral genomes. Indeed, some of the ICP4 foci must contain parental genomes, as they later develop into replication compartments (20). Since ICP0 precisely colocalizes with ND10 at early times (17, 30), it was expected that the ICP4 foci would be frequently juxtaposed to accumulations of ICP0, and this was found to be the case (20).
In this paper we report on several factors concerning the ICP4 foci that are formed early during infection. They appear very rapidly just inside the nuclear envelope, indicating that they assemble soon after the viral genome has entered the nucleus. ICP27 was also recruited into the ICP4 complexes at early times of infection, but their formation was not dependent on ICP0 during high-multiplicity productive infection. The association of many of the ICP4 complexes with ND10 was also very rapid, occurring as soon as ICP4 could be detected. Examination of cells at the edges of developing plaques or of thinly seeded cells soon after high-multiplicity infection indicated that ICP4 foci present in ICP0-negative virus infections appear to induce the formation of associated ND10-like complexes. This is not readily observed in wild-type virus infection because of the rapid dispersal and/or degradation of ND10 proteins induced by ICP0. Thus, the association of viral genomes with ND10 may be due not only to their deposition at preexisting ND10 but also to recruitment of cellular ND10 proteins to sites in close proximity to the incoming viral genomes. The cellular locations of viral proteins and nucleoprotein complexes play an important role in the progress of lytic infection, and these data indicate a dynamic interplay between viral and ND10 proteins that is controlled by both viral and cellular factors.
MATERIALS AND METHODS
Viruses and cells.
Parental virus HSV-1 strain 17 syn+ and viruses vECFP-ICP4 and vEYFP-ICP4 (expressing ECFP- and EYFP-linked ICP4, respectively) (20) were propagated and titrated in BHK cells. ICP0 deletion mutant dl1403 (47) was grown in BHK cells and titrated in U2OS cells; it was used at the stated multiplicities of infection (MOIs) based on its titer in U2OS cells. ICP27 deletion mutant 17 + 27-pR19lacZ (25) was propagated and titrated in BHK M49 complementing cells. Virus stocks were prepared from low-multiplicity input infections of the appropriate cell type. When the cells exhibited extensive cytopathic effect, the medium was harvested, clarified by low-speed centrifugation, and stored at 4°C. Virus stocks prepared in this manner were stable for several weeks and contained minimal amounts of cell debris. BHK cells were grown in Glasgow modified Eagle's medium containing 100 U of penicillin per ml and 100 μg of streptomycin per ml and supplemented with 10% newborn calf serum and 10% tryptose phosphate broth. Vero and U2OS cells were grown in Glasgow modified Eagle's medium or Dulbecco's modified Eagle's medium, respectively, supplemented with 10% fetal calf serum and antibiotics as described above. Human fetal foreskin fibroblast cells (HFFF-2; European Collection of Cell Cultures) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 1% glutamine, and antibiotics as described above. Baculovirus Ac.CMV.ECFP-PML expresses ECFP-linked PML from the human cytomegalovirus promoter in mammalian cells (46).
Construction of virus vEYFP-ICP27.
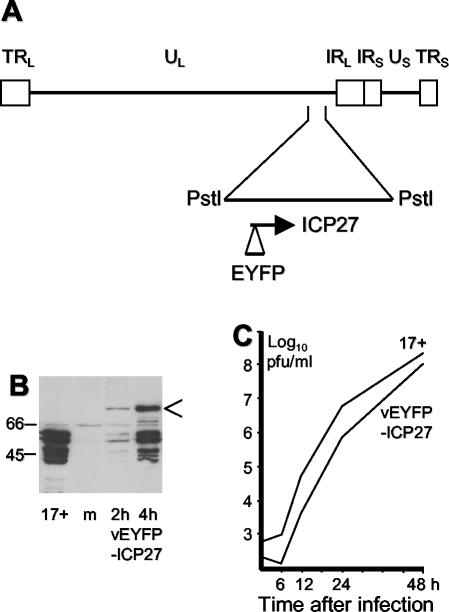
A 6-kb PstI HSV-1 genomic fragment containing the complete IE-2 gene was inserted into plasmid vector pTRE2pur (Clontech). The resultant plasmid was linearized at the DrdI site immediately 5′ of the ICP27 ATG initiation codon, the 3′ extensions were removed with DNA polymerase I Klenow fragment, and a similarly blunt-ended NheI-KpnI fragment containing the EYFP-coding region from pEYFP-C1 (Clontech) was inserted in the appropriate orientation to give plasmid pTRE2-EYFP-ICP27. This contains the EYFP-coding region inserted in frame immediately upstream of the ICP27 initiation codon, and it expresses an EYFP-ICP27 fusion protein from the IE-2 promoter. This plasmid was cotransfected with 17 + 27-pR19lacZ viral DNA into BHK cells, and the progeny virus was screened for EYFP fluorescent plaques. A stock of virus vEYFP-ICP27 was produced after plaque purification, and its genomic structure was checked by Southern blotting.
Antibodies.
The following antibodies were used: anti-ICP4 monoclonal antibody (MAb) 58S (44) and rabbit polyclonal serum r74 (11), anti-ICP0 MAb 11060 (18), anti-ICP8 MAb 7381 (a kind gift from Howard Marsden), and anti-ICP27 MAb H1113 (1) (purchased from the Goodwin Institute for Cancer Research). The sources of anti-PML MAb 5E10 and rabbit polyclonal antibody r8, of rabbit and rat anti-Sp100 polyclonal antibodies SpGH and Sp26, and of anti-hDaxx rabbit polyclonal antibody r1866 have been described previously (15). Anti-PML MAb PGM3 was purchased from Santa Cruz Technologies. Fluorescein isothiocyanate-conjugated sheep anti-mouse immunoglobulin G (IgG) was obtained from Sigma. Alexa 488-conjugated goat anti-rabbit and goat anti-mouse IgGs were purchased from Molecular Probes. Cy3- and Cy5-conjugated goat anti-mouse and goat anti-rabbit IgGs were obtained from Amersham.
Live-cell microscopy.
Live cell samples expressing autofluorescent proteins were prepared and examined as described previously (20). The live-cell microscopy system included mercury lamp illumination; a motorized excitation filter wheel to select 510- and 436-nm wavelengths to excite EYFP and ECFP, respectively; and fixed dichroic and emission filters configured for excitation and emission of both EYFP and ECFP. Thus, the distinction between ECFP and EYFP depends on the differences between their excitation rather than emission spectra. Excitation at 510 nm did not produce a signal from ECFP, but excitation at 436 nm produced a signal from EYFP of about 10% of that obtained at 510 nm. This potential overlap became important if the ECFP signal was weak compared to that of EYFP and was taken into account in the design of double-labeling experiments. While ECFP did not overlap into the EYFP channel, the signal from an ECFP fusion protein was weaker and the autofluorescence background was higher than with the corresponding EYFP fusion protein. Therefore, although clearer images could be obtained with EYFP fusion proteins, there was a risk of overlap if the ECFP signals were weak.
In certain circumstances we found a surprising difference between the behaviors of the ECFP- and EYFP-ICP4 fusion proteins. In transfected cells ECFP-ICP4 was diffusely distributed, but EYFP-ICP4 produced aggregates or foci of various sizes and intensities. Therefore, the EYFP fusion moiety changes the behavior of the ICP4 fusion protein in a way that the ECFP fusion does not. We do not know if this effect extends to other fusion proteins, and there was little difference between the distributions of EYFP-ICP4 and ECFP-ICP4 during lytic virus infection. The data that made use of EYFP-ICP4 were confirmed where possible by using ECFP-ICP4, with the exception of Fig. 2A to H (for which it is not possible with ECFP-ICP4 due to the overlap described above). All of the observations in live cells with vECFP-ICP4 and vEYFP-ICP27 have been compared extensively with those for the normal proteins in fixed cell samples.
FIG. 2.
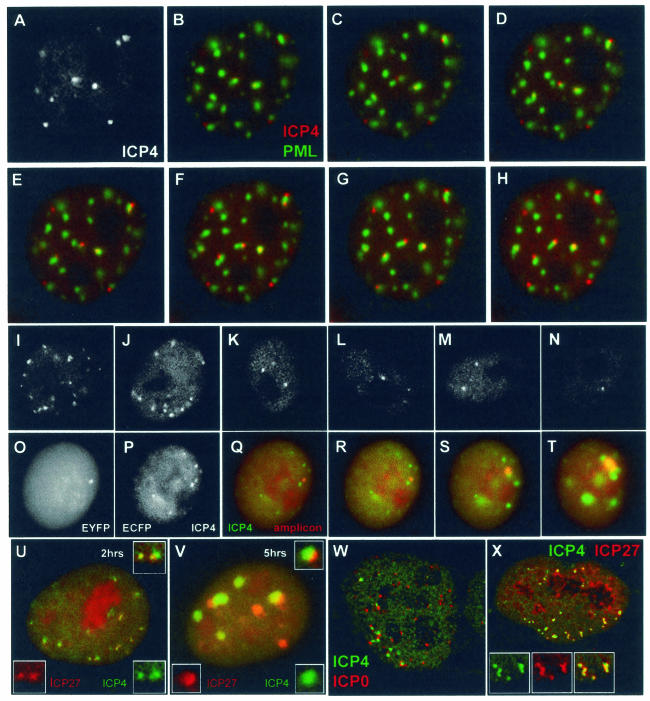
ICP4 foci associate rapidly with ND10, are proportional to input multiplicity, colocalize with amplicon genomes, and contain ICP27 at early times of infection. (A to H) Vero cells were infected with baculovirus Ac.CMV.ECFP-PML at a multiplicity of five insect cell PFU per Vero cell and then infected with vEYFP-ICP4 at 50 PFU per cell the next day. (A) Enhanced image of the EYFP signal (ICP4) of a cell 80 min after addition of virus. (B) Merged image of the same time point, showing PML (green) and ICP4 (red). (C to H) Images of the same cell captured at 3-min intervals. (I to N) Vero cells were infected with vECFP-ICP4 at 10 (I and J), 1 (K and L), or 0.1 (M and N) PFU per cell, and images were taken 2, 3, and 4 h, respectively, after addition of virus. (O to T) ICP4 and parental amplicon genomes colocalize. Vero cells were coinfected with vECFP-ICP4 (MOI of 5) and amplicon EYFPnlsTetR (MOI of <1), which allows the detection of amplicon genomes. Panels O and P show the separated EYFP (amplicon genomes) and ECFP (ICP4) channels of an image of a coinfected cell 4 h after addition of the virus mixture. The amplicon genome dot in panel O colocalizes with a small accumulation of ICP4 (P), as shown in the merged image (Q). Panels R, S, and T show merged images of amplicon genomes (red) and ICP4 (green) taken 45, 75, and 105 min after that in panel P. The development of the amplicon dot into a replication compartment confirms that it originally contained an amplicon genome. (U and V) Detection of ICP4 (green) and ICP27 (red) in live cells coinfected with vEYFP-ICP27 and vECFP-ICP4. At early times (U) ICP27 colocalizes with ICP4, but later, and especially when replication compartments have developed, there is greater relative separation between the proteins. The insets show the separated and merged channels of a selected region of the main image. (W) Detection of ICP4 foci (green) juxtaposed to ICP0 foci (red) in a fixed HFFF-2 cell 2 h after addition of HSV-1 strain 17. (X) Colocalization of ICP4 (green) and ICP27 (red) in a fixed HFFF-2 cell 2 h after addition of HSV-1 strain 17. The insets show the separated and merged channels of a selected region of the main image.
Confocal microscopy.
Cells on glass coverslips were washed with phosphate-buffered saline (PBS), fixed with formaldehyde (5% [vol/vol] in PBS containing 2% sucrose), and then permeabilized with 0.5% NP-40 in PBS with 10% sucrose. The coverslips were incubated for 1 h at room temperature with primary antibodies diluted in PBS containing 1% newborn calf serum and then washed several times before treatment with secondary antibodies in the same manner. The secondary antibodies used were Alexa 488-, 546-, or 633-conjugated goat anti-mouse or anti-rabbit IgG (Molecular Probes) or corresponding fluorescein isothiocyanate (Sigma) or Cy-dye (Amersham) reagents. The coverslips were mounted in Citifluor AF1 and examined with an LSM 510 confocal microscope (Zeiss Axioplan; ×63 objective lens; numerical aperture, 1.4) equipped with 458-, 488-, and 633-nm laser excitation lines. The emission signals were routed through appropriate band pass filters, and the data were collected with fourfold averaging at a resolution of 1,024 by 1,024 pixels with optical slices of between 0.5 and 1 μm. Image files were exported in TIF format for processing with Photoshop.
Some experiments involved the detection of signals with greatly differing intensities in the same sample, increasing the potential for optical or antibody overlap from the strong to the weak channel. If the green channel signal is strong compared to the red (488- and 546-nm excitation), simultaneous scanning leads to green-to-red overlap that can be mostly avoided by sequential scanning. If the red is strong compared to the green, since many fluorophores that are excited at 546 nm are also excited by the 488-nm laser line and since the emission from the red fluorophore can overlap into green band pass filter windows, red-to-green overlap is possible whether scanning is done sequentially or simultaneously. This problem can be alleviated by using 488-nm excitation of fluorescein isothiocyanate in combination with Cy5 (633-nm excitation, with emission greater than 650 nm), but this does not avoid the possibility of incomplete specificity of secondary antibodies. If the signal in one channel is very much greater than that in the other, there is a possibility that even the slightest secondary antibody specificity overlap will give a significant signal. The intense staining of viral proteins in replication compartments and the very weak residual signal from cellular proteins PML and Sp100, which have largely been degraded by the time replication compartments form, combined to produce considerable overlap problems. Double labeling of strain 17-infected samples always gave apparently greater levels of cellular proteins in viral replication compartments than if cells were stained solely for the cellular protein.
RESULTS
ICP4 forms nuclear foci at the earliest stages of infection.
ICP4 forms nuclear foci that are frequently associated with ND10 early during infection, some of which later develop into replication compartments (20). We set out to extend these findings by examining the dynamics and time course of ICP4 focus formation in live cells by using viruses vECFP-ICP4 and vEYFP-ICP4, which express ECFP- and EYFP-linked versions of ICP4 (20). Both viruses were analyzed in these experiments, but the spectral characteristics of the EYFP fusion allowed clearer images to be captured.
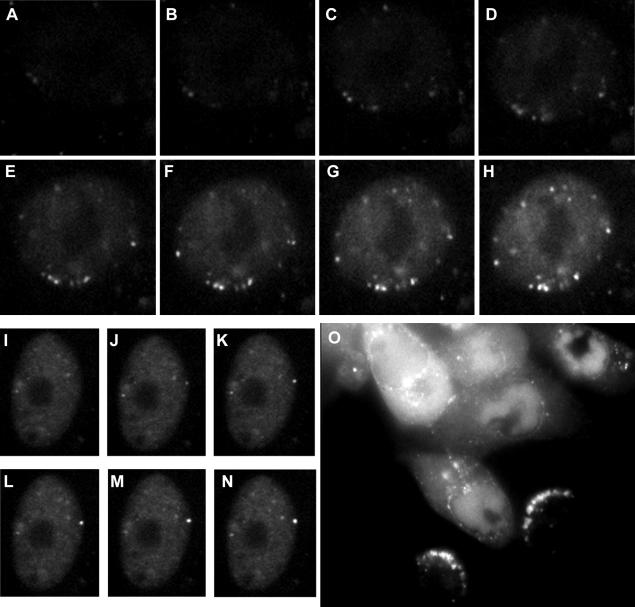
Vero cells were infected with virus vEYFP-ICP4 at an MOI of 50 PFU per cell, and image capture was initiated 35 min after a 5-min adsorption period. Figure 1A to H show a time course of a single cell nucleus at 3-min intervals. Faint foci of ICP4 are visible even in the first image of the sequence, despite there being insufficient diffusely distributed ICP4 to demarcate the nucleus. Later images in the sequence make clear that these initial foci are indeed inside the nucleus. These foci are the result of newly synthesized ICP4, since they did not appear in the presence of actinomycin D (data not shown). We have previously argued that at least some of the ICP4 foci are likely to comprise nucleoprotein complexes on viral DNA (although the formation of ICP4 foci at other sites cannot be excluded) (20), and their appearance before abundant expression of ICP4 suggests that the recruitment of ICP4 onto such complexes occurs extremely rapidly. Progressively more ICP4 foci appeared during the course of the illustrated short time sequence, while the diffusely distributed protein also increased in intensity.
FIG. 1.
Location of ICP4 as visualized by autofluorescence following infection by vEYFP-ICP4. (A to H) Vero cells were infected at 50 PFU per cell. The image in panel A was captured 40 min after addition of virus, and those in panels B to H were captured at 3-min intervals thereafter. (I to N) Vero cells were infected at 10 PFU per cell. The image in panel I was captured 85 min after addition of virus, and those in panels J to N were captured at 10-s intervals thereafter. (O) Vero cells were infected at low multiplicity and then incubated overnight to allow plaque development. This image shows heavily infected cells at late stages of infection (top left), cells with large replication compartments (center and top right), and newly infected cells with arcs of ICP4 complexes on the interior of the nuclear envelope on the side facing the center of the plaque.
A general feature was that many ICP4 foci appeared just inside the limits of the nuclear volume, as judged by the extent of the nuclear diffuse ICP4 signal. Sometimes this process was exceptionally rapid and dramatic. Figure 1I to N show a sequence of images taken at 10-s intervals that illustrate the appearance of a bright dot at the nuclear edge in less than a minute. The presence of ICP4 foci just inside the nuclear boundary was particularly apparent in newly infected cells at the edge of a developing plaque. Figure 1O shows a group of live Vero cells 1 day after infection with vEYFP-ICP4. The cells towards the top left of the image are heavily infected, with ICP4 present in large replication compartments or spread throughout the cell. The two cells in the lower right of the image are indicative of those relatively recently infected and show ICP4 foci in arcs inside the nuclear rim on the side closest to the parent plaque.
These observations fit very well with the contention that at least some of the ICP4 foci that appear early during infection represent nucleoprotein complexes assembling on parental viral genomes. After the viral particle has penetrated the cell, it is carried on microtubules toward the nucleus. The capsids associate with the nuclear envelope, and then the genome is uncoated and transported into the nucleus through the nuclear pore (8, 37, 45). The images of capsids encircling the outside of the nucleus (37, 45) parallel those of ICP4 foci just on the inside (Fig. 1 and 2). During plaque development viral particles will travel to a recipient cell from an adjacent infected cell, causing an arc of ICP4 foci facing the donor cell. During high-multiplicity infection with exogenous virus, many particles will alight on the surface of the cell above the nucleus, leading to formation of foci apparently in the central portion (but in all probability just under the upper surface of the nuclear envelope). Particles entering a cell from more distal locations will travel on microtubules to the surface of the nucleus that appears to form a lateral edge when viewed from the top. Therefore, the positioning of the initial ICP4 foci, and the replication compartments that subsequently develop, can reflect the direction from which the cell was infected.
ICP4 foci rapidly associate with ND10.
Previous data showed that the ICP4 foci that form early during infection have a high probability of being associated with ND10 (20). To investigate the dynamics of this association, ND10 in Vero cells was labeled by prior infection with a baculovirus expressing ECFP-linked PML (46) and then infected with vEYFP-ICP4 at an MOI of 50. Figure 2A to H show a sequence of images of a single cell starting 80 min after addition of the virus. The greyscale image in Fig. 2A is an enhanced copy of the red channel of the first image (Fig. 2B), showing ICP4 foci that are present but very faint in the colored version. The subsequent images, taken at 3-min intervals, illustrate that at the start of the sequence, when the ICP4 foci were relatively faint, many were already associated with ND10. Thereafter there is a limited amount of movement of both the ND10 and the ICP4 foci, but those present in close association remain so throughout the sequence. As the relative positions of associated foci can change, there is some independence in their movement. We conclude that the ICP4 foci and ND10 are separate structures that associate very rapidly and that once it is formed, the association is quite stable.
The number of ICP4 foci increases at higher-input MOIs.
The suggestion that at least some of the ICP4 foci that form early during infection represent nucleoprotein complexes on parental viral genomes implies that their number should reflect the input MOI. This was indeed the case. Vero cells infected with vECFP-ICP4 at an MOI of 10 (Fig. 2I and J [2 h postabsorption]) contained many more foci at early times of infection than those infected at an MOI of 1 (Fig. 2K and L [3 h postabsorption]) or of 0.1 (Fig. 2M and N [4 h postabsorption]). At an MOI of 0.1, the cells contained numbers of foci similar to those at an MOI of 1, except fewer cells in the population were positive, and at the lower multiplicities longer infection times were required to produce equivalent levels of ICP4. The number of ICP4 dots does not seem to be a simple consequence of the amount of ICP4 expressed, since (i) the images in Fig. 2I to N were taken at similar exposure settings and (ii) HFFF-2 cells infected with dl1403 at low MOIs can contain for extended periods levels of ICP4 equivalent to those in cells infected at much higher multiplicity without developing large numbers of ICP4 foci (see Fig. 6B) (14). As discussed previously (20), many factors can complicate an estimation of viral genome numbers in infected-cell nuclei in relation to input MOI, but these data show that the number of ICP4 foci varies with MOI, consistent with a significant proportion of them being formed on parental viral genomes. However, in most of the examples presented in this paper, the actual number of prominent ICP4 foci exceeds the nominal MOI. This could be due to several factors: (i) the number of virus particles capable of expressing ICP4 exceeds the PFU titer by a factor of about 3 (14), (ii) cells receiving more than the average number of particles become more heavily infected and therefore give stronger signals and tend to be selected for image acquisition, and (iii) the formation of ICP4 foci at sites other than on viral genomes cannot be excluded.
FIG.6.
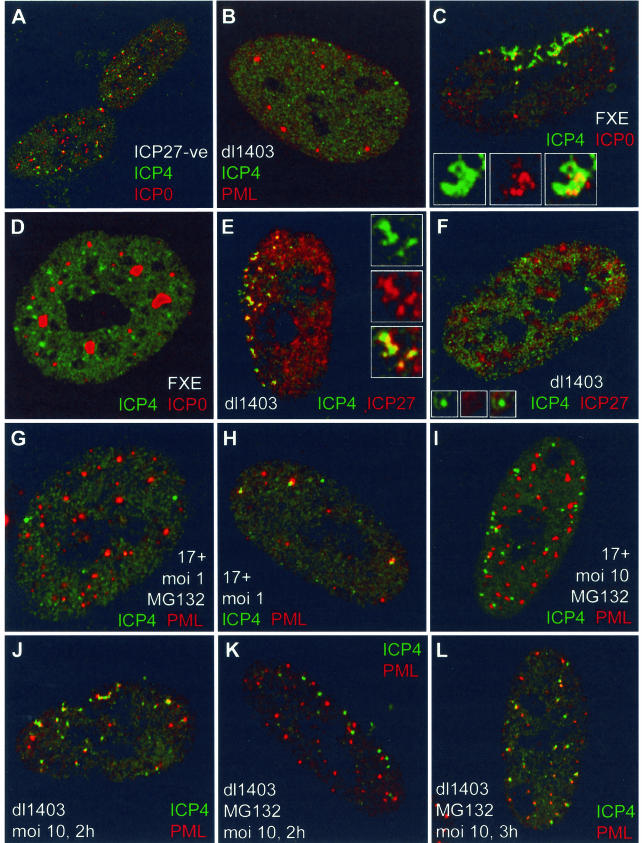
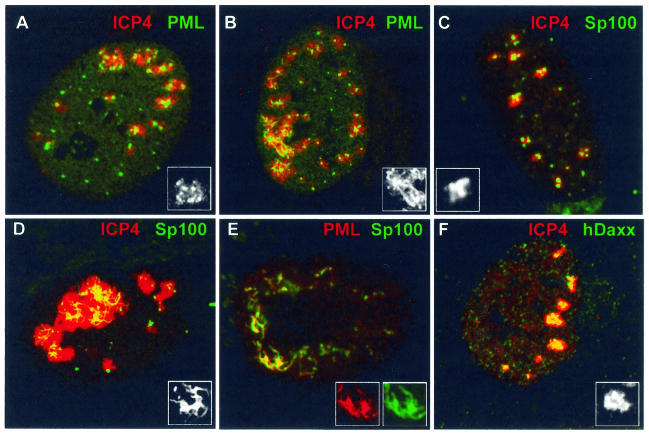
(A) ICP27 is not required for the formation of ICP4 complexes juxtaposed to structures containing ICP0. HFFF-2 cells were infected with virus 17+27-pR19lacZ (MOI of 10) and stained for ICP4 and ICP0 3 h later. Foci of ICP4 (green) were frequently associated with ICP0 (red) in the absence of ICP27. (B) ICP4 foci (green) were rarely associated with ND10 (PML) (red) in HFFF-2 cells nonproductively infected with dl1403 20 h after low-multiplicity infection. (C and D) ICP4 foci (green) were associated with mutant ICP0 (red) in HFFF-2 cells productively infected with virus FXE (C) but not in nonproductively infected cells 24 h after infection (D). Panel C shows a cell at the edge of a developing FXE plaque; panel D shows a nonproductively infected cell in the same sample. (E and F) The ICP4 foci (green) in HFFF-2 cells nonproductively infected with dl1403 contain reduced or undetectable amounts of ICP27 (red). Panel E shows a cell at the edge of a developing plaque, and panel F shows a nonproductively infected cell in the same sample. (G to L) MG132 (present at 10 μM throughout) diminishes the association of ICP4 foci (green) with ND10 (PML) (red). The details are noted on the figure and described in the text.
Direct evidence for recruitment of ICP4 onto parental amplicon genomes.
Direct evidence that at least some of the ICP4 foci that form early during infection contain parental viral genomes was obtained by examining viral amplicon genomes engineered to be detectable by binding of an autofluorescent tetracycline repressor fusion protein (EYFPnlsTetR) to TetO recognition sequences present within the amplicon (46). Figure 2O to T show a live Vero cell coinfected with an EYFPnlsTetR amplicon preparation at low multiplicity and with vECFP-ICP4 helper virus at an MOI of 5. Figure 2O, P, and Q show the EYFP, ECFP, and merged images of an infected cell with a single EYFP-labeled amplicon genome dot (Fig. 2O) and several ECFP-labeled ICP4 foci (many of which, in a manner analogous to that for the experiments above, reflect ICP4 complexes on helper virus genomes) (Fig. 2P); recruitment of ICP4 into the amplicon dot is demonstrated by the merged image (Fig. 2Q). Figure 2R, S, and T show a subsequent time course of the same cell, illustrating that the parental amplicon genome-ICP4 complex progresses to form an amplicon replication compartment. A number of the other ICP4 foci also develop into replication compartments, demonstrating that they contain helper virus genomes. These data identify the presence of a parental viral amplicon genome within an initial accumulation of ICP4. The separate characters of the amplicon- and helper-derived replication compartments, even when in very close proximity (Fig. 2T), underscore our previous observation that replication compartments are clonal in the sense that they are derived from individual ICP4 foci, and, contrary to a recent report (50), they do not merge even after they have expanded to fill large volumes of the nucleus (46).
Recruitment of ICP27 into the initial ICP4 foci.
The recruitment of quantities of ICP4 into what appear to be viral nucleoprotein complexes at very early times of infection poses the question of whether other viral IE proteins behave similarly. ICP27 is perhaps the most interesting in this regard, since it has been implicated in both transcriptional and posttranscriptional regulation and in interactions with both ICP4 and several cellular proteins (see references 23, 24, 43, and 52 and references therein). We have examined the localization of ICP27 in both live and fixed cells. Virus vEYFP-ICP27 was isolated by constructing a plasmid that expresses EYFP fused to the N terminus of ICP27 and then recombining the fusion protein open reading frame into the IE-2 gene locus in ICP27 deletion mutant virus 17+27-pR19lacZ (25). Expression of the fusion protein by vEYFP-ICP27 was confirmed by Western blotting (although some of the fusion protein appears to break down to a fragment similar in size to normal ICP27), and the virus replicated to a similar titer to HSV-1 strain 17 (Fig. 3). Coinfection of Vero cells with vEYFP-ICP27 and vECFP-ICP4 demonstrated that ICP27 colocalized with ICP4 in characteristic punctate foci at early times of infection (Fig. 2U). As time progressed, and particularly when the ICP4 foci began to develop into replication compartments, the degree of ICP27-ICP4 colocalization diminished. Instead, the ICP27 fusion protein accumulated in large foci separate from ICP4 and sometimes in areas close to the periphery of ICP4-defined replication compartments (Fig. 2V). While there was only limited accumulation of ICP27 in viral replication compartments in either Vero or HFFF cells, in NT2 cells much greater relative amounts of ICP27 were observed in these structures, illustrating that this property of ICP27 varies with cell type (data not shown).
FIG. 3.
Construction and characterization of virus vEYFP-ICP27. (A) Map of the HSV-1 genome showing the 6-kb PstI fragment that was subcloned for insertion of the EYFP open reading frame into the IE-2 gene at the N terminus of the ICP27-coding region. (B) Western blot showing expression of the EYFP-ICP27 fusion protein (arrow) in comparison with ICP27 expressed by HSV-1 strain 17 (17+). Lane m, mock-infected control. The fusion protein shows evidence of breakdown to smaller species. The positions of the appropriate molecular mass markers (in kilodaltons) are indicated on the left. (C) Multiple-cycle growth curve comparison of HSV-1 strain 17 and vEYFP-ICP27. BHK cells were infected at an initial MOI of 0.001, and then parallel samples were harvested at the indicated times and titrated on BHK cells.
Detection of ICP4 foci in fixed cells.
As formation of ICP4 foci at early times had not been described in detail in previous fixed-cell studies, we had surmised that these foci were more readily observed in live cells (20). However, costaining of ICP4 and ICP0 in fixed HFFF-2 cells at early times of HSV-1 strain 17 infection revealed that ICP4 foci were readily detectable by antibody staining, and, as in the live-cell study with autofluorescent fusion proteins (20), these were commonly juxtaposed to foci of ICP0 (Fig. 2W) and PML (see below). The juxtaposition of the ICP4 and ICP0 foci occurs because ICP0 precisely colocalizes with PML in ND10, whereas the ICP4 complexes associate with ND10 rather than colocalize with them. As in the live-cell experiment (Fig. 2A to H), association of ICP4 foci with PML could be observed as soon as the former could be detected, that is, by 1 h after virus adsorption (data not shown, but see Fig. 6H). Similarly, ICP27 also colocalized with ICP4 in characteristic foci at early times of infection in fixed-cell samples (Fig. 2X). These results demonstrate that these aspects of the localization of ICP4, ICP0, and ICP27 in live cells are not artifacts caused by the autofluorescent protein fusion. The detection of ICP4 foci in fixed cells opened the way to study the localization of endogenous cellular ND10 proteins with respect to the ICP4 complexes.
Evidence for de novo production of ND10-like complexes in association with viral nucleoprotein foci.
Numerous studies have observed the juxtaposition of ND10 and the parental genomes of several DNA viruses during the early stages of infection and then the later development of viral replication compartments in association with ND10 (12, 29). Because ND10 proteins are present in uninfected cells, it has been assumed that viral genomes are transported within the nucleus until they encounter an ND10 structure. However, it is also possible that ND10 complexes are formed de novo in association with an incoming viral nucleoprotein complex or that the ND10 structures themselves move to positions adjacent to the viral genomes. As the number of ND10 varies quite markedly from cell to cell, this question has been very difficult to approach. However, the directional entry of virus particles in a developing plaque leads to a highly asymmetric distribution of ICP4 complexes in newly infected cells (Fig. 1O), which poses the question of whether the ICP4 complexes that are congregated at one edge of the nucleus are also ND10 associated: in uninfected cells, ND10 appear to be randomly distributed throughout the nucleus.
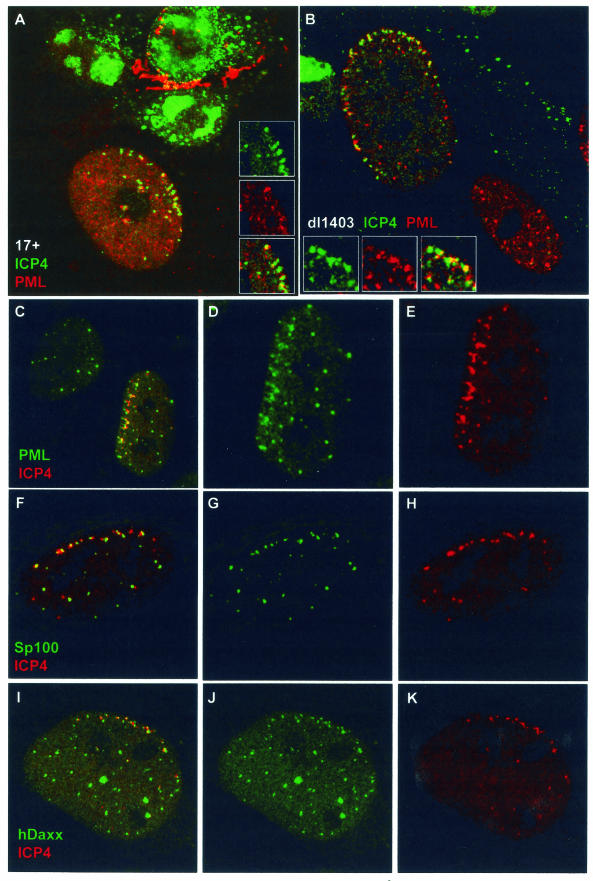
Vero and HFFF-2 human fibroblast cells were infected at low multiplicity with HSV-1 strain 17 (MOI of 0.001) and the ICP0-null mutant dl1403 (MOIs of 0.01 in Vero cells and 0.1 in HFFF-2 cells, based on PFU titers in U2OS cells) and then stained for ICP4 and ND10 proteins 24 h later. Similar results were obtained with both cell types. Many newly infected cells at the edges of developing strain 17 plaques contained arcs of ICP4 complexes near the nuclear periphery, some of which were associated with residual PML (Fig. 4A). However, because ICP0 induces the rapid dispersal or degradation of ND10 proteins, infections with strain 17 were not the best way to study this aspect of the dynamics of ND10 proteins. On the other hand, cells infected with dl1403 retained ND10 proteins, and in these infections accumulations of PML were clearly observed in association with asymmetrically distributed ICP4 complexes in both Vero cells (data not shown) and HFFF-2 cells (Fig. 4B). The cell in the lower right part of Fig. 4B is only lightly infected and shows the normal, apparently random nuclear distribution of PML in ND10, in marked contrast to its highly infected neighbor.
FIG.4.
Evidence for de novo formation of ND10-like structures in association with ICP4 complexes at the early stages of infection. (A) An HFFF-2 cell at the edge of a developing HSV-1 strain 17 plaque, showing ICP4 foci (green) commonly at the edge of the nucleus, some of which are in association with residual PML (red). The cells had been infected at an MOI of 0.001 and fixed and stained 24 h later, and then individual plaques were examined. (B) An HFFF-2 cell at the edge of a developing dl1403 plaque, showing extensive relocalization of PML into structures at the nuclear periphery. The cells had been infected at an MOI of 0.1 and fixed and stained 24 h later, and then individual plaques were examined. The lower cell is at an earlier stage of infection and shows a more normal distribution of PML. The insets in panels A and B show the separated and merged channels of a selected detailed area of each cell. (C to K) Evidence for de novo formation of ND10-like complexes containing PML, Sp100, and hDaxx in association with ICP4 complexes near the nuclear periphery at early times of high-multiplicity infections. HFFF-2 cells were infected with dl1403 at 10 PFU/cell and then stained for ICP4 (red; C to K) and PML (green; C to E), Sp100 (green; F to H), or hDaxx (green; I to K) 3 h after addition of the virus. The images show cells selected to illustrate the extensive recruitment of these three ND10 proteins into structures that are associated with ICP4 complexes near the nuclear periphery, in the absence of functional ICP0.
The distributions of the viral ICP4 complexes and ND10-like structures in these examples discount the possibility that viral genome-ND10 association relies exclusively on genome migration within the nucleus until an ND10 structure is encountered. At least in dl1403-infected cells with arcs of ICP4 complexes at the nuclear periphery, either preexisting ND10 migrates through the nucleus towards the incoming viral genomes or ND10-like complexes form de novo from proteins within the nucleoplasm. Although a minority of ND10 structures have been observed to migrate at rates of up to 5 μm per min within the nucleus, 88% of them exhibited either no movement or very restricted movement (34), a conclusion consistent with our own unpublished observations on ND10 movement in uninfected cells. On the other hand, at least a proportion of PML within ND10 is highly mobile, since fluorescence-recovery-after-photobleaching experiments have indicated that one-third of the original PML fluorescence recovers within 2 min after photobleaching of an ND10 structure (G. Maul, personal communication). Taken with the observation that the number of PML foci at the nuclear periphery is related to the number of ICP4 foci and is frequently much greater than that in neighboring uninfected cells (Fig. 4B to D), this indicates that it is likely that PML is being deposited in aggregates that are associated with the dl1403 ICP4 complexes. Deposition of PML from the nucleoplasm in this manner could restrict the amount of PML available for the normal dynamics of the protein in the preexisting ND10, leading to a net PML loss from these structures. If so, the end point of this process would be a situation such as that in Fig. 4B. A further observation that is consistent with de novo deposition is that many of the PML foci associated with ICP4 complexes at the nuclear periphery are smaller, fainter, and less distinct than the tightly punctate preexisting ND10. Therefore, we consider that the most likely explanation of the dramatic association of PML deposits with the large numbers of ICP4 foci at the nuclear periphery in high-MOI dl1403 infections is the de novo formation of ND10-like structures by aggregation of protein from within the nucleoplasm.
HFFF-2 cells were particularly suitable for these studies because similar effects could be observed in a proportion of cells at early times of high-multiplicity infections, particularly if the cells were seeded thinly. This is probably because the cytoplasm of HFFF-2 cells spreads over a large, rather elongated area. Accordingly, most virus particles will adsorb to the cell surface at sites far from the nucleus, and after penetration they will be carried on the microtubule network toward the microtubule organizing center at the nuclear periphery. This could produce an asymmetric distribution of genomes that enter the nucleus and, hence, lines of ICP4 complexes just inside the nuclear rim. An example of such a cell is shown in Fig. 4C next to an uninfected cell with a normal distribution of ND10 for comparison. The separated channels of the image of this cell are shown in Fig. 4D and E. In smaller, rounder, and more thickly seeded cells (such as Vero or HEp-2 cells), the region directly above the nucleus constitutes a much larger proportion of the cellular surface area, so a greater proportion of particles adsorb to the cell immediately above or close to the nucleus, obscuring the nuclear rim effect and any de novo induction or migration of ND10 complexes in high-multiplicity infections. The cell in Fig. 4C to E was selected to give a convincing demonstration and is perhaps an extreme example. However, the same effects could be seen, albeit less dramatically, in many HFFF-2 cells infected at high MOIs and extensively in cells in developing dl1403 plaques (Fig. 4B and data not shown).
In addition to PML, the major ND10 proteins Sp100 and hDaxx were also associated with ICP4 foci at the nuclear periphery and, again, in an asymmetric manner (Fig. 4F to K). These observations indicate that the ND10-like complexes at the nuclear periphery that are associated with ICP4 contain several ND10 proteins, although they were less intense and less discrete than the tight, regular punctate appearance of normal ND10. We conclude that the most likely explanation of these observations is that ND10-like complexes containing several ND10 proteins are being formed by the cell in response to the entry or activity of dl1403 viral nucleoprotein complexes. Because of the activity of ICP0, it is not possible to determine from current experimentation whether this process occurs at all, is not readily observed, or occurs only transiently in wild-type virus infections. If the same underlying processes do occur in wild-type virus infections, they could also be affected by multiplicity. Although the most likely explanation of the observations in Fig. 4 is de novo induction of ND10-like complexes, this conclusion cannot be extrapolated to all viral infection situations; this analysis does not exclude that migration of viral nucleoprotein complexes to ND10, or vice versa, can also occur.
Fate of ND10 protein components after development of viral replication compartments.
Many previous studies have shown that in the absence of ICP0, viral replication compartments begin to develop at sites associated with ND10 and that the ND10 appear to be stable. However, most of these studies dealt only with the first few hours of infection. During the present work on dl1403 infection of HFFF-2 cells, we observed that following the apparently de novo formation of ND10 complexes in association with ICP4 nucleoprotein complexes, the development of replication compartments was accompanied by partial dispersal of PML, Sp100, and hDaxx into the replication compartment region (Fig. 5A, C, and F). In a proportion of cells that had developed large replication compartments, PML and especially Sp100 were redistributed into string-like structures that were associated with the replication compartments (Fig. 5B and D). Double-labeling experiments indicated that these thread structures contained both PML and Sp100 (Fig. 5E), but hDaxx was present in only trace amounts (data not shown). Of these three ND10 proteins, hDaxx was the one most obviously recruited into small replication compartments early in dl1403 infection (Fig. 5F). In contrast to the tight association of PML, Sp100, and hDaxx in ND10 in uninfected cells, the redistribution of these proteins during dl1403 infection and replication compartment development resulted in partial or subtle separation of their signals (data not shown, but see Fig. 5E). These data indicate that ND10 proteins are in a highly dynamic state during HSV-1 infection, even in the absence of ICP0. They can initially associate to form novel ND10-like structures that are subject to further structural reorganization once viral replication compartments develop.
FIG. 5.
Partial disruption of ND10 and partial recruitment of ND10 proteins into viral replication compartments in dl1403-infected cells. HFFF-2 cells were infected with dl1403 (MOI of 10), fixed 5 h later, and stained for ICP4 (red; A to D and F) and PML (green; A and B), Sp100 (green; C and D), or hDaxx (green; F). (A) A cell with replication compartments that have expanded to a medium size, showing foci of PML associated with the periphery of the compartments and some diffuse PML staining within the compartment itself (see inset greyscale expansion of the PML signal from the top compartment). (B) Larger replication compartments are sometimes associated with or contain thread-like structures of PML. (C and D) Sp100 behaves in a manner similar to that for PML in medium-size (C) and large (D) replication compartments. The insets show details of the Sp100 channel from selected portions of each cell. (E) The string-like structures contain both PML (red) and Sp100 (green) in partial colocalization (also see insets), although some separation of the proteins was detected (not shown). (F) In contrast to PML, hDaxx did not readily form thread-like structures but instead was more closely associated with the dl1403 replication compartments, particularly those of small and medium sizes. The inset shows the greyscale expansion of the hDaxx signal from the most central compartment of the cell.
The detection of ND10 proteins within replication compartments in dl1403-infected cells raises the question of the relationship of ND10 proteins and replication compartments in wild-type HSV-1 infections. A number of previous studies have reported that certain isoforms of PML, Sp100, and other proteins such as p53 can be recruited into viral replication compartments (4, 5, 27, 28, 41, 53). We have repeated some of these studies with HFFF-2 cells, but there are complicating technical issues that are considered in detail in Materials and Methods. In summary, the low abundance of Sp100 and PML in wild-type virus-infected cells (after their ICP0-induced degradation) creates a situation where the amount of the viral protein marking the replication compartment (for example, ICP4 or ICP8) is vastly greater than that of residual PML or Sp100. This creates the opportunity for channel overlap artifacts at the optical or antibody specificity levels that we were unable to eliminate. We found that the most reliable method to determine whether ND10 proteins were present in replication compartments in strain 17-infected HFFF-2 cells was to stain parallel infected samples with single antibodies recognizing PML, Sp100, hDaxx, or ICP4. Screening of such singly stained coverslips indicated that whereas around 40% of the infected cells in the sample contained ICP4-defined replication compartments at 5 h postinfection, PML staining alone did not detect any such cells on a parallel infected coverslip (data not shown). Therefore, any recruitment of PML into strain 17 replication compartments is insufficient to make the latter visible. Similar negative results were observed in the parallel hDaxx-stained sample (data not shown). However, infected cells containing replication compartments could be identified by residual Sp100 staining alone, although the signals were weak (data not shown). Therefore, in agreement with a previous study (41), residual Sp100 can be recruited into HSV-1 replication compartments, but in HFFF-2 cells no PML was detectable in these structures by any of three independent antibodies that recognize the major spliced forms of the protein. Although the SUMO-1 modified forms of Sp100 are degraded during HSV-1 infection, its isoforms that are not so modified are relatively resistant to degradation (38); it is likely to be these forms that are found in replication compartments. In contrast, all PML isoforms are sensitive to degradation during HSV-1 infection of limited-passage human fibroblasts (38), and at the time of replication compartment development, detection of endogenous PML by immunofluorescence was extremely faint.
Although these conclusions are in apparent contradiction to earlier studies that indicated that PML could be recruited into replication compartments (4, 5, 27, 28), we note that the dl1403 results indicate that PML can be so recruited if it is not degraded. All isoforms of PML are highly sensitive to ICP0-induced degradation in HFFF-2 cells, but in certain other cells types PML (or particular isoforms of PML) may be degraded less rapidly. Any residual PML could be recruited into a developing replication compartment. Thus, this phenomenon is likely to be cell type dependent.
ICP27 is not required for the formation of ICP4 foci and their association with ICP0.
It has been observed that viral amplicon genome association with ND10 requires both ICP4 and ICP27 (49). However, ICP27 was not required for the formation of readily detectable ICP4 foci in HFFF-2 cells infected with ICP27-negative virus 17+27-pR19lacZ (Fig. 6A). The ICP4 foci were similarly associated with ICP0 accumulations in the absence and presence of ICP27 (compare Fig. 6A and 2W). Therefore, ICP27 is not required for the formation of ICP4 foci or for their association with structures containing ICP0 during a developing virus infection.
ICP4 foci are formed but are rarely associated with ND10 in nonproductively infected cells in the absence of ICP0.
Infection of Vero or HFFF-2 cells with ICP0-defective viruses at low MOIs results in many cells with nonproductive infections in which one or more of the other IE proteins are present in readily detectable quantities (6, 14). Such cells are defined as being the result of a nonproductive or stalled infection because they do not develop replication compartments (probably because of absent or incomplete early gene expression) and because they can persist for at least 48 h without initiating plaque formation (14). Figure 4 clearly shows that ICP0 is required neither for the formation of ICP4 foci nor for their association with existing or induced ND10 structures in productively infected cells. To investigate the formation and location of ICP4 complexes in nonproductive infections in the absence of ICP0, HFFF-2 cells were infected with dl1403 at a low MOI and then incubated for 24 h in the presence of human serum to prevent secondary infections from the few plaques that did develop. Staining for ICP4 revealed large numbers of cells that contained ICP4 and had not progressed to later stages of infection (14). Such cells contained small numbers of distinct ICP4 foci that in the great majority of cases were not associated with PML (Fig. 6B) or with Sp100 or hDaxx (data not shown). In this experiment, 10 cells were examined in detail; each contained two or three ICP4 foci, none of which were associated with PML in ND10 structures. In a replicate experiment, 3 out of 38 ICP4 foci were so associated. This is in contrast to the case for productively infected cells (Fig. 4B to F), in which the great majority of ICP4 foci had become associated with PML deposits by 2 to 4 h after infection.
Infection with ICP0 RING finger deletion mutant virus FXE allowed the simultaneous detection of ICP4 and mutant ICP0. Under productive high-MOI conditions, the mutant ICP0 FXE protein colocalized with PML (data not shown) and was found juxtaposed to ICP4 in characteristic structures at the periphery of nuclei of newly infected cells in plaques (Fig. 6C). This result is analogous to the juxtaposition of ICP0 and ICP4 foci at the early stages of infection with strain 17 (Fig. 2W). In low-MOI nonproductive FXE infections, however, although the mutant ICP0 protein remained colocalized with PML (data not shown), it was not associated with ICP4 foci at 24 h postinfection (Fig. 6D). In addition, unlike in productive dl1403 (Fig. 6E) and FXE (data not shown) infections in which ICP27 is readily recruited into the early ICP4 foci, ICP27 was not present in large quantities in the ICP4 foci in cells nonproductively infected with these viruses (Fig. 6F).
Therefore, although ICP0 is not required for ICP4 foci to associate with or recruit ND10-like complexes in cells productively infected at high MOI, in nonproductively infected cells under low-multiplicity conditions, the ICP4 foci are not juxtaposed to ND10 and they contain much reduced or undetectable levels of ICP27. Cells that harbor such nonproductive infections can therefore be characterized not only by failure to express a full complement of viral proteins (14) but also by the different distributions of ICP0 and ICP27 with respect to ICP4.
MG132 impedes but does not eliminate juxtaposition of ICP4 and ND10-like foci.
The proteasome inhibitor MG132 increases the number and prominence of ND10, leads to more stable colocalization of functional ICP0 in ND10, protects ND10 from the effects of ICP0, and causes a phenotype in HSV-1 strain 17 infections at low MOIs that mimics the lack of ICP0 (15, 16, 19). Therefore, we tested whether proteasome activity was involved in the relative dynamics of ICP4 foci and ND10 proteins. Untreated HFFF-2 cells were infected at a low MOI with strain 17 and stained for PML and ICP4 2 h later. As expected from previous results, in infected cells that retained ND10 at this time, the ICP4 foci were commonly associated with PML (Fig. 6H). In the presence of MG132, however, the majority of prominent ICP4 foci were not associated with PML (Fig. 6G), thus mimicking the situation in cells nonproductively infected with dl1403 (Fig. 6B). Even at an MOI of 10, ND10 structures stabilized by the inhibitor were far less commonly associated with ICP4 complexes than in a productive high-MOI infection in the absence of ICP0 (Fig. 6I; compare with Fig. 4C). The situation was similar in high-MOI dl1403 infections; ICP4 foci were juxtaposed to ND10 frequently in the absence of MG132 (Fig. 6J) but less so in its presence (Fig. 6K). This was not a total block, however, because later in infection the ICP4 foci became associated with ND10 in many drug-treated cells (Fig. 6L). This is consistent with the observation that the effects of MG132, like the lack of ICP0, can be overcome at high MOIs (19). The data indicate that the efficient association of ICP4 complexes with ND10 is dependent on cellular responses that require proteasome activity.
DISCUSSION
In this paper we present evidence that ICP4 is recruited rapidly onto parental HSV-1 viral genomes soon after they have been released into the nucleus. The detection of ICP27 colocalizing with ICP4 at the early stages of infection suggests that these foci represent viral nucleoprotein complexes that are biologically active and engaged in viral transcription. The components of these complexes must be dynamic, since ICP27 becomes less markedly colocalized with ICP4 as time progresses. We provide evidence that the complexes rapidly become associated with ND10; the presence of both ICP4 and ICP27 has parallels with the requirement for both of these proteins for efficient association of model amplicon genomes with ND10 (49). However, the distribution of ND10 proteins with respect to the ICP4 foci in cells newly infected with dl1403 at the periphery of a plaque, and in a proportion of HFFF-2 cells infected with dl1403 at high MOIs, strongly suggests that although some viral DNA complexes may associate with preexisting ND10, ND10-like complexes can be formed de novo in association with and probably in response to the assembly of active viral nucleoprotein complexes. An alternative explanation of the association of PML with numerous ICP4 foci at the nuclear periphery in high-multiplicity dl1403 infections is that preexisting ND10 structures migrate within the nucleus to the ICP4 complexes. For reasons explained in Results, we consider this to be unlikely. However, we have not yet been able to test directly for the de novo formation of ND10-like structures in a live-cell experiment such as that in Fig. 2. For a variety of reasons this is likely to be a technically challenging study, but it certainly deserves further investigation.
The suggestion that ND10-like structures can form de novo in response to viral infection merits an explanation of why it has not been observed in previous studies: (i) the phenomenon is unlikely be observed in wild-type virus infections because of the rapid disruption of ND10 induced by ICP0; (ii) it is much more likely to be observed in samples that include a clear marker for viral nucleoprotein complexes in the early stages of infection, and it has only recently been shown that ICP4 provides such a marker; (iii) it is most readily seen in newly infected cells in developing plaques, a situation not previously considered in this type of study; and (iv) once recognized in such plaque cells, it can be observed in a proportion of HFFF-2 cells in high-multiplicity infections when it is clear what the observer is searching for. This is the first study to investigate the distribution of ICP4 in plaques and HFFF-2 cells infected with an ICP0-negative virus, conditions that provide the clearest demonstration of the phenotype. As discussed above, we must emphasize that this analysis does not exclude the possibility that migration of viral genomes to existing ND10 can also occur.
Are the novel ND10-like structures (or ND10 migration) induced simply because of entry of the viral genome, or is the association of viral proteins and transcriptional activity required? Our attempts to approach this question by using actinomycin D and cycloheximide proved unsuccessful because both drugs caused changes in ND10 themselves, and cells displaying the phenotype at high MOIs are difficult to recognize unless the observer is guided by ICP4 distribution. The observation that the association does not occur in cells nonproductively infected with dl1403 at a low MOI suggests that the response requires more than viral genome entry or transcriptional activity and is in some way linked to whether the cell commits to productive infection. These findings present an alternative explanation of why the replication complexes of nuclear-replicating DNA viruses in general are so frequently found to be associated with ND10; the ND10-like structures can form in response to the development of the viral complexes. This conclusion is also consistent with previous studies that have suggested that viral transcription or replication increases the frequency of ND10 association (20, 48, 49) and that “foreign” DNA-protein complexes become associated with ND10 (51).
These observations provide additional considerations (but no resolution) to the debate as to whether ND10 proteins act as depots of cellular factors that are beneficial to viral infection or whether they constitute part of a defense mechanism that impedes viral gene expression. Several factors provide circumstantial evidence in support of the latter model: PML and Sp100 are both interferon-induced proteins; both these proteins and hDaxx have the ability to repress gene expression in model assays; ICP0-deficient viruses have a reduced probability of entering the lytic cycle and are hypersensitive to interferon (32, 33); HSV-1 triggers activation of interferon-responsive gene transcription, and ICP0 can inactivate this response (9, 31, 40); and finally, PML appears to be required for the sensitivity of ICP0-deficient viruses to the action of interferon (7). Our observation that ND10-like structures are likely to be induced in response to viral infection and that this process is eliminated by ICP0 appears at first sight to be more consistent with a repression than an activation role for ND10. However, there are several examples of transcription factors that are regulated by SUMO-1 modification, some of which can be associated with ND10 (3, 10, 21, 35, 36, 42). It is possible that the release of such factors from ND10 and their potential loss of SUMO-1 modification in response to ICP0, perhaps through the action of SUMO-specific proteases (2), could stimulate viral infection; the suggested de novo formation of ND10-like structures in association with viral nucleoprotein complexes could equally be regarded in this light. Thus, ND10 could have characteristics that have both negative and positive influences on viral infection, i.e., negative through sequestration of activators in an inactive form and positive through release of such factors mediated by ICP0.
It could be argued that ND10 proteins do not have a repressive role, as high-level expression of PML does not appear to impede HSV-1 infection (26). However, it is difficult to conduct this type of experiment under conditions in which the ICP0-dependent phenotype is exhibited. The defect of ICP0-deficient viruses becomes apparent in Vero cells only if the input MOI is less than 0.05 PFU (as measured in Vero cells) per cell, or about 0.5 Vero cell PFU in HFFF-2 cells (14). Even in low-MOI infections below these levels, there are far greater numbers of nonproductively infected cells that express viral IE proteins than cells that become committed to plaque formation (14). Thus, even if high-level expression of PML (for example) could completely overcome the stimulation of viral infection by ICP0, this effect would be seen only at very low MOIs, and even then the effects on viral gene expression would be of an intermediate nature.
The association of ICP4 foci with ND10 was greatly reduced or absent under conditions of nonproductive dl1403 infection or in cells infected with strain 17 at low MOIs in the presence of MG132. This would argue that viral nucleoprotein complex association with and recruitment of ND10 proteins occurs only under productive infection conditions and thus supports the activation model rather than the repression model for ND10. However, because the ICP4 foci that form in these cells do not develop into replication compartments, we cannot be certain that these foci contain viral genomes (although their numbers are consistent with the MOI). Furthermore, the fact that ICP4 foci are visible in these cells indicates that at least some viral gene expression has occurred. Quantitative analysis indicates that HFFF-2 cell cultures infected with dl1403 at low multiplicity must also contain a population of cells in which no ICP4 expression is detectable (14). The location of viral genomes in these cells remains unknown, so it possible that the repressed genomes are associated with ND10 under conditions in which the potential repressive nature of some ND10 components has become dominant. Thus, the role of ND10 and their constituent proteins in HSV-1 infection remains a difficult and complex problem, with many observations capable of being interpreted in favor of either activation or repression models.
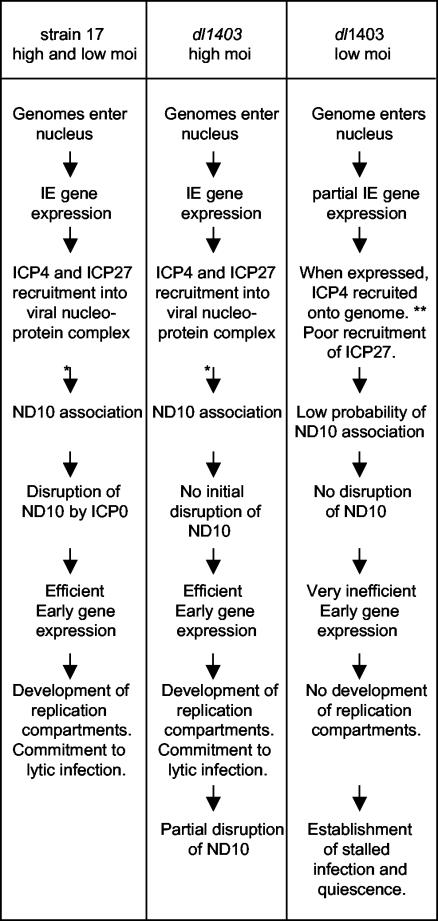
The data presented in this paper, taken with the detailed analysis of the phenotype of dl1403 in HFFF-2 cells (14), allow the various relationships between viral genomes, ICP4, ICP27, ICP0, and ND10 to be depicted in a partly speculative flow diagram (Fig. 7). Figure 7 summarizes the microscopy observations in conjunction with the functional implications concerning whether the infection progresses into the lytic cycle or becomes stalled and attains a quiescent state. The layout of Fig. 7 is intended to compare and contrast the events between the various infection scenarios, but it is not intended to define a precise order of events or their functional interdependence. At present, is not clear whether viral genome association with ND10 can occur prior to or only after initial IE gene transcription and recruitment of IE proteins. It should also be noted that not all cells infected with dl1403 at low MOIs express ICP4 (14). Crucially, it has not yet been established whether the ICP4 foci that form in low-MOI dl1403 infections and that are not associated with ND10 contain viral genomes and whether disruption of ND10 has a direct effect on the efficiency of early gene expression in low-MOI infections. These important questions, which are currently under study, lie at the heart of future understanding of the functional significance of viral genome association with ND10 in the biology of HSV-1.
FIG. 7.
A simplified, partly speculative flow diagram comparing the observed relationships between ICP4, ICP27, ICP0, viral genomes, and ND10 during high- and low-multiplicity infections in the presence and absence of ICP0. This summary is based on the results described in this paper and in a detailed study of the phenotype of ICP0-null mutant viruses (14). *, whether genome association with ND10 occurs efficiently before or after recruitment of ICP4 into viral nucleoprotein complexes is unclear; **, it is assumed that the low numbers of prominent ICP4 foci observed in low-MOI dl1403 infections in HFFF-2 cells represent ICP4 recruitment onto parental viral genomes in a manner similar to that deduced for high-MOI and strain 17 infections. This diagram is not intended to imply a rigid order or functional interdependence of events, particularly in the middle part of the low-MOI dl1403 column, but more to highlight the differences between productive and nonproductive infections in the presence and absence of ICP0.
Acknowledgments
This work was supported by the Medical Research Council and by grant G9826324 to J.B.C. G.S. was funded by a Marie Curie Fellowship of the European Community Framework 5 programme (contract HPMF-CT-2000-01078). C.L. was supported by a Medical Research Council studentship.
We are grateful for helpful discussions with Beate Sodeik and the communication of unpublished information by Gerd Maul.
REFERENCES
- 1.Ackermann, M., D. K. Braun, L. Pereira, and B. Roizman. 1984. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 52:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey, D., and P. O'Hare. 2002. Herpes simplex virus 1 ICP0 co-localizes with a SUMO-specific protease. J. Gen. Virol. 83:2951-2964. [DOI] [PubMed] [Google Scholar]
- 3.Bies, J., J. Markus, and L. Wolff. 2002. Covalent attachment of the SUMO-1 protein to the negative regulatory domain of the c-Myb transcription factor modifies its stability and transactivation capacity. J. Biol. Chem. 277:8999-9009. [DOI] [PubMed] [Google Scholar]
- 4.Burkham, J., D. M. Coen, C. B. Hwang, and S. K. Weller. 2001. Interactions of herpes simplex virus type 1 with ND10 and recruitment of PML to replication compartments. J. Virol. 75:2353-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkham, J., D. M. Coen, and S. K. Weller. 1998. ND10 protein PML is recruited to herpes simplex virus type 1 prereplicative sites and replication compartments in the presence of viral DNA polymerase. J. Virol. 72:10100-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai, W., and P. A. Schaffer. 1992. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 66:2904-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chee, A. V., P. Lopez, P. P. Pandolfi, and B. Roizman. 2003. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 77:7101-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohner, K., A. Wolfstein, U. Prank, C. Echeverri, D. Dujardin, R. Vallee, and B. Sodeik. 2002. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol. Biol. Cell 13:2795-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eidson, K. M., W. E. Hobbs, B. J. Manning, P. Carlson, and N. A. DeLuca. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eloranta, J. J., and H. C. Hurst. 2002. Transcription factor AP-2 interacts with the SUMO-conjugating enzyme UBC9 and is sumolated in vivo. J. Biol. Chem. 277:30798-30804. [DOI] [PubMed] [Google Scholar]
- 11.Everett, R., A. Cross, J. Tyler, and A. Orr. 1993. An epitope within the DNA-binding domain of the herpes simplex virus immediate early protein Vmw175 is conserved in the varicella-zoster virus gene 62 protein. J. Gen. Virol. 74:1955-1958. [DOI] [PubMed] [Google Scholar]
- 12.Everett, R. D. 2001. DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene 20:7266-7273. [DOI] [PubMed] [Google Scholar]
- 13.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 14.Everett, R. D., C. Boutell, and A. Orr. 2004. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 78:1763-1774. [DOI] [PMC free article] [PubMed]
- 15.Everett, R. D., W. C. Earnshaw, A. F. Pluta, T. Sternsdorf, A. M. Ainsztein, M. Carmena, S. Ruchaud, W. L. Hsu, and A. Orr. 1999. A dynamic connection between centromeres and ND10 proteins. J. Cell Sci. 112:3443-3454. [DOI] [PubMed] [Google Scholar]
- 16.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everett, R. D., and G. G. Maul. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 13:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everett, R. D., A. Orr, and M. Elliott. 1991. High level expression and purification of herpes simplex virus type 1 immediate early polypeptide Vmw110. Nucleic Acids Res. 19:6155-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everett, R. D., A. Orr, and C. M. Preston. 1998. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 17:7161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett, R. D., G. Sourvinos, and A. Orr. 2003. Recruitment of herpes simplex virus type 1 transcriptional regulatory protein ICP4 into foci juxtaposed to ND10 in live, infected cells. J. Virol. 77:3680-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girdwood, D., D. Bumpass, O. A. Vaughan, A. Thain, L. A. Anderson, A. W. Snowden, E. Garcia-Wilson, N. D. Perkins, and R. T. Hay. 2003. p300 transcriptional repression is mediated by SUMO modification. Mol. Cell 11:1043-1054. [DOI] [PubMed] [Google Scholar]
- 22.Ishov, A. M., R. M. Stenberg, and G. G. Maul. 1997. Human cytomegalovirus immediate early interaction with host nuclear structures: definition of an immediate transcript environment. J. Cell Biol. 138:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knipe, D. M., P. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus. 2001. Fields virology, 4th ed. Lippincott-Williams and Wilkins, Philadelphia, Pa.
- 24.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 20:5769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lilley, C. E., F. Groutsi, Z. Han, J. A. Palmer, P. N. Anderson, D. S. Latchman, and R. S. Coffin. 2001. Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J. Virol. 75:4343-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez, P., R. J. Jacob, and B. Roizman. 2002. Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus type 1 or its proteins. J. Virol. 76:9355-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukonis, C. J., J. Burkham, and S. K. Weller. 1997. Herpes simplex virus type 1 prereplicative sites are a heterogeneous population: only a subset are likely to be precursors to replication compartments. J. Virol. 71:4771-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukonis, C. J., and S. K. Weller. 1997. Formation of herpes simplex virus type 1 replication compartments by transfection: requirements and localization to nuclear domain 10. J. Virol. 71:2390-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maul, G. G. 1998. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays 20:660-667. [DOI] [PubMed] [Google Scholar]
- 30.Maul, G. G., and R. D. Everett. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 75:1223-1233. [DOI] [PubMed] [Google Scholar]
- 31.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 76:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muratani, M., D. Gerlich, S. M. Janicki, M. Gebhard, R. Eils, and D. L. Spector. 2002. Metabolic-energy-dependent movement of PML bodies within the mammalian cell nucleus. Nat. Cell Biol. 4:106-110. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa, K., and H. Yokosawa. 2002. PIAS3 induces SUMO-1 modification and transcriptional repression of IRF-1. FEBS Lett. 530:204. [DOI] [PubMed] [Google Scholar]
- 36.Nishida, T., and H. Yasuda. 2002. PIAS1 and PIASxalpha function as SUMO-E3 ligases toward androgen receptor and repress androgen receptor-dependent transcription. J. Biol. Chem. 277:41311-41317. [DOI] [PubMed] [Google Scholar]
- 37.Ojala, P. M., B. Sodeik, M. W. Ebersold, U. Kutay, and A. Helenius. 2000. Herpes simplex virus type 1 entry into host cells: reconstitution of capsid binding and uncoating at the nuclear pore complex in vitro. Mol. Cell. Biol. 20:4922-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkinson, J., and R. D. Everett. 2000. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 74:10006-10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Preston, C. M. 2000. Repression of viral transcription during herpes simplex virus latency. J. Gen. Virol. 81:1-19. [DOI] [PubMed] [Google Scholar]
- 40.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75:8909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puvion-Dutilleul, F., L. Venturini, M. C. Guillemin, H. de The, and E. Puvion. 1995. Sequestration of PML and Sp100 proteins in an intranuclear viral structure during herpes simplex virus type 1 infection. Exp. Cell Res. 221:448-461. [DOI] [PubMed] [Google Scholar]
- 42.Ross, S., J. L. Best, L. I. Zon, and G. Gill. 2002. SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Mol. Cell 10:831-842. [DOI] [PubMed] [Google Scholar]
- 43.Sciabica, K. S., Q. J. Dai, and R. M. Sandri-Goldin. 2003. ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. EMBO J. 22:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Showalter, S. D., M. Zweig, and B. Hampar. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sourvinos, G., and R. D. Everett. 2002. Visualization of parental HSV-1 genomes and replication compartments in association with ND10 in live infected cells. EMBO J. 21:4989-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 48.Tang, Q., P. Bell, P. Tegtmeyer, and G. G. Maul. 2000. Replication but not transcription of simian virus 40 DNA is dependent on nuclear domain 10. J. Virol. 74:9694-9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang, Q., L. Li, A. M. Ishov, V. Revol, A. L. Epstein, and G. G. Maul. 2003. Determination of minimum herpes simplex virus type 1 components necessary to localize transcriptionally active DNA to ND10. J. Virol. 77:5821-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor, T. J., E. E. McNamee, C. Day, and D. M. Knipe. 2003. Herpes simplex virus replication compartments can form by coalescence of smaller compartments. Virology 309:232-247. [DOI] [PubMed] [Google Scholar]
- 51.Tsukamoto, T., N. Hashiguchi, S. M. Janicki, T. Tumbar, A. S. Belmont, and D. L. Spector. 2000. Visualization of gene activity in living cells. Nat. Cell Biol. 2:871-878. [DOI] [PubMed] [Google Scholar]
- 52.Wadd, S., H. Bryant, O. Filhol, J. E. Scott, T. Y. Hsieh, R. D. Everett, and J. B. Clements. 1999. The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J. Biol. Chem. 274:28991-28998. [DOI] [PubMed] [Google Scholar]
- 53.Wilcock, D., and D. P. Lane. 1991. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature 349:429-431. [DOI] [PubMed] [Google Scholar]