Abstract
We examined the relationship between pressure and age-related changes in decision-making using a task where currently available rewards depend upon the participant’s previous history of choices. Optimal responding in this task requires the participant to learn how their current choices affect changes in the future rewards given for each option. Building upon the scaffolding theory of aging and cognition we predicted that when additional frontal resources are available, compensatory recruitment leads to increased monitoring and increased use of heuristic-based strategies, ultimately leading to better performance. Specifically, we predicted that scaffolding would result in an age-related performance advantage under no pressure conditions. We also predicted that, while younger adults would engage in scaffolding under pressure, older adults would not have additional resources available for increased scaffolding under pressure-packed conditions, leading to an age-related performance deficit. Both predictions were supported by the data. In addition, computational models were used to evaluate decision-making strategies employed by each participant group. As expected, older adults under no pressure conditions and younger adults under pressure showed increased use of heuristic-based strategies relative to older adults under pressure and younger adults under no pressure, respectively. These results are consistent with the notion that scaffolding can occur across the lifespan in the face of an environmental challenge.
Keywords: Aging, Decision-making, Scaffolding, Pressure, Win-Stay-Lose-Shift
Introduction
Decisions are an important and pervasive part of our lives, yet the decisions that we make are rarely made in pressure-free situations, and little is known about how pressure affects decision making as we age. The impact of decisions increases with age with older adults often holding important positions in government, industry, and society. In addition, personal retirement and medical decisions are often critical. Considering the increasing importance of decisions made by older adults, and the pressure under which these decisions are often made, it is important to understand how pressure impacts older adults’ decision-making as well as decision-making across the lifespan.
The existing literature examining age-related changes in decision-making under pressure-free conditions has yielded mixed results. Some studies find an age-related decision-making advantage (Agarwal, Driscoll, Gabaix, & Laibson, 2009; Peters, Hess, Vastfjall, & Auman, 2007; Worthy, Gorlick, Pacheco, Schnyer, & Maddox, 2011), whereas others find no difference or an age-related decision-making deficit (Christensen, Haroun, Schneiderman, & Jeste, 1995; Denburg et al., 2007; Denburg, Tranel, & Bechara, 2005; Jacoby, 1999; Mell et al., 2005; Mell et al., 2009; Samanez-Larkin, Kuhnen, Yoo, & Knutson, 2010). One line of thought is that some tasks used to assess age-related changes in decision-making are biased toward younger adults (Henninger, Madden & Huettel, 2010), whereas others are biased toward older adults. In particular, some research suggests that age-related advantages emerge when tasks require higher-order processing of relational dependencies between recent choices and available rewards in the environment (Blanchard-Fields, 2007; Grossman et al., 2010; Worthy et al., 2011). Even so, this may not always be the case. Older adult performance declines in some tasks under increased task demand most likely because additional processing resources are unavailable (Cappell, Gmeindl, & Reuter-Lorenz, 2010; Mattay el al., 2006; Reuter-Lorenz & Cappell, 2008; Reuter-Lorenz & Lustig, 2005). In the present research, we explore the hypothesis that older adults excel in tasks that require an understanding of relational dependencies in the reward environment (a history-dependent task), but only when enough cognitive resources are available.
Many tasks used to study decision-making strategies are history-independent, where the probabilities of obtaining rewards on a specific trial are pre-determined by the experimenter regardless of the choice history of the participant, often resulting in minimal age differences or age-related performance deficits (Denburg et al., 2005; Samanez-Larkin, Hollon, Carstensen & Knutson, 2008; Samanez-Larken et al., 2010). History-independent tasks are prevalent in the literature and include the Iowa Gambling Task (Denburg et al., 2005), the Behavioral Investment Allocation Strategy Task (Kuhnen & Knutson, 2005; Samanez-Larken et al., 2010), and the Monetary Incentive Delay Task (Samanez-Larkin et al., 2008).
Recent work from our lab revealed an age-related advantage in a history-dependent decision-making task (described below) where rewards available on the current trial were dependent on the previous sequence of choices (Worthy et al., 2011). Older adults were better fit by heuristic-based win-stay lose-shift strategies than younger adults both when it was advantageous (history-dependent tasks), and when it was disadvantageous (history-independent), indicating that the observed performance differences in decision making may be attributable to reliance on different strategies which help performance in history-dependent tasks but hurt performance in history-independent tasks (Worthy & Maddox, 2012).
Overview of the Task
The history-dependent task in the current study, the Mars Farming task, has been utilized in the decision-making literature to assess decision-making in older adults, younger adults, and individuals with depressive symptoms (Maddox, Gorlick, Worthy, & Beevers, 2012; Worthy, Otto, & Maddox, 2012; Worthy et al., 2011). In this experiment participants are given the task of farming oxygen on Mars by choosing which extraction system to use on each trial. Unbeknownst to the participants, one of these systems corresponds to a decreasing option and one of these systems corresponds to an increasing option. The decreasing option gives a larger immediate reward on each trial relative to the increasing option; however, the rewards available for both options increase proportionally to the number of times the increasing option has been selected in the previous ten trials (Figure 1). Thus, the reward values for both options depend on how often each option has recently been chosen, and the optimal strategy involves foregoing the option that gives a larger immediate reward (the decreasing option) in favor of the option that leads to larger delayed rewards (the increasing option). Optimal performance in this task can be achieved by utilizing heuristic-based strategies in which participants compare the reward that they receive on each trial to the reward that they receive on previous trials, modifying their behavior based on changes in the reward environment (Worthy et al., 2012; Worthy & Maddox, 2012). As the use of heuristic-based strategies is thought to be frontally-mediated (Ashby, Alfonso-Reese, Turken, & Waldron, 1998), we expect to see improved performance on this task in individuals who have adequate neural resources available to monitor the reward environment and engage in the use of heuristic-based strategies.
Figure 1.
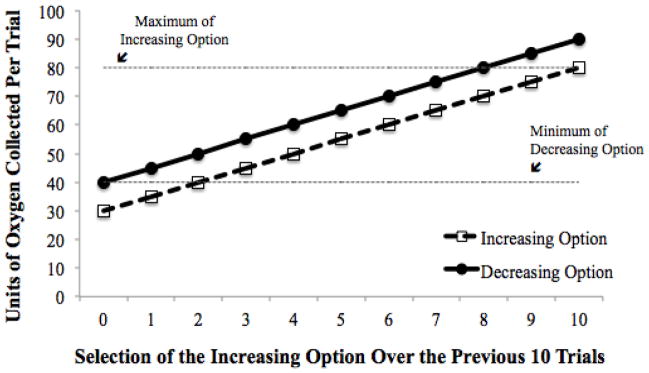
Rewards given for each option as a function of the number of times the increasing option was selected over the previous ten trials. Selecting the increasing option ten consecutive times will lead to a reward of 80 units of oxygen on each trial, whereas selecting the decreasing option ten consecutive times will lead to a reward of 40 units of oxygen on each trial.
Scaffolding Theory of Aging and Cognition (STAC)
Compensatory scaffolding, defined in the Scaffolding Theory of Aging and Cognition (STAC), suggests that an increase in age is associated with a compensatory shift in neural recruitment for a variety of cognitively demanding tasks (Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Cappell, 2008; Reuter-Lorenz & Lustig, 2005). Specifically, older adults show increased activation in prefrontal sites (Cabeza et al., 2004; Park & Reuter-Lorenz, 2009; Reuter-Lorenz et al., 2000). In the context of the current task, and under pressure-free conditions, we hypothesize that older adults will compensate for neural declines in other brain areas like the ventral striatum by recruiting additional frontal resources. This is predicted to result in increased use of frontal resources in forming an understanding of the underlying reward environment and an age-related performance advantage relative to younger adults. Although generally applied to aging, STAC also predicts that scaffolding can occur in younger adults as the brain’s normal response to challenge (Park & Reuter-Lorenz, 2009). Thus, under challenging conditions (such as pressure) younger adults might engage in scaffolding, leading to improved performance relative to younger adults under pressure-free conditions.
Effects of Pressure
Explicit Monitoring theory suggests that pressure causes an increase in explicit monitoring of one’s performance (Baumeister, 1984; Beilock, Kulp, Holt, & Carr, 2004; Gray, 2004). Monitoring theory predicts an increase in performance in cognitively demanding tasks, such as the Mars Farming task, for both younger and older adults under pressure relative to the no pressure condition. However, explicit monitoring theory does not take into account age-related neural changes that may affect the ability to accurately monitor one’s performance. In the next section we combine monitoring theory with the notion of scaffolding to generate a set of predictions regarding the interactive effects of age and pressure on history-dependent decision-making in the Mars Farming task.
The current experiment manipulates social pressure and participants are given a performance goal that, when met, will result in a monetary bonus for themselves and a partner. This mirrors real-world decision-making where the consequences of an individuals’ decision are felt by both themselves and others such as friends, family members, and colleagues or co-workers. This type of social pressure manipulation has been widely used and has been shown to induce both changes in behavior and an increase in reported feelings of pressure in a variety of cognitive tasks (e.g., Beilock & Carr, 2001, 2005; Beilock & DeCaro, 2007; Gray, 2004; Markman, Maddox & Worthy, 2006; Worthy, Markman & Maddox, 2009). To our knowledge this manipulation has not been used with older adults and this study provides additional information about susceptibility of older adults to social pressure induced by the worry of disappointing another person based on one’s own performance.
To date, much work concerning social pressure and aging has focused on social pressure to conform, reporting that older adults are less susceptible than younger adults to conformity (e.g. Pasupathi, 1999). Though this work does indicate that social pressure interacts with age, this work provides little insight into the effects of social pressure on performance during decision making. Other research examining the Trier Social Stress test (TSST) demonstrates that older adults experience a physiological response to social stress. Here stress is induced through social evaluation over a 20-minute period where participants are required to prepare a statement that will be presented to a panel of judges. A meta-analysis of the TSST in older and younger adults found a significant Hypothalamic-Pituitary-Adrenal axis stress response in both older and younger age groups (Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004). Thus, while older adults may be less likely to conform based on social pressure they are not immune to the physiological stress responses that arise from impending social stressors. Further, changes in the HPA axis have been demonstrated to affect decision making in younger and older adults. Mather, Gorlick & Lighthall (2009) found an interaction between stress-induced increases in cortisol and age in performance during risky decision making. Although older adult performance was impaired under stress, younger adult performance was not affected. While these studies do not manipulate “pressure” in the sense that they do not increase the importance of good or improved performance in a task (Baumeister, 1984), they provide evidence that social situational challenges increase stress and may have a differential effect on older and younger adult decision making performance (Additional discussion of the similarities and differences between pressure and stress is reserved for the General Discussion.)
Integrating Monitoring Theory and Scaffolding Hypotheses
We propose that pressure acts as an environmental challenge and induces scaffolding, or the recruitment of available frontal neural regions, but only when resources are available. This relatively small distinction is critical as it leads to qualitatively different predictions regarding the effects of pressure on older vs. younger adult decision-making. In younger adults, we propose that pressure increases the recruitment of frontal resources (through scaffolding), increasing performance in our history-dependent task. Because frontal resources are plentiful in younger adults, pressure leads to improved performance relative to pressure-free conditions. However, older adults already engage in scaffolding under pressure-free conditions and have no additional resources available to recruit when placed under pressure. Thus, we propose that pressure is unable to increase the recruitment of frontal resources (i.e., scaffolding) beyond what exists under pressure-free conditions. In the literature this is referred to as the “crunch” point. The compensation-related utilization of neural circuits hypothesis (CRUNCH) asserts that compensatory activation is effective at lower levels of demand, but that the aging brain cannot reach sufficient activation levels to accommodate higher demand (Cappell et al., 2010; Reuter-Lorenz & Cappell, 2008; Reuter-Lorenz & Lustig, 2005; Stern et al., 2012). We predict that the neural declines of aging will cause older adults to effectively choke under pressure, while younger adults will excel under pressure due to scaffolding.
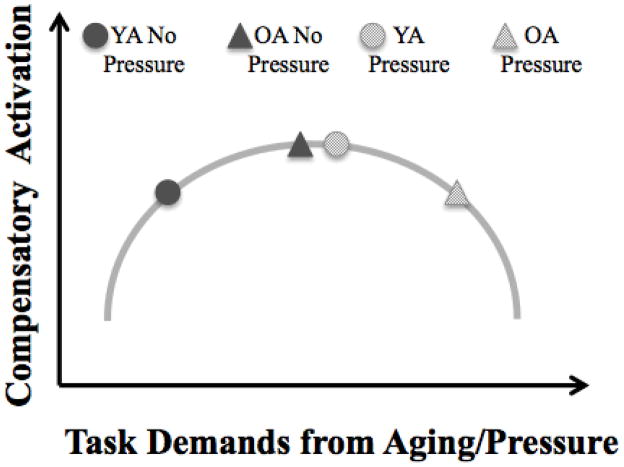
These predictions regarding the interactive effects of age and pressure on scaffolding are represented graphically in Figure 2. Pressure increases task demands and shifts activation to the right along the inverted U-shaped curve. Older adults under no pressure conditions experience roughly the same level of task demand as younger adults under pressure, and both are hypothesized to result in compensatory over-activation of frontal regions. Pressure forces older adults beyond the crunch point, leading to under-activation of frontal areas.
Figure 2.
Hypothesized relationship between task demands from aging/pressure and compensatory activation.
Method
Participants
Forty-two older adults (average age 67.40) from the greater Austin and College Station, Texas communities and 47 younger adults from the University of Texas and Texas A&M University communities were paid $10 per hour for their participation. Informed consent was obtained from all participants and the experiment was approved for ethics procedures using human participants.
Neuropsychological Testing Procedures
Older adults were given a series of standardized neuropsychological tests designed to assess general intellectual ability across attention (WAIS-III Digit Span, Wechsler, 1997; WAIS-III Vocabulary, Weschler, 1997), executive functioning (Trail Making Test A&B (TMT), Lezak, 1995; FAS; Wisconsin Card Sorting Task (WCST), Heaton, 1981), and memory (California Verbal Learning Test (CVLT), Delis, 1987). The tests were administered in one two-hour session.
Normative scores for each subject were calculated for each neuropsychological test using the standard age-appropriate published norms. Table 1 shows the means, standard deviations, and ranges of standardized z-scores on each test for older adults in both conditions. All WAIS subtest percentiles were calculated according to the testing instructions and then converted to standardized z-scores. The CVLT and WCST standardized T-scores were calculated according to testing directions then converted to standardized z-scores, and the TMT standard z-scores were calculated according to the testing instructions. Subjects were excluded from participation if they scored more than two standard deviations below the standardized mean on more than one neuropsychological test in the same area (memory, executive functioning, or attention). Only subjects who were within normal ranges were asked to participate in the experiment.
Table 1.
Z-scores Summary for each Neuropsychological Exam
| Neuropsychological Test | No Pressure | Pressure | ||
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Range | Mean (SD) | Range | |
| WAIS Vocabulary | 1.11 (0.69) | 0.0–2.3 | 1.28 (1.16) | −3.0-2.7 |
| Digit Span | 0.68 (1.10) | −1.0–3.0 | 0.39 (0.79) | −0.3–2.7 |
| CVLT Delayed Recall (Free) | 0.71 (0.82) | −1.0–2.0 | 0.54 (0.95) | −1.0–2.5 |
| CVLT Immediate Recall (Free) | 0.81 (0.87) | −0.5–2.0 | 0.61 (0.74) | −0.5–2.0 |
| CVLT Delayed Recall (Cued) | 0.48 (0.86) | −1.5–2.0 | 0.59 (0.81) | −1.0–2.0 |
| CVLT Immediate Recall (Cued) | 0.86 (0.73) | 0.0–2.5 | 0.61 (0.76) | −1.0–2.0 |
| CVLT Recognition False Positives | 0.07 (0.88) | −1.0–2.0 | −0.35 (0.85) | −1.0–2.5 |
| CVLT Recognition True Positives | 0.40 (0.70) | −1.0-1.0 | 0.02 (0.68) | −1.5-1.0 |
| FAS | 0.43 (0.91) | −0.8–2.6 | 0.28 (1.05) | −1.2-1.0 |
| Trails A | −0.80 (0.42) | −1.4-0.2 | −0.49 (0.54) | −1.4-0.9 |
| Trails B | −0.63 (0.35) | −1.1-0.2 | −0.58 (0.60) | −2.1-0.6 |
| WCST Errors | 0.20 (1.02) | −2.3–2.5 | 0.41 (0.77) | −0.7–2.5 |
| WCST Perseveration | 0.31 (0.87) | −1.6–2.5 | 0.46 (0.71) | −0.5–2.5 |
|
| ||||
| Demographic Information | No Pressure | Pressure | ||
|
| ||||
| Age | 67.52 (6.29) | 60–82 | 67.29 (5.65) | 61–83 |
| Years of Education | 17.79 (1.89) | 13.5–21 | 17.66 (1.68) | 13–20 |
Note: Mean z-scores for each exam with standard deviation in parenthesis and z-score range. Scores are separated by condition (pressure or no pressure).
Stimuli
The experiment was performed on PC computers using Matlab software with Psychtoolbox 2.54. Participants were given a hypothetical scenario that they would be testing two oxygen extraction systems on Mars with the goal of collecting enough oxygen to sustain life. Figure 3 shows a sample screen shot from the experiment. On each trial the participant chose between Extraction System A and Extraction System B and a bar representing the oxygen tank would show the amount of oxygen that had been extracted on that trial. The oxygen would then be moved to the larger cumulative tank and the next trial would begin. A line on the cumulative tank indicated the amount of oxygen needed to sustain life on Mars. The goal line was set at the equivalent of 16,000 units of oxygen, corresponding to selecting the increasing option on approximately 80% of trials. Participants performed a total of 5 consecutive 50-trial blocks, and were told nothing of the history-dependent nature of the reward structure.
Figure 3.
Sample screen shot from the experiment. Participants were told that they were testing two oxygen extraction systems. The oxygen extracted on each trial was shown in the “Current” tank then transferred to the “Cumulative” tank before the next trial began.
Pressure Manipulation
The no-pressure group was asked to do their best. The pressure group was told that they and their (fictitious) partner would both receive a monetary bonus if they both exceeded the bonus criterion (the goal line of 16,000 units), and that neither would receive the bonus if either of them failed. The pressure participants were then informed that their partner had already reached the criterion, so the bonus for both the participant and their partner depended on their performance.
Results
Behavioral results
We began by examining the effects of age and pressure on the amount of oxygen earned throughout the experiment (Figure 4). These data were subjected to a 2 Age × 2 Pressure ANOVA1 As predicted, there was a significant Age x Pressure interaction, F(1,85)=11.218, p<.001, partial-η2=.116, with no main effects of age or pressure emerging (p>.10). To decompose the interaction we conducted a priori comparisons within each group. Older adults collected more oxygen under no pressure than under pressure conditions, t(40)=2.324, p<.025, Cohen’s d=.735 (No Pressure M=15743; Pressure M= 14015), whereas younger adults collected more oxygen under pressure than under no pressure conditions, t(45)=−2.398, p<.021, Cohen’s d=.715 (No Pressure M=14618; Pressure M=16323). In addition, younger adults collected more oxygen than older adults under pressure conditions, t(45)=2.875, p<.006, Cohen’s d=.857, whereas older adults collected more oxygen than younger adults under no pressure conditions (although this effect was only marginally significant), t(40)=−1.825, p<.076, Cohen’s d=.578.
Figure 4.
Total oxygen collected in each age-pressure variant group across all 250 trials. Error bars represent standard error.
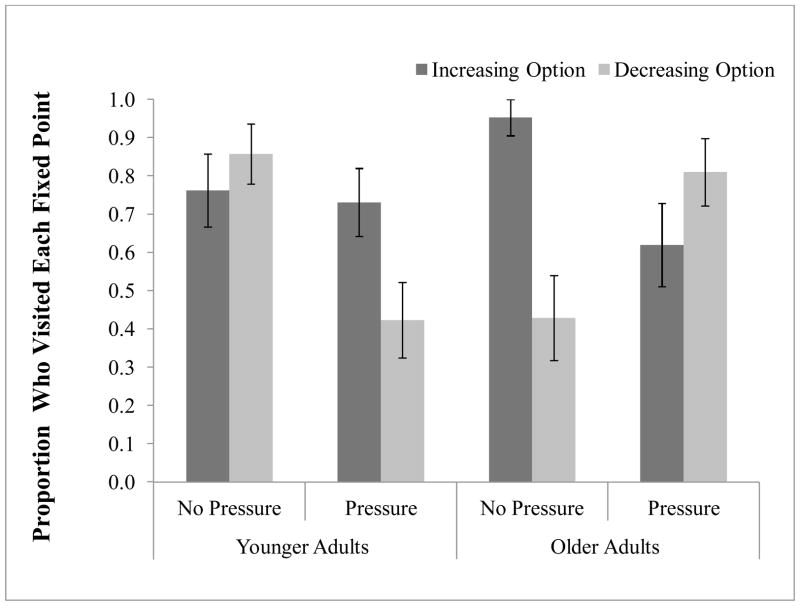
To examine participants’ responsiveness to the changing reward values as a function of their recent selections we calculated the proportion of participants in each condition who reached the end-state for each option—that is, participants who had at least one streak of 10 consecutive decreasing option selections and participants who had at least one streak of 10 consecutive increasing option selections (Figure 5). For the increasing option, reaching the end-state entailed reaching the maximum value for that option (80 units). For the decreasing option, reaching the end-state entailed reaching the minimum value for that option (40 units). Two-tailed binomial tests (Bonferroni corrected for multiple comparisons) were used to compare the proportion of participants who visited each end-state. A larger proportion of older adults in the no pressure condition relative to the pressure condition reached the increasing option’s end-state (p<.01), whereas there was no difference for younger adults under pressure vs. no pressure (p>.10).
Figure 5.
Proportion of subjects in each condition who reached the end-state of each option. The end-state was reached by selecting an option ten times consecutively, resulting in the most extreme and stable reward values. Error bars represent standard error.
A number of differences emerged in the proportion of participants who reached the decreasing option end-state. A larger proportion of older adults in the pressure condition relative to the no pressure condition reached the decreasing option end-state (p<.01), whereas a larger proportion of younger adults in the no pressure condition relative to the pressure condition reached the decreasing option end-state (p<.01). In addition, a larger proportion of older adults than younger adults reached the decreasing option end-state under pressure (p<.01), whereas a larger proportion of younger adults than older adults reached the decreasing option end-state under no pressure conditions (p<.01). These results indicate that participants in better performing groups were more sensitive to the decline in rewards resulting from selecting the decreasing option, as a greater percentage of participants in the better performing groups (OA no pressure & YA pressure) refrained from selecting the decreasing option repeatedly (for ten consecutive trials) and responded to the decline in reward before the decreasing option stabilized at it’s minimum value.
Relationship Between Neuropsychological Test Scores and Task Performance
Correlations between z-scores from each neuropsychological test and performance (units of oxygen collected) in the decision-making task were examined in older adults within each condition and across collapsed conditions. None of the neuropsychological measures were correlated with performance in the decision-making task. Additionally, there were no significant correlations between performance and participant age or years of education within the older adult groups.
Computational Modeling
Description of Models
One advantage of our approach is that the decision-making task is amenable to computational modeling that can provide additional insight into the decision-making behavior of our participants. We applied a series of computational models to the individual participants’ data on a trial-by-trial basis. The models included a heuristic-based Extended win-stay-lose-shift (WSLS) model, an Eligibility Trace Reinforcement Learning (RL) model, and a Baseline model.
WSLS models have been used extensively to model behavior in decision-making tasks (Otto, Taylor & Markman, 2011; Steyvers, Lee, & Wagenmakers, 2009; Worthy et al., 2012; Worthy & Maddox, 2012). The Basic WSLS model that is often fit to similar tasks assumes that participants compare the reward received on the present trial to the reward that they received on the previous trial. If the reward is greater than or equal to the reward on the previous trial it is a “win” trial and participants “stay” by picking the same option on the next trial with a probability estimated by the model. If the reward is less than the reward received on the previous trial it is a “loss” trial, and participants “shift” by picking the other option on the next trial, with a certain probability estimated by the model. Utilization of a WSLS strategy can lead to good performance in the Mars Farming task since performance relies on one’s ability to observe how rewards improve or decline across trials (Worthy et al., 2012). The Basic WSLS model has a total of two free parameters P(stay\win) and P(shift\loss). The probability of switching to the other option after a “win” is 1-P(stay\win), and the probability of staying with an option after a “loss” is 1-P(shift\loss).
The Extended WSLS model, which we fit to our data, expands on the Basic WSLS model by providing separate estimations of win-stay and lose-shift behavior after initial selection (one selection) or extended selection (multiple consecutive selections) of each option. The Extended WSLS model has a total of four free parameters, two “win-stay” and two “lose-shift” parameters, one of each type for initial selection trials and one of each type for extended selection trials. Initial selection trials are defined as trials for which the currently selected option is different from the option selected on the previous trial, and extended selection trials are defined as trials for which the currently selected option is the same as the option selected on the previous trial. Thus, the Extended WSLS model expands on the Basic WSLS model by accounting for reactions to a win or loss based on repeated selection (history-dependent changes) separately from reactions to initial wins or losses that result from switching to the other option2.
Although WSLS models have often been found to be a good fit for data from this task, we fit an additional reinforcement learning model to our data that assumes that participants develop expected reward values (EVs) for each option and probabilistically compare those value to determine a probability for selecting each option. The RL model we used is an Eligibility Trace (ET) model that allows credit for the rewards on each trial to be given to options that were chosen on previous trials by incorporating eligibility traces for recent actions. Expected values (EVs) are initialized at zero for each option and are updated for the each option according to the rule:
| (1) |
The recency parameter (α), 0≤α≤1, weighs the degree to which participants update the expected values based on their most recently received rewards. As α approaches 1 recent rewards are given greater weight in updating EVs. An α value of zero indicates that no learning took place and EVs were not updated from their initial starting point. Eligibility traces represent memories for recent actions (Bogacz, McClure, Li, Cohen & Montague., 2007; Neth, Sims, & Gray, 2006). In this model, each option is associated with a decaying trace that tracks how often the option has been chosen in the recent past.
The Eligibility Trace for each option j is represented in eq. 1 by λ. On each trial the ET for each option decays according to λj =λj · ζ, 0 ≤ ζ ≤ 1. However, each time an option is selected it’s ET increases by 1. Thus, the more often an option has been selected in the recent past, the higher it’s ET, and the bigger impact the reward prediction error will have in updating the EV for that option. This addition to the model allows for the reward received on each trial to be credited to not only the chosen option on the current trial, but to options chosen on previous trials. Thus, this assumption is quite appropriate for the task as past choices have a direct effect on the rewards received on each trial.
In addition to utilizing the EV terms to model choice behavior, we also included an autocorrelation term that models participants’ tendencies to perseverate on the same option. These terms have been used in previous reinforcement learning models and they often provide a much better fit to the data (Kovatch et al., 2012). Similar to the eligibility trace, the autocorrelation term, A, is incremented by 1 each time an option is chosen, A = A +1 and decays on each trial by decay parameter d, A = A · d. In this task a tendency to perseverate on the same option may lead to an increased awareness of how selecting each option affects rewards on future trials. Such a tendency could improve performance in the task (Otto, Gureckis, Markman & Love, 2009).
The expected values for each option and the autocorrelation term are used to determine the probabilities for selecting each option by the Softmax decision rule (Sutton & Barto, 1998):
| (2) |
Where θEV and θA are exploitation parameters that measure the relative contributions of the expected values (EV) and autocorrelation terms (A) to the choices made on each trial. Higher values of θEV indicate that the highest valued option is chosen more often, while a value of zero indicates that they are chosen equally often. Contrary to WSLS models, output from RL models has been correlated with striatal activity (Pagnoni, Zink, Montague, & Berns, 2002; Pessiglione, Seymour, Flandin, Dolan, & Frith, 2006; Hare, O’Doherty, Camerer, Schultz, & Rangel, 2008).
Finally, we also fit a baseline model, or null model, that assumes fixed choice probabilities (Gureckis & Love, 2009; Worthy & Maddox, 2012; Yechiam & Busemeyer, 2005). The baseline model has one free parameter, p(Increasing), that represents the probability of selecting one of the two options in our task on any given trial. The probability of selecting the decreasing option in this model is simply 1-p(Increasing). This model does not assume that participants learn from rewards given on each trial, yet it provides a good fit to the data when participants repeatedly choose the same option (Gureckis & Love, 2009).
We predicted that under normal conditions older adults would engage in more heuristic-based strategies relative to reinforcement learning strategies, reflected in increased fit of the Extended WSLS model compared to the ET model. These predictions are supported by previous findings (Worthy & Maddox, 2012) and are consistent with notion that older adults engage more frontal regions than younger adults under normal conditions. We also predicted that older adults should utilize heuristic-based (WSLS) strategies to a lesser degree under pressure than under normal conditions due to potential under-activation at increased levels of task demand (Reuter-Lorenz & Cappell, 2008). Younger adults, who are expected to engage in scaffolding under pressure, should also utilize heuristic-based WSLS strategies to a greater degree under pressure than younger adults under normal conditions.
Model Comparison Methods
We used Akaike’s Weights (Wagenmakers & Farrell, 2004) to compare the relative fits of the models. The Akaike’s Information Criterion (Akaike, 1974) is defined for each model j as:
| (3) |
Where Lj is the maximum likelihood for model j and Vj is the number of free parameters in the model. Notice that the AIC measure penalizes the model for each additional free parameter.
The AIC values were used to generate the Akaike Weight for each of the three models for each participant. The relative likelihood, L, of each model, j, is computed using the transform:
| (4) |
where Δj(AIC) represents the difference between the AIC for that model and the lowest AIC of all candidate models. The relative likelihoods of each candidate model are then normalized by dividing each of the likelihoods by the sum of all likelihoods for all k models:
| (5) |
These Akaike weights can be interpreted as the probability that the model is the best model for the data given the data set and the set of candidate models (Wagenmakers & Farrell, 2004).
Modeling Results
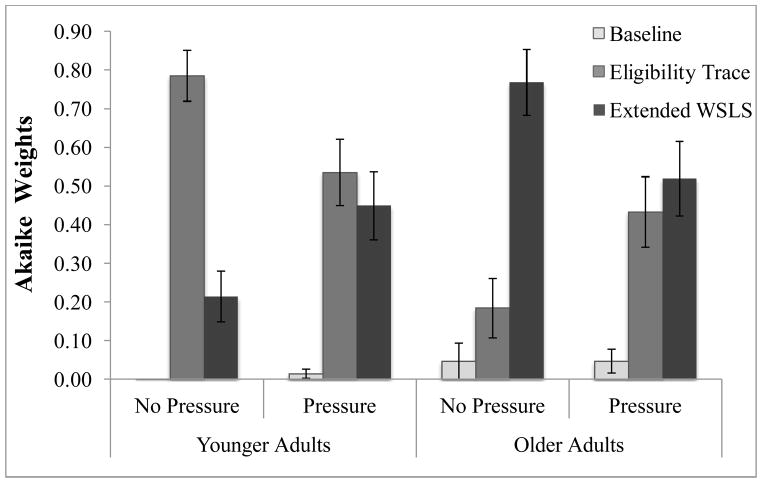
Figure 6 shows the average Akaike weights for participants in each condition. Akaike Weights were compared using 2 (Age) × 2 (Pressure) ANOVA. There was a significant Age x Pressure interaction for the Akaike weights for the Extended WSLS model, F(1,85)=8.776, p<.01, partial-η2=.094. Akaike Weights for the Extended WSLS model were higher for older adults under normal conditions than older adults under pressure, t(40)=2.122, p<.05, and higher for younger adults under pressure than younger adults under no pressure, t(45)=2.056, p<.05, indicating that older adults engaged in more heuristic-based strategies under normal conditions, while younger adults increased the use of these strategies under pressure. A main effect of age was also observed, F(1,85)=12.142 p<.001 partial-η2=.125. Older adults were better fit by the Extended WSLS (M=.632), which assumes a heuristic-based strategy, than younger adults (M=.333). The opposite pattern was observed for the ET model. An Age x Pressure interaction was also observed, F(1,85)=10.320, p<.01, partial-η2=.108; older adults under pressure were better fit by the ET model than older adults under normal conditions t(40)=2.313, p<.05, and younger adults were better fit by this model under normal conditions than younger adults under pressure, t(45)=−2.228, p<.05. A main effect of age was also observed, F(1.85)=16.964, p<.001, partial-η2= .166. Overall, younger adults were better fit by the ET model (M=.660) than older adults (M=.322). Akaike weights for the Baseline model were very low across all conditions, which suggests that participants were not simply responding randomly. This pattern of increased relative fit of heuristic-based models for older adults and increased relative fit of RL models for younger adults is consistent with previous work examining strategy use in younger and older adults (Worthy & Maddox, 2012).
Figure 6.
Akaike Weights of the baseline model, Eligibility Trace model, and Extended WSLS model. Higher Akaike weights indicate better fit. Error bars represent standard error.
Small-Scale Replication of the Age-Related Pressure Deficit
Replication is a critical pillar of the scientific method because it increases confidence in the robustness of experimental effects (e.g. Ioannidis, 2012; Makel, Plucker, & Hegarty, 2012). In the current study we examined age-related changes in history-dependent decision making as a function of the degree of pressure (no pressure vs. pressure). The current no pressure condition represents a direct replication of a previous study from our lab (Worthy, et al., 2011), and importantly the age-related history-dependent decision making advantage observed in Worthy et al. (2011) replicated here. The current pressure condition is novel and a small-scale replication is in order to instill confidence on our finding. Our small-scale replication included 7 older adults and 10 younger adults. Each participant completed a number of neuropsychological tests and met the same inclusion criteria outlined above.
The pattern of performance was consistent with the results from the larger study. Specifically, in each of the 5 50-trial blocks, younger adults obtained more points (2914, 3182, 3392, 3433, and 3550 in blocks 1 – 5, respectively) than older adults (2797, 2658, 3087, 3322, and 3165 in blocks 1 – 5, respectively), with overall performance being superior for younger (16474 points) than for older adults (15032). These block-by-block differences are highly significant based on a sign test (p< .001). We also fit the models described above to each participant’s data. In line with the study reported above, the Akaike weights for the Extended WSLS and ET model were similar for younger adults under pressure, ET=.44, WSLS=.56. The pattern of Akaike weights for older adults under pressure was also similar to those observed in the larger study (Baseline=.13, ET=.31, WSLS=.55).
Taken together, the behavioral and modeling results from the small-scale replication converge nicely with those observed in the larger study and lend strong support to the robustness of the age-related effects of pressure on history-dependent decision-making.
Discussion
We observed an interaction between age and pressure on performance in a history-dependent decision-making task. Older adults performed better than younger adults when they were not under pressure, while younger adults outperformed older adults when they were under pressure. This replicates our previous findings using the same history dependent task under no pressure conditions (Worthy et al., 2011), but extends the work by showing that pressure has differential effects on performance across the lifespan. The age-related performance deficit under pressure was observed in our study and in a small-scale replication lending support for the robustness of the effect. These differences in performance could be due to a shift in neural areas recruited during decision-making, predicted by the scaffolding theory of aging and cognition (STAC). Under the scaffolding theory, older adults are presumed to recruit additional neural resources to compensate for decline in other areas and across the lifespan in response to challenging and novel situations (Park and Reuter-Lorenz, 2009). The challenge imposed by the pressure manipulation may have caused scaffolding in younger adults, increasing recruitment of additional frontal resources for use in the task. Older adults, who regularly engage in compensatory scaffolding, may have been unable to recruit additional resources under pressure, reaching their “crunch” point (Reuter-Lorenz & Cappell, 2008) and experiencing a decline in performance relative to the no pressure condition. The results suggest that the previously observed age-related performance advantage (Worthy, et al., 2011) may be due to engagement of additional frontal regions to compensate for age-related decline. Thus, the older brain under no pressure may operate at a similar level as the younger brain under pressure.
We applied a heuristic-based Win-Stay Lose-Shift model and a reinforcement learning Eligibility Trace model to the data that allowed us to examine the strategies that younger and older adults were utilizing in each condition. Younger and older adults in their optimal performing conditions (younger adults in the pressure condition and older adults in the no pressure condition) both showed increased use of heuristic-based strategies relative to their lower performing counterparts. We attribute the observed difference in performance, and the difference in strategies, to the increased use of frontal regions predicted by STAC. We hypothesize that older adults were able to recruit these additional resources under normal task conditions, but were unable to do so when the combined effects of age and pressure caused them exceed their crunch point, beyond which there were no additional resources to recruit (Reuter-Lorenz & Cappell, 2008). In contrast, younger adults recruited additional neural resources when task demand increased due to a social pressure manipulation, resulting in increased utilization of heuristic-based strategies (WSLS) relative to the younger adults under no pressure conditions who relied heavily on reinforcement learning strategies.
The results of this study suggest that older adults can outperform younger adults in history dependent decision-making tasks, but that the advantage is dependent on the level of challenge or demands of the task. Our findings differ from those of previous studies on the behavioral effects of frontal compensation in older adults, which showed minimal age differences in performance at lower levels of task demand, but impaired performance of older adults at higher levels of demand (Cappell et al., 2010; Mattay el al., 2006). These findings indicate that the effects of frontal compensation on behavioral performance may vary for different task-types; while Cappell et al. (2010) and Mattay et al. (2006) employed verbal working memory tasks, our study focused on differences in decision-making. Engagement of striatally-mediated reward learning strategies results in suboptimal performance in history-dependent tasks such as the one used in this study (Worthy & Maddox, 2012). Thus, in addition to scaffolding, declines in areas such as the striatum may have contributed to the older adult advantage observed under normal conditions in this task by reducing older adults’ tendency to choose the immediately rewarding option. Future studies using neuroimaging and decision-making tasks with different optimal strategies should provide additional information about age-based differences in performance and the neural mechanisms implicated in decision-making under varying levels of task and motivational demands.
Pressure vs. Stress
One shortcoming of this study is that the observed effect of social partner pressure may not be generalizable to other forms of pressure. Additional work should focus on the effects of other types of pressure, such as time pressure, on decision-making performance. Furthermore, continued work on social partner pressure would benefit from quantifying the amount of pressure felt by participants and the HPA axis response experienced in younger and older adults. Conventional TSST pressure manipulations take much time (about 20 minutes) and resources (a panel of judges, film equipment) to administer. If the social partner pressure task elicits a similar HPA axis response to TSST, it could be an exciting new tool for social stress manipulations.
Hormones released after social stressors are known to affect prefrontal brain regions and dopaminergic pathways involved in decision-making (Moghaddam & Jackson, 2004; Wang et al., 2005), and as people age, prefrontal brain regions decline in volume and in dopamine transmission effectiveness (Raz, 2004; Volkow et al., 2000). Mather, Gorlick & Lighthall (2009) attributed the differential effects of stress observed in older and younger adults’ performance during decision-making to these declines, however, they observed no younger adult performance differences. Thus, it is possible that the effect of the social pressure manipulation may be fundamentally different than the effect of stress, or may have an additional component that enhances younger adult performance. Future studies can clarify this issue by analyzing the effect of established stress manipulations in this history-dependent decision making task.
Conclusion
Our results support the hypotheses that pressure and normal aging both lead to scaffolding whereby additional neural resources are recruited as a normal response to challenging situations. They are also consistent with the idea of a “crunch” point where there are no longer enough available resources to be recruited in situations requiring scaffolding. The additive effects of pressure and normal aging cause older adults to reach a crunch point and “choke” under pressure, even though they outperformed younger adults under no pressure. This is one of the first studies to examine how pressure affects older adults’ decision-making ability. The results suggest that pressure hinders older adults’ history-dependent decision-making ability, while it enhances history-dependent decision-making in younger adults.
Acknowledgments
This research was supported in part by a National Institutes of Health Grant R01 MH077708 and NIDA grant DA032457 to WTM, and in part by the Department of Defense (DoD) through a National Defense Science & Engineering Graduate Fellowship (NDSEG) to JAC. We thank Brittany Nix, Neda Abdul-Razzak, and Kirsten Smayda for their help with data collection.
Footnotes
For completeness we also examined the effect of block. As expected, significant learning resulted in all four conditions (main effect of block: F(4,82)= 13.404, p<.001, η2=.136), but block did not interact with age or pressure (p’s>.1).
The Basic WSLS model was also fit to each participant’s data to ensure that the best fitting heuristic-based model was used for our analysis. The Extended WSLS model provided a better fit, indicated by a lower average AIC (AIC Extended=192.64, AIC Basic=204.75).
References
- Agarwal S, Driscoll JC, Gabaix X, Laibson D. The age of reason: Financial decisions over the life-cycle and implications for regulation. Brookings Papers on Economic Activity. 2009;2:51–117. [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Baumeister RF. Choking under pressure: Self-consciousness and paradoxical effects of incentives on skillful performance. Journal of Personality and Social Psychology. 1984;46:610–620. doi: 10.1037//0022-3514.46.3.610. [DOI] [PubMed] [Google Scholar]
- Beilock SL, Carr TH. On the fragility of skilled performance: What governs choking under pressure? Journal of Experimental Psychology: General. 2001;130:701–725. [PubMed] [Google Scholar]
- Beilock SN, Carr TH. When high-powered people fail: Working memory and “choking under pressure” in math. Psychological Science. 2005;16:101–105. doi: 10.1111/j.0956-7976.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- Beilock SL, DeCaro MS. From poor performance to success under stress: Working memory, strategy selection, and mathematical problem solving under pressure. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2007;33:983–998. doi: 10.1037/0278-7393.33.6.983. [DOI] [PubMed] [Google Scholar]
- Beilock SL, Kulp CA, Holt LE, Carr TH. More on the fragility of performance: Choking under pressure in mathematical problem solving. Journal of Experimental Psychology: General. 2004;133:584–600. doi: 10.1037/0096-3445.133.4.584. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fields F. Everyday problem solving and emotion: An Adult Developmental Perspective. Current Directions in Psychological Science. 2007;16:26–31. [Google Scholar]
- Bogacz R, McClure SM, Li J, Cohen JD, Montague PR. Short-term memory traces for action bias in human reinforcement learning. Brain Research. 2007;1153:111–121. doi: 10.1016/j.brainres.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar S, Dolcos F, Prince SE, Buddle M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention, and episodic retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefrontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Haroun A, Schneiderman LJ, Jeste DV. Decision-making capacity for informed consent in the older population. Bulletin of the American Academy of Psychiatry and the Law. 1995;23:353–365. [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult Version Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Denburg NL, Cole CA, Hernandez M, Yamada TH, Tranel D, Bechara A, Wallace RB. The orbitofrontal cortex, real-world decision making, and normal aging. Annals of the New York Academy of Science. 2007;1121:480–498. doi: 10.1196/annals.1401.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43:1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Gray RG. Attending to the execution of a complex sensorimotor skill: Expertise differences, choking, slumps. Journal of Experimental Psychology: Applied. 2004;10:42–54. doi: 10.1037/1076-898X.10.1.42. [DOI] [PubMed] [Google Scholar]
- Grossman I, Na J, Varnum ME, Park DC, Kitayama S, Nisbett RE. Reasoning about social conflicts improves into old age. Proceedings of the National Academy of Sciences. 2010;107:7246–7250. doi: 10.1073/pnas.1001715107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureckis TM, Love BC. Learning in noise: Dynamic decision-making in a variable environment. Journal of Mathematical Psychology. 2009;53:180–193. doi: 10.1016/j.jmp.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. A Manual for the Wisconsin Card Sorting Test. Odessa, Florida: Psychological Assessment Resources; 1981. [Google Scholar]
- Henninger DE, Madden DJ, Huettel SA. Processing speed and memory mediate age-related differences in decision-making. Psychology and Aging. 2010;25:262–270. doi: 10.1037/a0019096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J. Why Science is Not Necessarily Self-Correcting. Perspectives on Psychological Science. 2012;7:645–654. doi: 10.1177/1745691612464056. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. Deceiving the elderly: Effects of accessibility bias in cued-recall performance. Cognitive Neuropsychology. 1999;16:417–436. [Google Scholar]
- Kovatch CK, Daw ND, Rudrauf D, Tranel D, O’Doherty JP, Adolphs R. Anterior prefrontal cortex contributes to action selection through tracking of recent reward trends. Journal of Neuroscience. 2012;32:8234–8442. doi: 10.1523/JNEUROSCI.5468-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk-taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- Maddox WT, Gorlick MA, Worthy DA, Beevers CG. Depressive symptoms enhance loss-minimization, but attenuate gain-maximization in history-dependent decision-making. Cognition. 2012;125:118–124. doi: 10.1016/j.cognition.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makel MC, Plucker J, Hegarty CB. Replications in psychology research: How often do they really occur? Perspectives on Psychological Science. 2012;7:537–542. doi: 10.1177/1745691612460688. [DOI] [PubMed] [Google Scholar]
- Markman AB, Maddox WT, Worthy DA. Choking and excelling under pressure. Psychological Science. 2006;17:944–948. doi: 10.1111/j.1467-9280.2006.01809.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Gorlick MA, Lighthall NR. To brake or accelerate when the light turns yellow? Stress reduces older adults’ risk taking in a driving game. Psychological Science. 2009;20:174–176. doi: 10.1111/j.1467-9280.2009.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hairiri AR, Berman KF, Das S, et al. Neurophysiological correlates of age-related changes in working memory capacity. Neuroscience Letters. 2006;392:82–27. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Mell T, Heekeren HR, Marschner A, Wartenburger I, Villringer A, Reischies FM. Effects of aging on stimulus-reward association learning. Neuropsychologia. 2005;43:554–563. doi: 10.1016/j.neuropsychologia.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Mell T, Wartenburger I, Marschner A, Villringer A, Reischies FM, Heekeren HR. Altered function of ventral striatum during reward-based decision-making in old age. Frontiers in Human Neuroscience. 2009;3:1–10. doi: 10.3389/neuro.09.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Jackson M. Effect of stress on prefrontal cortex function. Neurotoxicity Research. 2004;6:73–78. doi: 10.1007/BF03033299. [DOI] [PubMed] [Google Scholar]
- Neth H, Sims C, Gray W. Melioration dominates maximization: Stable suboptimal performance despite global feedback. In: Sun R, Miyake N, editors. Proceedings of the 28th annual meeting of the cognitive science society. Hillsdale, NJ: Lawrence Erlbaum Associates; 2006. [Google Scholar]
- Otto AR, Gureckis TM, Markman AB, Love BC. Navigating through abstract decision spaces: Evaluating the role of state generalization in a dynamic decision-making task. Psychonomic Bulletin & Review. 2009;16:957–963. doi: 10.3758/PBR.16.5.957. [DOI] [PubMed] [Google Scholar]
- Otto AR, Taylor EG, Markman AB. There are at least two kinds of probability matching: Evidence from a secondary task. Cognition. 2011;118:274–279. doi: 10.1016/j.cognition.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in the human ventral striatum locked to errors of reward prediction. Nature Neuroscience. 2002;5:97–98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupathi M. Age differences in response to conformity pressure for emotional and nonemotional material. Psychology and Aging. 1999;14:170–174. doi: 10.1037//0882-7974.14.1.170. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behavior in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E, Hess TM, Vastfjall D, Auman C. Adult age differences in dual information processes. Perspective on Psychological Science. 2007;2:1–23. doi: 10.1111/j.1745-6916.2007.00025.x. [DOI] [PubMed] [Google Scholar]
- Raz N. The aging brain: Structural changes and their implications for cognitive aging. In: Dixon RA, Backman L, Nilsson L-G, editors. New frontiers in cognitive aging. New York: Oxford University Press; 2004. pp. 115–133. [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith E, Hartley A, Miller A, Marshuetz C, Keoppe R. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: Reorganizing discoveries about the aging mind. Current Opinion in Neurobiology. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychological Science. 2008;19:320–323. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Kuhnen CK, Yoo DJ, Knutson B. Variability in nucleus accumbens activity mediates age-related suboptimal financial risk-taking. The Journal of Neuroscience. 2010;30:1426–1434. doi: 10.1523/JNEUROSCI.4902-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Rakitin BC, Habeck C, Gazes Y, Steffener J, Kumar A, Reuben A. Task difficulty modulates young-old differences in network expression. Brain Research. 2012;30:130–145. doi: 10.1016/j.brainres.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyvers M, Lee MD, Wagenmakers EJ. A Bayesian analysis of human decision making on bandit problems. Journal of Mathematical Psychology. 2009;53:168–179. [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge: MIT Press; 1998. [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. American Journal of Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences, USA. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers EJ, Farrell S. AIC model selection using Akaike weights. Psychonomic Bulletin and Review. 2004;11:192–196. doi: 10.3758/bf03206482. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio: Harcourt Brace & Company; 1997. [Google Scholar]
- Worthy DA, Otto AR, Maddox WT. Working-Memory Load and Temporal Myopia in Dynamic Decision-Making. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2012 doi: 10.1037/a0028146. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthy DA, Gorlick MA, Pacheco JL, Schnyer DM, Maddox WT. With age comes wisdom: decision-making in older and younger adults. Psychological Science. 2011;22:1375–1380. doi: 10.1177/0956797611420301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthy DA, Maddox WT. Age-based differences in strategy-use in choice tasks. Frontiers in Neuroscience. 2012;5:1–10. doi: 10.3389/fnins.2011.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthy DA, Markman AB, Maddox WT. Choking and excelling under pressure in experienced classifiers. Attention, Perception and Psychophysics. 2009;71:924–935. doi: 10.3758/APP.71.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Busemeyer JR. Comparison of basic assumptions embedded in learning models for experience based decision-making. Psychonomic Bulletin & Review. 2005;12:387–402. doi: 10.3758/bf03193783. [DOI] [PubMed] [Google Scholar]