Abstract
Neurofibromatosis type 1 (NF1) is a genetic disease that results from either heritable or spontaneous autosomal dominant mutations in the NF1 gene. A second-hit mutation precedes the predominant NF1 neoplasms, including myeloid leukemia, optic glioma, and plexiform neurofibroma formation. Despite this requisite NF1 loss of heterozygosity in the tumor cell of origin, non-tumorigenic cells contribute to both generalized and specific disease manifestations. In mouse models of plexiform neurofibroma formation, Nf1 haploinsufficient mast cells promote inflammation accelerating tumor formation and growth. These recruited mast cells, hematopoietic effector cells long known to permeate neurofibroma tissue, mediate key mitogenic signals promoting vascular in-growth, collagen deposition, and tumor growth. Thus, the plexiform neurofibroma microenvironment involves a tumor/stromal interaction with the hematopoietic system which depends, at the molecular level, on a stem cell factor/c-kit-mediated signaling axis. These observations parallel findings in other NF1 disease manifestations and have clear relevance toward medical neurofibroma management.
Keywords: Neurofibromatosis, NF1, mast cell, SCF, c-kit
Overview: NF1, neurofibromas, and mast cells
NF1
Neurofibromatosis type 1 (von Recklinghausen's disease) is a genetic disorder caused by autosomal dominant mutations in the NF1 gene, which encodes Neurofibromin, a protein that accelerates the intrinsic hydrolysis of Ras from its GTP- to GDP-bound conformation. The disease afflicts approximately 1 in 3500 persons worldwide in a pandemic fashion, and it is the most common genetic disorder with a predisposition to cancer (1). NF1 manifests with both non-tumorigenic and tumorigenic maladies, including learning disabilities, skeletal dysplasia, non-healing fractures (pseudarthrosis), myeloid leukemia, and tumors such as optic glioma and the namesake neurofibroma. The disease's hallmark signs include hyper-pigmented areas of the skin (café au lait macules) and hamartomas on the iris (Lisch nodules), which serve as important diagnostic criteria and may be observed in infancy or childhood of afflicted individuals (2, 3). Because prominent NF1 symptoms arise from neural crest-derived tissue (e.g. glia, Schwann cells, melanocytes), some reports have characterized NF1 as a disorder of the neural crest. However, NF1 pathologies arise in organs derived from all embryonic germ layers, and we should consider NF1 not only a tumor predisposition syndrome but also a systemic developmental disorder (4).
Neurofibromas
NF1-like cutaneous tumor syndromes appeared in the literature during the 18th century (5-7), and in the 1880s Friedrich von Recklinghausen published seminal observations detailing cutaneous tumors comprised of both neuronal and fibroblastic tissue (8). NF1's pathognomonic neurofibromas are slowly progressing, heterogeneous solid tumors comprised of Schwann cells, fibroblasts, vascular cells, and invading hematopoietic cells, predominantly degranulating mast cells (9-14)(Figure 1). Cutaneous and subcutaneous neurofibromas derive from small peripheral nerve branches during adolescence or adulthood and are found in nearly all individuals with NF1 (15). By comparison, plexiform neurofibromas afflict half or fewer individuals with NF1 and develop from cranial and large-peripheral nerve sheaths, possibly initiating during gestation or early infancy from abnormally differentiated nonmyelinating Schwann cells or their less-differentiated precursors (16, 17).
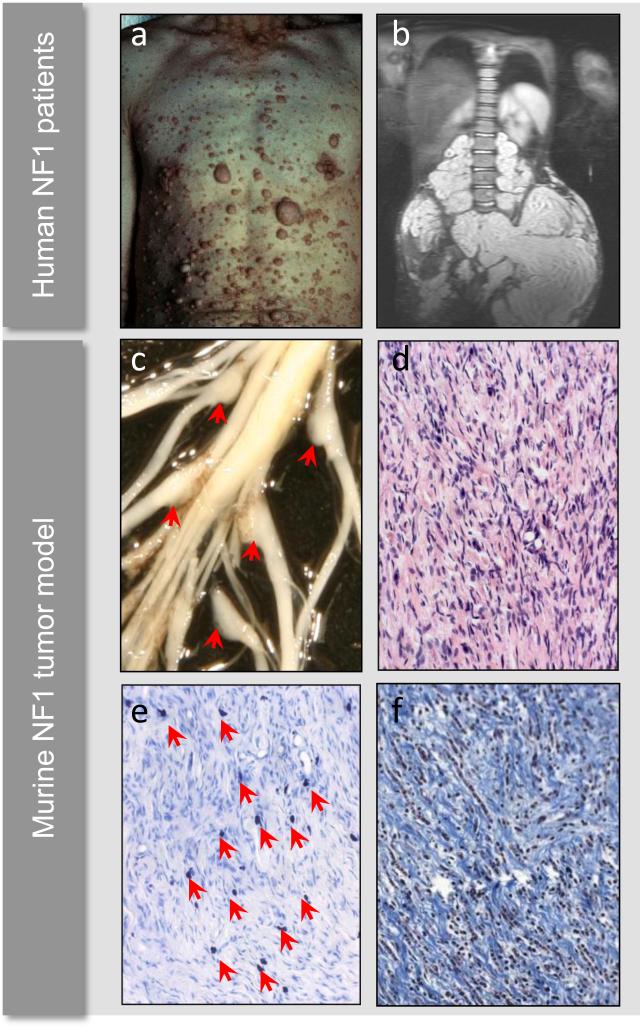
Figure 1. Cutaneous neurofibromas, plexiform neurofibromas, and histology.
Example of cutaneous neurofibromas covering the chest and abdomen of a patient with neurofibromatosis type 1 (a). MRI of a large plexiform neurofibroma compressing the spinal column and abdominal viscera (b). Murine models of NF1-associated plexiform neurofibroma development develop enlarged dorsal root ganglia (c, red arrows), which are histologically comprised of wavy Schwann cells, numerous fibroblasts, and a granular mast cell infiltrate (d). Alcian blue deeply stains mast cell granules (e, red arrows), and trichrome stain highlights the abundant collagenization typical of neurofibromas (e). Human photograph samples reproduced with permission from the Children's Tumor Foundation: www.ctf.org.
Plexiform neurofibromas are typically a lifelong source of disfigurement, disability, and mortality. In many cases, plexiform neurofibromas compress cranial nerves and/or peripheral nerve roots at the vertebral column and create an array of morbidity, including paresthesia, paralysis, drooling, sleeplessness, respiratory and gastrointestinal distress, blindness, and loss of bowel and bladder control (18, 19). A plexiform neurofibroma also has the potential to transform into a malignant peripheral nerve sheath tumor (MPNST), a highly morbid, metastatic cancer afflicting up to 10% of NF1 patients in their lifetime (20, 21).
Plexiform neurofibroma treatment consists primarily of symptom management and/or surgical resection. In many cases, the tumor's close involvement with vital nerve tissue, vasculature, or other viscera complicates surgery (18, 19, 22). Currently, the tumors have no medical therapy or cure, although several molecularly-targeted compounds are in preclinical or clinical testing (23-27). Problematically, nerve sheaths and heavily collagenized areas may resist drug bioavailability, complicating direct pharmacological inhibition of the tumorous mass. Therefore, therapeutic strategies targeting components of the tumor microenvironment, including vascular cells and invading mast cells, may prove viable alternatives (28). In this review, we discuss the therapy-relevant insights into the interactions between the tumor, the stroma, and the pathogenic mast cell.
Mast cells
Mast cells are granular hematopoietic cells that arise from common myeloid progenitors prior to granulocyte/monocyte lineage commitment (29). Mast cell precursors migrate from the bone marrow into the vasculature and enter dermal tissue where they mature into immune effector cells. Mast cells fight pathogens, protect against venoms and toxins, and may perform other immunomodulatory functions, both pro- and anti-inflammatory (30-33). While mast cells are predominantly known as the mediators of allergy and allergic asthma via IgE/FcεR pathways, they additionally depend on stem cell factor (SCF) signaling at the c-kit receptor tyrosine kinase for their generation and, in some contexts, pathophysiological activation (34-37). Indeed, mice naturally mutated at the c-kit receptor tyrosine kinase (W, or “white spotting locus” mutants) exhibit profoundly reduced numbers of tissue-resident mast cells (35).
The pro-inflammatory activities of recruited mast cells and other immune effector cells have been shown to sustain tumor microenvironments in various disease models (reviewed in (38-40)). In this inflammatory microenvironment hypothesis, tumorigenic cells recruit and co-opt the functions of non-tumorigenic hematopoietic cells via unchecked mitogenic and chemotactic signals. These recruited cells, in turn, coordinate vascular in-growth, collagen deposition, and the pathological inflammation promoting extracellular matrix remodeling, tumor expansion, invasion, and metastasis. Specifically, mast cells can synthesize and secrete matrix metalloproteinases (MMPs), various cytokines (e.g. IL-6 and TNF-α), and multiple mitogens (e.g. NGF, VEGF, and PDGF) (32, 33) with putative roles in tumor initiation, maintenance, and growth.
Mast cells have been associated with NF1 since 1911, when H. Greggio first noted les cellules granuleuses in neurofibroma tissue (14). Decades later, several investigators confirmed their presence using traditional histology and electron microscopy (9-13)). By the 1980s, mast cells were widely-recognized inflammatory effectors and hallmark histological features (albeit of unknown significance) of the neurofibroma. Vincent Riccardi hypothesized that mast cells may critically contribute to neurofibroma formation, proposing that mast cell degranulation explained his clinical observations of coincident pruritus and cutaneous neurofibroma formation (41). A small human study with a mast cell granule stabilizer (ketotifen) reduced pruritus and/or slowed neurofibroma growth (42), but a subsequent multiphase trial confirmed only anti-pruritic and analgesic effects, not neurofibroma reduction (43). These inquiries provided important evidence of aberrant mast cell degranulatory activity in neurofibroma tissue yet suggested that local inhibition of degranulation alone does not change overall disease course. As discussed in this review, recent biochemical, transplantation, and pharmacological studies have implicated a preponderant role for SCF-mediated mast cell gain-in-functions in orchestrating the neurofibroma microenvironment. This SCF/c-kit coordination of mast cell inflammation and tumor growth may inform a novel approach to NF1 therapeutics.
NF1 genetics
NF1 mutations
Discovery of the NF1 gene and its cloning have been fundamental to human diagnoses, to the evolving understanding of NF1 pathogenic mechanisms, and to the ongoing development of novel therapeutics. Of note, NF1's genetic identification was pre-requisite to the generation of accurate NF1 disease models in mice, biological tools which have greatly accelerated research into experimental therapeutics. A century after von Recklinghausen's seminal case reports, genetic linkage studies in NF1-afflicted families identified the pericentromeric region of chromosome 17 as the genomic region harboring the gene responsible for the disease (44, 45). Further studies in patients with translocations of chromosome 17 (46-49) facilitated the identification and full-length sequencing of the NF1 gene (50), which spans 350 kilobases of human chromosome 17 (17q11.2) and encodes 59 exons producing a 2818 amino acid protein (46, 51-53). Of note, human Neurofibromin and its mouse homolog share 98% identity at the protein level (54).
Approximately half of NF1 mutations in humans arise spontaneously (55), with the majority of mutations leading to premature truncation of the protein neurofibromin (56, 57). When NF1 mutations occur post-meiotically, individuals may exhibit segmental NF1 with manifestations confined regionally or to a subset of normally affected cell types (e.g. only pigmentation defects) (58). Different NF1 frameshift and point mutations do not necessarily correlate with phenotypic severity, although some studies have shown that microdeletions encompassing the entire NF1 locus (which account for less than 10% of mutations) associate with earlier onset and more profound disease manifestations (59, 60). Phenotypic variation tends to be high even within families, and pedigree analyses indicate that while NF1 mutations are fully penetrant, variation in genes independent of the NF1 locus critically modulates time-to-onset and course of the disease (61, 62). Parallel to the human data, different Nf1-mutant mouse strains exhibit both varied expression levels of Nf1 and variable susceptibility to different NF1-like disease manifestations (63). Overall, with the exception of the documented severity associated with NF1 locus-encompassing microdeletions and a uniquely mild phenotype associated with a 3-base pair deletion in exon 17 (64), particular genetic mutations or genomic variations which may correlate to specific disease outcomes are largely unknown.
NF1 encodes neurofibromin, a protein which functions, at least in part, as a p21ras (Ras) guanosine tri-phosphotase (GTP) activating protein (GAP) (65-69). Neurofibromin and other Ras-GAPs logarithmically accelerate the intrinsic hydrolysis of Ras-GTP to its inactive guanosine di-phosphate- (GDP)-bound conformation (70). In response to multiple mitogenic stimuli, active Ras-GTP orchestrates diverse protein signaling networks, including mitogen activated protein kinase- (MAPK)- and Akt-directed pathways (71-75). Hence, by accelerating the conversion of Ras-GTP to Ras-GDP, Neurofibromin negatively regulates Ras-dependent signaling cascades and, generally, serves to downregulate mitogenic events across diverse protein networks. In cases of NF1 heterozygosity or nullizygosity, as observed in somatic cells and in tumor cells of individuals with NF1, respectively, downstream Ras-mediated phosphorylation and transcriptional events can increase in duration and total output. This global upregulation of Ras-dependent activity in NF1/Nf1-disrupted tissue typically leads to cellular gain-in-functions, including enhanced proliferation, migration, and survival in multiple cell types (reviewed in (76-80). Of note, the specific Ras effectors potentiated by loss of NF1 may vary by cell and receptor type, and biochemical consequences in one cell-receptor system may or may not be observed in another.
NF1 gene dosage
Although NF1 is classified as a classical Knudson tumor suppressor gene, multiple studies have shown that NF1 heterozygosity critically modulates cell fate and function by altering Ras-dependent biochemical pathways in distinct cell types (reviewed in (79)). Moreover, physiological Ras activity regulates embryogenesis, early development, and normal tissue maintenance. Therefore, neurofibromin may be viewed not only as a tumor suppressor but also as a regulator of histiogenesis, cellular maintenance, and repair (4). Accordingly, NF1 is a disorder of both tumor predisposition and of developmental dysplasia.
While somatic cells in an individual with NF1 are heterozygous for NF1, loss of heterozygosity (LOH) in different cell types typically precedes hallmark hyperplastic, dysplastic, and neoplastic disease manifestations. LOH has been shown in human tissue samples and confirmed in NF1 mouse models of certain NF1 pathologies via multiple molecular techniques, coinciding with NF1's designation as a tumor suppressor gene. As examples, LOH in Schwann cells or their precursors permits neurofibroma formation (17, 81-83), LOH in skin-derived glial precursors precedes cutaneous neurofibroma formation (15), LOH in chromaffin cells initiates pheochromocytoma (84), LOH in melanocytes produces hyperpigmentation (e.g café-au-lait macules) (58, 85), LOH in myeloid progenitor cells induces myelomonocytic leukemia (86), and LOH in glia permits astrocytoma formation (87). Recent studies have also shown LOH in tissues extracted from tibial pseudarthrosis (88), although involved cell types and their exact genetic requirements are, as yet, not clearly documented (89, 90). (Table 1).
Table 1.
The cell of origin and known heterotypic interactions in multiple NF1 symptoms
| Neoplasia | Cell of origin | Other cells |
|---|---|---|
| Plexiform neurofibromas | Nf1-/- Schwann cells or their precursors | Nf1+/- mast cells |
| CNS gliomas | Nf1-/- astrocytes | Nf1+/- microglia |
| Dermal neurofibromas | Nf1-/- skin-derived glial precursors | Nf1+/- background/Uncleara |
| Pheochromocytomas | Nf1-/- chromaffin cells | Unclear |
| Early CNS gliomas | Nf1-/- astrocytes | Noneb |
| Early glial tumors | Nf1-/- glia | None |
| Myeloid leukemia | Nf1-/- myeloid cells | None |
| Other symptoms | ||
| Café-au-lait macules | Nf1-/- melanocytes | Unclear |
| Pseudoarthroses | Nf1-/- or Nf1+/- connective tissue | Unclear |
| Osteoporosis |
Nf1+/- osteoblasts Nf1+/- osteoclasts |
Unknownc |
| Cognitive deficits | Nf1+/- neurons | Unknown |
| Vascular disease |
Nf1+/- endothelia Nf1+/- vascular smooth muscle |
Unknown |
“Unclear” indicates that the data were derived from mice and/or human samples with a heterozygous Nf1/NF1 background but that a firm conclusion has not been made as to whether the condition requires this background.
“None” indicates that these symptoms can emerge despite wild-type supporting cells.
“Unknown” indicates that while Nf1 haploinsufficiency appears to be sufficient for pathogenesis, the possibility of undetected cooperating events cannot be ruled out. These data principally derive from studies in both mice and humans.
While it is unknown why some tissues are more susceptible to NF1 LOH and subsequent disease manifestation, a general hypothesis suggests that normal regulation of Ras signaling in certain cell types, for unclear reasons, depends more heavily on neurofibromin activity and/or expression levels than on the activity of other Ras-GAPs. Similarly, identical cell types located within discrete regions of an organ may demonstrate an increased dependence on NF1 expression and its Ras-GAP activity. Recent studies have shown that astrocytes and neuroglial stem cells in the optic nerve and brain stem express more neurofibromin and more readily manifest phenotypic consequences in its absence when compared to astrocytes from the frontal cortex (91, 92). These observations help explain the NF1-associated predisposition of astrocytoma to arise as optic glioma and not within the forebrain. In other NF1 pathologies, the mechanisms behind regional and tissue type-specific reliance on functional Neurofibromin is largely unknown, although insights in this area could impel future discovery of spatially- and/or temporally-targeted molecular therapies.
Individuals with NF1 also have an increased prevalence of multiple generalized manifestations which do not appear to require cell-specific biallelic inactivation of NF1, implicating a systemic gene dosage effect. These pathologies include skeletal and mesenchymal dysplasia (e.g. short stature, osteoporosis, and soft tissue malformation), disorders of neurocognitive development (e.g. retardation, spatial/visual coordination, and autism), and vascular pathologies (e.g. fistulae, infarcts, and aneurysms). Corresponding to these findings, biochemical and animal studies have shown various abnormalities of function in Nf1/NF1 heterozygous osteoblasts and osteoclasts (93-95), GABA neurons (96), endothelial cells (97, 98), and smooth muscle cells (99). These studies and others have additionally demonstrated increased risk for NF1-associated bone, neuronal, and vascular disease in Nf1 heterozygous mice. Hence, NF1 heterozygosity alone alters Ras-dependent pathways to a degree sufficient for the pathological alteration of normal developmental and homeostatic processes in multiple organ systems.
While NF1 heterozygosity may predispose NF1 patients to generalized deficiencies, NF1 heterozygous cells appear to also critically modulate neurofibroma growth and maintenance. In mice, Nf1 haploinsufficient mast cells and fibroblasts, major constituents of the heterogeneous plexiform neurofibroma, demonstrate multiple gain-in-function phenotypes that include enhanced proliferation, survival, migration, and cytokine production in response to specific stimuli (100, 101). These data parallel findings in Nf1 haploinsufficient microglia (102), which critically modulate the inflammatory microenvironment of NF1-associated optic glioma (103-105). In some mouse models of plexiform neurofibroma and optic glioma formation, tumorigenesis requires Nf1 haploinsufficiency in non-tumorigenic cells. Specifically, hematopoietic stem cell transplantation studies in the Nf1flox/flox; Krox20Cre and Nf1flox/flox; P0aCre models (discussed in detail below) have shown that neurofibroma genesis requires Nf1 haploinsufficiency and c-kit-mediated signaling in the hematopoietic compartment (24).
Thus, heterotypic interactions between the tumor, the hematopoietic system, and other stromal components critically promote and sustain the neurofibroma microenvironment. Moreover, Nf1/NF1 gene dosage (normal, heterozygous, and nullizygous) in discrete cell lineages differentially modulates cell fate, function, and, in some mouse models, disease outcome. These mouse models may illuminate the pathogenic mechanisms underpinning human genetic disease. While several NF1-associated diseases have been successfully modeled in mice, this review predominantly focuses on plexiform neurofibroma formation while making parallel observations in models of optic glioma formation. From the ongoing insights derived from human data and these mouse models, investigators can seek out novel molecular therapeutics targeting specific and essential interactions permitting disease pathogenesis.
NF1 murine tumor models
Nf1 traditional knockout
Nf1 knockout mice harbor a disruptive neomycin cassette in Nf1 exon 31 (Nf1+/n31) (106, 107), an exon site homologous to a “hotspot” of human NF1 mutations (108). The encoded neo cassette induces protein instability and degradation, leading to an approximate 50% reduction in total neurofibromin protein level. Nf1+/n31 (i.e. Nf1+/-) mice have a shortened lifespan and occasionally develop pheochromocytoma and leukemia, diseases found in increased incidence in humans with NF1. Other investigations have shown these mice to have neuronal deficiencies (96), increased risk for vascular pathology (109), and increased susceptibility to osteoporosis (95). However, Nf1+/- mice do not develop the pathognomic neurofibroma or hallmark café-au-lait macules and, by themselves, are not used as models of NF1-associated tumor formation.
The failure of Nf1+/- mice to develop neurofibromas – which individuals with NF1 near-universally develop – was initially puzzling, as humans, like the heterozygous mice, are born essentially NF1+/-. This phenotypic disparity may be ascribed to inherent differences between humans and mice: mice have shorter lifespans, different exposures and responses to toxins and carcinogens, and, perhaps, different susceptibilities to mutations in the normal Nf1 allele (106). Therefore, the chance of an Nf1 second-hit and concomitant neoplasia may be lower for the Nf1+/- mouse compared to a human born heterozygous at the NF1 locus.
Despite the absence of neurofibromas in the Nf1+/- mice, extracted leukemic myelocytes and pheochromocytoma tissue exhibit Nf1 LOH, providing strong evidence that tumorigenesis requires a second-hit in these tissues and supporting the classification of Nf1 as a tumor suppressor gene (106). Hence, many or all cell types and tissues with forced biallelic Nf1 inactivation could demonstrate aberrant and/or neoplastic phenotypes. However, mice born Nf1-/- die at embryonic day 13.5 secondary to defects in developing structures of the heart (107). In parallel to these findings, humans are never born nullizygous for NF1, presumably dying in utero. Due to the developmental requirement for at least one allele of NF1/Nf1, the investigation of biallelic Nf1 inactivation in adult mice must be performed using chimeric or conditional knockout models.
Indeed, chimeric Nf1-/- mice (mice with mixed Nf1+/+ and Nf1-/- somatic cells) provided direct evidence that neurofibroma genesis requires biallelic inactivation of Nf1. To create this model, investigators injected Nf1-/- embryonic stem cells into wild-type blastocysts during embryogenesis (81). Moderately chimeric animals developed several hallmark symptoms of NF1, including neuromotor defects, myelodysplasia, and neurofibromas in the tongue, limb, and along the dorsal root ganglia. By contrast, high Nf1-/- chimerism was lethal while low chimerism produced no immediately observable phenotype. In this study, Nf1-/- embryonic stem cells also carried a β-galactosidase transgene, and tumor histology revealed widespread β-gal expression. Conclusively, then, the neurofibromas arose specifically from the cell lineages developmentally derived from Nf1-/- embryonic stem cells.
Observations from this chimeric model, vis-à-vis findings from the Nf1 heterozygous mouse, validate biallelic Nf1 inactivation as a condition engendering and, most likely, required for tumorigenesis. Despite these critical insights, tumor formation in the chimera depends upon an undefined quantity of Nf1-/- cells (of all lineages) admixed with Nf1+/+ cells (also of all lineages). These genetic doses do not mimic human NF1 genetics, where, presumably, only the tumorigenic cells are essentially NF1-/- and the surrounding fibroblasts, pericytes, endothelial cells, and hematopoietic cells – i.e. the microenvironment – are essentially NF1+/-. While observations from the Nf1-/- chimera have accelerated the mechanistic understanding of neurofibroma pathogenesis, they do not allow delineation of the specific genetic conditions and heterotypic interactions underpinning tumor formation.
Nf1 conditional knockout
Cre-lox site-specific recombination technology permits tissue- and condition-specific gene disruption, allowing researchers to circumvent the embryonic lethality of traditional knockout constructs and to investigate protein function in specific cell lineages. To these ends, Zhu et al engineered a conditional Nf1 knockout construct targeted for disruption in a subset of Schwann cells (76, 82, 110). First, the investigators generated a mouse harboring flanking loxP (flox) sites at Nf1's exon 31 and 32 (Nf1flox/flox). LoxP is a 34 base pair recognition sequence for the bacteriophage-derived protein Cre recombinase, which mediates excision and recombination at these sites (111). The investigators then crossed this Nf1flox/flox mouse with a mouse carrying the Krox20Cre construct, which places Cre recombinase downstream of the promoter element for Krox20. Krox20 is an endogenous mammalian gene directing peripheral nerve myelination (112), and it robustly expresses in about 10% of Schwann cells and their precursors (82). In the Nf1flox/flox; Krox20Cre mouse, then, a subset of Schwann cells harbor biallelic Nf1 disruption while the bulk of somatic cells remain functionally wild-type.
However, Nf1flox/flox; Krox20Cre mice do not develop neurofibromas. Therefore, biallelic Nf1 inactivation in only a subset of Schwann cells and/or Schwann cell precursors is not sufficient to engender tumorigenesis. By contrast, plexiform neurofibromas readily and reliably form in Nf1flox; Krox20Cre mice additionally carrying the Nf1 knockout allele (Nf1flox/-; Krox20Cre). In this animal, a subset of Schwann cells are Nf1-deficient while other somatic cells are phenotypically Nf1+/- (i.e. Nf1flox/- but without Cre-mediated recombination). At about one year of age, the Nf1flox/-; Krox20Cre mouse develops dorsal root ganglia tumors grossly and histologically resembling plexiform neurofibromas. Histological examination of these tumors reveals wavy, dysplastic Schwann cells, abundant fibroblasts, collagenization, and an infiltration of degranulating mast cells. As observed in the Nf1flox/-; Krox20Cre model, then, plexiform neurofibroma genesis requires biallelic Nf1 inactivation in Schwann cells and heterotypic interactions with an Nf1 haploinsufficient stroma, which includes the fibroblasts, vascular cells, and mast cells long known to comprise the neurofibroma microenvironment.
Of note, widespread Nf1 deletion in glial precursor cells during embryognesis (~E12.5), as driven by the Nf1flox/flox; DhhCre model, permits plexiform neurofibroma genesis despite wild-type levels of neurofibromin in the surrounding cells of the microenvironment (17). These Nf1flox/flox; DhhCre derived plexiform neurofibromas exhibit dysplastic Schwann cell-like cells, aberrant collagenization, and an abundant infiltration of mast cells, just as the neurofibromas observed in the Nf1flox/-; Krox20Cre model. Thus, early and widespread biallelic inactivation of Nf1 circumvents the requirement for an Nf1 haploinsufficient microenvironment in plexiform neurofibroma formation (17). This model gives important insights into potential tumor cells of origin and provides a model for sporadic neurofibroma formation as observed in individuals without genetic neurofibromatosis type 1.
Neurofibroma models as parallels to models of astrocytoma formation
A mouse model of NF1-associated optic nerve glioma formation demonstrates a similar requirement for Nf1 haploinsufficiency in the tumor microenvironment (113). To generate this model, the Nf1flox/flox mice were intercrossed with a transgenic mouse carrying Cre recombinase downstream of the promoter element for glial fibrillary acidic protein (GFAP) gene, which drives Cre expression in astrocytes. While both Nf1+/- and Nf1flox/flox; GFAPCre mice demonstrate increased numbers of astrocytes (102, 114), the Nf1flox/flox; GFAPCre mice do not develop optic nerve gliomas. These findings directly parallel the Nf1flox/flox; Krox20Cre mice, which never develop plexiform neurofibromas (113, 114). As another direct parallel to the Krox20Cre tumor model, additional haploinsufficiency in the cellular background permits optic glioma formtion. At about one year of age, these Nf1flox/-; GFAPCre mice reliably demonstrate tumors of the optic chiasm histologically consistent with low-grade gliomas infiltrated by inflammatory microglia (113).
In another intriguing parallel, biallelic inactivation in neuroglial progenitor cells induces optic glioma formation independent of the Nf1 genetic dose in surrounding cells (87), just as in the Nf1flox/flox; DhhCre model of plexiform neurofibroma genesis. These mutant mice carry a transgenic construct expressing Cre recombinase under control of the human GFAP promoter (hGFAPCre), which has been shown to express in mature astrocytes as well as in the more primitive radial glial cell population. In this model, Nf1flox/-; hGFAPCre and Nf1flox/flox; hGFAPCre mice manifest similar disease phenotypes compared to their hGFAPCre-negative littermates, including increased numbers of astrocytes, increased proliferation of glial progenitor cells during brain development, hyperplasia of the optic nerve, and shortened lifespans (87).
Thus, multiple mouse models in two different NF1-associated pathologies demonstrate different Nf1 dosage requirements for tumorigenesis, seemingly dependent upon the cell-specific developmental timing of the gene's deletion. As a general observation, widespread biallelic inactivation of Nf1 in precursors of the putative tumor cell appears to bypass the requirement for haploinsufficiency of cells in the tumor microenvironment. Mechanistically, it is unclear if Nf1-inactivated precursor cells harbor an intrinsic proclivity toward neoplasm, if they respond more aggressively to recruited nontumorigenic cells, or if they are simply better at recruiting these cells. Above all, given the consistent observation of invading mast cells and microglia, these models all suggest pathogenic roles for hematopoietic effectors co-opted by tumorigenic cells.
Schwann cells, Nf1 haploinsufficient mast cells, and the tumor microenvironment hypothesis
The Nf1flox/-; Krox20Cre model, among others, approximates the genetic conditions expected in humans with NF1, wherein all body cells are NF1 heterozygous and only a small fraction of Schwann cells/Schwann cell precursors experience biallelic NF1 inactivation. Thus, heterozygous non-tumorigenic cells, including vascular cells, fibroblasts, and mast cells, likely contribute to tumor pathogenesis and maintenance in the human patient. Here, we focus on cell culture- and transplantation-based studies demonstrating phenotypic consequence of biochemical deregulation in non-tumorigenic cells as well as the critical importance of heterotypic interactions between the tumorigenic Nf1-/- Schwann cell, the recruited Nf1+/- mast cell, and other mitogenic events within the tumor stroma.
Schwann cells
That the Schwann cell is the tumor cell of origin in NF1-associated peripheral nerve tumors had long been a matter of controversy, with von Recklinghausen himself believing the tumors to be of a primary fibroblastic or connective tissue origin (see, for example, (115) for an early and detailed discussion of this debate). Today, preponderant molecular evidence implicates a Schwann cell or Schwann cell precursor cell as the tumor cell of origin. As foundational evidence, investigators had detected NF1 LOH in neurofibroma-derived Schwann cells but not fibroblasts (116-118), and Schwann cells cultured from neurofibromas demonstrate angiogenic and invasive phenotypes in tissue culture (119). Importantly, all characterized mouse models of plexiform neurofibroma formation depend upon biallelic Nf1 inactivation using Cre drivers primarily restricted to neural crest- and Schwann cell-directed cell lineages (15-17, 24, 82, 83).
However, questions still exist as to the precise stage of Schwann cell differentiation giving rise to the tumor (reviewed in ((120)). Plexiform neurofibromas probably develop congenitally, and, thus, neural crest stem cells or other progenitor cells could generate tumors during embryogenesis, although investigators largely have agreed that Nf1-deficient fetal neural crest stem cells cannot directly form a plexiform neurofibroma (83, 120). Moreover, aggregated observations from studies of the different mouse tumor models (Nf1flox/-; Wnt1Cre; Nf1flox/-; P0aCre, Nf1flox/-; Krox20Cre, and Nf1flox/-; 3.9PeriostinCre) implicate differentiated glia, including mature nonmyelinating Schwann cells (i.e. Remak bundles), as the likely tumor cells of origin (16, 24, 82, 83). However, given the observation from the Nf1flox/flox; DhhCre model, which expresses Cre at the late boundary cap cell stage, as well as the fact that P0aCre and Krox20Cre both express in Schwann cell precursors, tumorigenic events in these precursor cells or their progeny cannot be ruled out (17). Finally, Le and colleagues have created dermal and plexiform neurofibromas by autologous transplantation of neural crest-like stem cells derived from the skin (“skin-derived precursors” or “SKPs”) of adult Nf1flox/- mice carrying a tamoxifen-inducible Cre transgene (15). This study has shown that adult-type neural precursor cells can form tumors while demonstrating the importance of localized microenvironment cues, whereby a SKP can develop into a dermal neurofibroma or a plexiform neurofibroma depending on its engrafted location. Intriguingly, SKP-derived tumor formation is greatly accelerated in pregnant females versus males and non-pregnant females, suggesting that hormonal cues, as well as genetic and spatial, dictate tumor fate.
Based on these studies, firm conclusions cannot yet be made as to the exact tumor cell of origin, but these models and the multiple molecular studies in human tissues almost certainly rule out a fibroblast-derived neoplasia, as some once postulated. Likely, then, biallelic Nf1 inactivation at multiple stages of Schwann cell differentiation can initiate tumorigenesis with potentially disparate requirements for cooperative cues, whether genetic or environmental.
As a Schwann cell or Schwann cell-like cell is neurofibroma's tumorigenic cell, Nf1-/- cultured primary Schwann cells have provided critical insights into putative mechanisms of neurofibroma pathophysiology. An initial study demonstrated that Nf1-/- Schwann cells induce endothelial and fibroblastoid cell proliferation by secreting fibroblast growth factor (FGF-2), platelet-derived growth factor (PDGF), and midkine (MK) (121). These factors could potentially drive vascularization and collagenization of the growing tumor, thus suggesting a mechanism by which Nf1-/- Schwann cells orchestrate the nascent tumor microenvironment. A subsequent study found that Schwann cells derived from Nf1-/- mouse embryos proliferate more quickly than WT cells, display an irregular morphology in cell culture, and secrete approximately six times as much soluble SCF as Nf1+/- and WT Schwann cells (122). Conditioned media from these Nf1-/- Schwann cells promotes the chemotaxis of primary cultured mast cells, doing so at twice the rate for Nf1+/- mast cells versus WT mast cells. Recombinant SCF reproduces this chemotactic effect, and genetic disruption of the mast cell's c-kit receptor (via Nf1+/-; W mutations) or addition of c-kit receptor blocking antibodies abolishes Schwann cell-mediated chemotaxis. These data implicate a direct link between the Nf1-/- Schwann cell and the mast cell, one mediated mechanistically through the SCF/c-kit signaling axis. As further evidence of this signaling pathway's importance in humans with NF1, previous and subsequent studies have shown that neurofibroma tissues express SCF mRNA, that human neurofibroma-derived Schwann cells secrete high levels of SCF, and that serum from individuals with NF1 contains increased levels of SCF compared as compared to controls (122-125). When considered with the well-established histological phenotype of mast cell invasion and degranulation in tumor tissue (as well as the mast cell's dependence on c-kit for its cytopoiesis), these studies roundly implicate an SCF/c-kit signaling axis as a potential pathogenic mechanism.
Nf1 haploinsufficient mast cell phenotype
SCF regulates mast cell cytopoiesis, proliferation, survival, and cytokine synthesis, functions which Nf1 haploinsufficiency potentiates. In fact, the study of SCF-stimulated Nf1+/- mast cells provided foundational evidence that haploinsufficiency of the Nf1 “tumor suppressor” could modulate multi-lineage cell fate and function, in tissue culture and in vivo (100). In these experiments, Nf1+/- mice were intercrossed with mice naturally mutated at the c-kit receptor tyrosine kinase (W41). This naturally-occurring mutation at the W – or “white spotting” – locus reduces c-kit receptor tyrosine kinase activity by about 85%, leading to albinism and tissues largely absent of mast cells (34, 126-129). Ingram and colleagues found that Nf1+/-; W41/W41 intercrossed mice exhibit increased numbers of dermal and peritoneal mast cells compared to their W41/W41 counterparts. Likewise, Nf1+/- mice demonstrate increased numbers of dermal and peritoneal mast cells. Mast cells cultured from Nf1+/- and Nf1+/-; W41/W41 bone marrow progenitor cells display several SCF-mediated gain-in-functions, including proliferation at about twice the rate of WT and W41/W41 mast cells, respectively. Nf1+/- and Nf1+/-; W41/W41 bone marrow cells also form increased numbers of mast cell colonies ex vivo (100). In vivo, Nf1+/- mast cells and mast cell precursors demonstrate an exquisite hypersensitivity to low doses of soluble SCF, as measured by mast cell proliferation and recruitment using SCF-loaded micro-osmotic pumps implanted in dermal tissue (130). Moreover, Nf1+/-; W41/W41 animals have a black mottling of the W41/W41 mouse's normally predominant albino coat, showing that Nf1 haploinsufficiency restores c-kit-impaired melanocyte function (100), a neural crest-derived lineage that expresses neurofibromin and has relevance to human NF1 (e.g. café-au-lait macules)(Figure 2)(131). Biochemical inquiries further demonstrated increased latency and potency of GTP-bound Ras in SCF-stimulated Nf1+/- mast cells. Taken together, these data indicate that Nf1 haploinsufficiency restores c-kit-dependent kinase activity to a biologically relevant degree, alters mast cell cytopoiesis and functional potential, and modulates fate and function in multiple cell lineages in the live animal.
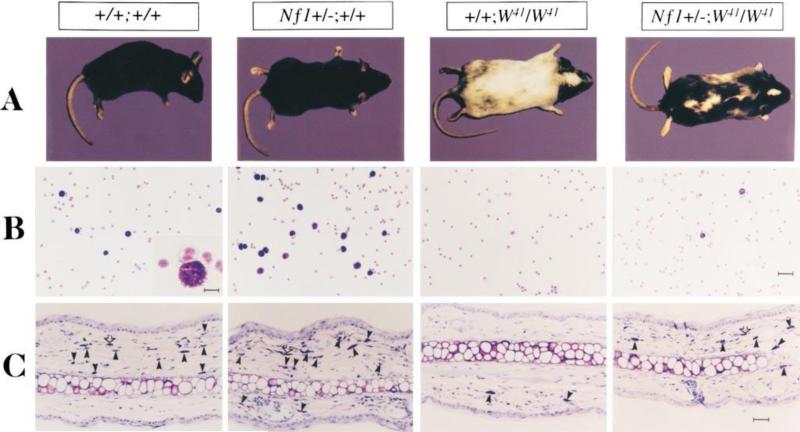
Figure 2. Effect of haploinsufficiency of Nf1 on coat color and total numbers of cutaneous and peritoneal mast cells.
(A) Coat color pattern of a representative mouse from each of the following genotypes: +/+;+/+, Nf1+/-;+/+, +/+;W41/W41, and Nf1+/-;W41/W41. Haploinsufficiency at Nf1partially corrects the coat color deficiency in mice homozygous for the W41 allele in a C57BL/6 genetic background. (B) Representative cytospins from peritoneal lavages stained for mast cells from individual mice of the four Nf1 and W genotypes. Peritoneal cells were stained with toluidine blue to quantify the total number of mast cells per peritoneal lavage. A higher magnification of a representative mast cell is shown in the inset of the wild-type mouse (original magnification: 3200). Bar (inset) 10 μm. Bar (far right) 30 μm. (C) Representative ear biopsies stained for cutaneous mast cells from individual mice of the four Nf1 and W genotypes. Specimens were stained with hematoxylin-eosin to assess routine histology, and with Giemsa to identify mast cells. Ear biopsies were stained with Fontana-Masson to differentiate melanin-containing cells from mast cells. Cutaneous mast cells (Giemsa-positive, Fontana-Masson–negative) were quantitated in a blinded fashion by counting the distal 5 mm of ears. Black arrows indicate Giemsa-positive mast cells, and open arrows indicate Fontana-Masson melanin–containing cells. Bar, 35 μm. © Ingram et al.,2000. Originally published in J. Exp.Med. 191: 181–188.
Nf1 haploinsufficient biochemistry in the SCF-stimulated mast cell
Subsequent studies have detailed the biochemical mechanisms modulating SCF-mediated gain-in-functions in the Nf1 haploinsufficient mast cell. Principally, these alterations arise from deregulated signaling events in Ras-dependent networks. In response to ligand binding at diverse cell surface receptors, Ras activates to its guanine triphosphate (GTP)-bound state and promotes phosphorylation in downstream protein networks, including those orchestrated by MAPKs and phosphoinositide-3-kinase (PI-3K)(72-75). Neurofibromin, which contains a highly-conserved GAP-related domain (GRD) with homology to the yeast gene products IRA1 and IRA2, logarithmically accelerates the intrinsic hydrolysis of active GTP-bound Ras to its GDP-bound state (47, 52, 65, 68, 76, 132). Generally, loss-of-function mutations in genes encoding Ras-GAPs promote cell growth, proliferation, migration, and survival (40). In myeloid progenitor cells, microglia, and mast cells, loss of one or both alleles of Nf1 leads to increased duration of Ras-GTP and the activity of specific effectors within Raf-Mek-Erk, PI-3K-Rac-Pak-P38, and PI-3K-Akt cascades (86, 100, 105, 122, 133-138).
Cell culture and in vivo studies of genetically disrupted mast cells indicate that the Raf-Mek-Erk pathway may primarily modulate SCF-mediated proliferation while the PI-3K-Rac2-Pak-p38 pathway controls F-actin dynamics and cellular motility.(130, 135, 136, 138-140). However, biochemical investigations have additionally shown that the PI-3K-dependent pathway reinforces the classical Raf-Mek-Erk cascade through the activity of the p21 activated kinases (Paks) (130, 136). Thus, PI-3K directly modulates SCF-mediated proliferation. In this schema, PI-3K-activated Rac2 induces Pak1 to phosphorylate Mek at serine 298 as well as Raf1 at serine 338, potentiating Raf1's phosphorylation of Mek at serine 217/222. These activities potentiate phosphorylation of the extracellular regulated kinases, Erk1 and Erk2. Erk1/2 phosphorylate cytoplasmic targets (e.g. p90rsk), translocate to the nucleus, and activate multiple mitogenic transcription factors (e.g. c-Fos, Elk1, C/EBP), although Erk-dependent transcriptional events and gene products are largely undocumented for the SCF-stimulated Nf1+/- mast cell. Ultimately, Erk activity appears to regulate SCF-mediated cell cycle progression, events potentiated in the Nf1+/- mast cell. Furthermore, these studies have suggested that SCF-mediated hyper-proliferation in the Nf1+/- mast cell directly relies on PI-3K-Rac2-Pak1's aberrant potentiation of Erk1/2.
In addition to promoting proliferation through reinforcement of Raf-Mek-Erk, SCF-dependent hyper-activation of PI-3K critically orchestrates Nf1+/- mast cell migration and survival (130, 136, 137). Genetic and chemical inhibitor- (PD98059, SB203580, LY294002)-based studies have described a PI-3K-dependent activation of Rac2 controlling SCF-mediated mast cell chemotaxis as well as Nf1-dependent gain-in-motility. Specifically, a Rac2-Pak1-p38 signaling pathway induces F-actin rearrangement and cell motility, although the precise downstream effectors remain unknown (136). These cytoskeletal observations directly correlate with a genetic study showing that Nf1+/- mast cells require PI-3K activity for Nf1-/- Schwann cell conditioned media-dependent degranulation (140). Moreover, SCF-induced PI-3K-Rac2 activity promotes Akt's modulation of Bcl-2 family proteins (e.g. BAD/Bcl-XL), increasing mast cell survival through suppression of pro-apoptotic pathways (139). Taken together, these biochemical data explain SCF-mediated gain-in-functions in the Nf1+/- mast cell, and they illuminate potential molecular therapeutic targets within Ras-dependent pathways. Figure 3 summarizes these pathways.
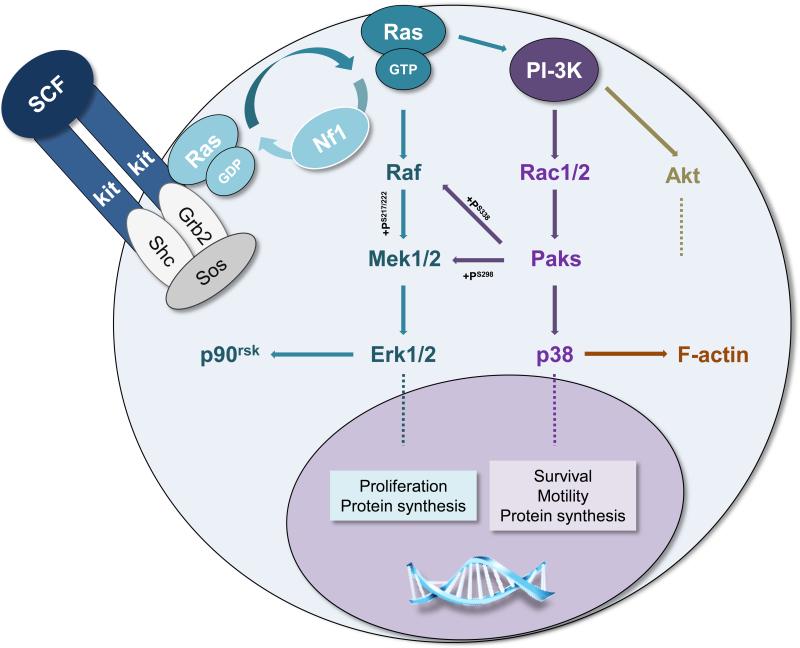
Figure 3. Hyperactive SCF/c-kit pathways in the Nf1+/- mast cell.
Kit-ligand (SCF) binding at the c-kit receptor tyrosine kinase induces receptor dimerization, activates Ras to its GTP-bound conformation, and induces Ras-Raf-Mek-Erk and PI-3K-Rac-Pak-p38 signaling pathways. While Mek-Erk signals may principally mediate mast cell proliferation, PI-3K mediates survival, motility and, through its Pak-dependent crosstalk with Raf-Mek, proliferation. Nf1 accelerates the intrinsic hydrolysis of Ras-GTP to inactive Ras-GDP and serves, at least in part, to negatively regulate Mek-Erk- and PI-3K-directed pathways. Although SCF-c-kit interactions initiate other molecular events (e.g. Akt-mTOR), this schematic highlights only those thus far shown to be hyperactivate in the Nf1+/- mast cell. Dashed lines indicate multiple downstream effectors not fully detailed.
The tumor microenvironment
Nf1-/- Schwann cells potently recruit normal and Nf1+/- mast cells via SCF-mediated activation of Ras pathways, but what role do these recruited mast cells play in the nascent and mature plexiform neurofibroma microenvironment? As a matter of definition, the plexiform neurofibroma microenvironment includes (but is perhaps not restricted to) tumorigenic Schwann cells and/or their precursors, recruited mast cells, other hematopoietic cells, pericytes, smooth muscle, and endothelial cells (i.e. vasculature), fibroblasts, and the extracellular matrix, including a large amount of collagen. Indeed, fibroblasts and fibroblast-secreted collagen are major constituents, and extracellular matrix material typically accounts for about half of dry neurofibroma weight (141). Given that SCF-stimulated mast cells can synthesize and secrete pleiotropic cytokines and growth factors, the mast cell could promote growth and activity in the tumor microenvironment through multiple hypothetical mechanisms, including nerve growth factor (NGF) stimulation of Schwann cells, vascular endothelial growth factor (VEGF) stimulation of vascular cells, and transforming growth factor beta (TGF-ß) stimulation of fibroblasts.
Fibroblasts
Accordingly, Yang and colleagues have experimentally demonstrated that SCF-stimulated Nf1+/- mast cells modulate multiple fibroblast functions through secreted TGF-ß (101). TGF-ß induces fibroblasts to migrate, proliferate, and synthesize collagen, well-documented and critical biological processes, especially during embryogenesis and inflammatory wound healing. SCF-stimulated mast cells not only secrete TGF-ß, but Nf1+/- mast cells generate 2.5 fold more TGF-ß compared to WT mast cells. In response to mast cell conditioned media, WT and Nf1+/- fibroblasts proliferate, migrate, produce collagen, and promote extracellular matrix remodeling, with Nf1+/- mast cell/Nf1+/- fibroblast interactions demonstrating the greatest biological activity. Yang et al's study directly demonstrated TGF-ß-dependence by disrupting the fibroblast gain-in-function phenotype through addition of TGF-ß blocking antibody to the Nf1+/- mast cell-conditioned media. Intriguingly, TGF-ß-stimulated fibroblasts derived from human neurofibroma samples demonstrated gain-in-functions similar to the murine Nf1+/- cultured fibroblasts, and re-introduction of the NF1 GAP-related domain restores normal activity. Mechanistically, TGF-ß induces hyperactivity of the non-receptor tyrosine kinase c-abl, and genetic or pharmacological suppression of c-abl corrects multiple fibroblast gain-in-function phenotypes. Correspondingly, imatinib mesylate (Gleevec©), an FDA-approved inhibitor of c-abl, suppresses collagen production and fibroblast migration/invasion in mice implanted subcutaneously with TGF-ß and Nf1+/- mast cell conditioned media. This study, in conjunction with previous studies of Schwann cells and mast cells, illuminates critical microenvironment interactions, whereby the Nf1-/- Schwann cell recruits the Nf1+/- mast cell which, in turn, promotes fibroblast proliferation and collagenization.
Vascular cells
Neurofibromas, like other human tumors, require the in-growth of new vessels for their expansion and metastasis (40, 142). An initial study found that Nf1-/- Schwann cells directly promote angiogenesis when engrafted on chorioallantoic membranes of post-fertilization chicken eggs, potentially through aberrant Ras-mediated expression of VEGF (143). An in vivo murine study corroborated these findings, as Nf1-/- Schwann cell-conditioned media implanted into mice induces angiogenesis, with Nf1+/- mice showing a greater angiogenic response than WT mice (97). Likewise, endothelial cells (ECs) cultured from Nf1+/- mice and humans with NF1 exhibit a heightened migratory and proliferative response to neurofibroma-derived and recombinant growth factors, including VEGF and basic fibroblast growth factor (bFGF). Both proliferation and migration in patient-derived and Nf1+/- ECs appear to require aberrant Mek-Erk activity, an interesting contrast to Nf1+/- mast cells, where Mek-Erk are largely dispensable for SCF-mediated migration. A concomitant study from the same laboratory found enhanced proliferation and migration in Nf1+/- vascular smooth muscle cells (VSMCs) in response to PDGF-BB (99). Intriguingly, Nf1+/- fibroblasts secrete five-fold higher concentrations of PDGF-BB than their WT counterparts, suggesting that the haploinsufficient fibroblast directly participates in promulgating the pathogenic microenvironment. As in ECs, this PDGF-BB-mediated potentiation of VSMC function requires hyperactivity in the Raf-Mek-Erk pathway. The investigators detected no Nf1 haploinsufficient enhancement of Akt activity in either ECs or VSMCs, a surprising finding given Ras's well-documented modulation of PI-3K in the mast cell and other cell types. Thus, Nf1 haploinsufficiency modifies biochemistry in a cell type-specific manner.
Other lineages
Macrophages and pericytes may also modulate plexiform neurofibroma genesis and maintenance, but their roles remain, as now, largely undocumented. Although mast cells comprise the majority of hematopoietic cells in the neurofibroma, macrophages account for about 10 to 15% of CD45+ cells, at least in murine models (24). And, in some cancers, tumor-associated macrophages (TAMs) mediate key angiogenic and mitogenic signals promoting tumor vascularization, remodeling, and growth (144). Therefore, macrophages emerge as a reasonable target for future investigations of pathogenic mechanisms in the neurofibroma microenvironment. Likewise, pericytes, which are less-differentiated cells supporting vascular and connective tissue growth, could modulate events in the microenvironment, including angiogenesis. Intriguingly, intracranial tumors in Nf1+/–; Trp53+/– mice secrete stromal-derived factor-1 (SDF1) which recruits endothelial precursor cells from the bone marrow to the tumor site (145). These recruited precursors can differentiate into either endothelial cells or pericytes, although this phenomenon has not been directly studied in plexiform neurofibroma formation.
In sum, complex heterotypic interactions underlie neurofibroma genesis and maintenance. As a global model, tumorigenic cells secrete SCF, recruiting inflammatory mast cells, which then promote fibroblast proliferation and collagenization. Tumorigenic cells and hyperactive fibroblasts can promote neo-angiogenesis, potentially through their stimulation of ECs and VSMCs via VEGF, bFGF, and PDGF-BB. Moreover, other cell types, including macrophages and pericytes, could modulate angiogenic and mitogenic events in the microenvironment, although their roles are relatively unknown. Figure 4 presents the microenvironment model, including both known and hypothetical interactions.
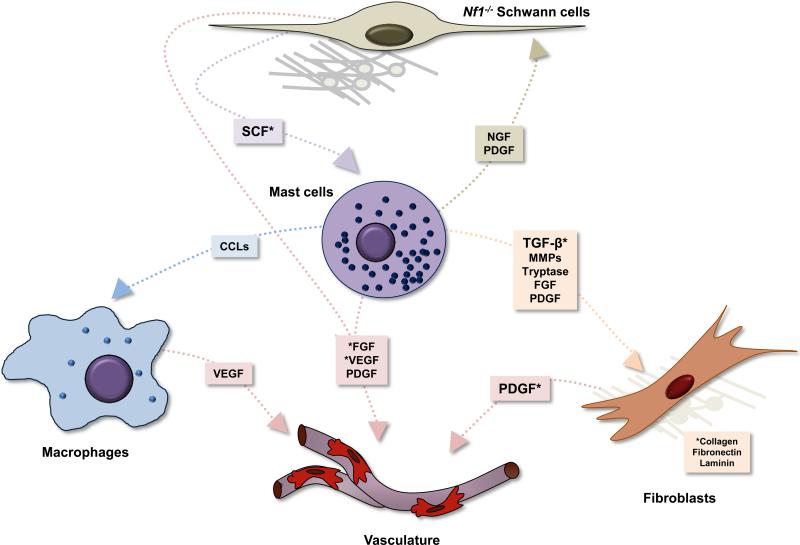
Figure 4. Hypothetical tumor/stromal/hematopoietic interactions in the neurofibroma microenvironment.
In this tumor microenvironment model, loss of heterozygosity in Schwann cells or their precursor leads to aberrant SCF production and secretion, the ligand for the c-kit receptor tyrosine kinase. SCF induces maturation, proliferation, and recruitment of mast cells from bone marrow progenitor cells. These SCF-activated mast cells generate and release multiple inflammatory cytokines and growth factors acting on macrophages, vasculature, fibroblasts, and the tumorigenic Schwann cells. TGF-β-stimulated fibroblasts, in turn, aberrantly proliferate and produce collagen, increasing tumor bulk and pressure. Schwann cells, fibroblasts, and macrophages may all directly contribute to vascularization through various growth factors. Asterisks denote heterotypic interactions with experimental validation.
Parallels to the optic glioma microenvironment
Mouse models of NF1-associated optic glioma formation have demonstrated the importance of heterotypic interactions between the tumorigenic astrocyte and gliomagen-elaborating microglia, hematopoietic effectors derived from monocyte precursor cells (reviewed in (146)). Neoplastic and pre-neoplastic astrocytes secrete multiple factors attracting and expanding microglia populations (146). Similar to the Nf1+/- mast cell, Nf1+/- microglia demonstrate multiple gain-in-functions, including enhanced macrophage-colony stimulated factor- (M-CSF)-mediated proliferation and production of hyaluronidase (105). This secreted hyaluronidase drives aberrant Nf1-/- astrocyte proliferation and correlates with glioma formation in vivo, both in mouse models and in humans with NF1. Mechanistically, Nf1+/- microglial gain-in-function appears to depend upon the aberrant activity of c-Jun-NH(2)-kinase (JNK) and not upon hyperactive Mek-Erk, p38, or PI-3K-Akt, reinforcing the notion that Nf1 haploinsufficiency modifies signaling in a cell type-specific manner (104). Nf1+/- microglia also express three-fold more CXCL12 than WT microglia, which, intriguingly, promotes astrocyte growth only in the context of Nf1 deficiency (CXCL12 induces apoptosis in normal astroycytes). This finding associates with aberrantly low cyclic AMP (cAMP) levels in the CXCL12-stimulated Nf1-deficient astrocyte (147), and forced suppression of cAMP can induce NF1-associated glioma formation in brain regions typically not susceptible to this tumor (148). Thus, optic glioma genesis demonstrates a direct parallel to neurofibroma genesis, whereby tumorigenic cells co-opt inflammatory cells which, in turn, enable tumor growth and maintenance through secreted mitogens.
The SCF/c-kit in plexiform neurofibroma formation and its therapeutic implications
Tumorigenesis requires an Nf1+/- hematopoietic system
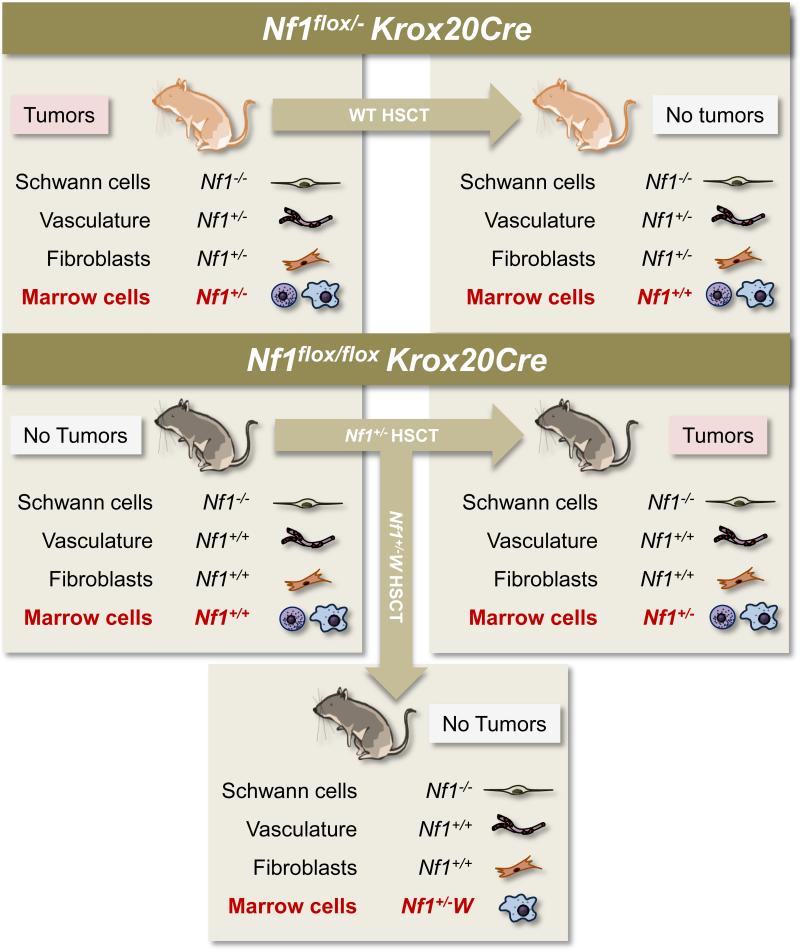
Given the requirement for an Nf1 haploinsufficient cellular background for plexiform neurofibroma formation in multiple mouse models combined with the various studies showing interactions between Nf1 disrupted Schwann cells, mast cells, fibroblasts, and endothelial cells, the question arises as to which haploinsufficient cell types specifically engender tumorigenesis. To address this question, Yang et al transplanted hematopoietic stem cells from Nf1+/- mice into lethally-irradiated Nf1flox/flox; Krox20Cre mice, a genetic status which, as discussed above, does not lead to tumor formation (24). This Nf1+/- hematopoietic stem cell transplant into Nf1flox/flox; Krox20Cre mice created animals with 5 to 10% of their Schwann cells and Schwann cell precursors biallelically inactivated for Nf1, their hematopoietic system haploinsufficient for Nf1, and all other cells phenotypically normal (i.e. Nf1flox/flox). Approximately six months after transplantation, the Nf1flox/flox; Krox20Cre mice reconstituted with an Nf1+/ hematopoietic system developed enlargements along their dorsal root ganglia histologically resembling plexiform neurofibromas. The tumor formation and increased mortality directly compared to the symptoms observed in the reliably tumorigenic Nf1flox/-; Krox20Cre mice. Figure 5 presents the schematics for hematopoietic stem cell transplantation into tumor model mice.
Figure 5. Transplant experiments in the Krox20Cre tumor model.
The left column shows the genetic status of Schwann cells, vascular cells, fibroblasts, and marrow cells in two different mice genotypes: the Nf1flox/-Krox20Cre tumor model and the Nf1flox/floxKrox20Cre model, which does not develop tumors. The arrows indicate the genotype of the hematopoietic stem cells transplanted into these two different genotypes of mice. “W” represents either W41 or Wv c-kit receptor tyrosine kinase deficient mutations. The right column and bottom box show the resultant genetic status of each cell lineage, post-transplant. As indicated, only those mice with Nf1-/- Schwann cells and c-kit-competent Nf1+/- bone marrow develop tumors. HSCT: hematopoietic stem cell transplant.
These transplant experiments indicate that Nf1 haploinsufficiency in the hematopoietic system permits tumorigenesis in Nf1 disrupted Schwann cells. However, these observations do not exclude the possibility that Nf1+/- fibroblasts, endothelial cells, and/or other stromal cells could permit tumorigenesis despite a genetically normal hematopoietic system. To test this hypothesis, Yang et al additionally transplanted wild-type hematopoietic stem cells into lethally-irradiated Nf1flox/-;Krox20Cre mice, creating mice with Nf1 disrupted Schwann cells, a wild-type hematopoietic system, and all other cells phenotypically Nf1+/-. These mice never developed tumors. Therefore, an Nf1+/- hematopoietic system is both required and sufficient for tumorigenesis in the Krox20Cre model.
Similar investigations have revealed that the P0aCre tumor model requires an Nf1+/- hematopoietic system and that an Nf1+/- hematopoietic system increases the penetrance and severity of tumors in the PeriostinCre model. Thus, in multiple murine plexiform neurofibroma models with clear relevance to the human genetic disease, pathogenesis hinges on the inflammatory cues granted by an Nf1 haploinsufficient hematopoietic system. We reiterate, however, that the early glial-driven DhhCre model does not require an Nf1 haploinsufficient hematopoietic system, and we emphasize that different mouse models may distinctly inform the varied constellation of NF1-associated pathologies observed in humans with or without heritable NF1.
Tumorigenesis requires high functioning c-kit in an Nf1+/- hematopoietic system
The Nf1+/- hematopoietic stem cell transplant experiments do not rule-out possible contributions from other Nf1+/- hematopoietic cells, such as macrophages and endothelial precursor cells. To test the hypothesis that tumor formation requires Nf1+/- mast cells, Yang and colleagues also transplanted Nf1+/-hematopoietic stem cellscarrying one of two naturally occurring c-kit receptor tyrosine kinase mutations, W41/W41 or Wv/Wv. These mutations reduce kinase activity 85% and 92-95%, respectively, leading to loss of coat color and failed mast cell cytopoiesis, as discussed above. In these transplant experiments, Nf1flox/flox; Krox20Cre mice reconstituted with an Nf1+/-; W41/W41 or Nf1+/-; Wv/Wv hematopoietic system protected against dorsal root ganglia hyperplasia and mast cell invasion, as compared with the Nf1flox/flox; Krox20Cre mice reconstituted with an Nf1+/--only hematopoietic system. Of note, because c-kit-mutated hematopoietic stem cells do not necessarily engraft as efficiently as wild-type hematopoietic stem cells, the authors used Southern blot analysis of myeloid colonies grown from bone marrow progenitor cells in Nf1+/-; W41/W41 or Nf1+/-; Wv/Wv recipients to assay engraftment efficiency. In the transplant recipients, approximately 95% of hematopoietic progenitors originated from the Nf1+/-; W donor stem cells, indicating successful engraftment. These data indicate that plexiform neurofibroma formation requires an Nf1 haploinsufficient and c-kit-competent hematopoietic system, and, in light of the other studies discussed above, roundly implicate the SCF/c-kit-dependent mast cell as a principal pathogenic effector underpinning neurofibroma genesis.
Pharmacological modulation of the SCF/c-kit axis
Imatinib mesylate (Gleevec©) inhibits multiple receptor and non-receptor tyrosine kinases, including c-kit, PDGF-β, and bcr/abl and carries FDA approval for use in children and adults (149). Imatinib mesylate is principally used to treat chronic myelogenous leukemia, other hematological cancers, and some solid tumors. Given the findings that plexiform neurofibroma genesis and/or maintenance requires c-kit-mediated signals, Yang et al used Imatinib mesylate to treat Nf1flox/-;Krox20cre mice with pre-existing tumors, as measured by fluoridinated deoxyglucose signals in positron emission tomography (FDG-PET). After a three-month course of treatment, volumetric analyses on FDG-PET demonstrated reduced tumor volume and metabolic activity and histology of the dorsal root ganglia showed fewer mast cells, more orderly patterned Schwann cells, increased numbers of apoptotic cells, and diminished cellular proliferation, as compared to the placebo cohort. In fact, the placebo cohort showed a small increase in glucose metabolism in affected areas, suggesting tumor growth throughout the three month treatment course.
Imatinib mesylate also successfully reduced a progressively growing plexiform neurofibroma in a pediatric patient with hallmark symptoms of NF1. By six months of age, the girl had developed a histologically identified neurofibroma involving the left floor of her mouth, tongue, mastoid bone, carotid artery and jugular vein. As the tumor was compressing her airway, she had symptoms of drooling, insomnia, and anorexia. The tumor's intimate involvement of vasculature and nerve tissue precluded surgical resection. Given the absence of treatment options and the results from the study of imatinib mesylate in the murine plexiform neurofibroma model, clinicians discussed the risks and potential benefits of this experimental therapeutic with her pediatrician and her parents, leading to initiation of medical therapy. Following three months of treatment with 350 mg/m2/dose of imatinib mesylate, the tumor diminished in size to about a third of its pre-treatment volume, as assessed by magnetic resonance imaging. Importantly, the patient's symptoms of drooling, insomnia, and anorexia resolved, and imatinib mesylate produced no apparent side effects. Based on these findings, a phase II trial of NF1 patients with plexiform neurofibromas has recently been completed and is being submitted for publication.
How does imatinib mesylate treatment reduce a plexiform neurofibroma?
A neurofibroma's maintenance depends upon continual Schwann cell proliferation and/or survival, mast cell invasion, angiogenesis, and fibroblast proliferation and secretion of collagen. A simplistic hypothesis would suggest that imatinib disrupts SCF/c-kit-mediated mast cell cytopoiesis and inflammation, interrupting a key link between the tumorigenic Schwann cell and the mitogenic cues required to sustain itself and its microenvironment. However, cooperating mechanisms of action likely exist. For one, imatinib's well-characterized inhibitory action on the c-abl non-receptor tyrosine kinase may additionally disrupt TGF-β-mediated signaling in biochemically deregulated fibroblasts comprising the tumor stroma, as suggested experimentally in studies of murine and patient-derived cells. Further, imatinib can inhibit signaling at the platelet-derived growth factor receptor (PDGFR), which experiments have shown neurofibroma-derived Schwann cells to express and to respond to imatinib treatment in tumor xenograft models (27). Similarly, vascular cells in the tumor microenvironment may also depend on PDGFR signaling (99). Accordingly, imatinib may operate on several cell-receptor systems important for tumor growth, although the data from transplantation-based and other studies compellingly suggest that disruption of the SCF/c-kit signaling axis is of primary importance.
Future targeted therapies
In addition to imatinib mesylate, then, novel agents targeting the c-kit receptor tyrosine kinase and other relevant receptors/kinases may be viable therapeutics for the medical treatment of plexiform neurofibromas. A deeper understanding of the multiple mechanisms doubtlessly underlying heterotypic tumor interactions, as well as uncovering associations between genomic variation and disease course, will permit treatments tailored to the specific way in which a plexiform neurofibroma manifests within the individual patient. In a medical era increasingly cognizant of the potentials of personalized medicine and receptor-specific therapeutics, we view the results from imatinib as a first step. Indeed, singular or combined use of novel antiangiogenic drugs (e.g. bevacizumab), targeted anti-inflammatory agents (e.g. infliximab), and other c-kit antibodies (e.g. sunitinib) could prove beneficial, perhaps in a case-specific manner. Moreover, pharmacological grade small molecule kinase inhibitors, which typically modulate intracellular effectors involved in multiple ligand-receptor systems, may be efficacious, given the observed hyperactivity of MAPK and PI-3K pathways in Nf1-deficient cell types. Of note, accurate murine models of tumorigenesis are currently facilitating the high-throughput testing of multiple experimental therapeutic agents.
Conclusion
Neurofibromin-deficient glial cells do not grow in isolation. Rather, they send mitogenic signals that co-opt endothelial cells, fibroblasts, and hematopoietic cells, orchestrating the tumor microenvironment. As experimentally demonstrated, SCF-recruited mast cells buttress nascent tumors and support mature microenvironments by promoting fibroblast expansion and collagen synthesis, and Schwann cells and fibroblasts secrete factors directly contributing to vascular in-growth. The SCF-stimulated mast cell may also release factors promoting recruitment and/or growth of endothelial cells (e.g. VEGF, PDGF, bFGF), other hematopoietic cells (e.g. IL-6, TNF-α, MCP-1), and Schwann cells (e.g. NFG, PDGF), but the importance of these signals remain largely unexamined. Thus, broadly, complex and cooperating events in tumor genesis are obscure, potentially including multiple ligand-receptor interactions across diverse cell types and the modulating influences of variation in other genes. Regardless, genetic dosage of NF1 is central to neurofibroma pathogenesis, with a requirement for NF1 nullizygosity in the tumorigenic cell and, in several disease models, Nf1 heterozygosity in the hematopoietic system. Whether or not this requirement for Nf1 heterozygosity holds special relevance for the tumor's medical treatment in humans is uncertain, although these insights have revealed the discrete importance of the SCF/c-kit signaling axis in modulating disease course. Above all, continued exploration of the genomic variance between individual phenotypes and a deeper delineation of heterotypic interactions underlying tumorigenesis will propel the development and application of targeted, effective medical therapies.
Acknowledgements
This work was supported, in part, by NIH-NCI/RO1 CA074177-11A1/D and P50 NS052606-04 (D.W.C.). K.S. was additionally supported by a predoctoral fellowship from National Institutes of Health Grant T32 CA111198.
Literature cited
- 1.Friedman J, Gutmann DH, Maccollin M, Riccardi VM. Neurofibromatosis: phenotype, natural history, and pathogenesis. The Johns Hopkins University Press; Baltimore: 1999. p. 381. [Google Scholar]
- 2.Lubs ML, Bauer MS, Formas ME, Djokic B. Lisch nodules in neurofibromatosis type 1. N Engl J Med. 1991;324:1264–6. doi: 10.1056/NEJM199105023241807. [DOI] [PubMed] [Google Scholar]
- 3.Riccardi VM. Neurofibromatosis: past, present, and future. N Engl J Med. 1991;324:1283–5. doi: 10.1056/NEJM199105023241812. [DOI] [PubMed] [Google Scholar]
- 4.Riccardi VM. Neurofibromatosis type 1 is a disorder of dysplasia: the importance of distinguishing features, consequences, and complications. Birth Defects Res A Clin Mol Teratol. 2010;88:9–14. doi: 10.1002/bdra.20616. [DOI] [PubMed] [Google Scholar]
- 5.Akenside M. Observations on cancers. Med Trans Coll Phys Lond. 1768;1:64–92. [Google Scholar]
- 6.Tilesius von Tilenau W. Historia pathologica singularis Cutis Turpitudinis: Jo Godofredi Rheinhardi viri L annorum. SL Crusius; Leipzig, Germany: 1793. [Google Scholar]
- 7.Morse RP. Neurofibromatosis type 1. Arch Neurol. 1999;56:364–5. doi: 10.1001/archneur.56.3.364. [DOI] [PubMed] [Google Scholar]
- 8.von Recklinghausen F. Uber die Multiplen Fibrome der Haut und ihre Beziehung zu Multiplen Neuromen. August Hirschwald; Berlin: 1882. [PubMed] [Google Scholar]
- 9.Gamble HJ, Goldby S. Mast cells in peripheral nerve trunks. Nature. 1961;189:766–7. doi: 10.1038/189766a0. [DOI] [PubMed] [Google Scholar]
- 10.Baroni C. On the Relationship of Mast Cells to Various Soft Tissue Tumours. Br J Cancer. 1964;18:686–91. doi: 10.1038/bjc.1964.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pineda A. Mast cells--their presence and ultrastructural characteristics in peripheral nerve tumors. Arch Neurol. 1965;13:372–82. doi: 10.1001/archneur.1965.00470040038006. [DOI] [PubMed] [Google Scholar]
- 12.Olsson Y. Mast cells in human peripheral nerve. Acta Neurol Scand. 1971;47:357–68. doi: 10.1111/j.1600-0404.1971.tb07490.x. [DOI] [PubMed] [Google Scholar]
- 13.Isaacson P. Mast cells in benign nerve sheath tumours. J Pathol. 1976;119:193–6. doi: 10.1002/path.1711190402. [DOI] [PubMed] [Google Scholar]
- 14.Greggio H. Les cellules granuleuses (Mastzellen) dans les tissus normaux et dans certaines maladies chirurgicales. Arch. Med. Exp. 1911;23:323–75. [Google Scholar]
- 15.Le LQ, Shipman T, Burns DK, Parada LF. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009;4:453–63. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng H, Chang L, Patel N, Yang J, Lowe L, Burns DK, Zhu Y. Induction of abnormal proliferation by nonmyelinating schwann cells triggers neurofibroma formation. Cancer Cell. 2008;13:117–28. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Williams JP, Rizvi TA, Kordich JJ, Witte D, Meijer D, Stemmer-Rachamimov AO, Cancelas JA, Ratner N. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13:105–16. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raffensperger J, Cohen R. Plexiform neurofibromas in childhood. J Pediatr Surg. 1972;7:144–51. doi: 10.1016/0022-3468(72)90488-5. [DOI] [PubMed] [Google Scholar]
- 19.Serletis D, Parkin P, Bouffet E, Shroff M, Drake JM, Rutka JT. Massive plexiform neurofibromas in childhood: natural history and management issues. J Neurosurg. 2007;106:363–7. doi: 10.3171/ped.2007.106.5.363. [DOI] [PubMed] [Google Scholar]
- 20.McCaughan JA, Holloway SM, Davidson R, Lam WW. Further evidence of the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J Med Genet. 2007;44:463–6. doi: 10.1136/jmg.2006.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–4. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavallaro G, Basile U, Polistena A, Giustini S, Arena R, Scorsi A, Zinnamosca L, Letizia C, Calvieri S, De Toma G. Surgical management of abdominal manifestations of type 1 neurofibromatosis: experience of a single center. Am Surg. 2010;76:389–96. [PubMed] [Google Scholar]
- 23.Wu J, Dombi E, Jousma E, Scott Dunn R, Lindquist D, Schnell BM, Kim MO, Kim A, Widemann BC, Cripe TP, Ratner N. Preclincial testing of Sorafenib and RAD001 in the Nf(flox/flox) ;DhhCre mouse model of plexiform neurofibroma using magnetic resonance imaging. Pediatr Blood Cancer. 2011 doi: 10.1002/pbc.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang FC, Ingram DA, Chen S, Zhu Y, Yuan J, Li X, Yang X, Knowles S, Horn W, Li Y, Zhang S, Yang Y, Vakili ST, Yu M, Burns D, Robertson K, Hutchins G, Parada LF, Clapp DW. Nf1-dependent tumors require a microenvironment containing Nf1+/-- and c-kit-dependent bone marrow. Cell. 2008;135:437–48. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakacki RI, Dombi E, Potter DM, Goldman S, Allen JC, Pollack IF, Widemann BC. Phase I trial of pegylated interferon-alpha-2b in young patients with plexiform neurofibromas. Neurology. 2011;76:265–72. doi: 10.1212/WNL.0b013e318207b031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widemann BC, Salzer WL, Arceci RJ, Blaney SM, Fox E, End D, Gillespie A, Whitcomb P, Palumbo JS, Pitney A, Jayaprakash N, Zannikos P, Balis FM. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol. 2006;24:507–16. doi: 10.1200/JCO.2005.03.8638. [DOI] [PubMed] [Google Scholar]
- 27.Demestre M, Herzberg J, Holtkamp N, Hagel C, Reuss D, Friedrich RE, Kluwe L, Von Deimling A, Mautner VF, Kurtz A. Imatinib mesylate (Glivec) inhibits Schwann cell viability and reduces the size of human plexiform neurofibroma in a xenograft model. J Neurooncol. 2010;98:11–9. doi: 10.1007/s11060-009-0049-4. [DOI] [PubMed] [Google Scholar]
- 28.Packer RJ, Rosser T. Therapy for plexiform neurofibromas in children with neurofibromatosis 1: an overview. J Child Neurol. 2002;17:638–41. doi: 10.1177/088307380201700816. discussion 46-51. [DOI] [PubMed] [Google Scholar]
- 29.Franco CB, Chen CC, Drukker M, Weissman IL, Galli SJ. Distinguishing mast cell and granulocyte differentiation at the single-cell level. Cell Stem Cell. 6:361–8. doi: 10.1016/j.stem.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–30. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 31.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–23. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–86. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galli SJ, Tsai M. Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci. 2008;49:7–19. doi: 10.1016/j.jdermsci.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–9. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 35.Galli SJ, Tsai M, Wershil BK. The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am J Pathol. 1993;142:965–74. [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci U S A. 1991;88:6382–6. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor AM, Galli SJ, Coleman JW. Stem-cell factor, the kit ligand, induces direct degranulation of rat peritoneal mast cells in vitro and in vivo: dependence of the in vitro effect on period of culture and comparisons of stem-cell factor with other mast cell-activating agents. Immunology. 1995;86:427–33. [PMC free article] [PubMed] [Google Scholar]
- 38.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J Exp Med. 2001;193:F23–6. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 41.Riccardi VM. Cutaneous manifestation of neurofibromatosis: cellular interaction, pigmentation, and mast cells. Birth Defects Orig Artic Ser. 1981;17:129–45. [PubMed] [Google Scholar]
- 42.Riccardi VM. Mast-cell stabilization to decrease neurofibroma growth. Preliminary experience with ketotifen. Arch Dermatol. 1987;123:1011–6. [PubMed] [Google Scholar]
- 43.Riccardi VM. A controlled multiphase trial of ketotifen to minimize neurofibroma-associated pain and itching. Arch Dermatol. 1993;129:577–81. [PubMed] [Google Scholar]
- 44.Barker D, Wright E, Nguyen K, Cannon L, Fain P, Goldgar D, Bishop DT, Carey J, Baty B, Kivlin J, et al. Gene for von Recklinghausen neurofibromatosis is in the pericentromeric region of chromosome 17. Science. 1987;236:1100–2. doi: 10.1126/science.3107130. [DOI] [PubMed] [Google Scholar]
- 45.Seizinger BR, Rouleau GA, Ozelius LJ, Lane AH, Faryniarz AG, Chao MV, Huson S, Korf BR, Parry DM, Pericak-Vance MA, et al. Genetic linkage of von Recklinghausen neurofibromatosis to the nerve growth factor receptor gene. Cell. 1987;49:589–94. doi: 10.1016/0092-8674(87)90534-4. [DOI] [PubMed] [Google Scholar]
- 46.Fountain JW, Wallace MR, Bruce MA, Seizinger BR, Menon AG, Gusella JF, Michels VV, Schmidt MA, Dewald GW, Collins FS. Physical mapping of a translocation breakpoint in neurofibromatosis. Science. 1989;244:1085–7. doi: 10.1126/science.2543076. [DOI] [PubMed] [Google Scholar]
- 47.Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, Brereton A, Nicholson J, Mitchell AL, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–6. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 48.Viskochil D, Buchberg AM, Xu G, Cawthon RM, Stevens J, Wolff RK, Culver M, Carey JC, Copeland NG, Jenkins NA, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–92. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- 49.Cawthon RM, Weiss R, Xu GF, Viskochil D, Culver M, Stevens J, Robertson M, Dunn D, Gesteland R, O'Connell P, et al. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990;62:193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- 50.Marchuk DA, Saulino AM, Tavakkol R, Swaroop M, Wallace MR, Andersen LB, Mitchell AL, Gutmann DH, Boguski M, Collins FS. cDNA cloning of the type 1 neurofibromatosis gene: complete sequence of the NF1 gene product. Genomics. 1991;11:931–40. doi: 10.1016/0888-7543(91)90017-9. [DOI] [PubMed] [Google Scholar]
- 51.Ledbetter DH, Rich DC, O'Connell P, Leppert M, Carey JC. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20–4. [PMC free article] [PubMed] [Google Scholar]
- 52.Fountain JW, Wallace MR, Brereton AM, O'Connell P, White RL, Rich DC, Ledbetter DH, Leach RJ, Fournier RE, Menon AG, et al. Physical mapping of the von Recklinghausen neurofibromatosis region on chromosome 17. Am J Hum Genet. 1989;44:58–67. [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, O'Connell P, Breidenbach HH, Cawthon R, Stevens J, Xu G, Neil S, Robertson M, White R, Viskochil D. Genomic organization of the neurofibromatosis 1 gene (NF1). Genomics. 1995;25:9–18. doi: 10.1016/0888-7543(95)80104-t. [DOI] [PubMed] [Google Scholar]
- 54.Shilyansky C, Lee YS, Silva AJ. Molecular and cellular mechanisms of learning disabilities: a focus on NF1. Annu Rev Neurosci. 2010;33:221–43. doi: 10.1146/annurev-neuro-060909-153215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sergeyev AS. On the mutation rate of neurofibromatosis. Humangenetik. 1975;28:129–38. doi: 10.1007/BF00735745. [DOI] [PubMed] [Google Scholar]
- 56.Park VM, Pivnick EK. Neurofibromatosis type 1 (NF1): a protein truncation assay yielding identification of mutations in 73% of patients. J Med Genet. 1998;35:813–20. doi: 10.1136/jmg.35.10.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Messiaen LM, Callens T, Mortier G, Beysen D, Vandenbroucke I, Van Roy N, Speleman F, Paepe AD. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat. 2000;15:541–55. doi: 10.1002/1098-1004(200006)15:6<541::AID-HUMU6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 58.Maertens O, De Schepper S, Vandesompele J, Brems H, Heyns I, Janssens S, Speleman F, Legius E, Messiaen L. Molecular dissection of isolated disease features in mosaic neurofibromatosis type 1. Am J Hum Genet. 2007;81:243–51. doi: 10.1086/519562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Praxedes LA, Pereira FM, Mazzeu JF, Costa SS, Bertola DR, Kim CA, Vianna-Morgante AM, Otto PA. An Illustrative Case of Neurofibromatosis Type 1 and NF1 Microdeletion. Mol Syndromol. 2010;1:133–5. doi: 10.1159/000319976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dorschner MO, Sybert VP, Weaver M, Pletcher BA, Stephens K. NF1 microdeletion breakpoints are clustered at flanking repetitive sequences. Hum Mol Genet. 2000;9:35–46. doi: 10.1093/hmg/9.1.35. [DOI] [PubMed] [Google Scholar]
- 61.Wiest V, Eisenbarth I, Schmegner C, Krone W, Assum G. Somatic NF1 mutation spectra in a family with neurofibromatosis type 1: toward a theory of genetic modifiers. Hum Mutat. 2003;22:423–7. doi: 10.1002/humu.10272. [DOI] [PubMed] [Google Scholar]
- 62.Sabbagh A, Pasmant E, Laurendeau I, Parfait B, Barbarot S, Guillot B, Combemale P, Ferkal S, Vidaud M, Aubourg P, Vidaud D, Wolkenstein P. Unravelling the genetic basis of variable clinical expression in neurofibromatosis 1. Hum Mol Genet. 2009;18:2768–78. doi: 10.1093/hmg/ddp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hawes JJ, Tuskan RG, Reilly KM. Nf1 expression is dependent on strain background: implications for tumor suppressor haploinsufficiency studies. Neurogenetics. 2007;8:121–30. doi: 10.1007/s10048-006-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Upadhyaya M, Huson SM, Davies M, Thomas N, Chuzhanova N, Giovannini S, Evans DG, Howard E, Kerr B, Griffiths S, Consoli C, Side L, Adams D, Pierpont M, Hachen R, Barnicoat A, Li H, Wallace P, Van Biervliet JP, Stevenson D, Viskochil D, Baralle D, Haan E, Riccardi V, Turnpenny P, Lazaro C, Messiaen L. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970-2972 delAAT): evidence of a clinically significant NF1 genotype-phenotype correlation. Am J Hum Genet. 2007;80:140–51. doi: 10.1086/510781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu GF, O'Connell P, Viskochil D, Cawthon R, Robertson M, Culver M, Dunn D, Stevens J, Gesteland R, White R, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 66.Gutmann DH, Collins FS. The neurofibromatosis type 1 gene and its protein product, neurofibromin. Neuron. 1993;10:335–43. doi: 10.1016/0896-6273(93)90324-k. [DOI] [PubMed] [Google Scholar]
- 67.Gutmann DH, Boguski M, Marchuk D, Wigler M, Collins FS, Ballester R. Analysis of the neurofibromatosis type 1 (NF1) GAP-related domain by site-directed mutagenesis. Oncogene. 1993;8:761–9. [PubMed] [Google Scholar]
- 68.Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H, Conroy L, Clark R, O'Connell P, Cawthon RM, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63:843–9. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- 69.DeClue JE, Papageorge AG, Fletcher JA, Diehl SR, Ratner N, Vass WC, Lowy DR. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992;69:265–73. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- 70.McCormick F. ras GTPase activating protein: signal transmitter and signal terminator. Cell. 1989;56:5–8. doi: 10.1016/0092-8674(89)90976-8. [DOI] [PubMed] [Google Scholar]
- 71.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–57. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–32. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 73.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–27. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 74.Hall A. The cellular functions of small GTP-binding proteins. Science. 1990;249:635–40. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- 75.Hall A. Signal transduction through small GTPases--a tale of two GAPs. Cell. 1992;69:389–91. doi: 10.1016/0092-8674(92)90441-e. [DOI] [PubMed] [Google Scholar]
- 76.Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene. 2007;26:4609–16. doi: 10.1038/sj.onc.1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dasgupta B, Gutmann DH. Neurofibromatosis 1: closing the GAP between mice and men. Curr Opin Genet Dev. 2003;13:20–7. doi: 10.1016/s0959-437x(02)00015-1. [DOI] [PubMed] [Google Scholar]
- 78.Costa RM, Silva AJ. Mouse models of neurofibromatosis type I: bridging the GAP. Trends Mol Med. 2003;9:19–23. doi: 10.1016/s1471-4914(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 79.Staser K, Yang FC, Clapp DW. Plexiform neurofibroma genesis: questions of Nf1 gene dose and hyperactive mast cells. Curr Opin Hematol. 2010;17:287–93. doi: 10.1097/MOH.0b013e328339511b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Staser K, Yang FC, Clapp DW. Mast cells and the neurofibroma microenvironment. Blood. 2010;116:157–64. doi: 10.1182/blood-2009-09-242875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–6. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- 82.Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–2. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Joseph NM, Mosher JT, Buchstaller J, Snider P, McKeever PE, Lim M, Conway SJ, Parada LF, Zhu Y, Morrison SJ. The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell. 2008;13:129–40. doi: 10.1016/j.ccr.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zinnamosca L, Petramala L, Cotesta D, Marinelli C, Schina M, Cianci R, Giustini S, Sciomer S, Anastasi E, Calvieri S, De Toma G, Letizia C. Neurofibromatosis type 1 (NF1) and pheochromocytoma: prevalence, clinical and cardiovascular aspects. Arch Dermatol Res. 2010 doi: 10.1007/s00403-010-1090-z. [DOI] [PubMed] [Google Scholar]
- 85.De Schepper S, Maertens O, Callens T, Naeyaert JM, Lambert J, Messiaen L. Somatic mutation analysis in NF1 cafe au lait spots reveals two NF1 hits in the melanocytes. J Invest Dermatol. 2008;128:1050–3. doi: 10.1038/sj.jid.5701095. [DOI] [PubMed] [Google Scholar]
- 86.Bollag G, Clapp DW, Shih S, Adler F, Zhang YY, Thompson P, Lange BJ, Freedman MH, McCormick F, Jacks T, Shannon K. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet. 1996;12:144–8. doi: 10.1038/ng0296-144. [DOI] [PubMed] [Google Scholar]
- 87.Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132:5577–88. doi: 10.1242/dev.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stevenson DA, Zhou H, Ashrafi S, Messiaen LM, Carey JC, D'Astous JL, Santora SD, Viskochil DH. Double inactivation of NF1 in tibial pseudarthrosis. Am J Hum Genet. 2006;79:143–8. doi: 10.1086/504441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakamoto A, Yoshida T, Yamamoto H, Oda Y, Tsuneyoshi M, Iwamoto Y. Congenital pseudarthrosis of the tibia: analysis of the histology and the NF1 gene. J Orthop Sci. 2007;12:361–5. doi: 10.1007/s00776-007-1142-1. [DOI] [PubMed] [Google Scholar]
- 90.Kolanczyk M, Kuhnisch J, Kossler N, Osswald M, Stumpp S, Thurisch B, Kornak U, Mundlos S. Modelling neurofibromatosis type 1 tibial dysplasia and its treatment with lovastatin. BMC Med. 2008;6:21. doi: 10.1186/1741-7015-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeh TH, Lee da Y, Gianino SM, Gutmann DH. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia. 2009;57:1239–49. doi: 10.1002/glia.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee da Y, Yeh TH, Emnett RJ, White CR, Gutmann DH. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev. 2010;24:2317–29. doi: 10.1101/gad.1957110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li H, Liu Y, Zhang Q, Jing Y, Chen S, Song Z, Yan J, Li Y, Wu X, Zhang X, Zhang Y, Case J, Yu M, Ingram DA, Yang FC. Ras dependent paracrine secretion of osteopontin by Nf1+/- osteoblasts promote osteoclast activation in a neurofibromatosis type I murine model. Pediatr Res. 2009;65:613–8. doi: 10.1203/PDR.0b013e3181a1c607. [DOI] [PubMed] [Google Scholar]
- 94.Yan J, Chen S, Zhang Y, Li X, Li Y, Wu X, Yuan J, Robling AG, Kapur R, Chan RJ, Yang FC. Rac1 mediates the osteoclast gains-in-function induced by haploinsufficiency of Nf1. Hum Mol Genet. 2008;17:936–48. doi: 10.1093/hmg/ddm366. [DOI] [PubMed] [Google Scholar]
- 95.Yang FC, Chen S, Robling AG, Yu X, Nebesio TD, Yan J, Morgan T, Li X, Yuan J, Hock J, Ingram DA, Clapp DW. Hyperactivation of p21ras and PI3K cooperate to alter murine and human neurofibromatosis type 1-haploinsufficient osteoclast functions. J Clin Invest. 2006;116:2880–91. doi: 10.1172/JCI29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–60. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Munchhof AM, Li F, White HA, Mead LE, Krier TR, Fenoglio A, Li X, Yuan J, Yang FC, Ingram DA. Neurofibroma-associated growth factors activate a distinct signaling network to alter the function of neurofibromin-deficient endothelial cells. Hum Mol Genet. 2006;15:1858–69. doi: 10.1093/hmg/ddl108. [DOI] [PubMed] [Google Scholar]
- 98.Lasater EA, Bessler WK, Mead LE, Horn WE, Clapp DW, Conway SJ, Ingram DA, Li F. Nf1+/- mice have increased neointima formation via hyperactivation of a Gleevec sensitive molecular pathway. Hum Mol Genet. 2008;17:2336–44. doi: 10.1093/hmg/ddn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li F, Munchhof AM, White HA, Mead LE, Krier TR, Fenoglio A, Chen S, Wu X, Cai S, Yang FC, Ingram DA. Neurofibromin is a novel regulator of RAS-induced signals in primary vascular smooth muscle cells. Hum Mol Genet. 2006;15:1921–30. doi: 10.1093/hmg/ddl114. [DOI] [PubMed] [Google Scholar]
- 100.Ingram DA, Yang FC, Travers JB, Wenning MJ, Hiatt K, New S, Hood A, Shannon K, Williams DA, Clapp DW. Genetic and biochemical evidence that haploinsufficiency of the Nf1 tumor suppressor gene modulates melanocyte and mast cell fates in vivo. J Exp Med. 2000;191:181–8. doi: 10.1084/jem.191.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang FC, Chen S, Clegg T, Li X, Morgan T, Estwick SA, Yuan J, Khalaf W, Burgin S, Travers J, Parada LF, Ingram DA, Clapp DW. Nf1+/- mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15:2421–37. doi: 10.1093/hmg/ddl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bajenaru ML, Donahoe J, Corral T, Reilly KM, Brophy S, Pellicer A, Gutmann DH. Neurofibromatosis 1 (NF1) heterozygosity results in a cell-autonomous growth advantage for astrocytes. Glia. 2001;33:314–23. doi: 10.1002/1098-1136(20010315)33:4<314::aid-glia1030>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 103.Simmons GW, Pong WW, Emnett RJ, White CR, Gianino SM, Rodriguez FJ, Gutmann DH. Neurofibromatosis-1 Heterozygosity Increases Microglia in a Spatially and Temporally Restricted Pattern Relevant to Mouse Optic Glioma Formation and Growth. J Neuropathol Exp Neurol. 2010 doi: 10.1097/NEN.0b013e3182032d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daginakatte GC, Gianino SM, Zhao NW, Parsadanian AS, Gutmann DH. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer Res. 2008;68:10358–66. doi: 10.1158/0008-5472.CAN-08-2506. [DOI] [PubMed] [Google Scholar]
- 105.Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16:1098–112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- 106.Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet. 1994;7:353–61. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- 107.Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW, Buchberg AM, Jenkins NA, Parada LF, Copeland NG. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8:1019–29. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- 108.Valero MC, Velasco E, Moreno F, Hernandez-Chico C. Characterization of four mutations in the neurofibromatosis type 1 gene by denaturing gradient gel electrophoresis (DGGE). Hum Mol Genet. 1994;3:639–41. doi: 10.1093/hmg/3.4.639. [DOI] [PubMed] [Google Scholar]
- 109.Lasater EA, Li F, Bessler WK, Estes ML, Vemula S, Hingtgen CM, Dinauer MC, Kapur R, Conway SJ, Ingram DA., Jr. Genetic and cellular evidence of vascular inflammation in neurofibromin-deficient mice and humans. J Clin Invest. 2010;120:859–70. doi: 10.1172/JCI41443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–76. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci U S A. 1988;85:5166–70. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–9. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- 113.Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–7. [PubMed] [Google Scholar]
- 114.Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol. 2002;22:5100–13. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bailey P, Herrmann JD. The role of the cells of Schwann in the formation of tumors of the peripheral nerves. Am J Pathol. 1938;14:1–38. 19. [PMC free article] [PubMed] [Google Scholar]
- 116.Kluwe L, Friedrich R, Mautner VF. Loss of NF1 allele in Schwann cells but not in fibroblasts derived from an NF1-associated neurofibroma. Genes Chromosomes Cancer. 1999;24:283–5. doi: 10.1002/(sici)1098-2264(199903)24:3<283::aid-gcc15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 117.Rutkowski JL, Wu K, Gutmann DH, Boyer PJ, Legius E. Genetic and cellular defects contributing to benign tumor formation in neurofibromatosis type 1. Hum Mol Genet. 2000;9:1059–66. doi: 10.1093/hmg/9.7.1059. [DOI] [PubMed] [Google Scholar]
- 118.Maertens O, Brems H, Vandesompele J, De Raedt T, Heyns I, Rosenbaum T, De Schepper S, De Paepe A, Mortier G, Janssens S, Speleman F, Legius E, Messiaen L. Comprehensive NF1 screening on cultured Schwann cells from neurofibromas. Hum Mutat. 2006;27:1030–40. doi: 10.1002/humu.20389. [DOI] [PubMed] [Google Scholar]
- 119.Sheela S, Riccardi VM, Ratner N. Angiogenic and invasive properties of neurofibroma Schwann cells. J Cell Biol. 1990;111:645–53. doi: 10.1083/jcb.111.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carroll SL, Ratner N. How does the Schwann cell lineage form tumors in NF1? Glia. 2008;56:1590–605. doi: 10.1002/glia.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mashour GA, Ratner N, Khan GA, Wang HL, Martuza RL, Kurtz A. The angiogenic factor midkine is aberrantly expressed in NF1-deficient Schwann cells and is a mitogen for neurofibroma-derived cells. Oncogene. 2001;20:97–105. doi: 10.1038/sj.onc.1204026. [DOI] [PubMed] [Google Scholar]
- 122.Yang FC, Ingram DA, Chen S, Hingtgen CM, Ratner N, Monk KR, Clegg T, White H, Mead L, Wenning MJ, Williams DA, Kapur R, Atkinson SJ, Clapp DW. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/- mast cells. J Clin Invest. 2003;112:1851–61. doi: 10.1172/JCI19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirota S, Nomura S, Asada H, Ito A, Morii E, Kitamura Y. Possible involvement of c-kit receptor and its ligand in increase of mast cells in neurofibroma tissues. Arch Pathol Lab Med. 1993;117:996–9. [PubMed] [Google Scholar]
- 124.Ryan JJ, Klein KA, Neuberger TJ, Leftwich JA, Westin EH, Kauma S, Fletcher JA, DeVries GH, Huff TF. Role for the stem cell factor/KIT complex in Schwann cell neoplasia and mast cell proliferation associated with neurofibromatosis. J Neurosci Res. 1994;37:415–32. doi: 10.1002/jnr.490370314. [DOI] [PubMed] [Google Scholar]
- 125.Mashour GA, Driever PH, Hartmann M, Drissel SN, Zhang T, Scharf B, Felderhoff-Muser U, Sakuma S, Friedrich RE, Martuza RL, Mautner VF, Kurtz A. Circulating growth factor levels are associated with tumorigenesis in neurofibromatosis type 1. Clin Cancer Res. 2004;10:5677–83. doi: 10.1158/1078-0432.CCR-03-0769. [DOI] [PubMed] [Google Scholar]
- 126.Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW, Wellner D, Leder P, Besmer P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–33. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 127.Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–24. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 128.Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990;9:1805–13. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tan JC, Nocka K, Ray P, Traktman P, Besmer P. The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science. 1990;247:209–12. doi: 10.1126/science.1688471. [DOI] [PubMed] [Google Scholar]
- 130.Ingram DA, Hiatt K, King AJ, Fisher L, Shivakumar R, Derstine C, Wenning MJ, Diaz B, Travers JB, Hood A, Marshall M, Williams DA, Clapp DW. Hyperactivation of p21(ras) and the hematopoietic-specific Rho GTPase, Rac2, cooperate to alter the proliferation of neurofibromin-deficient mast cells in vivo and in vitro. J Exp Med. 2001;194:57–69. doi: 10.1084/jem.194.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Malhotra R, Ratner N. Localization of neurofibromin to keratinocytes and melanocytes in developing rat and human skin. J Invest Dermatol. 1994;102:812–8. doi: 10.1111/1523-1747.ep12379925. [DOI] [PubMed] [Google Scholar]
- 132.Ballester R, Marchuk D, Boguski M, Saulino A, Letcher R, Wigler M, Collins F. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990;63:851–9. doi: 10.1016/0092-8674(90)90151-4. [DOI] [PubMed] [Google Scholar]
- 133.Zhang YY, Vik TA, Ryder JW, Srour EF, Jacks T, Shannon K, Clapp DW. Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J Exp Med. 1998;187:1893–902. doi: 10.1084/jem.187.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ingram DA, Zhang L, McCarthy J, Wenning MJ, Fisher L, Yang FC, Clapp DW, Kapur R. Lymphoproliferative defects in mice lacking the expression of neurofibromin: functional and biochemical consequences of Nf1 deficiency in T-cell development and function. Blood. 2002;100:3656–62. doi: 10.1182/blood-2002-03-0734. [DOI] [PubMed] [Google Scholar]
- 135.Khalaf WF, Yang FC, Chen S, White H, Bessler W, Ingram DA, Clapp DW. K-ras is critical for modulating multiple c-kit-mediated cellular functions in wild-type and Nf1+/- mast cells. J Immunol. 2007;178:2527–34. doi: 10.4049/jimmunol.178.4.2527. [DOI] [PubMed] [Google Scholar]
- 136.McDaniel AS, Allen JD, Park SJ, Jaffer ZM, Michels EG, Burgin SJ, Chen S, Bessler WK, Hofmann C, Ingram DA, Chernoff J, Clapp DW. Pak1 regulates multiple c-Kit mediated Ras-MAPK gain-in-function phenotypes in Nf1+/- mast cells. Blood. 2008;112:4646–54. doi: 10.1182/blood-2008-04-155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hiatt K, Ingram DA, Huddleston H, Spandau DF, Kapur R, Clapp DW. Loss of the nf1 tumor suppressor gene decreases fas antigen expression in myeloid cells. Am J Pathol. 2004;164:1471–9. doi: 10.1016/S0002-9440(10)63233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hiatt KK, Ingram DA, Zhang Y, Bollag G, Clapp DW. Neurofibromin GTPase-activating protein-related domains restore normal growth in Nf1-/- cells. J Biol Chem. 2001;276:7240–5. doi: 10.1074/jbc.M009202200. [DOI] [PubMed] [Google Scholar]
- 139.Yang FC, Kapur R, King AJ, Tao W, Kim C, Borneo J, Breese R, Marshall M, Dinauer MC, Williams DA. Rac2 stimulates Akt activation affecting BAD/Bcl-XL expression while mediating survival and actin function in primary mast cells. Immunity. 2000;12:557–68. doi: 10.1016/s1074-7613(00)80207-1. [DOI] [PubMed] [Google Scholar]
- 140.Chen S, Burgin S, McDaniel A, Li X, Yuan J, Chen M, Khalaf W, Clapp DW, Yang FC. Nf1-/- Schwann cell-conditioned medium modulates mast cell degranulation by c-Kit-mediated hyperactivation of phosphatidylinositol 3-kinase. Am J Pathol. 2010;177:3125–32. doi: 10.2353/ajpath.2010.100369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jaakkola S, Peltonen J, Riccardi V, Chu ML, Uitto J. Type 1 neurofibromatosis: selective expression of extracellular matrix genes by Schwann cells, perineurial cells, and fibroblasts in mixed cultures. J Clin Invest. 1989;84:253–61. doi: 10.1172/JCI114148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Arbiser JL, Flynn E, Barnhill RL. Analysis of vascularity of human neurofibromas. J Am Acad Dermatol. 1998;38:950–4. doi: 10.1016/s0190-9622(98)70158-6. [DOI] [PubMed] [Google Scholar]
- 143.Kim HA, Ling B, Ratner N. Nf1-deficient mouse Schwann cells are angiogenic and invasive and can be induced to hyperproliferate: reversion of some phenotypes by an inhibitor of farnesyl protein transferase. Mol Cell Biol. 1997;17:862–72. doi: 10.1128/mcb.17.2.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 145.Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–64. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 146.Pong WW, Gutmann DH. The ecology of brain tumors: lessons learned from neurofibromatosis-1. Oncogene. 2011;30:1135–46. doi: 10.1038/onc.2010.519. [DOI] [PubMed] [Google Scholar]
- 147.Warrington NM, Woerner BM, Daginakatte GC, Dasgupta B, Perry A, Gutmann DH, Rubin JB. Spatiotemporal differences in CXCL12 expression and cyclic AMP underlie the unique pattern of optic glioma growth in neurofibromatosis type 1. Cancer Res. 2007;67:8588–95. doi: 10.1158/0008-5472.CAN-06-2220. [DOI] [PubMed] [Google Scholar]
- 148.Warrington NM, Gianino SM, Jackson E, Goldhoff P, Garbow JR, Piwnica-Worms D, Gutmann DH, Rubin JB. Cyclic AMP suppression is sufficient to induce gliomagenesis in a mouse model of neurofibromatosis-1. Cancer Res. 2010;70:5717–27. doi: 10.1158/0008-5472.CAN-09-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808–17. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]