Abstract
This study aimed to investigate prospective changes to neurophysiologic function over 3 years in patients with well-controlled diabetes. Sixty-two subjects had neurologic examinations, symptom scores, autonomic testing, nerve conduction studies, quantitative sensory testing and laser-Doppler flowmetry at 18-month intervals for 3 years. During the study, there was a 1 μV decrease in sural amplitude (P<0.05), an increase in monofilament detection threshold of 0.36 grams (P<0.001) and a decrease in the axon-reflex vasodilation in the foot (P<0.005) and forearm (P<0.05). There was an increase in symptoms of distal hypersensitivity (P<0.005) but no change in neuropathy frequency or severity. Our findings suggest that laser-Doppler flowmetry, a test of small fiber function, can detect the largest neurophysiologic change over time in groups of patients with diabetes. Sural nerve amplitude and monofilament thresholds may be more effective at detecting change in individual patients. Other tests of neurophysiologic function may require longer periods of time and greater numbers of participants to detect a difference. We conclude that patients with well-controlled diabetes and optimal medical management of co-morbid risk factors have low rates of neuropathy development and progression although the clinical relevance of this finding to the general population of individuals with diabetes is unknown.
Keywords: diabetes, neuropathy, neurophysiology, natural history, small fiber
Introduction
Historically, neuropathy is seen in more than half of individuals with diabetes (DCCT Research Group 1988; Dyck et al. 1997). It is estimated that individuals with type 1 diabetes will develop neuropathy at rates of 1.3–2.4% per year according to the EDIC and DCCT trials, while individuals with type 2 diabetes develop neuropathy at rates approaching 5% per year (DCCT Research Group 1988; Ziegler et al. 1993; Partanen et al. 1995; Albers et al. 2010). Symptoms of neuropathy generally progress even more rapidly than examination findings (DCCT Research Group 1988; Ziegler et al. 1993; Albers et al. 2010). These longitudinal trials clearly establish the association between hyperglycemia and progression of complications. Improvements in glycemic control are now integrated into standard clinical practice and reduce the rates of neuropathy development (LV et al. 2002; Tesfaye et al. 2005). However, even more recent studies do not account for the many changes to standard clinical care. The addition of ACE-inhibitors, angiotensin receptor blockers and statin drugs may reduce the risks of neuropathy development, but to an unknown degree (Gaede et al. 2008).
Recent clinical trials of novel agents to prevent or reverse diabetic neuropathy were powered using data from historical trials. Unfortunately, these clinical trials have failed to show any meaningful improvement over placebo (Vinik et al. 2005; Bril et al. 2009). In hindsight, it is apparent that the placebo treated groups did not develop neuropathy, or progress in severity of neuropathy, at the expected rate (Casellini et al. 2007; Bril et al. 2009).
We have followed a cohort of well defined subjects with diabetes over 3 years (Gibbons et al. 2010). The objective of this study is to prospectively define the changes to neurophysiologic function over a 3 year interval in patients with diabetes using a comprehensive battery of neurophysiologic tests and examination scores.
Materials and Methods
We studied a cohort of individuals with diabetes longitudinally at 18 month intervals for 3 years. Subjects were recruited from a pool of patients followed at Joslin diabetes center, the BIDMC-Joslin Podiatry clinic, from local referrals and local patient recruitment. The study was conducted from January 2005 and testing continued through November 2010. The study protocol was approved by the Beth Israel Deaconess Medical Center Institutional Review Board and all subjects gave their written informed consent. Exclusion criteria included symptomatic peripheral arterial disease, congestive heart failure, cardiac arrhythmias, stroke, end stage renal failure, uncontrolled hypertension, severe hyperlipidemia, hepatitis, HIV, thyroid disease, chronic liver disease or other chronic medical condition requiring ongoing active treatment or other disease that could cause neuropathy. All subjects were studied at a single institution, with the same examiners at each visit. Each test was administered by a trained technician, in a random testing order, without knowledge of other test results to reduce bias. Detailed anthropomorphic measurements were taken at each visit, and peripheral venous blood was sent for routine hematology and chemistry (including complete hepatic, renal and other metabolic testing panels) under fasting conditions.
Symptoms and examination
Subjects completed the Neuropathy Symptom Score (NSS) questionnaire (Veves et al. 1993), and had physical examinations quantified by the Neuropathy Disability Score (NDS) (Veves et al. 1994; Donaghue et al. 1995; Gibbons et al. 2010). An NDS score of 0–2 was defined as ‘no neuropathy’ and scores >2 were defined as ‘neuropathy’. Semmes-Weinstein monofilaments (ranging from 2.83–10 grams, Stoelting Co, Wood Dale, IL) determined the cutaneous perception threshold at the plantar surface of both great toes (Kumar et al. 1991; Sosenko et al. 1999). The final monofilament score was reported as the average of both legs.
Quantitative sensory testing
Thermal testing was performed using a MEDOC TSAII thermal and vibratory analyzer (Medoc Ltd., Israel). The method of limits was used for detection of (1) warm, (2) cool, (3) heat-pain, (4) cold-pain detection thresholds on the right thenar eminence of the hand and dorsum of the foot and (5) vibration detection thresholds on the right thumb and right great toe (Hilz et al. 1999; Gibbons and Freeman 2004).
Nerve conduction studies
Nerve conduction studies (NCS) were performed using a Viking IIIP EMG instrument (Viasys Healthcare, Madison, WI) by the same trained technician for all patients. Peroneal and sural NCS were performed on the left leg using standard methodologies (Albers et al. 1996). Distances, response latencies, and amplitudes were measured using onset latencies and baseline to peak amplitudes. Tests were performed with a limb temperature ≥32°C.
Autonomic testing
Subjects had continuous beat-to-beat blood pressure and electrocardiography monitoring during heart rate response to deep respiration, the heart rate response to Valsalva maneuver and a passive 60-degree upright tilt table test (Gibbons and Freeman 2004). Medications known to affect autonomic function were held the day of testing.
Nerve axon reflex
The blood flow response to iontophoresis of 1% acetylcholine chloride was assessed within the direct (endothelium dependent) and axon-reflex (endothelium independent) mediated regions at the dorsum of the foot (Kilo et al. 2000; Caselli et al. 2003). We used two single point laser probes and a DRT4 Laser-Doppler Blood Flow Monitor (Moor Instruments, Millwey, Devon, England) as previously described (Hamdy et al. 2001).
Statistical analysis
Results for individual tests are expressed as means with standard deviations. Data are represented graphically as percent change from baseline over 36 months with 95% confidence intervals. Normalcy of the data was checked by Mardia’s multivariate normality test. Repeated measures ANOVA compared neurophysiologic responses over time when data was normally distributed. The effects of age, duration of diabetes, type of diabetes, systolic blood pressure, smoking history, cholesterol, triglyceride levels, glycosylated hemoglobin levels and body mass index were evaluated as covariates in the repeated measures ANOVA model against changes to individual neurophysiologic or examination outcomes as the dependent variables. The covariates analyzed in this study were an exploratory endpoint, and therefore we did not correct for multiple comparisons. However, we present the results with and without correction for multiple comparisons. Significance values were set at 0.05 (two sided). Significance values for multiple comparisons in the ANOVA model set at p<0.001. Statistical analysis was performed using SPSS version 15.0 (SPSS Inc. Chicago IL, USA). The size of the current study was not sufficient to apply statistical techniques for missing values; therefore only individuals that completed all 3 visits are reported in the current study.
Results
Demographics
A total of 105 subjects completed the initial visit, 74 completed the 18 month visit and 62 completed all 3 visits. There were no differences in the main demographic and testing variables of the subjects who withdrew compared to those that completed the study, and no differences in rates of neuropathy progression in the individuals that completed 18 months of the study compared to those that completed 36 months.
Only data from the sixty-two subjects that completed 3 visits over 36 months at 18 month intervals are included. Twelve individuals had type 1 diabetes (6 female) and 50 had type 2 diabetes (22 female). The general demographic information is listed in Table 1. Only 7 subjects reported regular alcohol use, with 6/7 drinking less than 1 drink per day, and 1 subject reporting 2 drinks per day. All others reported no regular alcohol use and no history of alcohol abuse. Individuals with type 1 diabetes had greater disease duration (29±12 vs. 12±9 years, P<0.001), with lower body mass index (26.3±4.3 vs. 35.4±11.5, P<0.01), lower systolic blood pressure (115±11 vs. 123±15, P=0,05), lower diastolic blood pressure (65±9 vs. 72±12, P<0.05) and lower triglycerides (85±48 mg/dl vs. 135±87 mg/dl, P<0.05) than individuals with type 2 diabetes. There were no significant differences in age, neuropathy scores, ankle-brachial index, creatinine, cholesterol or glycosylated hemoglobin A1C between individuals with type 1 and type 2 diabetes. There were no significant differences to medications over the course of the study, although doses and number of individuals on antihypertensive medications increased slightly.
Table 1.
Demographics
| No. in analysis | Baseline | 18 Months | 36 Months | P Value | |
|---|---|---|---|---|---|
| Age | 62 | 57.1±14 | 58.6±14 | 60.1±14 | |
| Sex M/F | 62 | 28/34 | 28/34 | 28/34 | |
| Height (m) | 62 | 168±9 | 168±9 | 168±9 | |
| Weight (kg) | 62 | 91±19 | 94±29 | 96±32 | NS |
| Body mass index (BMI) | 62 | 31.8±6.3 | 32.4±8.4 | 32.9±10.4 | NS |
| Systolic BP (mmHg) | 62 | 135±18 | 128±14 | 125±15 | p<0.001 |
| Diastolic BP (mmHg) | 62 | 74±10 | 70±8 | 68±8 | p<0.001 |
| Resting Heart Rate (bpm) | 62 | 70±12 | 68±11 | 69±13 | NS |
| Hemoglobin A1C (%) | 62 | 7.23±10.3 | 7.36±1.47 | 7.40±1.54 | NS |
| Fasting Glucose | 62 | 127±47 | 136±63 | 135±60 | NS |
| Total cholesterol (mmol/l) | 62 | 152±28 | 161±45 | 157±45 | NS |
| Triglycerides (mmol/L) | 62 | 110±56 | 132±98 | 125±84 | NS |
| HDL cholesterol (mmol/L) | 62 | 53±16 | 54±17 | 52±17 | NS |
| LDL cholesterol (mmol/L) | 62 | 81±29 | 83±37 | 80±34 | NS |
| Creatinine (mg/dL) | 62 | 0.98±0.32 | 1.04±0.37 | 1.07±0.44 | p<0.05 |
| Blood urea nitrogen (mg/dL) | 62 | 19.5±7.3 | 20.3±8.7 | 21.9±11.4 | NS |
| Ankle-brachial index | 62 | 1.13±0.19 | 1.07±0.14 | 1.08±.014 | NS |
| Active tobacco use (all historical tobacco use) | 62 | 8 (18) | 7 (18) | 5 (18) | NS |
| Statin drug use | 62 | 48 (77%) | 49 (79%) | 48 (77%) | NS |
| Ace inhibitor/ARB use | 62 | 46 (74%) | 52 (84%) | 51 (82%) | NS |
| Insulin use (T1DM/T2DM) | 62 | 12/12: 26/50 | 12/12: 29/50 | 12/12: 31/50 | NS |
The demographic results for each testing period are reported. Results for each test date compared by repeated measures ANOVA. bpm: beats per minute. BP: blood pressure. T1DM: type 1 diabetes. T2DM: type 2 diabetes.
Examination findings, laboratory studies and symptoms
Over 36 months, there were no substantive changes to physical exam findings, anthropomorphic measurements, symptom scores, hematology or chemistry panels. There was a slight increase in serum creatinine (0.98±0.32 baseline vs. 1.07±0.44, P<0.05). Systolic blood pressure declined during the same period (133±19 mmHg vs. 125±15 mmHg, P<0.05). All results are described in detail in Table 1.
The number of subjects with neuropathy at baseline evaluation (by NDS score >2) was 32 and after 36 months this number was 33 (P=NS). There were no changes to mean neuropathy symptom scores between testing periods, but the number of subjects reporting distal neuropathic pain (tingling, burning, shooting, aching pain or allodynia) increased from 20 at baseline to 34 after 36 months (P<0.005).
Quantitative sensory testing
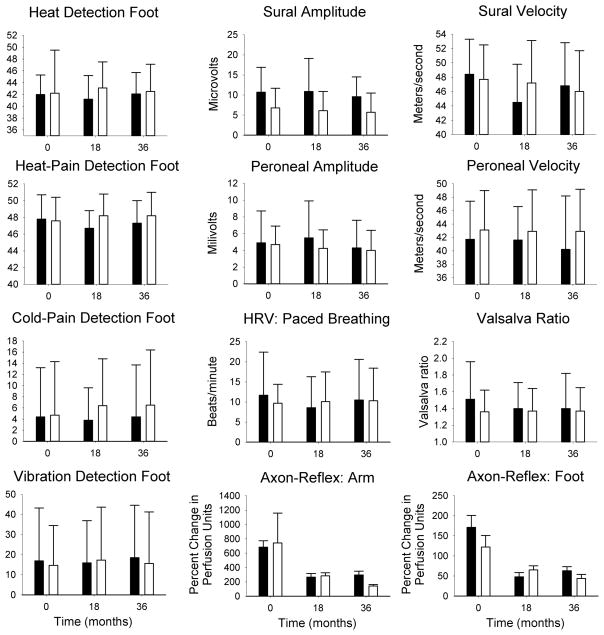
The average monofilament detection threshold worsened in the feet over a 36 month period (4.32±0.89 vs. 4.69±1.04, P<0.001) although no differences were noted between individuals with type 1 and type 2 diabetes. There were no significant changes to thermal, thermal pain or vibratory detection thresholds in the hands or feet over 36 months in individuals with type 1 or type 2 diabetes (Figure 1). The mean changes per year for all data are described in detail in Table 2.
Figure 1. Neurophysiologic test data.
Results for neurophysiologic test data in patients with type 1 diabetes (black bars) and type 2 diabetes (white bars) for baseline, 18 month and 36 month visits. There were no significant differences between individuals with type 1 and type 2 diabetes with the exception of sural amplitude (P<0.05 all visits). Values shown are mean ± standard deviation.
Table 2.
Results of neurophysiologic testing at each visit.
| Test | Number | Baseline | 18 Months | 36 Months | P Value | Yearly Change |
|---|---|---|---|---|---|---|
| Examination & Symptom Scores | ||||||
| Neuropathy % (NDS>2) | 62 | 32 | 31 | 33 | NS | |
| NDS score | 62 | 4.17±5.52 | 4.39±6.12 | 4.84±5.60 | NS | 0.22/yr |
| Neuropathy symptom score | 62 | 2.65±3.42 | 2.98±3.45 | 3.05±3.80 | NS | 0.13/yr |
| Distal neuropathic pain | 62 | 20 | 25 | 34 | p<0.005 | |
| Monofilament detection threshold (grams) | 62 | 4.32±0.89 | 4.46±0.91 | 4.69±1.04 | p<0.001 | 0.12/yr |
| Quantitative Sensory Testing | ||||||
| Vibration Thumb (JND) | 58 | 2.62±3.54 | 2.22±4.54 | 1.63±2.45 | p<0.05 | 0.3/yr |
| Vibration Toe (JND) | 58 | 14.6±23.4 | 14.5±23.5 | 16.1±25.9 | NS | 0.5/yr |
| Cold Detection Hand (°C) | 58 | 30.4±4.9 | 30.0±1.3 | 30.2±1.9 | NS | 0.07/yr |
| Cold Detection Foot (°C) | 58 | 21.5±10.2 | 23.9±7.9 | 23.8±9.1 | NS | 0.8/yr |
| Warm Detection Hand (°C) | 58 | 35.8±3.2 | 34.9±1.9 | 34.8±2.7 | NS | 0.3/yr |
| Warm Detection Foot (°C) | 58 | 42.0±6.8 | 42.6±4.3 | 42.4±4.2 | NS | 0.07/yr |
| Cold Pain Detection Hand (°C) | 58 | 5.2±7.1 | 7.7±7.2 | 7.8±8.1 | p<0.05 | 0.9/yr |
| Cold Pain Detection Foot (°C) | 58 | 4.5±9.3 | 6.3±8.3 | 6.3±9.9 | NS | 0.6/yr |
| Heap Pain Detection Hand (°C) | 58 | 45.6±3.9 | 44.7±3.8 | 45.6±5.4 | NS | 0/yr |
| Heat Pain Detection Foot (°C) | 58 | 47.7±6.6 | 47.7±2.5 | 48.1±2.9 | NS | 0.1/yr |
| Autonomic Testing | ||||||
| Heart Rate Variability(bpm) | 57 | 9.8±6.4 | 10.0±7.7 | 10.1±8.1 | NS | 0.1/yr |
| Valsalva Ratio | 52 | 1.40±0.34 | 1.39±0.29 | 1.40±0.32 | NS | 0/yr |
| Valsalva Blood Pressure Fall (mmHg) | 52 | 36±19 | 37±21 | 36±21 | NS | 0/yr |
| Valsalva Blood Pressure Recovery (mmHg) | 52 | 11±11 | 12±13 | 11±12 | NS | 0/yr |
| Valsalva Phase 4 overshoot (mmHg) | 52 | 20±31 | 20±30 | 19±26 | NS | 0.3/yr |
| Tilt Blood Pressure Change (mmHg) | 58 | 19±18 | 17±12 | 16±12 | NS | 1/yr |
| Tilt Heart Rate Change (bpm) | 58 | 12±9 | 12±8 | 13±9 | NS | 0.3/yr |
| Nerve Conduction Studies | ||||||
| Sural Amplitude (μV) | 57 | 7.6±5.4 | 7.3±5.8 | 6.6±5.1 | p<0.05 | 0.33/yr |
| Sural Velocity (m/sec) | 57 | 48.0±5.9 | 47.2±5.5 | 46.0±6.4 | NS | 0.67/yr |
| Peroneal Amplitude (mV) | 57 | 4.34±2.47 | 4.34±2.57 | 4.13±2.56 | NS | 0.07/yr |
| Peroneal Velocity (m/sec) | 57 | 42.9±5.9 | 42.8±5.9 | 41.9±6.9 | NS | 0.33/yr |
| Peroneal Distal Latency (ms) | 57 | 4.76±0.98 | 4.60±0.83 | 4.69±0.90 | NS | 0.07/yr |
| Laser Doppler Flowmetry | ||||||
| Arm Axon-Reflex % Change | 62 | 761±3119 | 285±362 | 164±230 | p<0.05 | 199%/yr |
| Foot Axon-Reflex % Change | 62 | 137±231 | 64±78 | 49±75 | p<0.005 | 29%/yr |
The results for each testing period are grouped by neurophysiologic modality. Results for each test date compared by repeated measures ANOVA, with P values denoting the difference between all 3 visits. Results listed in italics actually improved over the 3 year testing period. NDS: neuropathy disability score. JND: just noticeable difference. bpm: beats per minute.
Nerve conduction studies
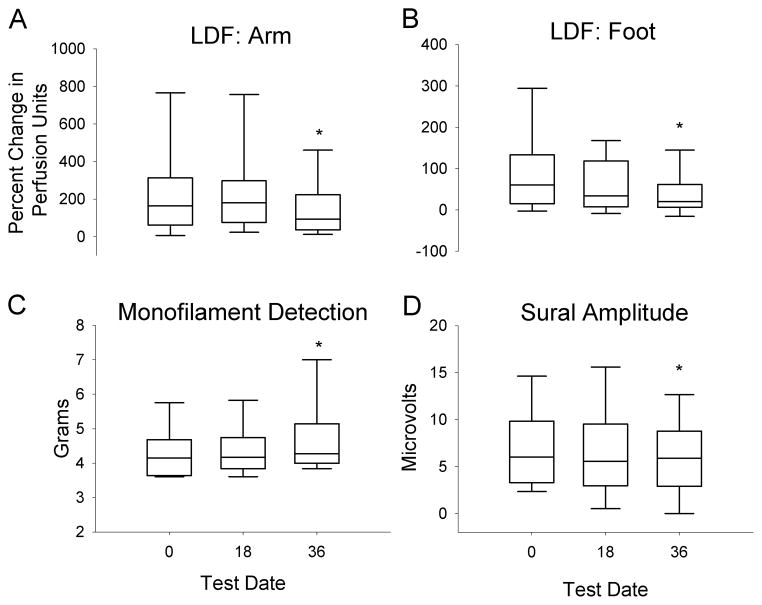
Sural nerve amplitudes were greater in those individuals with type 1 diabetes at all testing visits (P<0.05, Figure 1). There were no significant differences to sural velocity, peroneal amplitude or peroneal velocity over the course of the study in individuals with type 1 or type 2 diabetes, and no differences over time in combined data (Figures 1 & 2). Aggregate sural nerve amplitudes decreased by 1 microvolt over 3 years (P<0.05: Figure 2). The rate of change per year for all data is described in detail in Table 2.
Figure 2. Neurophysiologic outcomes.
Combined results for individuals with type 1 and type 2 diabetes are shown using box plots. There was a significant decrease in laser-Doppler flowmetry (LDF) at the arm (A) and leg (B) by the 36 month visit. There was a significant increase to monofilament detection thresholds (C) and a decrease to sural nerve amplitudes (D) over 36 months. The box plots represent the 25–75% range, with median values denoted by the center line. The 5–95% values are shown with whisker lines. * P<0.05.
Autonomic testing
There were no changes to sympathetic or parasympathetic function during the Valsalva maneuver and paced breathing over 36 months. At baseline, 17 subjects had orthostatic hypotension (sustained drop in SBP ≥20 mmHg during tilt), while at the final follow up visit 19 had orthostatic hypotension. The magnitude of the blood pressure fall during baseline and all follow up test visits was similar as was the heart rate response during tilt. There were no differences between individuals with type 1 and type 2 diabetes. The mean changes per year for all data are described in detail in Figure 1 and Table 2.
Nerve-axon reflex
There was a significant decrease in the axon reflex mediated vasodilation in the forearm, as measured by laser-Doppler flowmetry, over 36 months (P<0.01). There was a decrease in the axon-reflex mediated vasodilation in the foot over 36 months (P<0.005). The similar results were found for individuals with type 1 and type 2 diabetes (Figures 1 & 2). All results are described in detail in Table 2.
Covariate risks of disease progression
Although most measurable neurophysiologic parameters did not change to a significant degree during the course of the study, several factors were associated with worsening of individual neurophysiologic test results. Higher systolic blood pressure, history of smoking, longer duration of diabetes, type 2 diabetes, greater age, higher body mass index, higher A1C, higher cholesterol and triglycerides were all associated with worsening of at least 1 neurophysiologic parameter or exam score as reported in Table 3. With correction for multiple comparisons, smoking, diabetes duration, age, body mass index, cholesterol and triglycerides were still significant (Table 3).
Table 3.
Effects of individual covariates on decreasing neurophysiologic and examination function (expressed as F-statistics)
| Test | SBP | Smoking | DM type | DM Duration | Age | BMI | A1C | Cholesterol | Triglycerides |
|---|---|---|---|---|---|---|---|---|---|
| Quantitative Sensory Testing | |||||||||
| Vibration thumb | 0.8 | 11.7* | 0.2 | 0.1 | 0.4 | 0.5 | 0.3 | 1.3 | 0.1 |
| Vibration toe | 0.6 | 4.4 | 0.6 | 0.5 | 1.2 | 0.4 | 0.3 | 7.6 | 0.3 |
| Cold detection hand | 0.7 | 0.7 | 0.1 | 0 | 0.2 | 0.2 | 0.6 | 0 | 0 |
| Cold detection foot | 0.3 | 2.6 | 0 | 2.4 | 0.2 | 0 | 0.2 | 0.9 | 0.6 |
| Warm detection hand | 3.9 | 2.3 | 0.8 | 2.3 | 0.5 | 0 | 1.7 | 7.6 | 3.5 |
| Warm detection foot | 7.4 | 8.6* | 2.6 | 18.5* | 2.2 | 1.3 | 3.5 | 0.8 | 4.1 |
| Cold pain detection hand | 0.2 | 0 | 2.1 | 4.5 | 0.6 | 0.7 | 0.7 | 2.9 | 2.1 |
| Cold pain detection foot | 2.7 | 0.1 | 0.8 | 0 | 0.6 | 0 | 0 | 4.3 | 0 |
| Heap pain detection hand | 1.3 | 0.1 | 0.5 | 1.7 | 0.2 | 0.9 | 1.0 | 9.1* | 2.3 |
| Heat pain detection foot | 1.0 | 13.6* | 0.2 | 2.2 | 0.3 | 1.2 | 1.7 | 0.7 | 8.4* |
| Autonomic Testing | |||||||||
| Heart rate variability | 7.3 | 0 | 5.6 | 0.1 | 25.3* | 0 | 1.2 | 1.1 | 1.1 |
| Valsalva ratio | 0 | 0 | 2.6 | 0.7 | 41.2* | 0.1 | 4.3 | 0.1 | 0.1 |
| Valsalva blood pressure fall | 12.7* | 0.1 | 0 | 0 | 3.9 | 3.2 | 0.2 | 1.3 | 0.2 |
| Valsalva blood pressure Recovery | 0.1 | 1.3 | 0.4 | 0.1 | 8.7* | 1.2 | 1.7 | 1.8 | 0 |
| Valsalva phase 4 overshoot | 3.9 | 0 | 0.1 | 0.2 | 4.2 | 1.5 | 0.2 | 1.2 | 0.3 |
| Tilt blood pressure change | 12.0* | 6.8 | 1.5 | 0.4 | 6.4 | 0.3 | 6.9 | 2.3 | 4.2 |
| Tilt heart rate change | 0 | 0 | 0.1 | 3.0 | 8.7* | 0.2 | 1.1 | 0.5 | 0 |
| Nerve Conduction Studies | |||||||||
| Sural amplitude | 4.9 | 5.2 | 1.6 | 1.9 | 7.1 | 9.6* | 4.0 | 0.5 | 0.5 |
| Sural velocity | 7.1 | 4.4 | 0 | 4.5 | 1.0 | 0.3 | 0.8 | 3.9 | 0.3 |
| Peroneal amplitude | 5.3 | 3.8 | 0.6 | 2.9 | 0.4 | 0 | 0.2 | 1.1 | 0.1 |
| Peroneal velocity | 1.5 | 4.3 | 3.1 | 0.3 | 1.0 | 0.6 | 2.0 | 3.0 | 0.4 |
| Peroneal distal latency | 1.6 | 0.8 | 0.2 | 1.0 | 0.9 | 0.8 | 0 | 1.2 | 0.8 |
| Laser Doppler Imaging | |||||||||
| Arm %change indirect | 0.1 | 0.1 | 0 | 0.1 | 0.4 | 0 | 0 | 0.1 | 0.1 |
| Foot %change indirect | 0 | 0 | 0.4 | 0.4 | 7.7 | 2.5 | 0.1 | 0.5 | 0.1 |
| Examination & Symptoms | |||||||||
| NDS | 0.2 | 0.5 | 0 | 4.6 | 3.3 | 0.1 | 4.4 | 3.3 | 0.1 |
| NSS | 0.1 | 0.7 | 0.9 | 4.8 | 0 | 0.1 | 0 | 0.3 | 0.2 |
| Monofilament | 0 | 0.4 | 0.2 | 4.3 | 5.9 | 0.2 | 1.6 | 4.0 | 0.2 |
The results for each test visit were compared by repeated measured ANOVA with all covariates listed above. The results are expressed as F statistics, where larger numbers are more strongly correlated with declining neurophysiologic function. F-statistics > 3.7 are significant at p<0.05, and are shown in bold type;
F-statistics >8.1, significant at p<0.001 (i.e. significant with bonferroni correction for multiple comparisons). NDS: neuropathy disability score. NSS: neuropathy symptom score.
Discussion
In this prospective study of subjects with diabetes, we found that the majority of neurophysiologic tests did not appreciably change over a 36 month period in patients with diabetes. Those tests that detected progression of neuropathy over 36 months included 1) laser-Doppler flowmetry, 2) Semmes-Weinstein monofilaments and 3) the sural nerve amplitude. We found that other tests of neurophysiologic function and quantified examination scores did not detect a meaningful change during the course of this study. Those risk factors associated with neuropathy progression in individual neurophysiologic tests included smoking, age, blood pressure, duration of diabetes, body mass index, glucose control, cholesterol and triglyceride levels.
A unique aspect of this study is the comprehensive analysis of small, large and autonomic nerve fiber function over a 3 year period. The individuals in this study had stable diabetes, tended to be non-smokers and non-drinkers, had excellent control of their lipids and had decreasing blood pressure over the course of the study. These individuals maintained good (but not optimal) glucose control but had low rates of co-morbid risk factors and therefore reflect the current treatment guidelines for people with diabetes. These findings are important because the expected rate of change among many neurophysiologic tests is lower than previously reported and suggests that the natural history of diabetic neuropathy continues to evolve (DCCT Research Group 1988; Ziegler et al. 1993; Toyry et al. 1997; DCCT Research Group 1998; Albers et al. 2010; Pop-Busui et al. 2010).
Our findings highlight the limitations of standard nerve conduction studies in trials of diabetic neuropathy. Our study participants may not represent the ‘typical’ patient with diabetes and neuropathy seen in private practice, but may more closely reflect individuals that participate in pharmaceutical neuropathy trials. If so, nerve conduction studies may not represent a feasible primary endpoint for diabetic neuropathy trials and alternative outcomes need to be investigated.
The largest measurable change in neurophysiologic function was detected by laser-Doppler flowmetry. Unlike other neurophysiologic parameters, laser-Doppler flowmetry has a high degree of inherent variability in the measurement technique because it is susceptible to interference by changes in environment, activity and medication use. The variability of the technique does limit its use in individual subjects but may prove useful in research studies evaluating nociceptive C-fiber function between groups of sufficient size.
Our data contrast with prior reports of expected neuropathy progression during a similar time-frame and may highlight the changes to standard of care over the past decade (Partanen et al. 1995; Toyry et al. 1996a; Dyck et al. 1997; Toyry et al. 1997). Our subjects differed from participants in previous trials in a number of ways. All individuals in our trial had reasonably good control of their diabetes, especially in the context of the ACCORD trial, where intense control of glucose increased the mortality risk (Skyler et al. 2009; Nilsson 2010). In addition, seventy percent of our subjects were on either an angiotensin converting enzyme inhibitor or angiotensin II receptor blocker, and 75% of all subjects were taking a medication to lower their cholesterol (66% were on a statin). Our subjects maintained mean cholesterol levels of 160 mg/dl, triglyceride levels of 130 mg/dl and actually had lower systolic blood pressure at follow up. Only a small proportion of our study subjects had used tobacco or alcohol at all within the past 5 years, rates much lower than other longitudinal studies of diabetic neuropathy (Partanen et al. 1995, 1998; Albers et al. 2010). The cholesterol and triglyceride levels in our cohort were substantially lower than prior reports, including the DCCT and UKPDS studies, where neuropathy did progress over time (Partanen et al. 1995, 1998; Albers et al. 2010).
The risk factors associated with worsening neurophysiologic, examination or sensory symptoms over 36 months included systolic blood pressure, smoking, diabetes type, diabetes duration, age, body mass index, A1C, cholesterol and triglyceride levels. These risk factors were relatively well controlled in the majority of our subjects and likely contributed to the overall stability of their neuropathy. These findings are consistent with recent publications that highlight the multiple risk factors (in addition to glycemic control) that contribute to neuropathy progression (Tesfaye et al. 2005; Wiggin et al. 2009).
There are several limitations to the current project. Greater than expected subject dropout was noted due to a variety of factors, but the dropout was statistically random and did not appear to impact the results. In addition, structural outcomes, such as sural nerve biopsies or skin biopsies, were not available. Structural data could provide additional information on the diagnostic interpretation of individual test results. More detailed testing of each neurophysiologic parameter would have been of interest (i.e. a complete set of nerve conduction studies as well as an EMG), and sudomotor function testing, but was limited by practical consideration of patient participation. Our covariate analysis was an exploratory endpoint of the study, so we did not correct for multiple comparisons. If we had performed a more conservative analysis (p<0.001), smoking, diabetes duration, age, body mass index, cholesterol and triglycerides would still be considered significant covariates in our ANOVA model. In addition, we did not have a control group as a comparison. The unexpectedly low rate of neurophysiologic decline in our population highlights the need for additional control data to correctly power future studies of diabetic neuropathy.
In summary, our findings suggest a decrease in the rate of progression of diabetic neuropathy compared to older trials. Standard tests of neurophysiologic function, such as nerve conduction studies, may require extended periods of time before they can detect a measureable decline in individuals with diabetes. The clinical significance of these findings is still unknown and future studies incorporating longitudinal structural investigation of nerve fibers that complement the neurophysiologic measures are needed to understand the changing natural history of diabetic neuropathy.
Acknowledgments
This study was supported by the following research grants: NIH NINDS K23NS50209 (CHG) and NIH NINDS R01NS046710 (AV).
Footnotes
The authors have no conflicts of interest to disclose.
References
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 352(9131):837–853. [PubMed] [Google Scholar]
- Albers JW, Brown MB, et al. Nerve conduction measures in mild diabetic neuropathy in the Early Diabetes Intervention Trial: the effects of age, sex, type of diabetes, disease duration, and anthropometric factors. Tolrestat Study Group for the Early Diabetes Intervention Trial. Neurology. 1996;46(1):85–91. doi: 10.1212/wnl.46.1.85. [DOI] [PubMed] [Google Scholar]
- Albers JW, Herman WH, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33(5):1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril V, Hirose T, et al. Ranirestat for the management of diabetic sensorimotor polyneuropathy. Diabetes Care. 2009;32(7):1256–1260. doi: 10.2337/dc08-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli A, Rich J, et al. Role of C-nociceptive fibers in the nerve axon reflex-related vasodilation in diabetes. Neurology. 2003;60(2):297–300. doi: 10.1212/01.wnl.0000040250.31755.f9. [DOI] [PubMed] [Google Scholar]
- Casellini CM, Barlow PM, et al. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care. 2007;30(4):896–902. doi: 10.2337/dc06-1699. [DOI] [PubMed] [Google Scholar]
- DCCT Research Group. Factors in development of diabetic neuropathy. Baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. Diabetes. 1988;37:476–481. [PubMed] [Google Scholar]
- DCCT Research Group. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT) Diabetologia. 1998;41(4):416–423. doi: 10.1007/s001250050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghue VM, Giurini JM, et al. Variability in function measurements of three sensory foot nerves in neuropathic diabetic patients. Diabetes Res Clin Pract. 1995;29(1):37–42. doi: 10.1016/0168-8227(95)01107-o. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Davies JL, et al. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology. 1997;49(1):229–239. doi: 10.1212/wnl.49.1.229. [DOI] [PubMed] [Google Scholar]
- Gaede P, Lund-Andersen H, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- Gibbons C, Freeman R. The evaluation of small fiber function-autonomic and quantitative sensory testing. Neurol Clin. 2004;22(3):683–702. vii. doi: 10.1016/j.ncl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Freeman R, et al. Diabetic neuropathy: a cross-sectional study of the relationships among tests of neurophysiology. Diabetes Care. 2010;33(12):2629–2634. doi: 10.2337/dc10-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy O, Abou-Elenin K, et al. Contribution of nerve-axon reflex-related vasodilation to the total skin vasodilation in diabetic patients with and without neuropathy. Diabetes Care. 2001;24(2):344–349. doi: 10.2337/diacare.24.2.344. [DOI] [PubMed] [Google Scholar]
- Hilz MJ, Stemper B, et al. Quantitative thermal perception testing in adults. J Clin Neurophysiol. 1999;16(5):462–471. doi: 10.1097/00004691-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Kilo S, Berghoff M, et al. Neural and endothelial control of the microcirculation in diabetic peripheral neuropathy. Neurology. 2000;54(6):1246–1252. doi: 10.1212/wnl.54.6.1246. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fernando DJ, et al. Semmes-Weinstein monofilaments: a simple, effective and inexpensive screening device for identifying diabetic patients at risk of foot ulceration. Diabetes Res Clin Pract. 1991;13(1–2):63–67. doi: 10.1016/0168-8227(91)90034-b. [DOI] [PubMed] [Google Scholar]
- Low VA, Sandroni P, et al. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34(1):57–61. doi: 10.1002/mus.20551. [DOI] [PubMed] [Google Scholar]
- vdP-F LV, Valk GD, et al. Longitudinal assessment of the development of diabetic polyneuropathy and associated risk factors. Diabet Med. 2002;19(9):771–776. doi: 10.1046/j.1464-5491.2002.00778.x. [DOI] [PubMed] [Google Scholar]
- Malik R, Veves A, et al. Small Fiber Neuropathy: Role in the diagnosis of Diabetic Sensorimotor Polyneuropathy. Diabetes Metab Res Rev. 2011 doi: 10.1002/dmrr.1222. [DOI] [PubMed] [Google Scholar]
- Nilsson PM. ACCORD and Risk-Factor Control in Type 2 Diabetes. N Engl J Med. 2010;362(17):1628–1630. doi: 10.1056/NEJMe1002498. [DOI] [PubMed] [Google Scholar]
- Partanen J, Niskanen L, et al. Natural history of peripheral neuropathy in patients with non- insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(2):89–94. doi: 10.1056/NEJM199507133330203. [DOI] [PubMed] [Google Scholar]
- Pop-Busui R, Herman WH, et al. DCCT and EDIC studies in type 1 diabetes: lessons for diabetic neuropathy regarding metabolic memory and natural history. Curr Diab Rep. 2010;10(4):276–282. doi: 10.1007/s11892-010-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyler JS, Bergenstal R, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119(2):351–357. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- Sosenko JM, Sparling YH, et al. Use of the Semmes-Weinstein monofilament in the strong heart study. Risk factors for clinical neuropathy. Diabetes Care. 1999;22(10):1715–1721. doi: 10.2337/diacare.22.10.1715. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Chaturvedi N, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- Toyry JP, Niskanen LK, et al. Autonomic neuropathy predicts the development of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke. 1996a;27(8):1316–1318. doi: 10.1161/01.str.27.8.1316. [DOI] [PubMed] [Google Scholar]
- Toyry JP, Niskanen LK, et al. Occurrence, predictors, and clinical significance of autonomic neuropathy in NIDDM. Ten-year follow-up from the diagnosis. Diabetes. 1996b;45(3):308–315. doi: 10.2337/diab.45.3.308. [DOI] [PubMed] [Google Scholar]
- Toyry JP, Partanen JV, et al. Divergent development of autonomic and peripheral somatic neuropathies in NIDDM. Diabetologia. 1997;40(8):953–958. doi: 10.1007/s001250050773. [DOI] [PubMed] [Google Scholar]
- Veves A, Manes C, et al. Painful neuropathy and foot ulceration in diabetic patients. Diabetes Care. 1993;16(8):1187–1189. doi: 10.2337/diacare.16.8.1187. [DOI] [PubMed] [Google Scholar]
- Veves A, Uccioli L, et al. Comparison of risk factors for foot problems in diabetic patients attending teaching hospital outpatient clinics in four different European states. Diabet Med. 1994;11(7):709–713. doi: 10.1111/j.1464-5491.1994.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Vinik AI, Bril V, et al. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther. 2005;27(8):1164–1180. doi: 10.1016/j.clinthera.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Wiggin TD, Sullivan KA, et al. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58(7):1634–1640. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D, Gries FA, et al. The epidemiology of diabetic neuropathy. DiaCAN Multicenter Study Group. Diabet Med. 1993;10(Suppl 2):82S–86S. doi: 10.1111/j.1464-5491.1993.tb00208.x. [DOI] [PubMed] [Google Scholar]