Abstract
Background and Objectives
National treatment guidelines state that polysubstance users, including cocaine users, may not be appropriate candidates for office-based buprenorphine treatment. However, data to support this recommendation are sparse and conflicting, and the implications of this recommendation may include limiting the usefulness of buprenorphine treatment, as cocaine use is common among opioid-dependent individuals seeking buprenorphine treatment. We compared buprenorphine treatment outcomes (6-month treatment retention and self-reported opioid use over 6 months) in opioid-dependent cocaine users versus non-users who initiated buprenorphine treatment at an urban community health center.
Methods
We followed 87 participants over 6 months, collecting interview and medical record data. We used logistic regression models to test whether baseline cocaine use was associated with treatment retention and mixed effects non-linear models to test whether baseline cocaine use was associated with self-reported opioid use.
Results
At baseline, 39.1% reported cocaine use. In all participants, self-reported opioid use decreased from 89.7% to 27.4% over 6 months, and 6-month treatment retention was 54.5%. We found no significant difference in 6-month treatment retention (AOR=1.56, 95%CI=0.58–4.17, p=0.38) or self-reported opioid use (AOR=0.89, 95%CI=0.26–3.07, p=0.85) between cocaine users and non-users.
Conclusions and Scientific Significance
This study demonstrates that buprenorphine treatment retention is not worse in cocaine users than non-users, with clinically meaningful improvements in self-reported opioid use. These findings suggest that opioid-dependent cocaine users attain considerable benefits from office-based buprenorphine treatment and argue for the inclusion of these patients in office-based buprenorphine treatment programs.
Background
Opioid addiction continues to be a growing problem in the U.S. 1–4. To address this increasing need for opioid addiction treatment, the availability of buprenorphine treatment has steadily improved over the last several years 5, 6. Expanded physician experience, together with a growing body of clinical research on buprenorphine treatment, can now inform those elements of the original national treatment guidelines for buprenorphine treatment that lacked a sufficient evidence base. For example, current guidelines state that individuals who are using other non-opioid drugs (e.g., cocaine) may not be appropriate candidates for office-based buprenorphine treatment7. However, data supporting this recommendation are limited, despite polysubstance use, particularly cocaine use, being common in opioid-dependent people seeking buprenorphine treatment in office-based settings 8–14.
Few studies have specifically examined outcomes among opioid-dependent cocaine users receiving office-based buprenorphine treatment 11, 12. Other studies that have not focused primarily on cocaine use have examined general predictors of office-based buprenorphine treatment outcomes, and have included cocaine as a potential risk factor for negative outcomes 8–10, 14. Additional studies have examined buprenorphine treatment outcomes among cocaine users in drug treatment settings, rather than office-based settings 15–17. Findings from all these studies are somewhat conflicting—many demonstrate worse treatment outcomes in cocaine users than non-users, while some demonstrate no difference. It is not surprising that these studies did not consistently find poor treatment outcomes among cocaine users, as these studies varied in terms of treatment settings, treatment protocols (including how ongoing drug use is handled), patient populations, and definitions of outcomes.
Compared with studies that examined outcomes in opioid-dependent cocaine users receiving buprenorphine treatment, studies that examined outcomes in opioid-dependent cocaine users receiving methadone maintenance treatment have reported more consistent results. These latter studies reveal consistently poorer treatment outcomes among cocaine users than non-users, including poorer treatment retention and higher rates of opioid use 18–25. Given the differences between methadone treatment and buprenorphine treatment in terms of patient populations and treatment delivery, extrapolations of treatment outcomes observed in opioid-dependent cocaine users receiving methadone treatment to opioid-dependent cocaine users receiving office-based buprenorphine treatment may not be appropriate.
To examine whether cocaine use is associated with buprenorphine treatment outcomes in opioid-dependent individuals, we compared treatment retention and opioid use in cocaine users versus non-users who initiated office-based buprenorphine treatment. We hypothesized that compared to non-users, cocaine users would have poorer treatment retention and higher rates of opioid use.
Methods
We conducted an analysis of a longitudinal cohort study of opioid-dependent individuals who initiated buprenorphine treatment at an urban community health center. Original study aims were to identify factors that predict positive treatment outcomes in participants receiving office-based buprenorphine treatment that was integrated into primary care. Participants were followed for six months, and data collection included interviews and medical record extraction.
Setting
The study was conducted in a Bronx community health center from November 2004 to December 2009, immediately after a buprenorphine treatment program was established. (For details about the buprenorphine treatment program, see Cunningham et al.10). Briefly, six general internists work closely with a clinical pharmacologist to provide buprenorphine treatment in the context of general primary care medicine. No substance abuse counselors or support groups are available at the health center, but, two social workers are available to all health center patients, including those who received buprenorphine treatment.
If there is evidence of ongoing drug use despite buprenorphine treatment (e.g. repeatedly positive urine toxicology tests) or repeated problems with adherence to scheduled visits, physicians typically intensify treatment through more frequent visits and/or referrals for psychosocial support. For example, instead of monthly visits that typically occur when patients are in the maintenance phase of treatment, physicians may require visits every 1–2 weeks. Additionally, physicians may require patients to participate in off-site self-help groups, outpatient substance abuse treatment programs or psychiatric treatment. Termination of treatment typically occurs if there are known instances of buprenorphine diversion, refusal to accept or follow through with treatment intensification, or abusive behavior towards clinical staff.
Participants
Original study eligibility criteria included: 1) newly initiating buprenorphine treatment at the health center (defined as transferred from a hospital, rehabilitation, or detoxification facility within 7 days of starting buprenorphine medication or no prescribed buprenorphine in the previous 30 days), 2) HIV infection, and 3) English fluency. After securing additional funding, the last two criteria were revised in January 2007 to include participants who were HIV-positive or HIV-negative, and fluent in English or Spanish. To receive buprenorphine treatment at the health center, participants had to be at least 18 years old, dependent on opioids (per DSM-IV criteria 26), and insured by a health plan accepted at the health center or willing to pay for treatment on a sliding scale fee. Consistent with national guidelines 7, participants were excluded from receiving buprenorphine treatment if they were: 1) hypersensitive to buprenorphine or naloxone, 2) pregnant, 3) alcohol dependent (per DSM-IV criteria 26), 4) benzodiazepine dependent (per DSM-IV criteria 26), 5) with transaminase levels greater than five times normal, 6) known to have severe, untreated psychiatric illness, and 7) taking more than 60 mg of methadone daily in the past month. There was no treatment exclusion criterion related to cocaine use.
The study was registered in clinicaltrials.gov and was approved by the medical center’s institutional review board.
Data Collection
Participants were interviewed by a research assistant at baseline (prior to initiating buprenorphine treatment at the health center), and 1, 3, and 6 months. Interviews lasted 45–60 minutes and occurred in a private room at the health center. Interviews were conducted using audio computer-assisted self-interview (ACASI) technology in which questions were displayed on a computer while an audio recording of the question was played. Participants entered responses directly on the computer, which may result in more accurate reporting of sensitive behavior than other survey methods 27. Participants received $15 travel reimbursement for each interview. (See below for a description of interview questions.)
At the 6-month follow-up period, visit and prescription data were extracted from electronic medical records. Because urine toxicology tests are routinely collected as part of buprenorphine treatment at the health center, results of these tests were also available in medical records. However, because urine toxicology tests were conducted for clinical care rather than research, they were not collected in a standardized manner for all study participants. For example, patients who are retained in treatment or have more frequent visits are more likely to have a larger number of toxicology tests than those who drop out of treatment or have less frequent visits. Because of the great potential for bias in how urine toxicology testing was conducted among study participants, results of urine toxicology tests were not used in this analysis.
Dependent variables
Our two primary outcomes were buprenorphine treatment retention and self-reported opioid use. Treatment retention was examined 1, 3, and 6 months after participants initiated buprenorphine treatment. Participants were categorized as retained in treatment if they had either a medical visit or active buprenorphine prescription between day 30–60 for 1-month retention, between day 90–120 for 3-month retention, and between day 180–210 for 6-month retention. To be retained in treatment at 3 months, participants also had to be retained at 1 month, and to be retained in treatment at 6 months, participants had to be retained at both 1 and 3 months. All retention data were from medical records.
Self-reported opioid use was defined as self-report of any heroin, methadone (prescribed or non-prescribed), or opioid analgesic (prescribed or non-prescribed) use in the 30-day period prior to each interview during the 6-month follow-up period (at 1, 3, and 6 months). As mentioned above, urine toxicology tests were not used in the outcome measure.
Independent variable
Our main independent variable was cocaine use at baseline, defined as self-reported cocaine use in the 30-day period prior to the baseline interview. For this analysis, we focused on cocaine use at baseline (prior to initiating buprenorphine treatment), because at baseline (the time of clinical presentation) physicians in office-based settings make the important decision of whether they will offer buprenorphine treatment.
Other variables
Additional covariates collected via interviews included: age (continuous); gender (male, female); race/ethnicity (Hispanic, non-Hispanic black, non-Hispanic other); education (less than a high school diploma or GED, at least a high school diploma or GED); employment (employed, unemployed); housing status (stably housed defined as reporting living in an apartment or home, unstably housed defined as living in any other situation); history of incarceration for three or more days (yes, no); substance use in the 30 days prior to baseline (heroin; methadone; opioid analgesics; cocaine; sedatives, hypnotics, or tranquillizers); ever injected drugs (yes, no); and depressive symptoms (score of ≥16 from the Center for Epidemiologic Studies, Depression [CESD]28; score of <16 on the CESD). Demographic questions were from a buprenorphine/HIV multi-site study 29, and substance use questions were from the Addiction Severity Index (ASI) 30.
Data Analysis
Buprenorphine treatment retention and cocaine use
We first conducted simple chi square tests of those retained in treatment at 1, 3, and 6 months by baseline cocaine use. We then used logistic regression models to test whether baseline cocaine use was associated with 6-month treatment retention while adjusting for potential confounders. Confounding variables included in the full model were those that differed (p<0.20) between cocaine users and non-users (age, baseline opioid analgesic use, and history of incarceration).
Self-reported opioid use and cocaine use
We used mixed effects non-linear models to test whether baseline cocaine use was associated with self-reported opioid use over the 6 month follow-up period (measured at 1, 3, and 6 months) while adjusting for baseline opioid use, time, and potential confounders. Confounding variables included in the full model were those that differed (p<0.20) between cocaine users and non-users. Age, baseline opioid analgesic use, and history of incarceration all met this criterion, but baseline opioid analgesic use was not included in the full model due to collinearity.
Results
Participant characteristics
Of the 114 screened and eligible participants, 108 (94.7%) enrolled in the study. Of these, 3 withdrew from the study, 14 never initiated buprenorphine treatment, and 4 had no follow-up interviews. Thus, 87 participants are included in this analysis. From these 87 participants, 75 (86.2%) interviews were conducted at 1 month, 79 (90.8%) at 3 months, and 73 (83.9%) at 6 months.
Participants’ mean age was 43.5 years at baseline, and most were men (73.6%), Hispanic (73.2%), unemployed (69.0%), unstably housed (60.9%), with histories of incarceration (69.0%) and with histories of injection drug use (50.6%) (see Table). Regarding baseline opioid use (use within the 30-day period prior to the baseline interview), 67.8% reported using heroin, 52.9% methadone, and 26.4% opioid analgesics.
Table.
Characteristics associated with baseline cocaine use among participants receiving buprenorphine treatment
| Sociodemographic characteristics | Total N=87 n (%) | Baseline cocaine users N=34 of 87 n (%) | Baseline cocaine non-users N=53 of 87 n (%) |
|---|---|---|---|
|
| |||
| Age (mean years ± SD) | 43.5 ± 9.0 | 40.5 ± 8.8* | 45.5 ± 8.7 |
|
| |||
| Male | 64 (73.6) | 26 (76.5) | 38 (71.1) |
|
| |||
| Race/ethnicity1 | |||
| Hispanic | 60 (73.2) | 23 (69.7) | 37 (75.5) |
| Non-Hispanic black | 19 (23.2) | 9 (27.3) | 10 (20.4) |
| Non-Hispanic other | 3 (3.7) | 1 (3.0) | 2 (4.1) |
|
| |||
| English fluency | 53 (60.9) | 18 (52.9) | 35 (66.0) |
|
| |||
| High school diploma or GED | 57 (65.5) | 21 (61.7) | 36 (67.9) |
|
| |||
| Employed | 27 (31.0) | 10 (29.4) | 17 (32.1) |
|
| |||
| Stably housed | 34 (39.1) | 12 (35.3) | 22 (41.5) |
|
| |||
| Married | 26 (30.0) | 9 (26.5) | 17 (32.1) |
|
| |||
| Ever incarcerated | 60 (69.0) | 27 (79.4)** | 33 (62.3) |
|
| |||
| Clinical characteristics | |||
|
| |||
| Recent self-reported drug use2 | |||
|
| |||
| Heroin | 59 (76.8) | 27 (79.4) | 32 (60.4) |
|
| |||
| Methadone | 46 (52.9) | 17 (50.0) | 29 (54.7) |
|
| |||
| Opioid analgesics | 23 (26.4) | 15 (44.1)* | 8 (15.1) |
|
| |||
| Sedatives | 13 (14.9) | 5 (14.7) | 8 (15.1) |
|
| |||
| Ever injected drugs | 44 (50.6) | 20 (58.8) | 24 (45.3) |
|
| |||
| CESD ≥16 | 55 (63.2) | 20 (58.8) | 35 (66.0) |
|
| |||
| HIV-infected | 26 (29.9) | 11 (32.4) | 15 (28.3) |
Note: Column percentages are presented. CESD=Center for Epidemiology Studies, Depression (Radloff, 1977)
Race/ethnicity data are missing for 5 participants.
Recent self-reported drug use is defined as self-reported use of the drug within 30 days prior to study enrollment.
p<0.05
p<0.20
At baseline, 39.1% reported cocaine use prior to initiating buprenorphine treatment, ranging from using cocaine 1–25 days, with a median of 5 days in the previous 30 days. When examining sociodemographic and clinical characteristics, cocaine users versus non-users were younger (mean age in years ± SD: 40.5 ± 8.8 vs. 45.5 ± 8.7, p<0.05) and more likely to use opioid analgesics (44.1% vs. 15.1%, p<0.05). Over time, among all participants, cocaine use decreased significantly from 39.1% reporting cocaine use at baseline, to 33.3% at 1 month, 19.0% at 3 months, and 12.3% at 6 months (p<0.001 for trend test). Of the 53 participants without baseline cocaine use, only 4 (7.5%) reported cocaine use during the 6-month follow-up period after initiating buprenorphine treatment.
Buprenorphine treatment retention
Among all participants, treatment retention at 1, 3, and 6 months was 92.0%, 69.0% and 54.0%, respectively. There was no significant difference in treatment retention by cocaine use; 6-month treatment retention for cocaine users versus non-users was 58.8% vs. 50.9% (odds ratio [OR]=1.38, 95% Confidence Interval [CI]=0.58–3.28, p=0.47) (see Figure 1). In our model adjusted for age, baseline opioid analgesic use, and history of incarceration, this finding was unchanged (adjusted odds ratio [AOR]=1.56, 95%CI=0.58–4.17, p=0.38).
Figure 1.
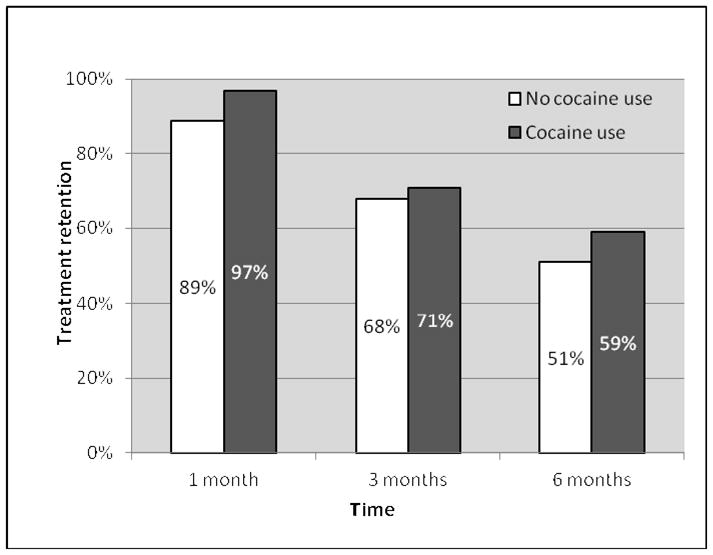
Buprenorphine treatment retention by baseline cocaine use
Self-reported Opioid use
Among all participants, self-reported opioid use decreased over time from 89.7% at baseline to 41.9% at 1 month, 32.9% at 3 months, and 27.4 % at 6 months. Similar patterns of consistent reductions in self-reported opioid use were observed in cocaine users and non-users (see Figure 2). There was no significant difference in self-reported opioid use in cocaine users versus non-users before adjusting for confounders (OR=0.90, 95%CI=0.28–2.92, p=0.87) or after adjusting for age and history of incarceration (AOR=0.89, 95%CI=0.26–3.07, p=0.85).
Figure 2.
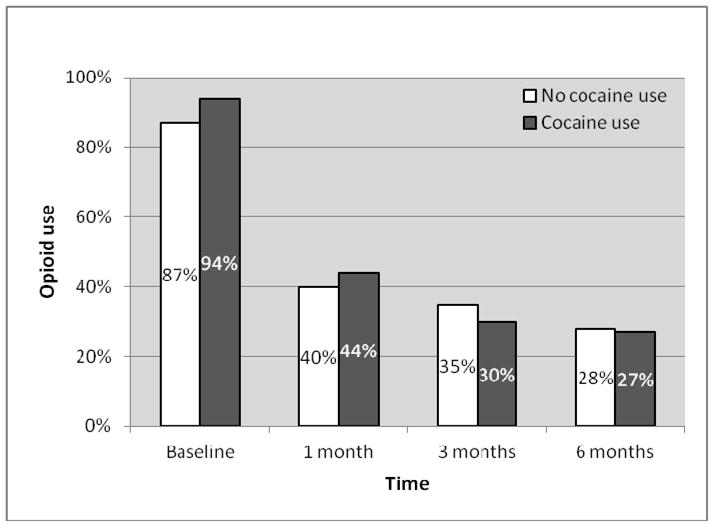
Self-reported opioid use among participants who initiated buprenorphine treatment by baseline cocaine use
Discussion
In our study of opioid-dependent participants who initiated office-based buprenorphine treatment in the Bronx, nearly 40% of participants reported using cocaine at the time they were seeking buprenorphine treatment. We found no significant difference in buprenorphine treatment retention or self-reported opioid use between cocaine users and non-users. Additionally, cocaine use significantly decreased over time.
Guidelines from the Center for Substance Abuse Treatment state that polysubstance users, including cocaine users, many not be good candidates for receiving office-based buprenorphine treatment 7. This is important because cocaine use is common in patients seeking office-based buprenorphine treatment 8–14. Our study and others’ have demonstrated that 24–66% of patients who received office-based buprenorphine treatment were cocaine users. Despite this, few studies have specifically examined how cocaine use is associated with outcomes in office-based buprenorphine treatment settings. The issues of whether or how to offer buprenorphine treatment to cocaine users is important and clinically relevant.
While other studies address the question of the potential impact of cocaine use on buprenorphine treatment, ours is one of few studies to specifically examine how cocaine use is associated with buprenorphine treatment outcomes in a “real world” office-based setting without a select patient population, intensive clinical protocols, or treatment discontinuation for evidence of ongoing drug use. In one study of HIV-infected patients only, no difference in 12-month buprenorphine treatment retention occurred between cocaine users versus non-users (48.7% vs. 49.0%); however, higher self-reported opioid use was significantly different between cocaine users versus non-users (OR=1.43)11. Although that multi-site study occurred in several real world HIV primary care settings, substantial differences between HIV-positive and –negative opioid-dependent populations may influence treatment outcomes. Another study in an office-based setting found lower treatment retention and higher opioid use in cocaine users than non-users 12, but this study excluded cocaine-dependent individuals, had strict clinical protocols (e.g. visits 1–3 times per week), and discharged patients for ongoing drug use. Thus, it is difficult to extrapolate those findings to more general patient populations and less intensive treatment protocols that are likely to be common with office-based buprenorphine treatment. Two studies that had general patient populations and did not have strict clinical protocols examined general predictors of outcomes with office-based buprenorphine treatment, including cocaine use. Similar to our study, these two studies found no differences in treatment outcomes between cocaine users and non-users 8, 9. Finally, our findings of a reduction in self-reported opioid use in cocaine users from 94% to 27% and 6-month treatment retention of 59% are consistent with studies in which cocaine users received buprenorphine treated in drug treatment settings 17, 31–33.
The findings from our study conflict with what would be expected based on several studies conducted in methadone maintenance treatment settings that demonstrate worse retention and/or higher risk of opioid use in cocaine users than non-users 18–25, 34. However, we note that there are substantial differences between methadone and buprenorphine in treatment delivery, regulations, and patient populations that might explain these differences. Although this question of the impact of cocaine use on opioid addiction treatment outcomes with buprenorphine versus methadone is important, this is challenging to study given these fundamental differences between treatment options.
Because cocaine use is common among people seeking opioid addiction treatment, it is important to further study how treatment outcomes among cocaine users receiving opioid addiction treatment can be optimized. Based on our findings and others’, providing buprenorphine treatment to cocaine users in office-based settings can lead to substantial and clinically meaningful improvements in opioid addiction.
Our study has limitations. One of our outcomes, opioid use, relied on self report which may not accurately portray ongoing substance use and is subject to recall bias. However, our other main outcome, treatment retention, relied on objective data from medical records, and findings were consistent with both outcomes. Our study was limited to one clinical site; thus, our findings may not be generalizable to other populations. We acknowledge that our small sample of 87 participants limited our power to detect significant differences between cocaine users and non-users. However, because our observed difference was in the opposite direction of what was expected (AOR of retention in treatment for cocaine users vs. non-users was 1.56), we believe that our negative finding is unlikely to be due to limited power.
In conclusion, among opioid-dependent individuals seeking office-based buprenorphine treatment in the Bronx, cocaine use was common. Our study demonstrated no significant difference in treatment outcomes in cocaine users versus non-users. These findings suggest that opioid-dependent cocaine users attain considerable benefits from office-based buprenorphine treatment and argue for the inclusion of these patients in office-based buprenorphine treatment programs.
Acknowledgments
This study was supported by the Health Resources and Services Administration, HIV/AIDS Bureau, Special Projects of National Significance, grant 6H97HA00247; the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-51519); NIH R25DA023021; and the Robert Wood Johnson Foundation’s Harold Amos Medical Faculty Development Program.
We thank Mia Brisbane and Johanna Rivera for their help conducting this research.
Footnotes
Declaration of Interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCE LIST
- 1.Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. J Pain. 2005;6(10):662–72. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Sung HE, Richter L, Vaughan R, Johnson PB, Thom B. Nonmedical use of prescription opioids among teenagers in the United States: trends and correlates. J Adolesc Health. 2005 Jul;37(1):44–51. doi: 10.1016/j.jadohealth.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies; NSDUH Series H-34, DHHS Publication No. SMA 08-4343; 2008. [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration Office of Applied Studies. DAWN Series D-30 DHHS Publication No SMA 08-4339. Rockville, MD: 2008. Drug Abuse Warning Network, 2006: National Estimates of Drug-Related Emergency Department Visits. [Google Scholar]
- 5.Fiellin DA. The first three years of buprenorphine in the United States: Experience to date and future directions. J Addict Med. 2007;1:62–7. doi: 10.1097/ADM.0b013e3180473c11. [DOI] [PubMed] [Google Scholar]
- 6.Arfken CL, Johanson CE, di MS, Schuster CR. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: National surveys of physicians. J Subst Abuse Treat. 2010;39(2):96–104. doi: 10.1016/j.jsat.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) Series 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. DHHS Publication No. (SMA) 04-3939. [PubMed] [Google Scholar]
- 8.Lee JD, Grossman E, DiRocco D, Gourevitch MN. Home buprenorphine/naloxone induction in primary care. J Gen Intern Med. 2009 Feb;24(2):226–32. doi: 10.1007/s11606-008-0866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. J Gen Intern Med. 2005 Nov;20(11):1038–41. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham C, Giovanniello A, Sacajiu G, et al. Buprenorphine treatment in an urban community health center: what to expect. Fam Med. 2008;40(7):500–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan LE, Botsko M, Cunningham CO, et al. The impact of cocaine use on outcomes in HIV-infected patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3182097576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan LE, Moore BA, O’Connor PG, et al. The Association between Cocaine Use and Treatment Outcomes in Patients Receiving Office-Based Buprenorphine/Naloxone for the Treatment of Opioid Dependence. Am J Addict. 2010 Jan 1;19(1):53–8. doi: 10.1111/j.1521-0391.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan LE, Barry D, Moore BA, et al. A Trial of Integrated Buprenorphine/Naloxone and HIV Clinical Care. Clin Infect Dis. 2006 Dec 15;43(Suppl 4):S184–S190. doi: 10.1086/508182. [DOI] [PubMed] [Google Scholar]
- 14.Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011 Mar 14;171(5):425–31. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schottenfeld RS, Pakes J, Ziedonis D, Kosten TR. Buprenorphine: dose-related effects on cocaine and opioid use in cocaine-abusing opioid-dependent humans. Biol Psychiatry. 1993 Jul 1;34(1–2):66–74. doi: 10.1016/0006-3223(93)90258-f. [DOI] [PubMed] [Google Scholar]
- 16.Schottenfeld RS, Pakes J, O’Connor P, Chawarski M, Oliveto A, Kosten TR. Thrice-weekly versus daily buprenorphine maintenance. Biol Psychiatry. 2000 Jun 15;47(12):1072–9. doi: 10.1016/s0006-3223(99)00270-x. [DOI] [PubMed] [Google Scholar]
- 17.Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. Am J Psychiatry. 2005 Feb;162(2):340–9. doi: 10.1176/appi.ajp.162.2.340. [DOI] [PubMed] [Google Scholar]
- 18.Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003;98(1):7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 19.Perez de los CJ, Trujols J, Ribalta E, Casas M. Cocaine use immediately prior to entry in an inpatient heroin detoxification unit as a predictor of discharges against medical advice. Am J Drug Alcohol Abuse. 1997 May;23(2):267–79. doi: 10.3109/00952999709040946. [DOI] [PubMed] [Google Scholar]
- 20.Hartel DM, Schoenbaum EE, Selwyn PA, et al. Heroin use during methadone maintenance treatment: the importance of methadone dose and cocaine use. Am J Public Health. 1995;85(1):83–8. doi: 10.2105/ajph.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magura S, Nwakeze PC, Demsky SY. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93(1):51–60. doi: 10.1046/j.1360-0443.1998.931516.x. [DOI] [PubMed] [Google Scholar]
- 22.Bovasso G, Cacciola J. The long-term outcomes of drug use by methadone maintenance patients. J Behav Health Serv Res. 2003;30(3):290–303. doi: 10.1007/BF02287318. [DOI] [PubMed] [Google Scholar]
- 23.Peles E, Schreiber S, Adelson M. 15-Year survival and retention of patients in a general hospital-affiliated methadone maintenance treatment (MMT) center in Israel. Drug Alcohol Depend. 2010 Mar 1;107(2–3):141–8. doi: 10.1016/j.drugalcdep.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Rowan-Szal GA, Chatham LR, Simpson DD. Importance of identifying cocaine and alcohol dependent methadone clients. Am J Addict. 2000;9(1):38–50. doi: 10.1080/10550490050172218. [DOI] [PubMed] [Google Scholar]
- 25.DeMaria PA, Sterling R, Weinstein SP. The effect of stimulant and sedative use on treatment outcome of patients admitted to methadone maintenance treatment. Am J Addict. 2000;9(2):145–53. doi: 10.1080/10550490050173217. [DOI] [PubMed] [Google Scholar]
- 26.Diagnostic and Statistical Manual of Mental Disorders. 4. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: increased reporting with computer survey technology. Science. 1998 May 8;280(5365):867–73. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 29.Weiss L, Egan JE, Botsko M, Netherland J, Fiellin DA, Finkelstein R. The BHIVES collaborative: organization and evaluation of a multisite demonstration of integrated buprenorphine/naloxone and HIV treatment. J Acquir Immune Defic Syndr. 2011 Mar 1;56(Suppl 1):S7–13. doi: 10.1097/QAI.0b013e3182097426. [DOI] [PubMed] [Google Scholar]
- 30.McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 31.Schottenfeld RS, Pakes JR, Kosten TR. Prognostic factors in Buprenorphine- versus methadone-maintained patients. J Nerv Ment Dis. 1998 Jan;186(1):35–43. doi: 10.1097/00005053-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Montoya ID, Gorelick DA, Preston KL, et al. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther. 2004;75(1):34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid-dependent cocaine users. Psychopharmacology (Berl) 1994;116(4):401–6. doi: 10.1007/BF02247469. [DOI] [PubMed] [Google Scholar]
- 34.Wasserman DA, Weinstein MG, Havassy BE, Hall SM. Factors associated with lapses to heroin use during methadone maintenance. Drug Alcohol Depend. 1998 Nov 1;52(3):183–92. doi: 10.1016/s0376-8716(98)00092-1. [DOI] [PubMed] [Google Scholar]


