Abstract
Context
Post-operative atrial fibrillation/flutter (AF) is one of the most common complications of cardiac surgery and significantly increases morbidity and healthcare utilization. A few small trials have evaluated whether long-chain n-3-polyunsaturated fatty acids (PUFA) reduce post-op AF, with mixed results.
Objective
To determine whether peri-operative n-3-PUFA supplementation reduces post-op AF.
Design
Randomized, double-blind, placebo-controlled, multinational, clinical trial.
Patients
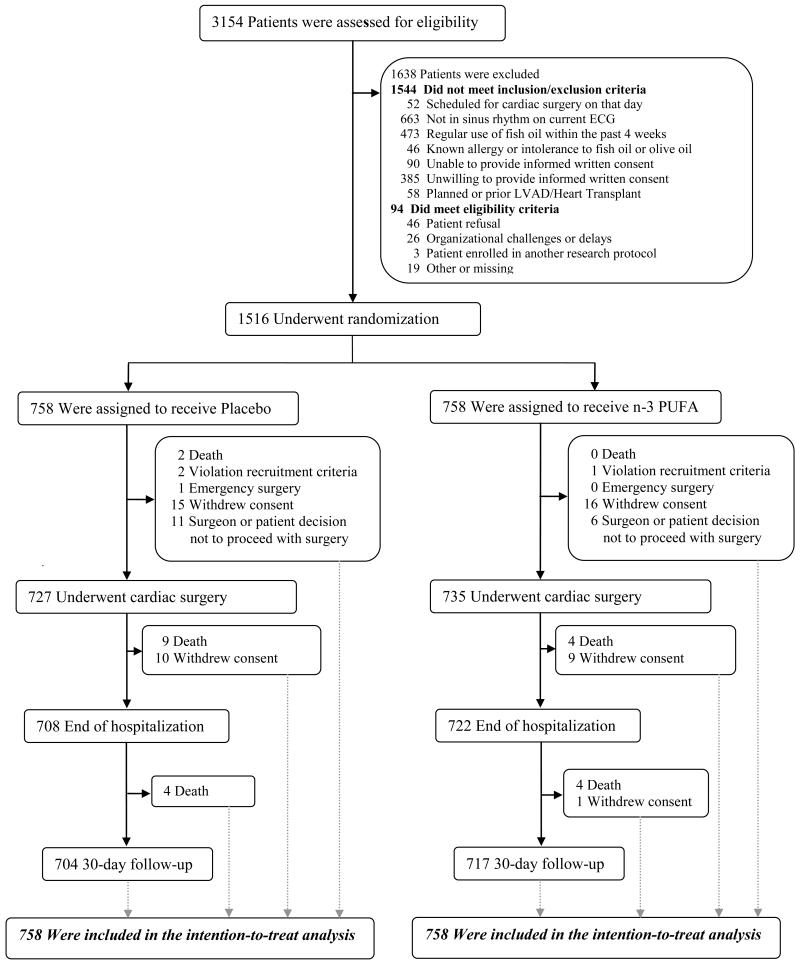
A total of 1,516 patients scheduled for cardiac surgery across 28 centers in the US, Italy, and Argentina, enrolled between Aug 2010 and Jun 2012. Inclusion criteria were broad; the main exclusions were regular use of fish oil or absence of sinus rhythm at enrollment. Forty-eight percent of screened patients and 94% of eligible patients were enrolled.
Intervention
Patients were randomized to receive fish oil (1 g capsules containing ≥840 mg n-3-PUFA as ethyl esters) or placebo, with pre-operative loading of 10g over 3-5 days (or 8g over 2 days) followed post-operatively by 2g/d until hospital discharge or post-op day10, whichever first.
Main Outcome Measures
The primary endpoint was occurrence of post-op AF >30 sec. We also evaluated post-op AF lasting >1hr, resulting in symptoms, or treated with cardioversion; other secondary post-op AF endpoints; other tachyarrhythmias; hospital utilization; and major adverse cardiovascular events, 30-day mortality, bleeding, and other adverse events. All endpoints and analyses plans were prespecified.
Results
At enrollment, mean±SD age was 64±13 years, 72.2% were male, and 51.8% had planned valvular surgery. The primary endpoint occurred in 233 (30.7%) and 227 (30.0%) patients assigned to placebo and n-3-PUFA, respectively (OR=0.96, 95%CI=0.77-1.20; P=0.74). None of the secondary endpoints were significantly different, including post-op AF that was sustained, symptomatic, or treated (n=231 [30.5%] vs. n=224 [29.6%], P=0.70) or number of post-op AF episodes per patient (1 episode: n=220 [29.0%] vs. n=217 [28.6%]; 2 episodes: n=156 [20.6%] vs. n=157 [20.7%]; 3+ episodes: n=18 [2.4%] vs. n=21 [2.8%]; P=0.73). n-3-PUFA was generally well-tolerated, with no evidence for increased risk of bleeding or serious adverse events.
Conclusions
In this large multinational trial among patients undergoing cardiac surgery, peri-operative supplementation with n-3-PUFA, compared to placebo, did not reduce the risk of post-operative AF.
INTRODUCTION
Post-operative atrial fibrillation or flutter (AF) occurs in ~1 of 3 patients undergoing cardiac surgery, and rates of this complication remain unchanged even with advances in surgical techniques, anesthetic procedures, and peri-operative care.1,2 Post-op AF can cause hemodynamic instability or symptoms requiring cardioversion or escalation of supportive therapies, and also renal and neurological complications. Antiarrhythmic and anticoagulant drugs are often required, both in-hospital and after discharge, that can cause side effects including bleeding. Post-op AF at discharge can cause palpitations, fatigue, and decreased exercise tolerance. Patients with post-op AF also have higher long-term mortality,1,2 at least partly attributable to embolic stroke.3
Post-op AF is also associated with greater intensive care unit stays, total hospital stays, and total hospital costs. 4-6 Even with use of beta-blockers and amiodarone, ~1 of 4 patients still develop post-op AF, with inconsistent improvements in mortality or resource utilization.7 Hence, new therapies are needed to prevent post-op AF and its associated morbidity and healthcare costs.
The accumulated evidence from observational studies and clinical trials suggests that habitual intake of fish or fish oil reduces risk of coronary death, possibly related to fewer primary ventricular arrhythmias.8,9 Experimental evidence supports direct and indirect antiarrhythmic effects of long-chain n-3 polyunsaturated fatty acids (n-3-PUFA) in fish oil, especially in the setting of acute ischemia.8 Yet, effects of n-3-PUFA on atrial arrhythmias such as post-op AF remain uncertain. In experimental studies and short-term clinical trials, n-3-PUFA favorably influence several risk factors for AF.8,10-12 Only small trials of n-3-PUFA supplementation to prevent post-op AF have been performed, with mixed results.13 We designed and implemented the Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) trial to determine whether peri-operative administration of oral n-3-PUFA reduces post-op AF in patients undergoing cardiac surgery.
METHODS
Study Design and Patients
OPERA was an investigator-initiated, randomized, double-blind, placebo-controlled, clinical trial across 28 centers in the US, Italy, and Argentina to test the primary hypothesis that peri-operative n-3-PUFA supplementation reduces the occurrence of post-op AF in 1,516 patients undergoing cardiac surgery (ClinicalTrials.gov NCT00970489). The detailed methods have been published.14 The design was pragmatic to maximize practical application and generalizability to real-world patients and clinical care. Inclusion criteria were broad (Supplementary Table 1), including age ≥18 years, being scheduled for cardiac surgery on the following day or later, and presence of sinus rhythm on the screening ECG. Exclusions were absence of sinus rhythm at screening, regular use of fish oil, known allergy or intolerance to fish oil or olive oil, being currently pregnant, existing or planned cardiac transplant or ventricular assist device, or being unable or unwilling to provide informed consent. Use of chronic or prophylactic anti-arrhythmic drugs, history of prior AF, and planned AF ablation were not exclusions, given similar or higher risk of post-op AF in these patients and no known biologic interaction that might reduce efficacy of n-3-PUFA in such patients. The study was approved by human subjects committees of all participating institutions and conducted according to international standards of Good Clinical Practice (FDA Title 21 part 312, International Conference on Harmonization guidelines). All patients provided informed written consent.
Intervention
Patients were block-randomized to receive 1 g n-3-PUFA capsules containing at least 840 mg of eicosapentaenoic acid (EPA, ~465 mg) plus docosahexaenoic acid (DHA, ~375 mg) as ethyl esters (Omacor, Pronova BioPharma, Norway) or matched placebo (olive oil) by means of computer-generated numbers, stratified by enrolling medical center and planned valve surgery (yes/no). Study drugs were prepared in identical-appearing capsules and specially coated to minimize taste differences. All investigators, patients, and providers were blinded to treatment assignment.
Patients received a total pre-operative loading dose of 10g, divided over 3-5 days (or 8g divided over 2 days) including the morning of surgery.14 For each patient, the loading dose was divided over the maximum number of days possible, based on the dated of enrollment and planned surgery. Flexibility in the loading days regimen maximized generalizability by allowing enrollment of most patients undergoing cardiac surgery, including those scheduled as early as the next day. Following cardiac surgery, patients received 2 g/d until hospital discharge or post-op day 10, whichever occurred sooner, at which time administrative censoring occurred for in-hospital follow-up.
The dosing was selected to balance potential efficacy vs. patient intolerance and risk. Cohort studies suggest that n-3-PUFA reduce risk of primary ventricular arrhythmias at low doses, e.g., 250-500 mg/d EPA+DHA;15 similar low dietary doses have been associated with lower incident AF in ambulatory adults;16 and in one small open-label trial, a 10g pre-op loading dose over 5 d followed by 2 g/d post-op reduced PoAF.17 n-3-PUFA supplementation alters circulating and tissue levels of EPA and DHA within days.18 Because n-3-PUFA persist in tissues for several days, a loading dose also provides some buffer in patients who might not tolerate oral medications for several days post-surgery. Higher doses could increase patient dyspepsia as well potential concern among treating physicians for risks such as bleeding.
For patients unable to tolerate oral medications post-operatively, the study drug could be administered via non-PVC nasogastric/gastric tube if present for clinical indications, or otherwise as soon as the patient was tolerating oral medications. Compliance was monitored by capsule count for outpatient loading and by hospital records for inpatient administration; as well as by changes in plasma phospholipid n-3-PUFA levels (see Covariates, below).
Centers were encouraged to utilize continuous electrocardiographic monitoring for at least 5 days post-surgery. Twelve-lead ECGs were recommended daily and more frequently at the discretion of the treating physicians for symptoms or clinically suspected arrhythmia. Clinical data (e.g., onset time, symptoms, treatments, duration) and confirmatory rhythm strips/12-lead ECGs were collected on all post-operative arrhythmias of at least 30 sec duration, including post-op AF and other tachyarrhythmias (Supplementary Table 2). Data on at least the first 3 suspected episodes of post-op AF were collected in each patient. All other treatments, including surgical and anesthetic procedures, medications including regular or prophylactic anti-arrhythmic drugs, and treatment of arrhythmias remained entirely at the discretion of the physicians caring for the patient. Current best-practice guidelines for prevention of post-op AF were strongly recommended to all Centers.19
Endpoints
The primary endpoint was the occurrence of post-op AF of at least 30 sec duration and documented by rhythm strip or 12-lead ECG (Supplementary Table 2). Secondary AF endpoints included post-op AF that was sustained (>1 hour), symptomatic, or treated with pharmacological or electrical cardioversion; post-op AF excluding atrial flutter; time to first post-op AF; and the number of post-op AF episodes per patient. OPERA also evaluated the total number of in-hospital days in which any post-op AF, including sustained post-op AF, was present; and the proportion of in-hospital days free of any post-op AF. All potential episodes of post-op AF and other tachyarrhythmias were reviewed and adjudicated by a centralized Events Committee of cardiac electrophysiologists. Additional endpoints included resource utilization, major adverse cardiovascular events (MACE), arterial thromboembolism, and 30-day mortality.
Safety Evaluation
Safety outcomes included adverse events and bleeding assessed by 24-hour chest tube output following surgery, total packed red blood cell transfusions, and composite bleeding indices (Supplementary Table 2). Potential adverse events were recorded and reported to the Steering Committee and the independent Data Safety and Monitoring Board (DSMB), as well as to the US Food and Drug Administration, European Medicine Agency, and Argentina National Administration of Drugs, Foods and Medical Devices. The Steering Committee monitored the progress of the trial, and the DSMB monitored both scientific integrity and patient safety throughout the trial and could recommend termination or other trial modifications at any time. In July 2011, the DSMB reviewed a detailed interim analysis prepared by a biostatistician not affiliated with the trial and recommended study continuation.
Covariates
Standardized data were collected on demographics, risk factors, major comorbidities, past medical/surgical history, anthropometry, lifestyle habits, outpatient and inpatient medications, and laboratory measures. Details of the surgical and anesthetic procedure were recorded. Daily follow-up and discharge information, including cardiac drug use and bleeding endpoints, was recorded. In a subset of 523 patients from centers participating in biologic studies, plasma EDTA was drawn at enrollment and on the morning of cardiac surgery, stored at −70°C, and shipped on dry ice to a central repository for long-term storage at −80°C. Plasma phospholipid n-3-PUFA (EPA+DPA+DHA) were measured as a percent of total fatty acids at the Fred Hutchinson Cancer Research Center (Seattle, Washington) using established methods,20 with coefficients of variation for EPA, DPA, and DHA <3%.
Statistical Analysis
All analyses were prespecified prior to closing of the study database. The main analysis was by intention-to-treat (ITT), including all patients according to treatment assigned at randomization. The primary endpoint was evaluated using Pearson chi-square (for two groups, equivalent to binomial test of proportions). Logistic regression was used to determine the odds ratio and 95% CI and for secondary multivariate analyses and tests of interaction. Survival analyses and the log-rank test were used for incident post-op AF and MACE, arterial thromboembolism, and mortality. In all analyses, missing values were not imputed, and only observed values were used. All patients who withdrew or died were included in all analyses until their date of death or withdrawal. A sensitivity analysis considered all patients who died, withdrew, or were otherwise lost to follow-up before hospital discharge or post-op day 10, whichever earlier, as having had post-op AF. Planned enrollment of 1,516 patients provided 90% power to detect a 25% reduction in post-op AF with two-tailed alpha=0.05, based on an estimated 30% event rate in controls and 5% drop-out. The control event rate was estimated from prior studies of post-op AF,1,2 and the 25% reduction as a reasonable minimum clinically meaningful risk reduction that was also considerably more conservative than earlier studies.17
Secondary multivariable analyses were prespecified, adjusted for age, sex, country, type of cardiac surgery, use of peri-operative antiarrhythmic drugs, and any baseline characteristics that were statistically different between treatment groups at P≤0.15. In addition to ITT analyses, we also secondarily evaluated (a) the surgical population, the subset of patients who were enrolled without protocol violation, received at least one dose of study drug, and underwent cardiac surgery; and (b) the adherent (on-treatment) population, the subset of the surgical population who took 80%+ of their loading dose (or 75%+ for patients loaded over 2 days) and also 80%+ of all assigned study pills over the course of the study until the onset of the primary endpoint or the end of assigned treatment, whichever first.
We hypothesized stronger efficacy of treatment in 3 prespecified subgroups: those with lower habitual oily fish consumption (<2 vs. ≥2 servings/week), lower enrollment plasma phospholipid n-3-PUFA levels (< vs. ≥4%, also evaluated continuously using semi-parametric restricted cubic splines), and greater number of actual study drug loading days (0 to 5 actual days, evaluated ordinally). Interaction by these subgroups was evaluated at two-tailed alpha=0.05. Other subgroup analyses (Supplementary Appendix) were also prespecified but considered exploratory (i.e., no hypothesized direction of interaction), and based on lower statistical power to detect interaction, were evaluated at two-tailed alpha=0.10. Analyses were performed using Stata 12.1 (College Station, TX).
RESULTS
Between Aug 2010 and Jun 2012, 1,516 patients were enrolled (Figure 1). Formal screening logs were maintained, and 48% of all screened patients and 94% of all eligible patient were enrolled. Ineligible patients were most often excluded because they were not in sinus rhythm (40.5%), were on fish oil (28.9%), or were unwilling to provide informed consent (23.5%). At baseline, mean±SD age was 64±13 y, 72.2% (n=1094) were male, and cardiovascular risk factors were common (Table 1). Fifty-two percent of patients (785 of 1516) had planned valve surgery; of the 756 who underwent valve surgery, 522 (69.0%) were aortic, 195 (25.8%) mitral, 30 (4.0%) aortic and mitral, and 9 (1.2%) other valves. The median (25th, 75th percentile) logistic EuroSCORE21,22 was 3.7 (1.9, 7.4). The treatment groups had evenly matched characteristics.
Figure 1. Screening, Randomization, and Follow-up.
Patients could be excluded based on more than one criterion; all are listed. No subjects were lost to follow-up.
Table 1. Baseline Characteristics of the OPERA Participants, According to Treatment Assignment.
| Placebo (N=758) |
n-3-PUFA (N=758) |
P value* | ||
|---|---|---|---|---|
| Age, years (SD) | 63.6 (12.4) | 63.8 (12.6) | 0.75 | |
| Male sex, n (%) | 543 (71.6) | 551 (72.7) | 0.65 | |
| Planned valve surgery, n (%) † | 389 (51.3) | 396 (52.2) | 0.72 | |
| Current smoking, n (%) | 96 (13.0) | 99 (13.5) | 0.78 | |
| Body mass index, kg/m2 (SD) | 28.4 (5.9) | 28.1 (5.4) | 0.30 | |
| Waist circumference, cm (SD) | 99.4 (13.5) | 98.8 (14.1) | 0.16 | |
| Hypertension, n (%) | 563 (74.9) | 572 (76.2) | 0.56 | |
| Dyslipidemia, n (%) | 477 (64.1) | 460 (61.7) | 0.33 | |
| Diabetes mellitus, n (%) | 199 (26.3) | 194 (25.7) | 0.78 | |
| Chronic obstructive pulmonary disease, n (%) | 90 (11.9) | 80 (10.6) | 0.42 | |
| Chronic renal failure, n (%) | 52 (6.9) | 44 (5.8) | 0.40 | |
| Coronary heart disease, n (%) ‡ | 288 (38.0) | 297 (39.2) | 0.64 | |
| Prior myocardial infarction, n (%) | 178 (23.6) | 188 (25.0) | 0.55 | |
| Prior percutaneous coronary intervention, n (%) |
99 (13.1) | 80 (10.6) | 0.13 | |
| Prior cardiac surgery, n (%) | 48 (6.3) | 45 (5.9) | 0.75 | |
| Coronary bypass, n (%) | 18 (2.4) | 17 (2.2) | 0.86 | |
| Valve surgery, n (%) | 27 (3.6) | 23 (3.0) | 0.57 | |
| Other cardiac surgery, n (%) | 12 (1.6) | 13 (1.7) | 0.84 | |
| Prior arrhythmias, n (%) | 99 (13.3) | 92 (12.5) | 0.64 | |
| Atrial fibrillation | 62 (8.4) | 52 (7.1) | 0.35 | |
| Other supraventricular tachycardia | 8 (1.1) | 12 (1.6) | 0.40 | |
| Ventricular tachycardia or fibrillation | 5 (0.7) | 7 (0.9) | 0.51 | |
| Other | 33 (4.5) | 22 (3.0) | 0.14 | |
| Congestive heart failure, n (%) | 212 (28.0) | 204 (27.0) | 0.66 | |
| NYHA Class, n (%) | I | 10 (1.3) | 4 (0.5) | |
| II | 104 (13.7) | 100 (13.2) | ||
| III | 61 (8.1) | 77 (10.2) | 0.16 | |
| IV | 12 (1.6) | 8 (1.1) | ||
| Not available | 25 (3.3) | 15 (2.0) | ||
| Ejection fraction, percent (SD) | 56.8 (11.3) | 56.6 (11.4) | 0.67 | |
| Left atrial diameter, mm (SD) | 42.2 (7.6) | 42.1 (7.8) | 0.77 | |
| Prior implantable cardiodefibrillator, n (%) | 11 (1.5) | 5 (0.7) | 0.13 | |
| Prior pacemaker, n (%) | 9 (1.2) | 11 (1.5) | 0.54 | |
| Prior cardiac resynchronization therapy, n (%) | 0 (0.0) | 1 (0.1) | 0.50 | |
| Prior AF ablation, n (%) | 6 (0.8) | 6 (0.8) | 1.00 | |
| EuroSCORE logistic, median (25th, 75th %) § | 3.6 (1.8, 7.2) | 3.7 (2.0, 7.5) | 0.64 | |
| EuroSCORE additive, median (25th, 75th %) § | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | 0.68 | |
| Medications at enrollment, n (%): | ||||
| Beta blocker | 433 (57.2) | 444 (58.6) | 0.57 | |
| Statin | 427 (56.3) | 436 (57.5) | 0.64 | |
| Angiotension converting enzyme- inhibitor |
274 (36.2) | 284 (37.5) | 0.59 | |
| Angiotensin receptor blocker | 103 (13.6) | 114 (15.0) | 0.42 | |
| Calcium channel blocker | 122 (16.1) | 132 (17.4) | 0.49 | |
| Loop diuretics | 168 (22.2) | 190 (25.1) | 0.19 | |
| Aldosterone antagonists | 37 (4.9) | 28 (3.7) | 0.25 | |
| Digitalis | 2 (0.3) | 14 (1.9) | 0.004 | |
| Antiplatelets or anticoagulants | 473 (62.4) | 455 (60.0) | 0.34 | |
| Antiplatelets | 407 (53.7) | 389 (51.3) | 0.36 | |
| Aspirin | 396 (52.2) | 378 (49.9) | 0.36 | |
| Clopidogrel | 53 (7.0) | 62 (8.2) | 0.38 | |
| Ticlopidine | 5 (0.7) | 4 (0.5) | 1.00 | |
| Others | 5 (0.7) | 4 (0.5) | 1.00 | |
| Anticoagulants | 129 (17.0) | 121 (16.0) | 0.58 | |
| Warfarin | 24 (3.2) | 17 (2.2) | 0.27 | |
| Heparin | 105 (13.9) | 103 (13.6) | 0.88 | |
| Others | 1 (0.1) | 1 (0.1) | 1.00 | |
| Amiodarone | 28 (3.7) | 30 (4.0) | 0.78 | |
| Other antiarrhythmics¥ | 12 (1.6) | 14 (1.9) | 0.69 | |
Values are mean (SD) for continuous variables and number (percent) for categorical variables.
Differences between treatment groups were evaluated using unpaired Students t-tests or Wilcoxon rank-sum tests, as appropriate, for continuous variables; and Pearson chi-square (or Fisher exact tests for cells<10) for categorical variables.
Of the 756 patients who underwent valve surgery, 522 (69.0%) underwent aortic valve surgery, 195 (25.8%) mitral valve surgery, 30 (4.0%) aortic and mitral valve surgery, and 9 (1.2%) other valve surgery; see Supplementary Table 3.
Prior myocardial infarction, coronary revascularization, or angina.
The EuroSCORE (European System for Cardiac Operative Risk Evaluation) incorporates 17 predictive factors about the patient, comorbidities, and the planned operation to calculate the risk of 30-day post-operative mortality.21,22 The EuroScore can be calculated using either a logistic or additive model, with higher scores indicating higher risk. The logistic model (theoretical range: 0 to 100) provides a score that is directly equivalent to the predicted 30-day mortality (percent). The additive model (theoretical range: 0 to 39) is a simplified version that approximates the predicted 30-day mortality. Values for the 17 individual components of these scores were also well balanced between treatment groups (Supplementary Table 4).
Including flecainide, propafenone, quinidine, disopyramide, or sotalol.
NYHA=New York Heart Association.
Following randomization and study drug loading, 96.4% of patients underwent cardiac surgery. Details of the surgical procedure and post-operative medications are in Supplementary Table 3. Post-operative medications were similar by treatment group and included beta-blockers in 76.9% and amiodarone in 36.9% of patients after cardiac surgery.
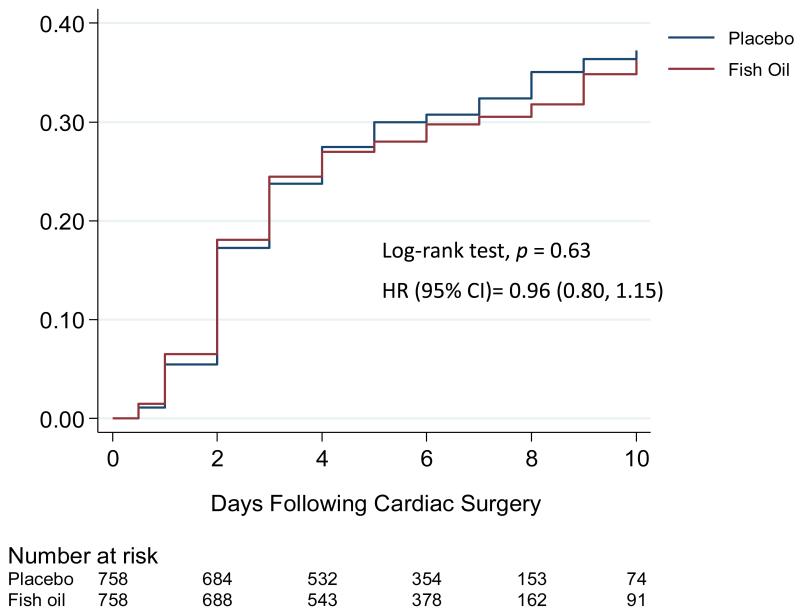
The primary endpoint occurred in 233 patients (30.7%) in the placebo group and 227 patients (30.0%) in the n-3-PUFA group (P=0.74). None of the secondary post-op AF endpoints or other arrhythmias were significantly different by treatment group (Table 2, Figure 2). In-hospital MACE occurred in 2.6 and 1.7% of patients in the placebo and PUFA groups, respectively (P=0.18). At 30 days, 15 (2.0%) and 8 (1.1%) of patients, respectively, had died (P=0.14). Incidence of the secondary endpoints of arterial thromboembolism and arterial thromboembolism or death was significantly lower in the n-3-PUFA group (P=0.047, P=0.01, respectively). The total number of days in the ICU/CCU, of telemetry monitoring, or total hospital stay did not differ significantly.
Table 2. Primary and Secondary Study Outcomes in OPERA, According to Treatment Assignment.
| Outcome | Placebo (n=758) |
n-3-PUFA (n=758) |
Odds Ratio or Hazard Ratio* (95% CI) |
P value† | |
|---|---|---|---|---|---|
|
Any first post-op AF, primary endpoint, n (%) ‡ |
233 (30.7) | 227 (30.0) | 0.96 (0.77, 1.20) | 0.74 | |
| Post-op AF, secondary endpoints | |||||
| Sustained, symptomatic, or treated post-op AF, n (%) § |
231 (30.5) | 224 (29.6) | 0.96 (0.77, 1.19) | 0.70 | |
| Post-op AF excluding flutter, n (%)∥ | 220 (29.0) | 217 (28.6) | 0.98 (0.79, 1.23) | 0.87 | |
| No. of post-op AF episodes, n (%) | 1 | 156 (20.6) | 157 (20.7) | ||
| 2 | 59 (7.8) | 49 (6.5) | n/a | 0.73 | |
| 3+ | 18 (2.4) | 21 (2.8) | |||
| Total in-hospital days with any post-op | 2.75 (2.1) | 2.84 (2.1) | n/a | 0.58 | |
| AF, mean (SD) ¥ | |||||
| Proportion of in-hospital days free of any post-op AF, percent |
89.0 | 88.7 | n/a | 0.88 | |
| Other arrhythmias, n (%) | |||||
| Other supraventricular tachycardia | 6 (0.8) | 11 (1.5) | 1.85 (0.68, 5.02) | 0.33 | |
| Ventricular tachycardia or fibrillation | 9 (1.2) | 5 (0.7) | 0.55 (0.18, 1.66) | 0.42 | |
| Other endpoints, n (%) | |||||
| MACE, in-hospital ¶ | 20 (2.6) | 13 (1.7) | 0.62 (0.31, 1.25) | 0.18 | |
| Myocardial infarction | 10 (1.3) | 10 (1.3) | 0.99 (0.41, 2.39) | 1.00 | |
| Stroke | 8 (1.1) | 4 (0.5) | 0.45 (0.13, 1.51) | 0.18 | |
| Cardiovascular death | 3 (0.4) | 0 (0.0) | n/a | 0.08 | |
| Arterial thromboembolism, 30 days | 13 (1.7) | 5 (0.7) | 0.37 (0.13-1.03) | 0.047 | |
| Arterial thromboembolism or death, 30 days |
27 (3.6) | 13 (1.7) | 0.43 (0.22-0.84) | 0.01 | |
| Total mortality, 30 days | 15 (2.0) | 8 (1.1) | 0.53 (0.23-1.26) | 0.14 | |
| - Cardiac arrhythmic | 0 (0.0) | 1 (0.1) | -- | 0.32 | |
| - Cardiac nonarrhythmic | 2 (0.3) | 0 (0.0) | -- | 0.16 | |
| - Vascular | 3 (0.4) | 0 (0.0) | -- | 0.08 | |
| - Noncardiovascular | 10 (1.3) | 7 (0.9) | 0.70 (0.27-1.84) | 0.47 | |
| Resource utilization, median (25th, 75th %) | |||||
| Total ICU/CCU stay, days | 2 (1, 3) | 2 (1, 3) | n/a | 0.38 | |
| Total telemetry monitoring, days | 6 (5, 7) | 6 (5, 7) | n/a | 0.39 | |
| Total hospital stay, days | 7 (5, 8) | 7 (5, 9) | n/a | 0.48 | |
All analyses were based on intention-to-treat. Values are odds ratios estimated using logistic regression for post-op AF and other arrhythmias; and hazard ratios estimated using Cox proportional hazards for other endpoints such as MACE.
Determined using Pearson chi-square (or Fisher exact tests for cells<10) for the primary post-op AF endpoint, the first two secondary post-op AF endpoints, and other tachyarrhythmias; Poisson regression for the total number of post-op AF events per patient and the total number of in-hospital days with one or more episodes of post-op AF; the Wilcoxon rank-sum test for the proportion of in-hospital days free of any post-op AF and days of resource utilization; and the log-rank test for MACE, arterial thromboembolism, and mortality endpoints.
The median (25th, 75th %) duration of the first post-op AF episode was 0.92 (0.17, 1.0) days in the placebo group and 1.0 (0.17, 1.0) days in the n-3-PUFA group (Wilcoxon rank-sum P=0.62).
A total of 661 post-op AF episodes occurred in 460 patients. Among these episodes, 8.9% were associated with dyspnea, chest pain, or hypotension requiring escalation of therapy; 76.7% were treated with electrical or pharmacological cardioversion (predominantly amiodarone); 87.4% lasted more than 1 hour; and 97.9% met one or more of these criteria.
Excluding atrial flutter and supraventricular tachycardia with some but not all characteristics consistent with atrial fibrillation.
Among the 460 patients with post-op AF. Among all 1,516 patients, the corresponding values were 0.8 (1.7) days in the placebo group and 0.8 (1.7) days in the n-3-PUFA group (P=0.93).
See Supplementary Table 2 for detailed definitions of all events. Analyses of individual MACE components include all subtypes of events occurring in any patient.
Post-op AF = post-operative atrial fibrillation or flutter. MACE = myocardial infarction, stroke, or cardiovascular death. ICU/CCU = intensive care unit/coronary care unit.
Figure 2. Kaplan Meier Incidence of Post-Operative Atrial Fibrillation, According to Treatment Group.
Most post-op AF events occurred between post-op days 1 to 4, peaking on day 2 (Supplementary Figure 1). Among all 661 episodes, 353 lasted less than 1 day (median duration=3.3 hours, 25th, 75%=1.2, 7.7 hours), and 308 lasted 1 day or longer (median duration=1.0 day, 25th, 75%=1.0, 3.0 days). There were no significant differences in duration by treatment (Wilcoxon rank-sum P=0.25). In the placebo and n-3-PUFA groups, respectively, 32 and 30 patients were discharged with persistent post-op AF.
In sensitivity analyses defining all patients who died or withdrew consent as having had post-op AF, 266 (35.1%) and 250 (33.0%) patients in the placebo and n-3-PUFA groups, respectively, developed post-op AF (P=0.39). Findings were similar in multivariable analyses adjusted for age, sex, country, type of cardiac surgery, peri-operative antiarrhythmic drugs, and baseline characteristics differing between groups (Supplementary Materials).
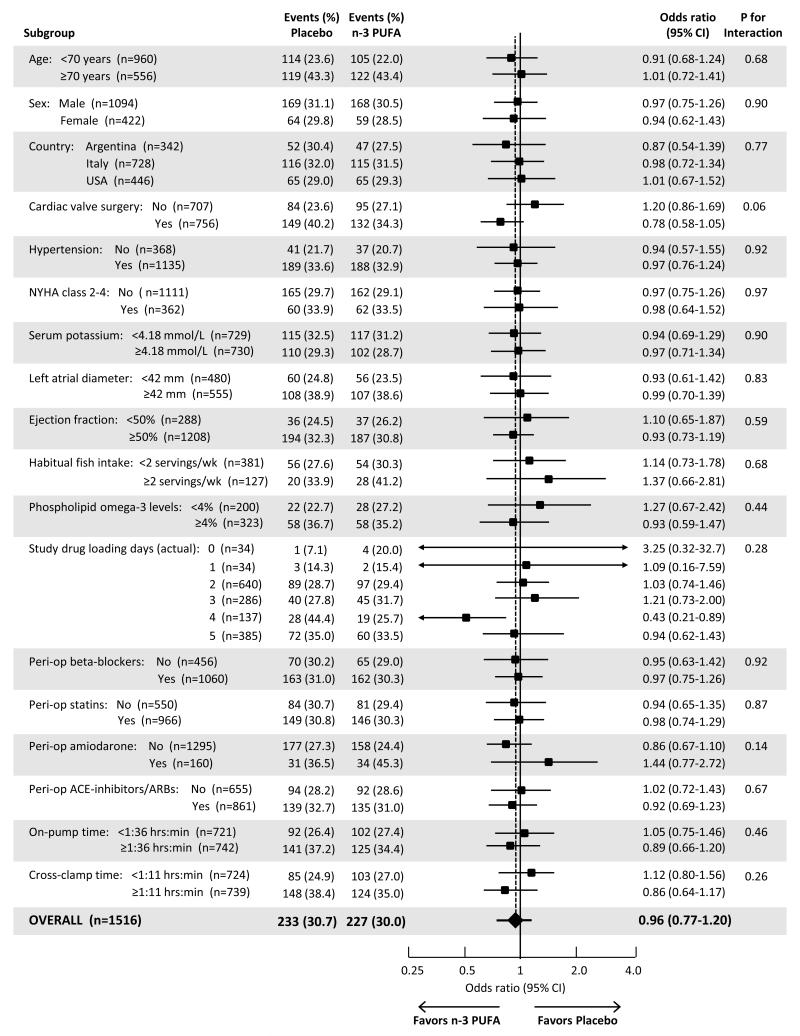
Effects of n-3-PUFA on the primary endpoint did not significantly differ among most prespecified subgroups (Figure 3). Potential interaction (prespecified alpha=0.10) was observed only by type of cardiac surgery (P-interaction=0.06), with patients undergoing valve surgery having a trend toward lower risk of post-op AF with n-3-PUFA treatment. In post-hoc analyses, risk of post-op AF with n-3-PUFA treatment was similar whether valve surgery was aortic or mitral (Supplementary Materials).
Figure 3. Efficacy of Omega-3 PUFA to Prevent Post-Operative AF, According to Prespecified Baseline Characteristics.
All characteristics are at enrollment except for study drug loading days, peri-operative medication use (defined as use at enrollment and/or the morning after cardiac surgery), on-pump time, and cross-clamp time. Only patients with complete data on the relevant stratifying variable were included in each subgroup analysis. Logistic regression was used to determine the odds ratio and 95% CI for the effect of treatment in each subgroup. The statistical significance of potential interaction was quantified using the Wald test for a multiplicative interaction term (treatment × stratifying variable).
In the predefined “surgical” population (underwent surgery, received at least one dose of study drug), 233 of 723 (32.2%) and 225 of 728 (30.9%) patients assigned to placebo and n-3-PUFA, respectively, developed post-op AF (P=0.60). In the further subset of adherent patients (taking 80%+ of assigned study drug), which included 591 of 723 patients (81.7%) assigned to placebo and 596 of 728 patients (81.9%) assigned to n-3-PUFA, 186 (31.5%) and 178 (29.9%) patients, respectively, developed post-op AF (P=0.55).
From the time of enrollment to the morning of cardiac surgery, plasma phospholipid n-3-PUFA concentrations increased ~40% in the n-3-PUFA group, from 4.65±1.44 to 6.40±1.70 percent of total fatty acids, while they remained unchanged in the placebo group (4.65±1.43 to 4.78±1.43) (P<0.001 for change in fatty acid levels by treatment group). Stratified by actual study drug loading days (1 to 5), phospholipid n-3-PUFA in the treatment group increased by 0.47, 1.11, 1.91, 2.55, and 2.30 percent of total fatty acids. Effects of n-3-PUFA treatment on post-op AF, however, were not significantly different by loading days (Figure 3).
Compared with placebo, patients in the n-3-PUFA group received significantly fewer packed red blood cell transfusions, including during surgery (P=0.002), post-surgery (P=0.008), and overall (P<0.001) (Supplementary Table 5). Other bleeding indices did not significantly differ by treatment. Minor adverse events commonly seen with fish oil, such as gastrointestinal upset, burping, and fish oil taste, occurred more commonly in the n-3-PUFA group. Adverse events requiring discontinuation of study drug and other serious adverse events were similar between groups (Supplementary Table 6).
DISCUSSION
This large, multinational, double-blind, placebo-controlled clinical trial found no evidence that peri-operative n-3-PUFA supplementation reduced post-op AF. Results were similar for various secondary endpoints, among different patient subgroups, and in various sensitivity analyses. Major strengths of OPERA include its large size and large numbers of events, which achieved anticipated statistical power. Our broad inclusion criteria and multinational enrollment each augment the generalizability of our findings.
In controlled trials lasting weeks to months, n-3-PUFA supplementation favorably influences several physiologic pathways related to AF, including blood pressure, systemic vascular resistance, heart rate, inflammation, endothelial function, left ventricular diastolic function, myocardial efficiency of oxygen use, and possibly vagal tone.8 Our design cannot exclude potential benefits of much longer durations (e.g., weeks to years) of n-3 PUFA supplementation for altering systemic physiology and risk of AF in other clinical contexts. Such prolonged durations of therapy would be impractical as a common preventive measure for most patients scheduled to undergo cardiac surgery. Based on the known benefits of n-3-PUFA on cardiovascular risk factors and physiologic pathways, a more promising strategy may be long-term consumption to reduce the primary incidence of AF among ambulatory elderly adults with hypertension or other risk factors;16 such an approach should be tested in appropriately designed and powered clinical trials.
Subgroup analyses did not detect any difference in efficacy depending on baseline fish consumption or circulating n-3-PUFA levels. Observational studies of fish consumption and coronary heart disease death in generally healthy populations suggest that some dietary n-3-PUFA (~250 mg/d EPA+DHA, or about 1-2 fish servings/week) is better than none, but that greater consumption may not substantially alter risk further.23 Our findings do not support such a dose-response for post-op AF. Findings were also similar in other subgroups, except for a suggestion of greater efficacy among patients undergoing valve surgery. Based on the multiple subgroups explored, this finding most plausibly results from chance and should be interpreted cautiously until evaluated prospectively in future studies.
Experimental studies suggest that n-3-PUFA have antiarrhythmic actions.24,25 Our findings provide no evidence that short-term n-3-PUFA supplementation provides clinically relevant anti-arrhythmic effects in the acute setting of cardiac surgery. Further, there is no consistent evidence that n-3-PUFA are effective anti-arrhythmic agents in the context of established cardiac arrhythmias, such as in patients with prior ventricular arrhythmias and implantable cardiodefibrillators or with prior established chronic or paroxysmal AF.26-32 Early clinical trials demonstrated that long-term fish33 or fish oil34,35 intake reduced the risk of cardiac death in patients with recent myocardial infarction, and the overall evidence from subsequent experiments, observational studies, and clinical trials continues to point toward a reduction in cardiac death as the principal cardiovascular benefit of long-term n-3-PUFA intake.8,9
Prior smaller trials found mixed effects of peri-operative n-3-PUFA on post-op AF.17,36-41 Two studies reported a reduction in post-op AF,17,39 but were small (<40 events each) and also open-label, i.e., neither placebo-controlled nor double-blind. Five other small placebo-controlled trials found no significant effects of n-3-PUFA on post-op AF,36-38,40,41 but the small numbers of events in each study (range: 24 to 91 events each) limited statistical power and made it difficult to draw strong conclusions about absence of effects. OPERA, comprising more subjects and AF events than all of these prior trials combined, provides the most definitive answer to this important research question. The variation in findings of prior small trials and especially studies without placebo-control highlights the importance of conducting large, appropriately powered, and placebo-controlled trials such as the present study.
Many drugs have been tested but failed to prevent post-op AF; others, such as beta-blockers and amiodarone, only partly reduce risk.2 The impact of cardiac surgery on neurohormonal, oxidative, and inflammatory activation and atrial remodeling may simply be too great to be countered by most drugs, including n-3-PUFA. Post-op AF remains an intractable and enigmatic complication of surgery. Our findings and those of prior studies highlight the need for meticulous investigation of the underlying physiologic, structural, and molecular underpinnings of post-op AF to allow novel targeted preventive and therapeutic interventions.
The secondary endpoints of arterial thromboembolism and arterial thromboembolism or death occurred less frequently in the n-3-PUFA group. However, numbers of events were small, and these findings should be viewed with caution until confirmed in future studies. n-3-PUFA supplementation was well-tolerated in this large and heterogeneous population of cardiac surgery patients, and there was no evidence that n-3-PUFA supplementation was unsafe. While there have been theoretical concerns that n-3-PUFA could aggravate bleeding, no increased hemorrhagic risk was seen across a variety of indices. Indeed, patients in the n-3-PUFA group required significantly fewer transfusions; this finding may be due to chance, but further supports absence of increased bleeding risk. These results confirm and extend the findings of prior smaller trials which found no evidence that n-3-PUFA supplementation increased clinical bleeding following cardiac surgery,17,36-40 surgical arterial endarterectomy,42,43 or coronary angioplasty.44 More than half the patients in OPERA were taking aspirin or other anticoagulants before cardiac surgery, similar to other trials in which n-3-PUFA was combined with aspirin or warfarin following cardiac surgery without any excess clinical bleeding.45
Limitations should be considered. Current best-practice guidelines for preventing PoAF were recommended to all Centers, which could have reduced the impact of any additional therapy on risk of PoAF. As would be reflected in clinical practice, patients were identified and assigned to receive n-3-PUFA over varying durations ranging from 2 to 5 days prior to surgery. Shorter durations could have been less effective. Subgroup analyses, however, did not detect significantly greater risk reduction with greater numbers of days of pre-op n-3-PUFA, providing little evidence that longer durations of loading make a difference, at least up to 5 days. The dose of n-3-PUFA may have been too low to produce a benefit, and we did not have available data on achieved myocardial levels of n-3-PUFA. Yet, circulating n-3-PUFA have systemic effects that could reduce AF risk,8 and phospholipid n-3-PUFA levels increased by an average of 40% by the time of surgery, providing novel evidence that even short-term supplementation significantly influences circulating levels. Compliance with study drug was high but not perfect. Our analysis restricted to adherent patients, however, was consistent with the main findings.
In summary, peri-operative n-3-PUFA supplementation did not reduce post-op AF in this large, adequately powered, placebo-controlled, multinational trial.
Supplementary Material
ACKNOWLEDGEMENTS
Data Safety and Monitoring Board: David M. Herrington, MD MHS (chair) (Wake Forest University School of Medicine, Winston-Salem, NC), Maria Mori Brooks, PhD (University of Pittsburgh, Pittsburgh, PA), Raffaele De Caterina, MD PhD (Università degli Studi “G. d’Annunzio”, Chieti, Italy), Marc Gillinov, MD (Cleveland Clinic Main Campus, Cleveland, OH), Luigi Padeletti, MD (University of Florence, Florence, Italy), Fabio Pellegrini, MS (Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy), Barbra Bluestone Rothschild, MD (The University of North Carolina at Chapel Hill, Chapel Hill, NC), Fernando Rubinstein, MD MPH (IECS-Instituto de Efectividad Clínica y Sanitaria, Buenos Aires, Argentina).
Biomarker and Cognitive Decline Ancillary Study: Pho Q. Diep, BS, Junitta B. Guzman, MS (Fred Hutchinson Cancer Research Center, Seattle, WA).
Main Data Coordinating Center, Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy: Laura Palmarini, PharmD, Stefania Sacchetti, PharmD, Anna V. Flamminio, Rosamaria Marfisi, MS, Marco Scarano, MS.
U.S. Clinical Coordinating Center, Harvard School of Public Health, Boston, MA: Ashkan Afshin, MD MPH, Hongyan Huang, MS PhD, Fadar Otite, MD MS.
Italy Centers
Ospedali Riuniti di Bergamo, Bergamo, Italy: Marilisa Ambrosio, BS, Annarita Lincesso, BS, Laura Pezzoli, BS.
Ospedali Riuniti and University of Trieste, Trieste, Italy: Marco Anzini, MD, Cosimo Carriere, MD, Rita Belfiore, MD.
Department of Surgical Sciences, University of Torino, Division of Cardiac Surgery, Torino, Italy: Guglielmo Fortunato, MD, Augusto Pellegrini, MD, Eliana Raviola, MD, Vincenzo Reale, MD, Paolo Sorrentino, MD.
GVM Care&Research – E.S. Health Science Foundation, Cotignola, Italy: Giulia Schiavina, MSB, Monica Negrosanti, BS.
Azienda Ospedaliera Ordine Mauriziano di Torino, Ospedale Mauriziano Umberto I, Torino, Italy: Giacomo Ravenni, MD.
Centro Cardiologico Monzino, I.R.C.C.S, Milano, Italy: Franco Moro, Marta Brambilla, PhD, Cristina Nava, MD, PhD, Monica Giroli, BS, Andrea Daprati, MD, Marco Gennari, MD, Dennis Ezra Puma Cusihuaman, MD.
GVM Care&Research – E.S. Health Science Foundation, Lecce, Italy: Barbara Spagnolo, PhD, Michela Francia, MS, Maurizio Stanca, MS.
Fondazione I.R.C.C.S. Policlinico S. Matteo, Pavia, Italy: Marco Paris, MD, Daniele Berwick, MD, Bruno Lusona, MD, Nicoletta Castiglione, MD.
Azienda Ospedaliero – Universitaria Santa Maria della Misericordia, Udine, Italy: Marzia De Biasio, MD, Cristian Daffarra, MD.
US Centers
Vanderbilt University, Nashville, TN, US: Rashid Ahmad, MD, Carol A. Meisch, RN BSN CCRP, Simon Maltais, MD, Jorge Balaguer, MD.
Wash University, St Louis, MO, US: Tamara Donahue, RN BSN.
Emory Healthcare, Atlanta, GA, US: Robert A. Guyton, MD, Vinod Thourani, MD, Michael Halkos, MD, Omar Lattouf, MD, Kim T. Baio, MSN, Samatha R. Levine, RN, Zachary E. Pitts, RN.
Sanford Health, Sanford USD Medical Center, Sioux Falls, SD, US: Kathy Janssen, Marilyn Ruhlman, RN BSN PA-C CCRP.
University of Arizona and Tucson Medical Center, Tucson, AZ, US: Sarah Sharp, BS.
State University of New York Downstate, Brooklyn, NY, US: Kristy Pang, BS RN.
Argentina Centers
Fundacion Favaloro, Buenos Aires, Argentina: Matías Nicolás Mungo, SC, Ada Liz Servián, RN.
Hospital Italiano, Buenos Aires, Argentina: Roberto Battellini, MD, Ricardo Marenchino, MD, Vadim Kotowicz, MD, Vicente Cesáreo, MD, Ricardo Sánchez, MD, Veronica Romero, SC, Silvia Avila, SC.
Hospital Espanol, Buenos Aires, Argentina: Fabian Donnini MD, Leticia Biancospino, BS.
Instituto de Cardiologia de Corrientes, Corrientes, Argentina: Romina Laurino, MD, Rodolfo Portalea, RN.
Analyses: Statistical analyses were performed by Dariush Mozaffarian at Harvard University and Maria Silleta and Marco Scarano at Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy. No separate compensation or funding was received for conducting the analyses. Dariush Mozaffarian and Roberto Marchioli had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: OPERA was an investigator-initiated, not-for-profit trial sponsored by the OPERA Investigators, who had full responsibility for study planning and conduct, curation of the study database, and discretion on data utilization, analysis, and publication. Financial support was provided by the National Heart, Lung, and Blood Institute, National Institutes of Health (RC2-HL101816), GlaxoSmithKline, Sigma Tau, and Pronova BioPharma, which also provided the study drug. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, GlaxoSmithKline, Sigma Tau, and Pronova BioPharma. ClinicalTrials.gov=NCT00970489.
Appendix
The OPERA Investigators – Authors
Drs. Mozaffarian and Marchioli contributed equally to all aspects of this manuscript, including serving as co-Principal Investigators of OPERA.
Steering Committee
Roberto Marchioli, MD (co-chair) (Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy), Dariush Mozaffarian, MD DrPH (co-chair) (Brigham and Women’s Hospital, Boston, MA), Timothy J. Gardner, MD (Christiana Care Health System, Newark, DE), Paolo Ferrazzi, MD (Ospedali Riuniti di Bergamo, Bergamo, Italy), Patrick T. O’Gara, MD (Brigham and Women’s Hospital, Boston, MA), Alejandro Macchia, MD (GESICA Foundation, Buenos Aires, Argentina), Massimo Santini, MD (Ospedale San Filippo Neri, Rome, Italy), Luigi Tavazzi, MD (Villa Maria Cecilia Hospital, Cotignola, Italy), Gianni Tognoni, MD (Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy), and the co-chairs of the Events and Biologic Studies Committees.
Events Committee
Richard L. Page, MD (co-chair) (University of Wisconsin, Madison, WI), Federico Lombardi, MD (co-chair) (University of Milan, Milan, Italy), Christine M. Albert, MD MPH (Brigham and Women’s Hospital, Boston, MA), Aldo P. Maggioni, MD (Centro Studi Associazione Nazionale Medici Cardiologi Ospedalieri, Florence, Italy), Katherine T. Murray, MD (Vanderbilt University School of Medicine, Nashville, TN).
Biologic Studies Committee
Roberto Latini, MD (co-chair) (Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy), Peter Libby, MD (co-chair) (Brigham and Women’s Hospital, Boston, MA), Bill Harris, PhD (Sanford School of Medicine, Sioux Falls, SD), Jeffery E. Saffitz, MD PhD (Harvard Medical School, Boston, MA), David Siscovick. MD MPH (University of Washington, Seattle, WA), Phyllis Stein, PhD (Washington University School of Medicine, St. Louis, MO), Domenico Corradi, MD (University of Parma, Parma, Italy), Serge Masson, MD (Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy).
Biomarker and Cognitive Decline Ancillary Study
Nancy J. Brown, MD, E. Wesley Ely, MD MPH, James C. Jackson, PsyD, Ayumi Shintani, PhD MPH, Ginger L. Milne, PhD (Vanderbilt University Medical School, Nashville, TN), Xiaoling Song, PhD (Fred Hutchinson Cancer Research Center, Seattle, WA), Frank W. Sellke, MD (Brown Medical School and Rhode Island Hospital, Providence, RI).
Main Data Coordinating Center, Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy
Maria G. Silletta, MS, Raffaella Pioggiarella PharmD, Lorenzo Marfisi, Meng.
U.S. Clinical Coordinating Center, Harvard School of Public Health, Boston, MA
Sarah L. King, BSN, Kristen E. Mills, MS, Adeyemi Ogunleye, MD SM, Namasha H. Schelling, BS ALM, Jason Wu, PhD.
Italy Centers
Ospedali Riuniti di Bergamo, Bergamo, Italy (197 patients enrolled)
Paolo Ferrazzi, MD (center PI), Caterina Simon, MD, Maria Iascone, BS PhD.
Ospedale S. Andrea,-Università La Sapienza, Roma, Italy (126 patients enrolled)
Riccardo Sinatra, MD (center PI), Umberto Benedetto, MD PhD.
Ospedali Riuniti and University of Trieste, Trieste, Italy (105 patients enrolled)
Lorella Dreas, MD (center PI), Aneta Aleksova MD MS.
Department of Surgical Sciences, University of Torino, Division of Cardiac Surgery, Torino, Italy (101 patients enrolled)
Mauro Rinaldi, (center PI), Stefano Salizzoni, MD, Giovanni Marchetto, MD.
GVM Care&Research - E.S. Health Science Foundation, Cotignola, Italy (62 patients enrolled)
Mauro Lamarra, MD (center PI), Marco Pagliaro, MD, Maria Cristina Jori, MD, Luca Dozza, MSB, Simone Calvi, MD.
Azienda Ospedaliera Ordine Mauriziano di Torino, Ospedale Mauriziano Umberto I, Torino, Italy (30 patients enrolled)
Riccardo Casabona, MD (center PI), Edoardo Zingarelli, MD, Roberto Flocco, MD.
Unit of Cardiac Surgery, Humanitas Clinical and Research Center, Rozzano, Italy (29 patients enrolled)
Alessandro Eusebio, MD (center PI), Giuseppe Raffa, MD, Giuseppe Tarelli, MD.
Centro Cardiologico Monzino I.R.C.C.S, Milano, Italy (24 patients enrolled)
Alessandro Parolari, MD PhD (center PI), Laura Cavallotti, MD, Veronica Miyasoedova, MD PhD, Federica Laguzzi, BS.
GVM Care&Research - E.S. Health Science Foundation, Lecce, Italy (19 patients enrolled)
Renato Gregorini, MD (center PI), Federica Mangia, MD.
Fondazione I.R.C.C.S. Policlinico S. Matteo, Pavia, Italy (15 patients enrolled)
Fabrizio Gazzoli, MD (center PI), Eliana Raviola, MD, Mario Viganò, MD.
Azienda Ospedaliero – Universitaria Santa Maria della Misericordia, Udine, Italy (12 patients enrolled)
Ugolino Livi (center PI) MD, Esmeralda Pompei, MD.
Ospedale San Bortolo, Vicenza, Italy (5 patients enrolled)
Loris Salvador, MD (center PI), Nicola Lamascese, MD, Massimo Bilotta, MD.
Niguarda Ca’ Granda Hospital, Department of Cardiac Surgery, Milan, Italy (3 patients enrolled)
Luigi Martinelli, MD (center PI), Aldo Cannata, MD.
US Centers
Vanderbilt University, Nashville, TN, US (99 patients enrolled)
Nancy J. Brown, MD (center PI), John Byrne, MD, Marzia Leacche, MD, Michael R. Petracek, MD, Stephen K. Ball, MD.
University of Texas Southwestern Medical Center Dallas, Dallas, TX, US (74 patients enrolled)
Michael E. Jessen, MD (center PI), Mary Weyant, RN BSN.
Wash University, St Louis, MO, US (66 patients enrolled)
Ralph J. Damiano, Jr., MD (center PI).
Rhode Island Hospital, Providence, RI, US (61 patients enrolled)
Frank W. Sellke, MD (center PI), Arun K. Singh, MD, Mary Jane McDonald, RN MS.
Brigham and Women’s Hospital, Boston, MA, US (54 patients enrolled)
R. Morton Bolman III, MD, (center PI), Debra A. Conboy, RN BSN, Anne Burgess, RN MSHI BSN.
Emory Healthcare, Atlanta, GA, US (45 patients enrolled)
John D. Puskas, MD (center PI).
Sanford Health, Sanford USD Medical Center, Sioux Falls, SD, US (31 patients enrolled)
John VanderWoude Jr., MD (center PI), Maria C. Bell, MD (center PI).
University of Arizona and Tucson Medical Center, Tucson, AZ, US (9 patients enrolled)
Gulshan Sethi, MD (center PI).
State University of New York Downstate, Brooklyn, NY, US (7 patients enrolled)
Daniel C. Lee, MD (center PI).
Argentina Centers
Fundacion Favaloro, Buenos Aires, Argentina (180 patients enrolled)
Roberto René Favaloro, MD (center PI), Alejandro Rubén Hershson, MD, Julio César Figal, MD.
Hospital Italiano, Buenos Aires, Argentina (59 patients enrolled)
Alberto Domenech, MD (center PI), Marcelo Halac, MD.
Hospital Espanol, Buenos Aires, Argentina (44 patients enrolled)
Liliana Noemí Nicolosi, MD (center PI), Claudio Gabriel Morós, MD, María del Carmen Rubio, MD, Richard Fuentes Suárez, MD.
Instituto de Cardiologia de Corrientes, Corrientes, Argentina (33 patients enrolled)
Horacio Cacheda, MD (center PI), Juan Pablo Casal, MD.
Clinica y Maternidad de Suizo, Buenos Aires, Argentina (23 patients enrolled)
Juan Carlos Medrano, MD (center PI), María Carla Cucurell, MD, Florencia Scattini, MD.
Instituto Fundacion de Lucha contra las Enfermedades Neurologicas en la Infancia, Buenos Aires, Argentina (3 patients enrolled)
Carlos Nojek, MD (center PI), Mariano Camporrotondo, MD.
Footnotes
See Appendix for the complete list of OPERA Investigators and institutions.
Contributor Information
Dariush Mozaffarian, Division of Cardiovascular Medicine and Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, and Departments of Epidemiology and Nutrition, Harvard School of Public Health, Boston, MA (DM).
Roberto Marchioli, Department of Clinical Pharmacology and Epidemiology, Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy.
Alejandro Macchia, GESICA Foundation, Buenos Aires, Argentina.
Maria G. Silletta, Department of Clinical Pharmacology and Epidemiology, Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy.
Paolo Ferrazzi, Ospedali Riuniti di Bergamo, Bergamo, Italy.
Timothy J. Gardner, The Center for Heart and Vascular Health, Christiana Care Health System, Newark, DE.
Roberto Latini, Department of Cardiovascular Research, Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy.
Peter Libby, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA.
Federico Lombardi, Department of Health Sciences, University of Milan, Milan, Italy.
Patrick T. O’Gara, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA.
Richard L. Page, Department of Medicine, University of Wisconsin, School of Medicine & Public Health, Madison, WI.
Luigi Tavazzi, GVM Hospitals of Care and Research, Villa Maria Cecilia Hospital, Cotignola, Italy.
Gianni Tognoni, Department of Clinical Pharmacology and Epidemiology, Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy.
REFERENCES
- 1.Hogue CW, Jr., Creswell LL, Gutterman DD, Fleisher LA. Epidemiology, mechanisms, and risks: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005 Aug;128(2 Suppl):9S–16S. doi: 10.1378/chest.128.2_suppl.9s. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell LB. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention and treatment of atrial fibrillation following cardiac surgery. Can. J. Cardiol. 2011 Jan-Feb;27(1):91–97. doi: 10.1016/j.cjca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Mariscalco G, Klersy C, Zanobini M, et al. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008 Oct 14;118(16):1612–1618. doi: 10.1161/CIRCULATIONAHA.108.777789. [DOI] [PubMed] [Google Scholar]
- 4.Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA. 1996 Jul 24-31;276(4):300–306. [PubMed] [Google Scholar]
- 5.Auer J, Weber T, Berent R, Ng CK, Lamm G, Eber B. Postoperative atrial fibrillation independently predicts prolongation of hospital stay after cardiac surgery. J. Cardiovasc. Surg. (Torino) 2005 Dec;46(6):583–588. [PubMed] [Google Scholar]
- 6.Hravnak M, Hoffman LA, Saul MI, Zullo TG, Whitman GR. Resource utilization related to atrial fibrillation after coronary artery bypass grafting. Am. J. Crit. Care. 2002 May;11(3):228–238. [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess DC, Kilborn MJ, Keech AC. Interventions for prevention of post-operative atrial fibrillation and its complications after cardiac surgery: a meta-analysis. Eur. Heart J. 2006 Dec;27(23):2846–2857. doi: 10.1093/eurheartj/ehl272. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011 Nov 8;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 9.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012 Sep 12;308(10):1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 10.Jahangiri A, Leifert WR, Patten GS, McMurchie EJ. Termination of asynchronous contractile activity in rat atrial myocytes by n-3 polyunsaturated fatty acids. Mol. Cell. Biochem. 2000 Mar;206(1-2):33–41. doi: 10.1023/a:1007025007403. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Sutherland F, Rosso R, et al. Effects of chronic omega-3 polyunsaturated fatty acid supplementation on human atrial electrophysiology. Heart Rhythm. 2011 Apr;8(4):562–568. doi: 10.1016/j.hrthm.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Sutherland F, Teh AW, et al. Effects of chronic omega-3 polyunsaturated fatty acid supplementation on human pulmonary vein and left atrial electrophysiology in paroxysmal atrial fibrillation. Am. J. Cardiol. 2011 Aug 15;108(4):531–535. doi: 10.1016/j.amjcard.2011.03.082. [DOI] [PubMed] [Google Scholar]
- 13.Khawaja O, Gaziano JM, Djousse L. A meta-analysis of omega-3 fatty acids and incidence of atrial fibrillation. J. Am. Coll. Nutr. 2012 Feb;31(1):4–13. doi: 10.1080/07315724.2012.10720003. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Marchioli R, Gardner T, et al. The Omega-3 fatty acids for prevention of post-operative atrial fibrillation (OPERA) trial--rationale and design. Am. Heart J. 2011 Jul;162(1):56–63 e53. doi: 10.1016/j.ahj.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease – Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011 doi: 10.1016/j.jacc.2011.06.063. In press. [DOI] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Psaty BM, Rimm EB, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004 Jul 27;110(4):368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calo L, Bianconi L, Colivicchi F, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J. Am. Coll. Cardiol. 2005 May 17;45(10):1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 18.Harris WS. Effects of n-3 Fatty Acids on Human Lipoprotein Metabolism. In: Frolich JC, von Schacky C, editors. Clinical Pharmacology, vol. 5. “Fish, Fish Oil, and Human Health.”. 1992. pp. 88–97. [Google Scholar]
- 19.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur. Heart J. 2010 Oct;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 20.Wu JH, Lemaitre RN, King IB, et al. Association of plasma phospholipid long-chain omega-3 fatty acids with incident atrial fibrillation in older adults: the Cardiovascular Health Study. Circulation. 2012 Mar 6;125(9):1084–1093. doi: 10.1161/CIRCULATIONAHA.111.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1999 Jul;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 22.Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur. Heart J. 2003 May;24(9):881–882. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006 Oct 18;296(15):1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 24.McLennan PL. Myocardial membrane fatty acids and the antiarrhythmic actions of dietary fish oil in animal models. Lipids. 2001;36(Suppl):S111–114. doi: 10.1007/s11745-001-0692-x. [DOI] [PubMed] [Google Scholar]
- 25.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003 Jun 3;107(21):2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 26.Brouwer IA, Raitt MH, Dullemeijer C, et al. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur. Heart J. 2009 Apr;30(7):820–826. doi: 10.1093/eurheartj/ehp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010 Nov 15;304(21):2363–2372. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- 28.Nodari S, Triggiani M, Campia U, et al. n-3 polyunsaturated fatty acids in the prevention of atrial fibrillation recurrences after electrical cardioversion: a prospective, randomized study. Circulation. 2011 Sep 6;124(10):1100–1106. doi: 10.1161/CIRCULATIONAHA.111.022194. [DOI] [PubMed] [Google Scholar]
- 29.Bianconi L, Calo L, Mennuni M, et al. n-3 polyunsaturated fatty acids for the prevention of arrhythmia recurrence after electrical cardioversion of chronic persistent atrial fibrillation: a randomized, double-blind, multicentre study. Europace. 2011 Feb;13(2):174–181. doi: 10.1093/europace/euq386. [DOI] [PubMed] [Google Scholar]
- 30.Ozaydin M, Erdogan D, Tayyar S, et al. N-3 polyunsaturated fatty acids administration does not reduce the recurrence rates of atrial fibrillation and inflammation after electrical cardioversion: a prospective randomized study. Anatolian J Cardiol. 2011 Jun;11(4):305–309. doi: 10.5152/akd.2011.080. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Sutherland F, Morton JB, et al. Long-term omega-3 polyunsaturated fatty acid supplementation reduces the recurrence of persistent atrial fibrillation after electrical cardioversion. Heart Rhythm. 2012 Apr;9(4):483–491. doi: 10.1016/j.hrthm.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 32.Finzi AA, Latini R, Barlera S, et al. Effects of n-3 polyunsaturated fatty acids on malignant ventricular arrhythmias in patients with chronic heart failure and implantable cardioverter-defibrillators: A substudy of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca (GISSI-HF) trial. Am. Heart J. 2011 Feb;161(2):338–343 e331. doi: 10.1016/j.ahj.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989 Sep 30;2(8666):757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 34.Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999 Aug 7;354(9177):447–455. [PubMed] [Google Scholar]
- 35.Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002 Apr 23;105(16):1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 36.Heidt MC, Vician M, Stracke SK, et al. Beneficial effects of intravenously administered N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a prospective randomized study. Thorac. Cardiovasc. Surg. 2009 Aug;57(5):276–280. doi: 10.1055/s-0029-1185301. [DOI] [PubMed] [Google Scholar]
- 37.Saravanan P, Bridgewater B, West AL, O’Neill SC, Calder PC, Davidson NC. Omega-3 fatty acid supplementation does not reduce risk of atrial fibrillation after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. Circ Arrhythm Electrophysiol. 2010 Feb 1;3(1):46–53. doi: 10.1161/CIRCEP.109.899633. [DOI] [PubMed] [Google Scholar]
- 38.Heidarsdottir R, Arnar DO, Skuladottir GV, et al. Does treatment with n-3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery? Europace. 2010 Mar;12(3):356–363. doi: 10.1093/europace/eup429. [DOI] [PubMed] [Google Scholar]
- 39.Sorice M, Tritto FP, Sordelli C, Gregorio R, Piazza L. N-3 polyunsaturated fatty acids reduces post-operative atrial fibrillation incidence in patients undergoing “on-pump” coronary artery bypass graft surgery. Monaldi Arch. Chest Dis. 2011 Jun;76(2):93–98. doi: 10.4081/monaldi.2011.196. [DOI] [PubMed] [Google Scholar]
- 40.Farquharson AL, Metcalf RG, Sanders P, et al. Effect of dietary fish oil on atrial fibrillation after cardiac surgery. Am. J. Cardiol. 2011 Sep 15;108(6):851–856. doi: 10.1016/j.amjcard.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 41.Sandesara CM, Chung MK, Van Wagoner DR, et al. A randomized, placebo-controlled trial of omega-3 fatty acids for inhibition of supraventricular arrhythmias after cardiac surgery: the FISH trial. J Am Heart Assoc. 2012;1:e000547. doi: 10.1161/JAHA.111.000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003 Feb 8;361(9356):477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 43.Rapp JH, Connor WE, Lin DS, Porter JM. Dietary eicosapentaenoic acid and docosahexaenoic acid from fish oil. Their incorporation into advanced human atherosclerotic plaques. Arterioscler Thromb. 1991 Jul-Aug;11(4):903–911. doi: 10.1161/01.atv.11.4.903. [DOI] [PubMed] [Google Scholar]
- 44.Reis GJ, Boucher TM, Sipperly ME, et al. Randomised trial of fish oil for prevention of restenosis after coronary angioplasty. Lancet. 1989 Jul 22;2(8656):177–181. doi: 10.1016/s0140-6736(89)90370-x. [DOI] [PubMed] [Google Scholar]
- 45.Eritsland J, Arnesen H, Seljeflot I, Kierulf P. Long-term effects of n-3 polyunsaturated fatty acids on haemostatic variables and bleeding episodes in patients with coronary artery disease. Blood Coagul Fibrinolysis. 1995 Feb;6(1):17–22. doi: 10.1097/00001721-199502000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.