Abstract
While activation of the hypothalamic-pituitary-adrenal (HPA) axis is an adaptive response to stress, excessive HPA axis reactivity may be an important marker of childhood vulnerability to psychopathology. Parenting, including parent affect during parent-child interactions, may play an important role in shaping the developing HPA system; however, the association of parent affect may be moderated by child factors, especially children’s emerging self-regulatory skills. We therefore tested the relationship between parent affectivity and 160 preschoolers’ cortisol reactivity during a laboratory visit, examining children’s effortful control (EC) as a moderator. Greater parent negative affectivity was related to greater initial and increasing cortisol over time, but only when children were low in EC. Higher parent positive affectivity was related to a higher baseline cortisol for children with low EC and lower baseline cortisol for children with high EC. Results indicate that children’s EC moderates the extent to which parent affect shapes stress reactive systems in early childhood.
Keywords: cortisol, parenting, effortful control, multi-level modeling
Cortisol reactivity to stress is an important marker of the hypothalamic-pituitary-adrenal (HPA) axis, a system that contributes to individual differences in vulnerabilities to psychopathology (Gunnar & Talge, 2008). The early environment appears to influence children’s cortisol reactivity (Gunnar & Donzella, 2002; Ouellet-Morin et al., 2008; Saridjan et al., 2010), thus potentially playing a causal role in elevating psychopathology risk via its influence on emerging individual differences in response to stress. In particular, available evidence suggests that parenting may be an especially critical factor (Blair et al., 2008; Murray, Halligan, Goodyer, & Herbert, 2010). For example, poor parental care has been linked to maladaptive cortisol functioning in animals (Coplan, Andrews, Rosenblum, Owens, Friedman, Gorman, & Nemeroff, 1996), infants (Bugental, Martorell, & Barraza, 2003) and preschool-aged children (Pendry & Adam, 2007).
With respect to more specific aspects of parenting, parent affect expressed during parent-child interaction may play an especially important role in children’s stress reactivity. Social referencing of parent affect by children is an adaptive behavior (Davies, 2011); for example, young children assess the safety of novel situations or persons in part by monitoring parental affective cues (Davies, 2011). Further, research indicates that children’s acceptance of a novel stranger or situation depends greatly on the affective tone that their parent conveys (Emde, 1992). Indeed, infants and young children frequently utilize both facial and vocal emotional cues from adults as a reference for how to respond to a variety of situations (Fernald, 1993; Camras & Sachs, 1991; Gunnar & Stone, 1984; Mumme, Fernald, & Herrera, 1996; Walker-Andrews, 1997; Walker-Andrews & Lennon, 1991). Considered as a whole, this literature suggests that parental affect may serve as an important cue for the activation of children’s psychophysiological responses to novelty and stress.
With specific respect to cortisol, a small literature speaks to this notion, with positive parenting, including positive affective tone when parents interact with children, moderating the influence of negative life events on children’s cortisol reactivity (Barry & Kochanska, 2010; Bugental, Martorell, & Barraza, 2003; Hagan et al., 2011; Pendry & Adam, 2007). This work suggests that parent affectivity may act in conjunction with other factors to shape children’s cortisol reactivity. In regard to parent negative affectivity, given its clear links to negative child outcomes (Carson & Parke, 1996, Kahen, Fainsilber Katz, & Gottman, 1994), surprisingly few studies have examined its relationship to child cortisol reactivity, although measures of parenting that likely capture aspects of parental NA (e.g., hostility) are associated with children’s cortisol reactivity (Dougherty, Klein, Rose, & Laptook, 2011). These findings, along with the larger literature implicating emotional tone during parent-child interaction as a critical element in child development and socialization (e.g., Eisenberg, Cumberland, & Spinrad, 1998), suggest that parents’ affectivity with their children may be an important determinant of children’s early emerging cortisol reactivity. This is particularly important as research has demonstrated clear stability in observed parental affect, both negative and positive, expressed during parent-child interactions from ages 2–5 (Feng, Shaw, Skuban, & Lane, 2007), a critical period for child development.
While very little research has examined potential moderators of caregiver affect on children’s cortisol reactivity, a large literature examining child outcomes more broadly defined shows that not all children exhibit the same degree of responsivity to early caregiving (Luthar, 2006; Masten, 2007). One factor that may play a role in moderating the effects of parenting on children’s cortisol reactivity is children’s self-regulatory abilities. More specifically, temperamental effortful control (EC) may play a key role in shaping risk for an array of negative outcomes (e.g., Belsky & Beaver, 2011; Carver, Johnson, & Joorman, 2008). This is not surprising as the capacity to effectively regulate one’s emotions and behavior is critical to adaptive development (Gottfredson & Hirschi, 1990; Vazsonyi & Huang, 2010). Psychopathologists have proposed that children’s emerging EC may moderate other forms of risk for psychopathology across development (e.g., Carver, Johnson, & Joorman, 2008; Kochanska et al 2009, Martel & Nigg, 2006; Verstraeten et al., 2009). For example, Verstraeten and colleagues found that, in a sample of 304 youth followed from grade seven to ten, child negative affect was associated with a ruminative response style and, in turn, with depressive symptoms, but only for children with low EC (2009). In addition, child EC has been shown to moderate the association between low levels of child guilt and later disruptive outcomes (Kochanska et al., 2009). This suggests the intriguing possibility that children’s EC might mitigate any risk for increased cortisol reactivity associated with parent affectivity, particularly negative affectivity. Main effects of EC on cortisol reactivity are also suggested by the literature indicating that EC serves as an important protective factor by influencing children’s negative emotional reactivity to stressful stimuli (Compas, Conner-Smith, & Jaser, 2004; Lengua, 2009; Spinrad et al., 2009).
With this literature in mind, we examined whether parental positive and negative affectivity during parent-child interactions was related to children’s cortisol reactivity during a standardized battery of stress-eliciting tasks. While previous work shows that early parenting is associated with children’s cortisol reactivity (Blair et al., 2006; Dougherty et al., 2011), considerable variability remains in children’s cortisol responses to stress, suggesting the possibility that child factors moderate the influence of parenting on children’s cortisol. As previous work highlights the role of children’s self-regulation as a marker of children’s responsivity to their early environments (Carver, Johnson, & Joorman, 2008; Kochanska et al., 2009, Martel & Nigg, 2006; Verstraeten et al., 2009), we posited that children with low levels of EC would display especially heightened cortisol reactivity in the context of parental negative affect. Although EC has several facets, including attentional regulation, inhibitory control, and activational control, we chose to examine one widely studied and readily observed behavioral manifestation of this trait; namely, inhibitory control, or the ability to plan and suppress inappropriate approach responses (Rothbart, 1989). We elected to focus on this specific aspect of EC as it has been linked to an array of important child outcomes (Carlson & Moses, 2001; Kochanska & Askan, 2006; Schachar, Mota, Logan, Tannock, & Klim, 2000).
With respect to positive parent affect, our hypotheses were more exploratory; children’s self-regulation skills might be less critical in the context of positive parenting behaviors. Hence, parent positive affect might be expected to show an association with reduced child cortisol that is unmoderated by children’s EC. However, we considered two additional, tentative hypotheses. First, children with high EC, by virtue of their more efficient self-regulatory skills, might be better positioned to reap the benefits of positive caregiving, thus showing especially low levels of some aspects of reactivity in the context of greater parent positive affect. Additionally, children with low EC might benefit from a less stimulating caregiving environment with respect to parent affectivity; if so, this would suggest that even positive aspects of parental affect might be associated with elevated cortisol in these children.
We tested these questions in a sample of preschool-aged children and their primary caregivers, using standardized observational measures of children’s EC and parent affectivity during parent-child interactions. We chose to study preschool-aged children because children’s EC skills show large and meaningful individual differences in EC at this age (Eisenberg, 2005; Kochanska, Murray, & Harlan, 2000; Posner & Rothbart, 1998).
Method
Participants
Participants were 160 children and their primary caregivers who were part of a larger study of temperament in preschool-aged children. Participants from the larger project were recruited from a commercial mailing list. Participants in our subsample identified themselves as Caucasian (N = 150; 93.7%), African-American (N = 2; 1.2%), Asian (N = 1; .6%), and mixed or other race (N = 7; 5.1%); ten participants identified themselves as Hispanic (6.2%). The majority of the families were middle class, as measured by Hollingshead’s Four Factor Index of Social Status (Hollingshead, 1975; M=46.14; SD=10.29). The mean age of parents was 36.5 years (SD = 3.8) for mothers and 38.7 years (SD = 4.6) for fathers. The mean age of child participants was 3.6 years (SD = .2), and 50.0% (N = 80) were female. The majority (77.9%) of the children came from two-parent homes, and 52.5% of the mothers worked outside the home part- or full-time; 16.7% worked more than 35 hours per week. Children were administered the Peabody Picture Vocabulary Test (PPVT; Dunn & Dunn, 1997) to screen for gross cognitive impairment and to assess task comprehension (M = 105.31, SD = 14.08, range = 61–139). Additionally, the videos of children with lower scores (albeit greater than 60) were further reviewed by trained study personnel to ensure that they appeared to understand the tasks; no children were excluded based on this review.
Procedure
All children attended a laboratory session approximately 2.5 hours in duration, during which they were videotaped while participating with a female experimenter in twelve standardized tasks selected from the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith, Reilly, Lemery, Longley & Prescott, 1995). Lab-TAB tasks are designed to elicit behavioral expressions of a broad range of emotional and other temperamental traits. Episodes were ordered so as to prevent carry-over effects in that no episodes presumed to evoke similar affective responses occurred consecutively, and each was followed by a brief play break. Two episodes were coded for EC and are described below. All other Lab-TAB episodes are described in detail elsewhere (Hayden et al., 2010).
Tower of patience
A female experimenter and child took turns building a tower using large cardboard blocks. The experimenter waited a series of increasing delays (5, 10, 15, 20, 30s) before placing her block on the tower, thus forcing the child to wait increasingly longer periods of time before being given a turn. Two towers were built over the course of the task.
Snack delay
The experimenter placed a chocolate candy underneath a transparent cup, telling the child that (s)he must wait until the experimenter rang a bell before picking up the cup and eating the candy. The experimenter adhered to a series of delays of increasing length (5, 10, 20, 30s), forcing the child to wait longer each time to eat the candy.
As an index of EC, each task was coded for prompting behavior (e.g., verbally prompting (e.g., “ring it” or “it’s your turn”) or physically prompting the experimenter to place their block or ring the bell (e.g., pointing at the block or bell; see Carlson, 2005, Kochanska, Murray, Jacques, Koenig & Vandegeest, 1996, and Kochanska & Knaack, 2003, for similar procedures). Prompts were averaged across each delay and then across tasks to create an aggregate EC scale. As more prompting reflects lower EC, scores were reverse-coded to ease interpretation. Coders were unaware of children’s parenting and cortisol reactivity. Raters had to reach at least 80% agreement with a “master” rater before coding independently. To examine interrater reliability, eight videotapes were independently coded by a second rater (ICC = .98).
Cortisol sampling procedure
Salivary cortisol was collected four times during the laboratory assessment. Saliva for cortisol assay was obtained by having the children dip 2 inch- long cotton dental rolls into small cups containing approximately .025 g of sugar-sweetened cherry Kool-Aid drink mix. Children were then instructed to chew the cotton rolls until they were saturated with saliva. Previous work shows that the use of Kool-Aid® does not compromise the quality of the assays when used sparingly as it does not significantly alter the pH of the saliva when used sparingly (Talge et al., 2005). In addition, its use promotes saliva flow and makes the sampling pleasant for the child. The collection of each sample took approximately 1–2 minutes. After each sample was collected, the saliva was expunged from the cotton roll into a labeled micro tube, and stored at −20°C until assayed.
The timing at which the laboratory cortisol samples were obtained was determined based on the presumed stress of the Lab-TAB episode and on previous studies using a similar paradigm with children near this age (Luby et al., 2003). The principle that cortisol levels are believed to reflect the level of stress experienced about 20–40 minutes prior (Dickerson & Kemeny, 2004) was also taken into account. Based on these considerations, the first sample was taken upon arrival to the laboratory after parents in the study had completed informed consent for both themselves and their child. Cortisol levels at the time of this sample reflect levels prior to the assessment, when the child was with a parent either at home or en route to the laboratory. While this was not hypothesized to be a particularly stressful time, such samples may still have relevance for children’s adaptive development as they may reflect ‘resting’ state cortisol levels (Gunnar & Talge, 2008), and will therefore be examined as a dependent variable in analyses. This sample will subsequently be referred to as baseline. The second sample was collected 30 minutes following the Stranger Approach task of the Lab-TAB, the most stressful episode in the battery, during which the child was separated from his/her parent and a stranger entered the room. The third salivary cortisol sample was taken 30 minutes after Transparent Box, a frustration-inducing task in which the child is unable to unlock a box with a desirable toy inside. The fourth and final sample was collected 20 minutes after the completion of all Lab-TAB tasks. The second, third, and fourth samples reflect cortisol reactivity to the tasks and therefore will be referred to as cortisol reactivity. To control for non-stress related elevations of cortisol, laboratory assessments were conducted at either 10 AM (69% of the assessments) or 2 PM. Families were instructed prior to coming to the laboratory that the child should not eat within one hour before their scheduled lab visit, and that children should avoid caffeine for at least two hours, and dairy for at least 15 minutes, prior to arrival.
Samples were assayed using a time-resolved fluorescence immunoassay with fluorometric end point detection (DELFIA). All samples were assayed in duplicate. Samples yielding values above 44 nanomoles per liter (nmol/L) were excluded, which applied to 4 laboratory samples from 4 different individuals. The correlation between duplicates was .99. The inter- and intra-assay coefficients of variation (CV) were between 7.1% – 9.0% and 4.0% –6.7%, respectively. As is typically found (Gunnar & Talge, 2008), the cortisol values in this sample were positively skewed. A log10 transformation of the raw cortisol values yielded unskewed cortisol values that were used in all analyses.
Parent affectivity
One hundred and forty-nine parent-child dyads completed an observational measure of parent-child interactions for preschool-aged children during a second laboratory visit approximately two weeks later. We used observational measures of parenting as these have been shown to have strong predictive validity for child outcomes (Zaslow et al., 2006). Children and a caregiver (typically the mother; N = 143; 96.0%) engaged in six tasks derived from the Teaching Tasks battery (Egeland, Weinfield, Hiester, Lawrence, Pierce, & Chippendale, 1995), which included book reading, block building, naming objects with wheels, matching shapes, completing a maze using an etch-sketch, and gift presentation. The battery (approximately 25 minutes in duration) was videotaped for subsequent coding on a number of parenting variables. The present study used two scales based on Weinfield, Egeland, and Ogawa (1998) that reflect parent affectivity during interaction with their child. The parental positive affectivity scale (range = 1–3) was based on the parent’s expression of positive regard and emotional support to the child and on general positive affectivity expressed during the battery (e.g., smiling, laughing, speaking in a pleasant tone). Higher scores indicate greater positive affectivity expressed throughout interaction with the child. The parental negative affectivity scale (range = 1–3) was based on the parent’s expression of negative regard and punitive behavior toward the child and on general negative affectivity (e.g., facial, bodily, and vocal expressions of anger, frustration, and or annoyance). Higher scores indicate greater negative affectivity expressed throughout interaction with the child. These two scales were significantly inversely correlated (r = −.35, p < .01).
Results
Table 1 presents correlations between mean cortisol levels at each sampling time and all major study variables. Cortisol levels at all four time points were highly positively correlated (rs = .29 – .83). Child EC scores were significantly associated with greater cortisol levels at the first and second samples post-stress. Parent negative affectivity expressed during the parent-child interaction task was also positively correlated with children’s cortisol reactivity levels. Child sex was negatively correlated with final cortisol levels, indicating that girls tended to have lower final cortisol levels than boys. Time of day was negatively correlated with baseline cortisol and the third sample taken post stress suggesting that children tested in the morning trended toward higher cortisol levels for these samples. Time of day was positively correlated with parent negative affectivity, Hollingshead scores, and child sex, indicating that children who were tested in the afternoon had parents who displayed more negative affectivity in interactions with them, were of a higher socio-economic status, and were more likely to be female.
Table 1.
Correlations among variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Baseline cortisol | -- | |||||||||||
| 2. Second cortisol sample | .62** | -- | ||||||||||
| 3. Third cortisol sample | .52** | .83** | -- | |||||||||
| 4. Fourth cortisol sample | .29** | .48** | .62** | -- | ||||||||
| 5. EC | −.09 | −.18* | −.19* | .00 | -- | |||||||
| 6. Parent Positive Affectivity | .02 | −.00 | .09 | .03 | −.03 | -- | ||||||
| 7. Parent Negative Affectivity | .01 | .23** | .23** | .11 | −.07 | −.35** | -- | |||||
| 8. PPVT | −.12 | −.02 | .04 | .06 | −.12 | .08 | −.09 | -- | ||||
| 9. Hollingshead | −.10 | −.07 | −.05 | −.13 | −.05 | .07 | −.07 | .02 | -- | |||
| 10. Child Sex | −.10 | −.07 | −.14 | −.17* | −.06 | −.02 | −.05 | −.07 | −.08 | -- | ||
| 11. Child Age in Months | −.12 | −.07 | −.01 | −.02 | −.12 | .12 | −.01 | .25** | .01 | .01 | -- | |
| 12. Time of Day | −.18* | −.05 | −.12 | −.24** | −.03 | −.06 | .02* | −.06 | .19* | .16* | .08 | -- |
| Mean (SD) |
.46 (.30) |
.43 (.31) |
.48 .28) |
.66 (.23) |
2.49 (.72) |
2.04 (.32) |
1.04 (.15) |
105.31 (14.08) |
46.14 (10.29) |
-- | 43.01 (2.83) |
-- |
Note: Cortisol levels are measured in nanomoles per liter (nmol/L)
Child Sex: Male = 1 and Female = 2
Time of Day: AM = 0 and PM = 1
p < .05,
p < .01.
Cortisol Level Comparisons Across Sample
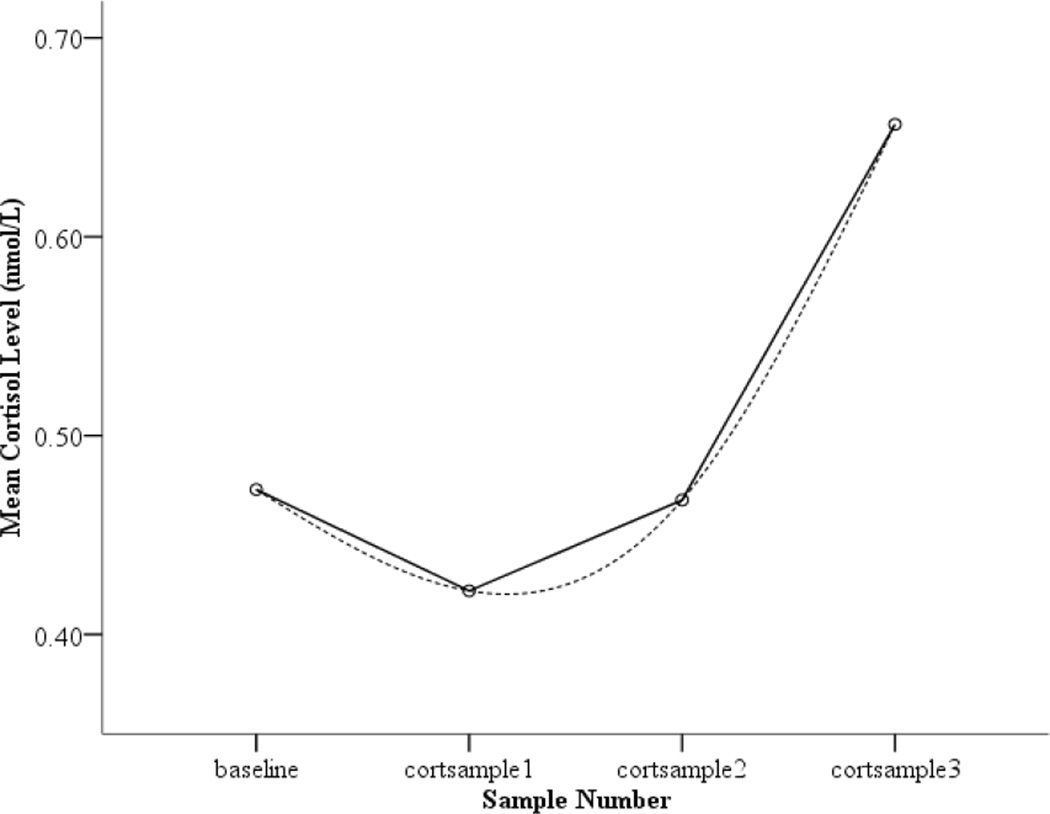
As reported in Dougherty, Klein, Congdon, Canli, and Hayden (2010), there was an observed decrease in child cortisol from baseline to the first sample taken after the beginning of the Lab-TAB. While counterintuitive, it is important to note that this pattern is frequently found in laboratory studies of cortisol reactivity in children (Gotlib, Joorman, Minor, & Cooney, 2006; Luby et al., 2003; Talge, Bruce, Donzella, & Gunnar, 2003), and may reflect stress-related increases related to anticipating the laboratory visit (Gunnar & Talge, 2008), such that the baseline sample is elevated and then declines. As evidenced by the significant positive quadratic effect (see Figure 1 for average trajectory of log 10 transformed data), average cortisol levels then began to increase steadily across the remaining two samples.
Figure 1.
Mean child cortisol level (nmol/L) as a function of cortisol sampling time. Figure displays data that has been log10 transformed.
Examination of Cortisol Trajectories
To examine overall cortisol trajectories for the sample and predictors of individual differences in these trajectories, we used multi-level modeling (MLM) conducted with HLM 6 (Scientific Software International Inc, IL). MLM has many advantages, including the ability to model data at two levels (Level 1, describing within-individual change over time; and Level 2, relating predictors to any interindividual differences in change), and the ability to account for missing values at Level 1 (Singer & Willett, 2003). For the Level 1 model, cortisol time points (baseline, first, second and third reactivity samples) were nested in the Level two variable, participant. Log10 transformed cortisol values were the dependent variable. Because cortisol levels show a diurnal pattern of variation, time of assessment (i.e., morning versus afternoon) was controlled for in all analyses.
Between-subjects predictors of individual change were modeled to allow examination of cortisol levels at each sampling time for each individual, while taking into account between-persons predictors. For these analyses, Level 2 predictors were time of day, child EC, and parent negative and positive affectivity during the parent-child interaction task. As study hypotheses focused on whether child EC moderated associations between parenting and child cortisol reactivity, two-way interactions between the relevant between-subject variables were the focus of analyses. Time was anchored at baseline (time = 0) so that the cortisol intercepts (β00) would reflect the average of individual’s cortisol levels at baseline. All Level 2 between-person variables were centered at their grand mean. MLM is equipped to handle missing data at Level 1 by estimating the trajectory based on existing data for that participant (two children were missing a baseline cortisol sample and two children were missing the final sample). At Level 2, parenting data was missing for 11 participants, and EC data was missing for 1 participant. These participants were excluded from analyses. The analytic sample did not differ from the full sample with regard to cortisol reactivity or basic demographic characteristics. Descriptive statistics for the full sample are reported throughout.
A quadratic equation was built to examine the effects of Level 2 variables on the intercept, instantaneous rate of change (henceforth referred to as slope), and curvature. As reported by Dougherty and colleagues (2010), significant variation in intercept, slope, and curvature was found, confirming the appropriateness of testing Level 2 predictors. As evidence that a quadratic model best fit the data, a chi-square test of the deviance statistics between unconditional linear and quadratic models indicated that adding a quadratic term to the model resulted in a significant improvement in model fit, supporting results from a graphical representation indicating that most children showed an initial decrease in cortisol from baseline followed by an increase (see Figure 1; X2 (1) = 36.03, p < .001).
To evaluate the model, the following function was specified to describe the data from each individual:
| (Equation 1) |
Main Effects and Interactions
To examine the main effects and two-way interactions of Level 2 predictors on the intercept, slope, and curvature for individuals’ cortisol trajectories, interaction terms were created by centering continuous variables, then multiplying the terms to reflect the product of Level 2 predictors of interest (Aiken & West, 1991). The grand mean centered Level 2 predictor variables were then entered in the quadratic growth model followed by the two-way interaction terms (see Table 2). All demographic predictors (child sex, PPVT scores, child age, time of day, and Hollingshead scores) were included in the initial model and those not significantly related to child cortisol intercept, slope, or curvature were removed to conserve power. Utilizing this process, only time of day was retained in final models.
Table 2.
HLM model effects
| Variable | Coefficient Intercept Coefficient (SE) |
t-value | Cortisol Instantaneous Rate of Change Coefficient (SD) |
t-value | Cortisol Curvature Coefficient (SD) |
t-value |
|---|---|---|---|---|---|---|
| Child EC and parent affectivity | ||||||
| Time of Day | −.111(.053) | −2.109* | .062(.055) | 1.120 | −.023(.016) | −1.432 |
| Parent Negative Affectivity | .214(.278) | .767 | .397(.126) | 3.144** | −.128(.044) | −2.942** |
| Parent Positive Affectivity | .108(.086) | 1.257 | .092(.082) | 1.112 | −.029(.023) | −1.256 |
| Child Effortful Control (EC) | −.036(.037) | −.981 | −.093(.040) | −2.363* | .036(.013) | 2.871** |
| EC X Parent Negative Affectivity | −.225(.289) | −.779 | −.291(.130) | −2.232* | .080(.044) | 1.857† |
| EC X Parent Positive Affectivity | −.440(.161) | −2.741** | .177(.160) | 1.108 | −.028(.042) | −.661 |
Time of Day: AM = 0 and PM = 1
< .05;
< .01;
< .10
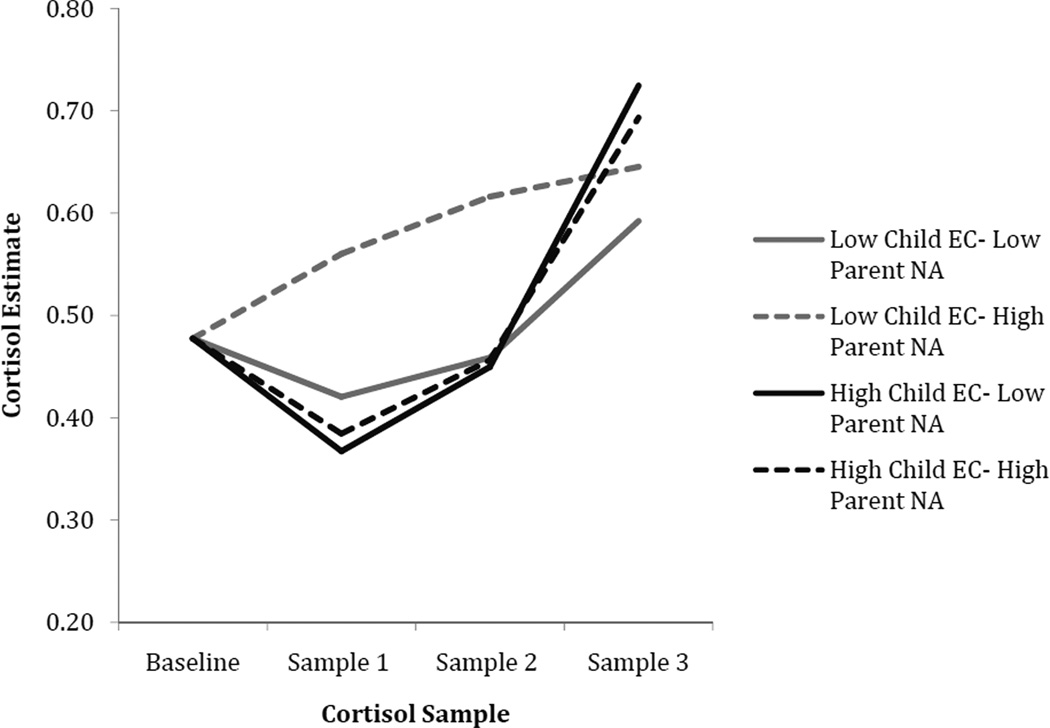
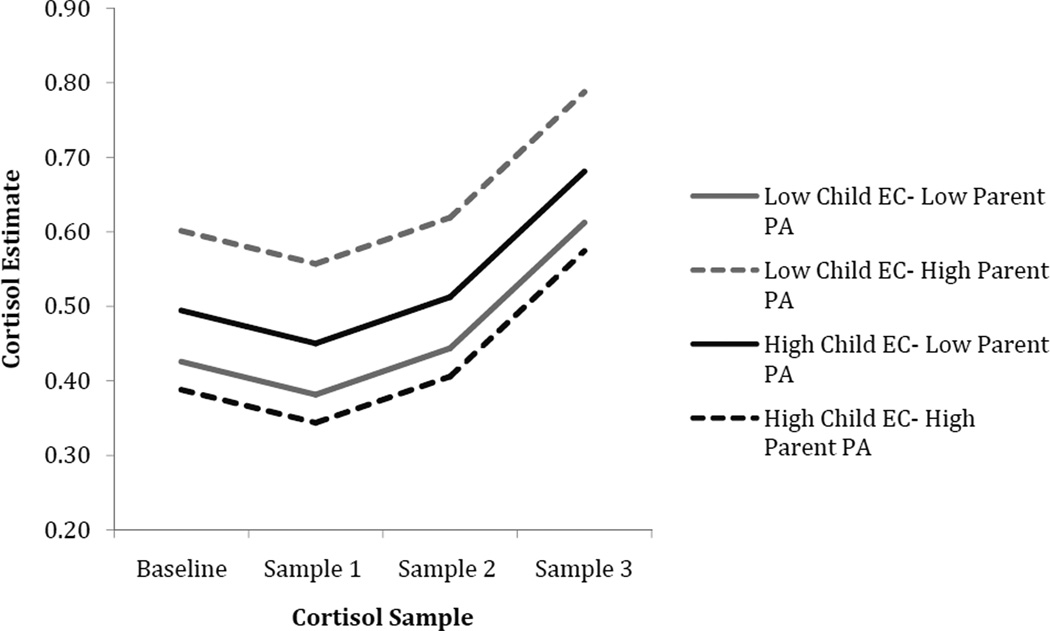
Main effects of time of day, parent negative affect, and EC were found in predicting cortisol intercept, slope, and curvature. Time of day was significantly associated with a lower cortisol intercept, suggesting that children tested in the afternoon had lower cortisol levels at the first assessment. The main effects of child EC and parent negative affectivity on cortisol were qualified by a significant interaction between these two variables in predicting cortisol slope and curvature, while EC and parent positive affectivity interacted to predict child cortisol intercepts only. To interpret the interactions between parent affectivity and EC, EC scores were centered at values one standard deviation above and below the mean so that model coefficients would reflect the effects of parent negative and positive affectivity when child EC was at high and low levels (see Figures 2 and 3). For children with high EC, greater parent positive affectivity was associated with a significantly lower cortisol intercept (intercept: unstandardized coefficient = −.208, SE = .085, t = −2.426, p = .017) compared to children with high EC and low parent positive affectivity. For children with low EC, high parent positive affectivity was associated with a significantly higher cortisol intercept (intercept: unstandardized coefficient = .423, SE = .183, t = 2.301, p = .023) compared to children with low EC and low parent positive affectivity.
Figure 2.
Cortisol as a function of child EC and parent negative affectivity. Note: parent affectivity 1 SD above and below the mean; EC = Effortful Control; NA = Negative Affectivity.
Figure 3.
Cortisol as a function of child EC and parent positive affectivity. Note: parent affectivity 1 SD above and below the mean; EC = Effortful Control; PA = Positive Affectivity.
With respect to parent negative affectivity, it was associated with a significantly steeper cortisol slope, and slower rate of curvature when children were low in EC (slope: unstandardized coefficient = .605, SE = .146, t = 4.146, p < .001; curvature: unstandardized coefficient = −.186, SE = .043, t = −4.290, p < .001). For children high in EC, parent negative affectivity was not significantly associated with any aspect of cortisol (ps > .26).
To further examine differences among cortisol levels at each time point for the EC-parent NA interaction, we re-centered our level 1 predictor time at each sample post-stress to examine interaction effects at the level of the intercept1. Consistent with the pattern suggested in Figures 2, these analyses showed a significant EC-parent NA interaction in predicting cortisol intercept at the second post-stress sample (p = .03). The interaction was at a trend level for intercept at the first post-stress sample (p = .06).
Discussion
While early parenting is associated with children’s cortisol reactivity (Blair et al., 2008; Dougherty et al., 2011), variability in children’s cortisol responses to stress suggests that child factors may moderate the influence of parenting on children’s cortisol. Children’s EC may be a marker of their responsivity to the early environments (Kochanska et al 2009, Martel & Nigg, 2006; Verstraeten et al., 2009). Consistent with this idea, we found that the effects of parent positive and negative affectivity predicted cortisol reactivity differently depending on children’s EC.
More specifically, we found that children lower in this trait showed a significantly steeper cortisol slope and slower rate of quadratic curvature when parent negative affectivity expressed during parent-child interactions was high. In other words, as hypothesized, children with lower EC showed a relatively pronounced cortisol response to laboratory stressors when their parents showed more negative emotions during parent-child interaction. Previous research has reported main effects of negative parenting behavior (Azar, Paquette, Zoccolillo, Baltzer, & Tremblay, 2007) and low levels of EC (Oldehinkel, Hartman, Nederhof, Riese, & Ormel, 2011) on children’s psychophysiological reactivity; however, our findings suggest further complexity in the relationship between parenting and child characteristics in predicting child cortisol function, and indicate that high parental negativity may exert the most profound impact when children have relatively low self-regulation. Also consistent with our hypotheses, greater cortisol reactivity in the context of high parent negative affectivity was not found for children with high EC, raising the possibility that greater self-regulation skills mitigate the effects of parental expressions of negative emotions on children’s cortisol. Such a process might be one means through which EC promotes adaptive child development (Gottfredson & Hirschi, 1990; Vazsonyi & Huang, 2010). This finding is also consistent with recent research demonstrating that EC moderates other risk factors across development (e.g., Carver, Johnson, & Joorman, 2008; Kochanska et al 2009, Martel & Nigg, 2006; Verstraeten et al., 2009).
A significant interaction was also found between parent positive affectivity and child EC such that for children with higher EC, greater parent positive affectivity was associated with lower baseline cortisol levels. Given the nature of the timing of collection of this sample, any effects must be interpreted with caution. However, this effect could mean that greater EC increases the extent to which children can derive benefits from parents’ expressions of positive affect. More specifically, children with high EC may have a greater capacity to internalize parental warmth expressed during interaction, resulting in more efficient regulation of stress coping. In contrast, for children with lower EC, greater parent positive affectivity was associated with a higher baseline cortisol levels. Baseline cortisol levels can be interpreted as a reflection of “resting state” cortisol activity (Gunnar & Talge, 2008). Alternatively, research has demonstrated that children’s cortisol samples obtained at arrival at a novel laboratory setting are elevated relative to samples obtained at home at the same time of day as a lab visit, which suggests that initial lab samples may reflect a response to novelty (Gunnar & Talge, 2008). Regardless, both heightened baseline cortisol and exaggerated reactivity to a relatively benign stressor, such as arrival at the lab, may be indicative of risk (Gunnar & Talge, 2008; Halligan, Herbert, Goodyer, & Murray, 2004). Our results suggest that lower parental emotional expression in general is associated low cortisol levels in children with low EC either at baseline (positive parent affect) or following stress (negative parent affect). Future research assessing cortisol reactivity using procedures to obtain more interpretable measures of baseline cortisol (e.g., Kryski, Smith, Sheikh, Singh, & Hayden, 2011) will be needed to better explore this possibility.
Our study had several strengths, including observational measures of parenting (Zaslow et al., 2006) which have good predictive validity for important child outcomes, and objective measures of child EC based on a coding scheme that showed good interrater reliability. However, a number of limitations should be acknowledged. While we focused on EC as a moderator of parental influences on cortisol reactivity, other temperament traits may also moderate responses to environmental factors (e.g., positive emotionality; Wichers et al. 2008)). Additionally, we examined only a narrow-band facet of EC (Rothbart & Bates, 2006) reflecting inhibitory control, despite the fact that other facets of this trait may also moderate contextual influences on cortisol reactivity. Replication of our findings in larger samples and with other EC measures is important to better understand the complexity of these interactions. Due to the cross-sectional nature of this study we are unable to claim directionality in our models. For example, it is possible that child cortisol reactivity may shape both EC development and parenting practices. Longitudinal studies are necessary to disentangle the direction of this relationship.
Another possible limitation of this study is the way in which cortisol reactivity was indexed. Variability in the time at which samples were collected (Dickerson & Kemeny, 2004) and child reactivity to the novelty of the laboratory testing conditions (Gunnar & Talge, 2008) may have reduced the accuracy with which children’s cortisol reactivity was assessed. For example, we found an average decrease in child cortisol from baseline to second sample. However, it is important to note that such a pattern is quite frequently reported in the literature (Gotlib, Joorman, Minor, & Cooney, 2006; Luby et al., 2003; Talge, Bruce, Donzella, & Gunnar, 2003); indeed, the majority of developmental studies of cortisol reactivity in children fail to find an increase in children’s cortisol in response to laboratory stressors (Gunnar et al., 2009). It has been posited that this may reflect developmental differences in how children’s HPA axis systems respond to stress, and that such hyporesponsivity may be the normative response to stress in early to middle childhood. Conversely, this pattern may also reflect methodological issues, such as children’s heightened reactivity to novel laboratory settings and procedures (Kryski et al., 2011). As cortisol reactivity and contextual factors that elicit it change over the course of development, studies exploring these relationships across development are warranted. Lastly, our sample was primarily Caucasian and middle class. While it is unclear what, if any, influence this had on the findings obtained, future work exploring these relationships in a more diverse sample is recommended as these findings cannot be generalized beyond the scope of a primarily Caucasian and middle class sample.
Overall, the results of this study indicate that child EC influences how parenting shapes children’s cortisol responses to stress. In order to more fully understand the development of psychophysiological indices of stress sensitivity, developing and testing models that include both intrinsic child factors and contextual variables is likely critical.
Acknowledgements
This work was supported by grants from the National Institute of Mental Health RO1 MH069942 (DNK) and F31 MH075484-01A2 (LRD), the Society for a Science of Clinical Psychology Dissertation Grant Award (LRD), the Emeritus Faculty Dissertation Award (LRD), the National Alliance for Research on Schizophrenia and Depression (EPH), the Government of Canada Vanier Canada Graduate Scholarship Program (KRK), and a GCRC Grant no. M01-RR10710 to Stony Brook University from the National Center for Research Resources. These funding agencies had no role in the study design, collection, analysis and interpretation of data, the writing of the report or in the decision to submit the paper for publication.
The authors would like to thank Bonnie Donzella and Megan R. Gunnar (University of Minnesota) for consultation and cortisol data collection procedures; and Andrea Gierens and the neuroendocrine laboratory and the University of Trier, Germany for assaying the salivary cortisol samples.
Footnotes
As the parent positive affectivity-child EC interaction was significant for the intercept only, we did not conduct similar additional analyses for that model.
Contributor Information
Katie R. Kryski, Email: kkryski2@uwo.ca.
Lea R. Dougherty, Email: ldougherty@psyc.umd.edu.
Margaret W. Dyson, Email: mdyson@notes.cc.sunysb.edu.
Thomas M. Olino, Email: olinotm@upmc.edu.
Rebecca S. Laptook, Email: Rebecca.Laptook@brown.edu.
Daniel N. Klein, Email: dklein@notes.cc.sunysb.edu.
Elizabeth P. Hayden, Email: ehayden@uwo.ca.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Azar R, Paquette D, Zoccolillo M, Baltzer F, Tremblay RE. The association of major depression, conduct disorder, and maternal overcontrol with a failure to show a cortisol buffered response in 4-month-old infants of teenage mothers. Biological Psychiatry. 2007;62:573–579. doi: 10.1016/j.biopsych.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Barry RA, Kochanska G. A longitudinal investigation of the affective environment in families with young children: From infancy to early school age. Emotion. 2010;10:237–249. doi: 10.1037/a0018485. 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Beaver M. Cumulative genetic plasticity, parenting, and adolescent self-regulation. Journal of Child Psychology and Psychiatry. 2011;52:619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT, et al. Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology. 2008;44:1095–1109. doi: 10.1037/0012-1649.44.4.1095. [DOI] [PubMed] [Google Scholar]
- Bugental DB, Martorell GA, Barraza V. The hormonal costs of subtle forms of infant maltreatment. Hormones and Behavior. 2003;43:237–244. doi: 10.1016/s0018-506x(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Carlson SM. Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- Camras LA, Sachs VB. Social referencing and caretaker expressive behavior in a day care setting. Infant Behavior and Development. 1991;14:27–36. [Google Scholar]
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s theory of mind. Child Development. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode model of self-regulation and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Connor-Smith J, Jaser SS. Temperament, stress reactivity, and coping: Implications for depression in childhood and adolescence. Journal of Clinical. Child and Adolescent Psychology. 2004;33:21–31. doi: 10.1207/S15374424JCCP3301_3. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotrophin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D, editor. Child development: A practitioner’s guide. New York, NY, US: Guilford Press; 2011. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Congdon E, Canli T, Hayden EP. Interaction between 5-HTTLPR and BDNF Val66Met polymorphisms on HPA axis reactivity in preschoolers. Biological Psychology. 2010;83:93–100. doi: 10.1016/j.biopsycho.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Rose S, Laptook RS. Hypothalamic-pituitary-adrenal axis reactivity in the preschool-age offspring of depressed parents: Moderation by early parenting. Psychological Science. 2011;22:650–658. doi: 10.1177/0956797611404084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L, Dunn D. Peabody Picture Vocabulary Test. 3rd edition. Circle Pines, Minnesota: American Guidance Service; 1997. [Google Scholar]
- Egeland B, Weinfield N, Hiester M, Lawrence C, Pierce S, Chippendale K. Teaching tasks administration and scoring manual. 1995. University of Minnesota. [Google Scholar]
- Eisenberg N. Temperamental Effortful Control (Self-Regulation) Arizona State University: Centre of Excellence for Early Childhood Development. 2005 Retrieved from http://www.child-encyclopedia.com/documents/EisenbergANGxp-Temperament.pdf.
- Eisenberg N, Cumberland A, Spinrad TL. Parental socialization of emotion. Psychological Inquiry. 1998;9:241–273. doi: 10.1207/s15327965pli0904_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emde RN. Social referencing research: Uncertainty, self, and the search for meaning. In: Emde RN, editor. Social referencing and the social construction of reality in infancy. New York, NY, US: Plenum Press; 1992. pp. 79–94. [Google Scholar]
- Feng X, Shaw DS, Skuban EM, Lane T. Emotional exchange in mother-child dyads: Stability, mutual influence, and associations with maternal depression and child problem behavior. Journal of Family Psychology. 2007;21:714–725. doi: 10.1037/0893-3200.21.4.714. [DOI] [PubMed] [Google Scholar]
- Fernald A. Approval and disapproval: Infant responsiveness to vocal affect in familiar and unfamiliar languages. Child Development. 1993;64:657–674. [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. Laboratory Temperament Assessment Battery: Preschool version. 1995. Unpublished manuscript. [Google Scholar]
- Gotlib I, Joormann J, Minor K, Cooney R. Cognitive and Biological Functioning in Children at Risk for Depression. In: Canli T, editor. Biology of personality and individual differences. New York, NY, US: Guilford Press; 2006. pp. 353–382. [Google Scholar]
- Gottfredson MR, Hirschi T. A general theory of crime. Stanford University Press; 1990. Retrieved from http://search.proquest.com/docview/617859635?accountid=15115. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM. Neuroendocrine measures in developmental research. New York, NY, US: Cambridge University Press; 2008. Retrieved from http://search.proquest.com/docview/622082833?accountid=15115. [Google Scholar]
- Gunnar MR, Stone C. The effects of positive maternal affect on infant responsiveness to pleasant, ambiguous, and fear-provoking toys. Child Development. 1984;55:1231–1236. [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MJ, Roubinov DS, Gress-Smith J, Luecken LJ, Sandler IN, Wolchik S. Positive parenting during childhood moderates the impact of recent negative events on cortisol activity in parentally bereaved youth. Psychopharmacology. 2011;214:231–238. doi: 10.1007/s00213-010-1889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Sheikh HI, Olino TM, Dougherty LR, Dyson MW, et al. The serotonin transporter promoter polymorphism and childhood positive and negative emotionality. Emotion. 2010;10:696–702. doi: 10.1037/a0019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. 1975. Unpublished manuscript. [Google Scholar]
- Kochanska G, Aksan N. Children’s conscience and self-regulation. Journal of Personality. 2006;74:1587–1618. doi: 10.1111/j.1467-6494.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Barry RA, Jimenez NB, Hollatz AL, Woodard J. Guilt and effortful control: Two mechanisms that prevent disruptive developmental trajectories. Journal of Personality and Social Psychology. 2009;97:322–333. doi: 10.1037/a0015471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Knaack A. EC as a personality characteristic of young children: Antecedents, correlates and consequences. Journal of Personality. 2003;71:1087–1112. doi: 10.1111/1467-6494.7106008. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray K, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents and implications for social development. Developmental Psychology. 2000;36:220–232. [PubMed] [Google Scholar]
- Kochanska G, Murray K, Jacques TY, Koenig AL, Vandegeest KA. Inhibitory control in young children and its role in emerging internalization. Child Development. 1996;67:490–507. [PubMed] [Google Scholar]
- Kryski KR, Smith HJ, Sheikh HI, Singh SM, Hayden EP. Assessing stress reactivity indexed via salivary cortisol in preschool-aged children. Psychoneuroendocrinology. 2011;36:1127–1136. doi: 10.1016/j.psyneuen.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Lengua LJ. Effortful control in the context of socioeconomic and psychosocial risk. Paper presented at the American Psychological Association’s fourth annual Science Leadership Conference Designing the Future: Innovations in Knowledge and Dissemination for Psychological Science; 2008, October; Tempe A.Z.. [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Archives of General Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Luthar SS. Resilience in development: A synthesis of research across five decades. Hoboken, NJ, US: John Wiley & Sons Inc.; 2006. Retrieved from http://search.proquest.com/docview/621139769?accountid=15115. [Google Scholar]
- Martel MM, Nigg JT. Child ADHD and personality/temperament traits of reactive and effortful control, resiliency, and emotionality. Journal of Child Psychology and Psychiatry. 2006;47:1175–1183. doi: 10.1111/j.1469-7610.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- Masten AS. Competence, resilience, and development in adolescence: Clues for prevention science. New York, NY, US: Oxford University Press; 2007. Retrieved from http://search.proquest.com/docview/621640681?accountid=15115. [Google Scholar]
- Mumme DL, Fernald A, Herrera C. Infants’ responses to facial and vocal emotional signals in a social referencing paradigm. Child Development. 1996;67:3219–3237. [PubMed] [Google Scholar]
- Murray L, Halligan SL, Goodyer I, Herbert J. Disturbances in early parenting of depressed mothers and cortisol secretion in offspring: A preliminary study. Journal of Affective Disorders. 2010;122:218–223. doi: 10.1016/j.jad.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Hartman CA, Nederhof E, Riese H, Ormel J. Effortful control as predictor of adolescents' psychological and physiological responses to a social stress test: The tracking adolescents' individual lives survey. Development and Psychopathology. 2011;23:679–688. doi: 10.1017/S0954579411000095. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Boivin M, Dionne G, Lupien SJ, Arsenault L, Barr RG, et al. Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Archives of General Psychiatry. 2008;65:211–218. doi: 10.1001/archgenpsychiatry.2007.27. [DOI] [PubMed] [Google Scholar]
- Pendry P, Adam EK. Associations between parents' marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. International Journal of Behavioral Development. 2007;31:218–231. [Google Scholar]
- Posner MI, Rothbart MK. Summary and commentary: Developing attentional skills. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 1998. Retrieved from http://search.proquest.com/docview/619349457?accountid=15115. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Rothbart MK, Bates JE, editors. Handbook of Child Psychology: Vol. 3, Social, emotional, and personality development. Hoboken, NJ, US: John Wiley & Sons Inc.; 2006. [Google Scholar]
- Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, et al. Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The generation R study. Hormones and Behavior. 2010;57:247–254. doi: 10.1016/j.yhbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Schachar R, Mota VL, Logan GD, Tannock R, Klim P. Confirmation of an inhibitory control deficit in attention deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2000;28:227–235. doi: 10.1023/a:1005140103162. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Spinrad TL, Eisenberg N, Granger DA, Eggum ND, Sallquist J, Haugen RG, et al. Individual differences in preschoolers' salivary cortisol and alpha-amylase reactivity: Relations to temperament and maladjustment. Hormones and Behavior. 2009;56(1):133–139. doi: 10.1016/j.yhbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Bruce J, Donzella B, Gunnar MR. Stability of physiological measures in young children; Poster presented at the International Society for Developmental Psychobiology; New Orleans, LA. 2003. [Google Scholar]
- Talge NM, Donzella B, Kryzer EM, Gierens A, Gunnar MR. It's not that bad: Error introduced by oral stimulants in salivary cortisol research. Developmental Psychobiology. 2005;47:369–376. doi: 10.1002/dev.20097. 369. [DOI] [PubMed] [Google Scholar]
- Vazsonyi AT, Huang L. Where self-control comes from: On the development of self-control and its relationship to deviance over time. Developmental Psychology. 2010;46(1):245–257. doi: 10.1037/a0016538. [DOI] [PubMed] [Google Scholar]
- Verstraeten K, Vasey MW, Raes F, Bijttebier P. Temperament and risk for depressive symptoms in adolescence: Mediation by rumination and moderation by effortful control. Journal of Abnormal Child Psychology: An Official Publication of the International Society for Research in Child and Adolescent Psychopathology. 2009;37:349–361. doi: 10.1007/s10802-008-9293-x. [DOI] [PubMed] [Google Scholar]
- Walker-Andrews AS. Infants’ perception of expressive behaviors: Differentiation of multimodal information. Psychological Bulletin. 1997;121:437–456. doi: 10.1037/0033-2909.121.3.437. [DOI] [PubMed] [Google Scholar]
- Walker-Andrews AS, Lennon E. Infants’ discrimination of vocal expressions: Contributions of auditory and visual information. Infant Behavior and Development. 1991;14:131–142. [Google Scholar]
- Weinfield NS, Egeland B, Ogawa JR. Affective quality of mother-child interaction. In: Zaslow M, Eldred C, editors. Parenting behavior in a sample of young mothers in poverty: Results of the New Chance Observational Study. New York: Manpower Demonstration Research Corporation; 1998. pp. 71–113. [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Myin-Germeys I, Schruers K, Mengelers R, et al. The psychology of psychiatric genetics: Evidence that positive emotions in females moderate genetic sensitivity to social stress associated with the BDNF Val66Met polymorphism. Journal of Abnormal Psychology. 2008;117:699–704. doi: 10.1037/a0012909. [DOI] [PubMed] [Google Scholar]
- Zaslow MJ, Weinfield NS, Gallagher M, Hair EC, Ogawa JR, Egeland B, et al. Longitudinal prediction of child outcomes from differing measures of parenting in a low-income sample. Developmental Psychology. 2006;42:27–37. doi: 10.1037/0012-1649.42.1.27. [DOI] [PubMed] [Google Scholar]