Abstract
Researchers have long been interested in whether particular temperamental traits in childhood connote risk for depressive disorders. For example, children characterized as having high negative emotionality (NE; sadness, fear, anger) and low positive emotionality (PE; anhedonia, listlessness, and lack of enthusiasm) are hypothesized to be at risk for depression. Few studies, however, have examined whether (and how) these two temperamental dimensions interact to confer risk. In a sample of 329 preschoolers, the present study addressed this question by examining the relation between PE and NE and asymmetry in resting EEG activity in frontal and posterior regions, which are putative biomarkers for depression. Using a laboratory battery to define temperament, we found an interaction of PE and NE on posterior asymmetry. Specifically, when PE was high, NE was associated with greater relative right activity. When PE was low, NE was not related to posterior asymmetry. These results were driven by differences in EEG activity in right posterior regions, an area associated with emotional processing and arousal, and were specific to girls. We found no relation between temperament and frontal asymmetry. These findings suggest that, at least for girls, PE and NE may have an interactive effect on risk for depression.
Quantitative studies of the structure of personality consistently report that positive emotionality (PE) and negative emotionality (NE) are two “superfactors” that account for the largest amount of variance in personality (Clark, Watson, & Mineka, 1994; Costa & McCrae, 1980; Watson & Tellegen, 1985). PE encompasses a tendency to experience affects such as joy, interest, and excitement. NE is characterized by a tendency to experience negative emotions such as sadness, anxiety, fear, and anger. PE and NE are hypothesized to be orthogonal dimensions (Clark & Watson, 1999; Watson & Tellegen, 1985); therefore, an individual can be high or low on one, the other, or both.
Both PE and NE have been hypothesized to play a role in the development of major depressive disorder (MDD; Clark & Watson, 1991; Shankman & Klein, 2003). Specifically, individuals who are high on NE and/or low on PE have been posited to be at increased risk for MDD. Consistent with this hypothesis, these two traits have been shown to predict onset of MDD (although the findings for NE are generally stronger) and NE has been shown to have a shared genetic liability with depression (Kendler et al., 1993; Kendler, Gatz, Gardner, & Pederson, 2006a, 2006b; Middeldorp, Cath, Van Dyck, & Boomsma, 2005; Ormel, Oldehinkel, & Vollebergh, 2004).
Although most investigations of personality and depression have focused on adults, a growing literature has examined the personality–depression association in preschool age children. Caspi, Moffitt, Newman and Silva (1996) reported that children rated as inhibited, socially reticent, and having difficulty concentrating at age 3 were more likely to develop mood disorders by age 21. Although Caspi et al. found that the inhibition–psychopathology link was specific to depression, others have reported that behavioral inhibition also predicts later anxiety disorders, particularly social phobia (Chronis-Tuscano et al., 2009; Fox, Henderson, Marshall, Nichols, & Ghera, 2005; Hirshfeld-Becker et al., 2008; Kagan & Snidman, 1999). Several studies from our lab have shown that low PE in preschool-aged children is associated with higher rates of maternal depression (Durbin, Klein, Hayden, Buckley, & Moerk, 2005) and predicted higher levels of depressotypic cognitive styles at age 7 and depressive symptoms at age 10 (Dougherty, Klein, Durbin, Hayden, & Olino, 2010; Hayden, Klein, Durbin, & Olino, 2006). Although there are important methodological and conceptual complexities in these and similar studies (e.g., equating temperament with adult personality, distinguishing temperament and psychopathology; Tackett, 2006), taken together, these lines of research suggest that low PE and high NE may represent temperamental precursors/risk factors for depression in preschool-age children.
Another approach to exploring the role of temperamental risk factors in depression is to examine whether NE and PE are associated with biological correlates of the condition. For example, we recently reported an association between low PE in preschool-aged children and elevated morning cortisol levels (Dougherty, Klein, Olino, Dyson, & Rose, 2009). Another biomarker for depression that has received a great deal of interest is an asymmetry of EEG recorded while at rest. Several studies have reported abnormal EEG asymmetries in adults with depression (Bruder et al., 1997; Henriques & Davidson, 1991; Shankman, Klein, Tenke, & Bruder, 2007), those who have recovered from depression (Gotlib, Ranganath, & Rosenfeld, 1998; Henriques & Davidson, 1990), and children with afamily history of depression (Bruder et al., 2005; Bruder, Tenke, Warner,& Weissman, 2007; Dawson, Frey, Panagiotides, Osterling, & Hessl, 1997; Jones, Field, Fox, Lundy, & Davalos, 1997; Tomarken, Dichter, Garber, & Simien, 2004). However, there have also been several inconsistencies in the literature. For example, Reid, Duke, and Allen (1998) failed to find an association between depression and resting frontal EEG asymmetry, a finding possibly due to the heterogeneity of depression (Bruder et al., 1997; Pizzagalli et al., 2002).
EEG asymmetries have been shown to have moderate stability in adults with depression (over 8 weeks, intraclass correlations [ICCs] = 0.41–9.77; over 16 weeks, ICCs = 0.33–0.85; Allen, Urry, Hitt, & Coan, 2004) and low to moderate stability in preschool-aged children (for frontal regions, ICCs = 0.21–9.48; for parietal regions, ICCs = 0.21–0.27; Jones, Field, Davalos, & Pickens, 1997; Vuga, Fox, Cohn, Kovacs, & George, 2008). Several studies have also reported that EEG asymmetries have a significant genetic component (Anokhin, Heath, & Myers, 2006; Smit, Posthuma, Boomsma, & DeGeus, 2007), although this has never been examined in young children. In addition, Pössel, Lo, Fritz, and Seemann (2008) reported that EEG asymmetries in adolescents (13–15 years old) predicted self-reported depression 12 months later. Taken together, these findings present suggestive evidence that EEG asymmetries may be a potential endophenotype for depression (Allen, 2009).
The EEG asymmetry–depression relationship is not uniform across all cortical areas and has been shown to differ at frontal versus posterior regions. Frontal EEG asymmetry has often been explained using the approach–withdrawal model (Davidson, 1994; Davidson et al., 2002), which posits that individuals predisposed to depression exhibit a dispositional tendency to be less likely to seek reward. Accordingly, this “low approach affective style” is associated with a frontal brain asymmetry due to decreased activity in the left frontal region.
Posterior EEG asymmetries have received less attention than anterior asymmetries in the depression literature. The posterior asymmetry in depression is posited to be the result of decreased activity in the right posterior region, which has been associated with decreased emotional arousal (Heller, Nitschke, & Miller, 1998) and general processing of emotional information (Bruder, 2003; Heller, 1990). Many studies have reported posterior EEG asymmetries in individuals with depression (Bruder et al., 1997; Kentgen et al., 2000; Reid et al., 1998), those who have recovered from depression (Henriques & Davidson, 1990), and college students with elevated depressive symptoms (Blackhart, Minnix, & Kline, 2006). However, several studies have produced contradictory findings. For example, Mathersul, Williams, Hopkinson, and Kemp (2008) found that, compared to controls, individuals with depression had relative increased right posterior activation. In addition, Pössel et al. (2008) found that relative increased right posterior activation predicted depression at a 12-month follow-up, even adjusting for baseline depressive and anxiety symptoms. Last, Metzger et al. (2004) found that although depressive symptoms were not associated with a posterior EEG asymmetry, they moderated the association between arousal and posterior asymmetry in that arousal had a stronger association with a relative right posterior asymmetry when depression was high.
Given that both temperamental emotionality traits and EEG asymmetries may tap somewhat stable predispositions for mood disorders with a genetic basis, it stands to reason that these two domains of risk may be related to one another. Several studies have found associations between these or related traits and EEG asymmetries in adults (Jacobs & Synder, 1996; Schmidtke & Heller, 2004; Tomarken, Davidson, Wheeler, & Doss, 1992) and young children (Calkins, Fox, & Marshall, 1996; Fox, Henderson, Rubin, Culkins, & Schmidt, 2001) for frontal and/or posterior regions. In a previous report, we also found an association between childhood PE and posterior asymmetry, but not frontal asymmetry (Shankman et al., 2005). Specifically, children classified using a standardized behavioral observation procedure (Goldsmith, Reilly, Lemery, Longley, & Prescott, 1995) as having low PE at age 3 exhibited a posterior EEG asymmetry of less relative right posterior activity at age 5–6.
Several methodological aspects of previous studies limit them as thorough tests of the relation between childhood temperamental/personality risk factors and EEG asymmetry. First, the sample sizes of previous reports are relatively small. Second, several reports are based on selecting groups that are high or low in dimensions of temperament (e.g., Shankman et al., 2005), and this limits the conclusions one can make about the full range of temperament traits. Third, several studies did not measure temperament and EEG at the same age (McManis, Kagan, Snidman, & Woodword, 2002; Shankman et al., 2005). Fourth, although this may not be a limitation, but instead may be a different approach to assessing temperament, some studies relied on self-report or parental report of personality rather than observational measures of personality/temperament (Tomarken, Davidson, Wheeler, & Kinney, 1992). Therefore, the present study will address these limitations by examining the association of EEG asymmetries and observationally based measures of PE and NE in over 300 preschool-aged children.
Given that both PE and NE are proposed to play a role in the development of depression, it is also important to examine whether the two dimensions have additive or interactive effects on risk instead of just exploring the separate effects of the two dimensions. This interaction could assume several possible forms (Gerhsuny & Sher, 1998). PE and NE may interact in that experiencing positive emotions may moderate (or buffer) the effects of stress reactivity and negative emotions on EEG asymmetry (Tugade & Fredrickson, 2004; Wichers et al., 2007). Alternatively, either low PE or high NE may be associated with greater risk than having both high PE and low NE (i.e., low risk on both dimensions), but the combination of low PE and high NE (i.e., high risk on both dimensions) may not confer greater risk than either trait alone. This would be consistent with the hypothesis that both low PE and high NE are sufficient to confer risk, although neither trait is necessary (Gershuny & Sher, 1998; Shankman & Klein, 2003). Another aim of the present study is to therefore determine whether and how the interaction of PE and NE is associated with EEG asymmetries.
Last, EEG asymmetry has also been shown to differ between men and women. Most investigations examining EEG asymmetry and depression have been comprised of female participants (Thibodeau, Jorgensen, & Kim, 2006). Of interest, in the few studies that have included at least a moderate number of men, depressed males’ EEG asymmetries differed from those of females (Jacobs & Snyder, 1996; Miller et al., 2002). Therefore, a final aim of this investigation is to examine whether preschool-aged boys and girls exhibit different patterns of association between temperament and EEG asymmetry.
Method
Participants
The sample consisted of 559 3-year-olds from a suburban community who participated in a larger study of child temperament and risk for psychopathology. Potential participants were identified using commercial mailing lists and screened by telephone. Children were included if they lived with at least one English-speaking biological parent and did not have any significant medical conditions or developmental disabilities.
All 559 children were invited to participate in the EEG portion of the study but 104 children refused, 9 parents refused, and 41 children failed to complete the EEG assessment. An additional 22 and 52 children were excluded, respectively, because they had too much artifact in their EEG data (see Data Analysis Section) or because they were sinistral (as indicated by performing at least three of six tasks [throwing a ball, drawing, pretending to cut with scissors, open a jar, kick a ball, look through a toy telescope] with their left hand/eye/foot). Finally, two more children were excluded who met DSM-IV criteria for a specific mood disorder, based on parent report from the Preschool Age Psychiatric Assessment (Egger, Ascher, & Angold, 1999). This yielded a final total of 329 children. Those who were in the final EEG analysis did not differ from those who were not on race; social status (Hollingshead, 1975); whether the child lived with both biological parents; whether the mother worked outside the home; Peabody Picture Vocabulary Test (PPVT; Dunn & Dunn, 1997) score; and child PE, NE, and anger. However, children in the final analysis did have higher fear and sadness ratings, were slightly older and were more likely to be female (p < .05).
The mean age of the final sample was 42.5 months (SD = 3.15), and 153 (46.5%) were male. The sample was predominantly (85.7%) Caucasian and middle class. On the Hollingshead (1975) Four Factor Index of Social Status, which is arguably the most widely used index of socioeconomic status (SES; Cirino et al., 2002), the sample’s mean score was 2.2 (SD = 0.90).1 The Hollingshead is a weighted average of the occupational prestige and educational level of both parents. The continuous score is often broken down into five categories, ranging from high (Class I) to low (Class V). In our sample, the proportions of the families in social Classes I–V were 21.0%, 45.3%, 25.2%, 7.6%, and 0.9%, respectively. Forty-six percent of fathers and 56.8% of mothers had at least a bachelor’s degree. The demographic characteristics of the sample were consistent with the larger county from which it was drawn. Most children (95.1%) lived with both biological parents, and 46.5% of the mothers worked outside the home part- or full-time. Children were of average cognitive ability as indexed by the PPVT (M=102.64, SD = 13.33).
Child temperament
Each child and a parent (95.1% mothers) visited the laboratory for a 2-hr observational assessment of temperament that included a standardized set of 12 episodes selected to elicit a range of temperament-relevant behaviors. Eleven episodes were from the Laboratory Temperament Assessment Battery (LAB-TAB; Goldsmith et al., 1995) and one was adapted from a LAB-TAB episode. The LAB-TAB is a standardized temperament assessment battery that has been used in numerous studies with preschoolers over the last 25 years (see http://psych.wisc.edu/goldsmith/researchers/GEO/lab_tab.htm). Using an independent sample, we previously reported moderate associations between LAB-TAB ratings and home observations, and moderate stability of laboratory ratings of temperament over four years (Durbin, Hayden, Kelin, & Olino, 2007). Each task was videotaped through a one-way mirror and later coded. Episodes were ordered so as to prevent carryover effects in that no episodes presumed to evoke similar affective responses occurred consecutively, and each was followed by a brief play break to allow the child to return to a baseline emotional state. The parent remained in the room with the child for all episodes except Stranger Approach and Box Empty (see below). The parent was instructed not to interact with the child (except in Pop-Up Snakes) and was seated facing at a right angle from the experimenter and child and given questionnaires to complete.
The episodes in order of presentation were (a) Risk Room: the child explored a set of novel and ambiguous stimuli, including a Halloween mask, balance beam, and black box; (b) Tower of Patience: the child and the experimenter alternated turns in building a tower, and the experimenter took increasing amounts of time before placing her block on the tower during each turn; (c) Arc of Toys: the child played independently with toys for 5 min before the experimenter asked the child to clean up the toys; (d) Stranger Approach: the child was left alone briefly in the room before a male accomplice entered, speaking to the child while slowly walking closer; (e) Make that Car Go: the child and the experimenter raced remote-controlled cars; (f) Transparent Box: the experimenter locked an attractive toy in a transparent box, leaving the child alone with a set of nonworking keys. After a few minutes, the experimenter returned and told the child that she had left the wrong set of keys. The child used the new keys to open the box and play with the toy. (g) Exploring New Objects: the child was given the opportunity to explore a set of novel and ambiguous stimuli, including a mechanical spider, a mechanical bird, and sticky water-filled soft gel balls; (h) Pop-up Snakes: the child and experimenter surprised the parent with a can of potato chips that actually contained coiled snakes; (i) Impossibly Perfect Green Circles: the experimenter repeatedly asked the child to draw a circle on a large piece of paper, mildly criticizing each attempt; (j) Popping Bubbles: the child and experimenter played with a bubble-shooting toy; (k) Snack Delay: the child was instructed to wait for the experimenter to ring a bell before eating a snack. The experimenter systematically increased the delay before ringing the bell; and (l) Box Empty: the child was given an elaborately wrapped box to open under the impression that a toy was inside. After the child discovered the box was empty, the experimenter returned with several toys for the child to keep.
Coding procedures
PE and NE were coded using an extensive manual developed for previous studies by Durbin et al. (2005, 2007). Discrete emotions (positive affect/exuberance, anger, sadness, and fear) were assessed by separately coding each display of facial, vocal, and bodily indicators of the emotion in each episode on a 3-point scale (ambiguous/low intensity, definite/moderate intensity, definite/high intensity). Coding of facial expressions and their intensity used the AFFEX system (Izard, Dougherty, & Hembree, 1989). For example, to assess positive affect, the intensity of each instance of smiling, positive verbalizations (e.g., a squeal of joy, or “that was fun!” stated in an enthusiastic tone), and bodily movements suggesting positive affect (e.g., jumping for joy; vigorous approach-oriented movements) were coded. Ratings were summed separately within each channel (facial, bodily, vocal) across the 12 episodes, standardized (z scored), and summed across the three channels to derive total scores for positive affect, fear, sadness, and anger. Thus, the affect codes reflect both the frequency and the intensity of the emotion. Following Durbin et al. (2005, 2007), interest was rated on a single global 4-point scale (none, low, moderate, and high) for each episode, based on the child’s comments about the activity and how engaged the child was in play. Interest ratings were then summed across the 12 episodes. PE consisted of the sum of the standardized total positive affect and interest variables (mean Z = 0.08, SD = 0.52). NE was the sum of the standardized total sadness, fear, and anger variables (mean Z = −0.03, SD = 0.51).
PE and NE had adequate internal consistency (α = 0.82 and 0.74, respectively; ICC = 0.89 and 0.82, respectively; N = 35). For the three facets of NE, the internal consistencies and interrater reliabilities (N = 35) were the following: anger, α = 0.68, ICC = 0.79; sadness, α = 0.81, ICC = 0.73; and fear, α = 0.63, ICC = 0.64. NE and its facets were positively skewed, so we natural log transformed these variables. Similar to our previous study on EEG and temperament (Shankman et al., 2005), PE and NE were relatively orthogonal (r = −.07, ns), and PE was also not correlated with any of the facets of NE (ps>.16). The two facets of PE (interest and positive affect) were highly correlated with each other (r = .65). In contrast, the three facets of NE had relatively low intercorrelations (rs =.08–.33); hence, we also analyzed the NE facets separately (sadness, anger, fear). Demographic variables were largely unrelated with PE, NE, and the three facets of NE, with the small exceptions of SES and paternal education being negatively correlated with anger (r = −.14, p < .01; r = −.12, p < .05, respectively) and girls displaying higher fear t (327) = 2.46, p < .05.
Procedures
EEG assessment
The child was shown the recording chamber, which was decorated to look like a space shuttle (adapted from Fox et al., 1995). The walls and ceiling of the room were lined with black fabric and covered with stickers of stars and planets and the recording chair was decorated to look like an astronaut command chair. The child was told that he/she would be pretending to be an astronaut and going on missions with an experimenter in the room.
Data were acquired using the Active Two system (Biosemi, Amsterdam, The Netherlands). A stretch Lycra cap was placed on the child’s head and 32 silver/silver chloride tipped electrodes arranged according to the international 10/20 labeling system (American Electroencephalographic Society, 1994). In addition, data were recorded from VEOG and HEOG (bipolar leads) to monitor vertical and horizontal eye movements, respectively. The offsets of all electrodes were between ±20 μV and both the EEG and EOG were sampled at a rate of 512 Hz, with high- and low-pass filters set at 0.16 and 40 Hz, respectively. All data was referenced offline to a nose reference. Because different reference montages can yield different results (see Hagemann, 2004; Tenke & Kayser, 2005), the EEG and EOG (epoched data) were also rereferenced offline to the average signal and reference was included as a repeated-measures factor in our analyses (see below). Although many studies have examined hemispheric asymmetries using a Cz reference (e.g., Dawson, Panagiotides, Klinger, & Spieker, 1997; Jones et al., 1997), there is evidence that this reference is problematic and that results obtained with it differ from those obtained using other reference montages (Allen, Coan, & Nazarian, 2004; Hagemann, 2004; Tenke & Kayser, 2005).
Scalp EEG was recorded while participants sat quietly in the recording chamber with an experimenter and, when necessary, their mother, during six 1-min blocks, with the order of the eyes open or closed conditions counterbalanced across subjects. Subjects were instructed to sit as still as possible during the recording period and to either fixate on a simple schematic of a space shuttle or sit quietly with their eyes closed until the experimenter indicated the end of the block. They were told that if they sat very still, they would receive a prize following each block.
EEG analyses
EEG files were converted from Biosemi to Neuroscan 4.1 (Charlotte, NC) formats using the program PolyRex (Kayser, 2003). The data were baseline corrected and then segmented into consecutive 1.024-s epochs every 0.512 s (50% overlap). We excluded from the analyses epochs that were contaminated with eye or movement artifacts by direct visual inspection of the raw epoched data (e.g., blink artifacts in frontal electrodes, obvious movement related artifacts in all channels, etc.). Given how difficult it is for preschool-aged children to sit still, direct visual inspection and manual artifact rejection is likely to be superior to automatic rejection or correction procedures (Hagemann, 2004; Somsen & van Beek, 1998). Children had an average of 85.84 (SD = 35.1) and 73.57 (SD = 37.1) epochs during the eyes open and closed conditions, respectively. The EEG was tapered over the entire 1.024-s epoch by a Hanning window to suppress spectral side lobes. By overlapping the epochs by 50%, data that were attenuated at the beginning and end of an epoch were preserved in adjacent (overlapping) epochs.
Power spectra were computed offline from the EEG data using a fast Fourier transform. Analyses focused on the alpha band to be consistent with prior EEG studies (Bruder et al., 1997; Jones et al., 1997) and because this band has been shown to be inversely correlated with other measures of brain activity such as positron emission tomography (Oishi et al., 2007; although see Tenke & Kayser, 2005). Moreover, it is unclear how to define the other frequency bands (delta, theta, beta) in children this age given the developmental changes in EEG frequency spectra (Fisch, 1999). For each electrode, alpha power was averaged for artifact-free epochs and then integrated over a 6- to 10-Hz band, the recommended frequency band to define alpha in children of this age (Marshall, Bar-Haim, & Fox, 2002). Visual inspection of a subset of data files revealed a discernable alpha peak in the middle of this range.
Statistical analyses
To normalize the data, each electrode was natural log transformed. We then created asymmetry scores (ln[left] – ln[right]) for homologous pairs in anterior (AF3/4, F3/4, F7/8) and posterior regions (PO3/4, P3/4, P7/8). Using the heuristic of alpha being inversely related to activity (Allen, Coan, et al., 2004), these asymmetry indices reflect the degree of relative right activity. EEG asymmetry studies typically focus on F3/4, F7/8, P3/4, and P7/8 (Allen, Coan, et al. 2004; Shankman et al., 2005); but we wanted to include these additional sites to examine the regional specificity of the findings. We also conducted hemisphere analyses to determine the contribution of each hemisphere to the asymmetry score.
To examine the relation between temperament and EEG asymmetry, we ran separate mixed linear models (SPSS 16.0; Coan, Allen, & McKnight, 2006; Stewart, Bismark, Towers, Coan, & Allen, 2009). Individual asymmetry scores for eyes (open, closed), reference (nose, average), and region (three regions listed above) were “stacked” resulting in a maximum of 12 cases for each anterior and posterior model. This approach allows for modeling of multiple within-subjects factors, and it is more powerful than traditional generalized linear model as it allows for missing observations (J. Allen & J. Coan, personal communication, April 2009). It is important to note that, because some children yielded clean data with their eyes open but not closed (and vice versa), this approach allowed us to include children who only had clean data in one “eyes” condition.
As expected, there was a main effect for eyes (more alpha when eyes were closed), but this factor did not interact with any of the temperament variables (ps > .62) for any of the analyses, suggesting that the findings did not vary by whether the child had their eyes open or closed. Thus, it was not included as a fixed effect in subsequent models, although it still was a repeated variable in the data structure. There were significant or marginally significant interactions between the temperament variables and region, and thus results are reported separately for the three regions within anterior and posterior sites. Although some might argue that including “anterior/posterior” (i.e., AF3/4, F3/4, F7/8 vs. PO3/4, P3/4, P7/8) as an additional repeated-measures factor would test the specificity of the effect, we feel that this would be inappropriate given that anterior and posterior asymmetries are proposed to reflect distinct mechanisms.
All temperament variables were centered as per Aiken and West’s (1991) guidelines on testing interactions. As outliers are particularly problematic for hypothesized interactions, we carefully screened our data and scatterplots for outliers. When outliers were removed, the below results were nearly identical.
In order to examine whether activity in right or left regions drove any asymmetry findings, we calculated alpha power scores for each of the hemispheres (e.g., P3 and P4). This was done by residualizing alpha power for each eyes condition and recording reference as a function of whole-head alpha power for that condition/reference, and then using these residualized scores as dependent variables. This approach removes overall differences in power due to anatomical factors such as scalp thickness while preserving topography (Allen, Coan, et al., 2004; Davidson, Jackson, & Larson, 2000).
Results
Anterior sites
To examine the relationship between temperament and frontal asymmetry, PE, NE, and their interaction were entered as between-subjects variables in separate models with each of the three anterior asymmetries as the dependent variables. The results are presented at the top of Table 1.
Table 1.
Associations between temperament variables and anterior and posterior EEG asymmetries
| Anterior EEG Asymmetry |
||||||
|---|---|---|---|---|---|---|
| F3/4 |
AF3/4 |
F7/8 |
||||
| Temperament Dimension | b | SE | b | SE | b | SE |
| NE | −0.008 | 0.007 | −0.003 | 0.005 | 0.004 | 0.009 |
| PE | −0.001 | 0.006 | 0.003 | 0.004 | 0.013† | 0.007 |
| NE×PE | −0.006 | 0.008 | −0.004 | 0.006 | −0.002 | 0.010 |
| Posterior EEG Asymmetry |
||||||
|---|---|---|---|---|---|---|
| P3/4 |
PO3/4 |
P7/8 |
||||
| b | SE | b | SE | b | SE | |
| NE | 0.030** | 0.010 | 0.028* | 0.012 | 0.059*** | 0.014 |
| PE | −0.023** | 0.008 | −0.040*** | 0.009 | −0.003 | 0.011 |
| NE×PE | 0.030** | 0.011 | 0.036* | 0.014 | −0.038* | 0.016 |
Note: The b values are unstandardized estimates of the association of the temperament dimension and EEG asymmetry. EEG asymmetries are coded such that higher values indicate greater relative right activity (e.g., ln[P3 alpha] – ln[P4 alpha]). NE, negative emotionality; PE, positive emotionality.
p < .10.
p < .05.
p < .01.
p < .001.
PE and NE were not significantly related to anterior asymmetries for any of the regions, although there was a trend for PE to be associated with the F78 asymmetry, with relative greater right activity associated with more PE (b = 0.013, SE = 0.007, p < .10). The interaction of PE and NE also was not related to any anterior EEG asymmetries.2
When reference (average reference vs. nose reference) was included as a repeated measure factor in the above models, the results remained the same, suggesting that the findings were not moderated by reference.
Posterior sites
We next repeated the above analyses but using the three posterior asymmetries as the dependent variables (see results on bottom of Table 1). These results indicate that both PE and NE were independently associated with posterior asymmetries in at least two of the three regions. Specifically, using the heuristic that alpha is inversely related to activity, NE was associated with P3/4, P7/8, and PO3/4 such that higher NE was related to greater relative right posterior activity. PE was associated with P3/4 and PO3/4 such that lower PE was related to greater relative right posterior activity.
The interaction of PE and NE was also related to P3/4, P7/8, and PO3/4, although the direction of the interaction term differed for P78 compared to the other two regions. However, the PE×NE interaction for P78 was moderated by reference at a trend level (PE × NE × reference: b = 0.057, SE = 0.032, p<.08), whereas the PE×NE interaction was not moderated by reference for the other two regions (both ps > .55). Follow-up analyses to this three-way interaction showed there was no PE×NE interaction in P7/8 for the nose reference (p > .56), but a PE×NE interaction for the average reference (p<.01). Given that data from lateral electrodes (such as P7/8) are affected by artifact more than medial electrodes, the pattern of results for main effects and interactions was different for P7/8 compared to the other two regions, and the asymmetry for P7/8 was only present in one reference, we have chosen to interpret the interactions for P3/4 and PO3/4, rather than P7/8.
To probe the P3/4 and PO3/4 interactions further, we employed the method proposed by Aiken and West (1991; also see Holmbeck, 2002). First, we computed two conditional moderators, high PE and low PE, for 1 SD above or below the mean, respectively. Second, we examined the simple slope of each of these regression lines. This approach is superior to doing a median split on PE as it is more powerful and allows for clearer interpretation of intercepts and slopes.
When PE was low, NE was unrelated to posterior asymmetry (P3/4: b = 0.002, SE = 0.015, ns; PO3/4: b = −0.005, SE = 0.017, ns). However, when PE was high, NE was related to posterior asymmetry such that increasing NE was associated with greater relative right activity (P3/4: b = 0.058, SE = 0.015, p < .001; PO3/4: b = 0.061, SE = 0.018, p < .01). In other words, NE was only correlated with posterior asymmetry for children with high PE.
Gender
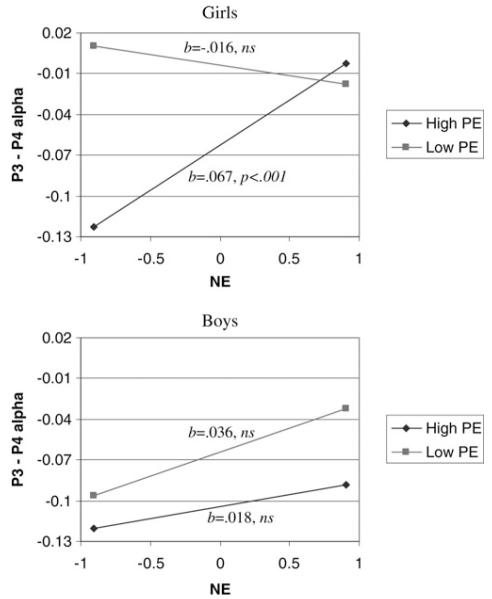
Given that previous studies have found that men and women at risk for depression exhibit different asymmetries (Jacobs & Snyder, 1996; Miller et al., 2002), we ran models with NE, PE, gender, and their interactions for P3/4, PO3/4, and P7/8 asymmetries. The PE×NE×Gender interactions were significant for P3/4 (b = −0.050, SE = 0.024, p < .05) and PO3/4 models (b = −0.102, SE = 0.034, p < .001), although not for the P7/8 model (b = −0.019, SE = 0.035, ns).3 For P3/4 and PO3/4, follow-up analyses demonstrated that the PE × NE interactions were not significant for boys (both ps > .17), but were significant for girls (both ps < .001). Figure 1 illustrates the PE × NE interaction for girls and boys separately for P3/4. These results suggest that the overall PE× NE interaction described above was driven by the effects in girls. For low PE girls, NE was unrelated to posterior asymmetry (b =− 0.016, SE = 0.016, ns), but for high PE girls, NE was related to posterior asymmetry (b=0.067, SE = 0.016, p < .001). The regression lines for boys were statistically flat. A similar pattern was found for PO3/4.
Figure 1.
The relation between negative emotionality (NE) and P3/4 asymmetry as moderated by positive emotionality (PE) for girls and boys presented separately. The dependent variable is coded such that higher values indicate greater relative right activity (e.g., ln[P3 alpha] – ln[P4 alpha]).
NE facets
We also explored which of the three facets of NE (sadness, fear, and anger) were driving the above PE×NE interactions for the P3/4 and PO3/4 asymmetries. For P3/4, there was a significant PE×Sadness interaction (b = 0.026, SE = 0.008, p < .01), a trend for PE×Fear (b = 0.017, SE = 0.009 p < .07), but no significant PE×Anger interaction (b = 0.005, SE = 0.008, ns). For PO3/4, there was a similar pattern of results, although slightly weaker. When reference was included in these models, the results were nearly identical and reference did not moderate the results. Also, similar to the above gender analyses for global NE, the results were driven by differences in females.
Hemisphere
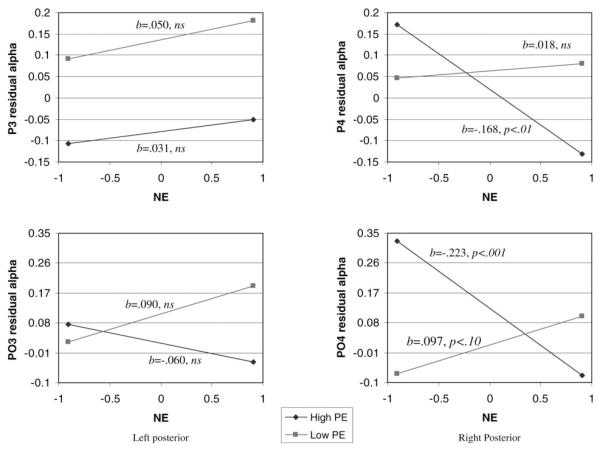
The previous results indicate that NE and PE interact to predict posterior asymmetries, but as the asymmetry is a difference score, these analyses cannot address whether the interaction is associated with activity in the right posterior, left posterior, or both hemispheres (Tomarken & Keener, 1998). Following Allen, Coan, et al. (2004), we residualized alpha activity in the right (P4, PO4) and left (P3, PO3) posterior sites as a function of whole-head alpha power and used these residualized scores as dependent variables in our PE×NE models. The PE×NE interaction was significantly associated with alpha power in P4 (b = −0.102, SE = 0.041 p < .05) and PO4 (b = −0.177, SE = 0.045 p < .001), but not P3 (b = −0.011, SE = 0.041 ns) and at a trend for PO3 (b = −0.086, SE = 0.045 p < .10). Figure 2 illustrates these results for these sites using the Aiken and West (1991) simple slope approach. For high PE children, as NE increased, right posterior activity increased (i.e., alpha decreased) in P4 (slope: b=−0.168, SE=0.053 p<.01) and PO4 (slope: b=−0.223, SE = 0.058 p < .001). For low PE children, NE was not associated with activity in P4 (slope: b=0.018, SE=0.050, ns) and was related to a trend for decreased activity in PO4 (slope: b = 0.097, SE = 0.054 p<.10). For the left posterior sites (P3 and PO3), the slopes were all statistically flat. Similar to the asymmetry analyses for gender, the findings were driven by PE×NE interactions for females, not males.
Figure 2.
Regression lines for relation between negative emotionality (NE) and left (P3 and PO3) and right (P4 and PO4) activity as moderated by positive emotionality (PE). The dependent variable is coded such that higher values indicate less activity (i.e., alpha is the inverse of activity).
Discussion
Researchers have long been interested in whether children with particular temperamental traits are at risk for depression. Using a large and well-characterized sample of preschoolers, this study examined whether individual differences on the temperamental dimensions of PE and NE predicted asymmetries in resting EEG activity in frontal and posterior regions, putative markers for risk for depression (Bruder et al., 1997; Davidson, 1998; Heller et al., 1998). We found that low PE and high NE independently predicted greater relative right posterior activity. In addition, we found that PE and NE interacted to predict a posterior asymmetry.
The direction of the interaction is particularly interesting. For children with greater PE, increasing levels of NE were related to greater relative right posterior activity. For children with lower levels of PE, variation in NE was unrelated to posterior asymmetry. It has been well documented that high levels of NE and (to a lesser extent) low levels of PE predict depression (Clark et al., 1994; Kendler et al., 2006a, 2006b) but the literature is unclear as to how (or if) the two traits interact to predict risk (Shankman & Klein, 2003). One possible interpretation of the present finding is that both low PE and high NE are sufficient for conferring risk for depression, although neither trait is necessary to confer risk. In addition, these results may suggest that children with both low PE and high NE have similar risk for depression compared to children who have either trait alone (although this last point may have been due to a ceiling effect on EEG asymmetry). Of interest, these results are consistent with other findings from this sample that examined the interaction of PE and NE in predicting parental history of depression (Olino, Klein, Dyson, Rose, & Durbin, 2010). That study found that compared to children with high PE and low NE, children with higher levels of NE, lower levels of PE, and the combination of high NE and low PE all had higher rates of parental depression, but these three latter subgroups did not differ from one another.
The interaction of PE and NE on posterior asymmetry appeared to be largely due to differences in right as opposed to left posterior regions. Neuropsychological and neurophysiological studies have often found that greater relative right posterior activity has been associated with increased emotional arousal and reduced processing of emotional stimuli (i.e., the “right hemisphere hypothesis;” Borod, 1992; Heller et al., 1998; Kayser, Bruder, Tenke, Stewart, & Quitkin, 2000). For example, a functional magnetic resonance imaging study (Killgore & Yurgelun-Todd, 2007) presented masked emotional faces to each hemisphere and concluded that right posterior regions may be involved in the processing of emotional stimuli regardless of valence even if the emotional stimuli are processed nonconsciously. Another study by McManis and colleagues (2002) found that 4-month-olds characterized as highlyemotionally “reactive” (i.e., greater motor activity and frequent fretting and crying) exhibited greater relative right parietal activity at age 10 compared to nonreactive infants. Taken together with our findings, it is possible that high NE and low PE temperaments may be associated with abnormalities in right posterior regions (and therefore risk for depression) because these children process affective stimuli abnormally and/or have difficulty regulating emotional arousal.
In contrast, the purported “right hemisphere hypothesis” has not always been supported and has often been criticized (Canli, Desmond, Zhao, Glover, & Gabrieli, 2001; Demaree, Everhart, Youngstrom, & Harrison, 2005). In addition, whereas our main effect for NE is consistent with the hypothesis that right posterior regions are associated with increased arousal, our main effect for PE suggests that low PE is also associated with increased arousal. Although this may have been due to the difficulty in interpreting main effects in the presence of the PE×NE interaction, one possible explanation is that our “at-rest” condition might not have been a true rest condition. In adult resting EEG studies, participants are typically instructed to relax and sit still. In order to ensure that our children sat still and yielded relatively artifact free data, we told our children that if they sat still, they would receive prizes. This may have inadvertently increased social pressure (Scullin & Bonner, 2006) and anxiety/negative affect in our low PE children and possibly increased their right posterior activity. A similar argument was made by Mathersul et al. (2008) in interpreting their finding that depressed mood was associated with abnormally high right posterior activity even compared to anxious mood. To fully test this hypothesis, future studies are needed in which EEG is recorded during different induced moods in order to elucidate the exact processes mediated by right posterior regions in low PE and high NE temperaments (Coan et al., 2006).
Our results also suggest that the PE×NE interaction was driven by the NE facet of sadness and (to a lesser extent) fear, not anger. The result for fear is consistent with findings that relative increased activity in right posterior regions is associated with anxiety disorders (Heller et al., 1998; Metzger et al., 2004; Nitschke, Heller, Palmeiri, & Miller, 1999), and may suggest that the posterior asymmetry observed in our study reflects risk for comorbid depression and anxiety (Bruder et al., 1997; Heller & Nitschke, 1998; although for contrary evidence, see Kentgen et al., 2000). This may also relate to the finding that childhood anxiety disorders have been shown to be precursors for particularly severe depression later in life (Reinherz, Paradis, Giaconia, Stashwick, & Fitzmaurice, 2003; Weissman et al., 2005). Regarding sadness, one may argue that this result is inconsistent with the view that sadness is a low arousal negative emotion (Russell, 1980). However, a study comparing the psychophysiological profiles of sadness and fear found that the two emotions yielded similar patterns on several indicators of arousal (Kreibig, Wilhelm, Roth, & Gross, 2007). In addition, sadness has been associated with increased adrenocortical activation (Ottowitz et al., 2004), a finding that may be particularly true in pre-schoolers (Lewis, Ramsay, & Sullivan, 2006) whose sadness expressions tend to be especially activated.
We also found that the PE×NE interaction on posterior asymmetry was specific to girls. Given that females are at far greater risk for depression than males (Nolen-Hoeksema & Hilt, 2009), it is important to look at whether temperament plays a role in this disparity (Hankin & Abrahmson, 1999). Of interest, there are conflicting results as to whether NE and PE have a similar relation to depression in boys versus girls (Chorpita, 2004; Jacques & Mash, 2004; Ollendick, Seligman, Goza, Byrd, & Singh 2003; Wetter & Hankin, 2009). Although speculative, it is possible that a posterior asymmetry may only connote risk for depression in girls and not boys. As we follow up this sample through adolescence, it will be important to examine whether EEG asymmetry can elucidate the gender disparities in depression that appear in adolescence (Hankin et al., 1998).
Similar to our previous report (Shankman et al., 2005), we did not find an association between temperament and frontal asymmetry. There are several possible explanations for this finding. First, frontal asymmetries may be more likely to be associated with depression/temperament in adult EEG studies because certain areas of the prefrontal cortex, such as the dorsolateral prefrontal cortex (Davidson et al., 2002), do not fully develop until adolescence. However, there is no evidence to suggest that the prefrontal cortex has to be fully developed in order to exhibit the hypothesized asymmetry. For example, Fox and colleagues have found differences in frontal EEG asymmetry between infants defined as criers versus noncriers (Davidson & Fox, 1989; Fox, Bell, & Jones, 1992) and in infants of depressed versus nondepressed mothers (Jones et al., 1997). However, these studies differed from the present study as they typically employed selected samples. Second, our failure to find a significant frontal asymmetry may be because this asymmetry is only low to moderately stable (Tomarken et al., 1992) and can be influenced by state variables (Blackhart, Kline, Donohue, LaRowe, & Joiner, 2002; Shankman et al., 2007). Third and finally, studies that have found frontal asymmetries in children at risk for depression defined risk as having a parent with depression (Dawson et al., 1999; Field, Pickens, Fox, & Nawrocki, 1995) while we defined it on the basis of child temperament in this and our previous report (Shankman et al., 2005).
This study had several strengths. First, the sample is quite large, particularly for a psychophysiological study in children. This allowed us to exclude left-handed children and examine interactions, which is often not done in EEG studies in children. Second, temperament was defined using a comprehensive laboratory observation measure (i.e., LAB-TAB; Goldsmith et al., 1995). Third, we examined the effect of recording reference (nose and average reference), a variable often used as an indicator of signal noise in EEG studies (Coan et al., 2006). As our primary results were not moderated by reference, this argues for the robustness of our findings.
The present study had several limitations as well. First, the children in the present sample had lower sadness and fear scores than the larger sample in our study. Our results may therefore not generalize to temperament in the general population. However, sadness and fear were significantly related to asymmetries in this sample, despite the more restricted range in this group relative to the larger sample. Second, as discussed above, because children were told that they would receive a prize if they would sit still, it may be difficult to compare our results to other studies in which EEG was recorded “at rest.” This, however, was necessitated by the young age of our sample in order to ensure relatively artifact-free data.
In summary, our results suggest that low PE and high NE interact in predicting a posterior asymmetry, and not an anterior asymmetry. Using EEG asymmetry as a marker for depression, the direction of the interaction suggests that children with low PE or high NE are at risk for depression and children with both temperamental traits are not at greater risk than those with only one. As we are following up this sample of preschoolers, it will be important to examine whether these temperamental traits continue to predict markers for depression as well as the development of the disorder. Such results would suggest that these temperamental traits may be good targets for preventative strategies (Garber, 2006; Karevold, Røysamb, Ystrom, & Mathiesen, 2009).
Footnotes
Given the growing interest in the neuroscience of SES (Hackman & Farah, 2009) and the preliminary evidence that SES may moderate the relation between a maternal history of depression and EEG asymmetry (Tomarken et al., 2004), we explored whether SES moderated the relation between temperament and EEG asymmetry. For anterior regions, SES did not moderate the relation between EEG asymmetry and PE, NE, or PE×NE, but there was a main effect for SES on asymmetry for F3/4 (b = 0.014, SE = 0.005, p<.05) and F7/8 (b= 0.015, SE=0.007, p<.01), indicating that greater relative right frontal activity was associated with lower SES. For posterior asymmetry, the only significant result was a PE×NE×SES interaction for P3/4 (b=−0.044, SE= 0.015, p<.01). Follow-up analyses indicated that both those with high (Hollingshead 1 or 2) or low (Hollingshead 3, 4, and 5) SES demonstrated significant or marginally significant PE×NE interactions for P3/4, although the effect was stronger for high SES individuals. In short, differences in SES did not appear to affect the results reported below.
None of the PE×NE×gender interactions for each of the three anterior regions was significant. Only one of the six two-way Temperament×Gender interactions was significant (PE×Gender for F3/4, p = 0.036). However, we are hesitant to interpret this effect because simple effects tests revealed that the effect for girls was not significant and there was only a trend for boys, in which boys with low PE had greater relative right frontal activity (p = .054).
Similar to our previous paper (Shankman et al., 2005), these models yielded significant main effects for gender with boys having greater relative activity in the right posterior hemisphere and girls have greater relative activity in the left. This is consistent with the controversial finding that males tend to rely more heavily on right hemisphere or visuospatial processing and females tend to make greater use of left hemisphere or verbal processing (see Hines, 2004).
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; Thousand Oaks, CA: 1991. [Google Scholar]
- Allen JJB. Frontal EEG asymmetry as an endophenotype for depression: A skeptic’s journey. Paper presented at the meeting of the Society for Psychophysiological Research; Berlin. Oct, 2009. [Google Scholar]
- Allen JJB, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Urry HL, Hitt SK, Coan JA. Stability of resting frontal EEG asymmetry across different clinical states of depression. Psychophysiology. 2004;41:269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- American Electroencephalographic Society American electroencephalographic society guidelines in electroencephalography, evoked potentials, and polysomnography. Journal of Clinical Neurophysiology. 1994;11:1–142. [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Myers E. Genetic and environmental influences on frontal EEG asymmetry: A twin study. Biological Psychology. 2006;71:289–295. doi: 10.1016/j.biopsycho.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhart GC, Kline JP, Donohue KF, LaRowe SD, Joiner TE. Affective responses to EEG preparation and their link to resting anterior EEG asymmetry. Personality and Individual Differences. 2002;32:167–174. [Google Scholar]
- Blackhart GC, Minnix JA, Kline JP. Can EEG asymmetry patterns predict future development of anxiety and depression? A preliminary study. *********Biological Psychology. 2006;72:46–50. doi: 10.1016/j.biopsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Borod JC. Interhemispheric and intrahemispheric control of emotion: A focus on unilateral brain damage. Journal of Consulting and Clinical Psychology. 1992;60:339–348. doi: 10.1037//0022-006x.60.3.339. [DOI] [PubMed] [Google Scholar]
- Bruder GE. Frontal and parietotemporal asymmetries in depressive disorders: Behavioral, electrophysiologic and neuroimaging findings. In: Hugdahl K, Davidson RJ, editors. The asymmetrical brain. MIT Press; Cambridge, MA: 2003. pp. 719–742. [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, et al. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biological Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Warner V, Nomura Y, Grillon C, Hille J, et al. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biological Psychiatry. 2005;57:328–335. doi: 10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Warner V, Weissman MM. Grandchildren at high and low risk for depression differ in EEG measures of regional brain asymmetry. Biological Psychiatry. 2007;62:1317–1323. doi: 10.1016/j.biopsych.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JDE. Hemispheric asymmetry for emotional stimuli detected with fMRI. NeuroReport. 1998;9:3233–3239. doi: 10.1097/00001756-199810050-00019. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Archives of General Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Chorpita BF. The tripartite model and dimensions of anxiety and depression: An examination of structure in a large school sample. Journal of Abnormal Child Psychology. 2004;30:177–190. doi: 10.1023/a:1014709417132. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD. Measuring socioeconomic status: Reliability and preliminary validity for different approaches. Assessment. 2002;9:145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Temperament: A new paradigm for trait psychology. In: Pervin LA, John OP, editors. Handbook of personality: Theory and research. 2nd ed Guilford Press; New York: 1999. pp. 399–423. [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103:103–116. [PubMed] [Google Scholar]
- Coan JA, Allen JJB, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Influence of extraversion and neuroticism on subjective well-being: Happy and unhappy people. Journal of Personality and Social Psychology. 1980;38:668–678. doi: 10.1037//0022-3514.38.4.668. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology. 1994;6:741–758. [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion. 1998;12:307–320. [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants response to maternal separation. Journal of Abnormal Psychology. 1989;98:127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2nd ed Cambridge University Press; New York: 2000. pp. 27–52. [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, et al. Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Self J, Panagiotides H, Hessl D, Yamada E, et al. Frontal brain electrical activity in infants of depressed and nondepressed mothers: Relation to variations in infant behavior. Development and Psychopathology. 1999;11:589–605. doi: 10.1017/s0954579499002229. [DOI] [PubMed] [Google Scholar]
- Dawson G, Panagiotides H, Klinger LG, Spieker S. Infants of depressed and nondepressed mothers exhibit differences in frontal brain electrical activity during the expression of negative emotions. Developmental Psychology. 1997;33:650–656. doi: 10.1037//0012-1649.33.4.650. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Everhart DE, Youngstrom EA, Harrison DW. Brain lateralization of emotional processing: Historical roots and a future incorporating “dominance. Behavioral and Cognitive Neuroscience Reviews. 2005;4:3–20. doi: 10.1177/1534582305276837. [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Durbin CE, Hayden EP, Olino TM. Temperamental positive and negative emotionality and children’s depressive symptoms: A longitudinal prospective study from age three to age ten. Journal of Social and Clinical Psychology. 2010;29:462–488. [Google Scholar]
- Dougherty LR, Klein DN, Olino TM, Dyson M, Rose S. Increased waking salivary cortisol and depression risk in preschoolers: The role of maternal history of melancholic depression and early child temperament. Journal of Child Psychology and Psychiatry. 2009;50:1495–1503. doi: 10.1111/j.1469-7610.2009.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3rd ed American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- Durbin CE, Hayden EP, Klein DN, Olino TM. Stability of laboratory-assessed temperamental emotionality traits from ages 3 to 7. Emotion. 2007;7:388–399. doi: 10.1037/1528-3542.7.2.388. [DOI] [PubMed] [Google Scholar]
- Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC. Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology. 2005;114:28–37. doi: 10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- Egger HL, Ascher B, Angold A. The Preschool Age Psychiatric Assessment. Version 1.1 Duke University Medical Center Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences; Durham, NC: 1999. [Google Scholar]
- Field T, Pickens J, Fox NA, Nawrocki T. Relative right frontal EEG activation in 3- to 6-month-old infants of “depressed” mothers. Developmental Psychology. 1995;31:358–363. [Google Scholar]
- Fisch BJ. Fisch and Spehlmann’s EEG primer: Basic principles of digital and analog EEG. Elsevier; Amsterdam: 1999. [Google Scholar]
- Fox NA, Bell MA, Jones NA. Individual differences in response to stress and cerebral asymmetry. Developmental Neuropsychology. 1992;8:161–184. [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, et al. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1770–1784. [PubMed] [Google Scholar]
- Garber J. Depression in children and adolescents: Linking risk research and prevention. American Journal of Preventive Medicine. 2006;31:S104–S125. doi: 10.1016/j.amepre.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Gershuny BS, Sher KJ. The relation between personality and anxiety: Findings from a 3-year prospective study. Journal of Abnormal Psychology. 1998;107:252–262. doi: 10.1037//0021-843x.107.2.252. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. Laboratory Temperament Assessment Battery: Preschool version. 1995. Unpublished manuscript.
- Gotlib IH, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition & Emotion. 1998;12:449–478. [Google Scholar]
- Hagemann D. Individual differences in anterior EEG asymmetry: Methodological problems and solutions. Biological Psychology. 2004;67:157–182. doi: 10.1016/j.biopsycho.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: Description and possible explanations. Annals of Medicine. 1999;31:372–379. doi: 10.3109/07853899908998794. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Durbin CE, Olino TM. Positive emotionality at age 3 predicts cognitive styles in 7-year-old children. Development and Psychopathology. 2006;18:409–423. doi: 10.1017/S0954579406060226. [DOI] [PubMed] [Google Scholar]
- Heller W. The neuropsychology of emotion: Developmental patterns and implications for psychopathology. In: Stein NL, Leventhal B, Trabasso T, editors. Psychological and biological approaches to emotion. Erlbaum; Hillsdale, NJ: 1990. pp. 167–211. [Google Scholar]
- Heller W, Nitschke JB, Miller GA. Lateralization in emotion and emotional disorders. Current Directions in Psychological Science. 1998;7:26–32. [Google Scholar]
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Hines M. Brain gender. Oxford University Press; New York: 2004. [Google Scholar]
- Hirschfeld-Becker DR, Micco J, Henin A, Bloomfield A, Biederman J, Rosenbaum J. Behavioral inhibition. Depression and Anxiety. 2008;25:357–367. doi: 10.1002/da.20490. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. 1975. Unpublished manuscript.
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Izard CE, Dougherty LM, Hembree EA. A system for identifying facial expressions by holistic judgment (AFFEX) University of Delaware Media Services; Newark, NJ: 1989. [Google Scholar]
- Jacobs GD, Snyder D. Frontal brain asymmetry predicts affective style in men. Behavioral Neuroscience. 1996;110:3–6. doi: 10.1037//0735-7044.110.1.3. [DOI] [PubMed] [Google Scholar]
- Jacques HAK, Mash EJ. A test of the tripartite model of anxiety and depression in elementary and high school boys and girls. Journal of Abnormal Child Psychology. 2004;32:13–25. doi: 10.1023/b:jacp.0000007577.38802.18. [DOI] [PubMed] [Google Scholar]
- Jones NA, Field T, Davalos M, Pickens J. EEG stability in infants/children of depressed mothers. Child Psychiatry & Human Development. 1997;28:59–70. doi: 10.1023/a:1025197101496. [DOI] [PubMed] [Google Scholar]
- Jones NA, Field T, Fox NA, Lundy B, Davalos M. EEG activation in 1-month-old infants of depressed mothers. Development and Psychopathology. 1997;9:491–505. doi: 10.1017/s0954579497001260. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Early childhood predictors of adult anxiety disorders. Biological Psychiatry. 1999;46:1536–1541. doi: 10.1016/s0006-3223(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Karevold E, Røysamb E, Ystrom E, Mathiesen KS. Predictors and pathways from infancy to symptoms of anxiety and depression in early adolescence. Developmental Psychology. 2009;45:1051–1060. doi: 10.1037/a0016123. [DOI] [PubMed] [Google Scholar]
- Kayser J. Polygraphic Recording Data Exchange—PolyRex. New York State Psychiatric Institute: Department of Biopsychology. 2003 Retrieved from http://psychophysiology.cpmc.columbia.edu/PolyRex.htm.
- Kayser J, Bruder GE, Tenke CE, Stewart JW, Quitkin FM. Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: Differences between depressed patients and healthy adults in P3 amplitude and asymmetry. International Journal of Psychophysiology. 2000;36:211–236. doi: 10.1016/s0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. Personality and major depression: A Swedish longitudinal, population-based twin study. Archives of General Psychiatry. 2006a;63:1113–1120. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. American Journal of Psychiatry. 2006b;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of personality and major depression in women. Archives of General Psychiatry. 1993;50:853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE. Electroencephalographic asymmetries in adolescents with major depression: Influence of comorbidity with anxiety disorders. Journal of Abnormal Psychology. 2000;109:797–802. doi: 10.1037//0021-843x.109.4.797. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. The right-hemisphere and valence hypotheses: Could they both be right (and sometimes left)? Social Cognitive and Affective Neuroscience. 2007;2:240–250. doi: 10.1093/scan/nsm020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD, Wilhelm FH, Roth WT, Gross JJ. Cardiovascular, electrodermal, and respiratory response patterns to fear- and sadness-inducing films. Psychophysiology. 2007;44:787–806. doi: 10.1111/j.1469-8986.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay DS, Sullivan MW. The relation of ANS and HPA activation to infant anger and sadness response to goal blockage. Developmental Psychobiology. 2006;48:397–405. doi: 10.1002/dev.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, Kemp AH. Investigating models of affect: Relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8:560–572. doi: 10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- McManis MH, Kagan J, Snidman NC, Woodward SA. EEG asymmetry, power, and temperament in children. Developmental Psychobiology. 2002;41:169–177. doi: 10.1002/dev.10053. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Paige SR, Carson MA, Lasko NB, Paulus LA, Pitman RK, et al. PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. Journal of Abnormal Psychology. 2004;113:324–329. doi: 10.1037/0021-843X.113.2.324. [DOI] [PubMed] [Google Scholar]
- Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI. The comorbidity of anxiety and depression in the perspective of genetic epidemiology: A review of twin and family studies. Psychological Medicine. 2005;35:611–624. doi: 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- Miller A, Fox NA, Cohn JF, Forbes EE, Sherrill JT, Kovacs M. Regional patterns of brain activity in adults with a history of childhood-onset depression: Gender differences and clinical variability. American Journal of Psychiatry. 2002;159:934–940. doi: 10.1176/appi.ajp.159.6.934. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Hilt LM. Gender differences in depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd ed Guilford Press; New York: 2009. pp. 386–404. [Google Scholar]
- Oishi N, Mima T, Ishii K, Bushara KO, Hiraoka T, Uekl Y, et al. Neural correlates of regional EEG power change. NeuroImage. 2007;36:1301–1312. doi: 10.1016/j.neuroimage.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: Associations in a large community sample. Journal of Abnormal Psychology. 2010;119:468–478. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollendick TH, Seligman LD, Goza AB, Byrd DA, Singh K. Anxiety and depression in children and adolescents: A factor-analytic examination of the tripartite model. Journal of Child and Family Studies. 2003;12:157–170. [Google Scholar]
- Ormel J, Oldehinkel AJ, Vollebergh W. Vulnerability before, during, and after a major depressive episode. Archives of General Psychiatry. 2004;61:990–996. doi: 10.1001/archpsyc.61.10.990. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Sirota A, Niaura R, Rauch SL, Brown WA. Neural and endocrine correlates of sadness in women: Implications for neural network regulation of HPA activity. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:446–455. doi: 10.1176/jnp.16.4.446. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Nitschke JB, Oakes TR, Hendrick AM, Horras KA, Larson CL, et al. Brain electrical tomography in depression: The importance of symptom severity, anxiety, and melancholic features. Biological Psychiatry. 2002;52:73–85. doi: 10.1016/s0006-3223(02)01313-6. [DOI] [PubMed] [Google Scholar]
- Pössel P, Lo H, Fritz A, Seemann S. A longitudinal study of cortical EEG activity in adolescents. Biological Psychology. 2008;78:173–178. doi: 10.1016/j.biopsycho.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Reid SA, Duke LM, Allen JJB. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. [PubMed] [Google Scholar]
- Reinherz HZ, Paradis AD, Giaconia RM, Stashwick CK, Fitzmaurice G. Childhood and adolescent predictors of major depression in the transition to adulthood. American Journal of Psychiatry. 2003;160:2141–2146. doi: 10.1176/appi.ajp.160.12.2141. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. [Google Scholar]
- Schmidtke JI, Heller W. Personality, affect and EEG: Predicting patterns of regional brain activity related to extraversion and neuroticism. Personality and Individual Differences. 2004;36:717–732. [Google Scholar]
- Scullin MH, Bonner K. Theory of mind, inhibitory control, and preschool-age children’s suggestibility in different interviewing contexts. Journal of Experimental Child Psychology. 2006;93:120–138. doi: 10.1016/j.jecp.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN. The relation between depression and anxiety: An evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clinical Psychology Review. 2003;23:605–637. doi: 10.1016/s0272-7358(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116:95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Tenke CE, Bruder GE, Durbin CE, Hayden EP, Klein DN. Low positive emotionality in young children: Association with EEG asymmetry. Development and Psychopathology. 2005;17:85–98. doi: 10.1017/s0954579405050054. [DOI] [PubMed] [Google Scholar]
- Smit DJA, Posthuma D, Boomsma DI, De Geus EJC. The relation between frontal EEG asymmetry and the risk for anxiety and depression. Biological Psychology. 2007;74:26–33. doi: 10.1016/j.biopsycho.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Somsen RJM, van Beek B. Ocular artifacts in children’s EEG: Selection is better than correction. Biological Psychology. 1998;48:281–300. doi: 10.1016/s0301-0511(98)00041-6. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Psychophysiology. 2009;46:S24–S24. [Google Scholar]
- Tackett JL. Evaluating models of the personality–psychopathology relationship in children and adolescents. Clinical Psychology Review. 2006;26:584–599. doi: 10.1016/j.cpr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Reference-free quantification of EEG spectra: Combining current source density (CSD) and frequency principal components analysis (fPCA) Clinical Neurophysiology. 2005;116:2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology. 1992;62:676–687. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology. 1992;29:576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Garber J, Simien C. Resting frontal brain activity: Linkages to maternal depression and socio-economic status among adolescents. Biological Psychology. 2004;67:77–102. doi: 10.1016/j.biopsycho.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Keener AD. Frontal brain asymmetry and depression: A self-regulatory perspective. Cognition & Emotion. 1998;12:387–420. [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology. 2004;86:320–333. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, Kovacs M, George CJ. Long-term stability of electroencephalographic asymmetry and power in 3 to 9 year-old children. International Journal of Psychophysiology. 2008;67:70–77. doi: 10.1016/j.ijpsycho.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98:219–235. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Nomura Y, Warner V, Verdeli H, Pilowsky DJ, et al. Families at high and low risk for depression: A 3-generation study. Archives of General Psychiatry. 2005;62:29–36. doi: 10.1001/archpsyc.62.1.29. [DOI] [PubMed] [Google Scholar]
- Wetter EK, Hankin BL. Mediational pathways through which positive and negative emotionality contribute to anhedonic symptoms of depression: A prospective study of adolescents. Journal of Abnormal Child Psychology. 2009;37:507–520. doi: 10.1007/s10802-009-9299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Myin-Germeys I, Jacobs N, Peeters F, Kenis G, Derom C, et al. Evidence that moment-to-moment variation in positive emotions buffer genetic risk for depression: A momentary assessment twin study. Acta Psychiatrica Scandinavica. 2007;115:451–457. doi: 10.1111/j.1600-0447.2006.00924.x. [DOI] [PubMed] [Google Scholar]