Abstract
Objective
Preschool and minority children have not been well represented in obesity treatment studies. This analysis of clinical obesity treatment was carried out within a diverse population of children 2–12 years to identify demographic characteristics associated with successful treatment.
Design and Methods
A medical record review captured BMI and demographics for children 2–12 years who began treatment during a 42 month period (n=479). Associations of BMI-Z change with child and family demographics were examined with logistic regression and time-to-event analysis.
Results
Treatment led to a mean BMI-Z decrease of 0.18. Half of children with follow-up (n=273) exceeded the a priori cut-off for successful treatment of −0.1 BMI-Z. Preschoolers and children of Spanish-speakers were more likely to succeed, (Adjusted OR: 5.8 [95% CI: 2.7–12.2] and 2.3 [95% CI: 1.1, 4.9]). The hazard ratio for treatment failure was 3.7 [95% CI: 2.1, 6.8] for children starting treatment at 6–12yrs compared to preschoolers, adjusted for other demographics.
Conclusions
This mode of treatment was more likely to succeed among children treated before school age and among children whose parents spoke only Spanish. Screening and treatment for obesity in preschoolers and Hispanic immigrant families deserve further prospective study.
Keywords: Pediatrics [H02.403.670]; Obesity [C18.654.726.500]; Obesity, Morbid [C18.654.726.500.700]; Socioeconomic Factors [N01.824]; Child [M01.060.406]; Child, Preschool [M01.060.406.448]; Hispanic Americans [M01.686.754.441]; Medical Informatics [L01.700]; Treatment Outcome [N05.715.360.575.575.800]; Outpatient Clinics, Hospital [N02.278.035.380]; Insurance Coverage [N03.219.521.576.265]
Introduction
Alongside efforts in public health and policy to reverse the childhood obesity epidemic, medical providers seek to play effective roles in prevention and treatment. Health care teams are called upon to provide resource-intensive treatment for children who are already obese, especially the severely obese who now comprise 4% of US children.(1) Health care providers seek strategies to use limited treatment resources in a cost-effective manner that is relevant to all demographic groups, especially those at highest risk. The medical referral model is frequently used to deliver multidisciplinary childhood obesity treatment consistent with Stages 3 and 4 of the 2007 Expert Committee Recommendations.(2) Examining the effectiveness of a frequently used referral model of treatment in a diverse population may identify demographic groups most successful in treatment. There may be justification to prioritize referral in certain demographic categories and the need for improved targeted treatment approaches may be identified in other groups.
The costs of obesity are compounded when it begins early in life, but little evidence exists for effective treatment in young children. Obesity with onset during the toddler and preschool years very frequently persists to adolescence and leads to more severe adult obesity.(3–6) Thus far, published randomized controlled trials of multidisciplinary obesity treatment have targeted children six years and older.(7) In 2010 the United States Preventive Services Task Force (USPSTF) recommended yearly screening for obesity using BMI in children 6 years and older with referral to high quality treatment programs.(8) Children less than 6 years old were not recommended for BMI screening by USPSTF, in contrast to the recommendations of professional societies.(9).
Most studies involving preschool children have targeted obesity prevention, and very few have evaluated treatment in this age group. However, 10.4% of US preschoolers are already obese, over one million American children may benefit from treatment for obesity before age 6 years.(10–12) A recent economic analysis conducted by researchers at the Johns Hopkins Bloomberg School of Public Health found that up to $2735 could be spent on a treatment intervention per obese child 2–6 years old and still realize net lifetime medical cost savings in this age group if treatment reduced obesity prevalence by only 1%. The authors further concluded that in their economic model, targeted treatment would be more cost effective than non-targeted prevention efforts in children 6 yrs and younger for the same level of prevalence reduction.(13)
Minorities have been under-represented in treatment studies, though large racial and ethnic disparities in childhood obesity exist.(8, 14) For example, one nationally representative study found 22% prevalence of obesity among four-year-old Hispanic children, compared to 16% in non-Hispanic white peers.(14) The two identified randomized-controlled trials testing obesity treatment in preschoolers have not examined effects specifically within Hispanic children or Spanish speaking caregivers, though Hispanics comprise 24% of the population under age five in the US.(12, 15, 16)
To assess treatment effectiveness in understudied groups, including preschoolers and Hispanic children, we examined demographic characteristics associated with BMI z-score (BMI-Z) change in a diverse group of children referred to a multidisciplinary weight management clinic, the GoodLIFE (Lifestyles Influencing Fitness and Eating) Clinic (GLC). We hypothesized that the mean decrease in BMI z-score for children returning for follow-up would exceed −0.10 BMI-Z, a cut-off reported as clinically significant in other studies.(7) (17, 18) We hypothesized that certain demographic characteristics would be associated with treatment success, including: age less than 6 years at first clinic visit because of greater parental control over the environment and greater malleability of lifestyle habits; private insurance as a marker of higher income and greater family resources; female gender because studies have suggested different gender-based perceptions of weight status and outcomes in weight management treatment;(16, 19) and absence of potential language, cultural, or distance barriers to treatment. We also sought to determine characteristics associated with return for follow-up.
Patients and Methods
Study Design
This was a retrospective cohort study of children referred by primary care providers to a multidisciplinary childhood obesity treatment clinic located at a tertiary-care children’s hospital. Anthropometric and demographic data were abstracted from an electronic medical record.
Study Setting
The GoodLIFE Clinic at Children’s Hospital Colorado treats obese children aged 0–12 years referred from Colorado and neighboring states. Since 1998, GLC has provided weight management treatment to a diverse population of clinically-referred obese children. During the period examined, the clinic model consisted of visits lasting 1–2 hours offered every 4–8 weeks. Treatment was provided by members of a multidisciplinary team: two pediatricians and a nurse practitioner with expertise in nutrition and obesity management, a registered dietitian, a child psychologist, and a certified child life specialist / recreational therapist. Treatment was family-centered and individualized. A professional medical interpreter was scheduled for all visits when the parent attending was not fluent in English.
Following an initial assessment by a medical provider, each family was recommended to have a follow-up visit with each of the other professionals on a rotating basis. Available appointments would allow each family to meet with each discipline multiple times within a year, but actual follow-up varied by the family’s adherence to the recommended visit schedule. When outstanding needs in a particular area were identified for a child, the treatment model allowed flexibility to spend more time or more frequent visits with a specialist in a particular discipline discussing medical, psychological, dietary, or activity issues as needed. The treatment team employed motivational interviewing and cognitive behavioral techniques focusing on goal-setting, self-monitoring, contingency management, and effective parenting. Families and GLC staff selected goals collaboratively from a menu of relevant diet, activity, and behavior changes created at each visit. Families chose from a list of tailored goals that focused on evidenced-based strategies for decreasing caloric intake and increasing energy expenditure including: the traffic light diet, tracking fruit and vegetable intake, elimination of sugar-sweetened beverages, moderation of portion sizes, avoidance of television viewing while eating, limitation of sedentary behaviors, and tracking of physical activity using charts and pedometers. On an as-needed basis, other goals included: sleep hygiene, treatment of obstructive sleep apnea, optimization of asthma care, training in parenting skills including differential attention and limit-setting for preschool children, or training in parenting skills to support school-age children in self-monitoring. Highly prescriptive reduced calorie diets with daily recording of intake were recommended to a small minority of patients with severe co-morbidity.
Treatment generally began with a medical provider visit. During a 60–120 minute initial visit, the medical provider discussed each family’s motivating factors and barriers to change, identified underlying medical conditions that could contribute to excess weight gain, and assessed for co-morbid conditions resulting from excess weight gain with history, review of systems, physical exam, and laboratory tests. The medical provider also introduced the concept of structured goal setting and self-monitoring using a daily log for health behavior changes and guided the family to select 1–3 behavior change goals (typically changes to diet, activity, sleep, or management of a co-morbid medical condition) to track before the next visit. Medical providers gave each family a 30-page ‘GLC Handbook’ which was a compilation of handouts describing how and why to make evidence-based dietary, activity, and behavioral changes. Return visits to the medical provider typically followed in a rotation with the other disciplines, but occasionally sooner if a co-morbidity required more urgent diagnostic or treatment follow-up such as type II diabetes or severe untreated obstructive sleep apnea.
Following the medical provider visit, a subsequent visit could occur with either the psychologist or dietitian for 45–60 minutes. The recreational therapist was also present at approximately half of these visits for an additional 15–30 minutes. The dietitian reviewed progress on the goals selected at prior visits, counseled in-depth on changes in eating patterns, diet content, and meal planning relevant to each family and collaboratively set new goals. With consultation from the medical providers to identify appropriate families, the dietitian instructed families of children with high morbidity and high readiness to change to follow a more structured diet. Diet options included the traffic light diet, a high-protein low-carbohydrate diet with less than 20–40 grams of carbohydrate per day, or daily meal planning to a strict calorie limit using daily diet records. Input of the family regarding the perceived feasibility and preference of one structured diet over another was used to selected particular diet plans for individual patients. Between 10–20% of patients followed a structured diet plan during the study period. The remainder focused on an approach of small incremental changes to diet and eating behaviors.
The psychologist also reviewed progress on multidisciplinary lifestyle change goals, reassessed readiness to change, and collaboratively set new lifestyle change goals. The focus of the psychologist visit included training in cognitive behavioral skills for monitoring goals, awareness of hunger/satiety for school-age children, and training in parenting skills for parents of preschoolers. Parenting skills discussed included limit-setting (often targeting screen time or sleep hygiene), effective discipline through differential attention and time-out, positive reinforcement of goal behaviors, and the technique of repeated exposure to healthy foods to increase acceptance. The psychologist’s intervention further varied based on the needs of each family, to include: assessment for psychological morbidity by validated screening tools and diagnostic interview, and referral for treatment of newly diagnosed disorders such as anxiety or depression. In cases of previously identified disorders, the psychologist obtained consent and communicated with psychiatrists, therapists, or school counselors about the interaction of weight status with the disorder and its treatment.
The recreational therapist visited with families at approximately half of the follow-up visits with the psychologist or dietitian. The rec therapist focused on identifying local resources for physical activity, creating home fitness plans, and setting activity goals. On visits when the recreational therapist was not present, other members of the multidisciplinary team set physical activity goals. For preschoolers she trained parents to foster developmentally appropriate active play and gave props such as balls, jump ropes, and dancing ribbons at the first visit. For older children, she focused on identifying structured activities most suitable for the child, self-monitoring with a pedometer and log given at first visit, and set goals for fitness at home using a pair of ‘Exercise Dice’ with a variety of calisthenics on one die and a number of repetitions on the other. When parents and other family members could be engaged, they were also invited to self-monitor using a pedometer and to participate with the child in fitness.
The model of treatment was in part dictated by the setting in a medical specialty clinic and dependence on insurance reimbursement to fund care, rather than a programmatic approach with non-clinical funding sources. Clinic treatment was not time-limited. Letters of consultation communicated treatment plans to primary care providers. Families were asked to schedule a follow-up appointment at the close of each encounter. Follow-up appointments were available at 1–2 month intervals. Families received a single reminder call prior to each visit. Other telephone follow-up or electronic communication with families between visits was not routine. Follow-up procedures did not differ by demographic group. Treatment recommendations including a menu of lifestyle change goals were communicated to all primary care providers in letters of consultation from GLC.
Patient Population
This analysis included children who were aged two to twelve years at an initial visit to the GLC between January 2005 and July 2009 (n=479). Children diagnosed with a genetic syndrome associated with obesity such as Prader-Willi Syndrome were excluded. BMI measurements were recorded from GLC follow-up (354 visits) through November 2009. Approximately one-third (n=162) of patients were referred from the hospital-based primary care clinic. In the subset with measurements preceding and following entry into GLC (n-122), comparison of BMI-Z trajectories within subjects pre- and post-treatment BMI was possible. Anthropometric data prior to a child’s first GLC visit were collected dating back to April 2004 for those children who had previously visited another Children’s Hospital clinic (464 visits.) In order to examine BMI-Z changes for the longest interval possible post-treatment, weight and height measurements were abstracted from subsequent visits to any other hospital-based clinic through November 2009 (590 visits).
Data Analysis and Definitions
Demographic characteristics as reported by the parents were abstracted from the electronic medical record at the first GLC visit. Variables extracted from the record included: the child’s race (White, Asian, Native American/Alaskan Native, or ‘other’), ethnicity (Hispanic or Non-Hispanic) as reported by the parent, language spoken by parents during the visit (English, Spanish, or other), gender, age at the visit (categorized as preschool 2–5 years vs. 6–12 years at first visit), insurance payer (Medicaid, Child Health Plan Plus (Medicaid extension), Tricare, and those receiving benefits from the hospital’s Indigent Care Program were categorized as ‘publicly insured’ vs. all private insurers), and zip code of the primary residence (categorized as those within 30 miles from GLC vs homes farther away.) Children with a BMI >99th percentile for age and gender based on Centers for Disease Control BMI references were categorized as severely obese. Mean BMI-Z change, proportion returning for follow-up, and associated demographic characteristics were examined using T-tests, chi-square, and logistic regression. Survival analysis using Kaplan Meier and Cox Proportional Hazards (PH) modeling examined outcomes by demographic group accounting for variable follow-up times (SAS 9.2 Cary, NC). Logistic and Proportional Hazards regressions tested hypotheses regarding the independent effects on treatment outcomes of dichotomous variables for gender, age 2–5 vs. 6–12 years, Spanish-only speaking parents vs. English speakers, Hispanic ethnicity, residence within 30 miles of clinic, private vs. public insurance, and severe obesity vs. BMI <99th percentile for age and gender.
Using the available data, including observed associations between primary variables of interest and potential confounders, the minimally detectable OR for the outcome of success vs. failure among the 265 children with follow-up BMI measurements was 2.3, with power of 0.8 and α=0.05.(20) All data were de-identified, and the study was judged exempt from human subject review by the Colorado Multiple Institution Review Board.
Results
Baseline Data
Demographic and anthropometric data on the cohort of 479 children who had an initial visit in GLC are depicted in Table 1. Race reported as “other” and Hispanic ethnicity were highly concordant, see footnote to Table 1. describing the race distribution. Publicly insured children were more likely to seek care at Children’s Hospital-based clinics and privately insured children were more likely to be lost to follow-up. Children with follow-up data had slightly higher BMI z-scores at baseline than those who were lost to follow-up, but both groups had a mean BMI-Z in the severely obese range (>99th percentile.)
TABLE 1.
Demographic and Anthropometric Characteristics of the Study Population
|
Variable |
Number in Category within total sample (% of total) n=479 |
Lost in Category / Total Lost (% Lost) n=223 |
Returned in Category / Total Returned (% Returned) n=256 |
p |
|
|---|---|---|---|---|---|
| Female | 244 (51%) | 114/214 (53%) | 130/265 (49%) | 0.36 | |
| Preschool 2–5y | 126 (26%) | 51/214 (24%) | 75/265 (28%) | 0.27 | |
| Spanish-Only Speaking Parent | 107 (22%) | 43/214 (20%) | 65/265 (25%) | 0.25 | |
| Hispanic Ethnicitya | 232 (48%) | 98/198 (49%) | 141/258 (55%) | 0.27 | |
| Local Resident - within 30 miles | 423 (88%) | 187/213 (88%) | 236/263 (90%) | 0.50 | |
| Private Insurer | 188 (39%) | 102/214 (48%) | 81/265 (31%) | 0.0001 | |
| Severely Obese BMI>99 percentile | 331 (69%) | 94/214 (44%) | 139/265 (52%) | 0.06 | |
| Range | Mean of Total Sample (SD) | Mean Lost (SD) | Mean Returned (SD) | p | |
| Age at 1st visit | 2–12y | 8.1yrs (2.9) | 8.2 (2.8) | 8.0 (3.0) | 0.49 |
| BMI Z at 1st GLC visit | 1.08–6.05 | 2.68 (0.76) | 2.58 (0.72) | 2.76 (0.78) | 0.01 |
| Range | Mean Time (SD) n = 122 |
Mean Time (SD) n = 256 |
|||
| Earliest BMI to 1st GLC visit | 2–39 months | 12.1 months (12.5) | |||
| 1st GLC visit to last BMI | 2–59 months | 12.4 months (13.6) | |||
Reported race of the total population was: 49% ‘other’ (94% of those selecting ‘other’ also reported Hispanic ethnicity), 38% white, 6% African American, 4% multiple races, 1% Asian, and <1% American Indian/Alaskan Native.
Aggregate BMI Change
BMI-Z change data were available for 256 (53%) of the 479 children who had an initial visit. The mean BMI-Z decrease −0.18 (standard deviation 0.37) was significantly greater than the a-priori cutoff for clinical significance −0.10 (p=0.0004). Of those with follow-up, 146 (57%) had a BMI-Z decrease of at least −0.10. An additional 72 children (28%) had a decrease in BMI-Z that did not exceed −0.10. Overall 85% of children with follow-up did not experience further BMI-Z increase. For the purposes of our conservative analysis, only the 57% with −0.10 or larger BMI-Z decrease were categorized as succeeding in treatment. Of those with follow-up data, 67% (n=172) returned to GLC for follow-up treatment, the remainder (n=84) had follow-up measurements in the hospital-based primary care setting only.
Comparison with Prior BMI Trajectory
In a subset of children with measurements both preceding and following initiation of GLC treatment (n=122) the mean BMI-Z trajectory (SD) changed from increasing at a rate of 0.08/year (0.95) to decreasing −0.32/year (0.58) during the 12 months before and after the start of treatment (p<0.0001 for rate change greater than −0.10/yr). The group with pre- and post-GLC follow-up was more likely to be of Hispanic ethnicity and to be publicly insured than those without both pre- and post-GLC follow-up (chi square p<0.05), but the groups did not differ in age, gender, parental language preference, distance from clinic, or severity of obesity.
Resolution of Obesity
Ten (4%) of the 256 children experienced resolution of obesity, resulting in a most recent BMI below the 95th percentile for age and gender, at a mean follow-up time of 16 (range 3–27) months. These children had a mean age of 9.8yrs (SD 1.9) and were less obese at baseline than the rest of the GLC population, with a mean BMI-Z of 1.79 (.34), corresponding to the 96.3rd percentile for BMI. Those children who experienced resolution of obesity had a mean decrease of −.46 BMI-Z (SD 0.5) with a range −0.12 to −1.43 BMI-Z.
Patient Characteristics Associated with Return for Follow-up
Patients who returned to GLC attended an average of five visits over twelve months (range 2–8). On average, this included two visits with the dietitian and one visit with each of the following: medical provider, psychologist, and recreational therapist. Patients in several demographic categories returned more frequently to GLC in bivariate analysis, including: preschool children, Spanish-speaking parents, publicly insured children, and the severely obese, though a multivariable logistic regression model did not identify significant predictors of return to GLC (Table 2). Similar analysis of demographic predictors of follow-up was carried out including all hospital-based clinics (Table 3). Compared to publicly insured patients, the privately insured were less likely to receive follow-up in any hospital-based clinic (44% vs. 62%) or GLC (29% vs 40%) (p<0.0001). In multivariable logistic regression privately insured children were half as likely to return to any hospital based clinic as publicly insured patients: aOR 0.5 [0.4, 0.8]. The majority (76%) of visits to hospital-based clinics other than GLC were to the hospital-based primary care clinic.
TABLE 2.
Associations of Demographic Characteristics with Return to GoodLIFE Clinic for Follow-Up
| Demographic Characteristic | Number Returning / Total by Category (%) |
Crude Odds Ratio Return to GLC (95% CI) |
p | Adj Odds Ratio Return to GLCa (95% CI) |
p |
|---|---|---|---|---|---|
| All Categories Returning to GLC | 172/479 (36%) | ||||
|
Gender Female vs. Male |
85/244 (35%) 87/235 (37%) |
0.91 (0.62, 1.32) | 0.62 | 0.94 (0.63, 1.39) | 0.75 |
|
Age at 1st GLC visit 2–5y (preschool) vs. 6–12 years |
55/126 (44%) 117/353 (33%) |
1.56 (1.03, 2.37) | 0.03 | 1.21 (0.75, 1.97) | 0.43 |
|
Language Spoken Spanish-only vs. English |
49/108 (45%) 123/371 (33%) |
1.67 (1.08, 2.59) | 0.02 | 1.41 (0.86, 2.08) | .19 |
|
Ethnicity Hispanic vs. Non-Hispanic missing = 23 |
97/239 (41%) 69/217 (32%) |
1.47 (.997, 2.15) |
0.051 |
1.04 (0.65, 1.67) |
0.87 |
|
Residence Local - within 30 miles vs. Distant >30 miles from clinic missing = 3 |
156/423 (37%) 15/53 (28%) |
1.48 (.079, 2.78) |
0.22 |
1.40 (0.73, 2.71) |
0.31 |
|
Insurance Private vs. Public |
53/183 (29%) 119/296 (40%) |
0.61 (0.41, 0.90) | 0.01 | 0.75 (0.48, 1.17) | 0.20 |
|
Degree of Obesity Severe BMI >99th percentile vs. BMI 95–99th percentile |
97/233 (42%) 75/246 (30%) |
1.63 (1.12, 2.37) | 0.01 | 1.34 (0.86, 2.08) | 0.20 |
Odds Ratio for return to GoodLIFE Clinic in fully adjusted model including: gender, age, language, ethnicity, residence, insurance, and degree of obesity
TABLE 3.
Associations of Demographic Characteristics with Return to any Children’s Hospital-Based Clinic
| Demographic Characteristic | Number Returning / Total by Category (%) |
Crude Odds Ratio for Return (95% CI) |
P | Adj Odds Ratio for Returna (95% CI) |
p |
|---|---|---|---|---|---|
|
All categories returning to Hospital-based Clinic |
265/479 (55%) | ||||
|
Gender Female vs. Male |
130/244 (53%) 135/235 (57%) |
0.84 (0.59, 1.21) | 0.36 | 0.87 (0.59, 1.28) | 0.47 |
|
Age at 1st GLC visit 2–5y (preschool) vs. 6–12 y |
75/126 (60%) 190/353 (54%) |
1.26 (0.84, 1.91) | 0.27 | 1.08 (0.66, 1.76) | 0.76 |
|
Language Spoken Spanish-only vs. English |
65/108 (60%) 200/371 (54%) |
1.29 (0.84, 2.00) | 0.25 | 1.01 (0.60, 1.69) | 0.98 |
|
Ethnicity Hispanic vs. Non-Hispanic missing = 23 |
141/239 (59%) 117/217 (54%) |
1.23 (0.85, 1.78) |
0.27 |
0.94 (0.59, 1.48) |
0.77 |
|
Residence Local - within 30 miles vs. Distant >30 miles from clinic missing= 3 |
236/423 (56%) 27/53 (51%) |
1.22 (0.69, 2.15) |
0.50 |
1.17 (0.63, 2.16) |
0.62 |
|
Insurance Private vs. Public |
81/183 (44%) 184/296 (62%) |
0.48 (0.33, 0.70) | <0.0001 | 0.53 (0.35, 0.81) | 0.004 |
|
Degree of Obesity Severe BMI>99th percentile vs. BMI 95–99th |
139/233 (60%) 126/246 (51%) |
1.41 (0.98, 2.02) | 0.06 | 1.20 (0.78, 1.85) | 0.41 |
Odds Ratio for return to any hospital-based clinic in fully adjusted model including: gender, age, language, ethnicity, residence, insurance, and degree of obesity
Patient Characteristics Associated with Success
Mean BMI-z change with treatment varied significantly in unadjusted analysis for several groups of interest. The mean BMI-Z change with GLC treatment among children starting treatment as preschoolers was −0.41 (standard deviation 0.53) compared to −0.10 (0.23) in children 6–12 years (p<0.0001). The mean BMI-Z change among children who had Spanish-only speaking parents was −0.29 (0.41) vs. −0.15 (0.35) in English speaking families (p=0.007). The mean BMI-Z change among children who were severely obese was −0.27 (0.43) vs −0.09 (0.27) in those who were not severely obese (p<0.0001).
Bivariate chi-square analysis of dichotomous variables indicated several possible confounding variables. Preschool age, Spanish speaking, and severe obesity had significant crude associations with treatment success (p<0.01). These variables were also associated with each other. Preschoolers were more likely to be severely obese and to have Spanish speaking parents (p<0.02). Likewise, Spanish speakers were more likely to return for care in the GoodLIFE clinic (p<0.01). Children with follow up were more likely to be preschoolers and to be severely obese (p<0.02).
In the multivariable logistic regression, success was strongly associated with age 2–5 years at first visit to GLC, adjusted for all other demographic characteristics and severity of obesity (Table 4). Children whose parents used a Spanish interpreter during the visit were also significantly more likely to succeed. Female gender, Hispanic ethnicity, private insurance, severe obesity, and residence greater than 30 miles from clinic were positively associated with treatment success, but were not significantly associated in the adjusted model. Interaction terms for the effects of age with severity, age with language, and severity with language were tested and were non-significant (data not shown).
Table 4.
Associations of Demographic Characteristics with Success in Treatment (BMI-Z −0.10 or more) among those with Follow-Up
| Demographic Characteristic | Number Succeeding / Total with Follow-up (%) N=265 |
Crude Odds Ratio for Success (95% CI) |
p | Adjusted Odds Ratio for Successa. (95% CI) |
p |
|---|---|---|---|---|---|
| All Categories Succeeding in Treatment | 152/265 (57%) | ||||
|
Age at 1st GLC visit 2–5y (preschool) vs. 6–12 y |
63/75 (84%) 89/190 (47%) |
5.96 (3.02, 11.76) | <0.0001 | 5.6 (2.59, 12.24) | <0.0001 |
|
Language Spoken Spanish-only vs. English |
46/65 (71%) 106/200 (53%) |
2.15 (1.18, 3.92) | 0.01 | 2.19 (1.04, 4.62) | 0.04 |
|
Gender Female vs. Male |
78/130 (60%) 74/135 (55%) |
1.24 (0.76, 2.01 | 0.39 | 1.37 (0.79, 2.37) | 0.27 |
|
Ethnicity Hispanic vs. Non-Hispanic missing = 7 |
87/141 (62%) 60/117 (51%) |
1.53 (0.93, 2.51) |
0.09 |
1.05 (0.55, 1.98) |
0.89 |
|
Residence Local - within 30 miles vs. Distant >30 miles from clinic missing = 2 |
130/236 (55%) 20/27 (74%) |
0.43 (0.17, 1.05) |
0.06 |
0.45 (0.17, 1.22) |
0.12 |
|
Insurance Private vs. Public |
49/81 (60%) 103/184 (56%) |
1.20 (0.71, 2.05) | 0.49 | 1.73 (0.92, 3.26) | 0.09 |
|
Degree of Obesity Severe BMI>99th percentile vs. BMI 95–99th |
94/139 (68%) 58/126 (46%) |
2.45 (1.49, 4.03) | 0.0004 | 1.36 (0.75, 2.47) | 0.31 |
Odds Ratio for successful BMI z-score reduction (−0.10) in fully adjusted model including: gender, age, language, ethnicity, residence, insurance, and degree of obesity
Time to Event Analysis
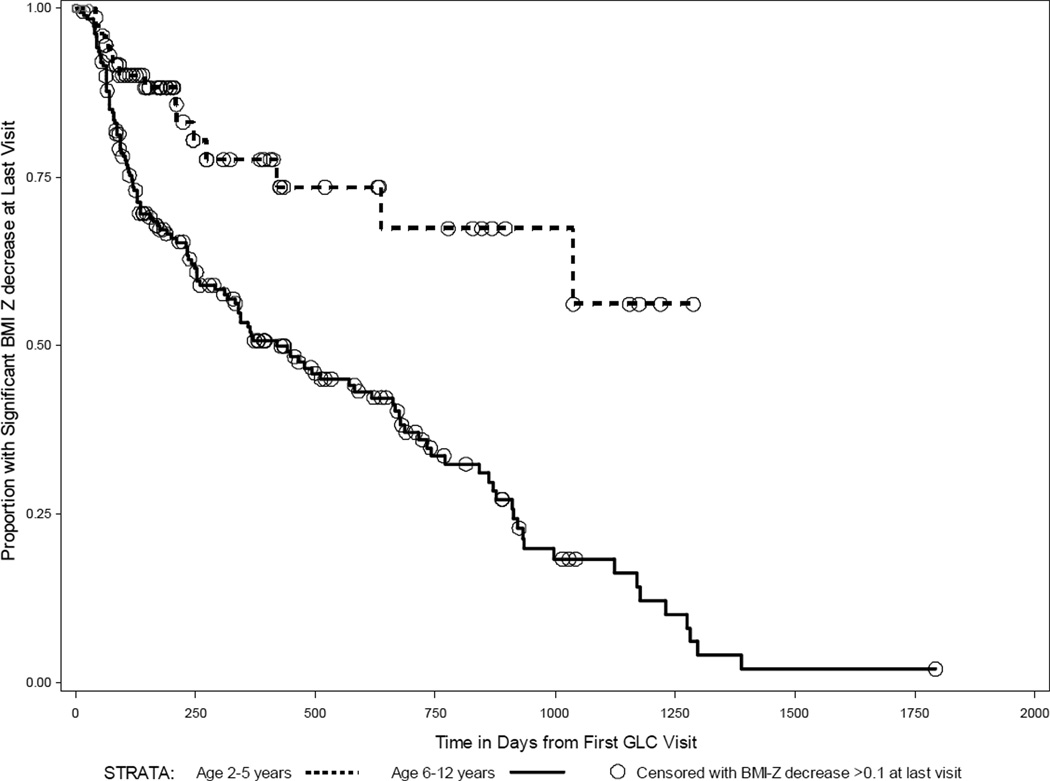
We employed time-to-event analysis to compare rates of treatment failure between demographic groups because we found a wide variability in the duration of follow-up. We performed this analysis for the 265 children for whom follow-up data was available. Kaplan-Meier survival curves were constructed to examine differences in treatment success between the two strata of each demographic group listed in tables 2–4. Figure 1 depicts curves by strata of ‘Age at First GLC Visit’: age 2–5years and 6–12 years. The vertical axis depicts the proportion of each age group with significant BMI-Z reduction (−0.10 or greater) at final measurement. The duration of follow-up in days is indicated on the horizontal axis. Downward steps of the survival curves represent children whose final BMI-Z was not at least −0.10 below baseline at final follow-up. Each circle represents a child who was successful in reducing BMI-Z at least −0.10 at the final measurement. Children who were successful at BMI-Z reduction at their final measurement were censored. Beyond the censoring point these children do not contribute to calculations of the proportion succeeding.
FIGURE 1.
Children with at least one follow-up visit included, n=265. Censoring occurred if BMI-Z was decreased at least −0.1 at last captured follow-up visit. Upper stratum in red: 2–5 years at start of GLC treatment. Lower stratum in blue: 6–12 yrs at start of treatment. Survival Distribution Function: proportion of each strata with a BMI-Z decrease of at least −0.10.
Age at First GLC Visit (2–5y vs. 6–12y) was the only demographic variable for which the log-rank test indicated a significant difference in success over time (gender, language, ethnicity, distance from clinic, insurance, and severity of obesity were all non-significant). Kaplan-Meier curves (Figure 1) for children who were preschoolers 2–5y vs. 6–12y at first GLC visit showed significantly lower failure rate for preschoolers (p<0.0001). The curves diverged early in the follow-up period and continued to separate further over time. After approximately one year of follow-up (370 days) 50% of the 6–12 year old children had failed treatment. In comparison, less than 25% of preschoolers failed within the first year. Cox Proportional Hazards Regression showed a hazard ratio of 3.7 (95% CI: 2.1, 6.8; p<0.0001) for treatment failure in school-aged children 6–12yrs compared to those who started treatment between 2–5years, controlled for the effects of gender, language, ethnicity, distance from clinic, insurance, and severity of obesity (other variables non-significant).
Discussion
Clinic treatment was successful overall, but the likelihood of success in treatment varied across demographic groups. Preschool age children were much more likely to succeed in treatment and to maintain success over a longer duration than children who were 6–12 years old at start of treatment. This outcome may be attributable to greater parental control over the diet and activity environment of younger children, greater ease in changing dietary or sedentary behavior habits that are not as longstanding, or other factors we were unable to measure. Rates of return for follow-up or mean number of visits per child did not differ significantly between preschool and school-aged children, suggesting that the difference in success rate by age was not mediated by a difference in treatment dose.
Children of Spanish-only speaking parents were also more likely to succeed, adjusting for age, ethnicity, and other demographics. Lack of English fluency may be interpreted as a marker of less acculturation. We hypothesize that dietary and activity habits remain in a state of transition for several years after immigration and may be changed more easily than the more entrenched habits of longer-term US residents. We cannot rule out that translation by a medical interpreter during the visit provided additional time for comprehension of treatment messages or enhanced cultural appropriateness. Our findings are reassuring that Spanish language and Hispanic cultural barriers did not impede success in GLC treatment as we originally hypothesized. Because childhood obesity and sequelae such as type II diabetes affect Hispanic children disproportionately,(21) outreach to help this community to maintain healthy lifestyle habits is important.
Our evaluation was limited by low rates of return for follow-up across demographic groups. These rates are similar to those of other medical referral-model obesity treatment programs.(22) Barriers to follow-up may include: inadequate frequency of visits to maintain engagement; low readiness to change of families at time of referral; limitation of insurance coverage; or other program and family characteristics we were unable to measure. The privately insured were significantly less likely to return for GoodLIFE care. Some private insurers specifically excluded or limited benefits for obesity care. Private insurers often reimburse the consulting physician but exclude the dietitian or psychologist treatment from covered benefits. Since limited insurance payment is a potentially modifiable factor that negatively impacts follow-up rates, it is worthwhile to examine the cost effectiveness of this treatment model. The cost of GLC treatment provided to a typical patient was approximately $1100 for five encounters per year, which is well below projected cut-offs for cost-effectiveness based on estimates of long-term medical cost savings for those seeking treatment, especially for the youngest patients.(13)
The preschoolers who are most likely to benefit from treatment are less likely to be screened and referred than older children.(23) Despite the deep and widespread impact of childhood obesity, many medical providers do not follow current recommendations for growth monitoring and obesity prevention guidance.(24) Opportunities for early detection of excess weight gain and intervention are often missed.(25–28) Approximately half of practicing pediatricians reported not using BMI charts to routinely monitor the weight status of children older than 2 years.(24) Physicians report hesitancy to screen for a condition such as childhood obesity for which they feel they cannot provide treatment and lack referral resources.(29, 30) Knowledge that treatment may be more effective when started at younger ages should influence primary care providers’ decisions to monitor growth with BMI at age 2 years and beyond.
The model of treatment reported here represents the highest level of expertise available within the medical referral model for obesity treatment.(2) This model may be capable of clinically significant improvements and long term cost-savings for obese patients, including the severely obese who are at highest risk of co-morbidity, but such a treatment model is likely to have insufficient reach to significantly impact obesity prevalence. Additional approaches involving screening, prevention, and treatment linking primary care, obesity specialists, and community resources are needed for the medical community to respond optimally to the epidemic of childhood obesity.(31)
Limitations
The observational design of this study dictates caution in interpreting the treatment effect of GLC in the absence of a randomized control group. Bearing in mind this limitation, within-subject comparison of BMI-Z trends before and after GLC treatment show marked reversals of BMI trajectories that on average were rapidly increasing prior to treatment. The effect seen in this clinically referred population biased towards the most severely obese children in a wide geographic area may differ from the effect of a similar intervention in less severely obese children. Given the caution required in interpreting the overall success rate, the most important findings of this study are the relative rates of success for preschoolers and Spanish speakers adjusted for severity of obesity, ethnicity, distance from clinic, insurance status, and gender.
The clinical data source imposed limitations on our analysis. Our dataset does not include follow-up information from clinics outside of the hospital network, so privately insured patients are under-represented in our outcomes analysis. Our ability to assess for possible confounding variables is limited to those available from the electronic medical record. Other characteristics may be associated with treatment success, including parental weight status or more detailed measures of socioeconomic status than insurance status. These factors have demonstrated associations with development of childhood obesity.(1, 5) The sample size limited our ability to detect significant odds ratios of magnitude below 2.3 in our logistic regression of success on patient demographic variables. Associations with smaller magnitude may be statistically significant in a larger study population and could prove clinically meaningful. Since the outcome of success in treatment was not a rare event, the odds ratios presented here are positively biased estimates of the relative risk.
Conclusion
Our data provide a strong rationale for further prospective and controlled study of obesity screening and treatment in children less than 6 years old. The data presented here suggest that even low-frequency treatment can be effective for children less than 6 years who are clinically referred to experts, beginning to fill a gap in the evidence supporting the rationale for BMI screening in this age group pointed out by the USPSTF. While limited by the observational design, the study has strengths, including a relatively large and diverse population of clinically referred patients treated for obesity in real-world conditions and without artificial incentives to participate in treatment.
Epidemiologic evidence indicating disparities in childhood obesity by race and ethnicity. These disparities and our data suggesting increased treatment success among less acculturated families, point out the need for further efforts to serve minority and immigrant communities. Children of less-acculturated Hispanic immigrants are vulnerable to obesity and appear more amenable to treatment once engaged than those whose parents have gained English fluency. Other large scale surveys of adults have reported that Spanish speakers have less access to healthcare resources, but are more likely to report favorable dietary intakes.(32, 33) Our data suggest that in this individualized model of clinical obesity care, Hispanic ethnicity and Spanish language did not create a barrier to obesity treatment. The associations found in our data should be explored in other populations testing similar treatment models.
Treatment programs across the US report long waiting lists and low reimbursement for the multidisciplinary teams (pediatric medical provider, dietitian, psychologist, fitness specialist, and social worker) required to treat severe pediatric obesity.(31) Evidence of clinical effectiveness may prompt insurers to increase reimbursement for childhood obesity screening and treatment. Increased availability of treatment with demonstrated effectiveness may in turn have a positive effect on clinician screening practices for obesity in the primary care setting. The medical community has an important role to play in reversing the childhood obesity epidemic including optimal screening and vigilant use of treatment resources. Future studies should focus on cost-effective methods to intervene in populations with highly prevalent obesity during the preschool years.
Acknowledgments
The authors wish to acknowledge the assistance of the University of Colorado School of Medicine, Children’s Outcomes Research Program for assistance in the preparation of this manuscript. The authors also acknowledge the support of the Department of Clinical Informatics at Children's Hospital Colorado for Daksha Ranade’s effort to obtain the data.
Dr Haemer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis and was responsible for study concept, design, and drafting of manuscript; Dr Haemer and Ms Ranade were responsible for acquisition of data; Drs Haemer, Krebs, and Barón were responsible for analysis and interpretation of data and critical revision of the manuscript for important intellectual content; Drs Haemer and Barón were responsible for statistical analysis; Dr Krebs obtained funding and was responsible for study supervision; and Drs Haemer and Ms Ranade were responsible for administrative, technical, or material support. All authors reviewed the final version of the manuscript.
Funded by the National Institutes of Health (NIH) Nutrition Training Grant - Dr Haemer (T32 DK07658-18) and Midcareer Investigator Award in Patient-Oriented Research Dr. Krebs (NIDDK 9K24 DK083772)
Glossary
Abbreviations
- BMI
Body Mass Index
- BMI-Z
Body Mass Index z-score
- GLC
The GoodLIFE Clinic
- USPSTF
United States Preventive Services Task Force
Footnotes
Competing interests: the authors have no competing interests
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and trends of severe obesity among US children and adolescents. Acad Pediatr. 2009 Sep-Oct;9(5):322–329. doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007 Dec;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 3.Harrington JW, Nguyen VQ, Paulson JF, Garland R, Pasquinelli L, Lewis D. Identifying the "tipping point" age for overweight pediatric patients. Clin Pediatr (Phila) Jul;49(7):638–643. doi: 10.1177/0009922809359418. [DOI] [PubMed] [Google Scholar]
- 4.Nader PR, O'Brien M, Houts R, Bradley R, Belsky J, Crosnoe R, et al. Identifying risk for obesity in early childhood. Pediatrics. 2006 Sep;118(3):e594–e601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- 5.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997 Sep 25;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001 Sep;108(3):712–718. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- 7.Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;1 doi: 10.1002/14651858.CD001872.pub2. CD001872. [DOI] [PubMed] [Google Scholar]
- 8.Barton M. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. Feb;125(2):361–367. doi: 10.1542/peds.2009-2037. [DOI] [PubMed] [Google Scholar]
- 9.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007 Dec;120(Suppl 4):S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 10.Monasta L, Batty GD, Macaluso A, Ronfani L, Lutje V, Bavcar A, et al. Interventions for the prevention of overweight and obesity in preschool children: a systematic review of randomized controlled trials. Obes Rev. May;12(5):e107–e118. doi: 10.1111/j.1467-789X.2010.00774.x. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of High Body Mass Index in US Children and Adolescents, 2007–2008. JAMA. 2010 Jan 20;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 12.US_Census_Bureau_Population_Division. US Census Bureau: Population Estimates, Vintage. [6-20-2011];2009 Available from: http://www.census.gov/popest/national/asrh/natasrh.html.
- 13.Ma S, Frick KD. A simulation of affordability and effectiveness of childhood obesity interventions. Acad Pediatr. Jul-Aug;11(4):342–350. doi: 10.1016/j.acap.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Anderson SE, Whitaker RC. Prevalence of obesity among US preschool children in different racial and ethnic groups. Arch Pediatr Adolesc Med. 2009 Apr;163(4):344–348. doi: 10.1001/archpediatrics.2009.18. [DOI] [PubMed] [Google Scholar]
- 15.Stark LJ, Spear S, Boles R, Kuhl E, Ratcliff M, Scharf C, et al. A pilot randomized controlled trial of a clinic and home-based behavioral intervention to decrease obesity in preschoolers. Obesity (Silver Spring) Jan;19(1):134–141. doi: 10.1038/oby.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taveras EM, Gortmaker SL, Hohman KH, Horan CM, Kleinman KP, Mitchell K, et al. Randomized Controlled Trial to Improve Primary Care to Prevent and Manage Childhood Obesity: The High Five for Kids Study. Arch Pediatr Adolesc Med. Apr;4 doi: 10.1001/archpediatrics.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Huerta M, Silverstein JH, et al. Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: outcomes from project STORY. Arch Pediatr Adolesc Med. 2008 Dec;162(12):1119–1125. doi: 10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estabrooks PA, Shoup JA, Gattshall M, Dandamudi P, Shetterly S, Xu S. Automated telephone counseling for parents of overweight children: a randomized controlled trial. Am J Prev Med. 2009 Jan;36(1):35–42. doi: 10.1016/j.amepre.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Ceballos N, Czyzewska M. Body image in Hispanic/Latino vs. European American adolescents: implications for treatment and prevention of obesity in underserved populations. J Health Care Poor Underserved. Aug;21(3):823–838. doi: 10.1353/hpu.0.0333. [DOI] [PubMed] [Google Scholar]
- 20.Kaysville, Utah: 2008. 2008 HJ. PASS, NCSS, LLC. www.ncss.com. [Google Scholar]
- 21.Dabelea D, Bell RA, D'Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. JAMA. 2007 Jun 27;297(24):2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 22.Hampl S, Paves H, Laubscher K, Eneli I. Patient engagement and attrition in pediatric obesity clinics and programs: results and recommendations. Pediatrics. Sep;128(Suppl 2):S59–S64. doi: 10.1542/peds.2011-0480E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien SH, Holubkov R, Reis EC. Identification, Evaluation, and Management of Obesity in an Academic Primary Care Center. Pediatrics. 2004 Aug 1;114(2):e154–e159. doi: 10.1542/peds.114.2.e154. 2004. [DOI] [PubMed] [Google Scholar]
- 24.Klein JD, Sesselberg TS, Johnson MS, O'Connor KG, Cook S, Coon M, et al. Adoption of body mass index guidelines for screening and counseling in pediatric practice. Pediatrics. Feb;125(2):265–272. doi: 10.1542/peds.2008-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Children and teens told by doctors that they were overweight--United States, 1999–2002. MMWR Morb Mortal Wkly Rep. 2005 Sep 2;54(34):848–849. [PubMed] [Google Scholar]
- 26.Dilley KJ, Martin LA, Sullivan C, Seshadri R, Binns HJ. Identification of overweight status is associated with higher rates of screening for comorbidities of overweight in pediatric primary care practice. Pediatrics. 2007 Jan;119(1):e148–e155. doi: 10.1542/peds.2005-2867. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien SH, Holubkov R, Reis EC. Identification, evaluation, and management of obesity in an academic primary care center. Pediatrics. 2004 Aug;114(2):e154–e159. doi: 10.1542/peds.114.2.e154. [DOI] [PubMed] [Google Scholar]
- 28.Voelker R. Improved use of BMI needed to screen children for overweight. JAMA. 2007 Jun 27;297(24):2684–2685. doi: 10.1001/jama.297.24.2684. [DOI] [PubMed] [Google Scholar]
- 29.Flower KB, Perrin EM, Viadro CI, Ammerman AS. Using body mass index to identify overweight children: barriers and facilitators in primary care. Ambul Pediatr. 2007 Jan-Feb;7(1):38–44. doi: 10.1016/j.ambp.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barlow SE, Richert M, Baker EA. Putting context in the statistics: paediatricians' experiences discussing obesity during office visits. Child Care Health Dev. 2007 Jul;33(4):416–423. doi: 10.1111/j.1365-2214.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 31.Slusser W, Staten K, Stephens K, Liu L, Yeh C, Armstrong S, et al. Payment for obesity services: examples and recommendations for stage 3 comprehensive multidisciplinary intervention programs for children and adolescents. Pediatrics. Sep;128(Suppl 2):S78–S85. doi: 10.1542/peds.2011-0480H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DuBard CA, Gizlice Z. Language spoken and differences in health status, access to care, and receipt of preventive services among US Hispanics. Am J Public Health. 2008 Nov;98(11):2021–2028. doi: 10.2105/AJPH.2007.119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimm KA, Blanck HM. Survey language preference as a predictor of meeting fruit and vegetable objectives among Hispanic adults in the United States, behavioral risk factor surveillance system 2009. Prev Chronic Dis. Nov;8(6):A133. [PMC free article] [PubMed] [Google Scholar]