Abstract
Objectives
Magnetic Resonance Elastography (MRE) can noninvasively sample tissue stiffness in-vivo. Renal fibrosis secondary to renal artery stenosis (RAS), which is aggravated in atherosclerotic RAS (ARAS), may increase its stiffness. An increase in cortical stiffness in-vivo can be masked by intrinsic hemodynamic determinants, while renal medullary stiffness is less dependent on renal hemodynamics. Therefore, this study tested the hypothesis that MRE-determined medullary stiffness would correspond to the histological degree of medullary fibrosis in stenotic kidneys in RAS and detect its exacerbation in ARAS.
Methods
Seventeen pigs were studied 10 weeks after induction of unilateral RAS (n=6), ARAS (n=5), or sham (n=6). Cortical and medullary stiffness was determined by 3D MRE, and renal perfusion and function using multi-detector computed-tomography. Kidney fibrosis was subsequently assessed ex-vivo using Masson’s trichrome staining.
Results
Renal stenotic cortex and medulla were significantly more fibrotic in RAS and ARAS compared to normal. However, MRE detected increased stiffness in RAS compared to normal (12.7±0.41kPa vs. 10.7±0.18kPa, p=0.004) only in the medulla, which was further increased in ARAS (16.6±1.3kPa, p=0.017 vs. RAS). MRE-derived medullary, but not cortical, stiffness significantly correlated with histological degree of fibrosis, although cortical and medullary fibrosis were correlated. Renal blood flow and function were similarly decreased in RAS and ARAS compared to normal.
Conclusion
Noninvasive 3D MRE detects increased renal medullary stiffness in RAS and ARAS in-vivo, which correlates with its fibrosis ex-vivo and may also reflect cortical fibrosis. Hence, MRE-derived medullary stiffness can potentially be useful to detect renal fibrosis and track disease progression.
Keywords: magnetic resonance elastography, renal artery stenosis, fibrosis
Introduction
Renal artery stenosis (RAS), a narrowing of the renal artery, causes reductions in renal blood flow (RBF), which often leads to structural changes, such as atrophy and fibrosis, in affected renal tissue. The affected kidney is also likely to become a pressor, drastically increasing blood pressure by activating the renin-angiotensin system. These effects might be exacerbated in atherosclerotic renal artery stenosis (ARAS), characterized by diffuse atherosclerosis and intrarenal microvascular disease1,2.
Parenchymal disease often changes the mechanical properties of the tissue. Magnetic resonance elastography (MRE) is an emerging MRI modality that can noninvasively quantify and visualize a tissue’s elasticity, which simulates tissue “palpation”. By passing acoustic shear waves through a target tissue and tracking their propagation using phase-contrast MRI, MRE measures the resulting transverse strain and derives the tissue shear modulus G or “stiffness,” resulting in a 3D stiffness map, or “elastogram.” These colorimetric elastograms allow for qualitative inspection and localization, while quantitative stiffness values can be obtained in defined regions of interest (ROI)3,4.
Importantly, tissue stiffness is determined by both its intrinsic and acquired mechanical properties (e.g. fibrosis), as well as by hemodynamic determinants of turgor. The hyper-perfused kidneys present a set of complications for MRE analysis which are less evident in less vascular organs, such as the brain5. Indeed, we have shown that acute reductions in RBF significantly alter renal cortical stiffness as determined by MRE, and that decreases in RBF due to chronic RAS can mask increases in the intrinsic stiffness of the renal cortex due to fibrosis6,7. Notably, the majority of the renal vasculature is located in the cortex, so that renal perfusion pressure likely drives a significant proportion of its stiffness in-vivo. In contrast, medullary perfusion is lower and regulated, in part, independently from the cortex. Thus, we hypothesized that while the degree of fibrosis in the medulla and cortex of stenotic kidneys would correlate, MRE analysis of medullary elasticity would be more sensitive to detecting changes in tissue stiffness compared to measures of cortical elasticity.
Materials and Methods
Animal Model
This study was approved by the Institutional Animal Care And Use Committee. Seventeen female pigs (50–60 kg) were fed normal pig chow (n=12) or an atherogenic diet of 2% high-cholesterol (Harlan Teklad, Madison, WI) (n=5) for six weeks. All animals were then anesthetized using an intra-muscular injection of xylazine (2mg/kg) and telazol (5mg/kg), and maintained using intravenous ketamine (11mg/kg/hr) and xylazine (1.8mg/kg/hr). To induce unilateral stenosis, a local-irritant coil was placed in the right renal artery of 6 pigs fed a normal diet (RAS) and the 5 pigs fed an atherogenic diet (ARAS) using fluoroscopy, as previously described8. The remaining normal pigs (n=6) underwent a sham procedure involving cannulation of the renal artery. Ten weeks later, all animals were again anesthetized and maintained via mechanical ventilation with isoflurane (1–2%) in atmospheric air to perform abdominal MRE. Pigs were again anesthetized a few days later to assess renal function and anatomy using multi-detector computed-tomography (MDCT) and to collect blood samples from the inferior vena cava to measure total and low density lipoprotein (LDL) cholesterol and serum creatinine. All pigs were euthanized three days later using 100mg/kg of sodium pentobarbital (Sleepaway®, Fort Dodge Laboratories, Inc., Fort Dodge, Iowa). The kidneys were harvested and preserved in formalin for ex-vivo analysis (Figure 1A).
Figure 1.
(A) Schematic of the experimental protocol used in renal artery stenosis (RAS), atherosclerotic RAS (ARAS), and sham animal models. (B) Depiction of MRE setup showing an active driver (located outside the scan room) connected to a passive pneumatic driver inside the MRI bore via flexible plastic tubing. (C) Representative co-localized elastogram, echo planar imaging (EPI) magnitude, and CT images of a normal pig. Medullary regions (arrows) were differentiated from the cortex in Magnitude images and verified by comparing to the corresponding MDCT images.
Magnetic Resonance Elastography
Pigs were placed on their dorsal surface within the bore of the MRI scanner (Signa TwinSpeed EXCITE 3T system, GE Healthcare, Waukeshau, WI). Scans were performed during 30-second breath holds. MRE pulse sequence parameters were as follows: TR/TE/FOV/imaging matrix/thickness/number of averages/shots/parallel encoding factor = (2200ms/64ms/34cm/96 × 96/3mm/1/2/2) interpolated to 256×256 images. A 3D stack of images was collected using 2D, transverse, multi-slice, flow-compensated, spin-echo, echo-planar imaging (EPI) in conjunction with motion sensitizing gradients (MEG) synchronized with the 120Hz vibrations produced by two passive pneumatic drivers placed on the animal’s dorsal surface, proximal to the kidneys. The passive drivers were connected to an active driver located outside the scan room via flexible plastic tubing (Figure 1B). Four time points were sampled per slice in all 3 directions. The MEG waveform, on each side of the spin-echo pulse, was 4 periods of a 120-Hz trapezoidal waveform with 0th and 1st moment nulling. The MEG alternated in amplitude and polarity during the scan to measure different components of the tissue displacement and was synchronized with the motion produced by the passive drivers. Motion was measured in six orthogonal directions (±x, ±y, ±z) in scanner coordinate system with a final motion sensitivity of 3.2μm/radian. Images were processed and analyzed using MRE/Wave© (MRI Research Lab, Mayo Clinic, Rochester, MN). Measurements of tissue displacement were used to derive shear wave speed and ultimately, shear modulus. Data were processed using phase unwrapping, curl filtration to exclude bulk motion, and a local spatial frequency estimation (LFE) algorithm to determine the shear wavelength at 120Hz. The product of shear wavelength and the vibration frequency was used to derive the shear wave speed, and the product of the tissue density (assumed to be 1000 kg/m3) and the square of the wave speed reported as the tissue stiffness.
Stiffness values were averaged within ROIs, manually traced around the renal medulla on the MR magnitude images from the EPI MRE acquisition and co-registered to the elastogram (Figure 1C). Medullary areas on EPI magnitude images were verified with the aid of contrast-enhanced CT images obtained during the vascular phase of contrast agent transit. Due to intrinsic distortion, EPI magnitude images are difficult to directly co-localize with MDCT images. This process was therefore performed indirectly, by first co-localizing T2-weighted images acquired with the same prescription as MRE and MDCT images, using a previously-described method9.
Inter-observer variability was assessed by the difference in stiffness values estimated by two independent observers (bias), as well as the standard deviation (STD) of these values, and reported as bias±STD. Moreover, the overall bias and the 95% confidence interval (2*STD) were determined.
Multi-detector computed tomography
In-vivo measurements of cortical and medullary renal volume and function were performed noninvasively using an MDCT scanner (Somatom Definition 64, Siemens Medical Solution, Forchheim, Germany) as we have previously shown10,11. Mean arterial pressure (MAP) was measured using an intra-arterial catheter. A central venous injection of contrast (iopamidol, 0.5ml/kg/2sec) was used to assess stenotic and contralateral kidney (CLK) RBF and glomerular filtration rate (GFR) after acquisition of 160 consecutive scans. Another injection of iopamidol (1ml/kg/5sec) was used to image 3D stacks of 5mm-thick kidney sections with 0.48mm pixel spacing for later volumetric analysis. Reconstruction and analysis of images was performed using Analyze™ (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). Time attenuation curves in the aorta, cortex, and medulla were generated and modeled using curve fitting algorithms from ROIs drawn on axial images10,11. Planimetry of the helical volume MDCT scans was used to calculate cortical and medullary volume. Siemens Syngo Applications Suite (Siemens, Germany) was used to quantify the degree of obstruction of the renal artery using 0.6mm thick MDCT scans with 0.48mm pixel spacing.
Histology
Cortical and medullary kidney tissue was embedded in paraffin and sectioned (5μm thick) for Masson’s trichrome staining. The degree of fibrosis in the renal cortex and medulla was semi-automatically quantified in 15–20 fields using a masking algorithm based on color thresholding and edge detection in AxioVision© (Carl Zeiss MicroImaging) and expressed as an average of percent fibrosis to total field area.
Tubular injury score
The level of injury in the tubules was assessed using a semi-quantitative method (0: no injury, 1: <10%, 2: 10–25%, 3: 26–50%, 4: 51–75% and 5: >75%) according to the tubular dilation, atrophy, cast formation, sluggish endothelial cells and thickening in the basal membrane using H&E slides (x40)12,13.
Statistics
Results for normally distributed values are expressed as mean±SEM (standard error). Hemodynamics Inter-group comparisons were performed using ANOVA followed by unpaired Student’s T-Test. Inter-group comparisons for fibrosis and stiffness were performed using non-parametric (Wilcoxon and Kruskal Wallis) tests, and data are shown as median (range). The relationship between histologically assessed fibrosis and MRE-derived tissue stiffness was investigated using a least-squares regression. Statistical significance was accepted at p≤0.05.
Results
The degree of stenosis was similar in RAS and ARAS, and MAP was elevated in both groups (p=0.03 and p<0.001, respectively) compared to Normal (Table 1). RBF was significantly reduced in both RAS and ARAS stenotic kidneys (p=0.03 and p=0.04) compared to Normal, as was stenotic-kidney GFR (p=0.01 and p<0.001), while serum creatinine levels did not differ among the groups. Total and LDL cholesterol were significantly higher in ARAS animals (p=0.003 and p=0.005) compared to Normal. Cortical and medullary volumes were significantly reduced in the stenotic RAS (p<0.001 and p<0.001, respectively) and ARAS (p=0.009 and p<0.001, respectively) kidneys compared to Normal (Table 1). CLK RBF and cortical volume were elevated in both RAS and ARAS.
Table 1.
Systemic characteristics and single-kidney hemodynamics and function in pigs with renal artery stenosis (RAS) or atherosclerotic RAS
| Normal | RAS | ARAS | |
|---|---|---|---|
| MAP (mmHg) | 96.9±3.9 | 115.4±8.9* | 145.3±11.9*† |
| Degree of Stenosis (%) | 0 | 95.3±4.1* | 94.4±5.6* |
| Total Cholesterol (mg/dL) | 73.5±3.4 | 110.8±19 | 640.6±104*† |
| LDL (mg/dL) | 25.6±2.6 | 30.7±1.5 | 417.8±83*† |
| Serum Creatinine (mg/dL) | 1.56±0.06 | 1.48±0.1 | 1.70±0.44 |
| Cortical Volume (cc): | |||
| STK (right) | 102.9±4.3 | 54.9±10.6* | 77.6±4.4* |
| CLK (left) | 100.3±4.1 | 119.9±7.0* | 148.4±7.2*† |
| Medullary Volume (cc): | |||
| STK (right) | 20.9±1.4 | 7.8±1.3* | 13.7±2.9* |
| CLK (left) | 21.2±1.6 | 19.4±3.6 | 25.1±2.7 |
| RBF (ml/min): | |||
| STK (right) | 470.3±83.5 | 221.3±66.5* | 243.1±28* |
| CLK (left) | 410.7±71.5 | 735.7±57.0* | 629.2±27.7* |
| GFR (ml/min): | |||
| STK (right) | 78.8±6.0 | 40.1±7.3* | 56.3±5.2* |
| CLK (left) | 76.8±7.1 | 96.0±7.9 | 98.3±19.2 |
p≤0.05 Vs. Normal,
p≤0.05 Vs. RAS
MAP: Mean Arterial Pressure, LDL: Low-Density Lipoprotein, RBF: Renal Blood Flow, GFR: Glomerular Filtration Rate, STK: stenotic Kidney, CLK: Contra-lateral Kidney
Cortex
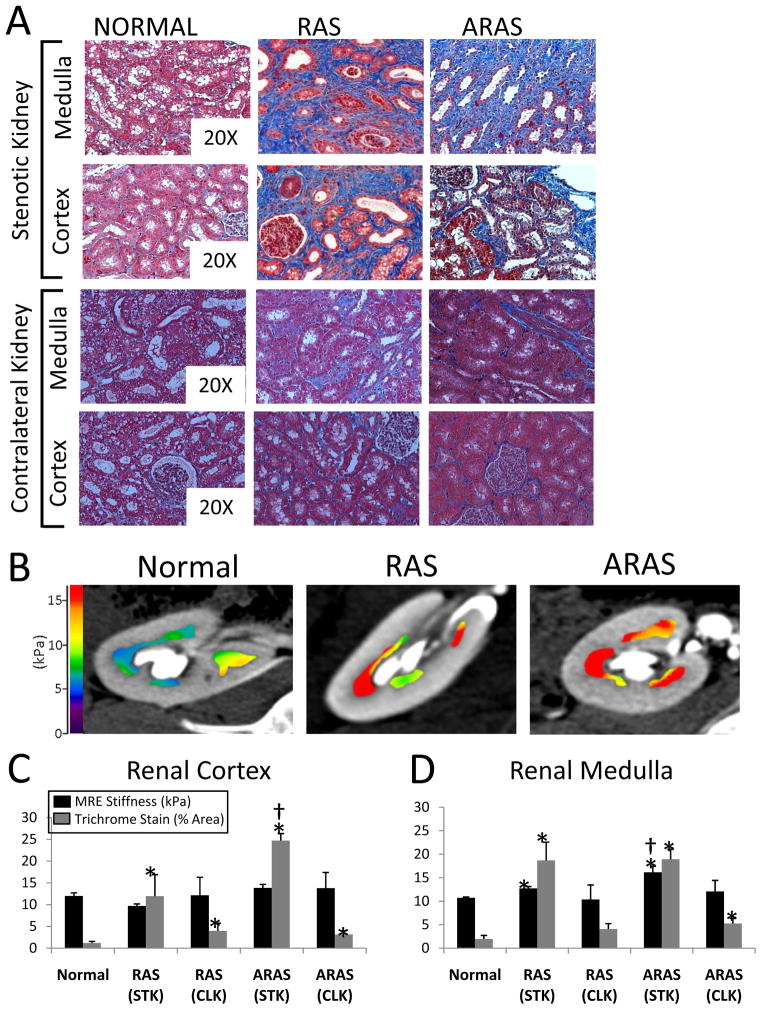
Cortical trichrome staining showed significantly higher fibrosis in stenotic RAS (p=0.004 vs. Normal) that was further elevated in ARAS kidneys (p=0.049 vs. RAS) (Figure 2A and 2C). CLK cortical fibrosis in RAS and in ARAS was significantly higher than Normal (p=0.025 and p=0.023, respectively), yet remained significantly lower than in the corresponding stenotic kidneys. MRE did not detect elevation in cortical stiffness in ARAS or RAS vs. Normal (Figure 2C). There was no significant correlation between degree of fibrosis and MRE-derived stiffness (Figure 3A, 3B) in the stenotic-kidney cortex. The CLK stiffness was not different than Normal and did not correlate with fibrosis.
Figure 2.
(A) Representative cortical and medullary trichrome-stained sections from normal, RAS, and ARAS stenotic and contralateral kidneys (20X magnification). (B) Representative MRE elastograms of Normal, RAS, and ARAS renal medulla overlaid on co-registered MDCT images. Cortical (C) and medullary (D) MRE-derived stiffness values and trichrome histology in normal, RAS, and ARAS groups. * p≤0.05 Vs. Normal, † p≤0.05 Vs. RAS
Figure 3.
Correlation between histologically assessed degree of fibrosis (trichrome stain) and MRE-derived tissue stiffness of renal medulla and cortex in the stenotic (A) and the contralateral (B) kidneys. (C) Correlation between cortical and medullary trichrome histology-derived degree of fibrosis.
Medulla
RAS and ARAS stenotic kidney medulla showed similar increases in fibrosis (trichrome staining) compared to Normal (p=0.004 and p=0.006, respectively) (Figure 2A and 2D). The RAS renal medulla was stiffer compared to normal (p=0.02), while the ARAS medulla was yet further stiffer (p=0.02 vs. RAS) as determined using MRE (Figure 2B,2D). The degree of medullary fibrosis significantly correlated with its MRE-derived stiffness (R2=0.28, p=0.03) (Figure 3A). In addition, a significant correlation between the ex-vivo cortical and medullary fibrosis was observed (R2=0.7, p<0.001) (Figure 3D). In CLK, the degree of medullary fibrosis was only elevated in ARAS and stiffness was neither different in RAS nor in ARAS than Normal.
Tubular injury score was significantly higher in stenotic RAS and ARAS kidneys vs. Normal and correlated with medullary stiffness (R2=0.3, p=0.025) (Figure 4).
Figure 4.
H&E staining tubular images (x20), and the semi-quantitative tubular score (A). This parameter showed a correlation with medullary stiffness in the stenotic kidney (B). * p≤0.05 Vs. Normal.
Inter-observer observer variability was investigated and among the all groups in the study. The bias±variability in in the individual groups (normal, RAS, and ARAS) were 0.3±1.1, 1.0±1.3 and 1.6±2.5 kPa, respectively. In all groups, the bias was 0.6 kPa and the 95% confidence interval assessed as 3.3 kPa.
Discussion
This study showed that both medullary and cortical fibrosis were elevated in stenotic RAS and ARAS kidneys compared to normal kidneys. However, the in-vivo MRE-derived medullary stiffness tracked more closely with the concomitant increases in fibrosis than did cortical stiffness. Therefore, this study suggests that MRE-derived medullary, but not cortical, stiffness might be a useful index of renal fibrosis in the stenotic kidney.
RAS decreases cortical turgor of the kidney through reductions in RBF, which also induce fibrogenesis1,2. As we have previously shown6, acute reductions in perfusion pressure by occlusion of the renal artery reduce the MRE-derived cortical stiffness. In chronic RAS, the decreased turgor is offset by increased cortical fibrosis, so that cortical elasticity remains close to normal. Renal parenchymal fibrosis is further exacerbated in ARAS compared to RAS kidneys, even in the face of similar degrees of renal artery occlusion, underscoring the contribution of atherogenic determinants other than hypoperfusion to the degree of nephropathy. These factors include cytokines, inflammatory mediators, and oxidative stress that can cause rarefaction of arterioles, loss of function, and fibrogenesis.14 However, because of the complexity of its hemodynamic regulation, detection of renal fibrosis in-vivo remains a challenge.
MRE is one of several promising novel imaging techniques capable of noninvasively detecting damaged renal tissue in-vivo. Diffusion weighted imaging (DWI), another promising technique, has also been used to detect renal functional and morphological changes15,16. This powerful MR-based method is sensitive to the presence of microstructures, which impose constrains on water molecules free diffusion. Apparent diffusion coefficient, a quantitative index calculated from DWI17, has been used as a marker of morphological change in renal tissue, but is not collagen-specific and has yet to be validated and standardized for clinical use18. Shear wave dispersion ultrasound vibrometry (SDUV) is similar in principle to MRE, but is limited to scanning a small, focal area and has thus far been validated only in the excised kidney in-vitro19. Like MRE, these methods are also vulnerable to changes in renal turgor pressure. Magnetic Resonance Spectroscopy (MRS) and Magnetization Transfer (MT) are exciting MRI techniques that might detect signal specific to collagen, its hydration characteristics, and macromolecular byproducts of fibrogenesis. While MRS and MT have been used successfully in the brain, organ dependency, prolonged scans, and limited breath-hold capacity in some patients may limit their clinical application in abdominal scanning20,21.
MRE has already been deployed for clinical use for detection of hepatic fibrosis, yet recent studies have shown that liver stiffness is also somewhat dependent on mesenteric blood flow22–24. Importantly, the current study shows that in the kidney these limitations can be circumvented because of regional differences in its constituent tissues. Roughly 90% of RBF is delivered to the cortex, whereas the medulla is less dependent on perfusion pressure1. Targeting the medullary tissue for MRE analysis takes advantage of the medullary ability to maintain relatively constant perfusion in the face of changes in RBF25.
Interestingly, MRE analysis not only detected increased medullary stiffness in stenotic RAS kidneys, but also detected magnified stiffness in the ARAS medulla, which was not detected in the cortex. Furthermore, the increase in cortical stiffness in ARAS kidneys compared to normal was rather modest (1.4kPa) as opposed to the striking (6.2kPa) increase observed in the renal medulla (Table 2). In fact, MRE also showed that cortical stiffness had slightly lower values in stenotic RAS kidneys, possibly due to hemodynamic effects6. Taken together, these observations suggest a threshold at which increasing intrinsic tissue stiffness caused by progressive fibrogenesis can override the masking effect of reduced RBF. These factors likely decrease the sensitivity of in-vivo methods to detect mild forms of cortical fibrosis. In contrast, medullary stiffness varied with disease severity and correlated significantly with tissue fibrosis. Nevertheless, the significant correlation between the degree of fibrosis in the stenotic kidneys cortex and medulla implies that medullary stiffness as assessed by MRE might, in some cases, serve as an adequate surrogate for cortical fibrosis. Also, because MRE-derived stiffness values correlate well with the degree of tissue damage, it could potentially be used to track disease progression longitudinally.
Table 2.
Stiffness and fibrosis in the cortex and the medulla of the stenotic kidney (STK) and the contra-lateral kidney (CLK) reported as median (range)
| Normal | RAS | ARAS | |||
|---|---|---|---|---|---|
|
| |||||
| STK | CLK | STK | CLK | ||
|
|
|||||
| Cortical Stiffness (kPa) | 12.2 (9.1–14.1) | 9.5 (8.3–11.2)* | 15.1 (6.4–18.6) | 13.6 (12.0–16.6)† | 11.5 (10.7–14.4) |
| Medullary Stiffness (kPa) | 11.0 (10.0– 13.0) | 12.3 (11.8–14.2)* | 11.5 (6.1–14.0) | 17.3 (12.5–19.8)*† | 12.4 (8.2–13.8) |
| Cortical Fibrosis (% area) | 1.0 (0.3–2.9) | 5.2 (3.6–32.1)** | 3.4 (2.1–7.2)* | 23.3 (21.1–29.7)*† | 3.3 (2.6–4.2)* |
| Medullary Fibrosis (% area) | 2.5 (0.4–6.6) | 15.8 (9.3–31.0)** | 4.3 (2.3–5.5) | 20.1 (10.9–22.7)* | 5.8 (4.1–6.5)* |
p<0.05 vs. Normal,
p<0.005 vs. Normal,
p≤0.05 Vs. RAS
Notably, the variability in medullary stiffness was greater at higher levels of tissue fibrosis. This may be a result of our limited sample size, but may also be attributed to the differential effect of the fibrosis on medullary atrophy and thereby edge effects. As acoustic waves cross barriers into different media (i.e. cortex to medulla), noise is produced near the interface. A smaller medulla increases the likelihood of sampling this noise. In addition, the degree of fibrosis observed in a single 5μm thick slice of tissue may not be representative of the entire kidney, yet was compared to 3D MRE data of the entire kidney. This may also explain why MRE, but not histology, detected a difference between stenotic RAS and ARAS medulla. Because it can sample the entire kidney, MRE may be more sensitive to changes in tissue structure than histology. Further studies will also need to improve and automate co-registration between the elastogram and anatomically accurate images. Severe tissue atrophy also renders MRE analysis difficult due to limitations in spatial resolution secondary to noise. Increased frequency of acoustic waves would improve the signal-to-noise ratio, but these short wavelength waves tend to dissipate quickly before reaching renal tissue. Increasing their amplitude is limited by the active driver’s maximum output power. Moreover, although RBF and GFR were not different between RAS and ARAS, future studies are needed to model the complex effects of RBF, GFR, fluid reabsorption, venous output, and MAP on turgor pressure in the kidney to utilize MRE for analysis of cortical tissue.
The main advantage of MRE compared to current clinical practice, kidney biopsy, is its non-invasiveness, and therefore minimal complications and lower cost. In addition, in kidney biopsy the sample size is limited and often not representative of the entire kidney, whereas MRE allows the study of the entire renal parenchyma and of both kidneys in the same session. On the other hand, MRE cannot provide a definite diagnosis, and has lower specificity and sensitivity. For example, in our study, tubular damage also correlated significantly with MRE-derived stiffness, although it often does co-exist with interstitial fibrosis. The effect of tissue cellularity on MRE-derived stiffness remains to be determined, but is likely smaller than the effect of interstitial fibrosis. Furthermore, MRE did not detect a slight increase in cortical fibrosis observed in the RAS and ARAS CLK, suggesting a threshold for its sensitivity. In addition, in the stenotic kidneys the degrees of stenosis and fibrosis were very significant. The ability of MRE to detect changes in tissue stiffness at earlier stages or smaller degrees of stenosis needs to be investigated in future studies.
In summary, noninvasive 3D MRE effectively detected increased medullary stiffness secondary to RAS and ARAS in-vivo, which might serve as a surrogate for cortical fibrosis in the post-stenotic kidney. Because MRE stiffness values correlate well with the degree of tissue damage, it could potentially be used to track disease progression as well. This study therefore supports development of MRE methods to detect and monitor kidney fibrosis in renovascular disease. Further studies are needed to examine the utility of this approach in humans.
Acknowledgments
This study was supported in part by NIH grants DK73608, HL77131, HL085307, and EB001981.
Bibliography
- 1.Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens. 2010 Nov;23(11):1159–1169. doi: 10.1038/ajh.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis. 2009 Nov-Dec;52(3):196–203. doi: 10.1016/j.pcad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annu Rev Biomed Eng. 2003;5:57–78. doi: 10.1146/annurev.bioeng.5.040202.121623. [DOI] [PubMed] [Google Scholar]
- 4.Yin M, Chen J, Glaser KJ, Talwalkar JA, Ehman RL. Abdominal magnetic resonance elastography. Top Magn Reson Imaging. 2009 Apr;20(2):79–87. doi: 10.1097/RMR.0b013e3181c4737e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Streitberger KJ, Sack I, Krefting D, et al. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. PLoS One. 2012;7(1):e29888. doi: 10.1371/journal.pone.0029888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner L, Yin M, Glaser KJ, et al. Noninvasive In vivo assessment of renal tissue elasticity during graded renal ischemia using MR elastography. Invest Radiol. 2011 Aug;46(8):509–514. doi: 10.1097/RLI.0b013e3182183a95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CU, Glockner JF, Glaser KJ, et al. MR Elastography in Renal Transplant Patients and Correlation with Renal Allograft Biopsy: A Feasibility Study. Acad Radiol. 2012 Apr 13; doi: 10.1016/j.acra.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol. 1999 Jul;10(7):1455–1465. doi: 10.1681/ASN.V1071455. [DOI] [PubMed] [Google Scholar]
- 9.Ebrahimi B, Gloviczki M, Woollard JR, Crane JA, Textor SC, Lerman LO. Compartmental analysis of renal BOLD MRI data: Introduction and validation. Invest Radiol. 2012;47(3):175–182. doi: 10.1097/RLI.0b013e318234e75b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol. 2001 Oct;281(4):F630–638. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 11.Daghini E, Primak AN, Chade AR, et al. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology. 2007 May;243(2):405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 12.Nangaku M, Alpers CE, Pippin J, et al. CD59 protects glomerular endothelial cells from immune-mediated thrombotic microangiopathy in rats. J Am Soc Nephrol. 1998 Apr;9(4):590–597. doi: 10.1681/ASN.V94590. [DOI] [PubMed] [Google Scholar]
- 13.Eirin A, Zhu XY, Krier JD, et al. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012 May;30(5):1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urbieta-Caceres VH, Lavi R, Zhu XY, et al. Early atherosclerosis aggravates the effect of renal artery stenosis on the swine kidney. Am J Physiol Renal Physiol. 2010 Jul;299(1):F135–140. doi: 10.1152/ajprenal.00159.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yildirim E, Kirbas I, Teksam M, Karadeli E, Gullu H, Ozer I. Diffusion-weighted MR imaging of kidneys in renal artery stenosis. Eur J Radiol. 2008 Jan;65(1):148–153. doi: 10.1016/j.ejrad.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Cova M, Squillaci E, Stacul F, et al. Diffusion-weighted MRI in the evaluation of renal lesions: preliminary results. Br J Radiol. 2004 Oct;77(922):851–857. doi: 10.1259/bjr/26525081. [DOI] [PubMed] [Google Scholar]
- 17.Chandarana H, Lee VS, Hecht E, Taouli B, Sigmund EE. Comparison of biexponential and monoexponential model of diffusion weighted imaging in evaluation of renal lesions: preliminary experience. Invest Radiol. 2011 May;46(5):285–291. doi: 10.1097/RLI.0b013e3181ffc485. [DOI] [PubMed] [Google Scholar]
- 18.Giannarini G, Petralia G, Thoeny HC. Potential and limitations of diffusion-weighted magnetic resonance imaging in kidney, prostate, and bladder cancer including pelvic lymph node staging: a critical analysis of the literature. Eur Urol. 2012 Feb;61(2):326–340. doi: 10.1016/j.eururo.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Amador C, Urban MW, Warner LV, Greenleaf JF. In vitro renal cortex elasticity and viscosity measurements with Shearwave Dispersion Ultrasound Vibrometry (SDUV) on swine kidney. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4428–4431. doi: 10.1109/IEMBS.2009.5332750. [DOI] [PubMed] [Google Scholar]
- 20.Rosenkrantz AB, Storey P, Gilet AG, et al. Magnetization transfer contrast-prepared MR imaging of the liver: inability to distinguish healthy from cirrhotic liver. Radiology. 2012 Jan;262(1):136–143. doi: 10.1148/radiol.11111043. [DOI] [PubMed] [Google Scholar]
- 21.Talwalkar JA, Yin M, Fidler JL, Sanderson SO, Kamath PS, Ehman RL. Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology. 2008 Jan;47(1):332–342. doi: 10.1002/hep.21972. [DOI] [PubMed] [Google Scholar]
- 22.Yin M, Woollard J, Wang X, et al. Quantitative assessment of hepatic fibrosis in an animal model with magnetic resonance elastography. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2007 Aug;58(2):346–353. doi: 10.1002/mrm.21286. [DOI] [PubMed] [Google Scholar]
- 23.Binkovitz LA, El-Youssef M, Glaser KJ, Yin M, Binkovitz AK, Ehman RL. Pediatric MR elastography of hepatic fibrosis: principles, technique and early clinical experience. Pediatr Radiol. 2012 Apr;42(4):402–409. doi: 10.1007/s00247-011-2298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hines CD, Lindstrom MJ, Varma AK, Reeder SB. Effects of postprandial state and mesenteric blood flow on the repeatability of MR elastography in asymptomatic subjects. J Magn Reson Imaging. 2011 Jan;33(1):239–244. doi: 10.1002/jmri.22354. [DOI] [PubMed] [Google Scholar]
- 25.Lerman LO, Taler SJ, Textor SC, Sheedy PF, 2nd, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int. 1996 Mar;49(3):846–854. doi: 10.1038/ki.1996.117. [DOI] [PubMed] [Google Scholar]