Abstract
The purpose of this study was to assess tendon metabolism and suture pull-out strength after simple tendon suture in a tissue culture model. One hundred and twelve flexor digitorum profundus tendons from 28 dogs were cultured for 7, 14, or 21 days with or without a static tensile load. In both groups increased levels of matrix metalloproteinase (MMP) mRNA was noted. Suture pull-out strength did not decrease during tissue culture. While the presence of a static load had no effect on the pull-out strength, it did affect MMP mRNA expression. This tissue culture model could be useful in studying the effect of factors on the tendon-suture interface.
Keywords: tissue culture, matrix metalloproteinases, tendon repair, suture strength
Introduction
In flexor tendon repair, it is important to maintain the strength of the suture repair in order to tolerate the repetitive force applied to the tendon during postoperative rehabilitation. The consequence of weakness at the tendon repair site is often tendon gapping, tendon rupture, or suture pull-out. The mechanical properties of repaired tendon have been reported from in vivo studies (Aoki et al., 1997; Boyer et al., 2001; Hitchcock et al., 1987; Silva et al., 1999; Strickland, 2000; Takai et al., 1991; Wada et al., 2001; Wagner et al., 1994; Winters et al., 1998; Zhao et al., 2005), which document a period of repair weakening in the first few weeks after repair. The cause of this weakening is thought to be due to collagen degradation at the repair site. Several studies have demonstrated that loading applied to the tendon is important in preventing this weakening in the early phases of tendon healing (Takai et al., 1991; Wada et al., 2001). The effect of the suture on this weakening is unknown.
In vivo models include many confounding factors that may influence the strength of the repaired tendon, such as inflammation and blood supply, as well as the tension applied to the tendon and repair technique. Therefore, in vivo models are not well adapted to study the specific issue of the suture-tendon interface. To address this limitation, we sought to develop a tissue culture model where the tendon-suture interface could be studied in isolation. A tissue culture model has many advantages in studying interactions of living tissue in a controlled environment. The physical and chemical milieu can be varied in precise ways. Such models are also parsimonious in their use of experimental animals.
The purpose of this study was to assess the effect of tissue culture on tendon metabolism and suture pull-out strength, in order to develop an ex vivo model for studying the tendon-suture interaction. We hypothesised that in this model the tendon would show evidence of catabolism and the pull-out strength of the suture would decrease with increasing time in tissue culture. We also hypothesised that application of a static tensile load during tissue culture would increase the holding strength of the suture and would change the pattern of gene expression related to tendon metabolism.
Materials and Methods
Tissue culture
Flexor digitorum profundus (FDP) tendons were harvested from 28 mixed-breed dogs weighing between 25 and 30kg. The dogs had been euthanised for other studies, not affecting these tendons, approved by our Institutional Animal Care and Use Committee. Immediately after euthanasia, the FDP tendons of the third and fourth toes of both hind paws were harvested under sterile conditions. Sixteen FDP tendons were used for each of seven groups, including day-0 (no culture), day 7-load, day 14-load, day 21-load, day 7-non-load, day 14-non-load, and day 21-non-load, for a total of 112 tendons (Table 1). All the loaded tendons were from right paws and all the unloaded tendons were from left paws. Previous studies have shown that there is no significant right-left difference in canine tendon material properties (Branson and Rogers, 2006; Poyser et al., 2006).
Table 1. Sample sizes of experimental groups and controls in this study.
| Evaluations | Experimental groups | Controls | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Culture with load | Culture without load | No culture | |||||
|
|
|
||||||
| Day 7 | Day 14 | Day 21 | Day 7 | Day 14 | Day 21 | Day 0 | |
| Pull-out | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 |
| PCR | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 |
| Histology | n = 2 | n = 2 | n = 2 | n = 2 | n = 2 | n = 2 | n = 2 |
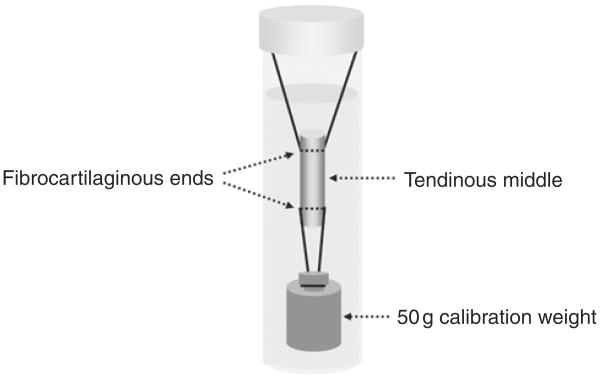
Segments 30 mm in length, centered on Okuda's zone D (Okuda et al., 1987), were harvested from each tendon. Before culture, the segments were washed three times using PBS in 50 mL polypropylene conical tubes (FALCON, Franklin Lakes, NJ, USA). In the group with load application, the tensile load was applied to the tendon by a stainless steel calibration weight of 50 g attached to the tendon end by a 3-0 Ethibond® suture (Ethicon Inc., Somerville, NJ, USA) (Fig 1). The 50 g weight was chosen because it was used in previous studies which analysed the effect of loading on the material properties of canine FDP tendons in tissue culture (Hannafin et al., 1995; Tanaka et al., 2005). In the group without load application, the tendons were incubated in the medium in the same manner but without the weight connected. The medium consisted of minimal essential medium (MEM) with Earle's salts (GIBCO, Grand Island, NY, USA), 10% fetal calf serum and 5% antibiotics (Antibiotic-Antimycotic, GIBCO, Grand Island, NY, USA).
Fig 1.
Method of tissue culture with static tensile load.
The tendons were incubated at 37°C with 5% CO2 and 95% air at 100% humidity. The medium was changed every other day. Thirty-two tendons (16 from each group, with and without load) were removed from culture at day 7, 14, and 21.
Pull-out test
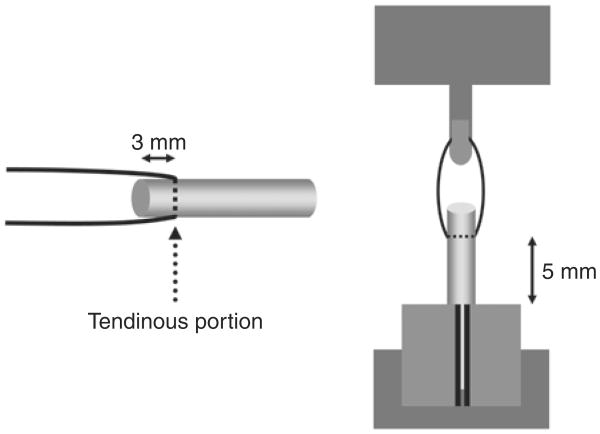
The maximum pull-out load and linear stiffness of the tendon were measured either immediately after euthanasia (Day 0, no culture), or after removal from culture (Day 7, 14, or 21) in seven tendons in each of the seven groups. Prior to testing, the width of the tendon was measured using a digital micro caliper (Mitsutoyo SC-6, Chiba, Japan) to eliminate the tendon diameter as a potential variable in breaking strength. Then, each specimen to be tested was sutured in the middle of the tendinous portion with a simple loop of 3-0 Ethibond® (Ethicon Inc., Somerville, NJ, USA) (Fig 2). The tendon was cut 3.0 mm distal to the suture site. The suture was attached to the tensile load transducer and the tendon was fixed to the bottom of the testing apparatus with a clamp. The length of the tendon from the sutured site to the clamped site was set at 5 mm (Fig 2). A 0.1N preload and 0.1 N–0.3N cyclic load were applied three times before testing. The suture construct was distracted at a rate of 0.2 mm per second. During testing, tendons were kept moist by using a saline spray.
Fig 2.
Schema of tendon suture (left) and pull-out measurement (right).
Gene expression
Gene expression was assessed using quantitative realtime reverse transcription-polymerase chain reaction (RT-PCR) in seven tendons in each of the seven groups. The tendons were put into liquid nitrogen and stored at −86°C. Each tendon segment was homogenised in a MIKRO DISMEMBRATOR (B. Braun Biotech International) at 1500 rpm for 30 minutes in 1.0 mL TRIzol® reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was extracted with chloroform and isopropyl alcohol, mixed with DNase I with Protector RNase inhibitor (Roche, Indianapolis, IN, USA) and purified with an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). The concentration of RNA was measured with a RiboGreen RNA Quantification Kit (Molecular Probes, Eugene, OR, USA). cDNA was synthesised using Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, USA) with the random primer. Quantitative PCR was performed with a LightCycler (Roche, Indianapolis, IN, USA) to study canine collagen type I, collagen type III, matrix metalloproteinases (MMP) −2, 3, 9, 13, and 14, tissue inhibitors of matrix metalloproteinase (TIMP) 1 and 2 and GAPDH. The expression of each gene was normalised to that of GAPDH. Primers were obtained from LightCycler Probe Design software 2.0 (Roche, Indianapolis, IN, USA) or the database of sequences in PubMed Nucleotide. The appropriate setting for PCR was determined by gel separation as 60°C of annealing temperature in 4.0 mM of MgCl2 and the sequences of the PCR product were confirmed by DNA sequencing in each case. The sequences of the primers we used are shown in Table 2.
Table 2. Primer sequences, the lengths of the amplified gene segments, and the conditions for the real-time PCR reactions.
| Gene | Forward primer sequence Reverse primer sequence | Length of amplified gene segment | Conditions for real-time PCR reactions |
|---|---|---|---|
| GAPDH | 5′-TATGATTCTACCCACGGCAA-3′ | 154 base pairs | [Mg2+] = 3.5mM |
| 5′-CAGTGGACTCCACAACATAC-3′ | Annealing temperature = 60°C | ||
| Collagen Type I | 5′-TGGTTCTCCTGGCAAAGAT-3′ | 232 base pairs | [Mg2+] = 3.5mM |
| 5′-ATCACCGGGTTCACCTTTA-3′ | Annealing temperature = 60°C | ||
| Collagen Type III | 5′-ACAGCAGCAAGCTATTGAT-3′ | 156 base pairs | [Mg2+] = 3.5mM |
| 5′-GGACAGTCTAATTCTTGTTCGT-3′ | Annealing temperature = 60°C | ||
| MMP2 | 5′-AGCTACTTCTTCAAGGGTG-3′ | 150 base pairs | [Mg2+] = 4.0mM |
| 5′-GTGTGCAGAAGGCAATG-3′ | Annealing temperature = 60°C | ||
| MMP3 | 5′-GGAGAGGCTGACATAAAGATT-3′ | 161 base pairs | [Mg2+] = 4.0mM |
| 5′-GATGTATCGCTTGTCCATTG-3′ | Annealing temperature = 60°C | ||
| MMP9 | 5′-TGCCTGAGACTGGAGAG-3′ | 159 base pairs | [Mg2+] = 4.0mM |
| 5′-GCAAGTCTTCCGAGTAGTT-3′ | Annealing temperature = 60°C | ||
| MMP13 | 5′-TACAACTTGTTCCTTGTCGC-3′ | 166 base pairs | [Mg2+] = 4.0mM |
| 5′-CTGGGCCATAGAGAGACT-3′ | Annealing temperature = 60°C | ||
| MMP14 | 5′-AAGCACTGGGTGTTTGAT-3′ | 169 base pairs | [Mg2+] = 4.0mM |
| 5′-GTTCCTCGTTGAAACGGT-3′ | Annealing temperature = 60°C | ||
| TIMP1 | 5′-CAGCGAGGAGTTTCTGG-3′ | 157 base pairs | [Mg2+] = 4.0mM |
| 5′-GGTAAACACTGTGCACCC-3′ | Annealing temperature = 60° C | ||
| TIMP2 | 5′-GAGGAAAGAAGG AGTATCTCATTG-3′ | 195 base pairs | [Mg2+]=4.0mM |
| 5′-CCGGAGACGAGATATAGC-3′ | Annealing temperature = 60° C |
Histological assessment
Fourteen tendons, two from each group, were used for the histological assessment. The tendons were fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned longitudinally at a thickness of 5 μm. The sections were stained with hematoxylin and eosin. The alignment of the tendon fibres and the distribution and number of cells were assessed by light microscopy.
Statistics
The results of the pull-out test (tendon width, maximum pull-out load and linear stiffness) and the PCR test were analysed statistically. Two types of analysis were performed. A one-way ANOVA and post hoc (Bonferroni/Dunn) tests were used for the analysis of results between the different time points, as these compared the results between specimens from different donor animals. A paired t-test was used for the analysis of the results between with and without load groups at each time point, as the specimens were indeed paired right-left from the same animals. Results of ANOVA and paired t-test with P values less than 0.05 were considered significant.
Results
Pull-out test
The tendon width was 3.1±0.1mm (Day 0), 3.1±0.2mm (Day 7 with load), 3.2±0.1mm (Day 7 without load), 3.2±0.2mm (Day 14 with load), 3.3±0.1mm (Day 14 without load), 3.1±0.2mm (Day 21 with load), 3.3±0.2mm (Day 21 without load). None of these differences were significant. The maximum failure load for suture pull-out was 8.0±2.0N (Day 0, average±standard deviation), 7.8±1.5N (Day 7 with load), 7.0±1.3N (Day 7 without load), 7.3±1.3N (Day 14 with load), 7.9±2.1N (Day 14 without load), 6.8±1.5N (Day 21 with load), 7.3±1.6N (Day 21 without load). All sutures pulled out from the tendons, without failure of the tendons at the clamping site. Overall, there was no significant difference in maximum failure force for the loaded and unloaded groups, and no significant difference was observed among the time points. Linear stiffness was 2.8±0.4N/mm (Day 0), 2.8±0.7N/mm (Day 7 with load), 2.6±0.7 N/mm (Day 7 without load), 2.4±0.6N/mm (Day 14 with load), 2.7±0.5N/mm (Day 14 without load), 2.6±0.6N/mm (Day 21 with load), 2.4±0.2N/mm (Day 21 without load). Again, there were no significant differences in stiffness in the loaded and unloaded groups and no significant differences among the time points. When the results were analysed at each time point, the maximum failure force and linear stiffness also showed no significant differences between loaded and unloaded groups.
Gene expression
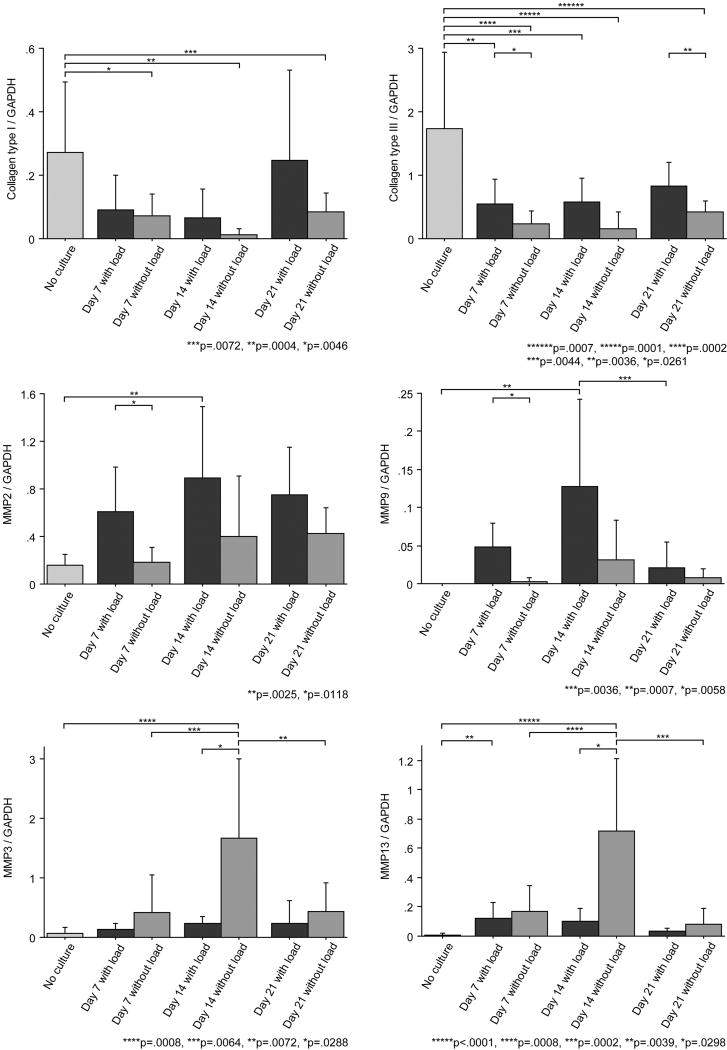
Collagen types I and III expression was decreased in all cultured tendons when compared to Day 0 uncultured tendons. Expression in unloaded tendons was significantly lower than control tendon expression at all time points. In the loaded tendons, collagen type III expression at day 7 and 14 was also significantly lower than that at that of control tendons. When comparing tendons with load and without load at each time point, collagen type III expression was significantly higher in tendons cultured under constant load than those without load at day 7 and 21 (Fig 3). However, there was no significant difference in type I collagen expression between loaded and unloaded tendons.
Fig 3.
Results of gene expression levels normalised to GAPDH.
MMP and TIMP expression
MMP2 and MMP9 had a similar pattern of gene expression. In the loaded tendons, expression of both MMP2 and MMP9 at day 14 was significantly higher than that of control tendons, and MMP2 and MMP9 expression with load was significantly higher than that without load at day 7. Gene expression of MMP2 and MMP9 in the non-loaded tendons was not significantly different from control tendons.
MMP3 and MMP13 had a similar pattern of gene expression. Without loading, both MMP3 and MMP13 expression at day 14 were significantly higher than those of control and day 7 and 21 tendons. Expression of MMP3 and MMP13 without loading was significantly higher than that of loaded tendons at day 14. MMP13 expression of loaded tendons at day 7 was significantly higher than that of the control, but the expression of MMP 3 and 14 was not significantly different when comparing cultured and uncultured tendons, with or without load.
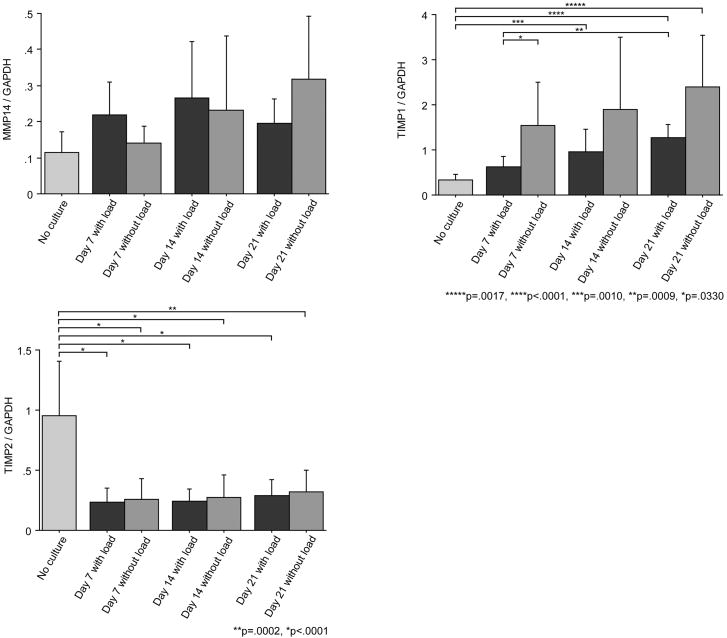
TIMP1 and TIMP2 showed different patterns of gene expressions. TIMP1 expressions during culture (Day 21 in both groups and Day 14 with load group) were higher than that of the control (Day 0), while TIMP2 expressions during culture were lower than that of the control (Day 0) (Fig 3).
Histological assessment
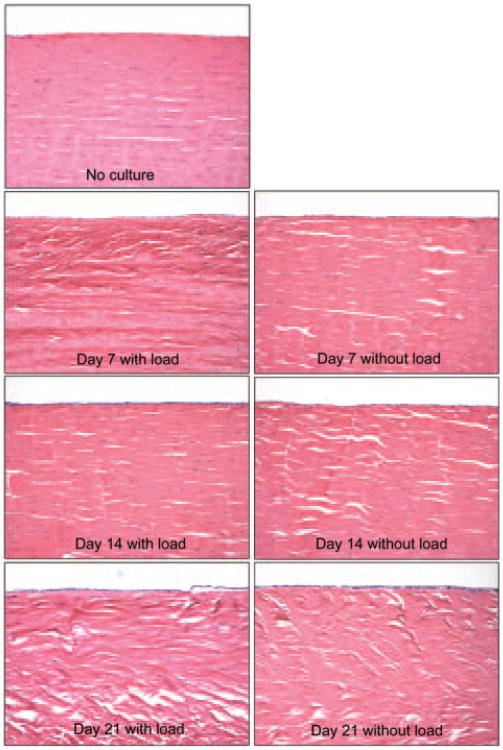
Although a quantitative assessment of the number of the cells was not performed, by gross observation the number of cells inside the tendon generally appeared to decrease with time in loaded and unloaded tendons, while on the surface of the tendon, the number of cells appeared to increase over time. The degree of the decrease in number of the cells inside the tendon and the increase in number of cells on the surface of the tendon seemed larger in the loaded tendons compared to the unloaded group. Over time the alignment of tendon fibres became irregular compared with the alignment of fresh tendon, especially in the unloaded tendons (Fig 4).
Fig 4.
Histological images. Original magnification ×100.
Discussion
Repair strength is one of the most important factors influencing the outcome after flexor tendon injury (Caulfield et al., 2008; Hoffmann et al., 2008; Navali and Rouhani, 2008; Tang, 2007). Many studies have demonstrated that repaired tendons commonly fail at the tendon suture interface (Lee, 1990; Pennington, 1979; Tanaka et al., 2004). Yet, while a catabolic tendon response after injury or overuse has been well documented with increasing MMP activity (Aoki et al., 2004; Arnoczky et al., 2007; Dovan et al., 2005; Oshiro et al., 2003; Riley, 2005) the effect of the catabolic activity on repair strength has been debated. Some in vivo studies have demonstrated that the repair strength within 3 weeks is not changed compared to the time 0 data (Aoki et al., 1997; Boyer et al., 2001; Silva et al., 1999; Tang et al., 2008; Wagner et al., 1994; Zhao et al., 2005). Other reports have noted that repair strength is decreased at 3 weeks compared to the initial repair strength if the repair tendons were immobilised, but not if they were mobilised (Hitchcock et al., 1987; Strickland, 2000; Wada et al., 2001). In the current study, although a catabolic response of the cultured tendon was observed, the holding strength at the tendon-suture interface was not significantly affected by time in culture or exposure to ongoing static load, simulating the baseline load of an immobilised tendon, experiencing only resting muscle tension (Tanaka et al., 2005).
MMPs are enzymes related to the degradation and remodeling of the extracellular matrix (ECM) (Sternlicht and Werb, 2001). MMP2 (gelatinase A) is secreted from fibroblasts and degrades denatured collagens. MMP9 (gelatinase B) also degrades denatured collagens and is secreted from inflammatory cells (Armstrong and Jude, 2002). MMP13 (collagenase 3) cleaves the triple helix of undenatured fibrillar collagens (Armstrong and Jude, 2002; Sternlicht and Werb, 2001). MMP3 (stromelysin 1) activates other MMPs and thus also plays an important role in ECM remodeling (Ireland et al., 2001). MMP14 exists on cell membranes and has been implicated as a key player in a proteinase cascade involving MMP2, MMP9, and MMP13 (Sternlicht and Werb, 2001).
Oshiro et al. (2003) reported that the expression of MMP2, MMP3, MMP9, MMP13, and MMP14 increased 2 weeks after repair in a rat flexor tendon model. This was associated with histological evidence of collagen degradation. Other studies have reported an increase in MMP2 (Hosaka et al., 2005), MMP9 (Hosaka et al., 2005; Jones et al., 2006), and MMP13 (Nomura et al., 2007) expression in models of chronic tendonitis.
In the current study, the expression of MMP2 and MMP9 in loaded tendons and MMP3 and MMP13 in unloaded tendons was significantly higher after 2 weeks in tissue culture than that of the fresh tendons. This suggests that both loaded and unloaded tendons experienced some degree of tendon catabolism during tissue culture. Within this overall effect, there were differences in specific MMPs that are of interest. In loaded tendons the MMPs that were increased were those with a primary effect on denatured collagen, while the MMPs whose expression increased in the unloaded tendons primarily affect undenatured collagen.
TIMPs are inhibitors of MMPs, and play an important role in regulating extracellular matrix turnover and tissue degradation. TIMP1 has been identified as a collagenase inhibitor, while TIMP2 is an important mediator of angiogenesis (Seo et al., 2003). Although TIMPs have been well studied in cell culture models, their expression has not been reported in an ex vivo tissue culture model such as ours. Our general impression from the current study is that collagen synthesis was down-regulated compared to the fresh tendon, while all MMPs were up-regulated, which indicated that matrix degradation was occurring. The increase in TIMP1 gene expression that we observed suggests a response to reduce collagen degradation. TIMP2, as a mediator of angiogenesis, might not play an important role in this ex vivo tissue culture model. The local balance of MMPs and TIMPs is likely to be of importance in the correct maintenance of tendon ECM and alterations to the synthetic-degradative equilibrium may underlie the degenerative changes observed in models of chronic tendon injury (Jones et al., 2006). Comparing loaded and unloaded tendons, there was a trend of increasing TIMP1 expression in unloaded tendons. Jones et al. (2006) reported a statistically significant difference in mRNA expression including higher levels of TIMP1 and lower levels of TIMP2 in ruptured human Achilles tendons. The gene expression patterns noted in that study (decreasing TIMP2 and increasing TIMP1), are similar to the findings in this study.
In the tendons cultured without load, the expression of collagen type I and collagen type III was significantly lower than in fresh tendon. However, the expression of collagen type I in loaded tendons was not significantly different from the fresh tendon. This might suggest that tensile load might help to stabilise collagen type I synthesis. However, this activity did not lead to any difference in suture pullout strength compared to the unloaded tendons. It is likely in our opinion that the pullout strength relates primarily to the collagen in the tendon at the time of animal sacrifice, and that 3 weeks in culture is too short a time for sufficient collagen catabolism to occur that might affect this native strength.
There are several limitations to the current study. First, we did not employ cyclic loading, which may have different effects compared to constant loading. Second, a single loop was used to evaluate the tendon holding strength, which differs from the suture techniques used clinically. However, as our goal was to investigate the effect of tendon catabolism on the strength of the tendon-suture interface, we believe that a single loop is a simpler yet still relevant model, in which the repair technique plays no role in determining the repair strength. Third, the degree of tendon catabolism in the tissue culture model may be different from the in vivo condition, due to the absence of vascular, inflammation and hormonal factors in vitro. Fourth, we did not analyse details of collagen metabolism such as the presence of collagen breakdown products, or any effect on collagen cross-linking on strength at the tendon-suture interface. Finally, we did not use immunohistochemistry staining to localise any cytokines or gene products in the tendon tissue.
Tendon catabolism was observed on qualitative histological analysis and by increasing levels of mRNA of matrix metalloproteinases (MMPs) in tissue culture. However, this catabolic response, detected at the molecular level, did not affect holding strength at the tendon/suture interface. The presence of a static load also had no effect on the pull-out strength, but had effects on the levels of mRNA of some MMPs. This tissue culture model would be useful in studying the effect of factors on the tendon-suture interface.
Acknowledgments
This study was funded by a grant from Mayo Foundation.
Footnotes
The online version of this article can be found at: http://jhs.sagepub.com/cgi/content/abstract/34/5/643
References
- Aoki M, Kubota H, Pruitt DL, Manske PR. Biomechanical and histologic characteristics of canine flexor tendon repair using early postoperative mobilization. J Hand Surg Am. 1997;22:107–14. doi: 10.1016/S0363-5023(05)80189-3. [DOI] [PubMed] [Google Scholar]
- Aoki M, Miyamoto S, Okamura K, Yamashita T, Ikada Y, Matsuda S. Tensile properties and biological response of poly(L-lactic acid) felt graft: an experimental trial for rotator-cuff reconstruction. J Biomed Mater Res B Appl Biomater. 2004;71:252–9. doi: 10.1002/jbm.b.30084. [DOI] [PubMed] [Google Scholar]
- Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc. 2002;92:12–8. doi: 10.7547/87507315-92-1-12. [DOI] [PubMed] [Google Scholar]
- Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sport Med. 2007;35:763–9. doi: 10.1177/0363546506296043. [DOI] [PubMed] [Google Scholar]
- Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Joint Surg Am. 2001;83:891–9. [PubMed] [Google Scholar]
- Branson NJ, Rogers LJ. Relationship between paw preference strength and noise phobia in Canis familiaris. J Comp Psychol. 2006;120:176–83. doi: 10.1037/0735-7036.120.3.176. [DOI] [PubMed] [Google Scholar]
- Caulfield RH, Maleki-Tabrizi A, Patel H, Coldham F, Mee S, Nanchahal J. Comparison of zones 1 to 4 flexor tendon repairs using absorbable and unabsorbable four-strand core sutures. J Hand Surg Eur. 2008;33:412–7. doi: 10.1177/1753193408090758. [DOI] [PubMed] [Google Scholar]
- Dovan TT, Ritty T, Ditsios K, Silva MJ, Kusano N, Gelberman RH. Flexor digitorum profundus tendon to bone tunnel repair: a vascularization and histologic study in canines. J Hand Surg Am. 2005;30:246–57. doi: 10.1016/j.jhsa.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13:907–14. doi: 10.1002/jor.1100130615. [DOI] [PubMed] [Google Scholar]
- Hitchcock TF, Light TR, Bunch WH, et al. The effect of immediate constrained digital motion on the strength of flexor tendon repairs in chickens. J Hand Surg Am. 1987;12:590–5. doi: 10.1016/s0363-5023(87)80213-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann GL, Büchler U, Vögelin E. Clinical results of flexor tendon repair in zone II using a six-strand double-loop technique compared with a two-strand technique. J Hand Surg Eur. 2008;33:418–23. doi: 10.1177/1753193408091570. [DOI] [PubMed] [Google Scholar]
- Hosaka Y, Ueda H, Yamasaki T, Suzuki D, Matsuda N, Takehana K. Structure and component alteration of rabbit Achilles tendon in tissue culture. Biomed Res. 2005;26:279–86. doi: 10.2220/biomedres.26.279. [DOI] [PubMed] [Google Scholar]
- Ireland D, Harrall R, Curry V, et al. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–69. doi: 10.1016/s0945-053x(01)00128-7. [DOI] [PubMed] [Google Scholar]
- Jones GC, Corps AN, Pennington CJ, et al. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006;54:832–42. doi: 10.1002/art.21672. [DOI] [PubMed] [Google Scholar]
- Lee H. Double loop locking suture: a technique of tendon repair for early active mobilization. Part II: Clinical experience. J Hand Surg Am. 1990;15:953–8. doi: 10.1016/0363-5023(90)90022-j. [DOI] [PubMed] [Google Scholar]
- Navali AM, Rouhani A. Zone 2 flexor tendon repair in young children: a comparative study of four-strand versus two-strand repair. J Hand Surg Eur. 2008;33:424–9. doi: 10.1177/1753193408090761. [DOI] [PubMed] [Google Scholar]
- Nomura M, Hosaka Y, Kasashima Y, et al. Active expression of matrix metalloproteinase-13 mRNA in the granulation tissue of equine superficial digital flexor tendinitis. J Vet Med Sci. 2007;69:637–9. doi: 10.1292/jvms.69.637. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Gorski JP, An KN, Amadio PC. Biochemical, histological, and biomechanical analyses of canine tendon. J Orthop Res. 1987;5:60–8. doi: 10.1002/jor.1100050109. [DOI] [PubMed] [Google Scholar]
- Oshiro W, Lou J, Xing X, Tu Y, Manske PR. Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg Am. 2003;28:814–23. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]
- Pennington DG. The locking loop tendon suture. Plast Reconstr Surg. 1979;63:648–52. doi: 10.1097/00006534-197905000-00007. [DOI] [PubMed] [Google Scholar]
- Poyser F, Caldwell C, Cobb M. Dog paw preference shows lability and sex differences. Behav Process. 2006;73:216–21. doi: 10.1016/j.beproc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Riley GP. Gene expression and matrix turnover in overused and damaged tendons. Scand J Med Sci Sports. 2005;15:241–51. doi: 10.1111/j.1600-0838.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- Seo DW, Li H, Guedez L, Wingfield PT, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–80. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Brodt MD, Boyer MI, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–83. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Ann Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg Am. 2000;25:214–35. doi: 10.1053/jhsu.2000.jhsu25a0214. [DOI] [PubMed] [Google Scholar]
- Takai S, Woo SL, Horibe S, Tung DK, Gelberman RH. The effects of frequency and duration of controlled passive mobilization on tendon healing. J Orthop Res. 1991;9:705–13. doi: 10.1002/jor.1100090510. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Amadio PC, Zhao C, Zobitz ME, An KN. Flexor digitorum profundus tendon tension during finger manipulation. J Hand Ther. 2005;18:330–8. doi: 10.1197/j.jht.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Amadio PC, Zhao C, Zobitz ME, Yang C, An KN. Gliding characteristics and gap formation for locking and grasping tendon repairs: a biomechanical study in a human cadaver model. J Hand Surg Am. 2004;29:6–14. doi: 10.1016/j.jhsa.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Tang JB. Indications, methods, postoperative motion and outcome evaluation of primary flexor tendon repairs in Zone 2. J Hand Surg Eur. 2007;32:118–29. doi: 10.1016/J.JHSB.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Tang JB, Cao Y, Zhu B, Xin KQ, Wang XT, Liu PY. Adeno-associated virus-2-mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength. an in vivo study. J Bone Joint Surg Am. 2008;90:1078–89. doi: 10.2106/JBJS.F.01188. [DOI] [PubMed] [Google Scholar]
- Wada A, Kubota H, Miyanishi K, Hatanaka H, Miura H, Iwamoto Y. Comparison of postoperative early active mobilization and immobilization in vivo utilizing a four-strand flexor tendon repair. J Hand Surg Br. 2001;26:301–6. doi: 10.1054/jhsb.2000.0547. [DOI] [PubMed] [Google Scholar]
- Wagner WF, Jr, Carroll Ct, Strickland JW, Heck DA, Toombs JP. A biomechanical comparison of techniques of flexor tendon repair. J Hand Surg Am. 1994;19:979–83. doi: 10.1016/0363-5023(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Winters SC, Gelberman RH, Woo SL, Chan SS, Grewal R, Seiler JG., 3rd The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: a biomechanical study in dogs. J Hand Surg Am. 1998;23:97–104. doi: 10.1016/s0363-5023(98)80096-8. [DOI] [PubMed] [Google Scholar]
- Zhao C, Amadio PC, Tanaka T, et al. Short-term assessment of optimal timing for postoperative rehabilitation after flexor digitorum profundus tendon repair in a canine model. J Hand Ther. 2005;18:322–9. doi: 10.1197/j.jht.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]