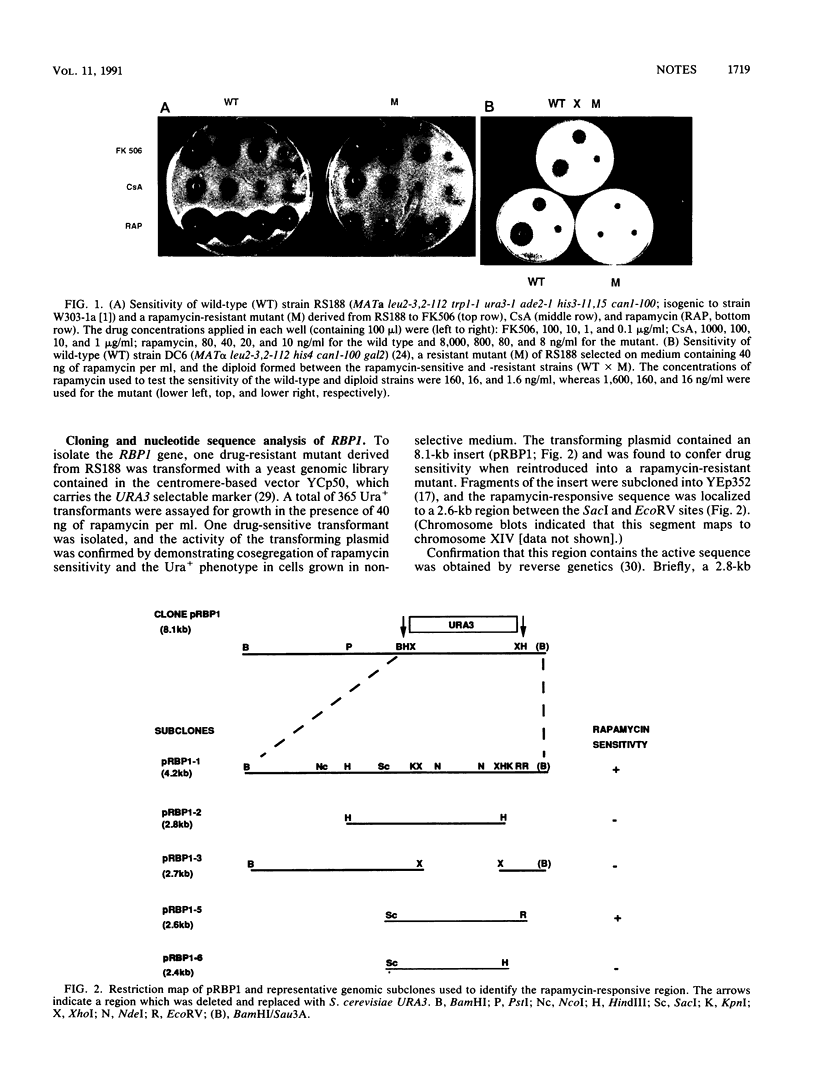
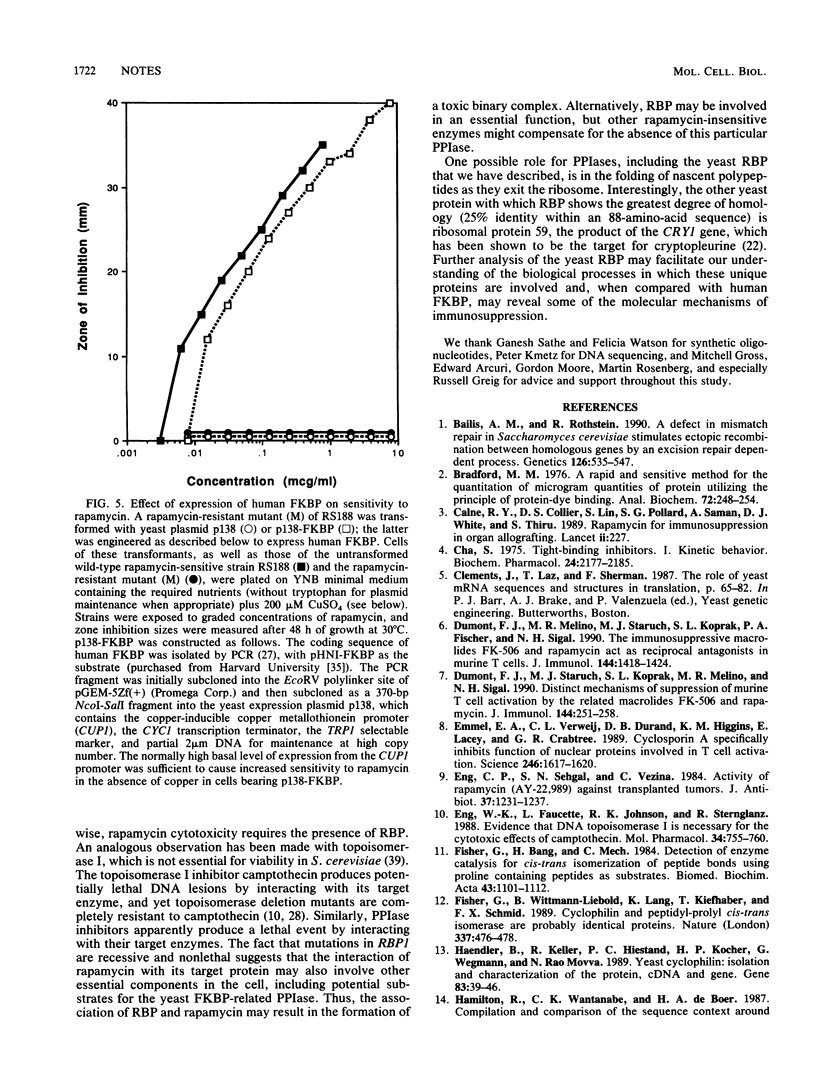
Abstract
Rapamycin is a macrolide antifungal agent with structural similarity to FK506. It exhibits potent immunosuppressive properties analogous to those of both FK506 and cyclosporin A (CsA). Unlike FK506 and CsA, however, rapamycin does not inhibit the transcription of early T-cell activation genes, including interleukin-2, but instead appears to block downstream events leading to T-cell activation. FK506 and CsA receptor proteins (FKBP and cyclophilin, respectively) have been identified and shown to be distinct members of a class of enzymes that possess peptidyl-prolyl cis-trans isomerase (PPIase) activity. Despite the apparent differences in their mode of action, rapamycin and FK506 act as reciprocal antagonists in vivo and compete for binding to FKBP. As a means of rapidly identifying a target protein for rapamycin in vivo, we selected and genetically characterized rapamycin-resistant mutants of Saccharomyces cerevisiae and isolated a yeast genomic fragment that confers drug sensitivity. We demonstrate that the resonse to rapamycin in yeast cells is mediated by a gene encoding a 114-amino-acid, approximately 13-kDa protein which has a high degree of sequence homology with human FKBP; we designated this gene RBP1 (for rapamycin-binding protein). The RBP1 protein (RBP) was expressed in Escherichia coli, purified to homogeneity, and shown to catalyze peptidyl-prolyl isomerization of a synthetic peptide substrate. PPIase activity was completely inhibited by rapamycin and FK506 but not by CsA, indicating that both macrolides bind to the recombinant protein. Expression of human FKBP in rapamycin-resistant mutants restored rapamycin sensitivity, indicating a functional equivalence between the yeast and human enzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailis A. M., Rothstein R. A defect in mismatch repair in Saccharomyces cerevisiae stimulates ectopic recombination between homeologous genes by an excision repair dependent process. Genetics. 1990 Nov;126(3):535–547. doi: 10.1093/genetics/126.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calne R. Y., Collier D. S., Lim S., Pollard S. G., Samaan A., White D. J., Thiru S. Rapamycin for immunosuppression in organ allografting. Lancet. 1989 Jul 22;2(8656):227–227. doi: 10.1016/s0140-6736(89)90417-0. [DOI] [PubMed] [Google Scholar]
- Cha S. Tight-binding inhibitors-I. Kinetic behavior. Biochem Pharmacol. 1975 Dec 1;24(23):2177–2185. doi: 10.1016/0006-2952(75)90050-7. [DOI] [PubMed] [Google Scholar]
- Dumont F. J., Melino M. R., Staruch M. J., Koprak S. L., Fischer P. A., Sigal N. H. The immunosuppressive macrolides FK-506 and rapamycin act as reciprocal antagonists in murine T cells. J Immunol. 1990 Feb 15;144(4):1418–1424. [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Koprak S. L., Melino M. R., Sigal N. H. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990 Jan 1;144(1):251–258. [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Eng C. P., Sehgal S. N., Vézina C. Activity of rapamycin (AY-22,989) against transplanted tumors. J Antibiot (Tokyo) 1984 Oct;37(10):1231–1237. doi: 10.7164/antibiotics.37.1231. [DOI] [PubMed] [Google Scholar]
- Eng W. K., Faucette L., Johnson R. K., Sternglanz R. Evidence that DNA topoisomerase I is necessary for the cytotoxic effects of camptothecin. Mol Pharmacol. 1988 Dec;34(6):755–760. [PubMed] [Google Scholar]
- Fischer G., Bang H., Mech C. Nachweis einer Enzymkatalyse für die cis-trans-Isomerisierung der Peptidbindung in prolinhaltigen Peptiden. Biomed Biochim Acta. 1984;43(10):1101–1111. [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Haendler B., Keller R., Hiestand P. C., Kocher H. P., Wegmann G., Movva N. R. Yeast cyclophilin: isolation and characterization of the protein, cDNA and gene. Gene. 1989 Nov 15;83(1):39–46. doi: 10.1016/0378-1119(89)90401-0. [DOI] [PubMed] [Google Scholar]
- Hamilton R., Watanabe C. K., de Boer H. A. Compilation and comparison of the sequence context around the AUG startcodons in Saccharomyces cerevisiae mRNAs. Nucleic Acids Res. 1987 Apr 24;15(8):3581–3593. doi: 10.1093/nar/15.8.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Houchens D. P., Ovejera A. A., Riblet S. M., Slagel D. E. Human brain tumor xenografts in nude mice as a chemotherapy model. Eur J Cancer Clin Oncol. 1983 Jun;19(6):799–805. doi: 10.1016/0277-5379(83)90012-3. [DOI] [PubMed] [Google Scholar]
- Kino T., Hatanaka H., Hashimoto M., Nishiyama M., Goto T., Okuhara M., Kohsaka M., Aoki H., Imanaka H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo) 1987 Sep;40(9):1249–1255. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- Koser P. L., Sylvester D., Livi G. P., Bergsma D. J. A second cyclophilin-related gene in Saccharomyces cerevisiae. Nucleic Acids Res. 1990 Mar 25;18(6):1643–1643. doi: 10.1093/nar/18.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J. C., Thompson J. R., Woolford J. L., Jr Structure and expression of the Saccharomyces cerevisiae CRY1 gene: a highly conserved ribosomal protein gene. Mol Cell Biol. 1987 May;7(5):1764–1775. doi: 10.1128/mcb.7.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Walsh C. T. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4028–4032. doi: 10.1073/pnas.87.11.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livi G. P., Hicks J. B., Klar A. J. The sum1-1 mutation affects silent mating-type gene transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Jan;10(1):409–412. doi: 10.1128/mcb.10.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki N., Sekiguchi F., Nishimaki J., Miwa K., Hayano T., Takahashi N., Suzuki M. Complementary DNA encoding the human T-cell FK506-binding protein, a peptidylprolyl cis-trans isomerase distinct from cyclophilin. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5440–5443. doi: 10.1073/pnas.87.14.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel R. R., Klicius J., Galet S. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can J Physiol Pharmacol. 1977 Feb;55(1):48–51. doi: 10.1139/y77-007. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nitiss J., Wang J. C. DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7501–7505. doi: 10.1073/pnas.85.20.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sawada S., Suzuki G., Kawase Y., Takaku F. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J Immunol. 1987 Sep 15;139(6):1797–1803. [PubMed] [Google Scholar]
- Sehgal S. N., Baker H., Vézina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975 Oct;28(10):727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- Shatzman A. R., Rosenberg M. The pAS vector system and its application to heterologous gene expression in Escherichia coli. Hepatology. 1987 Jan-Feb;7(1 Suppl):30S–35S. doi: 10.1002/hep.1840070706. [DOI] [PubMed] [Google Scholar]
- Siekierka J. J., Hung S. H., Poe M., Lin C. S., Sigal N. H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989 Oct 26;341(6244):755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- Standaert R. F., Galat A., Verdine G. L., Schreiber S. L. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990 Aug 16;346(6285):671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Woo J. Immunosuppressive properties of FK-506 and rapamycin. Lancet. 1989 Aug 19;2(8660):443–444. doi: 10.1016/s0140-6736(89)90616-8. [DOI] [PubMed] [Google Scholar]
- Thrash C., Bankier A. T., Barrell B. G., Sternglanz R. Cloning, characterization, and sequence of the yeast DNA topoisomerase I gene. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4374–4378. doi: 10.1073/pnas.82.13.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropschug M., Barthelmess I. B., Neupert W. Sensitivity to cyclosporin A is mediated by cyclophilin in Neurospora crassa and Saccharomyces cerevisiae. Nature. 1989 Dec 21;342(6252):953–955. doi: 10.1038/342953a0. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Wachter E., Mayer S., Schönbrunner E. R., Schmid F. X. Isolation and sequence of an FK506-binding protein from N. crassa which catalyses protein folding. Nature. 1990 Aug 16;346(6285):674–677. doi: 10.1038/346674a0. [DOI] [PubMed] [Google Scholar]
- Vézina C., Kudelski A., Sehgal S. N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975 Oct;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]