Abstract
There is much support for the role of Gamma-Aminobutyric acid (GABA) in the etiology of autism. Recent research has shown that hepatocyte growth factor (HGF) modulates GABAergic inhibition and seizure susceptibility. This study was designed to determine and correlate plasma levels of HGF, GABA, as well as symptom severity, in autistic children and neurotypical controls. Plasma from 48 autistic children and 29 neurotypical controls was assessed for HGF and GABA concentration using ELISAs. Symptom severity was assessed in these autistic individuals and compared to HGF and GABA concentrations. We previously reported that autistic children had significantly decreased levels of HGF. In this study, the same autistic children had significantly increased plasma levels of GABA (P = 0.002) and decreased HGF levels correlated with these increased GABA levels (r = 0.3; P = 0.05). High GABA levels correlated with increasing hyperactivity (r = 0.6; P = 0.0007) and impulsivity severity (r = 0.5; P = 0.007), tip toeing severity (r = 0.35; P = 0.03), light sensitivity (r = 0.4; P = 0.02), and tactile sensitivity (r = 0.4; P = 0.01). HGF levels did not correlate significantly with any symptom severity. These results suggest an association between HGF and GABA levels and suggest that plasma GABA levels are related to symptom severity in autistic children.
Keywords: GABA, HGF, autism, symptom severity
Introduction
Autism spectrum disorders (ASDs) comprise a complex and heterogeneous group of pathological conditions characterized by impaired social interactions, deficits in verbal and nonverbal communication, and a limited interest in the surrounding environment associated with stereotyped and repetitive behaviors.1
Evidence suggests that an impairment of GABAergic transmission contributes to the development of ASDs.2–5 While in the mature brain gamma-aminobutyric acid (GABA) acts as an inhibitory transmitter, during the embryonic and the perinatal period, it depolarizes targeted cells and triggers calcium influx, regulating different developmental processes from cell proliferation migration, differentiation, synapse maturation, and cell death.6
Hepatocyte growth factor (HGF), an 82 kDa, 674 amino acid residue heterodimeric glycoprotein, was originally isolated from rat platelets.7,8 This growth factor has also been called scatter factor, hepatopoietin A, and mammary growth factor.9 It is one of a small family of factors lacking significant homology with other known growth factors, while including an HGF-like factor known as macrophage stimulating protein (MSP).10–13 HGF has mitogenic, morphogenic, and motogenic effects on hepatocytes, as well as endothelial, mesenchymal, and hematopoietic cell types,12,14,15 and demonstrates noticeable species cross-reactivity.16
HGF regulates cell growth, cell motility, and morphogenesis by activating a tyrosine kinase-signaling cascade after binding to the proto-oncogenic c-Met receptor (translated by the MET gene). HGF is secreted by mesenchymal cells and, although it was first considered to exert biological effects only on specific target cells, it has since been demonstrated to mediate inflammatory responses to tissue injury, and regulate cell growth, cell motility, and morphogenesis in a wide variety of cells. Its ability to stimulate branching morphogenesis, cell migration, survival, and proliferation gives it a central role in angiogenesis, tissue regeneration, as well as tumorogenesis.7–13
Signaling by HGF has also been found to have anti-inflammatory, antifibrotic, and pro-regenerative activity on various types of tissue. But it seems to be particularly active in the nervous system, where it has been found to have neurotrophic and angiogenetic activity on central nervous system (CNS) neurons, promote both the survival of neurons and the regeneration of injured nerves, and function as a target-derived axonal chemoattractant, guiding axons to their target. As a result, it plays significant roles in the development of the CNS.17
GABA is the most abundant inhibitory neurotransmitter in the mammalian brain, where it is widely distributed.18 HGF has been shown to modulate GABAergic activity19 and enhance NMDA currents in the hippocampus.20
Specific evidence to support HGF modulation of GABA activity is data associated with a mouse model of autism, the uPAR−/− mouse, which displays a spatially selective defect in interneuron migration, such that the frontoparietal cortices of these mice show 50% less calbindin-positive interneurons (with a near absence of PV cells) whereas more caudal cortices are spared.21,22 These mice display autistic-like behaviors with increased anxiety and altered socialization, as well as interictal epileptiform EEG activity and an increased susceptibility to seizures.21,22 uPAR encodes a urokinase plasminogen activator (uPA) which is required for the proper processing of the HGF. In turn, HGF, through its receptor MET, has been shown to be a critical motogen for interneuron migration and is able to rescue the interneuron migration defect and seizure susceptibility of uPAR−/− mice.19,23 Interestingly, polymorphisms in the MET promoter have recently been described to confer an increased susceptibility to autism and this gene is included in one of the genomic sequences linked to autism susceptibility (7q31).24,25
Autism is a complex disorder and alterations in other GABAergic circuits, including the striatocortical circuits, likely contribute to this behavioral phenotype. Indeed, an interneuron-selective ablation of MET results in decreased cortical PV cells, but massively increased dorsal striatal PV interneurons, leading to a disruption in striatal-mediated procedural and reversal learning.26 Nonetheless, cortical and hippocampal GABAergic deficits certainly play a role in some of the cognitive-behavioral manifestations of autism, as well as in the associated susceptibility to seizures.
The aim of this study was to determine and correlate plasma levels of HGF, GABA, as well as symptom severity, in autistic children and neurotypical controls.
Materials and Methods
Enzyme linked immunosorbest assay (ELISA) was used to measure serum HGF and GABA (ELISA kits, R&D Systems, Minneapolis, Minn. and USCN Life Sciences, Wuhan, China).
All reagents and specimens were equilibrated to room temperature before the assay was performed. A 1:51 dilution of the patient samples was prepared by mixing 10 μL of the patient’s sera with 0.5 mL of Serum Diluent. One hundred microliters of calibrators (20–200 Eu/mL antibodies), positive and negative control serums, serum diluent alone, and diluted patient samples were added to the appropriate microwells of a microculture plate (each well contained affinity purified polyclonal IgG to HGF or GABA). Wells were incubated for 60 min (±5 min) at room temperature, then washed 4 times with wash buffer. One hundred microliters of pre-diluter anti-human IgG conjugated with HRP was added to all microwells, incubated for 30 min (±5 min) at room temperature, then washed 4 times with wash buffer. One hundred microliters of enzyme substrate was added to each microwell. After approximately 30 min at room temperature, the reaction was stopped by adding 50 μL of 1M sulfuric acid. The wells were then read at 405 nm with an ELISA reader (BioRad Laboratories, Inc., Hercules, CA, USA).
Plasma
All plasma, experimental and control, were treated in an identical fashion. Whole blood was drawn into tubes containing EDTA, then spun at 1500 rpm for 10 min. Plasma was removed and immediately frozen until thawed for use in ELISAs.
Subjects
Experimental and control
Plasma from consecutive individuals diagnosed with autism (n = 48; 42 male; mean age 8 years) and neurotypical controls (n = 29; 20 male; mean age 9.5 years) were all obtained from patients presenting at the Health Research Institute/Pfeiffer Treatment Center over a two-year period beginning in October 2008. The autistic individuals meet the DSM-IV criteria and many were diagnosed using The Autism Diagnostic Interview-Revised (ADI-R) before presenting for treatment at the Pfeiffer Treatment Center, Warrenville, Il.
Patient consent was obtained from all patients involved in this study and this study was approved by the Institutional Review Board of the Health Research Institute/Pfeiffer Treatment Center.
Severity of disease
An autism symptom severity questionnaire was used to evaluate symptoms. The questionnaire (Pfeiffer Questionnaire) asked parents or caregivers to assess the severity of the following symptoms: Awareness, Expressive Language, Receptive Language (Conversational), Pragmatic Language, Focus, Attention, Hyperactivity, Impulsivity, Perseveration, Fine Motor Skills, Gross Motor Skills, Hypotonia (low muscle tone), Tip Toeing, Rocking/Pacing, Stimming, Obsessions/Fixations, Eye Contact, Sound Sensitivity, Light Sensitivity, and Tactile Sensitivity. The symptoms were rated by parents/guardians on a scale of 0–5 (5 being the highest severity) for each of these behaviors.
Statistics
Inferential statistics were derived from unpaired t-test and odds ratios with 95% confidence intervals (http://studentsttest.com). Pearson moment correlation test was used to establish degree of correlation between groups (http://www.wessa.net/rwasp_correlation.wasp#output).
Results
We previously reported that autistic children had significantly decreased levels of HGF.27 In this study, Plasma from 48 autistic children and 29 neurotypical controls was assessed for GABA concentration using ELISAs. A random group of 29 of the above (N = 48) autistic children were also tested for HGF plasma concentration. Symptom severity was assessed in a random group of these autistic individuals and compared to HGF and GABA concentrations.
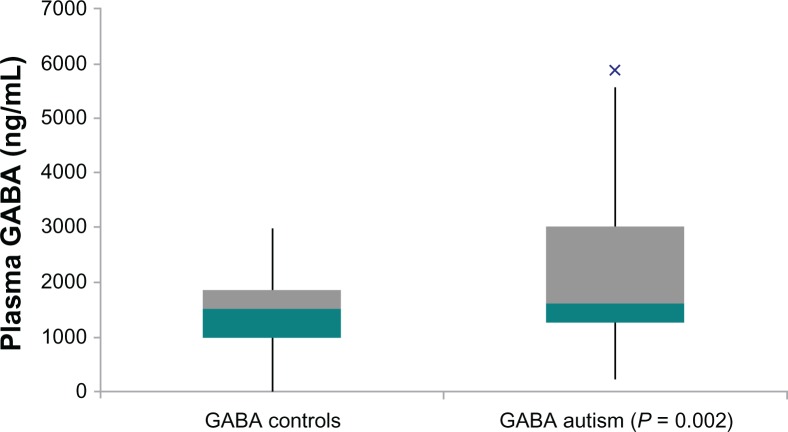
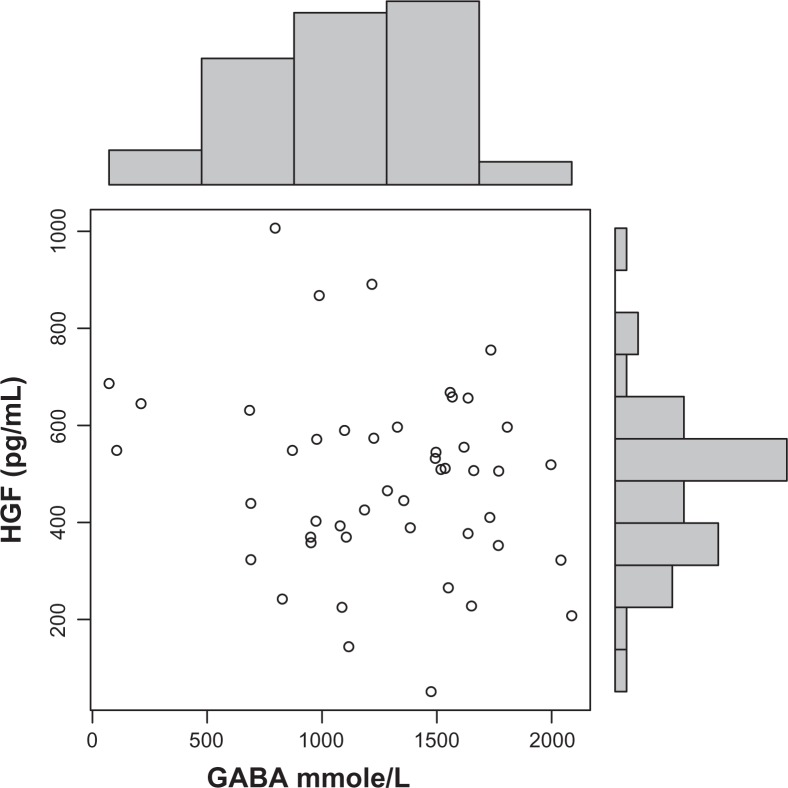
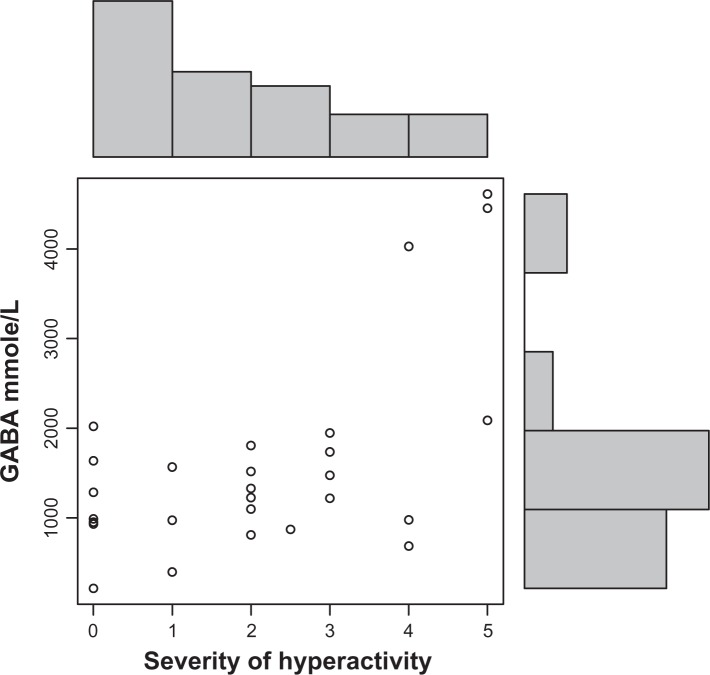
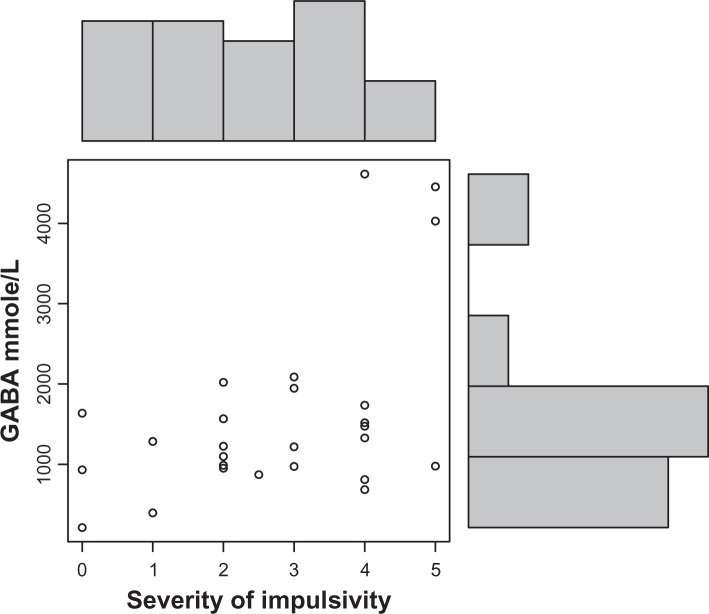
Autistic children in this study had significantly increased plasma levels of GABA (P = 0.002) (Fig. 1). Decreased HGF levels correlated with these increased GABA levels (r = 0.3; P = 0.05) (Fig. 4). High GABA levels correlated with increasing hyperactivity (r = 0.6; P = 0.0007) (Fig. 2), impulsivity severity (r = 0.5; P = 0.007) (Fig. 3), tip toeing severity (r = 0.35; P = 0.03), light sensitivity (r = 0.4; P = 0.02), and tactile sensitivity (r = 0.4; P = 0.01). HGF levels did not correlate significantly with any symptom severity (Table 1).
Figure 1.
Box and Whisker Plot: Plasma GABA concentration of autistic children significantly higher in autistic children compared to neurotypical, age-and gender similar controls.
Figure 4.
Decreased HGF levels correlate with increased GABA levels in autistic children (r = 0.3; P = 0.05).
Figure 2.
GABA plasma concentration correlates (r = 0.6; P = 0.0007) with severity of hyperactivity in autistic children. (O) represents GABA and severity level of hyperactivity (0–5; 5 is most severe).
Figure 3.
GABA plasma concentration correlates (r = 0.6; P = 0.007) with severity of impulsivity in autistic children. (O) represents GABA and severity level of impulsivity (0–5; 5 is most severe).
Table 1.
Relationship between symptom severity and GABA and HGF.
| HGF | GABA | |
|---|---|---|
| Symptom severity (0–5; 5 most severe behavior) | ||
| Awareness | ||
| r | −0.03 | 0.01 |
| P | 0.41 | 0.46 |
| Expressive language | ||
| r | −0.22 | −0.19 |
| P | 0.1 | 0.16 |
| Receptive language | ||
| r | −0.07 | −0.14 |
| P | 0.33 | 0.24 |
| Conversational language | ||
| r | −0.11 | −0.018 |
| P | 0.26 | 0.46 |
| Focus/attention | ||
| r | 0.14 | 0.328 |
| P | 0.2 | 0.043 |
| Hyperactivity | ||
| r | −0.1 | 0.57 |
| P | 0.27 | 0.0007 |
| Impulsivity | ||
| r | −0.01 | 0.46 |
| P | 0.47 | 0.007 |
| Perseveration | ||
| r | −0.15 | 0.13 |
| P | 0.19 | 0.25 |
| Fine motor skills | ||
| r | −0.05 | −0.06 |
| P | 0.37 | 0.36 |
| Gross motor skills | ||
| r | 0.13 | −0.3 |
| P | 0.22 | 0.06 |
| Hypotonia | ||
| r | −0.11 | −0.11 |
| P | 0.25 | 0.28 |
| Tip toeing | ||
| r | −0.05 | 0.348 |
| P | 0.38 | 0.03 |
| Rocking/pacing | ||
| r | −0.02 | −0.16 |
| P | 0.44 | 0.2 |
| Hand flapping/finger stimming | ||
| r | −0.06 | 0.187 |
| P | 0.34 | 0.16 |
| Obsession/fixation | ||
| r | −0.06 | 0.03 |
| P | 0.35 | 0.43 |
| Eye contact | ||
| r | 0.06 | −0.07 |
| P | 0.35 | 0.34 |
| Sound sensitivity | ||
| r | 0.06 | 0.22 |
| P | 0.34 | 0.12 |
| Light sensitivity | ||
| r | 0.09 | 0.39 |
| P | 0.29 | 0.02 |
| Tactile sensitivity | ||
| r | 0.002 | 0.415 |
| P | 0.49 | 0.01 |
Notes: GABA concentration correlates with severity of focus/attention, hyperactivity, impulsivity, tip toeing, light sensitivity and tactile sensitivity (shaded data).
Discussion
Histological, biochemical, and molecular approaches have demonstrated altered levels and distribution of GABA and GABA receptors in peripheral blood and plasma, as well as in the brain, including decreased GABA-A receptors and benzodiazepine binding sites in the hippocampal formation.28–30
Our data, demonstrating significantly high levels of GABA in the plasma of autistic children compared to controls, supports the work of others who have found elevated GABA levels in autistic children.28
To our knowledge, this is the first study to show a correlation between these high GABA levels and low levels of HGF, and, although the number of patients with measured symptom severity is low, our data shows a causal relationship between GABA and hyperactivity and impulsivity, prevalent in autistic behavior.
We did not find any correlation between HGF levels and symptom severity. This may suggest an indirect role for HGF in the etiology of the disease. It is conceivable that altered uPA and its receptor, uPAR, displayed in animal behavioral models of autism, are associated with altered behavior unrelated to altered HGF levels.
GABA is released by interneurons which contain the GABA synthesizing enzymes glutamic acid decarboxylase (GAD) 65 and GAD67, which convert the excitatory transmitter, glutamine, to GABA. GAD65 and GAD67 proteins are reduced in autistic parietal and cerebellar cortices31 and anti-GAD antibodies have been characterized in a pathogenetic model of autism involving Purkinje cell loss through.32 It is possible that specific altered behavior associated with hyperactivity is more closely associated with altered GAD, rather than the uPA/HGF system in autistic individuals.
GABA, the main inhibitory neurotransmitter in adulthood, is released by interneurons which contain the GABA synthesizing enzymes GAD65 and GAD67. GABAergic interneurons, which constitute a heterogeneous group of cells, differently classified in virtue of their anatomical, physiological, and molecular features,2 represent only 10%–15% of the total neuronal population. Nevertheless, they provide the functional balance, complexity, and computational architecture of neuronal circuits.3 They play a key role in regulating neuronal excitability via feedback and feed-forward inhibition. Axons of different inhibitory cells target different postsynaptic subcellular compartments, allowing them to selectively control the output of pyramidal cells,4 thus providing the temporal structure that orchestrates the activity of neuronal ensembles leading to coherent network oscillations.5
Considering the many facets of GABA activities, particularly during development, it is not surprising that disturbance of GABAergic signaling can result in aberrant information processing, as found in neurodevelopmental disorders such as ASDs.
Evidence suggests that GABAergic mechanisms play a role in the etiology of ASD. Future studies on the clinical relationship between GABA and behavior may provide the necessary data to formulate a coherent theory of GABA dysfunction. More trials of medication with known or suspected effects on GABA function are warranted.
Conclusions
We report a significant inverse relationship between plasma HGF and GABA levels in autistic children. This supports findings using a mouse model lacking the uPAR, demonstrating that decreased numbers of neocortical GABAergic interneurons would result in increased plasma GABA, spontaneous seizures, and reduced HGF.19
Although we found a significant relationship between HGF and GABA, we also found that the severity level of hyperactivity related symptoms correlated significantly with high levels of GABA, but none of the symptoms correlated with HGF levels. These results suggest that, despite the apparent regulatory effect of HGF on GABA levels, GABA levels, but not HGF levels, are associated with autistic behavior.
Acknowledgments
The author would like to acknowledge the financial support from the Autism Research Institute.
Footnotes
Author Contributions
AR carried out the immunoassays, participated in the design of the study and performed the statistical analysis. AR conceived of the study, and participated in its design and coordination. AR drafted and approved the final manuscript.
Funding
This research was funded by a grant from the Autism Research Institute.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the author has provided signed confirmation of compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DMS-IV-TR) American Psychiatric Association; Washington, DC, USA: 2000. [Google Scholar]
- 2.Petilla Interneuron Nomenclature Group. Ascoli GA, Alonso-Nanclares L, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9(7):557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8(9):673–86. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- 4.Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16(4):815–23. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 5.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562(Pt 1):9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3(9):715–27. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci U S A. 1986;83(17):6489–93. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura T, Nawa K, Ichihara A. Partial purification and characterization in hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122(3):1450–9. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki M, Nishio M, Sasaki T, Enami J. Identification of mouse mammary fibroblast-derived mammary growth factor as hepatocyte growth factor. Biochem Biophys Res Commun. 1994;199(2):772–9. doi: 10.1006/bbrc.1994.1296. [DOI] [PubMed] [Google Scholar]
- 10.Michalopoulos G, Houck KA, Dolan ML, Leutteke NC. Control of hepatocyte replication by two serum factors. Cancer Res. 1984;44(10):4414–9. [PubMed] [Google Scholar]
- 11.Thaler FJ, Michalopoulos G. Hepatopoietin A: partial characterization and trypsin activation of a hepatocyte growth factor. Cancer Res. 1985;45(6):2545–9. [PubMed] [Google Scholar]
- 12.Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129(5):1177–80. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weidner KM, Arakaki N, Hartmann G, et al. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991;88(16):7001–5. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comoglio PM, Graziani A. In: Guidebook to Cytokines and their Receptors. Nicola NA, editor. Oxford University Press; 1994. p. 182. [Google Scholar]
- 15.Comoglio PM, Graziani A. In: Guidebook to Cytokines and their Receptors. Nicola NA, editor. Oxford University Press; 1994. p. 185. [Google Scholar]
- 16.Grant DS, Kleinman HK, Goldberg ID, et al. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A. 1993;90(5):1937–41. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamanoue M, Takemoto N, Matsumoto K, Nakamura T, Nakajima K, Kohsaka S. Neurotrophic effect of hepatocyte growth factor on central nervous system neurons in vitro. J Neurosci Res. 1996;43(5):554–64. doi: 10.1002/(SICI)1097-4547(19960301)43:5<554::AID-JNR5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Zachmann M, Tocci P, Nyhan WL. The occurrence of gamma-aminobutyric acid in human tissues other than brain. J Biol Chem. 1966;241(6):1355–8. [PubMed] [Google Scholar]
- 19.Bae MH, Bissonette GB, Mars WM, et al. Hepatocyte growth factor (HGF) modulates GABAergic inhibition and seizure susceptibility. Exp Neurol. 2010;221(1):129–35. doi: 10.1016/j.expneurol.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akimoto M, Baba A, Ikeda-Matsuo Y, et al. Hepatocyte growth factor as an enhancer of nmda currents and synaptic plasticity in the hippocampus. Neuroscience. 2004;128(1):155–62. doi: 10.1016/j.neuroscience.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23(2):622–31. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004;27(7):400–6. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30(1):79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 24.Jackson PB, Boccuto L, Skinner C, et al. Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder. Autism Res. 2009;2(4):232–6. doi: 10.1002/aur.87. [DOI] [PubMed] [Google Scholar]
- 25.Campbell DB, Sutcliffe JS, Ebert PJ, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103(45):16834–9. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins GJ, Shahrokh M, Powell EM. Genetic disruption of Met signaling impairs GABAergic striatal development and cognition. Neuroscience. 2010;176:199–209. doi: 10.1016/j.neuroscience.2010.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo AJ, Krigsman A, Jepson B, Wakefield A. Decreased serum hepatocyte growth factor (HGF) in autistic children with severe gastrointestinal disease. Biomark Insights. 2009;4:181–90. doi: 10.4137/bmi.s3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhossche D, Applegate H, Abraham A, et al. Elevated plasma gamma-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for a GABA hypothesis of autism. Med Sci Monit. 2002;8(8):1–6. [PubMed] [Google Scholar]
- 29.Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord. 2001;31(6):537–43. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- 30.Rolf LH, Haarmann FY, Grotemeyer KH, Kehrer H. Serotonin and amino acid content in platelets of autistic children. Acta Psychiatr Scand. 1993;87(5):312–6. doi: 10.1111/j.1600-0447.1993.tb03378.x. [DOI] [PubMed] [Google Scholar]
- 31.Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52(8):805–10. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 32.Rout UK, Dhossche DM. A pathogenetic model of autism involving Purkinje cell loss through anti-GAD antibodies. Med Hypotheses. 2008;71(2):218–21. doi: 10.1016/j.mehy.2007.11.012. [DOI] [PubMed] [Google Scholar]