Abstract
White spot syndrome virus (WSSV) occurs worldwide and causes high mortality and considerable economic damage to the shrimp farming industry. No adequate treatments against this virus are available. It is generally accepted that invertebrates such as shrimp do not have an adaptive immune response system such as that present in vertebrates. As it has been demonstrated that shrimp surviving a WSSV infection have higher survival rates upon subsequent rechallenge, we investigated the potential of oral vaccination of shrimp with subunit vaccines consisting of WSSV virion envelope proteins. Penaeus monodon shrimp were fed food pellets coated with inactivated bacteria overexpressing two WSSV envelope proteins, VP19 and VP28. Vaccination with VP28 showed a significant lower cumulative mortality compared to vaccination with bacteria expressing the empty vectors after challenge via immersion (relative survival, 61%), while vaccination with VP19 provided no protection. To determine the onset and duration of protection, challenges were subsequently performed 3, 7, and 21 days after vaccination. A significantly higher survival was observed both 3 and 7 days postvaccination (relative survival, 64% and 77%, respectively), but the protection was reduced 21 days after the vaccination (relative survival, 29%). This suggests that contrary to current assumptions that invertebrates do not have a true adaptive immune system, a specific immune response and protection can be induced in P. monodon. These experiments open up new ways to benefit the WSSV-hampered shrimp farming industry.
White spot syndrome virus (WSSV) belongs to a new virus family, the Nimaviridae, and contains a large circular double-stranded DNA genome of 292,967 bp (22), but isolates with larger genomes have also been identified (AF440570) (27). WSSV virions are ellipsoid to bacilliform, enveloped particles with a distinctive tail-like appendage at one end and can be found throughout the body of infected shrimp. The virions contain one nucleocapsid with a typical striated appearance and five major and at least 13 minor proteins (6, 20, 21, 24).
WSSV, which was first discovered in southeast Asia around 1992, is currently the most serious viral pathogen of shrimp worldwide. It causes up to 100% mortality within 7 to 10 days in commercial shrimp farms, resulting in large economic losses to the shrimp farming industry (11). Shrimp culture has been a booming business since the beginning of the 1990s, and worldwide production was 1 million metric tons in 2002 (15). It is one of the few sources for economic development and provides well-paid employment in poor coastal areas (1).
Given the global environmental, the economic and sociological importance of shrimp farming, and the constraints of high-intensity cultivation, the development of vaccines against WSSV would be desirable. The idea of vaccinating shrimp, or invertebrates in general, seems to be unfeasible since they are assumed to lack an adaptive immune response and rely solely on innate immune responses (8). However, a recent study in the copepod Macrocyclops albidus showed that the defense system of this invertebrate species reacted more efficiently after a previous encounter with an antigenically similar parasite, implying that a specific memory may exist (9). Furthermore, immunostimulation and vaccination of shrimp with inactivated Vibrio spp. have been reported to provide some protection (2, 7, 19). Studies on the shrimp immune response to viral infections are limited, although the presence of virus-inhibiting proteins and specific upregulation of genes upon viral infection have been demonstrated (13, 14, 16). In vivo experiments with Penaeus japonicus demonstrated the presence of a quasi-immune response when survivors of both natural and experimental WSSV infections were rechallenged with WSSV (25). Plasma from the surviving infected shrimp could neutralize WSSV from 20 days up to 2 months after infection (26). These results suggest that some sort of adaptive immune response could exist in shrimp.
To investigate whether protection against WSSV could be induced in shrimp by vaccination, a subunit vaccine was generated with two major structural envelope proteins of WSSV, VP19 and VP28. Neutralization experiments with VP28 have shown it to be involved in the systemic infection of WSSV (23). An oral vaccination strategy was adopted because injection vaccinations are not practically feasible in shrimp farming. Inactivated bacteria overexpressing the WSSV envelope proteins VP19 and VP28 coated on food pellets were selected for delivery of the WSSV proteins. The onset and duration of the observed protection were further investigated by challenging shrimp at different time points after vaccination.
MATERIALS AND METHODS
Shrimp culture.
Healthy Penaeus monodon shrimp were imported as postlarvae from Malaysia and maintained in a recirculation system at the facility De Haar vissen at Wageningen University. Each shipment was tested for the presence of WSSV, monodon baculovirus, yellow head virus, Taura syndrome virus, and infectious hypodermal and hematopoietic necrosis virus by PCR. Prior to each experiment, shrimp were transferred to an experimental system located at the Laboratory of Virology at Wageningen University and stocked in 180-liter aquariums, each fitted with an individual filter system (Eheim, Germany), heating (Schego, Germany) to 28 ± 1°C, and continuous aeration. All experiments were performed in artificial seawater (Instant Ocean, Aquarium Systems) at a salinity of approximately 20 ppt.
WSSV stock.
The virus isolate used in this study originated from infected Penaeus monodon shrimp imported from Thailand in 1996. Virus stocks were generated in the crayfish Orconectes limosus and purified from freshly extracted hemolymph by sucrose gradient centrifugation as described by Van Hulten et al. (23). Virus samples were stored in aliquots at −80°C.
In vivo titration and WSSV challenge.
In order to mimic the natural route of infection and to ensure a constant and reproducible challenge pressure, shrimp were challenged via immersion. To determine the amount of virus required for the desired challenge pressure of approximately 75% mortality, a virus stock was prepared and titrated in vivo. Shrimp weighing approximately 1 g were immersed in different dilutions of WSSV for a 7-h incubation period. The shrimp were removed from the WSSV-containing water, rinsed, and moved to individual cages to prevent horizontal transmission by cannibalism. Mortality was recorded twice a day, and dead shrimp were tested for the presence of WSSV by PCR. WSSV challenge after vaccination was performed identically.
PCR analysis for WSSV.
Muscle tissue from the tails of shrimp was homogenized and mixed with 200 μl of 5% Chelex 100 resin (Bio-Rad) and 16 μl of 20-mg/ml proteinase K. This mixture was incubated overnight at 56°C, followed by 10 min at 95°C to inactivate the proteinase K. The samples were tested with two primer pairs. A 16S rRNA primer pair, 16S-FW1 (5′-GTG CGA AGG TAG CAT AAT C-3′ ) and 16S-RV1 (5′-CTG CTG CAA CAT AAG GAT AC-3′), amplifying a 414-bp fragment of shrimp ribosomal DNA, was used as an isolation control. A VP26 primer pair, VP26-FW1 (5′-ATG GAA TTT GGC AAC CTA ACA AAC CTG-3′) and VP26-RV1 (5′-GGG CTG TGA CGG TAG AGA TGA C-3′), amplifying part of the WSSV VP26 gene, was used to screen for WSSV-positive animals (12). Twenty-five cycles of amplification were performed at 30 s at 94°C, 30 s at 52°C, and 50 s at 72°C for both primer pairs.
Bacterial expression of VP28 and VP19.
Expression constructs were generated for VP28 and VP19 in fusion with His6 and maltose-binding protein (MBP) tags. The complete VP19 open reading frame was cloned as a BamHI/PstI fragment into the pMAL-C2 vector (New England Biolabs) after PCR amplification from the WSSV genome with primer VP19-FW1 (5′-CGG GAT CCA TGG CCA CCA CGA CTA A-3′) and primer VP19-RV1 (5′-GCC TGC AGC CTG ATG TTG TGT TTC TAT A-3′). Expression of the pMAL-C2-VP19 construct and an empty pMAL-C2 vector (control) was performed in Escherichia coli DH5α cells. A partial VP28 fragment (without the N-terminal hydrophobic region [Δ1-29]) was amplified from genomic WSSV DNA by PCR with primer VP28PF (3′-AAG GAT CCC ACA ACA CTG TGA CCA AG-5′) and primer VP28PR (3′-TAG CGG CCG CAA AAG CAC GAT TTA TTT AC-5′) and ligated into the BamHI and NotI sites of the pET28a vector (Novagen). Expression of the pET28a-VP28 construct and the pET28a vector (control) was performed in BL21 cells.
Protein production and inactivation.
The MBP-VP19, His6-VP28, and control proteins were overexpressed according to the manufacturer's instructions and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10) and Western blot (17) with a WSSV polyclonal antiserum (21). The bacterial concentration after inactivation was determined with a Beckman DU-7500 photo spectrometer, where an optical density at 600 nm of 1 equaled 109 bacteria per ml. The bacteria were inactivated in 0.5% formaldehyde, incubated for 15 min at 20°C, checked for inactivation levels, and stored at 4°C until further use.
Coating of feed pellets.
Commercial pellets weighing approximately 0.02 g (Coppens International) were each coated with approximately 108 inactivated bacteria or with phosphate-buffered saline for the positive and negative controls. The inactivated bacteria were centrifuged, washed twice in phosphate-buffered saline, and resuspended in phosphate-buffered saline. The bacteria were subsequently mixed with the food pellets and incubated on ice for 15 min to allow absorption of the bacterial suspension and coated with cod liver oil to prevent dispersion of the inactivated bacteria in the water.
Vaccination experiments.
In the vaccination experiments, groups of 15 shrimp were vaccinated by feeding coated food pellets for 7 days, as indicated in Table 1. For experiment 1, the vaccination was directly followed by a 7-h immersion challenge in WSSV-containing seawater of a predetermined dilution as described above. For experiment 2, the four groups were subdivided into three even subgroups after the 7 days of vaccination, which were subsequently challenged at 3, 14, and 21 days postvaccination.
TABLE 1.
Setup for vaccination experiments
| Expt. no | Group | Coating | No. of shrimp in group |
|---|---|---|---|
| 1 | VP19 | pMAL-VP19 | 15 |
| VP28 | pET28a-VP28 | 15 | |
| VP19 + VP28 | pMAL-VP19 + pET28a-VP28 | 15 | |
| pET + pMAL | pET28a + pMAL-C2 | 15 | |
| Positive control | PBS | 15 | |
| Negative control | PBS | 5 | |
| 2 | VP28 | pET28a-VP28 | 15 × 3 |
| pET | pET28a | 15 × 3 | |
| Positive control | PBS | 15 × 3 | |
| Negative control | PBS | 10 × 3 |
Statistical analysis.
Statistical analysis of the obtained time-mortality relationships was performed with Kaplan-Meier survival analysis (4) at a 5% confidence level. The protection against WSSV after vaccination was calculated as relative percent survival, calculated as (1 − vaccinated group mortality/control group mortality) × 100 (3).
RESULTS
Protein production and purification.
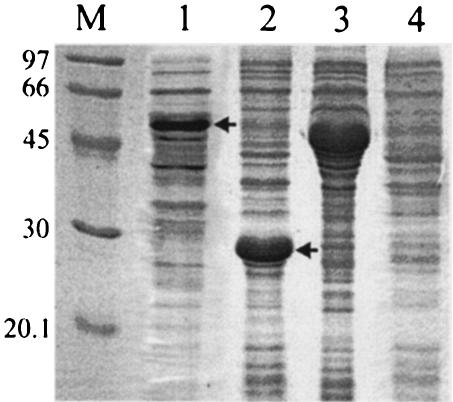
The two major WSSV envelope proteins VP19 and VP28 were selected for use as subunit vaccines. Multiple expressions were performed in E. coli, with complete and partial open reading frames and MBP and His6 tag fusions. The highest expression of VP28 was obtained in fusion with the His6 tag when the N-terminal hydrophobic domain was omitted. Expression for VP19 was generally lower and highest in fusion with MBP. Bands corresponding to the fusion proteins were observed at the expected heights (Fig. 1, lanes 1 and 2). The viral origin of the band was confirmed by Western analysis with anti-WSSV polyclonal antiserum (data not shown). Empty pMAL-C2 and pET28a vectors were overexpressed as control proteins according to the same protocols (Fig. 1, lanes 3 and 4).
FIG. 1.
Coomassie stained SDS-PAGE gel of E. coli-overexpressed VP19 in pMAL-c2, VP28 in pET28a, empty pMAL-C2, and empty pET28a. Lane M, size markers (kilodaltons). Lane 1, MBP-VP19 expression. Lane 2, His6-VP28 expression. Lane 3, pMAL-C2 expression. Lane 4, pET28a expression. Numbers on the left side indicate the positions of markers, and arrows indicate the MBP-VP19 and His6-VP28 overexpression products.
Vaccination experiments. (i) Experiment 1: VP19 and VP28 vaccination.
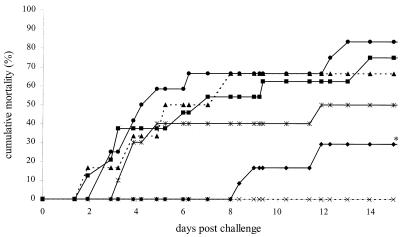
In this experiment, the vaccinating potential of WSSV envelope proteins VP19, VP28, and a mixture of both proteins via oral administration was tested. Four groups of 15 shrimp each were vaccinated as indicated in Table 1, directly followed by an immersion challenge. The resulting time-mortality relationship of this experiment is shown in Fig. 2. The positive control group and the group vaccinated with a mixture of the empty pMAL-C2 and pET28a vectors showed a cumulative mortality of 67% and 77%, respectively. The group vaccinated with VP19 also showed a high cumulative mortality of 83%, indicating that no protection could be obtained with this protein. However, vaccination with either VP28 alone or a mixture of VP28 and VP19 resulted in lower mortalities of 30% and 50% compared to the group vaccinated with the empty vectors (relative survival values of 61% and 31%, respectively). This mortality was significantly lower for the VP28-vaccinated group compared to the pMAL-C2/pET28a and positive control groups. Randomly selected survivors were checked for the presence of WSSV, and all tested negative.
FIG. 2.
Time-mortality relationship of vaccination experiment 1. Cumulative mortality rates of shrimp from the experimental groups vaccinated with VP19 (•), VP28 (⧫), VP19 plus VP28 (*), pET plus pMAL (▪), positive control (▴), and negative control (×) as indicated in Table 1 are plotted against the time after challenge. Lines marked with an asterisk are significantly different from the pET plus pMAL and positive control groups.
(ii) Experiment 2: onset and duration of vaccination with VP28.
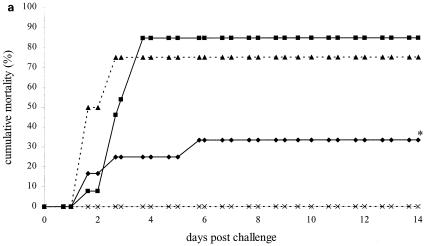
As the first experiment demonstrated that vaccination with VP28 resulted in higher survival upon WSSV challenge, the nature of this protection was analyzed further. Shrimp were vaccinated with WSSV envelope protein VP28, and three control groups were included, as indicated in Table 1. After vaccination, the four groups were subdivided into three even subgroups for subsequent challenge at three different time points. The first challenge was 3 days after vaccination had stopped, and the resulting time-mortality relationship is shown in Fig. 3a. The pET group showed a cumulative mortality of 85% and the positive control group a mortality of 75%. A significantly lower cumulative mortality (30%) was observed in the group fed VP28, resulting in relative survival of 64% and 59% compared to the pET and positive control groups, respectively. These results are consistent with an independent experiment (data not shown), where vaccination with VP28 resulted in relative survival values of 64% and 62% compared to the pET and positive control groups, respectively.
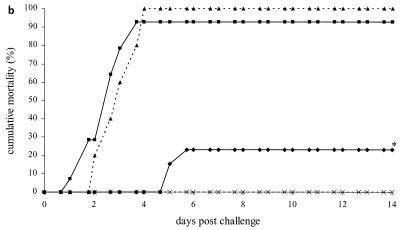
FIG. 3.
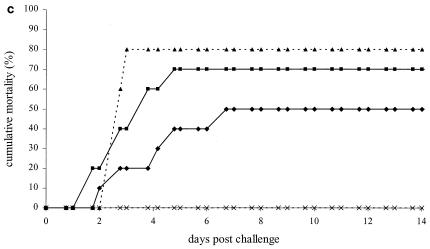
Time-mortality relationship of vaccination experiment 2. Shrimp were challenged 3 days (a), 7 days (b), and 21 days (c) after cessation of feeding coated food pellets. Cumulative mortality rates of shrimp from the experimental groups vaccinated with VP28 (⧫), pET (▪), positive control (▴), and negative control (×) as indicated in Table 1 are plotted against the time after challenge. Lines marked with an asterisk are significantly different from the pET and positive control groups.
For the second challenge, 7 days postvaccination, the group fed pET and the positive control group reached cumulative mortalities of 100% and 93%, respectively (Fig. 3b). The VP28 vaccination group showed a significantly lower mortality of 23%, resulting in relative survival values of 77% and 75% compared to the pET and positive control groups, respectively. Twenty-one days after the vaccinations, the third challenge was performed. This time, the pET and positive controls groups reached cumulative mortalities of 80 and 70%, respectively (Fig. 3c). The positive effect of feeding VP28 was reduced, and this group reached a cumulative mortality of 50% (not significantly different), resulting in relative survival values of 29% and 38% compared to the pET and positive control groups, respectively. The negative control groups showed no mortality. Randomly selected survivors from all groups were tested for WSSV, and all tested negative.
DISCUSSION
In the study presented here, we analyzed whether viral proteins can be used to elicit an immune response in shrimp, leading to protection against WSSV. To this end we used oral vaccination, as this is the only practical way to deliver potential vaccines to shrimp. The challenge with WSSV was performed by immersion, as the challenge pressure can be well controlled, contrary to challenge via infected tissue. In a natural situation, shrimp become infected through both oral and water-borne routes and the gills are thought to be a major point of viral entry (5, 18). We selected VP28 and VP19 for use in the crude subunit vaccines because they are the most exposed proteins abundantly present in the WSSV envelope and react strongly with polyclonal antibodies generated against complete virions in rabbits (21). As previous studies have shown that the major WSSV proteins are not glycosylated (24), bacteria were chosen for protein expression and as an antigen delivery vehicle because production for commercial applications is well established and cheap. Vaccination against bacterial diseases in shrimp with inactivated bacteria has been performed earlier (2, 7, 19), and a vaccine is commercially available (Norvax ShrimpVib, Intervet International BV). As it is generally believed that the inactivated bacteria induce a general immune stimulation in shrimp, the presence of bacteria in the subunit vaccine might by itself have a positive effect on shrimp survival upon WSSV challenge. However, none of the vaccines lacking VP28 provided protection against WSSV, indicating that protection was VP28 specific. Nonetheless, the presence of the bacterial proteins may still act as an adjuvant in the vaccination.
When a mixture of VP19 and VP28 was used, a lower relative survival value was obtained compared to the group vaccinated with VP28 alone. As the concentration of VP28 in the mixture was half that in the treatment with VP28 alone, this may suggest the existence of a dose-dependent response. Further experiments must elucidate whether the high protection found for up to 3 weeks after vaccination with VP28 can be extended or further improved by a longer vaccination period, different vaccination schemes with booster feeding, or optimization of the amount of vaccine. Challenge experiments with other shrimp-infective viruses such as yellow head virus and Taura syndrome virus may reveal whether the observed effect is virus specific and give us more insight into the processes involved in the immune response.
Previous experiments have indicated that VP28 plays an important role in the systemic infection of WSSV in shrimp, as it is possible to neutralize WSSV with VP28 antibodies (23). As protection against WSSV is maintained for up to 3 weeks after vaccination, it is unlikely that the presence of residual VP28 could block WSSV infection by blocking receptors needed by the virus to enter shrimp cells. The way in which the protection is obtained by the shrimp immune system remains to be resolved. Protection could, for example, be generated by prevention of entry of WSSV by secreted neutralizing substances or by blocking virus spread after entry. At the end of the vaccination experiment, survivors were checked for the presence of WSSV by one-step PCR, and all tested negative for WSSV, indicating that high levels of WSSV were not present in the shrimp. More sensitive nested PCRs could be used to monitor the entry and possible persistence of WSSV after challenge in vaccinated shrimp.
Altogether, these results suggest that a specific memory exists in invertebrates or more specifically in crustaceans, as the data obtained are in line with the results found for the copepod, which is a minute crustacean (9). This study is the first to show that oral vaccination of shrimp against WSSV is possible and opens the way for the design of practical strategies to control WSSV and other invertebrate pathogens.
Acknowledgments
We thank Angela Vermeesch for technical assistance and Sietze Leenstra of the facility de Haar vissen for maintaining and providing the shrimp.
This research was supported by Intervet International BV, Boxmeer, The Netherlands.
REFERENCES
- 1.Adger, W. N. 1998. Sustainability and social resilience in coastal resource use. CSERGE working paper GEC 97-23. Center for Social and Economic Research on the Global Environment. University of East Anglia and University College, London, England.
- 2.Alabi, A. O., D. A. Jones, and J. W. Latchford,. 1999. The efficacy of immersion as opposed to oral vaccination of Penaeus indicus larvae against Vibrio harveyi. Aquaculture 178:1-11. [Google Scholar]
- 3.Amend, D. F. 1981. Potency testing of fish vaccines, p. 447-454. In D. P. Anderson and W. Hennessen (ed.), Fish biologics: serodiagnostics and vaccines. S. Karger, Basel, Switzerland.
- 4.Bull, K., and D. J. Spiegelhalter. 1997. Tutorial in biostatistics: survival analysis in observational studies. Stat. Med. 16:1041-1074. [DOI] [PubMed] [Google Scholar]
- 5.Chang, P. S., C. F. Lo, Y. C. Wang, and G. H. Kou. 1996. Identification of white spot syndrome associated baculovirus (WSBV) target organs in the shrimp Penaeus monodon by in situ hybridization Dis. Aquat. Org. 27:131-139. [Google Scholar]
- 6.Huang, C., X. Zhang, Q. Lin, X. Xu, Z. H. Hu, and, C. L. Hew. 2002. Proteomic analysis of shrimp white spot syndrome viral proteins and characterization of a novel envelope protein VP466. Mol. Cell. Prot. 1:223-231. [DOI] [PubMed] [Google Scholar]
- 7.Itami, T., Y. Yan, and Y. Takahashi. 1989. Efficacy of vaccination against vibriosis in cultured kuruma prawns Penaeus japonicus. J. Aquat. Anim. Health 1:234-242. [Google Scholar]
- 8.Kimbrell, D. A. and, B. Beutler. 2001. The evolution and genetics of innate immunity. Nat. Rev. Gen. 2:256-267. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz, J., and K. Franz. 2003. Innate defence: evidence for memory in invertebrate immunity. Nature 425:37-38. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Lightner, D. V. 1996. A handbook of pathology and diagnostic procedures for diseases of penaeid shrimp. World Aquaculture Society, Baton Rouge, La.
- 12.Marks, H., M. Mennens, J. M. Vlak, and M. C. W. Van Hulten. 2003. Transcriptional analysis of the white spot syndrome major virion protein genes. J. Gen. Virol. 84:1517-1523. [DOI] [PubMed] [Google Scholar]
- 13.Pan, J., A. Kurosky, B. Xu, A. K. Chopra, D. H. Coppenhaver, I. P. Sigh, and S. Baron. 2000. Broad antiviral activity in tissue of crustaceans. Antiviral Res. 48:39-47. [DOI] [PubMed] [Google Scholar]
- 14.Rojtinnakorn, J., I. Hirono, T. Itami, Y. Takahashi, and T. Aoki. 2002. Gene expression in haemocytes of kuruma prawn, Penaeus japonicus, in response to infection with WSSV by EST approach. Fish Shellfish Immunol. 13:69-83. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberry, B. 2002. World shrimp farming 2002. Shrimp News International, San Diego, Calif.
- 16.Roux, M. M., A. Pain, K. R. Klimpel, and A. K. Dhar. 2002. The lipopolysaccharide and {beta}-1, 3-glucan binding protein gene is upregulated in white spot virus-infected shrimp (Penaeus stylirostris). J. Virol. 76:7140-7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Tan, L. T., S. Soon, K. L. Lee, M. Shariff, M. D. Hassan, and A. R. Omar. 2001. Quantitative analysis of an experimental white spot syndrome virus (WSSV) infection in Penaeus monodon Fabricius with competitive polymerase chain reaction. J. Fish Dis. 24:315-323. [Google Scholar]
- 19.Teunissen, O. S. P., R. Faber, G. H. R. Booms, T. Latscha, and J. H. Boon. 1998. Influence of vaccination on vibriosis resistance of the giant black tiger shrimp Penaeus monodon (Fabricius). Aquaculture 164:359-366. [Google Scholar]
- 20.Van Hulten, M. C. W., R. W. Goldbach, and J. M. Vlak. 2000a. Three functionally diverged major structural proteins of white spot syndrome virus evolved by gene duplication. J. Gen. Virol. 81:2525-2529. [DOI] [PubMed] [Google Scholar]
- 21.Van Hulten, M. C. W., M. Westenberg, S. D. Goodall, and J. M. Vlak. 2000b. Identification of two major virion protein genes of white spot syndrome virus of shrimp. Virology 266:227-236. [DOI] [PubMed] [Google Scholar]
- 22.Van Hulten, M. C. W., J. Witteveldt, S. Peters, N. Kloosterboer, R. Tarchini, M. Fiers, H. Sandbrink, R. Klein Lankhorst, and, J. M. Vlak. 2001. The white spot syndrome virus DNA genome sequence. Virology 286:7-22. [DOI] [PubMed] [Google Scholar]
- 23.Van Hulten, M. C. W., J. Witteveldt, M. Snippe, and J. M. Vlak. 2001. White spot syndrome virus envelope protein VP28 is involved in the systemic infection of shrimp. Virology 285:228-233. [DOI] [PubMed] [Google Scholar]
- 24.Van Hulten, M. C. W., M. Reijns, A. M. G. Vermeesch, F. Zandbergen, and J. M. Vlak. 2002. Identification of VP19 and VP15 of white spot syndrome virus (WSSV) and glycosylation of the WSSV major structural proteins. J. Gen. Virol. 83:257-265. [DOI] [PubMed] [Google Scholar]
- 25.Venegas, C. A., L. Nonaka, K. Mushiake, T. Nishizawa, and K. Muroga. 2000. Quasi-immune response of Penaeus japonicus to penaeid rod-shaped DNA virus (PRDV). Dis. Aquat. Org. 42:83-89. [DOI] [PubMed] [Google Scholar]
- 26.Wu, J. L., T. Nishioka, K. Mori, T. Nishizawa, and K. A. Muroga. 2002. Time-course study on the resistance of Penaeus japonicus induced by artificial infection with white spot syndrome virus. Fish Shellfish Immunol. 13:391-403. [DOI] [PubMed] [Google Scholar]
- 27.Yang, F., J. He, X. Lin, Q. Li, D. Pan, X. Zhang, and X. Xu. 2001. Complete genome sequence of the shrimp white spot bacilliform virus. J. Virol. 75:11811-11820. [DOI] [PMC free article] [PubMed] [Google Scholar]