Abstract
Background
Interactions of endothelial progenitor cells (EPCs) with vascular and blood cells contribute to vascular homeostasis. Although platelets promote the homing of EPCs to sites of vascular injury and their differentiation into endothelial cells, the functional consequences of such interactions on platelets remain unknown. Herein, we addressed the interactions between EPCs and platelets and their impact on platelet function and thrombus formation.
Methods and Results
Cultured on fibronectin in conditioned media, human peripheral blood mononuclear cells differentiated, within 10 days of culture, into EPCs, which uptake acetylated low-density lipoprotein, bind ulex-lectin, lack monocyte/leukocyte markers (CD14, P-selectin glycoprotein ligand-1, L-selectin), express progenitor/endothelial markers (CD34, vascular endothelial growth factor receptor-2, von Willebrand factor, and vascular endothelial cadherin), and proliferate in culture. These EPCs bound activated platelets via CD62P and inhibited its translocation, glycoprotein IIb/IIIa activation, aggregation, and adhesion to collagen, mainly via prostacyclin secretion. Indeed, this was associated with upregulation of cyclooxygenase-2 and inducible nitric oxide synthase. However, the effects on platelets in vitro were reversed by cyclooxygenase and cyclooxygenase-2 inhibition but not by nitric oxide or inducible nitric oxide synthase inhibition. Moreover, in a ferric chloride–induced murine arterial thrombosis model, injection of EPCs led to their incorporation into sites of injury and impaired thrombus formation, leading to an incomplete occlusion with 50% residual flow.
Conclusions
Peripheral blood mononuclear cell– derived EPCs bind platelets via CD62P and inhibit platelet activation, aggregation, adhesion to collagen, and thrombus formation, predominantly via upregulation of cyclooxygenase-2 and secretion of prostacyclin. These findings add new insights into the biology of EPCs and define their potential roles in regulating platelet function and thrombosis.
Keywords: progenitor cells, nitric oxide, platelets, prostaglandins, thrombosis
Endothelial progenitor cells (EPCs) are believed to contribute to vascular biology and hemostasis. Although EPCs have been localized in cord blood, bone marrow, and peripheral blood as well as in some regenerative tissues, controversy relative to their isolation, identification, and functionality still exists. Indeed, the term EPC has been employed to describe different populations of multipotent cells that have the capability to differentiate, depending on the environmental cues, into mature endothelial cells (ECs). These cells are extremely rare in peripheral blood, but their number can be markedly increased after treatment with mobilizing cytokines or vascular trauma.1 Nevertheless, cells with progenitor and endothelial phenotypes have been generated in vitro from peripheral blood mononuclear cells (PBMCs)2–10 and have shown potential therapeutic applications in vascular tissue engineering and cell-based therapy.5,11–14 The use of ex vivo expanded EPCs in animal and clinical studies has shown beneficial effects in reendothelialization and neovas-cularization, as well as in the prevention of hybrid graft thrombosis and rejection11,12,15 and late stent thrombosis.14,16 However, the mechanisms that regulate mobilization, migration, and differentiation of EPCs and their homing to sites of vascular injury are complex and involve several mediators and receptors, such as P-selectin glycoprotein ligand-1 (PSGL-1), α4 integrin, CXC chemokine receptor-2 and -4, and β1- and β2-integrins.17–20 Furthermore, it has been shown that the interaction of platelets with EPCs influences their chemotaxis, adhesion, activation, and differentiation into mature ECs during vascular repair.17,19,21–24 Collectively, these studies suggest the existence of a cross talk between EPCs and platelets in the regulation of each other’s function.
Platelet activation and adhesion to damaged blood vessels represent the first step in atherothrombosis.25 At sites of vascular injury, platelets roll and interact with various components of the subendothelial matrix via a number of adhesive receptors expressed on the platelet surface. This, in turn, induces signaling that leads to platelet aggregation through glycoprotein (GP) IIb/IIIa (αIIbβ3 integrin) activation and binding to fibrinogen.26 Platelet activation is accompanied by the translocation of P-selectin (CD62P), which contributes to the recruitment of other blood leukocytes.27 In this regard, CD62P has also been involved in the interaction of platelets with EPCs.17,21,22
Although platelets promote the homing of EPCs at sites of vascular injury and favor their differentiation into ECs, the functional consequences of such interactions on platelets remain unknown. In fact, EPCs secrete many vasoactive and angiogenic factors that may modulate vascular thrombosis and hemostasis. Accordingly, we hypothesized that they might influence platelet function. We therefore designed the present study to determine the impact and the mechanisms of action of EPCs on platelet function in vitro and in vivo. We found that human PBMC-derived EPCs bound platelets via CD62P and inhibited platelet activation, aggregation, and adhesion to collagen in vitro and thrombus formation in vivo, predominantly via upregulation of cyclooxygenase-2 (COX-2) and secretion of prostacyclin (prostaglandin I2 [PGI2]).
Methods
Methods are available in the online-only Data Supplement. This study has been approved by the human and animal ethical committees of the Montreal Heart Institute. All human subjects were healthy volunteers of either sex aged from 20 to 55 years. They gave informed consent and were free from any drugs that interfere with platelet function.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Characterization of PBMC-Derived EPCs
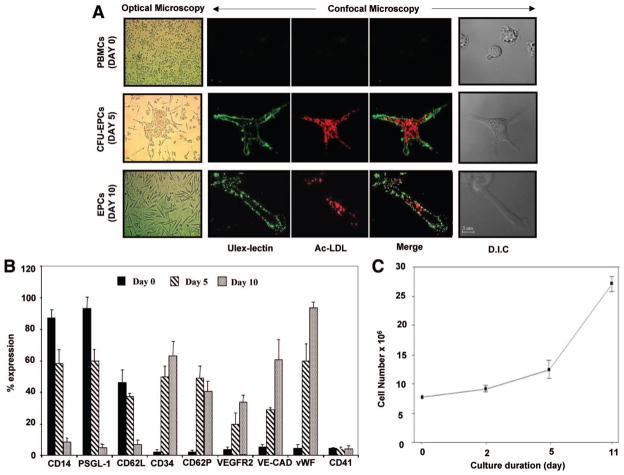
We first characterized the differentiation of PBMCs into EPCs according to their time-dependent appearance in culture, morphology, surface markers, proliferation rate, and functional properties. As shown in Figure 1A, human PBMCs differentiated after 5 days of culture into an adherent population characterized by a central cluster of round cells with few sprouts of elongated cells at the periphery defined as a colony-forming unit–EPC (CFU-EPC). The number of CFU-EPCs that derive from 5×106 PBMCs averaged 8±4 colonies per well. After 10 days, the cells formed a monolayer of spindle-shaped flat cells. Cells at both 5 and 10 days of culture were positive for acetylated low-density lipoprotein uptake and ulex-lectin binding (Figure 1A). To gain more insights into the nature of these ex vivo expanded cells, we used flow cytometry to quantify the expression of typical markers on the surface of cells during their differentiation. As shown in Figure 1B, freshly isolated PBMCs expressed predominantly the monocyte/leukocyte markers CD14 (87%), PSGL-1 or CD162 (93%), and L-selectin or CD62L (46%), whereas the progenitor and endothelial markers were missing. At day 5, the cells started to express progenitor/endothelial markers, whereas leukocyte markers were downregulated. After 10 days of culture, the progenitor/endothelial markers were highly expressed (63% CD34, 40% P-selectin, 33% vascular endothelial growth factor receptor-2 [VEGFR2], 85% vascular endothelial cadherin [VE-cadherin], and 97% von Willebrand factor), whereas the monocyte/leukocyte markers CD14, PSGL-1, and L-selectin were absent. The platelet marker CD41 was absent in all cell populations, indicating that these cells were devoid of platelet contamination. Moreover, cells after 10 days of culture were found to be highly proliferative, as shown in Figure 1C.
Figure 1.
Characteristics of cultured cells. A, Immediately after plating, optical microscopy observation showed that freshly isolated PBMCs with rounded morphology and irregular size appear either as single cells or as small clumps. At day 5, colonies are visible and scored as CFU-EPCs because they consist of a central core of round cells and elongated sprouting cells at the periphery. At day 10, cells form a flat monolayer of spindle-shaped cells characteristic of EPCs in culture (magnification ×20). Staining of freshly isolated PBMCs and 5- and 10- day cultured cells with ulex-lectin (green) and acetylated low-density lipoprotein (Ac-LDL) (red) under a laser-scanning confocal microscope is shown. Doubly stained cells appear after 5 days and remain positive up to 10 days of culture (bar=5 μm). D.I.C. indicates differential interference contrast. B, Histogram showing the expression of different cell surface markers as determined by flow cytometry. Single-color immunostaining of cultured cells was performed on day 0 (PBMCs) and days 5 and 10 with saturating concentrations of mouse anti-human PE-conjugated monoclonal antibodies directed against different mononuclear/leukocyte (CD14, PSGL-1, CD62L), progenitor (CD34, VEGFR2, CD62P), endothelial (VE-cadherin [VE-CAD], von Willebrand factor [vWF]), and platelet (CD41) cell surface markers (n=3 to 6 independent experiments). C, Growth capacity of PBMC-derived EPCs after 10 days of culture. On passaging, 10-day EPCs showed high proliferative potential (n=3).
EPCs Bind Platelets
The adhesive interactions between platelets and EPCs were determined by assessing the number of activated platelets that bound to 100 cells. As shown in Figure 2A, the binding averaged 157 platelets per 100 cells for freshly isolated PBMCs and decreased to 54 for EPCs at day 10 of culture. Inhibition of P-selectin significantly reduced platelet binding by 66% for PBMCs and 50% for EPCs, whereas the blockade of PSGL-1 was only effective in preventing the binding of platelets to PBMCs but not to EPCs. As control, the binding of platelets to PBMCs or EPCs was not affected by L-selectin blockade.
Figure 2.
EPCs bind and inhibit platelet activation. A, Bar graphs show the number of activated platelets per 100 cells. The binding of platelets to PBMCs and EPCs, in mixed cell populations, was determined with the use of a dual-labeling technique with saturating concentrations of monoclonal antibodies directed against platelet-CD41 (FITC) and PBMC-CD14 (PE) or EPC-CD34 (PE). The binding was assessed with and without blocking monoclonal antibodies directed against P-selectin (AK6, 10 μg/mL), PSGL-1 (KPL-1, 10 μg/mL), and L-selectin (Dreg-56, 10 μg/mL) for 5 minutes at 37°C. The fluorescence threshold was set to analyze only dual FITC-and PE-labeled cells, corresponding to CD14+ or CD34+ cells that exhibit platelet-CD41 fluorescence from 5000 events (n=6 to 7 independent experiments; *P<0.05, **P<0.01 vs control). Lower panels show flow cytometry plots from representative experiments illustrating platelet CD62P translocation (B) and GPIIb/IIIa activation (C) at baseline and after thrombin activation of platelets in the presence of the supernatant from PBMC or EPC populations. MFI indicates mean fluorescent intensity. D, Mean data and SEM for the mean fluorescent intensity of 3 to 9 independent experiments are presented. **P<0.01 vs control.
EPCs Inhibit Platelet Activation and Aggregation
We then investigated the impact of EPCs on thrombin-induced platelet activation by assessing the translocation of CD62P and activation of GPIIb/IIIa by flow cytometry. As shown in Figure 2B through 2D, platelet activation was completely inhibited in the presence of the supernatant of EPCs, as revealed by the absence of CD62P translocation and GPIIb/IIIa activation, whereas the supernatant of PBMCs had no significant effect on platelet activation.
Given that EPCs impair platelet activation, we sought to examine their functional impact on platelet aggregation in response to 2 physiological agonists, thrombin and collagen (Figure 3). Platelet aggregation was not affected by PBMCs but was significantly decreased by washed EPCs in response to both agonists (Figure 3A, 3C, 3D, and 3F). Moreover, platelet aggregation was completely inhibited by the supernatant of EPCs in response to both platelet agonists, whereas the supernatant of PBMCs had no significant effect (Figure 3B through 3E). Thus, the predominant effects of EPCs seem to be related to the release of platelet inhibitory factors that downregulate platelet activation and aggregation.
Figure 3.
Effect of EPCs on platelet aggregation. Representative tracings of thrombin-induced (A and B) and collagen-induced (D and E) platelet aggregation with or without PBMCs, EPCs, or their supernatants. Platelets alone (250×106/mL) or in the presence of 5×106/mL PBMCs, EPCs, or their supernatants were coincubated in a 4-channel lumiaggregometer under shear conditions (1000 rpm) at 37°C. Platelet aggregation was initiated by adding thrombin or collagen and then monitored for 5 minutes. C and F represent the mean data and SEM of 4 to 8 independent experiments, summarizing the effects of the different cell populations on thrombin- and collagen-induced platelet aggregation, respectively. *P<0.05, **P<0.01 vs control.
EPCs Inhibit Platelet Function via PGI2 Secretion
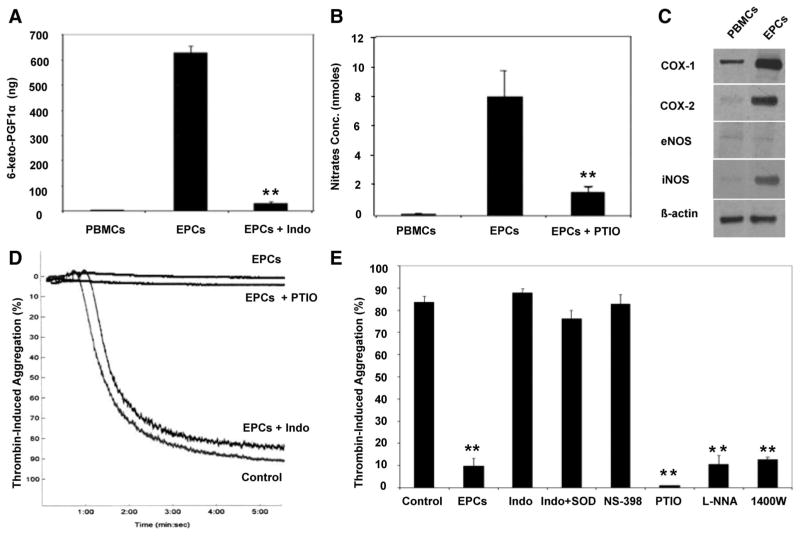
To reveal the factor(s) responsible for the inhibitory effects of EPCs on platelet activation and aggregation, we preincubated differentiating cells with COX and nitric oxide (NO) inhibitors because prostaglandins and NO constitute potent modulators of platelet function. These treatments did not affect the differentiation of PBMCs into EPCs and did not alter their proliferative capacities (data not shown). The culture supernatant from treated cells was collected and assessed for PGI2 and NO secretion and tested on platelet aggregation. As shown in Figure 4A and 4B, EPCs released high levels of PGI2 and NO. In contrast to EPCs, the amount of PGI2 and NO released by PBMCs was negligible. Moreover, the release of PGI2 and NO was totally prevented when differentiated EPCs were cultured in the presence of indomethacin (a COX inhibitor) or 2-phenyl-4,4,5,5,-tetramethylimidazoline-1-oxyl 3-oxide (PTIO) (a NO chelator), respectively. To further validate the release of PGI2 and NO from EPCs, we assessed the expression of the constitutive and inducible forms of COX (COX-1 and COX-2) and NO synthase (NOS) (endothelial NOS [eNOS] and inducible NOS [iNOS] isoforms) in cultured EPCs, in comparison to PBMCs, by Western blot. As shown in Figure 4C, COX-1 was detectable in freshly isolated PBMCs (day 0) and increased in EPCs at day 10. However, COX-2 was specifically upregulated after the differentiation of PBMCs into EPCs. Similarly, iNOS was poorly expressed in PBMCs and profoundly increased in EPCs, whereas eNOS was barely detectable in both cell populations. Interestingly, the antiaggregatory or inhibitory effect of EPCs on platelets was reversed by incubating cultured EPCs with indomethacin (even in the presence of superoxide dismutase) or NS-398 (a COX-2 inhibitor) but not with PTIO, NG-nitro-L-arginine (a NOS inhibitor), or 1400W (an iNOS inhibitor) (Figure 4D and 4E).
Figure 4.
EPCs secrete PGI2 and NO and upregulate COX-2 and iNOS. Release of PGI2 (A) and NO (B) from PBMCs and EPCs pre-treated or not with indomethacin (Indo) (a COX inhibitor) or PTIO (a NO chelator) was assessed in the supernatants by radioimmunoas-say and nitrate/nitrite fluorometric assay, respectively (n=3; **P<0.01 vs EPCs). C, Expression of COX and NOS isoforms in PBMC and EPC populations by Western blot. β-Actin was used as an internal control. The blots shown are representative of 3 independent experiments. D, Representative tracings of thrombin-induced platelet aggregation with the supernatant of EPCs pretreated or not with indomethacin or PTIO, showing the reversible effect of indomethacin, but not PTIO, on EPC-induced platelet aggregation inhibition. E, The mean data of thrombin-induced platelet aggregation are presented. The antiaggregatory or inhibitory effect of EPCs on platelets was reversed by incubating cultured EPCs with indomethacin (even in the presence of superoxide dismutase [SOD]) or NS-398 (a COX-2 inhibitor) but not with PTIO, NG-nitro-L-arginine (L-NNA, a NOS inhibitor), or 1400W (an iNOS inhibitor) (n=3 to 6; **P<0.01 vs control).
Taken together, these data indicate that, although EPCs specifically upregulate the expression of COX-2 and iNOS and release PGI2 and NO, their main effect on platelet activation and aggregation appears to be PGI2 dependent.
EPCs Reduce Platelet Adhesion Under Flow
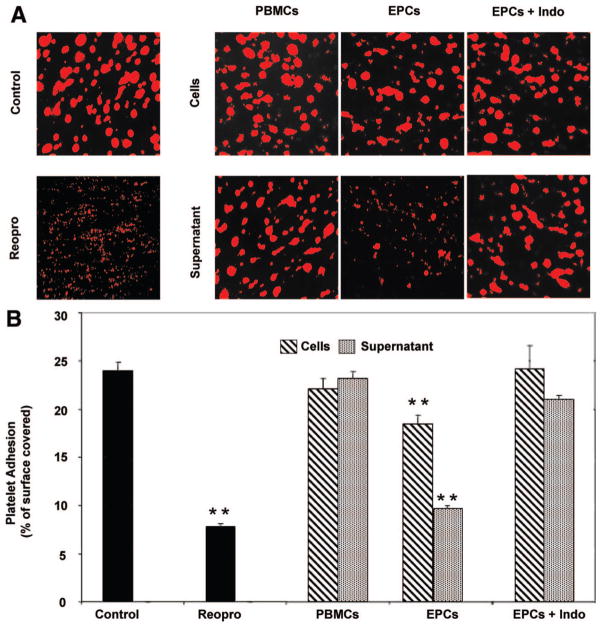
Because EPCs inhibit activation and aggregation of washed platelets, we then assessed their impact on platelet adhesion to collagen in a whole blood perfusion system. As shown in Figure 5, perfusion of whole blood resulted in almost 25% coverage of the collagen surface with platelets, which was reduced to 7% by a GPIIb/IIIa antagonist, Reopro (positive control). Coincubation of blood with PBMCs or their respective supernatant had no significant effect on platelet adhesion. In contrast, the presence of EPCs or their supernatant reduced significantly platelet adhesion by 23% and 53%, respectively. The effects of EPCs and their supernatant were reversed, once again, by pretreating EPCs with the COX inhibitor indomethacin. Hence, the effects of EPCs on activation and aggregation of isolated platelets were also highlighted in a more physiological approach of platelet adhesion under flow in whole blood.
Figure 5.
EPCs reduce platelet adhesion to collagen under flow. A, Representative fluorescent photomicrographs of platelet adhesion in whole blood on collagen-coated surfaces in the presence of PBMCs, EPCs, or their supernatants preincubated or not with indomethacin (Indo). Blood samples pre-treated with culture media or with an anti-GPIIb/IIIa antagonist (Reopro) were used as controls. Glass capillaries were coated overnight at 4°C with collagen. Two milliliters of sodium citrate anticoagulated blood was mixed with 200 μL of cells (500×103) or their supernatant followed by incubation with rhodamine for 15 minutes at 37°C. Stained samples were then perfused simultaneously at a shear rate of 300/s. B, The mean data of platelet adhesion presented as the percentage of capillary surface covered by platelets (n=3 to 6; **P<0.01 vs control).
EPCs Incorporate at the Sites of Mouse Carotid Injury and Inhibit Thrombus Formation
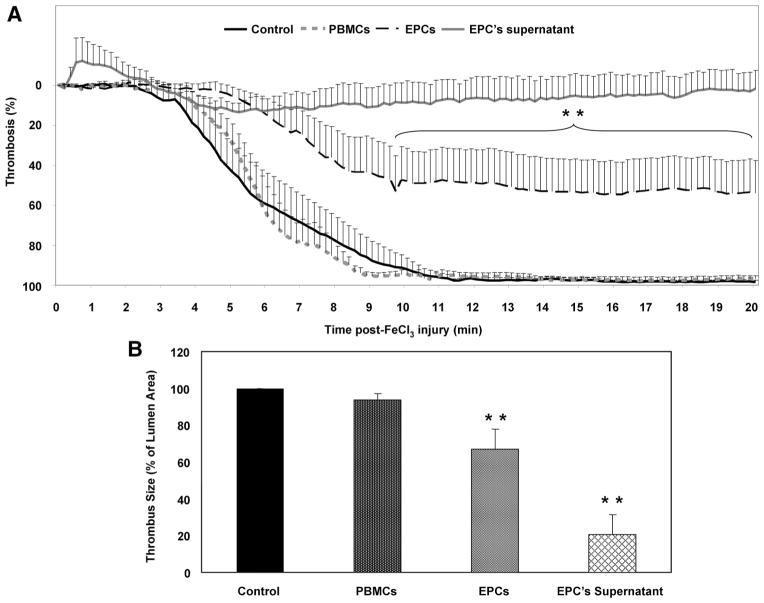
Having shown the impact of EPCs on activation and aggregation of washed platelets and on adhesion in whole blood in vitro, we hypothesized that they may impair platelet function in vivo. Therefore, we examined the effect of EPCs on thrombus formation by injecting freshly isolated PBMCs and cultured EPCs or their supernatant intravenously into mice 15 minutes before FeCl3-induced carotid arterial injury. As shown in Figure 6A, mice that received PBMCs had blood flows similar to those of control mice, which were characterized by complete occlusion of the arteries ≈10 minutes after FeCl3-induced injury. In contrast, injection of EPCs or their supernatant significantly impaired thrombus formation, leading to an incomplete occlusion with 50% and 100% residual flow, respectively.
Figure 6.
EPCs reduce thrombus formation in vivo. A, PBMCs and culture-derived EPCs (500×103 cells) or their supernatant were injected 15 minutes before FeCl3-induced injury of mouse carotid arteries, and thrombus formation was monitored by residual blood flow measurements. Control experiments were done with culture media alone. After a stabilization period and application of FeCl3 for 3 minutes, blood flow was monitored continuously for 20 minutes after FeCl3 injury. Tracings represent the mean data of 8 mice in the control and EPCs groups, 6 in the PBMCs, and 3 in the supernatant of the EPC group (**P<0.01 vs control and PBMCs). B, Bar graphs show the mean data of thrombus size measured on cross-sectional area of arterial thrombi and expressed as percentage of lumen area. ** P<0.01 vs control and PBMCs.
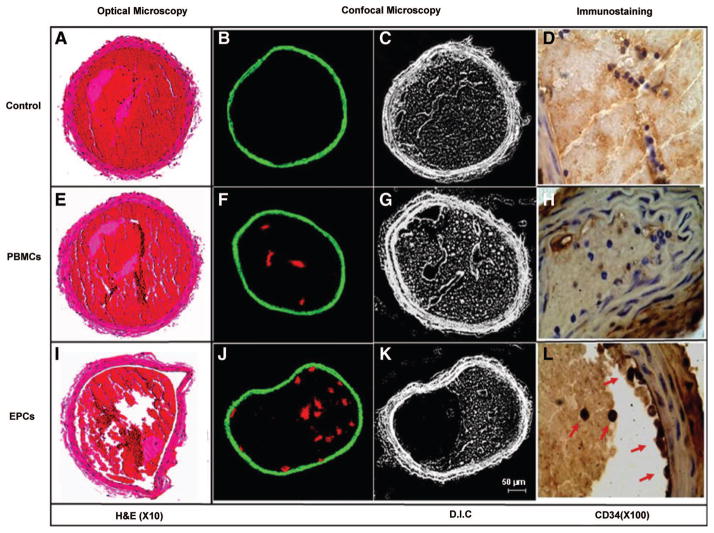
To further analyze the characteristics of the formed thrombi, the arteries were fixed immediately after blood flow measurements and were subjected to fluorescence confocal microscopy and histological and immunohistochemical analyses. The circumference of the arteries and thrombi was measured by computer-assisted planimetry, and the thrombus size was reported as percentage of total lumen area. Compared with control- and PBMC-treated arteries, which showed complete occlusion (Figure 6B and Figure 7A and 7E), the arterial thrombus formed in EPC-treated mice was partially occlusive (Figure 7I) and was significantly reduced by 80% and 33% by the supernatant of EPCs and EPCs, respectively (Figure 6B). In another set of experiments, PBMCs and EPCs were labeled with a fluorescent cell tracker before their intravenous injection and were identified in the luminal aspect of the thrombi as a cluster of red fluorescent clumps (Figure 7F and 7J). Injured arteries from control mice, used as negative control, were devoid from any staining (Figure 7B). This was also revealed in sections of injured arteries immunostained with an anti-CD34 antibody (Figure 7D, 7H, 7L). In control- and PBMC-treated mice, the injured carotids were negative for CD34+ cells. In contrast, focal accumulation of CD34+ cells, representing 10±2 cells per thrombus cross-sectional area, was observed within the thrombus and along the vascular wall in the carotids of EPC-treated mice.
Figure 7.
EPCs are recruited into arterial thrombi in vivo and reduce thrombus formation. Representative histological transverse sections of FeCl3-injured mouse carotid arteries, stained with hematoxylin-eosin (H&E) and observed by optical microscopy, are shown (left). Arterial thrombus was completely occlusive in control- and PBMC-treated mice (A and E), whereas it was partially occlusive in arteries from EPC-treated mice (I) (magnification ×10). Middle panels show confocal imaging of mouse carotid arteries after vascular injury. PBMCs and EPCs were labeled with intracellular fluorescence marker (DiL), injected before arterial injury, and assessed by confocal fluorescence on cryostat cross sections (F and J). Injured arteries from untreated mice were used as negative control (B). The corresponding differential interference contrast (D.I.C.) of the identical sections is shown on the right (C, G, K) (bar=50 μm). Right panels show CD34 immunostaining of the injured arteries. CD34-positive cells appeared only in the EPC-treated mice, confirming the recruitment of EPCs to the luminal aspect of arterial thrombi in vivo (arrows in L). The cells in D and H are CD34 negative (magnification ×100).
Discussion
The present study provides novel evidence for the regulation of platelet function by EPCs. We found (1) that human PBMC-derived EPCs bound platelets via CD62P and (2) that PBMC-derived EPCs inhibited platelet activation, aggregation, and adhesion to collagen in vitro and thrombus formation in vivo, predominantly via upregulation of COX-2 and secretion of PGI2. This study reveals a new biological role for EPCs in controlling platelet function, which may in turn limit thrombogenesis and maintain hemostasis at the sites of vascular injury.
It is well established that mobilization and recruitment of EPCs from bone marrow contribute to cell-based therapy and vascular repair. Thus far, therapeutic application of EPCs is still limited by the paucity of available cells. The “ex vivo” outgrowth of EPCs is, therefore, very important to ensure that an adequate cell number is available for their characterization and therapeutic application. Indeed, it has been shown that peripheral blood contains a complex assortment of progenitor cells that possess the ability to differentiate into EPCs in vitro.28 –30 In this connection, we succeeded in adequately differentiating EPCs from PBMCs. The morphology of these cells changed from a population of irregular and small rounded cells to a flattened, spindle-shaped population that forms CFU-EPCs during the differentiation process, uptakes Dil–acetylated low-density lipoprotein, and binds ulex-lectin. Additionally, the expression pattern of surface markers of cultured cells changed during differentiation, as demonstrated by a decrease in pan-monocyte/leukocyte markers and an increase in progenitor/endothelial markers, as well as by their capacity to proliferate after 10 days of culture. Such a subtype of differentiated EPCs displayed some of the morphological, phenotypical, and functional characteristics of late EPCs that derived from the adherent population of PBMCs when plated on collagen or fibronectin in the presence of endothelial-specific growth media.9,10,30,31
Accumulating evidence suggests that platelets play an important role not only in hemostasis and thrombosis but also in inflammation and tissue repair through paracrine mechanisms or direct interactions with other blood cells.25,32 Recently, it has been shown that platelets promote the homing and differentiation of EPCs at sites of vascular injury.17–24 Conversely, EPCs may influence platelet function and modulate their thrombogenic properties during vascular repair. To analyze and characterize the action of EPCs on platelet function, we first showed that platelets can bind EPCs but to a lower extent than the binding to PBMCs. The decrease in binding during the differentiation process may be explained by the dynamic changes in the expression of cell surface markers, as well as by the upregulation of platelet inhibiting factors, throughout the process of PBMC differentiation into EPCs. Indeed, we observed a decrease in the expression of PSGL-1 and an increase in the secretion of PGI2 and NO, thus providing a possible explanation for the significant decrease in the binding of activated platelets to EPCs. We also showed that PBMC-derived EPCs bind activated platelets in a CD62P-dependent manner, thus confirming previous findings demonstrating that the interaction between platelets and EPCs occurs, in part, via CD62P and its high-affinity receptor PSGL-1.17,19,21–24 In addition to PSGL-1, other mediators such as stromal cell– derived factor-1α, CXC chemokine receptor-2 and -4, and β1- and β2-integrins participate in homing of EPCs at sites of vascular injury.17–20
Moreover, incubation of platelets with the releasate of EPCs resulted in a profound inhibition of platelet activation, as revealed by the inhibition of CD62P translocation and GPIIb/IIIa activation, both of which are involved in the formation and stabilization of platelet thrombus.33,34 The functional impact of EPCs on platelets was also highlighted by their inhibitory effects on thrombin- and collagen-induced aggregation of washed platelets and adhesion to collagen under flow in whole blood. The functional impact of EPCs on platelets was further depicted in a mouse arterial thrombosis model, in which injection of EPCs impaired thrombus mass by 33%, leading to an incomplete occlusion with 50% residual flow. Interestingly, the supernatant of the EPCs was more efficacious than EPCs in inhibiting thrombus formation in vivo, thus confirming the in vitro findings that the main immediate antithrombotic effect of EPCs is related to the release of platelet inhibitory factors. These antiplatelet properties may ultimately lead to the development of novel EPC-derived antithrombotic therapies.
These findings may be of important physiopathological relevance because the acute vascular response to injury involves the adhesion, activation, and aggregation of platelets, followed by the recruitment of other platelets, leukocytes, and possibly circulating EPCs. The recruitment of circulating EPCs at the site of injury may contribute (1) to attenuate the acute thrombotic response by inhibiting platelet function and (2) to promote reendothelialization and vascular repair. This may explain previous observations showing that ex vivo expanded EPCs seeded onto the lumen of a small-diameter vascular graft and implanted in vivo have a much lower chance of thrombosis and subsequent rejection compared with uncoated controls.11,12 Moreover, the use of EPC capture stents during percutaneous coronary intervention reduces the incidence of late stent thrombosis.14,16 Thus, EPCs modulate platelet function and appear to play an important role in the management of thrombotic reactions.
The fundamental role of EPCs on platelets may be facilitated primarily by the secretion of thromboresistant factors such as PGI2 and NO, which are well-known antiplatelet mediators. In fact, we have shown that PBMC-derived EPCs specifically upregulate COX-2 and iNOS during their differentiation and produce PGI2 and NO. In the present study, however, we identify PGI2 as the principal mediator involved in the inhibitory action of EPCs on platelet function. This is based on our findings that, in contrast to PBMCs, which constitutively expressed COX-1 but did not produce PGI2, EPCs specifically upregulated COX-2 and produced PGI2. Additionally, the effects of EPCs on platelet function were reversed by a pan-COX inhibitor (indomethacin) even in the presence of superoxide dismutase, which ruled out an implication of NO in this phenomenon. Indeed, the effects of EPCs were also reversed by a specific COX-2 inhibitor (NS-398), whereas NO scavenging with PTIO, NOS inhibition with NG-nitro-L-arginine, or iNOS inhibition with 1400W was without any significant effects. Thus, PGI2 most likely represents the major EPC-derived platelet inhibitor. The high levels of NO released from EPCs could be correlated with a high expression of iNOS, whereas eNOS expression was negligible in EPCs. Our findings are in accordance with a recent study showing that iNOS, but not eNOS, is detectable in peripheral blood– derived EPCs.35 The low expression of eNOS in EPCs derived from peripheral blood may be explained by the fact that they are not fully matured ECs and are preferentially prone to produce NO in a monocytic/macrophage fashion through iNOS upregulation. This may suggest that the level of NO production in EPCs via iNOS is not sufficient to affect platelet function. Recently, Marjanovic et al36 have shown that NO synthesis during platelet activation not only involves eNOS but is also mediated by the constitutively expressed platelet iNOS. iNOS-derived NO is important in promoting platelet activation, both ex vivo and in vivo, via a cGMP-dependent mechanism. Although our data point to the lack of effects of EPC-derived NO on platelet aggregation, the contribution of platelet-derived NO and its signaling pathways, which are believed to play biphasic roles in platelet function, on the effects of EPCs remained to be explored. Moreover, the role of iNOS in EPCs appears to be important in angiogenesis, perhaps by promoting the recruitment of EPCs near the damaged endothelium.37 Taken together, this may explain, in part, our observation that the inhibitory effects of EPCs on platelet function in vitro were predominantly PGI2 dependent but not NO dependent. This does not negate, however, the role of EPC-derived NO as a regulatory factor involved in the secondary phase of vascular repair, including angiogenesis.37
Our data demonstrate that EPCs regulate platelet function via upregulation of COX-2 and a PGI2-dependent inhibition of platelet activation, aggregation, adhesion, and thrombus formation. In addition to the well-documented roles of EPCs in angiogenesis and vascular repair, our findings highlight a new biological role for EPCs in regulating platelet function, which may, in turn, limit thrombogenesis and maintain hemostasis at sites of vascular injury.
CLINICAL PERSPECTIVE.
Endothelial progenitor cells (EPCs) represent a promising therapeutic approach for the treatment of cardiovascular diseases. However, comprehensive delineation of the biology of these cells and the manner in which they interact with other blood and vascular cells is critical to fully understand their potential therapeutic properties. Herein, we addressed the interactions between EPCs and platelets and their impact on platelet function and thrombus formation, which may be relevant to the management of atherothrombosis during acute coronary syndromes and after percutaneous coronary interventions. We found that human peripheral blood monocyte– derived EPCs in culture bound platelets via CD62P and inhibited platelet activation, aggregation, and adhesion to collagen in vitro, mainly via upregulation of cyclooxygenase-2 and secretion of prostacyclin. Moreover, in a murine arterial thrombosis model, injection of EPCs led to their incorporation into sites of injury and impaired thrombus formation, leading to an incomplete occlusion. In addition to the well-documented roles of EPCs in angiogenesis and vascular repair, our findings highlight a new biological role for EPCs in regulating platelet function, which may, in turn, limit thrombogenesis while maintaining hemostasis at sites of vascular injury. These antiplatelet properties may ultimately lead to the development of novel EPC-derived antithrombotic therapies.
Acknowledgments
We are grateful to M. Louis Villeneuve for his assistance with confocal microscopy.
Sources of Funding
This study was supported by grants from the Canadian Institute for Health Research (MOP-82767 to Y.M.; IAP-73374 to E.T. and Y.M.; and RMF-79023 to M.T. and Y.M.).
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.109.894642/DC1.
Disclosures
None.
References
- 1.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 4.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 5.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 7.Romagnani P, Lasagni L, Romagnani S. Peripheral blood as a source of stem cells for regenerative medicine. Expert Opin Biol Ther. 2006;6:193–202. doi: 10.1517/14712598.6.3.193. [DOI] [PubMed] [Google Scholar]
- 8.Elsheikh E, Uzunel M, He Z, Holgersson J, Nowak G, Sumitran-Holgersson S. Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood. 2005;106:2347–2355. doi: 10.1182/blood-2005-04-1407. [DOI] [PubMed] [Google Scholar]
- 9.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 10.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirota T, He H, Yasui H, Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9:127–136. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 13.Werner N, Nickenig G. Clinical and therapeutical implications of EPC biology in atherosclerosis. J Cell Mol Med. 2006;10:318–332. doi: 10.1111/j.1582-4934.2006.tb00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Co M, Tay E, Lee CH, Poh KK, Low A, Lim J, Lim IH, Lim YT, Tan HC. Use of endothelial progenitor cell capture stent (Genous Bio-Engineered R Stent) during primary percutaneous coronary intervention in acute myocardial infarction: intermediate- to long-term clinical follow-up. Am Heart J. 2008;155:128–132. doi: 10.1016/j.ahj.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Shirota T, Yasui H, Shimokawa H, Matsuda T. Fabrication of endothelial progenitor cell (EPC)-seeded intravascular stent devices and in vitro endothelialization on hybrid vascular tissue. Biomaterials. 2003;24:2295–2302. doi: 10.1016/s0142-9612(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 16.Miglionico M, Patti G, D’Ambrosio A, Di Sciascio G. Percutaneous coronary intervention utilizing a new endothelial progenitor cells antibody-coated stent: a prospective single-center registry in high-risk patients. Catheter Cardiovasc Interv. 2008;71:600– 604. doi: 10.1002/ccd.21437. [DOI] [PubMed] [Google Scholar]
- 17.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, Schulz C, Kerstan S, Rudelius M, Seidl S, Sorge F, Langer H, Peluso M, Goyal P, Vestweber D, Emambokus NR, Busch DH, Frampton J, Gawaz M. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, Urbich C, Peters T, Scharffetter-Kochanek K, Zeiher AM, Chavakis T, Dimmeler S. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, Vestweber D, Massberg S, Schonberger T, Pfisterer I, Hatzopoulos AK, Gawaz M. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res. 2006;98:e2– e10. doi: 10.1161/01.RES.0000201285.87524.9e. [DOI] [PubMed] [Google Scholar]
- 20.Hristov M, Zernecke A, Bidzhekov K, Liehn EA, Shagdarsuren E, Ludwig A, Weber C. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res. 2007;100:590–597. doi: 10.1161/01.RES.0000259043.42571.68. [DOI] [PubMed] [Google Scholar]
- 21.Lev EI, Estrov Z, Aboulfatova K, Harris D, Granada JF, Alviar C, Kleiman NS, Dong JF. Potential role of activated platelets in homing of human endothelial progenitor cells to subendothelial matrix. Thromb Haemost. 2006;96:498–504. [PubMed] [Google Scholar]
- 22.Daub K, Langer H, Seizer P, Stellos K, May AE, Goyal P, Bigalke B, Schonberger T, Geisler T, Siegel-Axel D, Oostendorp RA, Lindemann S, Gawaz M. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB J. 2006;20:2559–2561. doi: 10.1096/fj.06-6265fje. [DOI] [PubMed] [Google Scholar]
- 23.Stellos K, Gawaz M. Platelet interaction with progenitor cells: potential implications for regenerative medicine. Thromb Haemost. 2007;98:922–929. doi: 10.1160/th07-02-0147. [DOI] [PubMed] [Google Scholar]
- 24.de Boer HC, Verseyden C, Ulfman LH, Zwaginga JJ, Bot I, Biessen EA, Rabelink TJ, van Zonneveld AJ. Fibrin and activated platelets cooperatively guide stem cells to a vascular injury and promote differentiation towards an endothelial cell phenotype. Arterioscler Thromb Vasc Biol. 2006;26:1653–1659. doi: 10.1161/01.ATV.0000222982.55731.f1. [DOI] [PubMed] [Google Scholar]
- 25.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 26.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 27.Theoret JF, Bienvenu JG, Kumar A, Merhi Y. P-selectin antagonism with recombinant P-selectin glycoprotein ligand-1 (rPSGL-Ig) inhibits circulating activated platelet binding to neutrophils induced by damaged arterial surfaces. J Pharmacol Exp Ther. 2001;298:658– 664. [PubMed] [Google Scholar]
- 28.Schatteman GC, Awad O. Hemangioblasts, angioblasts, and adult endothelial cell progenitors. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:13–21. doi: 10.1002/ar.a.10131. [DOI] [PubMed] [Google Scholar]
- 29.Balbarini A, Barsotti MC, Di Stefano R, Leone A, Santoni T. Circulating endothelial progenitor cells characterization, function and relationship with cardiovascular risk factors. Curr Pharm Des. 2007;13:1699–1713. doi: 10.2174/138161207780831329. [DOI] [PubMed] [Google Scholar]
- 30.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–1149. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caron A, Theoret JF, Mousa SA, Merhi Y. Anti-platelet effects of GPIIb/IIIa and P-selectin antagonism, platelet activation, and binding to neutrophils. J Cardiovasc Pharmacol. 2002;40:296–306. doi: 10.1097/00005344-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Theoret JF, Chahrour W, Yacoub D, Merhi Y. Recombinant P-selectin glycoprotein-ligand-1 delays thrombin-induced platelet aggregation: a new role for P-selectin in early aggregation. Br J Pharmacol. 2006;148:299–305. doi: 10.1038/sj.bjp.0706734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muscari C, Gamberini C, Carboni M, Basile I, Farruggia G, Bonafe F, Giordano E, Caldarera CM, Guarnieri C. Different expression of NOS isoforms in early endothelial progenitor cells derived from peripheral and cord blood. J Cell Biochem. 2007;102:992–1001. doi: 10.1002/jcb.21338. [DOI] [PubMed] [Google Scholar]
- 36.Marjanovic JA, Stojanovic A, Brovkovych VM, Skidgel RA, Du X. Signaling-mediated functional activation of inducible nitric-oxide synthase and its role in stimulating platelet activation. J Biol Chem. 2008;283:28827–28834. doi: 10.1074/jbc.M801646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayr U, Zou Y, Zhang Z, Dietrich H, Hu Y, Xu Q. Accelerated arteriosclerosis of vein grafts in inducible NO synthase(−/−) mice is related to decreased endothelial progenitor cell repair. Circ Res. 2006;98:412– 420. doi: 10.1161/01.RES.0000201957.09227.6d. [DOI] [PubMed] [Google Scholar]